Abstract
Rac GTPase activating protein 1 (RacGAP1) can regulate cytokinesis and cell differentiation. The oncogenic role of RacGAP1 has been partially studied in gastric cancer, colorectal cancer, and breast cancer. In the present study, we endeavor to evaluate its expression and functions in epithelial ovarian cancer (EOC). We retrospectively collected the clinicopathological information of 117 patients who underwent curative surgery for EOC. Expression of RacGAP1 protein in primary tumor tissues was evaluated by immunohistochemistry, which was significantly associated with tumor pathological grade, tumor stage, and lymph node metastasis. Patients with lower RacGAP1 level had a longer survival time and lower recurrence risk. Multivariate results identified the independent prognostic role of RacGAP1 for both recurrence and survival in EOC patients. Cellular studies showed that RacGAP1 can positively regulate the activation of RhoA and Erk proteins. In addition, wound healing assay and Transwell assay found that RacGAP1 can up‐regulate the migration and invasion process of EOC cells, respectively. In all, our results not only confirmed the prognostic role of RacGAP1 for recurrence and survival in EOC patients, but also highlighted its possible potency for drug development.
Keywords: epithelial ovarian cancer, invasion, prognosis, proliferation, RacGAP1
1. INTRODUCTION
Ovarian cancer is one of the most lethal gynecological malignancies, and during the past decades its incidence has been increasing.1 More than 70% of ovarian cancer patients are diagnosed at an advanced stage as a result of its asymptomatic characteristics and lack of reliable early diagnostic detection.2 Epithelial ovarian cancer (EOC) accounts for approximately 80% of all ovarian cancers.3 Despite great achievements in the development of therapeutic methods, the 5‐year survival rate of EOC patients remains at approximately 30%.4 In fact, most EOC patients will ultimately develop disease recurrence.5 EOC originates from malignant transformation of the ovarian surface epithelium as a result of an accumulation of genetic changes.6 Therefore, understanding the molecular mechanisms of EOC progression and searching for novel biomarkers is critical for identifying early‐stage patients, providing novel drug targets, and improving patients’ clinical outcomes.
Rho family small GTPases are intracellular signaling molecules belonging to the Ras superfamily, including RhoA, Rac1, and Cdc42.7 When GTP‐bound, Rho‐GTPases are active and can regulate cellular responses such as cytokinesis, the cell cycle, survival and migration.8, 9 Therefore, Rho‐GTPases have been implicated in tumorigenesis.10 As GTP is necessary for the activity of Rho‐GTPases, factors regulating GTP/GDP exchange are important in controlling the functions of Rho‐GTPases. Thus, the GTPase‐activating proteins (RhoGAP), which stimulate the intrinsic GTPase activity of Rho proteins and return them to an inactive GDP‐bound state,11 are generally presumed to inhibit tumorigenesis.12 Intriguingly, one of the specific RhoGAP, RacGAP1 (also named MgcRacGAP) that can inactivate Rac and Cdc42 proteins in male germ cells13 was reported to drive tumor growth.
RacGAP1 is essential for cytokinesis,14 and knockout of RACGAP1 results in the formation of multinucleated cells and failure of cytokinesis.15, 16 In addition, RacGAP1 was found to be involved in the growth and differentiation of hematopoietic cells.17 These observations have subsequently attracted attention from oncologists and researchers for its potential role in malignancies.18 Later studies identified the up‐regulation of RACGAP1 in bladder cancer,19 gastric cancer,20 colorectal cancer21 and uterine carcinosarcoma,22 implying its possible role in promoting tumor progression. Moreover, the expression level of RacGAP1 was reported to be correlated with the early recurrence of hepatocellular carcinoma.23
However, the expression and functions of RacGAP1 in EOC have not been investigated. In the present study, we tested its protein expression level in clinical EOC tissues and verified its association with overall survival time and disease recurrence. Statistical analyses showed its independent predictive role in the clinical outcomes of EOC patients. Moreover, we conducted cellular studies and showed that RacGAP1 can positively regulate activation of RhoA and Erk proteins, which may subsequently enhance the migration and invasion capacities of EOC cells.
2. MATERIALS AND METHODS
2.1. Patients and tissue samples
A total of 117 human epithelial ovarian tumor tissue samples which were selected randomly from patients undergoing surgery between January 2008 to January 2015 in the Affiliated Hospital of North Sichuan Medical College were used for this study. None of the patients enrolled in our study received any preoperative chemotherapy. All tissues were routinely fixed in 10% formalin and embedded in paraffin. Pathological parameters, including age, histological type, pathological grade, tumor location, lymph node metastasis, International Federation of Gynecology and Obstetrics (FIGO) stage, status of ascetic fluid, serum CA‐125 level, p53 mutation, disease occurrence and survival data, were retrospectively collected for all the 117 EOC cases.
2.2. Immunohistochemical (IHC) staining and IHC evaluation
IHC staining was carried out as described previously.24 Briefly, the paraffin blocks were cut into 4‐μm sections, deparaffinized and then heated in antigen retrieval solution (sodium citrate, pH 6.0) in a microwave for 15 minutes, followed by incubation in H2O2 for another 10 minutes. The slide sections were further incubated with primary rabbit anti‐RacGAP1 antibody (ab180802; Abcam, Cambridge, UK) at 4°C overnight. As a negative control, slide sections were incubated with non‐immune serum immunoglobulin instead of primary antibody. Immunoperoxidase staining was finally carried out using EliVision™ plus Kit (Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer's instructions.
Expression level of RacGAP1 was evaluated by integrating the proportion of positive tumor cells and the intensity of positive staining. Proportion of positive tumor cells was defined as 0 (<10% positive cells), 1 (11%‐25% positive cells), 2 (26%‐50% positive cells), 3 (51%‐75% positive cells), and 4 (76%‐100% positive cells). Staining intensity was recorded as 0 (negative staining), 1 (lightly yellow), 2 (deep yellow), and 3 (yellow brown). An immunostaining index (ISI) for each stain was calculated by multiplying the scores of proportion and intensity, ranging from 0 to 12. Median ISI, 6.0, was set as the cut‐off to distinguish RacGAP1 into high or low expression. IHC staining of p‐Erk protein (Phospho‐Thr202/Tyr204, Cat. 4370; Cell Signaling Technology, Danvers, MA, USA) was also carried out and assessed with the same criteria.
2.3. Cell culture and transfection
Human EOC cell lines Caov‐3 and OV‐90 cells were purchased from ATCC (Manassas, VA, USA). OV‐90 cells were cultured in DMEM‐F12 medium, whereas Caov‐3 cells were cultured in DMEM medium, both supplemented with 10% FBS and antibiotics (100 U penicillin G, 100 μg/mL streptomycin sulfate). All cells were maintained at 37°C in 5% CO2 atmosphere.
The plasmid constructs that express RacGAP1 (pcDNA‐RacGAP1), and the negative control vector plasmid (pcDNA) were purchased from GenScript (Nanjing, China). siRNA that targeted RacGAP1 and a scrambled negative control were purchased from GenePharma (Shanghai, China) with the following sequences:
RacGAP1 siRNA #1: 5′‐GGU CUG ACU GAG ACA GGC CUG UAU A‐3′; RacGAP1 siRNA #2: 5′‐GCC AAG AAC UGA GAC AGA CAG UGU G‐3′; RacGAP1 siRNA #3: 5′‐CAA CUA AGC GAG GAG CAA A‐3′;21 Scrambled control siRNA: 5′‐UUC UCC GAA CGU GUA CGU‐3′.
Cells were cultured 24 hours prior to transfection. Then, the cells were transiently transfected with corresponding siRNA or pcDNA using Lipofectamine 2000 Transfection Reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's instructions. After 48 hours, cells transfected with siRNA or pcDNA were harvested for Western blotting to test transfection efficiency. The oligo with highest silencing efficiency was selected for further experiment.
2.4. Western blotting
Total cell lysates were obtained by homogenization in RIPA lysis buffer (50 mmol/L Tris HCl, 150 mmol/L NaCl, 1% NP‐40, 0.5% sodium deoxycholate, 1 mmol/L EDTA, 0.1% SDS) supplemented with 1 mmol/L Na3VO4, 5 mmol/L NaF, 50 μg/mL aprotinin and leupeptin. The suspension was centrifuged at 13 800 g for 10 minutes to discard the precipitate. Protein concentrations were determined by Bradford assay (Bio‐Rad, Hercules, CA, USA), and 20 μg total protein was resolved by 10% SDS‐PAGE for immunoblotting analysis. Electrotransfer was conducted to transfer proteins onto PVDF membranes, followed by blocking and probing with the indicated primary antibodies at 4°C overnight. After incubation with the HRP‐conjugated secondary antibodies (Santa Cruz Biotechnologies, Santa Cruz, CA, USA), immunoreactivity was detected by enhanced chemiluminescence (Thermo Fisher Scientific).
2.5. Migration and invasion assays
Migration ability of EOC cells was evaluated by using a wound healing assay as previously described with minor modifications.25 Transfected cells were plated in 6‐well plates and left to grow until 100% confluence. Confluent cells were maintained under the same culture conditions for another 24 hours to induce density arrest and minimize cellular proliferation. A linear wound was created in the confluent monolayer using a 10‐μL pipette tip. After washing to remove the floating cells and replacement with fresh cell culture medium, cells were cultured in the same condition. Wound diameters were recorded immediately (time 0) and thereafter at 24 and 48 hours, respectively. Each experiment was carried out in triplicate.
Cell invasion was assessed using 24‐well Transwell chambers (Corning, NY, USA) with a pore size of 8 μm (Kurabo, Osaka, Japan). Transwell upper chambers were pre‐coated with solubilized Matrigel (BD Biosciences, San Jose, CA, USA). EOC cells were pre‐starved in DMEM containing 0.5% FBS for 24 hours, then the cells (2.0 × 105 per chamber) were seeded in the upper well of Transwell chambers and incubated for 24 hours at 37°C in 5% CO2. The invaded cells in the lower surface of the filter were fixed and stained with Giemsa staining solution, and counted under a microscope. Cells from at least five fields were counted for each chamber. Each experiment was carried out in triplicate.
2.6. Statistical analysis
Associations of RacGAP1 expression with histopathological features were evaluated by c 2‐test. Kaplan‐Meier method was used to display survival data, and Cox regression model was used to identify the independent variables. All experimental data from three independent experiments were analyzed by χ2‐test or Student's t test and results were expressed as mean ± standard deviation. P‐value <.05 were considered statistically significant. All statistical tests were conducted by Prism 5 software (GraphPad) and SPSS 19.0 software (SPSS Inc., Chicago, IL, USA).
2.7. Ethics
This research complied with the Helsinki Declaration and was approved by the Ethics Committee of North Sichuan Medical College. Written informed consents were obtained from each patient or immediate family members.
3. RESULTS
3.1. Patient information
In the present study, we recruited 117 EOC samples for evaluation of RacGAP1 protein expression. Clinicopathological characteristics of the patients are shown in Table 1. Briefly, mean age of the patients at surgery was 61 years (ranging from 43 to 77 years). Eighty‐six patients (73.5%) were diagnosed with serous carcinoma as the major histological type, followed by mucinous carcinoma (19 cases, 16.2%), endometrioid adenocarcinoma (9 cases, 7.7%), and clear cell carcinoma (3 cases, 2.6%). Most of the patients had moderate (64 cases, 54.7%) or poor (49 cases, 41.9%) differentiation. Sixty‐two patients (53.0%) had lymph node‐metastasized disease and 59 patients (50.4%) had FIGO stage IV at the time of diagnosis. Seventy‐four patients (63.2%) had disease relapse and 75 patients (64.1%) died by the end of follow up (October 2016). Mean and median overall survivals (OS) were 50.0 and 60.0 months, respectively. Mean and median disease‐free survivals (DFS) were 46.0 and 56.0 months, respectively. Five‐year OS and 5‐year DFS were 49.36% and 42.41%, respectively.
Table 1.
Clinicopathological characteristics of EOC patients
| Variable | Cases (n = 117) | RacGAP1 expression | P‐value χ2‐test | |
|---|---|---|---|---|
| Low (n = 49) (%) | High (n = 68) (%) | |||
| Age (y) | ||||
| ≤61 | 66 | 30 (45.5) | 36 (54.5) | .373 |
| >61 | 51 | 19 (37.3) | 32 (62.7) | |
| Histological type | ||||
| Serous adenocarcinoma | 86 | 35 (40.7) | 51 (59.3) | .726 |
| Endometrioid adenocarcinoma | 9 | 3 (33.3) | 6 (66.7) | |
| Mucinous adenocarcinoma | 19 | 10 (52.6) | 9 (47.4) | |
| Clear cell carcinoma | 3 | 1 (33.3) | 2 (66.7) | |
| Pathological grade | ||||
| Well | 4 | 3 (75.0) | 1 (25.0) | .029a |
| Moderate | 64 | 32 (50.0) | 32 (50.0) | |
| Poor | 49 | 14 (28.6) | 35 (71.4) | |
| Tumor location | ||||
| Unilateral | 78 | 34 (43.6) | 44 (56.4) | .596 |
| Bilateral | 39 | 15 (38.5) | 24 (61.5) | |
| Lymph node metastasis | ||||
| Negative | 55 | 32 (58.2) | 23 (41.8) | .001a |
| Positive | 62 | 17 (27.4) | 45 (72.6) | |
| FIGO stage | ||||
| I/II | 44 | 28 (63.6) | 16 (36.4) | <.001a |
| III | 14 | 2 (14.3) | 12 (85.7) | |
| IV | 59 | 19 (32.2) | 40 (67.8) | |
| Tumor cells in ascetic fluid | ||||
| Absent | 69 | 35 (50.7) | 34 (49.3) | .020a |
| Present | 48 | 14 (29.2) | 34 (70.8) | |
| Serum CA‐125 level (U/mL) | ||||
| ≤800 | 67 | 32 (47.8) | 35 (52.2) | .136 |
| >800 | 50 | 17 (34.0) | 33 (66.0) | |
| p53 mutation | ||||
| Negative | 67 | 30 (44.8) | 37 (55.2) | .462 |
| Positive | 50 | 19 (38.0) | 31 (62.0) | |
| Recurrence | ||||
| Absent | 43 | 21 (48.8) | 22 (51.2) | .245 |
| Present | 74 | 28 (37.8) | 46 (62.2) | |
| Vital status | ||||
| Alive | 42 | 24 (57.1) | 18 (42.9) | .012a |
| Dead | 75 | 25 (33.3) | 50 (66.7) | |
P < .05.
EOC, epithelial ovarian cancer; FIGO, International Federation of Gynecology and Obstetrics; RacGAP1, Rac GTPase‐activating protein 1.
3.2. RacGAP1 expression and its correlation with clinical characteristics
RacGAP1 is mainly located in the cell nucleus, with slight expression in the cytoplasm (Figure 1A,B). Using the classification method described before, RacGAP1 was defined as low expression in 41.9% (49/117) of cases and high expression in 58.1% (68/117) of cases, respectively. Associations between IHC staining and clinicopathological characteristics are shown in Table 1. High expression of RacGAP1 was significantly correlated with poor differentiation (P = .029, Figure 1C), positive lymph node metastasis (P = .001, Figure 1D), advanced FIGO stage (P < .001, Figure 1E) and existence of tumor cells in ascetic fluid (P = .020, Figure 1F). There was no statistical difference in the expression of RacGAP1 among different tumor types (P = .726).
Figure 1.
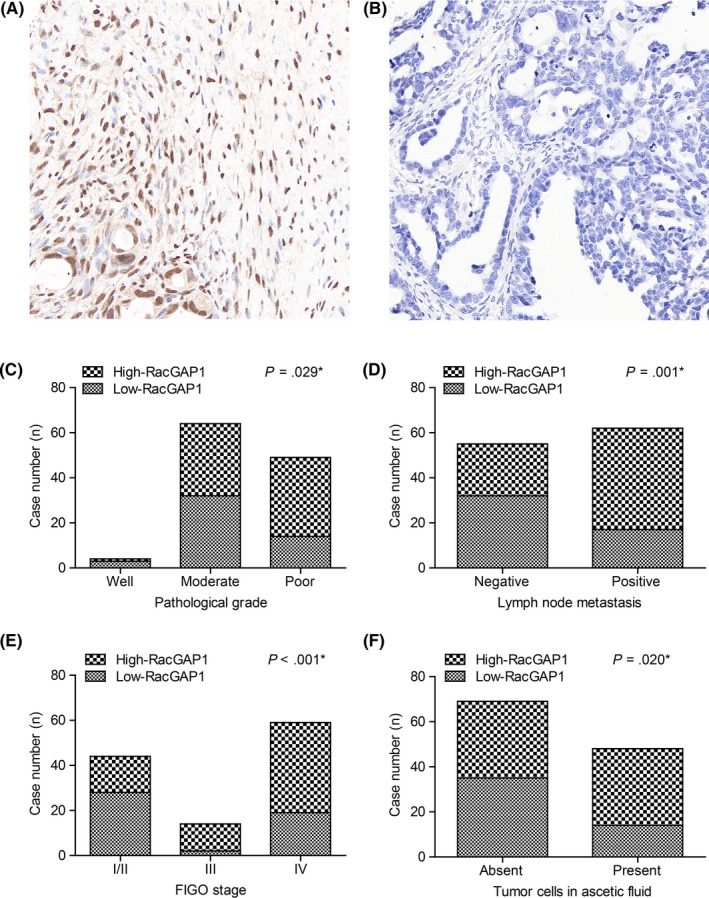
Expression of Rac GTPase activating protein 1 (RacGAP1) in epithelial ovarian cancer (EOC) tissues and its correlations with clinicopathological parameters. A, Representative high expression of RacGAP1 in the nucleus of EOC cells. B, Representative low expression of RacGAP1 in EOC tissue samples. Proportion of high‐RacGAP1 expression is higher in patients with (C) poor differentiation grade, (D) positive lymph node metastasis, (E) advanced International Federation of Gynecology and Obstetrics (FIGO) stage, and (F) positive tumor cells in ascetic fluid. Data were analyzed by χ2‐test
3.3. RacGAP1 indicates poor OS and progression‐free survival (PFS)
To further explore the role of RacGAP1 expression as well as clinicopathological parameters in the clinical outcomes of EOC patients, Kaplan‐Meier survival analyses were first carried out (Table 2). EOC patients with higher RacGAP1 expression had a shorter OS in comparison with those with lower RacGAP1 expression (75.0 ± 2.3 vs 44.0 ± 4.8 months, P < .001, Figure 2A). Other prognostic factors include lymph node metastasis, FIGO stage, and serum CA‐125 level (Figure 2B‐D). Neither pathological differentiation grade nor histological type of EOC showed statistical significance (Table 2). Moreover, subsequent multivariate COX proportional hazards analysis determined the independence of the prognostic value of RacGAP1 expression on the OS of EOC patients (HR = 2.063, 95% CI = 1.237‐3.439, P = .006, Table 2). Also, positive lymph node metastasis and advanced FIGO stages were both independently correlated with shorter OS (P = .048 and P = .031, respectively).
Table 2.
Prognostic parameters for OS in EOC patients by univariate and multivariate Cox proportional hazard model analyses
| Variable | Cases (n = 117) | Univariate analysis | Univariate P‐value | Multivariate analysis | Multivariate P‐value | ||
|---|---|---|---|---|---|---|---|
| 5‐y OS (%) | Median OS (mo) | HR | 95% CI | ||||
| Age (y) | |||||||
| ≤61 | 66 | 50.9 | 62.0 ± 5.6 | .942 | NA | NA | NA |
| >61 | 51 | 46.9 | 60.0 ± 8.8 | ||||
| Histological type | |||||||
| Serous | 86 | 50.0 | 60.0 ± 7.3 | .375 | NA | NA | NA |
| Non‐serous | 31 | 47.9 | 60.0 ± 5.1 | ||||
| Pathological grade | |||||||
| Well/Moderate | 68 | 54.4 | 69.0 ± 6.1 | .163 | NA | NA | NA |
| Poor | 49 | 42.1 | 55.0 ± 6.1 | ||||
| Tumor location | |||||||
| Unilateral | 78 | 49.6 | 60.0 ± 5.1 | .950 | NA | NA | NA |
| Bilateral | 39 | 47.7 | 60.0 ± 18.4 | ||||
| Lymph node metastasis | |||||||
| Negative | 55 | 78.1 | 76.0 ± 1.8 | <.001a | 2.205 | 1.008‐4.823 | .048a |
| Positive | 62 | 22.2 | 40.0 ± 6.8 | ||||
| FIGO stage | |||||||
| I/II | 44 | 85.6 | 77.0 ± 2.2 | <.001a | 2.767 | 1.097‐6.974 | .031a |
| III/IV | 73 | 24.8 | 44.0 ± 5.4 | ||||
| Tumor cells in ascetic fluid | |||||||
| Absent | 69 | 46.4 | 59.0 ± 4.6 | .860 | NA | NA | NA |
| Present | 48 | 52.5 | 64.0 ± 7.7 | ||||
| Serum CA‐125 level (U/mL) | |||||||
| ≤800 | 67 | 58.4 | 69.0 ± 5.1 | .036a | 1.494 | 0.903‐2.472 | .118 |
| >800 | 50 | 34.7 | 49.0 ± 4.4 | ||||
| p53 mutation | |||||||
| Negative | 67 | 50.7 | 62.0 ± 5.5 | .432 | NA | NA | NA |
| Positive | 50 | 47.6 | 59.0 ± 7.2 | ||||
| RacGAP1 expression | |||||||
| Low | 49 | 70.9 | 75.0 ± 2.3 | <.001a | 2.063 | 1.237‐3.439 | .006a |
| High | 68 | 33.1 | 44.0 ± 4.8 | ||||
P < .05.
CI, confidence interval; EOC, epithelial ovarian cancer; FIGO, International Federation of Gynecology and Obstetrics; HR, hazard ratio; NA, not applicable; OS, overall survival; RacGAP1, Rac GTPase‐activating protein 1.
Figure 2.
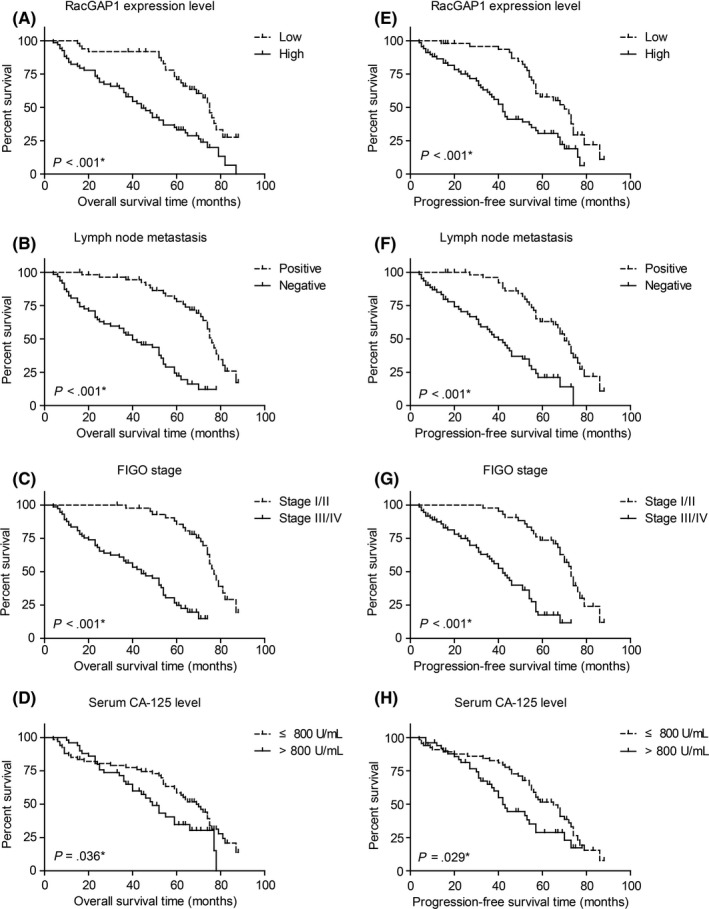
Kaplan‐Meier curves for overall survival and progression‐free survival according to Rac GTPase activating protein 1 (RacGAP1) expression and different clinicopathological features in epithelial ovarian cancer (EOC) patients. Patients with (A) higher RacGAP1 expression level, (B) positive lymph node metastasis, (C) advanced International Federation of Gynecology and Obstetrics (FIGO) stage and (D) higher serum CA‐125 level exhibited unfavorable overall survival. E‐H, Similarly, progression‐free survival was also evaluated by plotting Kaplan‐Meier curves. All statistical significance was compared by using log‐rank test
We also analyzed the PFS of the cohort by univariate and multivariate analyses (Table 3). In univariate Kaplan‐Meier survival results, higher RacGAP1 expression (P < .001, Figure 2E), positive lymph node status (P < .001, Figure 2F), advanced FIGO stage (P < .001, Figure 2G) and higher serum CA‐125 level (P = .029, Figure 2H) revealed unfavorable PFS for EOC patients. In the COX multivariate regression models, higher RacGAP1 expression was also correlated with shorter DFS (HR = 2.007, 95% CI = 1.217‐3.309, P = .006) in EOC patients. Other unfavorable independent factors for PFS include positive lymph node metastasis and advanced FIGO stage (P = .147 and P = .030, respectively).
Table 3.
Prognostic parameters for PFS in EOC patients by univariate and multivariate Cox proportional hazard model analyses
| Variable | Cases (n = 117) | Univariate analysis | Univariate P‐value | Multivariate analysis | Multivariate P‐value | ||
|---|---|---|---|---|---|---|---|
| 3‐y PFS (%) | Median PFS (mo) | HR | 95% CI | ||||
| Age (y) | |||||||
| ≤61 | 66 | 75.5 | 57.0 ± 4.6 | .434 | NA | NA | NA |
| >61 | 51 | 74.8 | 55.0 ± 2.1 | ||||
| Histological type | |||||||
| Serous | 86 | 72.7 | 54.0 ± 4.0 | .741 | NA | NA | NA |
| Non‐serous | 31 | 82.3 | 58.0 ± 7.8 | ||||
| Pathological grade | |||||||
| Well/Moderate | 68 | 81.2 | 57.0 ± 6.2 | .552 | NA | NA | NA |
| Poor | 49 | 66.5 | 54.0 ± 6.2 | ||||
| Tumor location | |||||||
| Unilateral | 78 | 79.6 | 55.0 ± 3.2 | .581 | NA | NA | NA |
| Bilateral | 39 | 66.0 | 57.0 ± 1.9 | ||||
| Lymph node metastasis | |||||||
| Negative | 55 | 97.7 | 70.0 ± 2.3 | <.001a | 1.760 | 0.820‐3.775 | .147a |
| Positive | 62 | 59.6 | 40.0 ± 4.5 | ||||
| FIGO stage | |||||||
| I/II | 44 | 85.6 | 73.0 ± 1.9 | <.001a | 2.599 | 1.095‐6.172 | .030a |
| III/IV | 73 | 24.8 | 42.0 ± 3.6 | ||||
| Tumor cells in ascetic fluid | |||||||
| Absent | 69 | 81.2 | 57.0 ± 1.8 | .500 | NA | NA | NA |
| Present | 48 | 66.9 | 55.0 ± 5.6 | ||||
| Serum CA‐125 level (U/mL) | |||||||
| ≤800 | 67 | 82.7 | 65.0 ± 5.1 | .029a | 1.480 | 0.905‐2.418 | .118 |
| >800 | 50 | 64.9 | 42.0 ± 3.0 | ||||
| p53 mutation | |||||||
| Negative | 67 | 72.6 | 57.0 ± 7.4 | .811 | NA | NA | NA |
| Positive | 50 | 78.8 | 54.0 ± 1.7 | ||||
| RacGAP1 expression | |||||||
| Low | 49 | 95.7 | 70.0 ± 3.7 | <.001a | 2.007 | 1.217‐3.309 | .006a |
| High | 68 | 59.6 | 42.0 ± 2.5 | ||||
P < .05.
CI, confidence interval; EOC, epithelial ovarian cancer; FIGO, International Federation of Gynecology and Obstetrics; HR, hazard ratio; NA, not applicable; PFS, progression‐free survival; RacGAP1, Rac GTPase‐activating protein 1.
3.4. RacGAP1 is involved in the process of cell migration and invasion
Clinical results showed that endogenous RacGAP1 expression was significantly correlated with lymph node metastasis, thus prompting us to investigate whether EOC cell migration and invasion can be affected by RacGAP1. We first tested the efficiency of RNAi in OV‐90 cells (Figure 3A), and selected siRNA#3 for further experiments. As shown in Figure 3B, migration ability was inhibited in OV‐90 by RacGAP1‐siRNA, whereas overexpression of RacGAP1 promoted cell migration. In addition, Transwell assay with Matrigel demonstrated that the invasiveness of the OV‐90 cells transfected with RacGAP1‐siRNA was dramatically decreased compared to that with scrambled siRNA control (Figure 3C). To confirm the effects of RacGAP1 in EOC cells, we chose another EOC cell line, Caov‐3 cells, to further test the migration and invasion capacities. Similarly, both migration and invasion were inhibited upon knockdown of RacGAP1 (Figure 3D,E). Taken together, these results indicated that RacGAP1 was a positive regulator of EOC progression.
Figure 3.
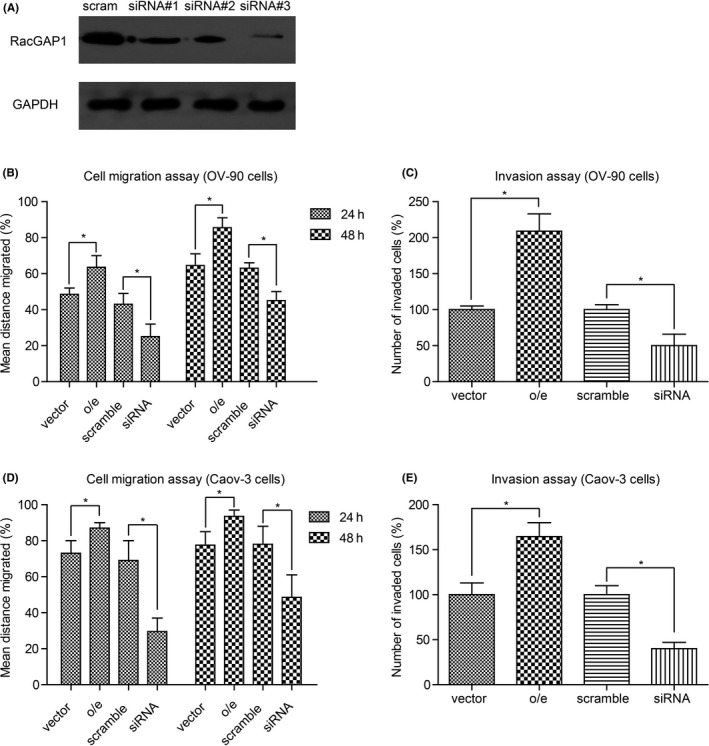
Role of Rac GTPase activating protein 1 (RacGAP1) in promoting epithelial ovarian cancer (EOC) cell migration and invasion. A, RNAi efficiency of RacGAP1‐siRNAs was tested by Western blotting, using scrambled siRNA (scram) as the negative control. B, Migration and (C) invasion capacities of OV‐90 cells were upregulated upon RacGAP1‐overexpression (o/e), whereas RacGAP1‐siRNA inhibited cell malignancy. D,E, The same experiments were carried out on another serous EOC cell line, the Caov‐3 cell line, and showed consistent results. All experimental data were from three independent experiments and analyzed by Student's t test. Bar, mean ± standard deviation. *P < .05
However, the possible mechanism of RacGAP1 as an oncoprotein in EOC is still unknown. We carried out immunoblotting assays in the transfected cells to test changes of downstream molecules (Figure 4A). As expected, RacGAP1 can deactivate Rac1 protein by decreasing the level of GTP‐Rac1. Interestingly, the active form of RhoA protein, GTP‐RhoA, was up‐regulated in EOC cells overexpressing RacGAP1. In contrast, knockdown of RacGAP1 inhibited the level of GTP‐RhoA. Similarly, the activity of Erk protein, a downstream effector of RhoA, was positively correlated with RacGAP1 expression. As Erk activation is important in regulating tumor cell migration and invasion, we next tested the protein level of p‐Erk in EOC tissues and found that p‐Erk was highly expressed in 66 patients (66/117, 56.4%), which mainly existed in the cytoplasm (Figure 4B). Furthermore, there was a significant positive correlation between RacGAP1 and p‐Erk expressions in clinical EOC tumor tissues as determined by IHC analysis (χ2‐test, P = .033, Figure 4C).
Figure 4.
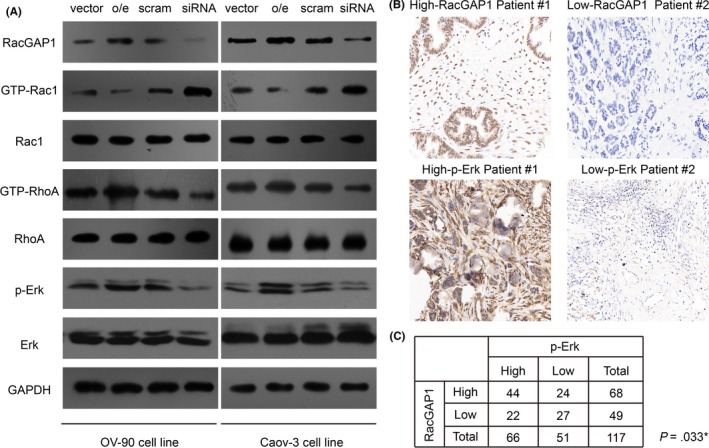
Rac GTPase activating protein 1 (RacGAP1) positively regulate activation of RhoA and Erk proteins. A, Overexpression of RacGAP1 inhibited GTP‐Rac1 expression, whereas it enhanced GTP‐RhoA and p‐Erk levels. Namely, knockdown of RacGAP1 by siRNA increased GTP‐Rac1 level while it attenuated GTP‐RhoA and p‐Erk expression. B, Representative expression of RacGAP1 and p‐Erk in clinical epithelial ovarian cancer (EOC) tissue samples, which showed significant statistical correlations as revealed by χ2‐test (P = .003, [C])
4. DISCUSSION
Although the expression of RacGAP1 in several tumor types has been reported, its detailed effects seem distinct among different malignancies. RacGAP1 mRNA level was shown to be significantly correlated with tumor size, clinical grade, histological type and prognosis in high‐grade meningiomas.18 In uterine carcinosarcoma, RacGAP1 also promoted cell motility and invasion by regulating STAT3 phosphorylation and survivin expression.22 Similarly, patients with positive RacGAP1 protein expression at the invasive front of gastric cancer showed poorer prognosis than those without it.20 Interestingly, colorectal cancer patients had opposite prognoses depending on the site of RacGAP1 expression,26 in that patients with high nuclear RacGAP1 expression had poor outcomes, whereas those with high cytoplasmic RacGAP1 expression had favorable prognoses. It is fascinating that different localization of RacGAP1 may drive distinct signaling pathways and opposite effects. However, based on our initial observations, we did not find strong expression of RacGAP1 in the cytoplasm of EOC cells, nor was statistical significance identified. Therefore, RacGAP1 can play distinct roles in cancer depending on their spatial regulation and cancer type context.
Besides the predictive role of RacGAP1 in cancers, it is also supposedly helpful in developing novel chemotherapy drugs. In head and neck squamous cell carcinoma (HNSCC) cells, doxorubicin resistance was reported to be mediated by RacGAP1‐dependent activation of AKT. Knockdown of RacGAP1 in HNSCC cells showed slower growth and sensitized the cytotoxic actions of doxorubicin in mice models.27 Further experiments are essential to verify its role in the drug resistance of other tumor types, including EOC.
Moreover, expression of RacGAP1 in vascular endothelial cells was also involved in tumor progression. Overexpression of RacGAP1 triggered focal adhesion formation and cytoskeletal rearrangement of endothelial cells, whereas depletion of RacGAP1 in endothelial cells attenuated melanoma cell trans‐endothelial migration.28 Zhang et al suggested that endothelial RacGAP1 may play critical roles in the pathogenic processes of cancer by regulating endothelial permeability.28
Our study focused on evaluating the expression and function of RacGAP1 in EOC cells, and found that RacGAP1 was statistically correlated with lymph node metastasis. To confirm our clinical findings, we carried out functional experiments by RacGAP1 knockdown or overexpression in EOC cells, which showed the role of RacGAP1 in promoting cell migration and invasion. In order to determine the possible mechanisms, we tested the expression of several common effectors of RhoGAP in EOC cells. RacGAP1 deactivated Rac1 protein by decreasing the level of GTP‐Rac1. However, the level of GTP‐RhoA was up‐regulated upon RacGAP1 overexpression. It is not known how RacGAP1 enhances RhoA protein activation, one hypothesis is that it may function indirectly by Rac‐RhoA interplay29 because suppressing Rac activity is sufficient to permit RhoA activation.30 In addition, phosphorylation of Erk protein was also positively regulated by RacGAP1 expression.
To better explain our results, we gathered resected EOC tissue samples and carried out IHC analysis. As expected, IHC data showed a positive correlation between RacGAP1 and p‐Erk expression levels. However, we must keep in mind that the statistical correlation is not enough to define infallible signaling pathways, and further experiments are necessary to better elucidate the functional mechanisms.
In summary, our clinical and molecular findings showed that a subset of EOC patients have RacGAP1 overexpression which is associated with advanced tumor stages and poor clinical outcomes. Thus, RacGAP1 may be a novel clinical biomarker for predicting the prognosis of EOC. This is the first report to suggest a relationship between RacGAP1 and prognosis of EOC patients, and further prospective trials in clinical settings would be worthwhile. Our study may also be helpful in exploring novel therapeutic targets and improving treatment outcomes.
CONFLICT OF INTEREST
Authors declare no conflicts of interest for this article.
Wang C, Wang W, Liu Y, Yong M, Yang Y, Zhou H. Rac GTPase activating protein 1 promotes oncogenic progression of epithelial ovarian cancer. Cancer Sci. 2018;109:84–93. https://doi.org/10.1111/cas.13434
CW and WW authors contributed equally to this work.
Contributor Information
Wenxia Wang, Email: wangwx1210@163.com.
Honggui Zhou, Email: talusedu@163.com, Email: hongguizhou@163.com.
REFERENCES
- 1. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69‐90. [DOI] [PubMed] [Google Scholar]
- 2. Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9‐29. [DOI] [PubMed] [Google Scholar]
- 3. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10‐29. [DOI] [PubMed] [Google Scholar]
- 4. Anuradha S, Webb PM, Blomfield P, et al. Survival of Australian women with invasive epithelial ovarian cancer: a population‐based study. Med J Aust. 2014;201:283‐288. [DOI] [PubMed] [Google Scholar]
- 5. Jelovac D, Armstrong DK. Recent progress in the diagnosis and treatment of ovarian cancer. CA Cancer J Clin. 2011;61:183‐203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Aunoble B, Sanches R, Didier E, Bignon YJ. Major oncogenes and tumor suppressor genes involved in epithelial ovarian cancer (review). Int J Oncol. 2000;16:567‐576. [DOI] [PubMed] [Google Scholar]
- 7. Wennerberg K, Rossman KL, Der CJ. The Ras superfamily at a glance. J Cell Sci. 2005;118(Pt 5):843‐846. [DOI] [PubMed] [Google Scholar]
- 8. Minoshima Y, Kawashima T, Hirose K, et al. Phosphorylation by aurora B converts MgcRacGAP to a RhoGAP during cytokinesis. Dev Cell. 2003;4:549‐560. [DOI] [PubMed] [Google Scholar]
- 9. Ridley AJ. Rho GTPase signalling in cell migration. Curr Opin Cell Biol. 2015;36:103‐112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Orgaz JL, Herraiz C, Sanz‐Moreno V. Rho GTPases modulate malignant transformation of tumor cells. Small GTPases. 2014;5:e29019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Csepanyi‐Komi R, Safar D, Grosz V, Tarjan ZL, Ligeti E. In silico tissue‐distribution of human Rho family GTPase activating proteins. Small GTPases. 2013;4:90‐101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vigil D, Cherfils J, Rossman KL, Der CJ. Ras superfamily GEFs and GAPs: validated and tractable targets for cancer therapy? Nat Rev Cancer. 2010;10:842‐857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Toure A, Dorseuil O, Morin L, et al. MgcRacGAP, a new human GTPase‐activating protein for Rac and Cdc42 similar to Drosophila rotundRacGAP gene product, is expressed in male germ cells. J Biol Chem. 1998;273:6019‐6023. [DOI] [PubMed] [Google Scholar]
- 14. Hirose K, Kawashima T, Iwamoto I, Nosaka T, Kitamura T. MgcRacGAP is involved in cytokinesis through associating with mitotic spindle and midbody. J Biol Chem. 2001;276:5821‐5828. [DOI] [PubMed] [Google Scholar]
- 15. Niiya F, Xie X, Lee KS, Inoue H, Miki T. Inhibition of cyclin‐dependent kinase 1 induces cytokinesis without chromosome segregation in an ECT2 and MgcRacGAP‐dependent manner. J Biol Chem. 2005;280:36502‐36509. [DOI] [PubMed] [Google Scholar]
- 16. Kamijo K, Ohara N, Abe M, et al. Dissecting the role of Rho‐mediated signaling in contractile ring formation. Mol Biol Cell. 2006;17:43‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kawashima T, Hirose K, Satoh T, et al. MgcRacGAP is involved in the control of growth and differentiation of hematopoietic cells. Blood. 2000;96:2116‐2124. [PubMed] [Google Scholar]
- 18. Ke HL, Ke RH, Li ST, Li B, Lu HT, Wang XQ. Expression of RACGAP1 in high grade meningiomas: a potential role in cancer progression. J Neurooncol. 2013;113:327‐332. [DOI] [PubMed] [Google Scholar]
- 19. Stone R II, Sabichi AL, Gill J, et al. Identification of genes correlated with early‐stage bladder cancer progression. Cancer Prev Res (Phila). 2010;3:776‐786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Saigusa S, Tanaka K, Mohri Y, et al. Clinical significance of RacGAP1 expression at the invasive front of gastric cancer. Gastric Cancer. 2015;18:84‐92. [DOI] [PubMed] [Google Scholar]
- 21. Imaoka H, Toiyama Y, Saigusa S, et al. RacGAP1 expression, increasing tumor malignant potential, as a predictive biomarker for lymph node metastasis and poor prognosis in colorectal cancer. Carcinogenesis. 2015;36:346‐354. [DOI] [PubMed] [Google Scholar]
- 22. Mi S, Lin M, Brouwer‐Visser J, et al. RNA‐seq Identification of RACGAP1 as a metastatic driver in uterine carcinosarcoma. Clin Cancer Res. 2016;22:4676‐4686. [DOI] [PubMed] [Google Scholar]
- 23. Wang SM, Ooi LL, Hui KM. Upregulation of Rac GTPase‐activating protein 1 is significantly associated with the early recurrence of human hepatocellular carcinoma. Clin Cancer Res. 2011;17:6040‐6051. [DOI] [PubMed] [Google Scholar]
- 24. Hou S, Du P, Wang P, Wang C, Liu P, Liu H. Significance of MNK1 in prognostic prediction and chemotherapy development of epithelial ovarian cancer. Clin Transl Oncol. 2017;19:1107‐1116. [DOI] [PubMed] [Google Scholar]
- 25. Liu H, Zhang Q, Li K, et al. Prognostic significance of USP33 in advanced colorectal cancer patients: new insights into beta‐arrestin‐dependent ERK signaling. Oncotarget. 2016;7:81223‐81240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yeh CM, Sung WW, Lai HW, et al. Opposing prognostic roles of nuclear and cytoplasmic RACGAP1 expression in colorectal cancer patients. Hum Pathol. 2016;47:45‐51. [DOI] [PubMed] [Google Scholar]
- 27. Hazar‐Rethinam M, de Long LM, Gannon OM, et al. RacGAP1 is a novel downstream effector of E2F7‐dependent resistance to doxorubicin and is prognostic for overall survival in squamous cell carcinoma. Mol Cancer Ther. 2015;14:1939‐1950. [DOI] [PubMed] [Google Scholar]
- 28. Zhang P, Bai H, Fu C, et al. RacGAP1‐driven focal adhesion formation promotes melanoma transendothelial migration through mediating adherens junction disassembly. Biochem Biophys Res Commun. 2015;459:1‐9. [DOI] [PubMed] [Google Scholar]
- 29. Sordella R, Van Aelst L. Dialogue between RhoA/ROCK and members of the Par complex in cell polarity. Dev Cell. 2008;14:150‐152. [DOI] [PubMed] [Google Scholar]
- 30. Guilluy C, Garcia‐Mata R, Burridge K. Rho protein crosstalk: another social network? Trends Cell Biol. 2011;21:718‐726. [DOI] [PMC free article] [PubMed] [Google Scholar]


