Abstract
Suppression of bone metastasis can improve patient quality of life. Current drugs for bone metastasis have been shown to prolong progression‐free survival but not overall survival; therefore, other potential therapeutic targets for bone metastasis should be investigated. Cell‐surface antigens, such as CD24, have been recently shown to be involved in the metastasis of various cancers. However, whether CD24 plays a role in bone metastasis of lung cancer remains unknown. To observe metastasis of lung cancer cells by imaging technology, we introduced a near‐infrared fluorescent protein, iRFP720, into a bone‐seeking subclone established from lung cancer cells, HARA‐B4 cells. The anchorage‐independent growth of these cells was then evaluated by colony formation assays. We also compared cancer cell tropism to bone tissue with HARA‐B4 cells in the presence or absence of CD24 by cell adhesion assays. To clarify the role of CD24 in bone metastasis, we intracardially injected CD24‐knockdown HARA‐B4 cells into mice and monitored metastasis through detection of iRFP720 using an in vivo imaging system. CD24‐knockdown HARA‐B4 cells in vitro showed reduced anchorage‐independent growth and cancer cell tropism to bone. Bone metastasis was diminished in mice inoculated with CD24‐knockdown HARA‐B4 cells, which was rescued by add‐back of CD24 in cells. Our findings indicate that iRFP720 is effective for in vivo imaging analysis of bone metastasis and that downregulation of CD24 suppresses bone metastasis of lung cancer cells. These findings collectively indicate that CD24 may be considered a promising new therapeutic candidate for the prevention of bone metastasis of lung cancer.
Keywords: bone metastasis, CD24, HARA‐B4, iRFP720, lung cancer
1. INTRODUCTION
The metastasis of cancer to bone is linked to several skeletal‐related events (SRE), including pathological fracture, spinal cord compression, surgical treatment, radiation treatment, and hypercalcemia, all of which deteriorate quality of life (QOL). Therefore, it is important to develop an effective management strategy for bone metastasis patients.
Recently, bisphosphonate1 and denosumab2 were developed to inhibit osteoclastic bone resorption, based on the molecular mechanism of bone metastasis. Although these drugs delay bone metastasis progression, they have not been shown to improve patient survival rate. In this context, improved drugs to treat bone metastasis are eagerly desired. Identifying a factor involved in bone metastasis would significantly contribute to the development of such a therapeutic target. In particular, recent drug development research targeting surface antigen molecules have proven successful.3
CD24 is a protein required for intercellular adhesion and cell differentiation, and is expressed on lymphocytes, epitheliocytes, and neuron precursor cells, where it plays an important role in neuron maturity. Recent studies have shown that CD24 is expressed in various cancer types, including pancreatic cancer, cerebral tumors, lung cancer, hepatocellular carcinoma, ovarian cancer, and breast cancer.4 Furthermore, CD24 may play a role in the metastasis of breast cancer,5, 6, 7 cervical cancer,4 gastric cancer,8 and bladder cancer,9, 10 further implicating it as a potential drug target. However, the role of CD24 in lung cancer and, in particular, its bone metastasis, remains obscure. Distant metastasis is common for lung cancer and the prognosis of such patients is usually poor, with almost no effective therapeutic method.11
To obtain experimental in vitro and in vivo evidence for the effect of CD24 in suppressing bone metastasis progression in lung cancer, we evaluated the role of CD24 in bone metastasis of lung cancer using HARA cells, derived from human lung squamous cell carcinoma, and HARA‐B4 cells, a bone‐seeking subclone established from HARA.
2. MATERIALS AND METHODS
2.1. Cell culture
The human squamous lung cancer cell line HARA and its bone‐seeking subclone, HARA‐B4, were obtained from the JCRB Cell Bank (Osaka, Japan). Cells were cultured in RPMI with 10% FBS and 1% penicillin–streptomycin (Nacalai Tesque, Kyoto, Japan). The osteosarcoma cell lines, MG‐63 and SAOS‐2, were obtained from Tohoku University (Sendai, Miyagi, Japan). To induce osteoblast differentiation, MG‐63 and SAOS‐2 cells were grown to confluence and cultured in osteoblastogenic medium containing α‐minimum essential medium with 20% FBS, 2% penicillin–streptomycin, 1.08 mg/mL β‐glycerol phosphate, 0.05 mg/mL ascorbic acid, and 80 nmol/L dexamethasone (Sigma‐Aldrich, St. Louis, MO, USA). Bone marrow stromal cells (Osteogenesis Culture Kit) was obtained from Cosmo Bio (Tokyo, Japan). Cells were cultured in the growth medium according to guidelines. The osteoblast‐like cell line MC3T3E1 was provided by Dr. Homare Eda (Biochemistry, Jikei University School of Medicine, Tokyo, Japan). Cells were cultured in α‐minimum essential medium, the same as MG‐63 cells. The mouse embryonic fibroblasts cell line NIH3T3 were provided by Dr. Noriyuki Murai (Molecular Biology, Jikei University School of Medicine, Tokyo, Japan). Cells was cultured in DMEM with 10% FBS and 1% penicillin–streptomycin. All cell lines were maintained in an incubator at 5% CO2 and 37°C. To induce anoikis, apoptosis due to inadequate cell adhesion, HARA and HARA‐B4 cells were cultured in ultra‐low attachment dishes (Corning, NY, USA) in RPMI under serum‐free conditions and incubated at 37°C and 5% CO2 for 2 weeks.
2.2. Quantitative real‐time PCR analysis
Total RNA was isolated using TRIsure (Nippon Genetics, Tokyo, Japan). cDNA was synthesized from total RNA using ReverTra Ace reverse transcriptase (Toyobo, Osaka, Japan) and oligo‐dT15. Quantitative real‐time PCR was carried out using primer sequences listed in Table S1, the KAPA SYBR FAST ABI Prism qPCR Kit (Kapa Biosystems, Wilmington, MA, USA), and PicoReal96 (Thermo Fisher Scientific, Kanagawa, Japan) according to the manufacturers’ instructions. Gene expression was normalized to that of the input control (GADPH).
2.3. Plasmid, transfection, and virus transduction
The near‐infrared fluorescent protein 720 (iRFP720) sequence was amplified from piRFP720‐N1 (Addgene, Cambridge, MA, USA) using primer sequences listed in Table S1. The amplified product was inserted into a pMXs‐IRES‐Puro retroviral vector (#RTB‐014; Cell Biolabs, San Diego, CA, USA), and the modified plasmid was transfected into PlatA retroviral packaging cells using PEI‐MAX (Polysciences, Warrington, PA, USA) and Opti‐MEM (Invitrogen, Carlsbad, CA, USA). After 48 hours, the virus‐containing supernatant was passed through a 0.45‐μm filter and applied to HARA‐B4 cells with 10 μg/mL polybrene (Sigma‐Aldrich). On day 2 following infection, puromycin (10 mg/mL) was added to the cultures for selection. A stable HARA‐B4 cell line expressing iRFP was established by sorting positive cells through a MoFlo XDP cell sorter (Beckman Coulter, Brea, CA, USA), and the collected cells were maintained in puromycin. HARA‐B4 cells that stably expressed iRFP720, named iRFP720‐HARA‐B4, had high fluorescence intensity, and were suitable for FACS and in vivo imaging system (IVIS) analysis.
To detect cell‐surface CD24 expression, flow cytometry was carried out according to standard procedures. Cells were harvested and resuspended in 2 × 105/100 μL FACS buffer (1% FBS in PBS). Each cell solution (100 μL) was incubated with 5 μL anti‐CD24 antibody (Miltenyi Biotec, Bergisch Gladbach, Germany) or 0.5 μL isotype control (Miltenyi Biotec) for 30 minutes at 4°C. After washing with FACS buffer, samples were analyzed using MACS Quant (Miltenyi Biotec). The shRNA target sequences were designed using the Oligoengine workstation (DNA Engine, Seattle, WA, USA) (Table S2). To generate stable shRNA‐CD24 cell lines, we used two different shRNAs against CD24 to control for non‐specific effects. As these shRNAs were designed to target the 3′‐UTR, the non‐coding region, they were not expected to disturb CD24 overexpression. The shRNAs against luciferase or CD24 were annealed and cloned into a pSUPER‐puro‐vector (Addgene), and Xba1‐Pac1 fragments were inserted into an FG12 lentiviral vector (Addgene) containing GFP as an infection marker, according to the manufacturer's instructions. The full‐length cDNA for CD24 was subcloned into the retroviral vector, pMXs‐IRES‐Hyg, the plasmid was introduced into PlatA cells, as described above, and the supernatant was applied to shCD24 #1 and #2 cells with 10 μg/mL polybrene. On day 2 following infection, hygromycin (25 μg/mL) was added to the cultures for selection. The resulting stable cell lines were called iRFP720‐HARA‐B4/shCD24 + rCD24 #1 and #2.
2.4. Anchorage‐independent proliferation in agar matrix
For analysis of anchorage‐independent cell growth in vitro, soft agar colony formation assays were carried out according to previous studies12 with slight modifications. Briefly, the bottom layer contained 2.5 mL of 1× soft agar matrix solution (Funakoshi, Tokyo, Japan) and 10% FBS in DMEM, and the top layer contained 1.5 mL of 1× soft agar matrix solution, 10% FBS in DMEM, and 1 × 104 cells. All assays were carried out in 6‐well plates. The plates were incubated at 5% CO2 and 37°C for 2 weeks to assess cell proliferation in soft agar. Colonies were stained using nitro blue tetrazolium solution (Nacalai Tesque) and counted by microscopic inspection using ImageJ (https://imagej.nih.gov/ij/).
2.5. Cell adhesion assay
MG‐63, SAOS‐2, the mouse primary osteoblasts, MC3T3E1, and NIH3T3 cells were grown to confluence and cultured as described in the “Cell culture” section. Osteoblast differentiation was assessed by measuring osteoblast differentiation markers by real‐time PCR, as described previously.13 The cell adhesion assay was undertaken as described previously.14 Briefly, 3 × 103 HARA‐B4 cells (containing GFP from the FG12 vector) were overlaid directly onto confluent MG‐63 cells in a dish and incubated for 30 minutes at 5% CO2 and 37°C. The cells were gently washed in PBS and fixed with 4% paraformaldehyde in PBS. The GFP‐expressing cells that adhered to the dish were counted (total cell count for each of four square fields for four wells, average of n = 3).
2.6. Cell proliferation
The proliferation assay was undertaken using the Hemacolor assay kit (Merck, Darmstadt, Germany). The supernatant of cell monolayers cultured in 96‐well plates was removed and the cells were quickly air dried. The monolayers were fixed with solution 1 (50 μL/well) for 30 seconds, and stained with solution 2 (80‐100 μL/well) for 60 seconds followed by solution 3 (80‐100 μL/well) for another 60 seconds. The plates were washed with water three times, filled with water again and left to sit for 5 minutes before the water was removed and the plates were air dried. Stain extraction was carried out using 0.5% SDS (Sigma‐Aldrich) dissolved in PBS (0.2 mL/well) for 90 minutes and the change in absorbance was measured at 600 or 630 nm using a microplate reader, as described previously.15
2.7. Animal studies and in vivo imaging
Our animal experiment protocol was approved by the Institutional Animal Care and Use Committee of Jikei University (Tokyo, Japan) (No. 26‐054) and the studies were carried out in accordance with the Guidelines for the Proper Conduct of Animal Experiments of the Science Council of Japan (2006). Mice were kept in a pathogen‐free room at 25 ± 3°C and treated according to the NIH guidelines. For intracardiac injections, 5‐week‐old male nude mice (BALB/cA Jcl‐nu/nu; CLEA, Tokyo, Japan) were anesthetized with an ip, injection of 0.75 mg/kg medetomidine (Orion, Espoo, Finland), 4 mg/kg midazolam (Astellas, Tokyo, Japan) and 5 mg/kg butorphanol tartrate (Meiji Seika Pharma, Tokyo, Japan). Subsequently, 2 × 106 iRFP720‐HARA‐B4 cells were injected into the left cardiac ventricle and the mice were given the medetomidine antagonist, atipamezole (Orion), for recovery. Five weeks after injection, the development of metastases in bones was monitored using the IVIS Lumina in vivo imaging system and Living Image Software Version 4.0 (Perkin Elmer, Waltham, MA, USA). For IVIS, animals were anesthetized using 2% isoflurane by inhalation and fluorescence from iRFP720‐HARA‐B4 cells was detected using a 710‐nm excitation and 810‐875 nm emission filter for indocyanine green. Analysis was carried out on images from IVIS and computerized tomography (CT) scans (Latheta LCT‐200; Hitachi, Tokyo, Japan).
2.8. Histopathological examination
Areas on the spine and hind leg bones of mice with fluorescence signal were fixed in 10% neutral buffered formalin then decalcified with K‐CX (FALMA, Tokyo, Japan) for approximately 24 hours before paraffin embedding and processing on regular slides. Sectioned tissue was stained with H&E and examined using an Olympus BH2 microscope with a DP20 digital camera (Olympus, Tokyo, Japan).
2.9. Statistical analysis
Results are presented as mean ± SD. Group comparisons were carried out using the parametric Student's t‐test. Differences at P < .05 were considered significant.
3. RESULTS
3.1. CD24 is highly expressed in bone‐directional lung cancer cells
Previous studies have shown that CD24 is associated with poor prognosis of several cancers.4, 16, 17 Consistent with this, meta‐analysis data indicate that the overall survival rate of patients with high CD24 expression is lower than that for those with low CD24 expression for breast, bladder, and lung cancer (Figure 1). However, the role of CD24 in lung cancer, in particular, its bone metastasis, remains unknown. Therefore, to examine whether CD24 plays a role in bone metastasis of lung cancer, we used HARA‐B4 cells, which are a bone‐seeking subclone established from HARA cells, a human lung squamous cell carcinoma cell line, as a bone metastasis model. HARA‐B4 cells show increased anchorage‐independent growth, a feature of malignant cells forming tumors in vivo, which is associated with highly metastatic cancer cells.18, 19 CD24 expression in HARA‐B4 cells was significantly higher than in HARA cells (Figure 2A). This result was consistent with microarray data (GSE29367 at NCBI Gene Expression Omnibus: https://www.ncbi.nlm.nih.gov/geo/). We subsequently cultured HARA and HARA‐B4 cells for 2 weeks in low attachment dishes and examined cell viability under anchorage‐independent conditions. CD24 expression was confirmed at 1, 3, and 6 days after incubation by real‐time PCR. HARA‐B4 cells were more viable in anchorage‐independent conditions than HARA cells (Figure 2B). In this regard, CD24 expression was significantly higher in HARA‐B4 than HARA cells (Figure 2C) under normal and anchorage‐independent conditions. Moreover, CD24 expression was higher in HARA‐B4 cells in the anchorage‐independent condition than in the normal condition. These findings indicate that CD24 plays an important role in anchorage‐independent growth.
Figure 1.
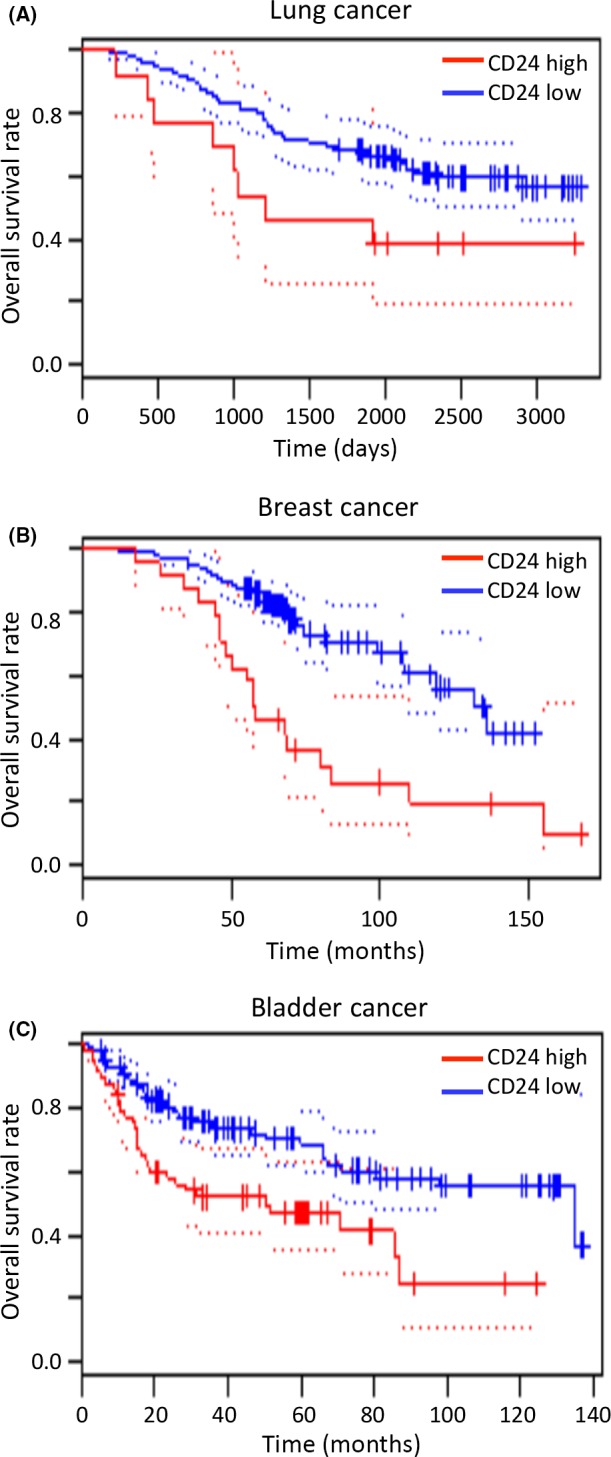
Data from PrognoScan (http://www.prognoscan.org/) indicates that CD24 is associated with poor prognosis of cancer. The overall survival curves of patients with lung cancer (A), breast cancer (B), and bladder cancer (C) with high and low expression of CD24
Figure 2.
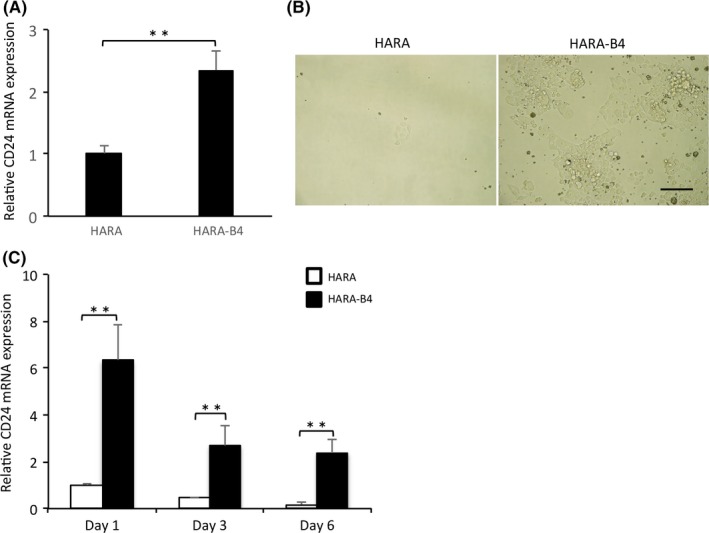
CD24 is involved in the anchorage‐independent growth of lung cancer cells. A, CD24 mRNA expression in HARA and HARA‐B4 cells. Data are shown as relative values based on that in HARA. B, Phase contrast images showing the survival of HARA (left) and HARA‐B4 (right) cells under normal conditions for 1 wk after 2‐wk culture in ultra‐low attachment culture conditions. Scale bar = 50 μm. C, CD24 mRNA expression in HARA and HARA‐B4 cells at the indicated days under anchorage‐independent conditions. Data are shown as relative values based on that in HARA cells at day 1, and are presented as the mean ± SD (n = 3). **P < .01
3.2. Generation of HARA‐B4 cells expressing iRFP
To investigate the mechanisms underlying bone metastasis in lung cancer cells, we generated a stable cell line by introducing an expression vector containing iRFP720 into HARA‐B4 cells. Expression of iRFP720 was confirmed by FACS analysis (Figure S1A). The iRFP720 protein can be readily detected in vivo20 because its absorption in mammalian tissue is minimal and does not require luminescent material. Therefore, it is suitable for deep tissue whole‐body imaging21 and monitoring the kinetics and emergence of bone metastatic lesion by the IVIS spectrum.
3.3. CD24 knockdown leads to suppression of anchorage‐independent growth and bone seeking
To evaluate the association between CD24 and bone metastasis, CD24 shRNA or negative control shRNA were transfected into iRFP720‐HARA‐B4 cells to generate iRFP720‐HARA‐B4/shCD24 (shCD24) or iRFP720‐HARA‐B4/shLuciferase (shLuc) cells, respectively. Subsequently, shRNA‐resistant CD24 was transfected into shCD24 HARA‐B4 cells to generate iRFP720‐HARA‐B4/shCD24 + rCD24 (resistant) cells. The expression of CD24 in each of these cell lines was confirmed by quantitative PCR and FACS analysis (Figure S1B,C). Anchorage‐independent growth of shLuc, shCD24, and resistant cells was evaluated using soft agar colony formation assays. Two weeks after plating, all three cell types colonized in the soft agar medium; however, the number and size of shCD24 cell colonies were reduced compared to those of shLuc cells (Figure 3A). The number of colonies formed by shCD24 #1 and shCD24 #2 cells were reduced by 72% and 92%, respectively (Figure 3B). Inhibition of colony formation by shCD24 expression was reversed in resistant cells (Figure 3A), with recovery to 61% and 72% in resistant #1 and resistant #2 cells, respectively. These data suggest that CD24 is involved in the anchorage‐independent growth of HARA‐B4 cells. We also investigated the impact of CD24 expression on the proliferation motility of HARA‐B4 cells using the Hemacolor assay. The shLuc, shCD24, and resistant cells were seeded and cell colonies were counted after 1, 2, 3, and 4 days. There was no significant difference in the cell proliferation rate among these cell lines (Figure S2A). Therefore, the difference in colony formation was independent of cell proliferation, suggesting that CD24 is not associated with the regulation of cell proliferation. To investigate a role for CD24 in the control of cancer cell tropism to bone, we examined whether CD24 regulates HARA‐B4 adhesion to osteoblasts. This was achieved by measuring the difference in adhesion between CD24‐expressing and ‐knockdown cells with the osteosarcoma cell line, MG‐63, using the cell adhesion assay. MG‐63 cells were cultured in osteoblastogenic medium and osteoblast differentiation was induced, which was confirmed by assessment of osteoblast differentiation markers by real‐time PCR (Figure S2B). The cell adhesion assay showed that significantly fewer shCD24 #1 and shCD24 #2 cells adhered to the confluent monolayer of MG‐63 cells compared to shLuc cells. The decreased adhesion showed by shCD24‐expressing cells was markedly reversed in resistant cells. Similar results were obtained with another osteosarcoma cell line, SAOS‐2 (Figure S2C). These results suggest that CD24 might regulate the adhesion of lung cancer cells to osteoblasts (Figure 3C). In addition, the cell adhesion assay was carried out using the mouse primary osteoblasts (primary OB) and osteoblast‐like cell line MC3T3E1 for positive controls, and mouse embryonic fibroblast cell line NIH3T3 for a negative control. First, bone marrow stromal cells were cultured in osteogenesis culture medium according to guidelines to induce the primary OB differentiation, and MC3T3E1 cells were cultured in osteoblastogenic medium to induce osteoblast differentiation, which was confirmed by assessment of osteoblast differentiation markers by real‐time PCR (Figure S3A). Next, to investigate the bone tropism of HARA‐B4 cells, we examined whether HARA‐B4 regulates adhesion to the primary OB, MC3T3E1, and NIH3T3 cells using the cell adhesion assay (Figure S3B). The result indicated that the bone tropism in HARA‐B4 was significantly different between positive controls (primary OB and MC3T3E1 cells) and a negative control (NIH3T3 cells). Therefore, CD24 may contribute to the anchorage‐independent growth and bone‐seeking functions.
Figure 3.
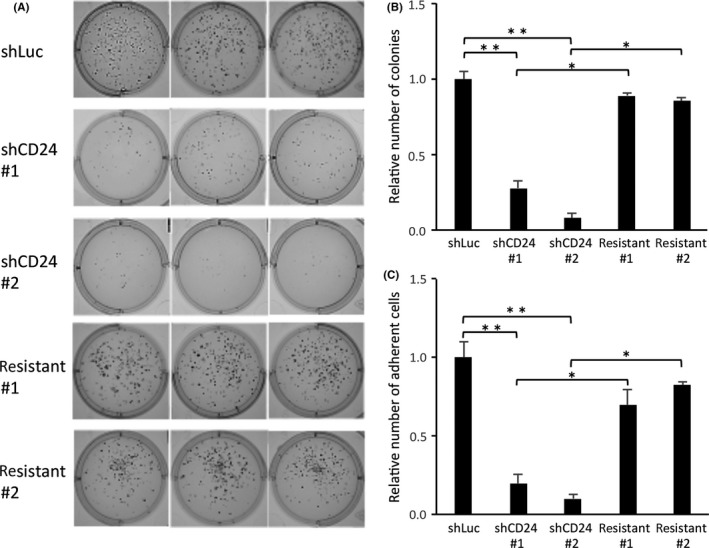
CD24 knockdown suppresses anchorage‐independent growth and bone seeking. A, Anchorage‐independent proliferation of HARA‐B4 cells that were infected with lentivirus encoding shLuc, shRNA against CD24, or shRNA‐resistant CD24. Cells were cultured for 2 wk in medium containing 1× soft agar matrix solution and examined by microscopy. Each data represents three independent experiments. B, The number of colonies formed by HARA‐B4 cells was counted under microscopic inspection using ImageJ software. C, MG‐63 and SAOS‐2 cells were induced to undergo osteoblast differentiation. GFP‐expressing cells (GFP was expressed in the shRNA vector in HARA‐B4 cells) that adhered to MG‐63 cells were counted under microscopic inspection (total cell count for each of four square fields for four wells). Data are shown as relative values based on that in shLuc, and are presented as the mean ± SD (n = 3). *P < .05, **P < .01
3.4. CD24 suppresses bone metastasis in vivo
To characterize the role of CD24 in bone metastasis of HARA‐B4 cells in vivo, shLuc, shCD24, and resistant cells (2 × 105 cells) were inoculated into the left cardiac ventricle of male nude mice and whole body imaging was undertaken using IVIS (Figure 4A). Expression of iRFP720 was observed in bone from all nude mice inoculated with shLuc cells, three of five with shCD24 cells, and four of five with resistant cells (Figure 4B). There tended to be more bone metastases in the spine, ribs, and hind legs (Figure 4B), which is consistent with a previous study.22 Although muscle metastases were also frequently noted, little metastasis was observed in other organs, consistent with a previous study.12 The total number of metastatic sites was 46, 5, and 37 for shLuc, shCD24, and resistant cell‐inoculated mice, respectively (Table 1), indicating that shLuc‐ and resistant cell‐inoculated mice showed significantly more metastases than shCD24 cell‐inoculated mice (Figure 4C, Table S3). Computed tomography images confirmed osteolysis in most of the individual bone metastases in the 5 weeks after injection (Figures 4D, S4). Furthermore, H&E staining confirmed that infiltrating tumor cells were present in the medullary cavity in bone metastatic specimens from shLuc‐ and resistant cell‐inoculated mice (Figure 4E). These results indicate that knockdown of CD24 markedly reduces bone metastasis of HARA‐B4 cells in vivo.
Figure 4.
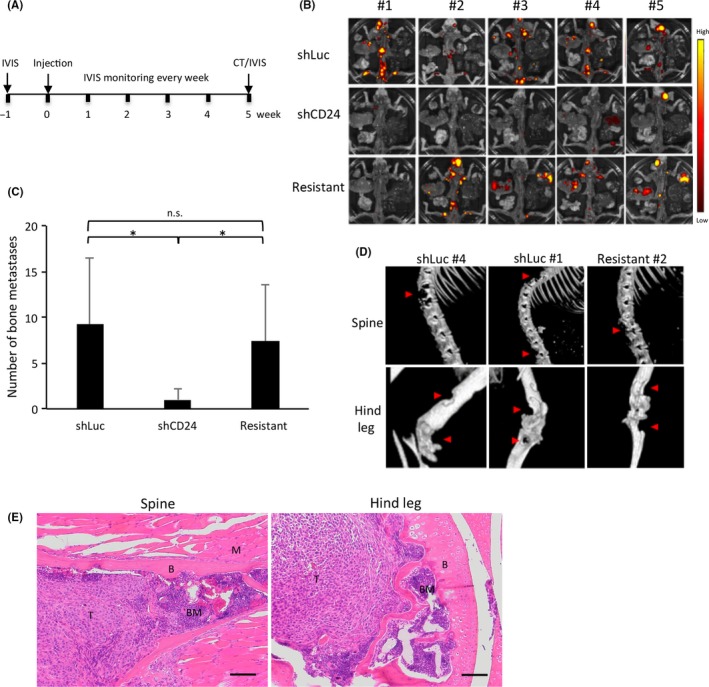
CD24 knockdown reduces bone metastases in vivo. A, Timeline of the procedure used for animal studies. Cells were injected into the left cardiac ventricle of male nude mice to induce bone metastases. After 5 wk, bone metastatic lesions were imaged by computed tomography and near‐infrared fluorescent protein (iRFP)‐positive cells were examined by an in vivo imaging system (n = 5/group). B, Luminescent images of bone metastasis in mice 5 wk after inoculation with iRFP‐labeled HARA‐B4 cells. Color scale range is from 5 × 107 to 2 × 108 p/s/cm2/sr. C, Number of total bone metastases per mice among shLuc, shCD24, and resistant groups. Data are presented as mean ± SD (n = 5). *P < .05. D, Representative computed tomography images of bone metastases in shLuc, shCD24, and resistant cell‐inoculated mice at 5 wk after iRFP‐labeled HARA‐B4 cells were injected intracardially. Osteolytic lesions in the spine (top) and hind leg (bottom) are indicated by red arrows. E, H&E staining of bone metastasis lesions of iRFP720‐HARA‐B4/shLuc cells from the spine (left) and hind leg (right) 5 wk after inoculation. Scale bar = 100 μmol/L. B, bone; BM, bone marrow; M, muscle; n.s., not significant; T, tumor cells
Table 1.
Total number of bone metastases in male nude mice inoculated with shLuc, shCD24, or resistant cells, determined by an in vivo imaging system
| shLuc | shCD24 | Resistant | |
|---|---|---|---|
| Skull | 6 | 1 | 6 |
| Spine | 15 | 1 | 6 |
| Ribs | 10 | 1 | 14 |
| Hind leg | 11 | 1 | 8 |
| Pelvis | 4 | 1 | 3 |
| Total | 46 | 5 | 37 |
4. DISCUSSION
In this study, we showed that CD24 is highly expressed in a bone metastatic lung cancer cell line and under metastatic conditions. CD24 promotes the bone metastasis of lung cancer cells in vitro and in vivo by promoting anchorage‐independent growth and adhesion. These results provide evidence for the potential of CD24 as a therapeutic candidate for the prevention of bone metastasis of lung cancer.
We used a parental cell line, HARA, prepared from a human lung cancer (lung squamous cell carcinoma) cell line, and a bone‐seeking subclone, HARA‐B4, established from HARA. HARA‐B4 cells are a highly specific bone metastasis model of lung cancer, which were previously shown to have high bone‐seeking characteristics and low frequency of metastasis to organs other than bone.12 As our data indicated that CD24 gene expression was significantly higher in HARA‐B4 than HARA cells, and under non‐anchorage‐dependent conditions, and previous reports suggested a potential association between CD24 and metastasis or cancer growth,4, 5, 6, 8, 9 we hypothesized that CD24 might play a role in bone metastasis.
This study is the first to show a role for CD24 in the bone metastasis of lung cancer cells. Although CD24 has been linked to metastatic invasion in several cancers, including breast cancer, evidence specifically for metastasis to bone has remained obscure. Our colony formation assay showed that HARA‐B4 cells showed increased anchorage‐independent growth, which is a feature of tumor‐forming malignant cells in vivo and is associated with highly metastatic cancer cells.18, 19 Furthermore, the anchorage‐independent growth of HARA‐B4 cells was reduced in shCD24 cells and was rescued by resistant cells. Consistent with previous results, the Hemacolor assay showed no differences in cell growth ability, indicating that CD24 is not involved in the regulation of cell growth.8 In a cell adhesion assay with an osteosarcoma cell line, MG‐63, shCD24 cells adhered significantly less to osteoblasts compared to normal HARA‐B4 cells. This effect was reversed in CD24‐resistant cells, suggesting a CD24‐specific role in regulating the adhesion of lung cancer cells to osteoblasts. These findings provide evidence for a potential correlation between CD24 expression and tropism of lung cancer cells to bone tissue, which may lead to the acquisition and enhancement of bone metastatic ability. The role of CD‐24 in bone metastasis was further validated in our in vivo findings; mice inoculated with shCD24 cells showed decreased bone metastasis, which was reversed in mice inoculated with resistant cells, revealing CD24‐specific effects.
Recent progress in cancer treatment has led to an increase in the incidence of bone metastases in all cancers due to the augmented survival rate of patients. The bone metastasis itself does not often become the cause of death. However, when metastasis becomes worse and comes to cause SRE, patients’ activity falls and their QOL remarkably decreases. As a result, patients become bedridden, and aspiration‐related pneumonia or whole body weakening have an influence on their prognosis. Indeed, there is a report that bone metastasis in progressive prostate and breast cancer patients with SRE shortens the duration of survival.23, 24 Thus, the bone metastasis itself is not direct, but participates in the survival rate. Therefore, to maintain patients’ QOL, it is important to improve management strategies for cancer patients. Research from the past 20 years has revealed the molecular mechanisms involved in metastasis formation with osteoclasts, which has been shown to play a prominent role in this process. Thus, drugs targeting osteoclasts have been developed for the treatment of bone metastasis, and have contributed to improving the QOL of patients with bone metastasis by delaying and reducing the occurrence of SRE in combination with systemic therapy. Although these drugs are widely used in clinical settings, their clinical usefulness is limited because they delay bone metastasis progression without improving patient survival. Thus, to develop novel drugs that further improve the QOL of patients with bone metastasis is eagerly demanded. This can arise through the identification of new factors involved in bone metastasis. In this regard, our results suggest that CD24 might constitute such a potential drug target.
In vivo experiments indicated that bone metastasis can be tracked using iRFP720. The iRFP720 protein is easy to handle and has much better tissue permeability than other fluorescent proteins due to its long wavelength fluorescence, and is minimally absorbed by tissue.20 With an excitation/emission maxima at 690/713 nm, iRFP is stably expressed and emits a clear signal for detecting deep visceral lesions compared to far‐red fluorescent proteins.25 Furthermore, iRFP720 with excitation at >700 nm and emission at 810‐875 nm enables it to be detected with high specificity, with reduced technical complexity and costs compared to luciferin‐based bioluminescence imaging. Therefore, iRFP720 is useful for in vivo imaging analysis of bone metastasis.
CD24, a glycosyl‐phosphatidylinositol‐anchored sialoglycoprotein, is a membrane protein involved in intercellular adhesion and cell differentiation. We confirmed that CD24 is involved in bone metastasis of cancer cells, suggesting a possible correlation between CD24 and tropism to bone tissue of lung cancer cells. CD24 has also been linked to tumor metastasis and invasion through its function as a ligand for P‐selectin and adhesion receptors in activated endothelial cells and platelets.26 Most cancer cells can bind to platelets through CD24‐mediated binding to P‐selectin, which thereby promotes their escape into the bloodstream and increases their metastasis.27 A recent study suggested that CD24 expression was critical for tumor growth and migration,10 and CD24 expression reduces stromal cell‐derived factor‐1‐mediated cell migration and signaling through C‐X‐C motif chemokine receptor 4.6 These factors might also participate in the bone metastasis mechanism by CD24, therefore, it is necessary to progress the search of molecules associated with CD24. Previous studies have also shown that overexpression of CD24 contributes to cell growth and invasion in vitro.28 Immunohistochemical studies have linked overexpression of CD24 with pathological grade, lymph node metastasis, and subsequent clinicopathological features, such as poor prognosis of cervical,4 bladder,16, 17 and prostate cancers.10 CD24 is also associated with cancer metastasis in bladder9, 10 and breast cancers.7 Although our results suggest that CD24 plays a pivotal role in the bone metastasis of a single lung cancer cell line, future studies should verify these findings with other cell lines. Additionally, studies are required to verify whether CD24 functions directly or indirectly to promote bone metastasis using drugs that target CD24. Importantly, inhibition of CD24 by mAbs and siRNAs have been reported to reduce primary tumor growth and metastasis of colon, pancreas, and bladder cancers,29, 30 suggesting that bone metastasis of lung cancer can be suppressed by drugs targeting CD24.
In conclusion, we have shown that CD24 is highly expressed in a bone metastatic lung cancer cell line, promotes anchorage‐independent growth and adhesion in vitro, and that CD24‐knockdown suppressed bone metastasis of lung cancer cells in vivo. Our results provide evidence that CD24 may be considered a promising therapeutic candidate for bone metastasis of lung cancer.
CONFLICT OF INTEREST
The authors have no conflict of interest.
Supporting information
ACKNOWLEDGMENTS
We thank Drs. Homare Eda and Mariko Honda for their advice on experiments, and Dr. Minori Kamada for help with the cell‐sorting analysis. We also thank DMC Corp. for providing English language editing. This work was supported by grants from the Japan Society for the Promotion of Science (KAKENHI Grant Nos. JP26290041 and JP17H03584), the Takeda Science Foundation, the Vehicle Racing Commemorative Foundation, and a research grant from the Princess Takamatsu Cancer Research Fund.
Okabe H, Aoki K, Yogosawa S, Saito M, Marumo K, Yoshida K. Downregulation of CD24 suppresses bone metastasis of lung cancer. Cancer Sci. 2018;109:112–120. https://doi.org/10.1111/cas.13435
Funding information
Japan Society for the Promotion of Science; Takeda Science Foundation; Vehicle Racing Commemorative Foundation; Princess Takamatsu Cancer Research Fund.
REFERENCES
- 1. Coleman R, Cook R, Hirsh V, Major P, Lipton A. Zoledronic acid use in cancer patients: more than just supportive care? Cancer. 2011;117:11‐23. [DOI] [PubMed] [Google Scholar]
- 2. Lacey DL, Boyle WJ, Simonet WS, et al. Bench to bedside: elucidation of the OPG‐RANK‐RANKL pathway and the development of denosumab. Nat Rev Drug Discov. 2012;11:401‐419. [DOI] [PubMed] [Google Scholar]
- 3. Scott AM, Wolchok JD, Old LJ. Antibody therapy of cancer. Nat Rev Cancer. 2012;12:278‐287. [DOI] [PubMed] [Google Scholar]
- 4. Tanaka T, Terai Y, Kogata Y, et al. CD24 expression as a marker for predicting clinical outcome and invasive activity in uterine cervical cancer. Oncol Rep. 2015;34:2282‐2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Baumann P, Cremers N, Kroese F, et al. CD24 expression causes the acquisition of multiple cellular properties associated with tumor growth and metastasis. Cancer Res. 2005;65:10783‐10793. [DOI] [PubMed] [Google Scholar]
- 6. Schabath H, Runz S, Joumaa S, Altevogt P. CD24 affects CXCR4 function in pre‐B lymphocytes and breast carcinoma cells. J Cell Sci. 2006;119:314‐325. [DOI] [PubMed] [Google Scholar]
- 7. Vazquez‐Martin A, Oliveras‐Ferraros C, Cufi S, et al. The anti‐diabetic drug metformin suppresses the metastasis‐associated protein CD24 in MDA‐MB‐468 triple‐negative breast cancer cells. Oncol Rep. 2011;25:135‐140. [PubMed] [Google Scholar]
- 8. Takahashi M, Nakajima M, Ogata H, et al. CD24 expression is associated with progression of gastric cancer. Hepatogastroenterology. 2013;60:653‐658. [DOI] [PubMed] [Google Scholar]
- 9. Overdevest JB, Thomas S, Kristiansen G, Hansel DE, Smith SC, Theodorescu D. CD24 offers a therapeutic target for control of bladder cancer metastasis based on a requirement for lung colonization. Cancer Res. 2011;71:3802‐3811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Thomas S, Harding MA, Smith SC, et al. CD24 is an effector of HIF‐1‐driven primary tumor growth and metastasis. Cancer Res. 2012;72:5600‐5612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Li X, Liu X, Gao F, Yin X. Crizotinib treatment in a lung adenocarcinoma harboring ALK fusion gene with bone marrow metastasis: case report and literature review. Zhongguo Fei Ai Za Zhi. 2015;18:85‐88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Takiguchi S, Korenaga N, Inoue K, et al. Involvement of CXCL14 in osteolytic bone metastasis from lung cancer. Int J Oncol. 2014;44:1316‐1324. [DOI] [PubMed] [Google Scholar]
- 13. Eda H, Santo L, Wein MN, et al. Regulation of sclerostin expression in multiple myeloma by Dkk‐1: a potential therapeutic strategy for myeloma bone disease. J Bone Miner Res. 2016;31:1225‐1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tang KD, Holzapfel BM, Liu J, et al. Tie‐2 regulates the stemness and metastatic properties of prostate cancer cells. Oncotarget. 2015;7:2572‐2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Keisari Y. A colorimetric microtiter assay for the quantitation of cytokine activity on adherent cells in tissue culture. J Immunol Methods. 1992;146:155‐161. [DOI] [PubMed] [Google Scholar]
- 16. Smith SC, Oxford G, Wu Z, et al. The metastasis‐associated gene CD24 is regulated by Ral GTPase and is a mediator of cell proliferation and survival in human cancer. Cancer Res. 2006;66:1917‐1922. [DOI] [PubMed] [Google Scholar]
- 17. Overdevest JB, Knubel KH, Duex JE, et al. CD24 expression is important in male urothelial tumorigenesis and metastasis in mice and is androgen regulated. Proc Natl Acad Sci U S A. 2012;109:E3588‐E3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chiarugi P, Giannoni E. Anoikis: a necessary death program for anchorage‐dependent cells. Biochem Pharmacol. 2008;76:1352‐1364. [DOI] [PubMed] [Google Scholar]
- 19. Taddei ML, Giannoni E, Fiaschi T, Chiarugi P. Anoikis: an emerging hallmark in health and diseases. J Pathol. 2012;226:380‐393. [DOI] [PubMed] [Google Scholar]
- 20. Shcherbakova DM, Verkhusha VV. Near‐infrared fluorescent proteins for multicolor in vivo imaging. Nat Methods. 2013;10:751‐754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rice WL, Shcherbakova DM, Verkhusha VV, Kumar AT. In vivo tomographic imaging of deep‐seated cancer using fluorescence lifetime contrast. Cancer Res. 2015;75:1236‐1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wood SL, Pernemalm M, Crosbie PA, Whetton AD. The role of the tumor‐microenvironment in lung cancer‐metastasis and its relationship to potential therapeutic targets. Cancer Treat Rev. 2014;40:558‐566. [DOI] [PubMed] [Google Scholar]
- 23. Norgaard M, Jensen AO, Jacobsen JB, Cetin K, Fryzek JP, Sorensen HT. Skeletal related events, bone metastasis and survival of prostate cancer: a population based cohort study in Denmark (1999 to 2007). J Urol. 2010;184:162‐167. [DOI] [PubMed] [Google Scholar]
- 24. Yong M, Jensen AO, Jacobsen JB, Norgaard M, Fryzek JP, Sorensen HT. Survival in breast cancer patients with bone metastases and skeletal‐related events: a population‐based cohort study in Denmark (1999‐2007). Breast Cancer Res Treat. 2011;129:495‐503. [DOI] [PubMed] [Google Scholar]
- 25. Filonov GS, Piatkevich KD, Ting LM, Zhang J, Kim K, Verkhusha VV. Bright and stable near‐infrared fluorescent protein for in vivo imaging. Nat Biotechnol. 2011;29:757‐761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jaggupilli A, Elkord E. Significance of CD44 and CD24 as cancer stem cell markers: an enduring ambiguity. Clin Dev Immunol. 2012;2012:708036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Newman H, Shapira S, Spierer O, et al. Involvement of CD24 in angiogenesis in a mouse model of oxygen‐induced retinopathy. Curr Eye Res. 2012;37:532‐539. [DOI] [PubMed] [Google Scholar]
- 28. Shi Y, Gong HL, Zhou L, Tian J, Wang Y. CD24: a novel cancer biomarker in laryngeal squamous cell carcinoma. ORL J Otorhinolaryngol Relat Spec. 2012;74:78‐85. [DOI] [PubMed] [Google Scholar]
- 29. Smith SC, Oxford G, Baras AS, et al. Expression of ral GTPases, their effectors, and activators in human bladder cancer. Clin Cancer Res. 2007;13:3803‐3813. [DOI] [PubMed] [Google Scholar]
- 30. Sagiv E, Starr A, Rozovski U, et al. Targeting CD24 for treatment of colorectal and pancreatic cancer by monoclonal antibodies or small interfering RNA. Cancer Res. 2008;68:2803‐2812. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


