Abstract
Xantohumol, a prenylated chalcone from hops (Humulus lupulus L.), has been shown to inhibit proliferation in some cancers. However, little is known regarding the effects of xanthohumol in pancreatic cancer. We have previously reported that activation of the transcription factor nuclear factor‐κB (NF‐κB) plays a key role in angiogenesis in pancreatic cancer. In this study, we investigated whether xanthohumol inhibited angiogenesis by blocking NF‐κB activation in pancreatic cancer in vitro and in vivo. We initially confirmed that xanthohumol significantly inhibited proliferation and NF‐κB activation in pancreatic cancer cell lines. Next, we demonstrated that xanthohumol significantly suppressed the expression of vascular endothelial growth factor (VEGF) and interleukin‐8 (IL‐8) at both the mRNA and protein levels in pancreatic cancer cell lines. We also found that coculture with BxPC‐3 cells significantly enhanced tube formation in human umbilical vein endothelial cells, and treatment with xanthohumol significantly blocked this effect. In vivo, the volume of BxPC‐3 subcutaneous xenograft tumors was significantly reduced in mice treated with weekly intraperitoneal injections of xanthohumol. Immunohistochemistry revealed that xanthohumol inhibited Ki‐67 expression, CD31‐positive microvessel density, NF‐κB p65 expression, and VEGF and IL‐8 levels. Taken together, these results showed, for the first time, that xanthohumol inhibited angiogenesis by suppressing NF‐κB activity in pancreatic cancer. Accordingly, xanthohumol may represent a novel therapeutic agent for the management of pancreatic cancer.
Keywords: angiogenesis, interleukin‐8, NF‐κB, pancreatic cancer, xanthohumol
1. INTRODUCTION
Pancreatic cancer is the third leading cause of cancer‐related death in the USA. In 2016, there were an estimated 53 070 new cases of pancreatic cancer and 41 780 pancreatic cancer‐related deaths.1 The death rates for patients with pancreatic cancer are increasing, and pancreatic cancer is predicted to be the second leading cause of cancer‐related death in the USA by 20302 Since 1997, gemcitabine therapy has become the standard treatment for pancreatic cancer.3 Compared with single‐agent gemcitabine, FORFIRINOX and gemcitabine with nab‐paclitaxel have recently been shown to be associated with improved survival;4, 5 however, the effects of this drug combination are largely palliative, and these regimens show increased toxicity. The 5‐year relative survival rate for pancreatic cancer in the USA is only 8%.1 Therefore, there is an urgent need for the development of new therapeutic compounds with low toxicity.
Natural compounds are generally considered to be safe and have been shown to have anti‐inflammatory and anticancer effects. Xanthohumol, a prenylated chalcone, is the principal flavonoid found in the hop plant (Humulus lupulus L.). Xanthohumol has been shown to inhibit the growth of different types of human cancer cells, including breast, colon, hepatocellular, ovarian, pancreatic and prostate cancer cells as well as leukemia cells.6, 7, 8, 9, 10, 11, 12, 13, 14 In addition, xanthohumol has been shown to induce both caspase‐dependent and caspase‐independent apoptosis8, 15 and to inhibit invasion7 and angiogenesis.16 However, the mechanisms through which xanthohumol mediates these effects are not fully understood. Xanthohumol has been shown to inhibit nuclear factor‐kB (NF‐κB) activation.17, 18 In addition, xanthohumol has been shown to have a low toxicity profile and high bioavailability, and orally administered xanthohumol does not affect major organ function in vivo.19, 20, 21
Nuclear factor‐kB is a transcription factor that is associated with cell proliferation, invasion, angiogenesis and metastasis in multiple types of cancer.22 In pancreatic cancer, NF‐κB has also been shown to play a major role in angiogenesis, which is essential for tumor growth and metastasis.23, 24 In our previous studies, we found that angiogenesis is essential for metastasis of pancreatic cancer to the liver.25, 26 Moreover, NF‐κB has been shown to be constitutively activated in pancreatic cancer cells.27 These findings suggest that agents inhibiting NF‐κB activation could reduce angiogenesis in pancreatic cancer. In previous studies, vascular endothelial growth factor (VEGF) and interleukin‐8 (IL‐8) have been identified as key mediators of angiogenesis in pancreatic tumors.28, 29, 30, 31 Indeed, we previously demonstrated that suppression of NF‐κB signaling reduces the production of both VEGF and IL‐8, which are major angiogenic factors, in pancreatic cancer.32 However, the effects of xanthohumol in pancreatic cancer have not been clearly elucidated.
In this study, we initially confirmed that low‐dose xanthohumol inhibited NF‐κB signaling. We next investigated whether xanthohumol suppressed angiogenesis in pancreatic cancer. We found that xanthohumol blocked angiogenesis in pancreatic cancer by reducing both NF‐κB activity and consequent production of angiogenic factors, in vitro and in vivo. This is the first report demonstrating the effects of xanthohumol on pancreatic cancer angiogenesis. In addition, our results suggest that xanthohumol may be useful in treating pancreatic cancer.
2. MATERIALS AND METHODS
2.1. Reagents
Xanthohumol and DMSO were purchased from Sigma‐Aldrich (St. Louis, MO, USA). Xanthohumol was dissolved in DMSO into stock concentrations of 50 mmol/L. Rabbit monoclonal antibodies against Ki67, NF‐κB p65 and VEGF and rabbit polyclonal antibodies against CD31 and IL‐8 were purchased from Abcam plc (Cambridge, UK). Neutralizing monoclonal anti human VEGF and IL‐8 antibody were provided by R&D Systems (Abingdon, UK).
2.2. Cell lines and cell culture
The human pancreatic adenocarcinoma cell lines BxPC‐3, MIA PaCa‐2 and AsPC‐1 were obtained from ATCC (Rockville, MD, USA). BxPC‐3 and AsPC‐1 cells were cultured in Roswell Park Memorial Institute (RPMI‐1640) medium (Sigma Aldrich), and MIA PaCa‐2 cells were cultured in DMEM supplemented with 10% FBS, 10 000 U/mL penicillin, 10 mg/mL streptomycin and 25 μg/mL amphotericin B (Sigma Aldrich) in a 37°C humidified incubator with 5% CO2.
2.3. Cell proliferation assay
The proliferation assay was performed using a Premix WST‐1 Cell Proliferation Assay System (Takara Bio, Japan) according to the manufacturer's instructions. BxPC‐3 (3 × 103), MIA PaCa‐2 (2 × 103) and AsPC‐1 (1 × 103) cells were seeded into each well of a 96‐well plate in a total volume of 100 μL and cultured for 1 day. The cells were then treated with various concentrations (0‐50 μmol/L) of xanthohumol. After 72 hours of incubation, absorbance was measured at 450 nm using a SpectraMax 340 spectrophotometer (Molecular Devices, Sunnyvale, CA, USA).
2.4. Real‐time quantitative RT‐PCR
Total RNA was extracted from cell pellets using an RNeasy Plus Mini Kit (Qiagen, Tokyo, Japan), and total RNA (1 μg) was reverse transcribed using Super Script III First‐Strand Synthesis SuperMix for quantitative RT‐PCR (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's instructions. Quantitative RT‐PCR was carried out using TaqMan Fast Advanced Master Mix (Thermo Fisher Scientific, Waltham, MA, USA) and TaqMan Gene Expression Assays for VEGF (Hs00900055_m1), IL‐8 (Hs01553824_g1), and β‐actin (Hs99999903_m1; Applied Biosystems, Foster City, CA, USA) with an Applied Biosystems 7900HT Fast Real‐Time PCR System (Applied Biosystems). PCR conditions were as follows: fast mode at 95°C for 20 seconds, followed by 40 cycles at 95°C for 1 second and 60°C for 20 seconds. The relative expression levels of VEGF and IL‐8 were normalized to the expression of β‐actin in each sample.
2.5. Analysis of vascular endothelial growth factor and interleukin‐8 levels by ELISA
BxPC‐3 (5 × 104), AsPC‐1 (5 × 104) and MIA PaCa‐2 (2 × 104) cells were seeded into 6‐well plates containing medium with 10% FBS overnight. The medium was then changed, and the cells were cultured for an additional 72 hours in the presence of different concentrations of xanthohumol (0‐5 μmol/L) with 5% FBS. After incubation, the culture medium was collected and centrifuged at 400 g for 5 minutes to remove particles. The supernatants were frozen at −80°C until use. The concentrations of VEGF and IL‐8 were determined using ELISA kits (R&D Systems, Minneapolis, MN, USA) according to the manufacturer's instructions.
2.6. Protein extraction and nuclear factor‐kB p65 activity assays
BxPC‐3, AsPC‐1 and MIA PaCa‐2 cells were seeded in 100‐mm dishes with 10% FBS and cultured to approximately 70% confluence. The cells were then treated with different concentrations of xanthohumol (0‐25 μmol/L) with 2% FBS for 24 hours and the cells were stimulated with or without tumor necrosis factor (TNF)‐α (10 ng/mL) for 30 minutes before the end of the incubation. After the indicated treatment, nuclear extracts were obtained from cells using a Nuclear Extraction kit (Active Motif, Carlsbad, CA, USA). The concentrations of proteins in the nuclear extracts were measured using a Pierce Bradford Protein Assay Kit (Thermo Fisher). The nuclear extracts were stored at −80°C until use. The activity of NF‐κB was measured using a Trans AM NF‐κB p65/p50 Transcription Factor Assay Kit (Active Motif) according to the manufacturer's instructions. Five micrograms of nuclear extracts were used in the NF‐κB activity assay.
2.7. Angiogenesis assay
The influence of VEGF and IL‐8 on tube formation by HUVEC in the presence of pancreatic cancer cells was investigated in triplicate using an Angiogenesis Kit (Kurabo, Osaka, Japan) according to the manufacturer's instructions. We cocultured pancreatic cancer cells (BxPC‐3), HUVEC and fibroblasts using a double‐chamber method in 24‐well plates. BxPC‐3 cells (2 × 103 cells) were seeded into transwell chambers and allowed to adhere overnight. The chambers were then placed into the HUVEC/fibroblast coculture system. The basal medium only (control) or basal medium containing anti‐VEGF antibody (10 μg/mL) or anti‐IL‐8 antibody (10 μg/mL) was added and changed every 3 days. Cells were cultured for a total of 11 days. HUVEC were then stained with anti‐CD31 antibody according to the manufacturer's protocols. The tube formation area was measured quantitatively over 15 different fields for each condition using an image analyzer (Kurabo). The influence of xanthohumol on pancreatic cancer‐induced tube formation was examined in triplicate using an Angiogenesis Kit (Kurabo) according to the manufacturer's instructions. Pancreatic cancer cells (BxPC‐3) were cocultured with HUVEC and fibroblasts using a double chamber method in 24‐well plates. BxPC‐3 cells (2 × 103 cells) were seeded into transwell chambers and cultured for 1 day; the chambers were then placed into the HUVEC/fibroblast coculture system. The optimized medium was added with various concentrations of xanthohumol (0‐1 μmol/L), and medium was refreshed every 3 days. Cells were cultured for 11 days, and the HUVEC tube formation was evaluated as described above.
2.8. Animals
Female BALB/c nu‐nu mice (6 weeks old) were purchased from Charles River Laboratories (Sulzbach, Germany). The animals were housed in standard Plexiglas cages (5 per cage) in a room maintained at constant temperature and humidity with a 12‐hour light/dark cycle. Mice were fed regular autoclaved chow and provided with water ad libitum. All experiments were conducted according to the Guidelines for Animal Experiments of the Nagoya City University Graduate School of Medical Sciences and approved by the Animal Care and Use Committee of the Nagoya City University Graduate School of Medical Sciences.
2.9. Experimental protocol
BxPC‐3 human pancreatic cancer cells (5 × 106 cells in 100 μL Hank's balanced salt solution) were injected subcutaneously into the right flank of each mouse. Tumor volume was measured once a week with a caliper. When the tumor size reached a volume of approximately 100 mm3, the mice were randomly divided into 2 groups (5 mice per group); mice in group I were not treated, whereas mice in group II were given xanthohumol (10 mg/kg/wk). We calculated the tumor volume according to the following formula: tumor volume (mm3) = (A × B2)/2, where A and B represented the longest and the shortest diameters in millimeters, respectively. The mice were injected intraperitoneally with DMSO or xanthohumol once a week for 5 weeks and then sacrificed 1 week later. Finally, the tumors were harvested from mice and fixed in formaldehyde for further analysis.
2.10. Histopathological examination
Subcutaneous xenograft tumors were fixed with paraformaldehyde and embedded in paraffin. The sections were mounted on 3‐amino‐propyltriethoxylsilane‐coated slides. Dewaxed paraffin sections were placed in a microwave (10 minutes, 600 watts) to recover antigens before staining. Antibodies used were as follows: rabbit monoclonal anti‐Ki67 antibodies (ab92742; 1:500), rabbit polyclonal anti‐CD31 antibodies (ab124432; 1:1000), rabbit monoclonal anti‐NF‐κB p65 antibodies (ab32536; 1:100), rabbit monoclonal anti‐VEGFA antibodies (ab52917; 1:100) and rabbit polyclonal anti‐IL‐8 antibodies (ab7747; 1:100; all from Abcam), followed by biotin‐conjugated secondary antibodies. Peroxidase‐conjugated streptavidin was used with 3,3‐diaminobenzidine tetrahydrochloride (DAB; Biocare Medical, Concord, CA, USA) as the chromogen for detection. Hematoxylin was used for nuclear counterstaining.
Proliferation was quantified by determining the percentage of Ki67‐positive cells. Results were expressed as the mean number of Ki67‐positive cells ± the SD per high‐power field. Ten fields were examined and counted from 3 tumors for each of the 2 treatment groups. For quantification of microvessel density (MVD) in sections stained for CD31, any distinct area of positive staining for CD31 was counted as a single vessel. Results were expressed as the mean number of vessels ± SD per high‐power field. Ten high‐power fields were examined and counted from 3 tumors for each of the 2 treatment groups. For quantification of activation of NF‐κB p65, the numbers of nuclear‐positive cells were counted. Nuclear translocation of p65 was considered a marker of NF‐κB activation. The numbers of NF‐κB p65‐positive (in nuclear), VEGF‐positive and IL‐8‐positive cells were expressed as percentages of the total number of cells that were randomly counted in 10 high‐power fields from 3 tumors of each of the 2 treatment groups for quantitative analysis.
2.11. Statistical analysis
All experimental data were presented as the mean ± SD. Multiple group comparisons were performed using 1‐way ANOVA with post‐hoc Dunnett tests for subsequent individual group comparisons. Comparisons between 2 groups were performed using Student's t‐tests. Results with P‐values of <.05 were considered significant.
3. RESULTS
3.1. Xanthohumol inhibited proliferation in pancreatic cancer cells
The effects of xanthohumol on the proliferation of human pancreatic cancer cells (BxPC‐3, AsPC‐1 and MIA PaCa‐2 cells) were examined using WST‐1 assays after incubation with various concentrations of xanthohumol (0‐25 μmol/L) for 72 hours. As shown in Figure 1A, xanthohumol (>10 μmol/L) significantly inhibited the proliferation of all 3 pancreatic cancer cell lines in a concentration‐dependent manner.
Figure 1.
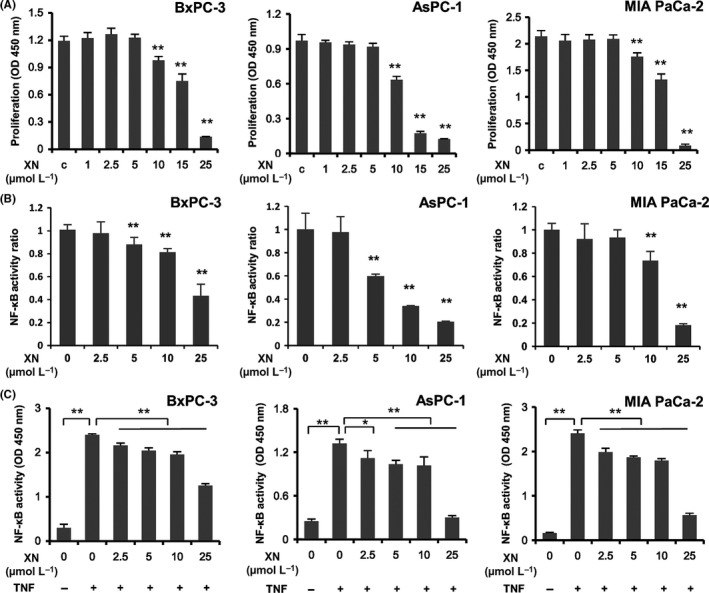
A, Effects of xanthohumol on the proliferation of pancreatic cancer cell lines. Pancreatic cancer cell lines (BxPC‐3, AsPC‐1 and MIA PaCa‐2) were treated with xanthohumol at the indicated concentrations for 72 h, and cytotoxicity was measured using WST‐1 assays. Values are expressed as means ± SD (n = 6; *P < .05; **P < .01 compared with the control in all cell lines). XN, xanthohumol; C, control. B, C, Xanthohumol inhibited NF‐κB activity in pancreatic cancer cell lines. Pancreatic cancer cell lines (BxPC‐3, AsPC‐1 and MIA PaCa‐2) were treated with xanthohumol at the indicated concentrations for 24 h (B) and then stimulated with TNF‐α (10 ng/mL) for 30 min (C). The activity of NF‐κB in nuclear extracts was measured using Trans AM NF‐κB p65/p50 Transcription Factor Assays. Values are expressed as means ± SD (n = 3; *P < .05; **P < .01 compared with the control in all cell lines). XN, xanthohumol
3.2. Xanthohumol inhibited nuclear factor‐kB activation in pancreatic cancer cells
To examine the effects of xanthohumol on the activity of NF‐κB, we performed Trans AM NF‐κB p65/p50 Transcription Factor Assays. As shown in Figure 1B,C, treatment with various concentrations of xanthohumol for 24 hours significantly inhibited NF‐κB activation under treatment with or without TNF‐α in all 3 pancreatic cancer cell lines in a concentration‐dependent manner. Interestingly, xanthohumol decreased the activity of NF‐κB when used at a low concentration that did not affect the proliferation of the pancreatic cancer cells.
3.3. Xanthohumol suppressed VEGF and IL‐8 mRNA expression in pancreatic cancer cells
To determine whether xanthohumol inhibited VEGF and IL‐8 mRNA expression, we performed real‐time qPCR. As shown in Figure 2, VEGF and IL‐8 mRNA levels were significantly suppressed by xanthohumol treatment in a concentration‐dependent manner.
Figure 2.
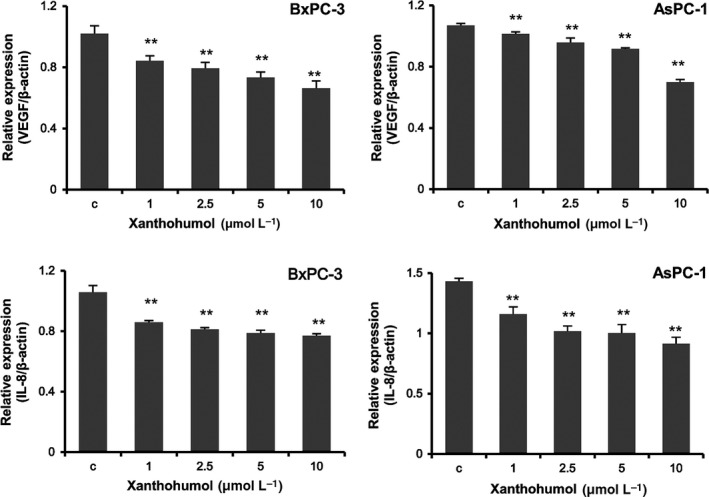
Xanthohumol suppressed VEGF and IL‐8 mRNA expression in pancreatic cancer cells. Pancreatic cancer cell lines (BxPC‐3 and AsPC‐1) were treated with xanthohumol at the indicated concentrations for 48 h. VEGF and IL‐8 mRNA levels were measured using real‐time qPCR (normalized to β‐actin expression). Values are expressed as means ± SDs (n = 3; *P < .05; **P < .01 compared with the control in all cell lines). C, control
3.4. Xanthohumol suppressed the secretion of vascular endothelial growth factor and interleukin‐8 in pancreatic cancer cells
To determine whether the reduction in mRNA expression led to a reduction in protein expression, we measured the concentrations of secreted VEGF and IL‐8 after treatment with xanthohumol using ELISA. As shown in Figure 3, xanthohumol significantly inhibited the secretion of VEGF and IL‐8 in a concentration‐dependent manner (>1 μmol/L).
Figure 3.
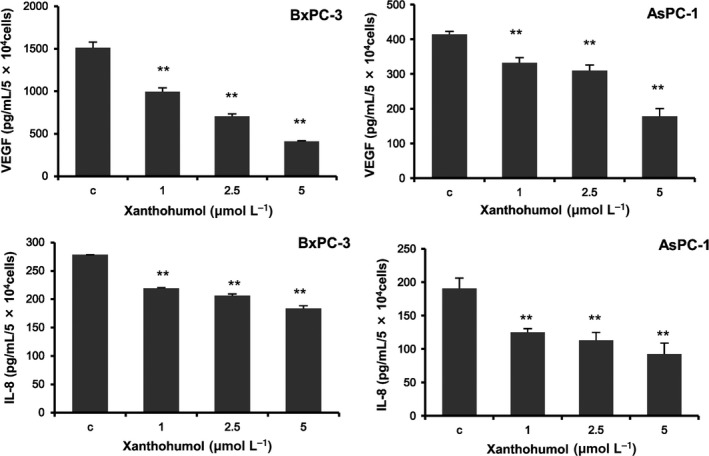
Xantohumol suppressed the secretion of VEGF and IL‐8 in pancreatic cancer cell lines. Pancreatic cancer cell lines (BxPC‐3 and AsPC‐1) were treated with xanthohumol at the indicated concentrations for 72 h, and the supernatants were then collected. The concentrations of secreted VEGF and IL‐8 were measured using ELISA. Values are expressed as means ± SD (n = 3; *P < .05; **P < .01 compared with the control in all cell lines). C, control
3.5. Xanthohumol inhibited tube formation by HUVEC in coculture with BxPC‐3 cells
To examine the effects of BxPC‐3‐derived VEGF and IL‐8 on tube formation by HUVEC, we performed in vitro angiogenesis assays using neutralizing antibody against VEGF and IL‐8. As shown in Figure 4, anti‐VEGF and anti‐IL‐8 antibody suppressed tube formation induced by BxPC‐3 cells. To examine the effects of xanthohumol on tube formation by HUVEC, we performed in vitro angiogenesis assays. As shown in Figure 4, coculture with BxPC‐3 cells significantly enhanced tube formation in HUVEC, and this enhancement was significantly inhibited by treatment with xanthohumol in a concentration‐dependent manner (>0.5 μmol/L).
Figure 4.
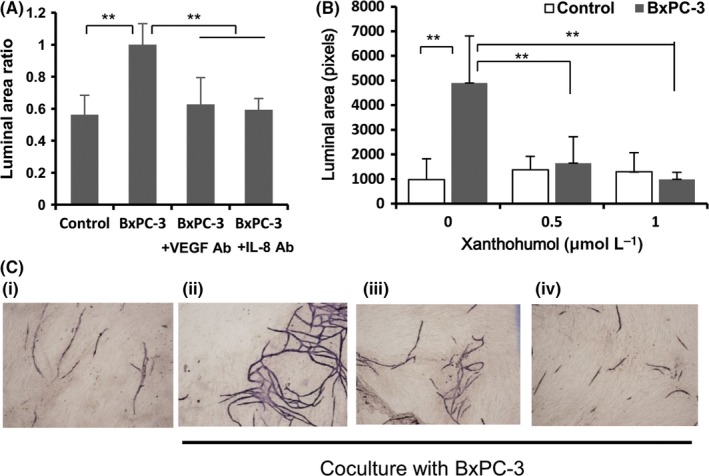
Xanthohumol inhibited BxPC‐3‐induced tube formation by HUVEC in vitro. A, Effects of anti‐VEGF (10 μg/mL) and anti‐IL‐8 (10 μg/mL) antibody on tube formation by HUVEC. B, Effects of xanthohumol on tube formation by HUVEC. Tube formation assays were performed using an angiogenesis kit. Values are expressed as means ± SD (n = 3; *P < .05; **P < .01 compared with the untreated group). C, (i) control; (ii) coculture with BxPC‐3 cells; (iii) coculture with BxPC‐3 cells treated with xanthohumol (0.5 μmol/L); and (iv) coculture with BxPC‐3 cells treated with xanthohumol (1 μmol/L). Representative images are shown (40×)
3.6. Xanthohumol suppressed tumor growth and angiogenesis in a subcutaneous xenograft model
To determine the antitumor effects of xanthohumol in vivo, a BxPC‐3 subcutaneous xenograft model was developed in nude mice. Once the tumors were developed, xanthohumol (10 mg/kg) or vehicle was administered weekly by intraperitoneal injection for 5 weeks. As shown in Figure 5, xanthohumol significantly inhibited tumor growth 21 days after treatment (Figure 5B,C). Moreover, loss of body weight was not observed, and body weights were similar between the 2 groups (Figure 5D).
Figure 5.
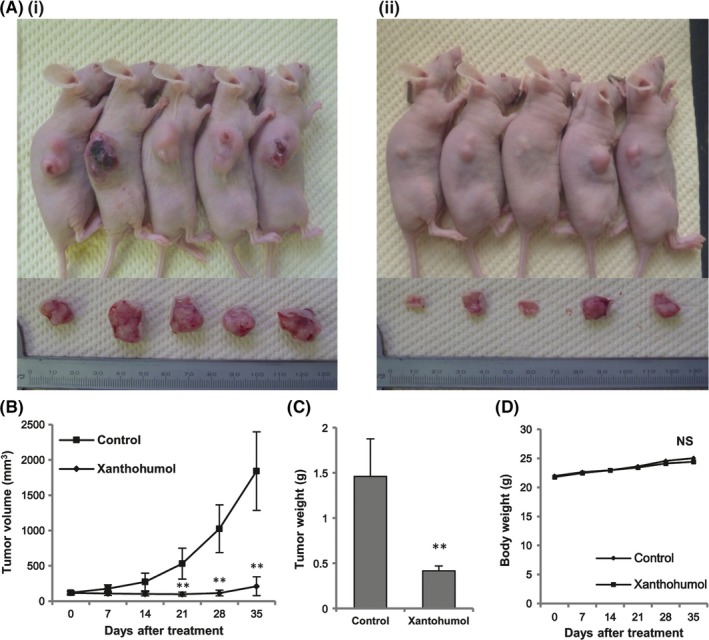
Xanthohumol inhibited tumor growth in a subcutaneous xenograft model of pancreatic cancer. BxPC‐3 cells (5 × 106 cells in 100 μL Hank's balanced salt solution) were injected subcutaneously, and tumors were developed. Mice were divided into 2 groups: untreated (group I) and treated with xanthohumol (10 mg/kg, ip, injection, weekly; group II). A, Images of mice and solid tumors removed from each group: (i) control and (ii) xanthohumol. B, Measurement of tumor volumes in each group once per week. C, Measurement of tumor weights in nude mice after sacrifice. D, Measurement of body weights in each group once per week. Values in (B), (C) and (D) represent the means ± SD of each group (n = 5; *P < .05, **P < .01 compared with the untreated group). NS, not significant
3.7. Xanthohumol inhibited proliferation and angiogenesis and reduced the expression of nuclear factor‐kB p65, vascular endothelial growth factor and interleukin‐8
To determine the effects of xanthohumol on proliferation and angiogenesis, we examined the expression of Ki‐67, the cell proliferation marker, and CD31, an MVD marker, in tumor tissue. As shown in Figure 6, xanthohumol significantly downregulated the expression of Ki‐67 and CD31. We next examined both the activation of NF‐κB p65 and the expression of angiogenic factor (VEGF and IL‐8). As shown in Figures 6 and 7, xanthohumol significantly decreased both the activation of NF‐κB p65 and the expression of VEGF and IL‐8 in tumor tissues. As shown in Figure 7, xanthohumol significantly suppressed VEGF and IL‐8 mRNA expression in pancreatic cancer tumors.
Figure 6.
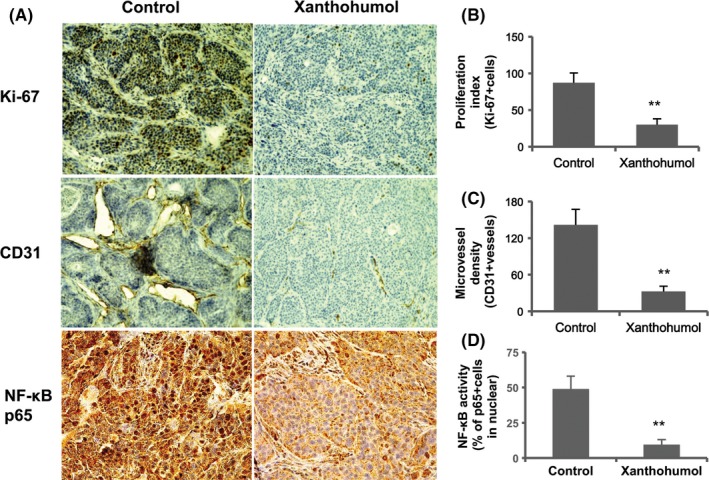
Xanthohumol inhibited proliferation and angiogenesis and reduced the activation of NF‐κB subunit p65 in pancreatic cancer tumors. A, Representative immunohistochemical analysis. Representative images are shown (200×: Ki‐67 and CD31, 400×: p65). B, Quantification of Ki‐67‐positive cells. C, Quantification of microvessel density. D, Quantification of the activation of NF‐κB p65. Values in (B)‐(D) represent the means ± SD of each group. *P < .05, **P < .01 compared with the untreated group
Figure 7.
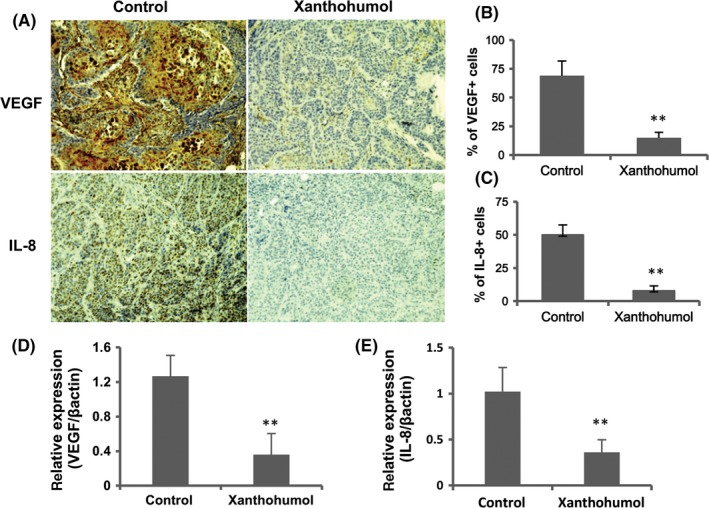
Xanthohumol reduced the expression of VEGF and IL‐8 in pancreatic cancer tumors. A, Representative immunohistochemical analysis. Representative images are shown (200×). B, Quantification of VEGF. C, Quantification of IL‐8. D, E, Xanthohumol suppressed VEGF and IL‐8 mRNA expression in pancreatic cancer tumors. Values in (B)‐(E) represent the means ± SD of each group. *P < .05, **P < .01 compared with the untreated group
4. DISCUSSION
The aim of this study was to determine whether xanthohumol, a flavonoid compound isolated from the hop plant (Humulus lupulus L.), could inhibit angiogenesis by blocking NF‐κB activation in pancreatic cancer. Our data demonstrated that xanthohumol inhibited both proliferation and NF‐κB activation and suppressed the expression of VEGF and IL‐8 in various human pancreatic cancer cell lines. In addition, we confirmed that xanthohumol suppressed tube formation, which was induced by pancreatic cancer, in an in vitro angiogenesis model. Finally, xanthohumol inhibited tumor growth and angiogenesis in a subcutaneous xenograft model in vivo.
Previous studies have shown that most pancreatic cancer cells (approximately 70%) exhibit constitutive activation of NF‐κB.23 Moreover, NF‐κB is thought to have an important role in the regulation of apoptosis, oncogenesis and angiogenesis.23, 33 Indeed, we previously reported that blockade of NF‐κB activity by the proteasome inhibitor MG132 inhibited the expression of the pro‐angiogenic molecules VEGF and IL‐8 and suppressed cancer‐induced angiogenesis.32 These reports were consistent with our results demonstrating that xanthohumol inhibited the expression of VEGF and IL‐8 by blocking NF‐κB activation in pancreatic cancer.
The aggressive growth and metastasis of solid tumors, including pancreatic cancer, depend on angiogenesis, which is mediated by angiogenic factors derived from tumor and stromal cells.34 Various pro‐angiogenic molecules secreted by pancreatic cells, including VEGF and IL‐8, have been identified as key mediators of pancreatic cancer angiogenesis.28, 30 Thus, it is reasonable to assume that inhibiting NF‐κB activation, which leads to suppression of VEGF and IL‐8 expression, would suppress angiogenesis in pancreatic cancer. In our study, we demonstrated that xanthohumol suppressed cancer‐induced tube formation in HUVEC in an in vitro angiogenesis assay.
Importantly, this is the first report showing that xanthohumol inhibited angiogenesis by blocking NF‐κB activation in pancreatic cancer. We hypothesized that xanthohumol may not only have direct antiproliferative effects on pancreatic cancer cells at higher concentrations but may also have anti‐angiogenic effects through suppression of VEGF and IL‐8 expression in pancreatic cancer cells at lower concentrations. Xanthohumol, at concentrations >10 μmol/L, inhibited proliferation in pancreatic cancer cells in our in vitro analysis. However, when used at concentrations of <5 μmol/L, xanthohumol inhibited NF‐κB‐dependent angiogenic activities in pancreatic cancer cells. At this concentration, we did not observe any cytotoxicity in WST‐1 assays in pancreatic cancer cells. Thus, we concluded that xanthohumol affected pancreatic cancer‐induced angiogenesis through downregulation of VEGF and IL‐8 production, which was specific and mediated through NF‐κB inactivation.
In addition to our in vitro results, our findings showed that xanthohumol significantly inhibited tumor growth in a subcutaneous xenograft model. Our immunohistochemistry analysis showed that xanthohumol suppressed 2 major markers: the proliferation marker Ki‐67 and the MVD marker CD31. We also demonstrated that xanthohumol downregulated both the activation of NF‐κB p65 and the expression of angiogenic factor (VEGF and IL‐8). Thus, our results suggested that xanthohumol inhibited angiogenesis in pancreatic cancer by downregulating these angiogenic proteins in vivo. Because the focus of this study is to examine the efficacy of xanthohumol on pancreatic cancer growth and tumorigenesis from the standpoint of angiogenesis, we used a subcutaneous injection model and successfully demonstrated that xanthohumol could inhibit pancreatic cancer growth by blocking NF‐kB and its downstream angiogenic targets (VEGF and IL‐8) in vitro and in vivo. Because our next focus is the efficacy of xanthohumol on pancreatic cancer metastasis, in the near future, we intend to perform an intra‐splenic liver metastasis assay using 2 cancer cell lines.
In this study, we focused on the effects of natural products, which are generally regarded as safe and nontoxic. Indeed, our findings demonstrated that xanthohumol did not affect the body weights of nude mice, consistent with the results of previous studies. In a previous report, orally administered xanthohumol was shown to have no adverse effects on the functions of major organs19 and did not affect female fertility or the development of offspring of Sprague‐Dawley rats.20 Chemicals that inhibit NF‐κB activation, such as bortezomib (a protease inhibitor), have various side effects (eg, acute lung injury, peripheral neuropathy, tumor lysis syndrome and myelosuppression). Therefore, it is very difficult to use these drugs in combination with chemotherapies for treating pancreatic cancer.35, 36 However, because we showed that xanthohumol has few adverse effects, xanthohumol may not be harmful to humans.
Overall, our results showed, for the first time, that xanthohumol inhibited angiogenesis by blocking NF‐κB and its downstream targets, leading to the inhibition of angiogenesis, even at low concentrations. Because low‐dose xanthohumol did not block normal physiological function, use of the natural product xanthohumol may be safer and may show reduced toxicity compared with other available treatments. Therefore, we concluded that xanthohumol may have significant potential as an effective therapy for pancreatic cancer.
CONFLICT OF INTEREST
The authors declare no potential conflicts of interest.
ACKNOWLEDGMENTS
This work was supported by a grant from the Japan Society for the Promotion of Science (KAKENHI Grant Number 17 K10708).
Saito K, Matsuo Y, Imafuji H, et al. Xanthohumol inhibits angiogenesis by suppressing nuclear factor‐κB activation in pancreatic cancer. Cancer Sci. 2018;109:132–140. https://doi.org/10.1111/cas.13441
Funding information
Japan Society for the Promotion of Science: KAKENHI Grant Number 17 K10708.
REFERENCES
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7‐30. [DOI] [PubMed] [Google Scholar]
- 2. Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913‐2921. [DOI] [PubMed] [Google Scholar]
- 3. Burris HA 3rd, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first‐line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403‐2413. [DOI] [PubMed] [Google Scholar]
- 4. Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817‐1825. [DOI] [PubMed] [Google Scholar]
- 5. Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab‐paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691‐1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gerhauser C, Alt A, Heiss E, et al. Cancer chemopreventive activity of xanthohumol, a natural product derived from hop. Mol Cancer Ther. 2002;1:959‐969. [PubMed] [Google Scholar]
- 7. Vanhoecke B, Derycke L, Van Marck V, Depypere H, De Keukeleire D, Bracke M. Antiinvasive effect of xanthohumol, a prenylated chalcone present in hops (Humulus lupulus L.) and beer. Int J Cancer. 2005;117:889‐895. [DOI] [PubMed] [Google Scholar]
- 8. Pan L, Becker H, Gerhauser C. Xanthohumol induces apoptosis in cultured 40‐16 human colon cancer cells by activation of the death receptor‐ and mitochondrial pathway. Mol Nutr Food Res. 2005;49:837‐843. [DOI] [PubMed] [Google Scholar]
- 9. Dorn C, Weiss TS, Heilmann J, Hellerbrand C. Xanthohumol, a prenylated chalcone derived from hops, inhibits proliferation, migration and interleukin‐8 expression of hepatocellular carcinoma cells. Int J Oncol. 2010;36:435‐441. [PubMed] [Google Scholar]
- 10. Miranda CL, Stevens JF, Helmrich A, et al. Antiproliferative and cytotoxic effects of prenylated flavonoids from hops (Humulus lupulus) in human cancer cell lines. Food Chem Toxicol. 1999;37:271‐285. [DOI] [PubMed] [Google Scholar]
- 11. Kunnimalaiyaan S, Trevino J, Tsai S, Gamblin TC, Kunnimalaiyaan M. Xanthohumol‐mediated suppression of Notch1 signaling is associated with antitumor activity in human pancreatic cancer cells. Mol Cancer Ther. 2015;14:1395‐1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vene R, Benelli R, Minghelli S, Astigiano S, Tosetti F, Ferrari N. Xanthohumol impairs human prostate cancer cell growth and invasion and diminishes the incidence and progression of advanced tumors in TRAMP mice. Mol Med. 2012;18:1292‐1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Harikumar KB, Kunnumakkara AB, Ahn KS, et al. Modification of the cysteine residues in IkappaBalpha kinase and NF‐kappaB (p65) by xanthohumol leads to suppression of NF‐kappaB‐regulated gene products and potentiation of apoptosis in leukemia cells. Blood. 2009;113:2003‐2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Monteghirfo S, Tosetti F, Ambrosini C, et al. Antileukemia effects of xanthohumol in Bcr/Abl‐transformed cells involve nuclear factor‐kappaB and p53 modulation. Mol Cancer Ther. 2008;7:2692‐2702. [DOI] [PubMed] [Google Scholar]
- 15. Delmulle L, Vanden Berghe T, Keukeleire DD, Vandenabeele P. Treatment of PC‐3 and DU145 prostate cancer cells by prenylflavonoids from hop (Humulus lupulus L.) induces a caspase‐independent form of cell death. Phytother Res. 2008;22:197‐203. [DOI] [PubMed] [Google Scholar]
- 16. Albini A, Dell'Eva R, Vene R, et al. Mechanisms of the antiangiogenic activity by the hop flavonoid xanthohumol: NF‐kappaB and Akt as targets. FASEB J. 2006;20:527‐529. [DOI] [PubMed] [Google Scholar]
- 17. Dell'Eva R, Ambrosini C, Vannini N, Piaggio G, Albini A, Ferrari N. AKT/NF‐kappaB inhibitor xanthohumol targets cell growth and angiogenesis in hematologic malignancies. Cancer. 2007;110:2007‐2011. [DOI] [PubMed] [Google Scholar]
- 18. Colgate EC, Miranda CL, Stevens JF, Bray TM, Ho E. Xanthohumol, a prenylflavonoid derived from hops induces apoptosis and inhibits NF‐kappaB activation in prostate epithelial cells. Cancer Lett. 2007;246:201‐209. [DOI] [PubMed] [Google Scholar]
- 19. Vanhoecke BW, Delporte F, Van Braeckel E, et al. A safety study of oral tangeretin and xanthohumol administration to laboratory mice. In Vivo. 2005;19:103‐107. [PubMed] [Google Scholar]
- 20. Hussong R, Frank N, Knauft J, et al. A safety study of oral xanthohumol administration and its influence on fertility in Sprague Dawley rats. Mol Nutr Food Res. 2005;49:861‐867. [DOI] [PubMed] [Google Scholar]
- 21. Hanske L, Hussong R, Frank N, Gerhauser C, Blaut M, Braune A. Xanthohumol does not affect the composition of rat intestinal microbiota. Mol Nutr Food Res. 2005;49:868‐873. [DOI] [PubMed] [Google Scholar]
- 22. Aggarwal BB. Nuclear factor‐kappaB: the enemy within. Cancer Cell. 2004;6:203‐208. [DOI] [PubMed] [Google Scholar]
- 23. Xiong HQ, Abbruzzese JL, Lin E, Wang L, Zheng L, Xie K. NF‐kappaB activity blockade impairs the angiogenic potential of human pancreatic cancer cells. Int J Cancer. 2004;108:181‐188. [DOI] [PubMed] [Google Scholar]
- 24. Fujioka S, Sclabas GM, Schmidt C, et al. Function of nuclear factor kappaB in pancreatic cancer metastasis. Clin Cancer Res. 2003;9:346‐354. [PubMed] [Google Scholar]
- 25. Matsuo Y, Sawai H, Funahashi H, et al. Enhanced angiogenesis due to inflammatory cytokines from pancreatic cancer cell lines and relation to metastatic potential. Pancreas. 2004;28:344‐352. [DOI] [PubMed] [Google Scholar]
- 26. Matsuo Y, Sawai H, Ochi N, et al. Interleukin‐1alpha secreted by pancreatic cancer cells promotes angiogenesis and its therapeutic implications. J Surg Res. 2009;153:274‐281. [DOI] [PubMed] [Google Scholar]
- 27. Wang W, Abbruzzese JL, Evans DB, Larry L, Cleary KR, Chiao PJ. The nuclear factor‐kappa B RelA transcription factor is constitutively activated in human pancreatic adenocarcinoma cells. Clin Cancer Res. 1999;5:119‐127. [PubMed] [Google Scholar]
- 28. Itakura J, Ishiwata T, Friess H, et al. Enhanced expression of vascular endothelial growth factor in human pancreatic cancer correlates with local disease progression. Clin Cancer Res. 1997;3:1309‐1316. [PubMed] [Google Scholar]
- 29. Shi Q, Le X, Abbruzzese JL, et al. Constitutive Sp1 activity is essential for differential constitutive expression of vascular endothelial growth factor in human pancreatic adenocarcinoma. Cancer Res. 2001;61:4143‐4154. [PubMed] [Google Scholar]
- 30. Shi Q, Abbruzzese JL, Huang S, Fidler IJ, Xiong Q, Xie K. Constitutive and inducible interleukin 8 expression by hypoxia and acidosis renders human pancreatic cancer cells more tumorigenic and metastatic. Clin Cancer Res. 1999;5:3711‐3721. [PubMed] [Google Scholar]
- 31. Matsuo Y, Ochi N, Sawai H, et al. CXCL8/IL‐8 and CXCL12/SDF‐1alpha co‐operatively promote invasiveness and angiogenesis in pancreatic cancer. Int J Cancer. 2009;124:853‐861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Matsuo Y, Sawai H, Ochi N, et al. Proteasome inhibitor MG132 inhibits angiogenesis in pancreatic cancer by blocking NF‐kappaB activity. Dig Dis Sci. 2010;55:1167‐1176. [DOI] [PubMed] [Google Scholar]
- 33. Shi Q, Le X, Wang B, et al. Regulation of vascular endothelial growth factor expression by acidosis in human cancer cells. Oncogene. 2001;20:3751‐3756. [DOI] [PubMed] [Google Scholar]
- 34. Folkman J. Angiogenesis and angiogenesis inhibition: an overview. EXS. 1997;79:1‐8. [DOI] [PubMed] [Google Scholar]
- 35. Alberts SR, Foster NR, Morton RF, et al. PS‐341 and gemcitabine in patients with metastatic pancreatic adenocarcinoma: a North Central Cancer Treatment Group (NCCTG) randomized phase II study. Ann Oncol. 2005;16:1654‐1661. [DOI] [PubMed] [Google Scholar]
- 36. San Miguel J, Blade J, Boccadoro M, et al. A practical update on the use of bortezomib in the management of multiple myeloma. Oncologist. 2006;11:51‐61. [DOI] [PubMed] [Google Scholar]


