Abstract
In the phase III RADIANT‐4 study, everolimus improved median progression‐free survival (PFS) by 7.1 months in patients with advanced, progressive, well‐differentiated (grade 1 or grade 2), non‐functional lung or gastrointestinal neuroendocrine tumors (NETs) vs placebo (hazard ratio, 0.48; 95% confidence interval [CI], 0.35‐0.67; P < .00001). This exploratory analysis reports the outcomes of the subgroup of patients with lung NETs. In RADIANT‐4, patients were randomized (2:1) to everolimus 10 mg/d or placebo, both with best supportive care. This is a post hoc analysis of the lung subgroup with PFS, by central radiology review, as the primary endpoint; secondary endpoints included objective response rate and safety measures. Ninety of the 302 patients enrolled in the study had primary lung NET (everolimus, n = 63; placebo, n = 27). Median PFS (95% CI) by central review was 9.2 (6.8‐10.9) months in the everolimus arm vs 3.6 (1.9‐5.1) months in the placebo arm (hazard ratio, 0.50; 95% CI, 0.28‐0.88). More patients who received everolimus (58%) experienced tumor shrinkage compared with placebo (13%). Most frequently reported (≥5% incidence) grade 3‐4 drug‐related adverse events (everolimus vs. placebo) included stomatitis (11% vs. 0%), hyperglycemia (10% vs. 0%), and any infections (8% vs. 0%). In patients with advanced, progressive, well‐differentiated, non‐functional lung NET, treatment with everolimus was associated with a median PFS improvement of 5.6 months, with a safety profile similar to that of the overall RADIANT‐4 cohort. These results support the use of everolimus in patients with advanced, non‐functional lung NET. The trial is registered with ClinicalTrials.gov (no. NCT01524783).
Keywords: everolimus, lung carcinoid, neuroendocrine tumors, progression‐free survival, RADIANT‐4
Abbreviations
- AC
atypical carcinoid
- AE
adverse event
- CI
confidence interval
- GI
gastrointestinal
- HR
hazard ratio
- NET
neuroendocrine tumor
- OS
overall survival
- PFS
progression‐free survival
- PRRT
peptide receptor radionuclide therapy
- SSA
somatostatin analog
- TC
typical carcinoid
1. INTRODUCTION
Neuroendocrine tumors are a diverse group of malignancies arising from neuroendocrine cells throughout the body.1 The majority (>90%) of NETs originating from the lungs are non‐hormone secreting (i.e., non‐functional).2 Lung carcinoids (otherwise called lung NETs) represent 22%‐27% of all NETs3, 4 and approximately 1%‐2% of all primary lung cancers.5 The annual age‐adjusted incidence of lung NETs increased approximately 5‐fold from 1973 to 2004.1 Neuroendocrine neoplasms originating in the lungs are categorized into well‐differentiated (low grade to intermediate grade), otherwise termed as NETs, and poorly differentiated (high grade), named neuroendocrine carcinomas. The well‐differentiated subgroup includes TC and AC, comprising 2% and 0.2% of all lung cancers, respectively.6 Approximately 4% of TC and 26% of AC develop distant metastases.7 According to an analysis of the Surveillance, Epidemiology and End Results registry, the 5‐year survival rate in patients with well‐differentiated lung NETs with distant metastases is 27%.1
Platinum and etoposide represent a relatively standard treatment in poorly differentiated lung neuroendocrine carcinoma, such as large‐cell neuroendocrine carcinoma and small‐cell lung cancer.8, 9 By contrast, therapeutic approaches are more varied for well‐differentiated lung NETs, with several different potentially active therapies being explored in this patient group, including SSAs,10 PRRT,11, 12 molecular targeted agents,13, 14, 15 and some chemotherapeutics such as alkylating agents, oxaliplatin, and fluoropyrimidines.16, 17, 18 However, substantial evidence regarding the efficacy of these agents as antiproliferative therapies is lacking. Therefore, no antitumor therapy has yet been approved for patients with lung NETs.
Everolimus, an oral selective inhibitor of mTOR, a key component that mediates cell growth, proliferation, and survival, has shown antitumor activity in patients with advanced NETs.19, 20, 21 In the phase III RADIANT‐4 study, comprising of patients with advanced, progressive, well‐differentiated, non‐functional lung or GI NET (n = 302), everolimus showed an improvement in median PFS of 7.1 months and a 52% reduction in the risk of disease progression or death (HR, 0.48; 95% CI, 0.35‐0.67; P < .00001) compared with placebo.22 The present analysis aimed to further explore the efficacy and safety of everolimus in the lung subgroup of the RADIANT‐4 study cohort.
2. MATERIALS AND METHODS
2.1. Study design
RADIANT‐4 was a prospective, double‐blind, randomized, parallel‐group, placebo‐controlled, multicenter phase III study in which everolimus 10 mg/d was compared with placebo, both with best supportive care, in patients with advanced, progressive, well‐differentiated (grade 1 or grade 2), non‐functional lung or GI NET with no history of or active symptoms of carcinoid syndrome (NCT01524783). Details of the study design were published previously.22 Of the 388 patients screened, 302 were randomized in a 2:1 ratio to receive everolimus or placebo. Patients were treated until radiologically documented disease progression, intolerable toxicity, death, withdrawal of consent, or loss to follow‐up. Patients were prospectively stratified based on prior SSA therapy (yes or no), tumor origin (stratum A vs. B; based on prognostic level, stratum A [better prognosis]: appendix, cecum, jejunum, ileum, duodenum, or NET of unknown primary; stratum B [worse prognosis]: lung, stomach, rectum, and colon except cecum), and WHO performance status (0 vs. 1). Crossover was not allowed prior to primary analyses. If the primary analyses results indicated a statistically significant improvement in PFS, the remaining patients in the placebo arm could be provided with the option to crossover to open‐label treatment with everolimus, based on the feedback from the data monitoring committee.
2.2. Patients
Adult patients (≥18 years) with pathologically confirmed, advanced (unresectable or metastatic), well‐differentiated (grade 1 or grade 2 per 2010 WHO gastroenteropancreatic NET classification),23, 24 non‐functional NET of the lung or GI origin were eligible for enrollment if radiologically progressing and enrolled within 6 months from diagnosis of progression before randomization. In addition to the treatment‐naïve patients, patients who discontinued previous treatments like SSA (for tumor control), interferon, or one line of chemotherapy for ≥4 weeks and PRRT for ≥6 months prior to randomization were allowed, if they had disease progression after their last treatment. Patients with poorly differentiated or high‐grade neuroendocrine carcinoma were excluded from the study. Also, patients with pancreatic NET, or carcinoid syndrome, history of one or more lines of chemotherapy, targeted therapy, therapy with mTOR inhibitors, hepatic intra‐arterial embolization within 6 months, or cryoablation or radiofrequency ablation of hepatic metastases within 2 months of randomization, or chronic treatment with corticosteroids or other immunosuppressive agents were excluded from the study. The study was carried out in accordance with Good Clinical Practice, the ethical principles outlined in the Declaration of Helsinki, and local regulations. An independent ethics committee or institutional review board at each participating center reviewed and approved the study and all protocol amendments. All patients provided written informed consent. An independent data monitoring committee provided ongoing oversight of safety and study conduct.
2.3. Assessment
All patients who underwent randomization were assessed for efficacy by cross‐sectional imaging with multiphasic computed tomography or MRI every 8 weeks during the first 12 months and every 12 weeks thereafter. Adverse events were assessed as per the NCI's CTCAE version 4.03 (http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf).
2.4. Statistical analysis
This is an exploratory subgroup analysis of patients with lung NET as a primary site enrolled in the RADIANT‐4 study. The full analysis set (all randomized patients) was used for all efficacy analyses. Sample size calculations were not undertaken for the cohort of patients described in this report and so this analysis was not powered to compare the treatment groups. Progression‐free survival, by central radiology review, was analyzed using Kaplan‐Meier methods. Hazard ratios and corresponding CIs were estimated using an unstratified Cox proportional hazards model. All patients who received on or more doses of study drug and who had one or more post‐baseline safety assessments were included in the safety cohort.
3. RESULTS
3.1. Patient characteristics
Of the 302 patients in the RADIANT‐4 study, 90 patients had advanced, progressive, well‐differentiated, non‐functional NET of lung origin and were included in this analysis; of them, 63 received everolimus and 27 received placebo. In the everolimus arm, one patient was not treated due to withdrawal of consent. Therefore, the safety cohort in the everolimus arm was comprised of 62 patients. The majority of the patients were men (52.2%), Caucasian (85.6%), and had a WHO performance status 0 (71.1%). Prior antineoplastic therapies included SSA, chemotherapy, and radiotherapy, including PRRT. Approximately 40% of the patients in each arm had received prior SSA for tumor control. Among them, >80% of the patients in each arm had received octreotide long‐acting release (85.2% of patients in the everolimus arm vs. 81.8% of patients in the placebo arm). The proportion of patients who had undergone surgery for primary tumor or metastatic lesions was lower in the everolimus arm (52.4%) compared with those in the placebo arm (66.7%). The proportion of patients who had received no prior treatment was similar in the two treatment groups (14.3% vs. 11.1%). Baseline demographics and characteristics by treatment arm are described in Table 1.
Table 1.
Baseline demographics and disease characteristics of patients with advanced, progressive, well‐differentiated, non‐functional lung neuroendocrine tumors (NET) treated with everolimus or placebo
| Characteristics | Patients with lung NET (n = 90) | |
|---|---|---|
| Everolimus n = 63 | Placebo n = 27 | |
| Age, years, median (range) | 67.0 (34‐86) | 61.0 (24‐80) |
| Male, n (%) | 32 (50.8) | 15 (55.6) |
| WHO performance status, n (%)a | ||
| 0 | 46 (73.0) | 18 (66.7) |
| 1 | 16 (25.4) | 9 (33.3) |
| Race, n (%) | ||
| Caucasian | 53 (84.1) | 24 (88.9) |
| Asian | 7 (11.1) | 2 (7.4) |
| Othersb | 3 (4.8) | 1 (3.7) |
| Stage IV at initial diagnosis, n (%) | 38 (60.3) | 9 (33.3) |
| Median time from initial diagnosis to randomization, months (range) | 25.8 (2.2‐258.4) | 37.5 (3.7‐303.3) |
| Liver tumor burden | ||
| None | 14 (22.2) | 5 (18.5) |
| >0%‐10% | 33 (52.4) | 17 (63.0) |
| >10%‐25% | 10 (15.9) | 2 (7.4) |
| >25% | 6 (9.5) | 3 (11.1) |
| Metastatic extent of disease, n (%) | ||
| Hepatic (with or without other organ) involvementc | 43 (68.3) | 20 (74.1) |
| Extrahepatic | 20 (31.7) | 7 (25.9) |
| Prior antineoplastic therapy | ||
| Yes | 56 (85.7) | 24 (88.9) |
| Nod | 9 (14.3) | 3 (11.1) |
| Prior treatments, n (%) | ||
| Surgery | 33 (52.4) | 18 (66.7) |
| Somatostatin analogs | 27 (42.9) | 11 (40.7) |
| Chemotherapy | 25 (39.7) | 13 (48.1) |
| Radiotherapy including peptide receptor radionuclide therapy | 25 (39.7) | 13 (48.1) |
In everolimus arm, one patient had WHO performance status 2.
Including Black people and Others.
Only hepatic involvement (everolimus vs. placebo, n [%]: 13 [20.6%] vs. 2 [7.4%]).
Patients with no prior antitumor medication, surgery, or radiotherapy, and no prior somatostatin analog therapy.
Either tumor morphology or grade of malignancy was collected for the lung NET patient group (n = 90). Tumors were defined as “well” or “moderately” differentiated based on local pathology assessment. Among the patients in whom tumor morphology was reported (n = 38), the majority were reported to have well‐differentiated NET (n = 32; 84.2%). Moderately differentiated NET was reported in 6 patients (15.8%). Considering the lack of consensus among pathologists on the precise definition, patients with moderately differentiated NETs were eligible for inclusion in this study provided that a poorly differentiated high‐grade neuroendocrine carcinoma was ruled out, consistent with the exclusion criteria of the study protocol. Among the patients in whom tumor grade was reported (n = 51), majority (86.2%) had an intermediate grade NET. In the patients in whom Ki‐67 percentage was reported (n = 63), it varied from 0% to 60% (median, 8%). Three patients in the everolimus arm had a Ki‐67 index >20% (30%, 50%, and 60%). The investigators confirmed the eligibility of these three patients based on well‐differentiated tumor morphology as reviewed by the local pathologist.
3.2. Treatment
At the data cut‐off date (November 28, 2014), 47 patients (74.6%) in the everolimus arm and 24 patients (88.9%) in the placebo arm discontinued the study treatment. Primary reasons for treatment discontinuation included disease progression (36.5% on everolimus vs. 74.1% on placebo), AEs (30.2% vs. 11.1%), and withdrawal of consent (6.3% vs. 0.0%).
The median duration of treatment was 41.8 weeks (range, 0.7‐118.1 weeks) for everolimus vs 14.3 weeks (range, 4.1‐91.0 weeks) for placebo. In the everolimus arm, 43 patients (69.4%) had one or more adjustments (reduction or interruption) of everolimus dosage. These dose adjustments were primarily attributable to AEs (69.4%). In the placebo arm (n = 27), 8 patients (29.6%) had one or more adjustments (reduction or interruption) of dosage and 18.5% of dose adjustments were due to AEs.
3.3. Efficacy
The median PFS, by central review, was 9.2 months (95% CI, 6.8‐10.9) for patients receiving everolimus compared with 3.6 months (95% CI, 1.9‐5.1) for those receiving placebo. Everolimus improved the median PFS by 5.6 months and was associated with a 50% reduction in the risk of disease progression or death (HR, 0.50; 95% CI, 0.28‐0.88) (Figure 1A). By local investigator assessment (Figure 1B), median PFS was 13.8 months (95% CI, 9.3‐22.2) for patients receiving everolimus compared with 3.5 months (95% CI, 1.9‐5.6) for those receiving placebo (HR, 0.23; 95% CI, 0.13‐0.41). In addition, more patients who received everolimus (57.9%) experienced tumor shrinkage compared to those receiving placebo (13.0%). Details of the overall response and tumor shrinkage are presented in Figure 2. Partial response was observed in one patient in each treatment arm; no patients in either arm showed complete response. Stable disease as best response was observed in 50 patients (79.4%) in the everolimus arm and 15 patients (55.6%) in the placebo arm. Disease control rate in the everolimus arm was 81.0% compared with 59.3% in the placebo arm. Everolimus improved the median PFS by approximately 5.5 months vs placebo in all the subgroups assessed, including the type of prior treatment received (Table 2).
Figure 1.
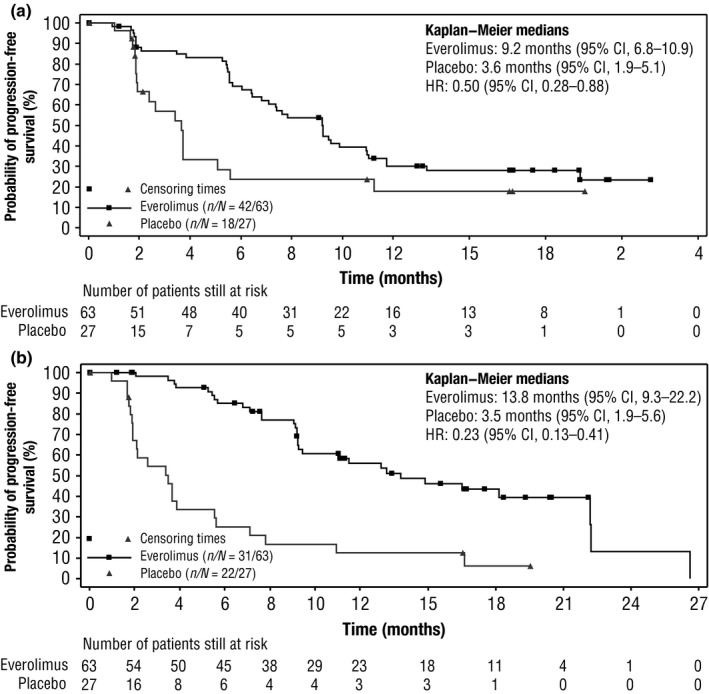
Progression‐free survival in patients with lung neuroendocrine tumors. Hazard ratio (HR) values presented are based on unstratified Cox regression analysis. A, Central radiology review. B, Local investigator review. CI, confidence interval
Figure 2.
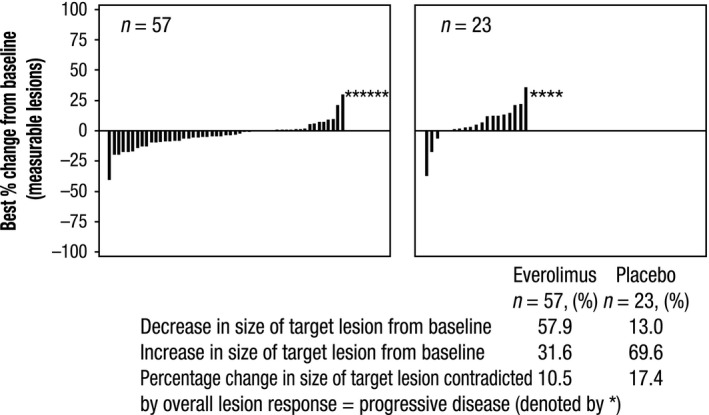
Best percentage change from baseline in lung neuroendocrine tumor response. *Change in size of target lesion contradicted by lesion response of progressive disease. Patients for whom the best percentage change in target lesions was not available and patients for whom the best percentage change was contradicted by overall lesion = unknown were excluded from the analyses
Table 2.
Progression‐free survival (PFS) treatment effect in patients with advanced, progressive, well‐differentiated, non‐functional lung neuroendocrine tumors treated with everolimus or placebo, based on central radiology review, grouped by prior therapies
| Prior therapy | Median PFS, months (95% CI) | |
|---|---|---|
| Everolimus (n = 63) | Placebo (n = 27) | |
| Overall lung subgroup (n = 90) | 9.2 (6.8‐10.9) | 3.6 (1.9‐5.1) |
| Prior chemotherapy (n = 38) | 8.5 (5.6‐11.7) | 2.9 (1.8‐3.7) |
| No prior chemotherapy (n = 52) | 9.2 (6.0‐NE) | 3.7 (1.7‐NE) |
| Prior SSA (n = 38) | 9.5 (6.0‐11.7) | 3.7 (1.0‐11.2) |
| No prior SSA (n = 52) | 9.2 (5.6‐11.0) | 3.6 (1.9‐5.6) |
| Prior radiotherapya (n = 38) | 9.2 (5.7‐NE) | 3.0 (1.9‐5.1) |
| No prior radiotherapy (n = 52) | 9.2 (6.0‐9.9) | 3.7 (1.7‐NE) |
| Any prior therapy (n = 78) | 9.2 (6.0‐11.0) | 3.4 (1.9‐5.1) |
| No prior therapy (n = 12) | 9.7 (0.9‐NE) | 3.6 (1.7‐NE) |
CI, confidence interval; NE, not evaluable; SSA, somatostatin analog.
Includes peptide receptor radionuclide therapy.
3.4. Safety
The most common AEs in the lung NET subgroup, irrespective of the study drug relationship (≥20% incidence in either arm), were stomatitis (53.2% with everolimus vs. 18.5% with placebo), infections (62.9% vs. 37.0%), peripheral edema (38.7% vs. 0.0%), fatigue (37.1% vs. 29.6%), diarrhea (37.1% vs. 7.4%), rash (35.5% vs. 3.7%), pyrexia (30.6% vs. 7.4%), cough (29.0% vs. 22.2%), asthenia (27.4% vs. 0.0%), dyspnea (25.8% vs. 18.5%), decreased weight (25.8% vs. 14.8%), decreased appetite (24.2% vs. 11.1%), nausea (22.6% vs. 14.8%), and anemia (21.0% vs. 3.7%). The most frequent grade 3‐4 AEs irrespective of the study drug relationship (≥5% incidence in either arm) were stomatitis (11.3% vs. 0.0%), hyperglycemia (9.7% vs. 0.0%), diarrhea (6.5% vs. 0.0%), hypophosphatemia (6.5% vs. 0.0%), dyspnea (4.8% vs. 7.4%), and hypertension (0.0% vs. 7.4%).
The most frequently reported (≥20% incidence in either arm) drug‐related any grade AEs (everolimus vs. placebo) were stomatitis (53.2% vs. 18.5%), rash (35.5% vs. 3.7%), fatigue (32.3% vs. 22.2%), peripheral edema (27.4% vs. 0.0%), diarrhea (25.8% vs. 7.4%), infections (22.6% vs. 3.7%), asthenia (22.6% vs. 0.0%), anemia (21.0% vs. 3.7%), and decreased appetite (21.0% vs. 7.4%) (Table 3). The drug‐related AEs associated with infections in the lung (everolimus vs. placebo) were respiratory tract infection (including lower and upper respiratory tract, bronchus, and lung infections; 9 patients [14.5%] vs. 1 patient [3.7%]) and pneumonia (3 patients [4.8%] vs. 0 [0.0%]). Of these, grade 3 or 4 drug‐related infections in the lung were respiratory tract infection (including lower and upper respiratory tract infections; 3 patients [4.8%] vs. 0 [0.0%]) and pneumonia (2 patients [3.2%] vs. 0 [0.0%]). Non‐infectious pneumonitis occurred in 8 patients receiving everolimus (12.9%; grade 3 in 1 patient, grade 2 in 6 patients, and grade 1 in 1 patient [highest grade is reported here]; all were suspected to be drug‐related, 6 patients required dose adjustment or interruption, 3 patients received concomitant medication, 1 patient required hospitalization, 2 patients did not require any intervention [grade 3 and grade 1], and none discontinued) vs 1 patient receiving placebo (3.7%; grade 2; suspected to be drug‐related, required dose adjustment or interruption and concomitant medication). All 9 patients (including the patient of the placebo arm) with pneumonitis had stable disease as their best response per central radiology review. The most frequent grade 3‐4 drug‐related AEs (≥5% incidence in either arm) were stomatitis, hyperglycemia, and infections (Table 3).
Table 3.
Drug‐related adverse events in the lung subgroup of of patients with advanced, progressive, well‐differentiated, non‐functional neuroendocrine tumors (NET) treated with everolimus or placebo (≥10% incidence in either arm)
| Preferred term, n (%) | Patients with lung NET (n = 90) | |||
|---|---|---|---|---|
| Everolimus n = 62a | Placebo n = 27 | |||
| All grades | Grade 3 or 4 | All grades | Grade 3 or 4 | |
| Stomatitisb | ||||
| Totalc , d | 38 (61.3) | 7 (11.3) | 7 (25.9) | 0 (0.0) |
| Stomatitis | 33 (53.2) | 7 (11.3) | 5 (18.5) | 0 (0.0) |
| Mouth ulceration | 4 (6.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Aphthous stomatitis | 2 (3.2) | 0 (0.0) | 2 (7.4) | 0 (0.0) |
| Glossitis | 2 (3.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Rash | 22 (35.5) | 0 (0.0) | 1 (3.7) | 0 (0.0) |
| Fatigue | 20 (32.3) | 2 (3.2) | 6 (22.2) | 0 (0.0) |
| Peripheral edema | 17 (27.4) | 2 (3.2) | 0 (0.0) | 0 (0.0) |
| Diarrhea | 16 (25.8) | 3 (4.8) | 2 (7.4) | 0 (0.0) |
| Infectionse | 14 (22.6) | 5 (7.1) | 1 (3.7) | 0 (0.0) |
| Asthenia | 14 (22.6) | 1 (1.6) | 0 (0.0) | 0 (0.0) |
| Anemia | 13 (21.0) | 2 (3.2) | 1 (3.7) | 0 (0.0) |
| Decreased appetite | 13 (21.0) | 0 (0.0) | 2 (7.4) | 0 (0.0) |
| Nausea | 12 (19.4) | 2 (3.2) | 3 (11.1) | 0 (0.0) |
| Pyrexia | 12 (19.4) | 2 (3.2) | 1 (3.7) | 0 (0.0) |
| Hyperglycemia | 11 (17.7) | 6 (9.8) | 2 (7.4) | 0 (0.0) |
| Dyspnea | 9 (14.5) | 1 (1.6) | 3 (11.1) | 1 (3.7) |
| Non‐infectious pneumonitis | 8 (12.9) | 1 (1.6) | 1 (3.7) | 0 (0.0) |
| Dysgeusia | 8 (12.9) | 0 (0.0) | 1 (3.7) | 0 (0.0) |
| Cough | 8 (12.9) | 0 (0.0) | 1 (3.7) | 0 (0.0) |
| Pruritus | 7 (11.3) | 1 (1.6) | 0 (0.0) | 0 (0.0) |
| Dry mouth | 7 (11.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Weight decreased | 5 (8.1) | 1 (1.6) | 3 (11.1) | 0 (0.0) |
In the everolimus arm, one patient withdrew consent.
Includes stomatitis, aphthous stomatitis, mouth ulceration, and glossitis.
Represents the total number of patients with stomatitis that includes mouth and stoma‐derived adverse events.
A patient with multiple adverse events within a category is counted only once in the “Total” row.
Includes all infections.
There were two on‐treatment deaths in each arm. In the everolimus arm, one of the two deaths was considered to be related to the primary disease and/or to disease progression, and the other was attributed to “cardiac failure”, which was not suspected by the investigator to be related to the study medication. In the placebo arm, one death was considered to be related to the primary disease and/or to disease progression and the other death was attributed to “dyspnea”, which was suspected by the investigator to be related to the study medication. The AEs observed in this subgroup of patients with lung NET were similar to those reported in the overall RADIANT‐4 patient group.
4. DISCUSSION
There is a large unmet medical need for patients with advanced lung NETs. Currently there is no standard antitumor systemic treatment available for these malignancies. In this exploratory analysis of the RADIANT‐4 study, everolimus was associated with a clinically meaningful improvement of 5.6 months in the median PFS compared to placebo, by central review, in patients with advanced, progressive, well‐differentiated, non‐functional lung NET, with 50% risk‐reduction in disease progression or death. By investigator assessment, median PFS was prolonged by a clinically meaningful time period of 10.3 months in the patients treated with everolimus compared to placebo, with 77% risk‐reduction in disease progression or death. These results strongly support the results of the central review.
Based on the results obtained in the RADIANT‐4 study, everolimus has been approved worldwide for the treatment of patients with advanced, progressive, well‐differentiated, non‐functional (GI and lung) NET.22 Therefore, everolimus represents the first agent ever approved for non‐functioning lung NET. At primary diagnosis, 52.2% of patients were diagnosed at stage IV; the majority of the remaining patients progressed to stage IV within a median time period of 28.8 months. The relatively short time for progression from initial diagnosis suggests that lung NET is not an indolent disease. Although data on different therapies for patients with lung NET have been reported from retrospective or single‐arm phase II prospective trials, no published data from a phase III trial are currently available, apart from the data for everolimus.19, 22 A randomized, double‐blind, phase III study (SPINET, NCT02683941) evaluating the efficacy and safety of lanreotide Autogel vs placebo is ongoing in patients with well‐differentiated, metastatic, and/or unresectable, typical or atypical lung carcinoids. Eligibility of patients with lung NET according to the AC and TC definitions, as reported in the WHO classification, was not mandatory for enrollment in the RADIANT‐4 study. To classify a tumor as TC or AC, the surgical primary tumor sample is usually required by the pathologist. However, in the metastatic setting (as in the RADIANT‐4 cohort), percutaneous biopsies or primary tumor endobronchial ultrasound‐guided fine needle aspiration biopsies of metastatic or primary sites represented the vast majority of histological material available, making it very difficult to classify the lung NET as TC or AC. Therefore, well‐differentiated tumor morphology, possibly with a thyroid transcription factor 1‐positive and caudal type homeobox 2‐negative immunohistochemistry, combined with a consistent clinical picture and clinical history supported the diagnosis of lung NET in RADIANT‐4 in the majority of cases.25, 26
The improvement in median PFS (5.6 months) observed in patients with lung NET treated with everolimus is consistent with the improvement in median PFS (7.1 months) observed in the overall RADIANT‐4 cohort. The reduction in the risk of disease progression or death in the lung NET subgroup (50%) is similar to that seen in the total population in the RADIANT‐4 study (52%). More patients in the everolimus arm experienced tumor shrinkage in the RADIANT‐4 cohort as well as in the lung subgroup (63.6% and 57.9% in the everolimus arm vs. 25.9% and 13.0% in the placebo arm); these results are consistent with the cytoreductive properties of everolimus.22 The data from our analysis supports the preliminary evidence of efficacy that was observed when everolimus was added to octreotide in patients with functional lung NET.19 The results from the three‐arm, randomized phase II LUNA trial (n = 112), comparing pasireotide in combination with everolimus vs everolimus alone and pasireotide alone in patients with typical and atypical lung or thymic carcinoids further support the efficacy of everolimus in this setting.27 The LUNA study achieved the preplanned statistical objective of a 9‐month PFS rate >20% in all three arms. The observed 9‐month PFS rates were 39.0%, 33.3%, and 58.5% with a median PFS of 8.5, 12.5, and 11.8 months in pasireotide alone, everolimus alone, and combination arms, respectively.
The preplanned first interim OS analysis (at data cut‐off date of November 28, 2014) was carried out after a total of 70 deaths and indicated that everolimus may be associated with a reduction in the risk of death (HR, 0.64; 95% CI, 0.40‐1.05; one‐sided P = .037, whereas the boundary for statistical significance was .0002).22 The results of a planned second interim analysis of OS (at data cut‐off date of November 30, 2015), undertaken after a total of 101 deaths (53% of the total targeted 191 deaths for final OS analysis), continued to suggest a positive trend for survival benefit with everolimus, although statistical significance was not achieved.28 Overall survival was immature at the interim analyses. Therefore, the OS in subgroups will be analyzed when the final OS analysis will be undertaken after 191 deaths have occurred in the overall RADIANT‐4 cohort. At the primary analysis (at data cut‐off date of November 28, 2014), 27 OS events occurred in 90 patients with lung NET (10 in the placebo arm [n = 27] and 17 in the everolimus arm [n = 63]).
Everolimus toxicity was manageable with no new safety signals. In patients with lung NET, drug‐related pneumonitis is of special interest; however, all patients in this lung subgroup who had grade 1‐3 drug‐related non‐infectious pneumonitis were effectively managed with dose modifications, without requiring treatment discontinuation. The AEs were similar to those reported in the overall RADIANT‐4 cohort22 and was consistent with the known safety profile of everolimus.21
In conclusion, although this is an exploratory subgroup analysis of a randomized phase III trial and the study was not powered for a comparison between subgroups, it represents the largest series of lung NET patients ever included in a phase III trial. These data indicate that treatment with everolimus can result in a clinically meaningful improvement in PFS in patients with advanced, progressive, well‐differentiated, non‐functional NET originating in the lung. Based on the preliminary evidence19 and the clinically meaningful results from the subgroup analysis of patients with lung NET in the RADIANT‐4 study,22 everolimus should be recommended for patients with advanced progressive lung NET as reported in the 2016 European Neuroendocrine Tumor Society guidelines.9
CONFLICT OF INTEREST
Nicola Fazio received honoraria from Novartis, Ipsen, and Pfizer, and his institution received research funding from Novartis and Merck Serono. Gianfranco Delle Fave received honoraria and research funding from Novartis. Margot Tesselaar received research funding from Novartis. Eric Van Cutsem received research funding from Novartis, Ipsen, Sanofi, Roche, Bayer, and Boehringer. Jonathan Strosberg received research funding from Novartis and Ipsen and his institution received research funding from Novartis. Maurizio Voi and Fabian Herbst are employees of Novartis and have stock ownership from Novartis. Lida Bubuteishvili‐Pacaud and Antonia Ridolfi are employees of Novartis. Simron Singh received research funding from Novartis. Marianne Pavel received honoraria from Novartis and her institution received research funding from Novartis. Juan W. Valle received honoraria and travel reimbursement from Novartis, and his institution received research funding from Novartis. James C. Yao received honoraria and research funding from Novartis. The study was designed under the responsibility of Novartis Pharmaceutical Corporation, in conjunction with the steering committee. The study was funded by Novartis Pharmaceutical Corporation. Everolimus was provided by Novartis Pharmaceutical Corporation. Novartis Pharmaceutical Corporation collected and analyzed the data and contributed to the interpretation of the study. All authors had full access to all of the data in the study and had final responsibility for the decision to submit for publication. Roberto Buzzoni, Edward Wolin, Paola Tomassetti, Jiri Tomasek, and Matthew H. Kulke have no conflict of interest.
ACKNOWLEDGMENTS
The authors acknowledge the patients, their families, and their caregivers, as well as all the investigators. The RADIANT‐4 study group is grateful to all the physicians who contributed to the present study, and the worldwide network of research nurses, trial coordinators, and operations staff for their contributions. We appreciate the support of the colleagues of the RADIANT‐4 Study Team who contributed to the presented data. In addition, we thank Rajasree Solipuram, PhD, Novartis Healthcare Pvt. Ltd. for providing medical editorial assistance with this manuscript.
Fazio N, Buzzoni R, Delle Fave G, et al. Everolimus in advanced, progressive, well‐differentiated, non‐functional neuroendocrine tumors: RADIANT‐4 lung subgroup analysis. Cancer Sci. 2018;109:174–181. https://doi.org/10.1111/cas.13427
Funding information
Novartis Pharmaceuticals Corporation.
This research was presented in part at the Annual European Neuroendocrine Tumors Society (ENETS) Conference, March 9‐11, 2016 in Barcelona, Spain and the European Society of Medical Oncology (ESMO) Congress, October 7‐11, 2016, Copenhagen, Denmark.
REFERENCES
- 1. Yao JC, Hassan M, Phan A, et al One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26:3063–3072. [DOI] [PubMed] [Google Scholar]
- 2. Detterbeck FC. Clinical presentation and evaluation of neuroendocrine tumors of the lung. Thorac Surg Clin. 2014;24:267–276. [DOI] [PubMed] [Google Scholar]
- 3. Taal BG, Visser O. Epidemiology of neuroendocrine tumours. Neuroendocrinology. 2004;80(Suppl 1):3–7. [DOI] [PubMed] [Google Scholar]
- 4. Rekhtman N. Neuroendocrine tumors of the lung: an update. Arch Pathol Lab Med. 2010;134:1628–1638. [DOI] [PubMed] [Google Scholar]
- 5. Oberg K, Hellman P, Ferolla P, Papotti M, Group EGW. Neuroendocrine bronchial and thymic tumors: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow‐up. Ann Oncol. 2012;23(Suppl 7):vii120–vii123. [DOI] [PubMed] [Google Scholar]
- 6. Granberg D. Neuroendocrine tumors of the lung In:Yalcin S, Öberg K, eds. Neuroendocrine Tumors: Diagnosis and Management. Berlin, Heidelberg:Springer‐Verlag;2015:143–164. [Google Scholar]
- 7. Filosso PL, Ferolla P, Guerrera F, et al Multidisciplinary management of advanced lung neuroendocrine tumors. J Thorac Dis. 2015;7:S163–S171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Krug LM, Kris MG, Rosenzweig K, et al Cancer of the Lung: small cell and other neuroendocrine tumors of the lung In:DeVita VT, Lawrence TS, Rosenberg SA, Weinberg RA, Depinho RA, eds. Cancer Principles and Practice of Oncology. Alphen aan den Rijn:Wolters Kluwer, Lippincott Williams and Wilkins;2008:946–971. [Google Scholar]
- 9. Pavel M, O'Toole D, Costa F, et al ENETS consensus guidelines update for the management of distant metastatic disease of intestinal, pancreatic, bronchial neuroendocrine neoplasms (NEN) and NEN of unknown primary site. Neuroendocrinology. 2016;103:172–185. [DOI] [PubMed] [Google Scholar]
- 10. Filosso PL, Ruffini E, Oliaro A, Papalia E, Donati G, Rena O. Long‐term survival of atypical bronchial carcinoids with liver metastases, treated with octreotide. Eur J Cardiothorac Surg. 2002;21:913–917. [DOI] [PubMed] [Google Scholar]
- 11. Waldherr C, Pless M, Maecke HR, Haldemann A, Mueller‐Brand J. The clinical value of [90Y‐DOTA]‐D‐Phe1‐Tyr3‐octreotide (90Y‐DOTATOC) in the treatment of neuroendocrine tumours: a clinical phase II study. Ann Oncol. 2001;12:941–945. [DOI] [PubMed] [Google Scholar]
- 12. Mariniello A, Bodei L, Tinelli C, et al Long‐term results of PRRT in advanced bronchopulmonary carcinoid. Eur J Nucl Med Mol Imaging. 2016;43:441–452. [DOI] [PubMed] [Google Scholar]
- 13. Kulke MH, Lenz HJ, Meropol NJ, et al Activity of sunitinib in patients with advanced neuroendocrine tumors. J Clin Oncol. 2008;26:3403–3410. [DOI] [PubMed] [Google Scholar]
- 14. Grande E, Capdevila J, Castellano D, et al Pazopanib in pretreated advanced neuroendocrine tumors: a phase II, open‐label trial of the Spanish Task Force Group for Neuroendocrine Tumors (GETNE). Ann Oncol. 2015;26:1987–1993. [DOI] [PubMed] [Google Scholar]
- 15. Yao JC, Phan A, Hoff PM, et al Targeting vascular endothelial growth factor in advanced carcinoid tumor: a random assignment phase II study of depot octreotide with bevacizumab and pegylated interferon alpha‐2b. J Clin Oncol. 2008;26:1316–1323. [DOI] [PubMed] [Google Scholar]
- 16. Brizzi MP, Berruti A, Ferrero A, et al Continuous 5‐fluorouracil infusion plus long acting octreotide in advanced well‐differentiated neuroendocrine carcinomas. A phase II trial of the Piemonte oncology network. BMC Cancer. 2009;9:388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bajetta E, Catena L, Procopio G, et al Are capecitabine and oxaliplatin (XELOX) suitable treatments for progressing low‐grade and high‐grade neuroendocrine tumours? Cancer Chemother Pharmacol. 2007;59:637–642. [DOI] [PubMed] [Google Scholar]
- 18. Ekeblad S, Sundin A, Janson ET, et al Temozolomide as monotherapy is effective in treatment of advanced malignant neuroendocrine tumors. Clin Cancer Res. 2007;13:2986–2991. [DOI] [PubMed] [Google Scholar]
- 19. Fazio N, Granberg D, Grossman A, et al Everolimus plus octreotide long‐acting repeatable in patients with advanced lung neuroendocrine tumors: analysis of the phase 3, randomized, placebo‐controlled RADIANT‐2 study. Chest. 2013;143:955–962. [DOI] [PubMed] [Google Scholar]
- 20. Yao JC, Lombard‐Bohas C, Baudin E, et al Daily oral everolimus activity in patients with metastatic pancreatic neuroendocrine tumors after failure of cytotoxic chemotherapy: a phase II trial. J Clin Oncol. 2010;28:69–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yao JC, Shah MH, Ito T, et al Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med. 2011;364:514–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yao JC, Fazio N, Singh S, et al Everolimus for the treatment of advanced, non‐functional neuroendocrine tumours of the lung or gastrointestinal tract (RADIANT‐4): a randomised, placebo‐controlled, phase 3 study. Lancet. 2016;387:968–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Klimstra DS, Modlin IR, Coppola D, Lloyd RV, Suster S. The pathologic classification of neuroendocrine tumors: a review of nomenclature, grading, and staging systems. Pancreas. 2010;39:707–712. [DOI] [PubMed] [Google Scholar]
- 24. Rindi G, Arnold R, Bosman FT, et al Nomenclature and classification of neuroendocrine neoplasm of the digestive system In:Bosman FT, Carniero F, Hruban RH, Theise ND, eds. WHO Classification of Tumours of the Digestive System, 4th edn Lyon:International Agency for Research on Cancer;2010:13–14. [Google Scholar]
- 25. Hendifar AE, Marchevsky AM, Tuli R. Neuroendocrine tumors of the lung: current challenges and advances in the diagnosis and management of well‐differentiated disease. J Thorac Oncol. 2017;12:425–436. [DOI] [PubMed] [Google Scholar]
- 26. Pelosi GFA, Cossa M, et al What clinicians are asking pathologists when dealing with lung neuroendocrine neoplasms? Semin Diagn Pathol. 2015;32:469–479. [DOI] [PubMed] [Google Scholar]
- 27. Ferolla P, Brizzi MP, Meyer T, et al Efficacy and safety of pasireotide LAR or everolimus alone, or in combination in patients with advanced carcinoids (NET) of the lung/thymus: results from the randomized, phase 2 LUNA study. Ann Oncol. 2016;27:136–148. [Google Scholar]
- 28. Yao JC, Fazio N, Singh S, et al Everolimus (EVE) in advanced, nonfunctional, well‐differentiated neuroendocrine tumors (NET) of gastrointestinal (GI) or lung origin: second interim overall survival (OS) results from the RADIANT‐4 study. J Clin Oncol. 2016;34:4090. [Google Scholar]


