Abstract
Tyrosine kinase inhibitors (TKI) improve the prognosis of patients with chronic myelogenous leukemia (CML) by inducing substantial deep molecular responses (DMR); some patients have successfully discontinued TKI therapy after maintaining DMR for ≥1 year. In this cessation study, we investigated the optimal conditions for dasatinib discontinuation in patients who maintained DMR for ≥2 years. This study included 54 patients with CML who were enrolled in a D‐STOP multicenter prospective trial, had achieved DMR, and had discontinued dasatinib after 2‐year consolidation. Peripheral lymphocyte profiles were analyzed by flow cytometry. The estimated 12‐month treatment‐free survival (TFS) was 62.9% (95% confidence interval: 48.5%‐74.2%). During dasatinib consolidation, the percentage of total lymphocytes and numbers of CD3− CD56+ natural killer (NK) cells, CD16+ CD56+ NK cells and CD56+ CD57+ NK‐large granular lymphocytes (LGL) were significantly higher in patients with molecular relapse after discontinuation but remained unchanged in patients without molecular relapse for >7 months. At the end of consolidation, patients whose total lymphocytes comprised <41% CD3− CD56+ NK cells, <35% CD16+ CD56+ NK cells, or <27% CD56+ CD57+ NK‐LGL cells had higher TFS relative to other patients (77% vs 18%; P < .0008; 76% vs 10%; P < .0001; 84% vs 46%; P = .0059, respectively). The increase in the number of these NK cells occurred only during dasatinib consolidation. In patients with DMR, dasatinib discontinuation after 2‐year consolidation can lead to high TFS. This outcome depends significantly on a smaller increase in NK cells during dasatinib consolidation.
Keywords: chronic myelogenous leukemia, dasatinib, natural killer, stop, tyrosine kinase inhibitors
1. INTRODUCTION
Chronic myelogenous leukemia (CML) is caused by the BCR‐ABL1 fusion gene on the Philadelphia chromosome. This leukemia tends to transform into an acute form and was, therefore, historically associated with a poor prognosis.1, 2 However, the advent of tyrosine kinase inhibitor (TKI) imatinib, which inhibits functions of BCR‐ABL1 protein, has dramatically improved the prognosis of CML patients.3 More recently, second‐generation TKI, such as dasatinib and nilotinib, have been found to induce faster and deeper molecular responses compared to imatinib.4, 5 Most patients who receive TKI therapy survive for more than a decade. Recent data have revealed that an increasing number of patients may gradually achieve a deep molecular response (DMR) if they continue to use second‐generation TKI for several years.6, 7 However, increasing concern surrounds the long‐term adverse effects and financial burden associated with TKI.8, 9, 10, 11, 12
To address these concerns, the possibility of imatinib cessation has been extensively studied. In a prospective study first presented by a French group (STIM study), approximately 39% of CML patients who maintained DMR for ≥2 years while using first‐line imatinib could safely stop taking the drug after >6 months,13 although patients who had received therapy for >5 years or had a low Sokal score were significantly more successful. An Australian group also found that imatinib could be stopped after a 2‐year DMR consolidation period (TWISTER trial).14 Regarding second‐generation TKI, a Japanese prospective study demonstrated that approximately 49% of CML patients who achieved DMR for >1 year during second‐line dasatinib therapy could successfully discontinue treatment for >6 months,15 and attempts to discontinue nilotinib yielded similar observations.16, 17 Therefore, the second‐generation TKI appear to better enable chronic‐phase CML patients to achieve therapy discontinuation.
The optimal conditions for successful TKI discontinuation regarding the type of TKI, depth of DMR, duration and amount of TKI administration or consolidation after DMR, and criteria for molecular relapse are yet to be determined. Currently, several of the additional TKI discontinuation trials needed to elucidate these conditions are under way.18 Dasatinib induces increases in the populations of lymphocytes, large granular lymphocytes (LGL), natural killer (NK) cells and cytotoxic T cells, as well as a decrease in regulatory T cells (Tregs), especially in patients with good clinical responses,19, 20 and a recent report found an association between an expanded NK cell population and successful TKI cessation.15, 21 Therefore, the potential association between dasatinib‐induced lymphocyte changes in number and successful discontinuation of dasatinib is an area of growing interest.
In the ongoing Japanese multicenter prospective D‐STOP trial, we have attempted to discontinue dasatinib following a 2‐year consolidation in patients with CML who had achieved DMR. During this period, we monitored the peripheral lymphocyte profiles via flow cytometry to investigate their associations with successful discontinuation. Here we show that dasatinib specifically increased NK cells during consolidation, but not during discontinuation, and demonstrate that a lower percentage of NK cells during consolidation significantly increases the likelihood that a patient will achieve a longer treatment‐free survival (TFS).
2. MATERIALS AND METHODS
2.1. Study design and patients
The D‐STOP trial (NCT01627132) is a multicenter, single‐arm, phase 2 study conducted over 22 centers in Japan. Figure 1 presents the study schema for D‐STOP. Patients diagnosed with chronic‐phase CML and who achieved DMR after receiving any TKI were eligible for 2‐year dasatinib consolidation therapy with the intent to maintain DMR. The inclusion criteria were as follows: age ≥15 years, performance status (ECOG score) of 0‐2, and no severe primary organ dysfunction involving the liver, kidney or lungs. All previous treatments were permitted except for allogeneic hematopoietic stem‐cell transplantation. The exclusion criteria were as follows: other chromosomal abnormalities in addition to the Philadelphia chromosome, a history of BCR‐ABL1 mutation, and/or other active malignant disorders. The D‐STOP trial was approved by the ethics committees of the participating institutes. All participants provided written informed consent in accordance with the Declaration of Helsinki.
Figure 1.
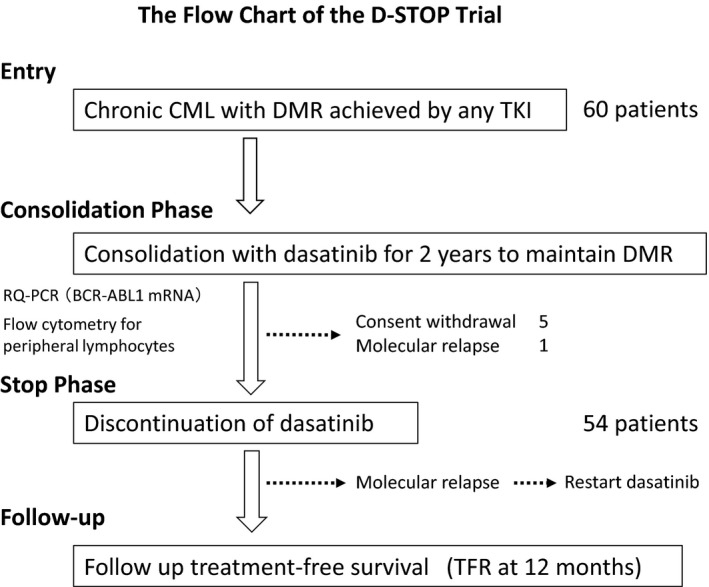
Flow chart of the D‐STOP trial. CML, chronic myelogenous leukemia; DMR, deep molecular response; RQ‐PCR, real‐time quantitative PCR; TFR, treatment‐free survival
2.2. Real‐time quantitative RT‐PCR
During the dasatinib‐consolidation phase, real‐time quantitative RT‐PCR (RQ‐PCR) analyses were performed every 3 months in the central laboratory (Bio Medical Laboratories [BML], Tokyo, Japan) to measure molecular responses19 according to the BCR‐ABL1 International Scale (IS) and the laboratory's conversion factor,22 as previously described.15 In summary, ABL1 was used as an internal control, and the cut‐off corresponded to BCR‐ABL1 of 0.0069% IS or molecular response of 4.0 (detectable disease with a BCR‐ABL1 <0.01% IS or undetectable disease in cDNA with >10 000 ABL1 transcripts).23 Patients with DMR confirmation every 3 months during a 2‐year dasatinib consolidation period subsequently entered the discontinuation phase. Following dasatinib cessation, DMR were monitored by RQ‐PCR every month for the first year (clinical cut‐off), followed by every 3 months for the remaining 2 years (total = 3 years).
If BCR‐ABL1 >0.0069% IS was detected at any point during the consolidation or discontinuation phase, an additional RQ‐PCR was performed within 1 month. Two successive BCR‐ABL1 positive results confirmed a molecular relapse. For relapses occurring during the discontinuation phase, dasatinib was restarted at the previously effective dose. After dasatinib recommencement, the response was assessed by RQ‐PCR at 1, 3, 6 and 12 months. Dasatinib dose reduction was permitted at any time in response to adverse events based on physicians’ judgment.
2.3. Flow cytometric analysis
The whole peripheral blood lymphocyte profiles were established by the central laboratory (BML) before and after 3, 6, 12 and 24 months of dasatinib consolidation. Blood samples were collected more than a few hours after dasatinib intake. Peripheral white blood cell counts were measured using an automated cell count analyzer. The flow cytometry methods have been previously described.15, 19, 24 In brief, the lymphocyte fraction was determined using forward‐scatter vs side‐scatter gating (Figure S1A), and immunophenotypic examinations were performed using two‐color or three‐color flow cytometry on a FACSCalibur system with CellQuest software, version 3.3 (Becton Dickinson, Franklin Lakes, NJ, USA). All antibodies used in this study were purchased from Becton Dickinson. The defined lymphocyte subsets comprised NK cells (CD3− CD56+ and CD16+ CD56+ cells), NK‐cell LGL (NK‐LGL, CD56+ CD57+), helper T cells (CD4+ CD8−), cytotoxic T cells (CD4− CD8+), T‐cell LGL (T‐LGL) (CD3+ CD57+)19 and regulatory T cells (Tregs) (CD4+ CD25+ CD127dim/−)25 (Figure S1B).
2.4. Definition of the endpoint
The primary endpoint was the 12‐month rate of TFS, defined as the time from the discontinuation of dasatinib to the date of molecular relapse. To establish the predictive factors associated with treatment‐free remission, we evaluated patients by sex, age at discontinuation, Sokal risk score at diagnosis, duration of BCR‐ABL1 transcript negativity before consolidation, duration of TKI therapy before consolidation, total dose of dasatinib, and type of TKI used when DMR was achieved. We also assessed the above‐described lymphocyte subsets before and after 3, 6, 12 and 24 months of dasatinib consolidation while in TFS. Safety was evaluated throughout the consolidation period and adverse events were classified using the Common Terminology Criteria for Adverse Events, version 4.0.
2.5. Statistical analysis
In this study, a sample size of at least 50 patients was determined to demonstrate that patients who discontinued dasatinib remained in TFS at a power >80% when compared with data from a previous study.13 Each continuous variable was separated into two groups using the cut‐off points calculated by the concordance index.
A Kaplan–Meier analysis was used to calculate the proportion of patients in treatment‐free remission, and a log‐rank test was used to statistically compare the stratified groups (two or more). Cox proportional hazards analysis of significant predictors in the univariate analysis was used to calculate the factors contributing to successful discontinuation. Strongly correlated explanatory variables were independently entered into the Cox regression model. Factors significant in at least one of the tested models were considered possible independent predictors of relapse risk.
Receiver operating characteristic (ROC) curves were generated to determine the cut‐off values of lymphocytes (%), NK cells (% and counts) and clinical data for the Kaplan–Meier analysis. Optimal thresholds along ROC curves were determined by searching for plausible values where the sum of the sensitivity and specificity were maximized. A P‐value <.05 was considered significant. The statistical software EZR on R commander (R Project for Statistical Computing, Vienna, Austria) was used for statistical analysis calculations.26
3. RESULTS
3.1. Treatment‐free survival after dasatinib discontinuation
As shown in Figure 1, 60 patients with a confirmed DMR between 1 February 2012 and 31 January 2014 were enrolled into the dasatinib consolidation phase. Of these patients, 6 were excluded during consolidation because of consent withdrawal or fluctuations in the BCR‐ABL1 transcript levels suggestive of a molecular relapse. Safety analyses revealed no severe (grade ≥3) treatment‐related toxic effects during the consolidation phase. A total of 54 patients (32 male, 22 female) were included in the dasatinib‐discontinuation (STOP) phase (Table 1).
Table 1.
Characteristics of the patients
| Age at discontinuation (y) | 56 (27‐84) |
| Sex | |
| Male | 32 |
| Female | 22 |
| Performance status | |
| ≤1 | 54 |
| Sokal score at diagnosisa | |
| Low | 24 |
| Intermediate | 11 |
| High | 6 |
| Type of TKI when achieving DMR | |
| Imatinib | 34 |
| Dasatinib | 19 |
| Unknown | 1 |
| BCR‐ABL(−) duration (mo) | 51 (24‐173) |
| Duration of TKI treatment (mo) | 92 (36‐177) |
DMR, deep molecular response; TKI, tyrosine kinase inhibitor.
Sokal scores were not available for 13 patients.
The median age at treatment discontinuation was 56 (range: 27‐84) years. The median duration of TKI treatment was 92 (36‐177) months, and the median duration of BCR‐ABL1 negativity before treatment cessation was 51 (24‐173) months. Patients were followed up for a median of 16 (3‐24) months after discontinuation. In total, 34, 19 and 1 patient used imatinib, dasatinib and an unknown agent, respectively, at the time of DMR achievement before consolidation. No patient received interferon‐alpha.
Regarding the clinical endpoint, the estimated overall probabilities of TFS were 68.5% (95% confidence interval [CI]: 54.3‐79.1) at 6 months and 62.9% (95% CI: 48.5‐74.2) at 12 months (Figure 2). Twenty patients experienced relapses during discontinuation phase, and most relapses occurred within 6 (and maximum 7) months. The loss of major molecular response (MMR) was observed in 20% of the relapsed patients. All relapsed patients responded to dasatinib within 3 months of recommencing treatment. MMR was achieved within 6 months in all patients and DMR within 12 months in 16 of the 19 patients evaluable within this period. No loss of a hematologic response, complete cytogenetic response or progression to advanced disease was observed.
Figure 2.
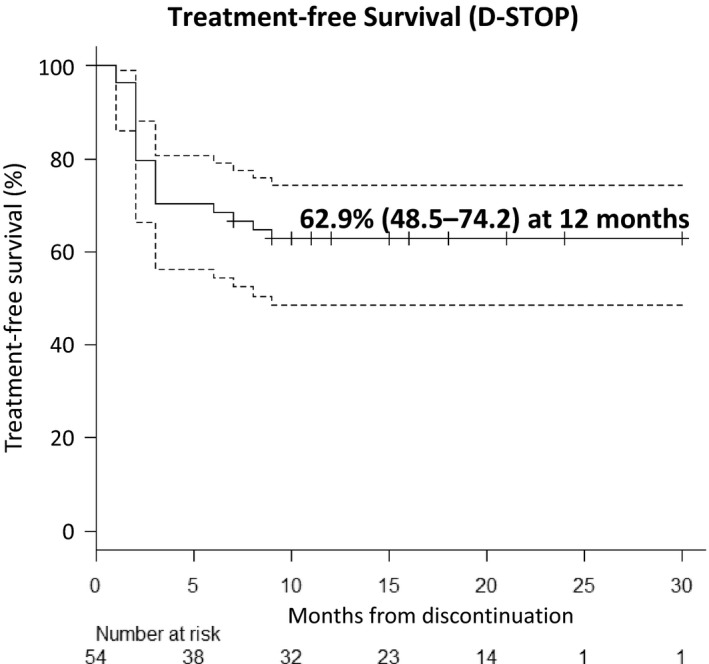
Estimated treatment‐free survival in the D‐STOP trial. The estimated treatment‐free survival of the patients who discontinued dasatinib (y‐axis) and the duration (months) of discontinuation (x‐axis) are shown. The dotted line indicates the 95% confidence interval
The clinical factors that affected molecular relapse during the discontinuation phase were analyzed (Figure 3). However, no significant associations of the 12‐month TFS rate were observed with patient characteristics such as sex (Figure 3A), Sokal score at diagnosis (Figure 3B), BCR‐ABL1 mRNA negative duration (Figure 3C), duration of TKI treatment (Figure 3D), age at discontinuation (Figure 3E), total dose of dasatinib (Figure 3F) and type of TKI used when DMR was achieved (Figure 3G).
Figure 3.
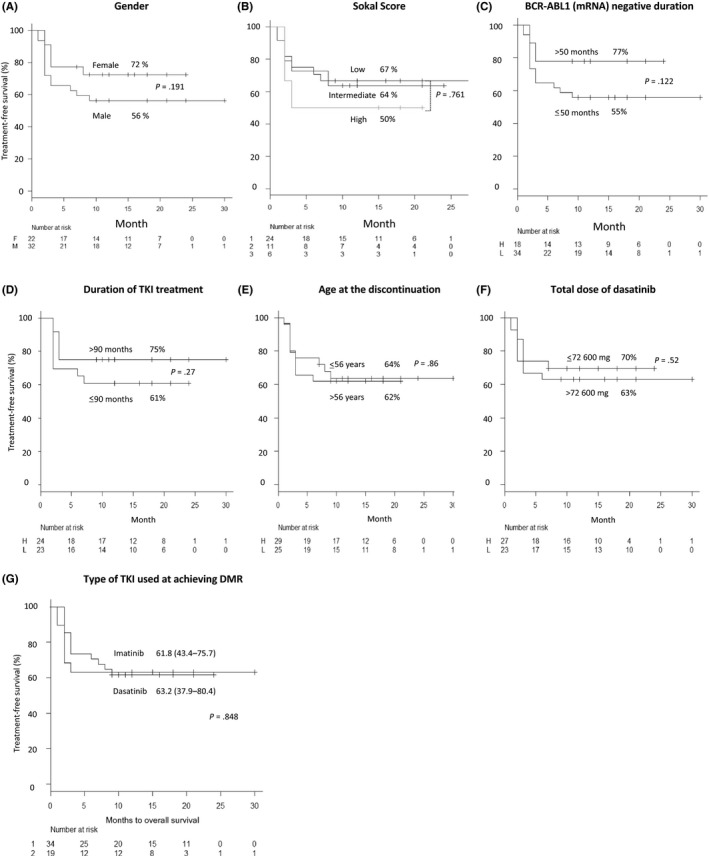
Estimated treatment‐free survival according to patients’ characteristics. The estimated treatment‐free survival rates according to patient characteristics, including sex (A), Sokal score (B), BCR‐ABL1 mRNA‐negative duration (C), duration of tyrosine kinase inhibitor (TKI) treatment (D), age at discontinuation (E), total dose of dasatinib (F), and the type of TKI when achieving a deep molecular response (G) are presented along the y‐axis. In all figures, the duration (months) of discontinuation is shown along the x‐axis. The cut‐off levels of each type of clinical data were determined using receiver operating characteristic (ROC) curve analyses as described in the Methods
At present, the last patient discontinued dasatinib more than 24 months ago and the actual late TFS rate at 24 months was calculated as 57%. This is consistent with the results of previous reports, which indicated that the most molecular relapses occur within 6 months after cessation, with a small percentage of patients experiencing late relapse.27, 28 We will report our final results at 3‐year follow‐up.
3.2. Clinical data during consolidation therapy
Physicians were free to determine the dose of dasatinib during the consolidation phase. Of the 60 patients who entered the consolidation phase, 35% and 52% experienced transient suspension and reduction of dasatinib, respectively. The etiologies of the drug suspensions included 20 non‐hematological adverse events (G2–3) (13 pleural effusions, 1 chronic heart failure, 1 elongation of corrected QT in ECG, 1 renal failure, 1 hematochezia, 1 diarrhea, 1 general fatigue and 1 unknown) and 2 hematological adverse events (≥G3) (anemia and pancytopenia). The etiologies of the dose reductions included 31 non‐hematological adverse events (G2–3) (13 pleural effusions, 1 elongation of corrected QT in ECG, 1 liver dysfunction, 1 renal failure, 1 hematochezia, 2 diarrhea, 1 general fatigue, 2 alopecia, 1 fever, 1 appetite loss, and others) and 3 hematological adverse events (≥G3) (1 anemia, 1 thrombocytopenia and 1 pancytopenia). Pleural effusions were the major reasons for dose change. No patient withdrew from the study due to adverse events. Thirty‐four patients switched from imatinib to dasatinib at the start of consolidation. Patients switching to dasatinib for the first time during consolidation took 80.1% of the scheduled dose (100 mg/d) (n = 30).
3.3. Lymphocyte profiles during consolidation therapy
We divided patients in the D‐STOP trial into S (successful) and F (failed) groups, which, respectively, comprised those who discontinued dasatinib and remained in remission for >7 months (n = 34) and those who relapsed during the observation period (n = 20). The absolute lymphocyte counts did not differ significantly between S and F groups before or after dasatinib consolidation therapy (P = .25 and .24, respectively) (data not shown). However, the relative lymphocyte count after consolidation increased significantly relative to the baseline values in F group (P = .0091*) (Figure 4A(i)).
Figure 4.
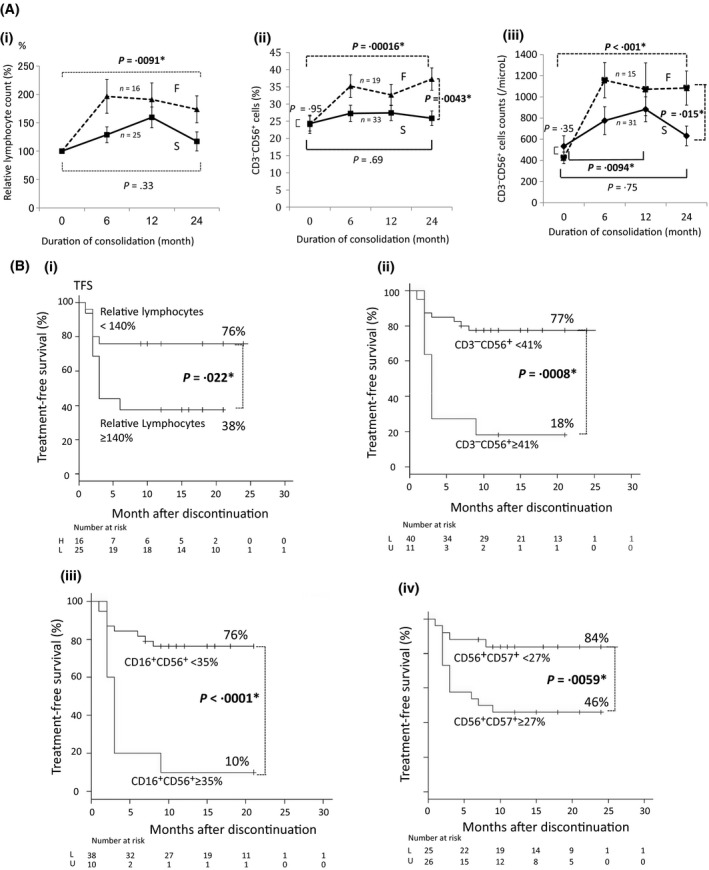
Changes in the numbers of total lymphocytes and natural killer (NK) cell subsets during consolidation therapy with dasatinib after achieving a deep molecular response. In all figures, the solid line indicates the successful (S) group, which comprised patients who achieved successful discontinuation without relapse for >7 mo (n = 34); the dotted line denotes the failed (F) group, which comprised patients with a molecular relapse during the observation period (n = 20). Data are presented as averages ± SE. The x‐axis indicates the duration of consolidation (months). The following data are presented on the y‐axes: (A(i)) Relative proportion of lymphocytes (%) compared to the baseline levels just before consolidation. (A(ii)) Proportion (%) of CD3− CD56+ NK cells during consolidation. (A(iii)) Absolute number of CD3− CD56+ NK cells during consolidation. (B(i)) Treatment‐free survival (TFS) rates of patients with lymphocyte counts ≥140% or <140% relative to the baseline at the end of consolidation. The cut‐off level was determined using a receiver operating characteristic (ROC) curve analysis as described in the Methods. (B(ii)) TFS rates of the patients with CD3− CD56+ NK cell proportions (%) ≥41% or <41% at the end of consolidation. The cut‐off level was determined using an ROC curve analysis as described in the Methods. (B(iii)) TFS rates of the patients with CD16+ CD56+ NK cell proportions (%) of ≥35% or <35% at the end of consolidation. The cut‐off level was determined using an ROC curve analysis as described in the Methods. (B(iv)) TFS rates of the patients with CD56+ CD57+ NK‐LGL proportions (%) of ≥27% or <27% at the end of consolidation. The cut‐off level was determined using an ROC curve analysis as described in the Methods
We further assessed the proportion (%) of each lymphocyte subset among the total lymphocyte population and the absolute cell counts using flow cytometry. The proportion of CD3− CD56+ NK cells did not differ between S and F groups before consolidation (P = .95); by contrast, this value increased significantly in F group during the consolidation period (P = .00016*) but remained stable in S group (P = .69). After consolidation, both the proportion (Figure 4A(ii)) and absolute number of CD3− CD56+ NK cells (Figure 4A(iii)) were significantly higher in F group than in S group (P = .0043* and P = .015*, respectively). In the S group, this NK cell population transiently increased at 12 months (P = .0094*) and returned to baseline levels after consolidation (P = .75), resulting in a significant difference between the two groups after consolidation (Figure 4A(iii)).
Regarding CD16+ CD56+ NK cells, the proportions among total lymphocytes and cell counts in both groups exhibited similar changes during the consolidation period (Figure S2A‐1,A‐2). The proportions of CD56+ CD57+ NK‐LGL cells among total lymphocytes did not differ significantly between the two groups before consolidation (P = .52); during the consolidation period, this proportion increased in F group (P = .0009*) while remaining stable in S group (P = .36). After consolidation, the proportion of CD56+ CD57+ NK‐LGL cells was significantly higher in F group compared with S group (P = .0051*) (Figure S2B‐1). The absolute CD56+ CD57+ cell count also increased during the consolidation period in F group (P = .0004*), whereas in S group this value increased transiently at 12 months (P = .0011*) but returned to the baseline level after 24 months (P = .46) (Figure S2B‐2).
Based on these observations, we our results were as follows. The patients with a relative lymphocyte change <140% (as determined by ROC analysis) after consolidation had a significantly higher TFS rate (76%) than those with a change >140% (38%) (P = .022*) (Figure 4B(i)). The difference of CD3− CD56+ proportions also led to a marked disparity in the TFS rates at 12 months between the patients with CD3− CD56+ proportions of <41% and ≥41% (ROC analysis) among total lymphocytes at the end of consolidation (77% vs 18%, respectively; P = .0008*) (Figure 4B(ii)). A marked difference in TFS was also observed between patients with a CD16+ CD56+ proportion of <35% vs those with a proportion ≥35% (ROC analysis) after consolidation (76% vs 10%, respectively; P < .0001*) (Figure 4B(iii)). A significant difference in the TFS at 12 months between patients with a CD56+ CD57+ NK‐LGL proportion of <27% (ROC analysis) and those with a proportion of ≥27% after consolidation was also observed (84% vs 46%, respectively; P = .0059*) (Figure 4B(iv)). Based on these results, we concluded that the increases in NK and NK‐LGL cells during the consolidation period correlated negatively with successful discontinuation.
Multivariate analysis was performed using factors such as sex, Sokal score, BCR‐ABL‐1 negative duration, duration of TKI treatment, patient age, total dasatinib dose, switching from imatinib to dasatinib before consolidation, the existence of imatinib resistance and levels of CD56− CD3+ NK cells (<41%) at the end of consolidation. In the univariate analysis, factors with P‐values of ≥.30 were excluded. Lower levels of CD3− CD56+ NK cells at the end of consolidation were an independent predictive factor for TFS (Figure S3). The final results will be reported at 3‐year follow‐up.
Regarding the cytotoxic T (CD4− CD8+), T‐LGL (CD3+ CD57+) and regulatory T (Tregs; CD4+ CD25+ CD127dim/−) cell subsets, no significant differences in the proportions among total lymphocytes or absolute cell counts were observed between S and F groups before or after consolidation (Figure S4A‐1, A‐2, B‐1, B‐2, D‐1 and D‐2). However, F group exhibited significant increases in cytotoxic T cells (Figure S4A‐2, P = .0009*) and T‐LGL (Figure S4B‐2, P = .028*) during consolidation. In contrast, the S group exhibited only a significant decrease in Tregs (Figure S4D‐2, P = .035*).
The proportion of helper T cells (CD4+ CD8−) among total lymphocytes was significantly lower in F group than in S group after consolidation (Figure S4C‐1). However, as the absolute cell counts did not differ between the two groups during consolidation (Figure S4C‐2), the difference appeared to arise from a relative increase in total lymphocytes in F group (Figure 4A(i)) and was not considered important. In summary, small and occasionally significant changes in T cell subsets were observed.
3.4. Changes in natural killer cell populations throughout the dasatinib discontinuation study
Rapid decreases in the lymphocyte, CD3− CD56+ NK cell and CD56+ CD57+ NK‐LGL populations were observed after dasatinib discontinuation, and no significant differences remained between the S and F groups after 3 months (Figure 5A). We note that although NK cell counts may also have shifted over time, we did not collect additional data.
Figure 5.
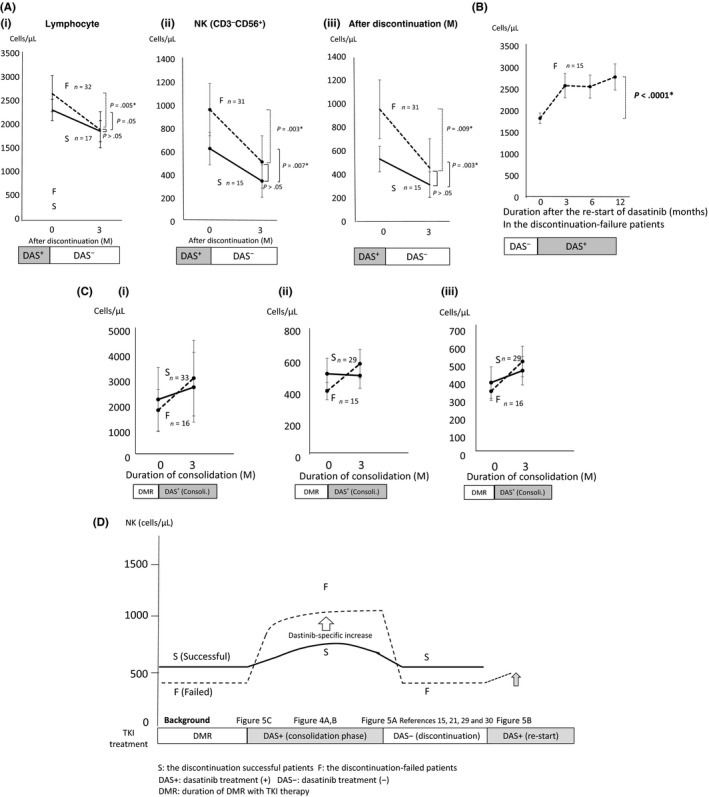
Natural killer (NK) cell changes throughout the dasatinib‐discontinuation study. (A) (i) Lymphocytes, (ii) NK cells (CD3− CD56+) and (iii) NK‐large granular lymphocytes (NK‐LGL; CD57+ CD56+) significantly decreased rapidly after dasatinib discontinuation in both the successful (S) and failed (F) groups (x‐axis: months after discontinuation of dasatinib, y‐axis: counts of lymphocytes, CD3− CD56+ NK cells or CD56+ CD57+ NK‐LGL). Cell counts of lymphocytes, NK and NK‐LGL did not differ significantly between patient groups after 3 mo (P = .89, P = .064 and P = .065, respectively). (B) A rapid and significant increase in lymphocytes was observed after 12 mo of dasatinib re‐start for F patients with molecular relapses (P < .0001*) (x‐axis: months after dasatinib re‐start, y‐axis: lymphocyte counts). (C) The average counts of (i) lymphocytes, (ii) NK cells (CD3− CD56+) and (iii) NK‐LGL (CD57+ CD56+) were lower in F patients (n = 20) relative to S patients (n = 34) before dasatinib‐consolidation; this pattern reversed after 3 mo of consolidation (x‐axis: months after dasatinib consolidation, y‐axis: lymphocyte counts). (D) The suggested hypothesis of NK cell transition during the dasatinib discontinuation study (x‐axis: treatments including the period of deep molecular response [DMR] induced by tyrosine kinase inhibitor [TKI] therapy [DMR], dasatinib consolidation therapy {DAS+ (consolidation phase)}, dasatinib discontinuation {DAS− (discontinuation)} and dasatinib re‐treatment for molecular relapses in discontinuation‐failed patients {DAS+ (re‐start)}, y‐axis: NK cell counts). Dasatinib discontinuation‐successful patients (S) are indicated by the solid line; failed (F) patients are indicated by the dotted line. Patients who achieved DMR before consolidation therapy and were discontinuation‐successful tended to have a higher average NK cell count (as in C). During dasatinib consolidation, NK cells increased specifically in response to dasatinib much more in the F group than in the S group, as shown in Figure 4A,B. A similar increase may not occur during imatinib consolidation.23 However, NK cells rapidly decreased to the basal level after discontinuation, as in Figure 5A. During discontinuation, NK cells were higher in group S, as previously reported.15, 21, 29, 30 Furthermore, lymphocytes increased again after re‐starting dasatinib in the F group, further supporting a specific effect of dasatinib (B). Consoli., consolidation with dasatinib; DAS+, duration with dasatinib treatment; DAS−, duration without dasatinib treatment; DAS→DAS, patients who continued dasatinib before and during consolidation; DMR, duration of DMR with TKI therapy; F, failed group; IM→DAS, patients who switched imatinib to dasatinib just before consolidation; S, successful group
In F group, the lymphocyte population increased significantly after dasatinib re‐treatment for a molecular relapse (Figure 5B), indicating that dasatinib specifically increased NK cells. Before consolidation, S group tended to have a higher average NK cell count compared with F group; however, this trend reversed after 3 months (Figure 5C). When we determined the cut‐off level before consolidation, patients with a higher than average NK cell count tended to have higher TFS (data not shown), whereas during consolidation, F group was more likely to exhibit a larger dasatinib‐specific lymphocyte increase (%) from the baseline (Figure 4A(i–iii)).
At the start of consolidation, some patients switched from imatinib to dasatinib. These patients were specific to the D‐STOP study and were not included in previous cessation studies. These patients did not affect the TRF rate (Figure 1G). However, the relative increase in lymphocyte levels in the failed patients during consolidation (157.6 ± 26.3%) tended to be higher than that in patients who continued dasatinib (117.4 ± 13.7%). Therefore, such a patient population might emphasize our results over those of other studies.13, 14, 15, 21
We considered the underlying mechanism of these NK cell changes during dasatinib discontinuation (Figure 5D). Before consolidation, NK cells tended to be higher in discontinuation‐successful patients (Figure 5C). Although these cells increased to a great extent in the discontinuation‐failed patients during consolidation (Figure 4A,B), the population contracted rapidly after discontinuation (Figure 5A). During discontinuation, NK cell numbers are reportedly higher among successful patients.15, 21, 29, 30 We observed that in the failed patients, the lymphocyte population increased again after re‐starting dasatinib, suggesting a direct effect of TKI on this cell population (Figure 5B).
4. DISCUSSION
The results of this study demonstrate the feasibility of achieving a high TFS rate with dasatinib consolidation for ≥2 years after achieving DMR; the dasatinib‐induced increase in NK cells during consolidation was found to correlate negatively with successful discontinuation.
We compared the D‐STOP and DADI trials,15 which included similar numbers of patients (63 and 54, respectively) with similar median ages (59 [24‐84] and 56 [27‐84]) years, respectively, to evaluate favorable conditions for dasatinib discontinuation. Both trials defined DMR at discontinuation and molecular relapse using RQ‐PCR data obtained at BML.15, 22 Patients in the DADI trial achieved DMR during second‐line dasatinib therapy after first‐line imatinib therapy.15 By contrast, patients in the D‐STOP trial underwent dasatinib consolidation after achieving DMR via treatment with any TKI, although the majority of patients had initially used imatinib (34 patients) or dasatinib (19 patients). Interestingly, the TFS rates of imatinib‐treated and dasatinib‐treated patients were extremely similar, with no significant differences (Figure 3G), indicating that the type of TKI used to achieve DMR was not important for successful discontinuation. The most significant difference between 2 studies was the duration of consolidation, which was 2 years in the D‐STOP but only 1 year in the DADI trial.15 In the D‐STOP study, the rate of imatinib resistance was 14.8% (8/54), which was lower than that in the DADI study (20.6%, 13/63).15 As imatinib resistance was a predictive factor for poor TFS in DADI, this may affect the TFS in the D‐STOP study. In contrast, the estimated TFS at 12 months in imatinib‐resistant patients and others were 62.5% (95% CI, 22.9‐86.1) and 62.9% (95% CI, 47.3‐75.1) (P = .94), respectively, in the D‐STOP study. These were not significantly different, and further analysis is necessary. The different TFS observed in D‐STOP (62.9% at 12 months) relative to DADI (49% at 6 months) might be attributable to these factors.
We previously reported that a relative increase in the levels of NK cells with initial dasatinib was associated with higher DMR achievement. Increased levels of NK cells may function to exclude CML cells before achieving DMR.19 Such patients with increased levels of NK cells could achieve DMR and enter a cessation‐study such as the D‐STOP. After the achievement of DMR, levels of NK cells may continue to increase or stabilize during consolidation. The D‐STOP trial indicated that patients with stable NK cells during consolidation were more successful in cessation.
After achieving DMR, NK cells may function differently for the immune surveillance to prevent molecular relapses. This was indicated by the previous reports that stated that the patients with successful discontinuation had larger and more functional NK cells than the failed patients.15, 21, 29, 30
To explain the greater increase in NK cells following dasatinib consolidation in the failed patients during DMR in the D‐STOP trial, we first hypothesized that NK cells of the successful patients and those of the failed patients responded differently to dasatinib during DMR. NK cells in the failed patients who had lower immune surveillance capability may respond more and increase after dasatinib administration. The increase in NK cells seemed dasatinib‐specific and might be attributable to the inhibition of multiple kinases, such as c‐KIT, PDGF, SRC and Src family kinases, in addition to ABL1.31 Although SRC has been reported to be associated with NK cell function and signal transduction,32 the reason underlying dasatinib‐induced specific increase in NK cells during consolidation in the failed patients remains unclear. Differences in the immune functions, maturation, and genomic as well as epigenomic structures of NK cells of the successful patients and failed patients is a topic for future research.
Another hypothesis is that the increase in NK cells during DMR occurs through the off‐target effects of dasatinib only when residual CML cells with high expansion capacity are excluded by NK cells, even though present in a small number (<MR4). Therefore, levels of NK cells stabilize when there are few residual CML cells with high expansion capacity, as is observed in the successful patients. In contrast, the levels of NK cells may increase with residual CML cells with high expansion capacity, such as imatinib‐resistant CML cells to be excluded by NK cells, as observed in the failed patients.
Such an increase in NK cells during consolidation was also induced specifically by dasatinib in DADI (Figure S3 in Imagawa15); however, similar increases were not reported for imatinib cessation. Levels of NK cells were high in successful patients during discontinuation;15, 21, 29, 30 however, in the D‐STOP study, they were high during the consolidation phase in those who failed (Figure 5A). The levels of NK cells should be reversed before and after dasatinib cessation. In Figure 5, we proposed the possibility of reversing the levels of NK cells before and after dasatinib cessation. After 3 months, the sample size was not sufficient because data collection was not scheduled for all patients, and data could not be collected from those who relapsed in <3 months and restarted dasatinib with further rise in the lymphocyte levels (Figure 5B).
In conclusion, we have demonstrated that a 2‐year consolidation with dasatinib after achieving DMR was feasible and yielded a high TFS rate after discontinuation. However, despite earlier reports that a larger NK cell population during discontinuation correlated with improved TFS,15, 21, 27, 28 we observed that a transient increase in NK cells during dasatinib consolidation was unfavorable for successful discontinuation.
D‐STOP showed that an increase in the levels of NK cells during dasatinib consolidation was negatively correlated with successful cessation. Therefore, patients with stable levels of NK cells with dasatinib consolidation after DMR may be good candidates for discontinuation. These findings may provide new insights into optimal TKI cessation.
DISCLOSURE STATEMENT
T.K. received lecture fees from Bristol‐Myers Squibb, Novartis and Pfizer. C.N. received honoraria from Bristol‐Myers Squibb, Pfizer and Novartis, and grants from Bristol‐Myers Squibb and Pfizer. K.N. received research funding from Novartis. C.Y. received honoraria and speakers bureau from Bristol‐Myers Squibb and Pfizer, and honoraria, speakers bureau and grants from Otsuka. K.M. received honoraria from Celgene. S.M. received honoraria from Bristol‐Myers Squibb. J.S. received remuneration from Yakult Honsha. I.K. received research grants from Bristol‐Myers Squibb, and honoraria from Bristol‐Myers Squibb, Novartis, Celgene and Pfizer.
Supporting information
ACKNOWLEDGMENTS
This study was supported by the Epidemiological and Clinical Research Information Network (ECRIN) and Bristol‐Myers Squibb (BMS). We thank Yumi Miyashita at ECRIN for collecting the data and Narutaka Sakurada and Yoshinori Yamamoto at BML for performing flow cytometry and RQ‐PCR. We thank Ishida Tadao (Department of Gastroenterology, Rheumatology and Clinical Immunology, Sapporo Medical University, Sapporo), Hiroyuki Sugawara (Department of Hematology, Sumitomo Hospital, Osaka), Sakae Tanosaki (Department of Hematology, Tokyo Doai Memorial Hospital, Tokyo), Masayuki Koizumi (Department of Hematology, Asahi Chuo Hospital, Chiba), Megumi Akiyama (Department of Hematology, Yokosuka Kyosai Hospital, Kanagawa), Hiroshi Inoue (Department of Hematology, Inoue Memorial Hospital, Chiba), Iwai Kazuya (Department of Hematology, Shizuoka City Shizuoka Hospital, Shizuoka) and Akira Ohwada (Department of Hematology, Tokyo Metropolitan Bokutoh Hospital, Tokyo) for participating in this study. We are grateful to Professor Junia V. Melo for critically commenting on and editing the manuscript.
Kumagai T, Nakaseko C, Nishiwaki K, et al. Dasatinib cessation after deep molecular response exceeding 2 years and natural killer cell transition during dasatinib consolidation. Cancer Sci. 2018;109:182–192. https://doi.org/10.1111/cas.13430
REFERENCES
- 1. Faderl S, Talpaz M, Estrov Z, O'Brien S, Kurzrock R, Kantarjian HM. The biology of chronic myeloid leukemia. N Engl J Med. 1999;341:164‐172. [DOI] [PubMed] [Google Scholar]
- 2. Lugo TG, Pendergast AM, Muller AJ, Witte ON. Tyrosine kinase activity and transformation potency of bcr‐abl oncogene products. Science. 1990;247:1079‐1082. [DOI] [PubMed] [Google Scholar]
- 3. Druker BJ, Guilhot F, O'Brien SG, et al. Five‐year follow‐up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med. 2006;355:2408‐2417. [DOI] [PubMed] [Google Scholar]
- 4. Kantarjian H, Shah NP, Hochhaus A, et al. Dasatinib versus imatinib in newly diagnosed chronic‐phase chronic myeloid leukemia. N Engl J Med. 2010;362:2260‐2270. [DOI] [PubMed] [Google Scholar]
- 5. Saglio G, Kim DW, Issaragrisil S, et al. ENESTnd investigators. Nilotinib versus imatinib fornewly diagnosed chronic myeloid leukemia. N Engl J Med. 2010;362:2251‐2259. [DOI] [PubMed] [Google Scholar]
- 6. Cortes JE, Saglio G, Kantarjian HM, et al. A. Final 5‐year study results of DASISION: the dasatinib versus imatinib study in treatment‐naïve chronic myeloid leukemia patients trial. J Clin Oncol. 2016;34:2333‐2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hochhaus A, Saglio G, Hughes TP, et al. Long‐term benefits and risks of frontline nilotinib vs imatinib for chronic myeloid leukemia in chronic phase: 5‐year update of the randomized ENESTnd trial. Leukemia. 2016;30:1044‐1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Padula WV, Larson RA, Dusetzina SB, et al. Cost‐effectiveness of tyrosine kinase inhibitor treatment strategies for chronic myeloid leukemia in chronic phase after generic entry of imatinib in the United States. J Natl Cancer Inst. 2016;108:djw003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rochau U, Sroczynski G, Wolf D, et al. Cost‐effectiveness of the sequential application of tyrosine kinase inhibitors for the treatment of chronic myeloid leukemia. Leuk Lymphoma. 2015;56:2315‐2325. [DOI] [PubMed] [Google Scholar]
- 10. Efficace F, Cannella L. The value of quality of life assessment in chronic myeloid leukemia patients receiving tyrosine kinase inhibitors. Hematology Am Soc Hematol Educ Program. 2016;2016:170‐179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cortes J, Kantarjian H. Chronic myeloid leukemia: sequencing of TKI therapies. Hematology Am Soc Hematol Educ Program. 2016;2016:164‐169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Steegmann JL, Baccarani M, Breccia M, et al. European LeukemiaNet recommendations for the management and avoidance of adverse events of treatment in chronic myeloid leukaemia. Leukemia. 2016;30:1648‐1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mahon FX, Réa D, Guilhot J, et al. Discontinuation of imatinib in patients with chronic myeloid leukaemia who have maintained complete molecular remission for at least 2 years: the prospective, multicentre Stop Imatinib (STIM) trial. Lancet Oncol. 2010;11:1029‐1035. [DOI] [PubMed] [Google Scholar]
- 14. Ross DM, Branford S, Seymour JF, et al. Safety and efficacy of imatinib cessation for CML patients with stable undetectable minimal residual disease: results from the TWISTER study. Blood. 2013;122:515‐522. [DOI] [PubMed] [Google Scholar]
- 15. Imagawa J, Tanaka H, Okada M, et al. Discontinuation of dasatinib in patients with chronic myeloid leukaemia who have maintained deep molecular response for longer than 1 year (DADI trial): a multicentre phase 2 trial. Lancet Haematol. 2015;2:e528‐e535. [DOI] [PubMed] [Google Scholar]
- 16. Mahon FX, Richter J, Guilhot J, et al. Interim analysis of a pan European stop tyrosine kinase inhibitor trial in chronic myeloid leukemia: the EURO‐SKI study. Blood. 2014;124:151.24993879 [Google Scholar]
- 17. Mahon FX, Baccarani M, Mauro MJ, et al. Treatment‐free remission (TFR) following nilotinib (NIL) in patients (pts) with chronic myeloid leukemia in chronic phase (CML‐CP): ENESTfreedom, ENESTop, ENESTgoal, and ENESTpath. J Clin Oncol. 2014;32:TPS7124. [Google Scholar]
- 18. Saußele S, Richter J, Hochhaus A, Mahon FX. The concept of treatment‐free remission in chronic myeloid leukemia. Leukemia. 2016;30:1638‐1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kumagai T, Matsuki E, Inokuchi K, et al. Relative increase in lymphocytes from as early as 1 month predicts improved response to dasatinib in chronic‐phase chronic myelogenous leukemia. Int J Hematol. 2014;99:41‐52. [DOI] [PubMed] [Google Scholar]
- 20. Mustjoki S, Ekblom M, Arstila TP, et al. Clonal expansion of T/NK‐cells during tyrosine kinase inhibitor dasatinib therapy. Leukemia. 2009;23:1398‐1405. [DOI] [PubMed] [Google Scholar]
- 21. Ilander M, Olsson‐Strömberg U, Schlums H, et al. Increased proportion of mature NK cells is associated with successful imatinib discontinuation in chronic myeloid leukemia. Leukemia. 2017;31:1108‐1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yoshida C, Fletcher L, Ohashi K, et al. Harmonization of molecular monitoring of chronic myeloid leukemia therapy in Japan. Int J Clin Oncol. 2012;17:584‐589. [DOI] [PubMed] [Google Scholar]
- 23. Cross NC, White HE, Colomer D, et al. Laboratory recommendations for scoring deep molecular responses following treatment for chronic myeloid leukemia. Leukemia. 2015;29:999‐1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Iriyama N, Fujisawa S, Yoshida C, et al. Early cytotoxic lymphocyte expansion contributes to a deep molecular response to dasatinib in patients with newly diagnosed chronic myeloid leukemia in the chronic phase: results of the D‐first study. Am J Hematol. 2015;90:819‐824. [DOI] [PubMed] [Google Scholar]
- 25. Liu W, Putnam AL, Xu‐Yu Z, et al. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4 + T reg cells. J Exp Med. 2006;203:1701‐1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kanda Y. Investigation of the freely available easy‐to‐use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48:452‐458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Etienne G, Guilhot J, Rea D, et al. Long‐term follow‐up of the French Stop Imatinib (STIM1) study in patients with chronic myeloid leukemia. J Clin Oncol. 2017;35:298‐305. [DOI] [PubMed] [Google Scholar]
- 28. Rea D, Nicolini FE, Tulliez M, et al. Discontinuation of dasatinib or nilotinib in chronic myeloid leukemia: interim analysis of the STOP 2G‐TKI study. Blood. 2017;129:846‐854. [DOI] [PubMed] [Google Scholar]
- 29. Hughes A, Clarson J, Tang C, et al. CML patients with deep molecular responses to TKI have restored immune effectors and decreased PD‐1 and immune suppressors. Blood. 2017;129:1166‐1176. [DOI] [PubMed] [Google Scholar]
- 30. Ohyashiki K, Katagiri S, Tauchi T, et al. Increased natural killer cells and decreased CD3(+)CD8(+)CD62L(+) T cells in CML patients who sustained complete molecular remission after discontinuation of imatinib. Br J Haematol. 2012;157:254‐256. [DOI] [PubMed] [Google Scholar]
- 31. Weisberg E, Manley PW, Cowan‐Jacob SW, Hochhaus A, Griffin JD. Second generation inhibitors of BCR‐ABL for the treatment of imatinib‐resistant chronic myeloid leukaemia. Nat Rev Cancer. 2007;7:345‐356. [DOI] [PubMed] [Google Scholar]
- 32. Hassold N, Seystahl K, Kempf K, et al. Enhancement of natural killer cell effector functions against selected lymphoma and leukemia cell lines by dasatinib. Int J Cancer. 2012;131:E916‐E927. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


