Abstract
Background
Nasal cavity tumors are usually diagnosed late, when they already have infiltrated adjacent tissues thus requiring very aggressive treatments with serious side effects. Here we use electrochemotherapy (ECT), a well demonstrated treatment modality for superficial tumors.
Materials and methods
In the case of deep-seated tumors, the main limitation of ECT is reaching the tumor with an appropriate electric field. To overcome this limitation we introduce the single needle electrode (SiNE), a minimally invasive device that can deliver an appropriate electric field with a simple procedure. Twenty-one canine patients with spontaneous tumors were selected, eleven were treated using the SiNE with ECT, and ten with surgery plus adjuvant chemotherapy as a control group.
Results
In the SiNE group, 27% achieved a complete response, 64% had a partial response, and 9% had a stable disease. This means that 91% of objective responses were obtained. The mean overall survival was 16.86 months (4–32 months, median 16.5 months), with a survival rate significantly higher (p = 0.0008) when compared with control group. The only side effect observed was the inflammation of the treated nasal passage, which was controlled with corticosteroid therapy for one week. One year after the treatment, 60% of the canine of the SiNE group vs. 10% of the control group remained alive, and after the 32 months follow-up, the survival rate were 30% and 0%, respectively.
Conclusions
ECT with the SiNE can be safely used in canine to treat nasal tumors with encouraging results.
Key words: electrochemotherapy, nasal cavity, canine, cancer, electrochemotherapy
Introduction
Spontaneous tumors of the nasal cavity and paranasal sinuses in canine patients account for 1% of all neoplasms. Their etiology is unknown, but animals living in urban areas are at an increased risk, due to the nasal filtering of pollutants. Also, exposure to tobacco smoke and fossil fuel combustion products increase the risk of this type of cancer. The average age of dogs with nasal cancer is around 10 years, predominantly in medium and large breeds. Common clinical signs include epistaxis, mucopurulent nasal discharge, facial deformity, sneezing, dyspnea, local pain, stertorous breathing, exophthalmos, and ocular discharge.1
The diagnostic procedures are the same as in human patients and involve high-resolution imaging procedures such as Computed Tomography (CT) scans or Magnetic Resonance Imaging (MRI) to determine the extension of the tumor and evaluating the involvement of neighboring structures such as the palate, the retro-orbital area, the sinuses, and the cranial vault.1 The most common histologies are adenocarcinoma, squamous cell carcinoma and sarcomas.1,2 Metastases are rare at the time of presentation, yet they are present at the time of death in around 40–50% of the cases. Lungs and lymph nodes are common metastatic sites.1
Electrochemotherapy (ECT) is a new treatment modality which has been gaining territory in oncology since the Standard Operating Procedures were published in 2006.3,4,5 ECT consists of the application of an electric field to a tumor in order to increase the uptake of bleomycin that was previously administered (locally or intravenously) at a very low dose6; alternatively, cisplatin can be used locally with equally good results.7 ECT is mainly indicated for cutaneous and subcutaneous tumors of any histology. As cell membrane permeabilization is produced by a physical phenomenon known as electroporation, it affects all cells, regardless of the histology of the tumor. Recently, a meta-analysis of ECT clinical studies in human oncology showed that the overall objective response (OR) rate vary from 62.6% to 82.2% depending also on the route of administration of the drug, being either intravenous or intratumoural.8 Great efforts are being made to extend ECT to non-cutaneous locations such as the liver9, the brain10 and the bones.11 Moreover, an endoscopic electrode was developed to treat the colon.12 For deep-seated tumors, ECT is performed in an intraoperative manner, thus requiring the patient to be exposed to the risks and complications of an open surgery. Here we explore ECT for treating nasal cavity tumors by means of the Single Needle Electrode (SiNE).
The aim of this work is to perform a validation study of the treatment of nasal cavity tumors using the SiNE with ECT in dogs and compare the overall survival with the standard treatment available in our setting, i.e.: surgery plus adjuvant chemotherapy.
Materials and methods
Consent was obtained from the dog’s owner to perform the treatment and for using the dog’s image in this scientific work. In all cases, all recommendations from the Consejo Profesional de Médicos Veterinarios de Buenos Aires (Buenos Aires Veterinary Council) were observed, as well as the relevant local legislation in Argentina, Act No. 14072 which governs Veterinary Medicine practice. Recommendations from Campana et al.13 for reporting clinical studies on ECT were followed.
The SiNE pictured in Figure 1 is an electrode specifically designed in our lab for deep-seated tumor treatment with ECT. It consists of an insulated needle with an anode in one side and a cathode in the other. This design provides an electric field of cylindrical shape around it, strong enough to produce the electroporation of the cells.
Figure 1.
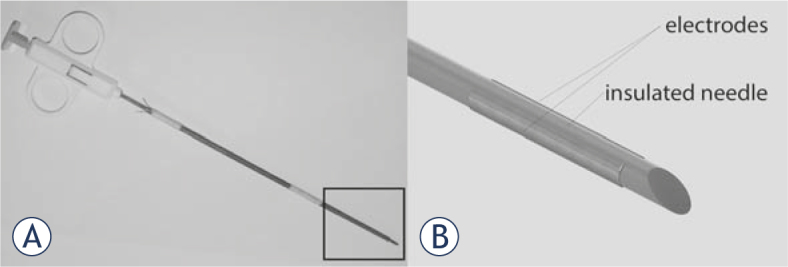
The SiNE. In (A) the prototype, in (B) the scheme of the tip, zoomed from the squared area in A. The electrode consists of a needle isolated surrounded by two parallel plaques at each side.
Twenty-one patients were selected, eleven for the SiNE group and ten for the control group. The patients for the SiNE group were selected according to the following inclusion criteria.
Canine patient with confirmed diagnosis of nasal duct tumor.
Life expectancy of more than 1 month.
Surgery refusal by the owner.
The control group was formed with ten patients whose owners accepted surgery plus adjuvant chemotherapy.
In the SiNE group, rhinoscopy was performed using a flexible or a rigid endoscope of variable diameters according to patient size; nasal neoplasia was diagnosed on the basis of histological findings. A complete blood count, serum biochemical profile, coagulation profile, abdominal ultrasound, and 3-view thoracic radiographs were performed. A CT/MRI was performed to determine the size and extension of the tumor. The patients were staged using Adam’s staging system (Table 1).
Table 1.
Adam’s modified staging system
| Stage | Tumor characteristics |
|---|---|
| T1 | Confined to one nasal passage, paranasal sinus, or frontal sinus with no bony involvement. |
| T2 | Any bony involvement, but no evidence of orbital, subcutaneous, or submucosal mass. |
| T3 | Involvement of orbit or subcutaneous or submucosal mass. |
| T4 | Tumor extension into nasopharynx or cribriform plate. |
In this set of patients (Table 2), 7 were males and 4 females. Four of the males were neutered, and 3 of the females were spayed. The median age of the dogs starting ECT treatment was 9 years (range of 6–13 years). The mean weight was 26 kg (range of 3.5–48 kg). Labrador Retriever (n = 3) and Golden Retriever (n = 3) were the most common breeds in this study. The other breeds were Collie, Old English Mastiff, Miniature Pincher, Spanish Breton, and a crossbreed dog. The clinical findings revealed sneezing in 11 dogs (100%), nasal discharge in 9 dogs (81%), epistaxis in 6 dogs (55%), exophthalmos in 2 dogs (18%), neurological symptoms in 2 dogs (18%) and facial deformity in 1 dog (9%). Regarding clinical Adam’s staging, there was 1 dog with T1 disease (9%), 6 dogs with T2 disease (55%), 2 dogs with T3 disease (18%) and 2 dogs with T4 disease (18%). No dogs had regional lymph nodes or distant metastasis at initial presentation. None of the patients had previous treatments.
Table 2.
SiNE group patient details and treatment outcome
| Patient | Breed | Histology | Adam’s stage | Response | Clinical Evaluation |
|---|---|---|---|---|---|
| 1 | Golden Retriever | Adenocarcinoma grade 3 | T2 | PR | Partial reduction of clinical signs, with a persistent minimum nasal discharge. |
| 2 | Miniature Pinscher | Adenocarcinoma grade 2 | T2 | CR | Complete resolution of clinical signs. |
| 3 | Labrador Retriever | Solid carcinoma grade 3 | T3 | PR | Partial reduction of clinical signs, with persistence of ocular discharge. Death by euthanasia due to distal radius metastasis. |
| 4 | Old English Mastiff | Adenocarcinoma grade 2 | T4 | PR | Complete Resolution of clinical signs. |
| 5 | Crossbreed | Squamous cell carcinoma | T2 | PR | Partial reduction of clinical signs with persistence of minimum nasal discharge. |
| 6 | Labrador Retriever | Round cell sarcoma | T3 | PR | Complete resolution of clinical signs. |
| 7 | Collie | Sticker sarcoma | T1 | CR | Complete resolution of clinical signs. |
| 8 | Golden Retriever | Squamous cell carcinoma | T2 | PR | Partial reduction of clinical signs, sneezing remains every day with occasional nasal discharge. Death by euthanasia based on the owner’s decision. |
| 9 | Spanish Breton | Adenocarcinoma grade 2 | T4 | SD | No reduction of clinical signs was observed, sneezing remained every day with continuous nasal discharge and persistent inflammation of the subcutaneous tissue and paranasal sinuses. Death by euthanasia based on poor quality of life. |
| 10 | Labrador Retriever | Squamous cell carcinoma | T2 | PR | Partial reduction of clinical symptoms with occasional sneezing. |
| 11 | Golden Retriever | Fibrosarcoma | T2 | CR | Complete resolution of clinical signs. |
The treatment was performed under general anesthesia with premedication with intramuscular administration of xylazine (Xylazine 100®, Richmond, Buenos Aires, Argentina) 0.5 mg/kg and tramadol (Tramadol®, John Martin, Buenos Aires, Argentina) 2 mg/kg. The induction was made with intravenous administration of propofol (Propofol Gemepe®, Gemepe, Buenos Aires, Argentina) 3 mg/kg. The maintenance was performed with inhaled isofluorane (Zuflax®, Richmond, Buenos Aires, Argentina) 2–3% and intravenous fentanyl (Fentanyl Gemepe®, Gemepe, Buenos Aires, Argentina) 2 mcg/kg. This anesthesia scheme provided adequate comfort during the treatment. Prophylactic antibiotics and meloxicam (Meloxivet®, John Martin, Buenos Aires, Argentina) 0.2 mg/kg were administered orally for analgesia after the treatment according to the needs of each patient.
The ECT procedure consisted of an intravenous bolus of bleomycin (Blocamicina®, Gador, Buenos Aires, Argentina) at a dose of 15 U/m2 of body surface area in 30–45 seconds, followed by the delivery of the electric pulses eight minutes later to allow drug distribution according to the Standard Operating Procedures published by Mir et al. [14].
The SiNE was inserted deep into the nasal fossa and a train of pulses was delivered, see Figure 2. For that purpose, a BTX ECM 830 Harvard Apparatus (Holliston, MA, USA) was used. The electric pulses consisted of thirty-two square pulses of 300V, 100 µs long at 1 Hz. The electrode was rotated 90 degrees clockwise and the pulses were repeated. After the rotation, the electrode was removed 2 cm and the whole procedure was repeated until complete coverage of the nasal duct. It was not possible to treat safety margins due to the location of the tumors.
Figure 2.
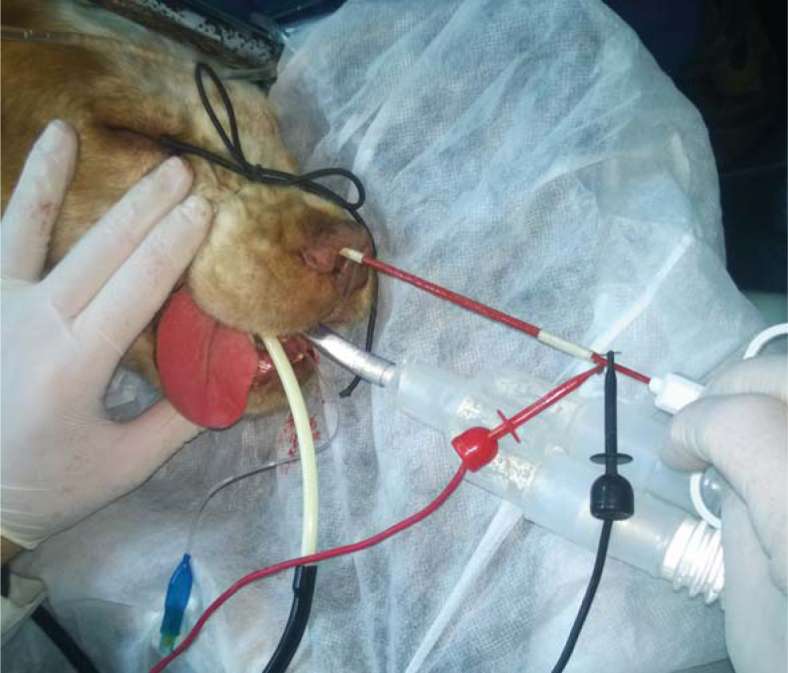
Picture of patient 9 during the treatment. The SiNE is inserted in the nasal fossa.
In order to prevent the SiNE from inadvertently passing through the cribriform plate during the insertion, it was marked to show the maximum insertion depth (following CT/MRI measurements performed before the treatment).
A single ECT treatment session was performed, and no other oncological treatment was indicated previously, concomitantly or afterward.
For the assessment of treatment results, follow-up was performed within 7, 15 and 30 days; during each visit, a complete clinical examination was performed. A second CT/MRI was obtained 60 days after the treatment to determine results. The patients were followed up for 32 months and responses reported according to WHO’s Criteria.15
The control group consisted of 10 cases, 5 males, and 5 females, whose owners accepted surgery as treatment (Table 3). One of the males and three of the females were neutered. The median age of the dogs was 10 years (range of 6–14 years). The mean weight was 22.7 kg (range of 5.6–34 kg). Crossbreed (n = 4) was the most common breed, other breeds were Puddle, Akita Inu, Boxer, Labrador Retriever, Fox Terrier and Cocker Spaniel. The clinical findings revealed sneezing in 10 dogs (100%), weight loss in 7 dogs (70%), epistaxis in 6 dogs (60%), hyporexia in 6 dogs (60%), facial deformity in 4 dogs (40%) and neurological symptoms in 1 dog (10%). Regarding Adam’s staging, there was 1 dog with T1 disease (10%), 5 dogs with T2 disease (50%), 3 dogs with T3 disease (30%) and 1 dog with T4 disease (10%). No dog had regional lymph nodes or distant metastases.
Table 3.
Control group patient details
| Patient | Breed | Histology | Adam’s stage | Cause of death |
|---|---|---|---|---|
| 1 | Puddle | Tubulopapillary carcinoma | T1 | Euthanasia due to local progression. |
| 2 | Crossbreed | Carcinoma | T2 | Euthanasia due to local progression. |
| 3 | Akita Inu | Chondrosarcoma | T2 | Euthanasia due to local progression to central nervous system |
| 4 | Crossbreed | Adenocarcinoma | T3 | Death due to local progression. |
| 5 | Boxer | Carcinoma | T4 | Death due to lung metastasis. |
| 6 | Crossbreed | Squamous cell carcinoma | T2 | Euthanasia due to breathing difficulty. |
| 7 | Crossbreed | Carcinoma | T2 | Euthanasia. |
| 8 | Labrador Retriever | Carcinoma | T3 | Euthanasia due to uncontrollable pain. |
| 9 | Fox Terrier | Solid carcinoma | T2 | Euthanasia. |
| 10 | Cocker Spaniel | Adenocarcinoma | T3 | Euthanasia due to local progression and pain. |
Surgery plus adjuvant chemotherapy was performed since it is the standard treatment available in our setting. The surgical procedure consisted of a dorsal/ventral uni/bilateral rhinotomy depending on the surgeon’s decision, and according to tumor localization and extension. The anesthesia procedure was identical to the one mentioned above. The patients remained in observation for 24 hours with intravenous butorphanol (Torbutrol Plus®, Alpha Farma, Buenos Aires, Argentina) 0.1 mg/kg every 4 hours for one day and oral meloxicam (Meloxivet®, John Martin, Buenos Aires, Argentina) 0.2 mg/kg every 24 hours for 10 days for analgesia. The adjuvant chemotherapy consisted of intravenous carboplatin (Carboplatino Delta Farma®, Sidus, Buenos Aires, Argentina) 300 mg/m2 of body surface area every 21 days.
Regarding the statistical analysis, Kaplan–Meier curves for survival were compared by the log-rank test with an alpha of 0.05. Age and body weight were compared between groups with the Mann-Whitney U test, with an alpha of 0.05.
Results
From the 21 patients selected, 11 were treated using the SiNE with ECT (SiNE group), and 10 were treated with surgery plus adjuvant chemotherapy (control group). The SiNE and control groups did not show significant differences in age or bodyweight (p = 0.46 and p = 0.13, respectively).
In the SiNE group, out of the 11 patients: 27% (3/11) achieved a complete response (CR), 64% (7/11) had a partial response (PR), and 9% (1/11) had a stable disease (SD). In the nasal duct treated, tumor reduction remained stable and the lesions did not grow again during the follow-up period. In the first two patients only one nasal duct was treated, in the non-treated duct, the tumor continued growing according to its natural history. For this reason and because of the highly satisfactory results obtained in the treated duct, both nasal passages were treated from patient number 3 onwards. The only side effect observed was the inflammation of the treated nasal passage, which was very well controlled with corticosteroid therapy for one week. The bleeding through the nasal fossa ceased immediately after the procedure.
The patients remained in follow-up for 32 months, with no relapses in the treated area. This means that 91% objective responses (OR) were obtained. Figure 3, pictures the CT scan of patient 11 showing a complete response obtained with the treatment.
Figure 3.
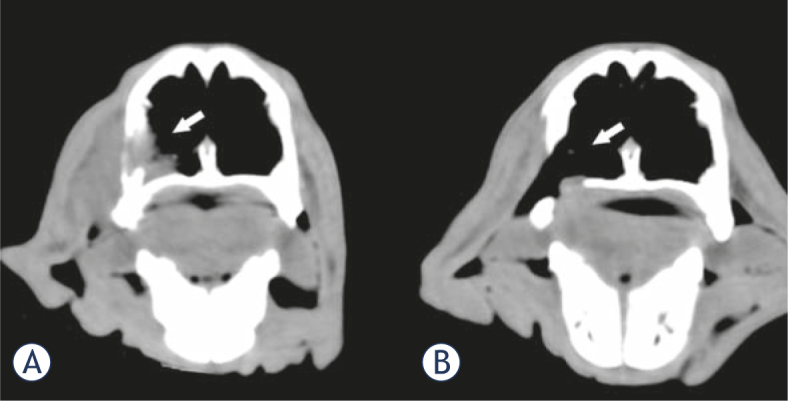
CT image of patient 11 before and after the treatment. (A) The tumor can be seen occupying the lateral inferior part of the left nasal passage (white arrow). (B) Two months after a single treatment a CR was obtained; as readily observed, the tumor tissue is absent leaving a defect in the nasal wall (white arrow).
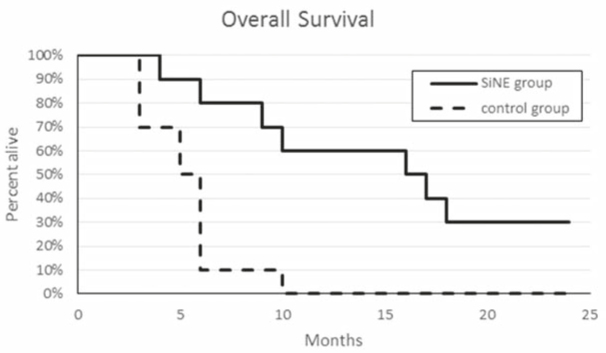
The Kaplan-Meier graph for overall survival in both groups can be seen in Figure 4. The overall survival of the SiNE group was significantly higher (p = 0.0008) than in the control group, with a mean survival time of 16.86 months (4–32 months, median 16.5 months). In the control group, a mean survival time of 5.3 months (3–10 months, median 5.5 months) was obtained. One year after the treatment, 60% of the patients of the SiNE group vs. 10% of the control group remained alive, and after the 32 months follow-up, the survival rate for the SiNE group is 30% vs. 0% for the control group.
Figure 4.
Kaplan-Meier graph of the outcome of dogs with nasal tumors treated using the SiNE with ECT procedure (SiNE group) versus the dogs treated with surgery plus adjuvant chemotherapy (control group).
Discussion
In an effort to provide a satisfactory treatment modality to canine patients with nasal tumors, and advance in the area of deep-seated tumor treatment using ECT, we developed the SiNE and a protocol for its implementation. We compared the results of this new treatment modality with the standard treatment modality in our setting: surgery plus chemotherapy.
When treating deep-seated tumors, the standard ECT procedure consists of inserting multiple needles to cover the whole tumor with an appropriate electric field. This procedure is very complex and prone to fail because the misplacement of the needles leaves areas of the tumor untreated. In some cases, open surgery is needed for placing the needles correctly16, such as in the case reported by Suzuki et al.17, where the results were very good though the procedure required open surgery and the filling of the nasal cavity with gel to improve conductivity. These was not needed in our case since the electrode is inserted through the nasal orifice and, secretions and blood present in the nasal duct replaced conductive gel. The most important advantage of the SiNE against standard electrodes is that only a single needle is inserted, thus reducing tissue intrusion and simplifying treatment planning and its guidance by imaging procedures. The disadvantage is that it is restricted to small tumors when compared with multiple needles.
A high percentage of objective responses was obtained. Most of them were partial responses (64%), mainly because these tumors are highly infiltrative, making them very difficult to treat. In these cases, tumor tissue was still seen in CT/MRI scans. This could be due to the fact that the electric field was not enough to cover all the tumor, leaving it untreated, or more likely because those tumor cells are quiescent. In the latter case, bleomycin already acted on those cells by cleaving the DNA strands, and they will die when cell division is attempted. If the electric field would not have been strong enough, we would have seen a rapid growth of the residual tumoral tissue, at least, comparable to the non-treated passage, which did not happen. Although a fraction of the tumor remained, a considerable improvement in the quality of life was achieved due to the reduction in the clinical symptoms. The patients who achieved a complete response (27%), had a complete palliation of the symptoms as was expected.
Regarding short-term side effects, the inflammation and pain of the treated nasal passage proved to be non-problematic and easily manageable with medication. Regarding the bleeding during the procedure it ceased immediately after the treatment was completed, probably because of the “vascular lock” phenomenon, which consists of the sudden stop of blood flow in the area exposed to the electric field used in ECT procedures.3 We observed no long-term side effects.
The gold standard treatment is surgery plus adjuvant radiotherapy.18 Although surgery is usually rejected by the owner due to mutilating results, frequently it is impossible to perform, and even procedures such as rhinotomy, can result in acute and chronic morbidity. Hypofractionated or orthovoltage radiation therapy have many side effects such as oral mucositis, rhinitis, moist desquamation, keratoconjunctivitis, and blepharitis. These symptoms can last for up to 8 weeks after therapy, even with medication, but if they persist, esophagostomy or gastrostomy tubes may be needed in the short term for an adequate nourishment of the animal. Long-term side effects include cataracts, keratoconjunctivitis, anterior uvea hemorrhage, brain necrosis, seizures, optic nerve degeneration, osteonecrosis, and fibrosis. Some of these complications are generally non-treatable.19,20
Our results show that mean survival with SiNE group was 16.86 months (4–32 months) with 60% of survival at 1 year and a 30% at 2 years. This is very encouraging since it is better when compared with the standard treatment option in our setting (surgery plus chemotherapy). Also, it is similar to the best treatments described in the literature. As mentioned before, the treatment of choice for nasal tumors is surgery plus radiotherapy. Unfortunately, due to the facilities and resources required, this option is not locally available for veterinary patients. In fact, this situation is replicated in the vast majority of centers around the world. In relation to the data available for this treatment in the literature we found that for radiotherapy alone in its different modalities, the median survival reported is around 8 months.21,22 The combination of radiation therapy followed by surgery when it is possible seems to have better results in the overall median survival (47.7 months).23 The new modalities like intensity-modulated radiation therapy (IMRT) report a median survival of 446 d (14.9 months) with 50% of survival at 1 year and a 25% at 2 years.24
The prolonged survival in our study may be partially due to the lack of need for euthanasia as the general condition of the patients greatly improved. Further research is needed to confirm this hypothesis. It remains to be seen whether the combination of treatments can improve outcomes. The combination of ECT using the SiNE with radiotherapy or systemic chemotherapy may enable the reduction of the dose in later treatments, reducing their side effects.25,26
Canine patients with spontaneous tumors are excellent models for preclinical validation. In particular, nasal tumors in dogs behave in a similar way to nasal tumors in humans.27 Dogs are exposed to similar environmental carcinogens. They develop tumors with the same histologies having the same interactions between tumor and stroma to those found in human patients; thus, the tumor grows in the context of an intact immune system that has been developed during its lifetime and exposed to different antigens. They show spontaneous metastasis and resistance to different forms of therapy. They have similar druggable targets, angiogenic pathways, and mechanisms of apoptosis. This enables a more accurate assessment of therapeutic approaches compared to rodent models. Other advantages of dogs, particularly for the purpose of this work, is that their size allows the use of the typical imaging procedures performed in humans; there is no need for miniaturization of the electrodes, and it is possible to repeat tissue and fluid sampling over time with more tissue for analysis than that available in rodent models. Also, typical forms of treatment used in humans can be performed in dogs to compare the results of the treatment.27,28
In human patients the situation is similar to canine patients, nasal tumors are a complex pathology in which current treatments do not often provide satisfactory results. The variety of tumor types, its proximity to critical anatomical structures and the late development of symptoms make malignant tumors of the paranasal sinuses and nasal cavity a complex problem. Diagnostic procedures and treatment modalities are also very similar to canine patients.29 Therefore, new therapeutic approaches that improve outcomes and minimize adverse effects are required. These tumors are rare and patients are often asymptomatic until late in the course of the disease. In the United States, nasal tumors account for only 0.2–0.8% of all cancers diagnosed annually. The incidence reported is 0.3–1 per 100,000 populations. They occur most commonly in the fifth decade of life, although they can occur at any age.29 These tumors are usually diagnosed when airway obstruction symptoms are present and the disease is at an advanced stage. Early and late symptoms are similar to those in canine patients.30 These regions are affected by a wide variety of tumor types31, with very similar histologies.32,33,34
In a study conducted by Dulguerov et al. the 5-year survival rate was 40%, and the local control rate was 59%. The prognosis depends on tumor histology, localization, tumor stage, and treatment modality.35 There is a consensus that complete surgical resection should be performed if possible. Surgery and postoperative radiation therapy may result in improved local control, absolute survival and complications when compared with radiation therapy alone.36 Chen et al. have not found improvements in disease control or overall survival for patients treated with radiotherapy in the last five decades.37 Nevertheless, new forms of radiotherapy reduce complications38, and intensity-modulated radiation therapy particularly promises better outcomes when compared to conventional radiotherapy.39 The role of chemotherapy in the neoadjuvant setting and/or postoperatively with irradiation is not clearly defined, but it is generally used for the most advanced cases and requires further investigation.34,40
In such a sensitive region, the proximity of critical normal anatomical structures restricts in many cases the possibility to adequately perform surgery and apply effective radiotherapy.38 Sometimes a tumor is resecable, but the patient’s clinical status may preclude surgery or, very often, patients reject it.41 For these reasons, also in human patients, new approaches for treating nasal tumors are needed.
Besides obvious differences between canine and human nasal tumors, this work shows that nasal tumors can be effectively treated with ECT and further studies should be conducted to validate this treatment in human patients.
Treating nasal tumors in human or canine patients using the ECT with the SiNE could be useful in selected cases where first-line treatments are not possible. It can be especially used to palliate symptoms in patients with short life expectancy, or a poor clinical condition. In any case, it can be performed as a cytoreductive treatment to simplify surgery and does not preclude other treatments.42,43,44 Therefore, it remains to be seen whether the use of the SiNE followed by surgery will improve survival, as happens with radiotherapy.
In conclusion, deep nasal tumors in canine patients can be successfully treated by ECT, and it can be safely and effectively performed using the SiNE, especially in tumors that are difficult or impossible to reach with conventional electrodes.
Acknowledgements
F Maglietti holds a scholarship from CONICET, N Olaiz and G Marshall are researchers from CONICET, S Michinski is a CPA-CONICET. This work is supported by grants from Consejo Nacional de Investigaciones Científicas y Técnicas, www.conicet.gov.ar, (PIP 379/2012/2016 and STAN 599/12) and Universidad de Buenos Aires, www.uba.ar, (UBACyT 2014/2017). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. This was proofread by YasminTranslations. com.
Disclosure: No potential conflicts of interest were disclosed.
Author contributions
FM developed the electrode, worked on the production of the prototype, treated the patients and worked in the preparation of this manuscript. MT selected and treated the patients and collaborated on the preparation of the manuscript. NO was in charge of provision of study material and equipment setup. SM collaborated on the treatment of the patients and the preparation of the manuscript. GM coordinated the group and collaborated on the preparation of the manuscript.
References
- 1.Withrow SJ, Vail DM, Page R. Withrow and MacEwen’s small animal clinical oncology. Elsevier Health Sciences; 2013. [DOI] [Google Scholar]
- 2.Madewell B, Priester W, Gillette E, Snyder S.. Neoplasms of the nasal passages and paranasal sinuses in domesticated animals as reported by 13 veterinary colleges. Am J Vet Res. 1976;37:851–6. [PubMed] [Google Scholar]
- 3.Marty M, Sersa G, Garbay JR, Gehl J, Collins CG, Snoj M. et al. Electrochemotherapy –An easy, highly effective and safe treatment of cutaneous and subcutaneous metastases: Results of ESOPE (European Standard Operating Procedures of Electrochemotherapy) study. Eur J Cancer Suppl. 2006;4:3–13. doi: 10.1016/j.ejcsup.2006.08.002. [DOI] [Google Scholar]
- 4.Cemazar M, Tamzali Y, Sersa G, Tozon N, Mir LM, Miklavcic D. et al. Electrochemotherapy in veterinary oncology. J Vet Int Med. 2008;22:826–31. doi: 10.1111/j.1939-1676.2008.0117.x. [DOI] [PubMed] [Google Scholar]
- 5.Impellizeri J, Aurisicchio L, Forde P, Soden D.. Electroporation in veterinary oncology. Vet J. 2016;217:18–25. doi: 10.1016/j.tvjl.2016.05.015. [DOI] [PubMed] [Google Scholar]
- 6.Kotnik T, Kramar P, Pucihar G, Miklavcic D, Tarek M.. Cell membrane electroporation-Part 1: The phenomenon. Electrical Insulation Magazine, IEEE. 2012;28:14–23. doi: 10.1109/MEI.2012.6268438. [DOI] [Google Scholar]
- 7.Tamzali Y, Borde L, Rols M, Golzio M, Lyazrhi F, Teissie J.. Successful treatment of equine sarcoids with cisplatin electrochemotherapy: a retrospective study of 48 cases. Equine Vet J. 2012;44:214–20. doi: 10.1111/j.2042-3306.2011.00425.x. [DOI] [PubMed] [Google Scholar]
- 8.Mali B, Jarm T, Snoj M, Sersa G, Miklavcic D.. Antitumor effectiveness of electrochemotherapy: a systematic review and meta-analysis. EJSO. 2013;39:4–16. doi: 10.1016/j.ejso.2012.08.016. https://doi.org/10.1016/j.ejso.2012.08.016. [DOI] [PubMed] [Google Scholar]
- 9.Edhemovic I, Brecelj E, Gasljevic G, Marolt Music M, Gorjup V, Mali B. et al. Intraoperative electrochemotherapy of colorectal liver metastases. J Surg Oncol. 2014;110:320–7. doi: 10.1002/jso.23625. [DOI] [PubMed] [Google Scholar]
- 10.Linnert M, Agerholm-Larsen B, Mahmood F, Iversen HK, Gehl J. Tumors of the Central Nervous System. Vol. 12. Springer; 2014. Treatment of brain tumors: Electrochemotherapy; pp. 247–59. [DOI] [Google Scholar]
- 11.Fini M, Salamanna F, Parrilli A, Martini L, Cadossi M, Maglio M. et al. Electrochemotherapy is effective in the treatment of rat bone metastases. Clin Exp Met. 2013;30:1033–45. doi: 10.1007/s10585-013-9601-x. [DOI] [PubMed] [Google Scholar]
- 12.Soden DM, Larkin JO, Collins CG, Tangney M, Aarons S, Piggott J. et al. Successful application of targeted electrochemotherapy using novel flexible electrodes and low dose bleomycin to solid tumours. Cancer Lett. 2006;232:300–10. doi: 10.1016/j.canlet.2005.03.057. [DOI] [PubMed] [Google Scholar]
- 13.Campana LG, Clover AJP, Valpione S, Quaglino P, Gehl J, Kunte C. et al. Recommendations for improving the quality of reporting clinical electrochemotherapy studies based on qualitative systematic review. Radiol Oncol. 2016;50:1–13. doi: 10.1515/raon-2016-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mir LM, Gehl J, Sersa G, Collins CG, Garbay JR, Billard V. et al. Standard operating procedures of the electrochemotherapy: instructions for the use of bleomycin or cisplatin administered either systemically or locally and electric pulses delivered by the Cliniporator TM by means of invasive or non-invasive electrodes. Eur J Cancer Suppl. 2006;4:14–25. doi: 10.1016/j.ejcsup.2006.08.003. [DOI] [Google Scholar]
- 15.WHO handbook for reporting results of cancer treatment. Geneva: WHO; 1979. World Healt Organization. [Google Scholar]
- 16.Miklavcic D, Mali B, Kos B, Heller R, Sersa G.. Electrochemotherapy: from the drawing board into medical practice. BioMed Engineering OnLine. 2014;13:29. doi: 10.1186/1475-925X-13-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suzuki DO, Berkenbrock JA, de Oliveira KD, Freytag JO, Rangel MM.. Novel application for electrochemotherapy: Immersion of nasal cavity in dog. Artif Organs. 2017;41:767–73. doi: 10.1111/aor.12858. [DOI] [PubMed] [Google Scholar]
- 18.Adams W, Withrow S, Walshaw R, Turrell J, Evans S, Walker M. et al. Radiotherapy of malignant nasal tumors in 67 dogs. J Am Vet Med Assoc. 1987;191:311–5. [PubMed] [Google Scholar]
- 19.Maruo T, Shida T, Fukuyama Y, Hosaka S, Noda M, Ito T. et al. Retrospective study of canine nasal tumor treated with hypofractionated radiotherapy. J Vet Med Sci. 2011;73:193–197. doi: 10.1292/jvms.10-0194. [DOI] [PubMed] [Google Scholar]
- 20.Dah-Renn F, Daiki K, Ai Watabe YE, Endo Y, Kadosawa T.. Prognostic utility of apoptosis index, Ki-67 and survivin expression in dogs with nasal carcinoma treated with orthovoltage radiation therapy. J Vet Med Sci. 2014;76:1505. doi: 10.1292/jvms.14-0245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Northrup NC, Etue SM, Ruslander DM, Rassnick KM, Hutto DL, Bengtson A. et al. Retrospective study of orthovoltage radiation therapy for nasal tumors in 42 dogs. J Vet Int Med. 2001;15:183–9. doi: 10.1111/j.1939-1676.2001.tb02309.x. [DOI] [PubMed] [Google Scholar]
- 22.Kubicek L, Milner R, An Q, Kow K, Chang M, Cooke K. et al. Outcomes and prognostic factors associated with canine sinonasal tumors treated with curative intent cone-based stereotactic radiosurgery (1999–2013) Vet Radiol Ultrasound. 2016;57:331–40. doi: 10.1111/vru.12349. [DOI] [PubMed] [Google Scholar]
- 23.Adams WM, Bjorling DE, McAnulty JF, Green EM, Forrest LJ, Vail DM.. Outcome of accelerated radiotherapy alone or accelerated radiotherapy followed by exenteration of the nasal cavity in dogs with intranasal neoplasia: 53 cases (1990–2002) J Am Vet Med Assoc. 2005;227:936–41. doi: 10.2460/javma.2005.227.936. [DOI] [PubMed] [Google Scholar]
- 24.Hunley DW, Mauldin GN, Shiomitsu K, Mauldin GE.. Clinical outcome in dogs with nasal tumors treated with intensity-modulated radiation therapy. Can Vet J. 2010;51:293. [PMC free article] [PubMed] [Google Scholar]
- 25.Domanico R, Trapasso S, Santoro M, Pingitore D, Allegra E.. Electrochemotherapy in combination with chemoradiotherapy in the treatment of oral carcinomas in advanced stages of disease: efficacy, safety, and clinical outcomes in a small number of selected cases. Drug Design Development and Therapy. 2015;9:1185. doi: 10.2147/DDDT.S75752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Raeisi E, Aghamiri S, Bandi A, Rahmatpour N, Firoozabadi S, Kafi-Abad S. et al. The antitumor efficiency of combined electrochemotherapy and single dose irradiation on a breast cancer tumor model. Radiol Oncol. 2012;46:226–32. doi: 10.2478/v10019-012-0035-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spugnini EP, Fanciulli M, Citro G, Baldi A.. Preclinical models in electrochemotherapy: the role of veterinary patients. Future Oncology. 2012;8:829–37. doi: 10.2217/fon.12.64. [DOI] [PubMed] [Google Scholar]
- 28.London CA.. Abstract SY28–01: Spontaneous cancer in dogs: opportunities for preclinical evaluation of novel therapies. Cancer Res. 2011;71(8):SY28–01. doi: 10.1158/1538-7445.AM2011-SY28-01. [DOI] [Google Scholar]
- 29.Holland JF, Hong WK. Hong WK, Bast R, Hait WN, Kufe DW, Pollock RE, Weichselbaum RR, Holland-Frei cancer medicine 8. Shelton: People’s Medical Publishing House; 2010. [Google Scholar]
- 30.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, AJCC cancer staging manual. Vol. 649. New York: Springer; 2010. [DOI] [Google Scholar]
- 31.Iezzoni JC, Mills SE.. “Undifferentiated” small round cell tumors of the sinonasal tract: differential diagnosis update. Am J Clin Pathol. 2005;124(1):S110–21. doi: 10.1309/59RBT2RK6LQE4YHB. [DOI] [PubMed] [Google Scholar]
- 32.Marcus DM, Marcus RP, Prabhu RS, Owonikoko TK, Lawson DH, Switchenko J. et al. Rising incidence of mucosal melanoma of the head and neck in the United States. J Skin Cancer. 2012;2012:231693. doi: 10.1155/2012/231693.S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith SR, Som P, Fahmy A, Lawson W, Sacks S, Brandwein M.. A clinicopathological study of sinonasal neuroendocrine carcinoma and sinonasal undifferentiated carcinoma. Laryngoscope. 2000;110:1617–22. doi: 10.1097/00005537-200010000-00007. [DOI] [PubMed] [Google Scholar]
- 34.Mourad WF, Hauerstock D, Shourbaji RA, Hu KS, Culliney B, Li Z. et al. Trimodality management of sinonasal undifferentiated carcinoma and review of the literature. Am J Clin Oncol. 2013;36:584–8. doi: 10.1097/COC.0b013e31825eb3a5. [DOI] [PubMed] [Google Scholar]
- 35.Dulguerov P, Jacobsen MS, Allal AS, Lehmann W, Calcaterra T.. Nasal and paranasal sinus carcinoma: are we making progress? Cancer. 2001;92:3012–29. doi: 10.1002/1097-0142(20011215)92:12. [DOI] [PubMed] [Google Scholar]
- 36.Katz TS, Mendenhall WM, Morris CG, Amdur RJ, Hinerman RW, Villaret DB.. Malignant tumors of the nasal cavity and paranasal sinuses. Head & Neck. 2002;24:821–9. doi: 10.1002/hed.10143. [DOI] [PubMed] [Google Scholar]
- 37.Chen AM, Daly ME, Bucci MK, Xia P, Akazawa C, Quivey JM. et al. Carcinomas of the paranasal sinuses and nasal cavity treated with radiotherapy at a single institution over five decades: are we making improvement? Int J Rad Oncol Biol Phys. 2007;9:141–7. doi: 10.1016/j.ijrobp.2007.02.031. [DOI] [PubMed] [Google Scholar]
- 38.Porceddu S, Martin J, Shanker G, Weih L, Russell C, Rischin D. et al. Paranasal sinus tumors: Peter MacCallum Cancer Institute experience. Head Neck. 2004;26:322–30. doi: 10.1002/hed.10388. [DOI] [PubMed] [Google Scholar]
- 39.Dirix P, Vanstraelen B, Jorissen M, Vander Poorten V, Nuyts S.. Intensity-modulated radiotherapy for sinonasal cancer: improved outcome compared to conventional radiotherapy. Int J Radiol Oncol Biol Phys. 2010;78:998–1004. doi: 10.1016/j.ijrobp.2009.09.067. [DOI] [PubMed] [Google Scholar]
- 40.Sohrabi S, Drabick JJ, Crist H, Goldenberg D, Sheehan JM, Mackley HB.. Neoadjuvant concurrent chemoradiation for advanced esthesioneuroblastoma: a case series and review of the literature. J Clin Oncol. 2011;29:e358–61. doi: 10.1200/JCO.2010.30.9278. [DOI] [PubMed] [Google Scholar]
- 41.Pfister DG, Ang KK, Brizel DM, Burtness BA, Busse PM, Caudell JJ. et al. Head and neck cancers, version 2. 2013. J Natl Compr Canc Netw. 2013;11:917–23. doi: 10.6004/jnccn.2013.0113. [DOI] [PubMed] [Google Scholar]
- 42.Spugnini EP, Citro G, Baldi A.. Adjuvant electrochemotherapy in veterinary patients: a model for the planning of future therapies in humans. J Exp Clin Cancer Res. 2009;28:1. doi: 10.1186/1756-9966-28-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lowe R, Gavazza A, Impellizeri J, Soden D, Lubas G.. The treatment of canine mast cell tumours with electrochemotherapy with or without surgical excision. Vet Comp Oncol. 2017;15:775–84. doi: 10.1111/vco.12217. [DOI] [PubMed] [Google Scholar]
- 44.Spugnini E, Vincenzi B, Citro G, Dotsinsky I, Mudrov T, Baldi A.. Evaluation of Cisplatin as an electrochemotherapy agent for the treatment of incompletely excised mast cell tumors in dogs. J Vet Int Med. 2011;25:407–411. doi: 10.1111/j.1939-1676.2011.0678.x. [DOI] [PubMed] [Google Scholar]