Abstract
Introduction
Deformable image registration (DIR) is used to modify structures according to anatomical changes for observing the dosimetric effect. In this study, megavoltage computed tomography (MVCT) images were used to generate cumulative doses for nasopharyngeal cancer (NPC) patients by various DIR methods. The performance of the multiple DIR methods was analysed, and the impact of dose accumulation was assessed.
Patients and methods
The study consisted of five NPC patients treated with a helical tomotherapy unit. The weekly MVCT images at the 1st, 6th, 11th, 16th, 21st, 26th, and 31st fractions were used to assess the dose accumulation by the four DIR methods. The cumulative dose deviations from the initial treatment plan were analysed, and correlations of these variations with the anatomic changes and DIR methods were explored.
Results
The target dose received a slightly different result from the initial plan at the end of the treatment. The organ dose differences increased as the treatment progressed to 6.8% (range: 2.2 to 10.9%), 15.2% (range: -1.7 to 36.3%), and 6.4% (range: -1.6 to 13.2%) for the right parotid, the left parotid, and the spinal cord, respectively. The mean uncertainty values to estimate the accumulated doses for all the DIR methods were 0.21 ± 0.11 Gy (target dose), 1.99 ± 0.76 Gy (right parotid), 1.19 ± 0.24 Gy (left parotid), and 0.41 ± 0.04 Gy (spinal cord).
Conclusions
Accuracy of the DIR methods affects the estimation of dose accumulation on both the target dose and the organ dose. The DIR methods provide an adequate dose estimation technique for observation as a result of inter-fractional anatomic changes and are beneficial for adaptive treatment strategies.
Key words: deformable image registration, dose accumulation, nasopharyngeal cancer, MVCT, helical tomotherapy
Introduction
Modern radiation therapy has the ability to utilize multimodality imaging technologies for disease definition, patient setup, and treatment assessment.1 Helical megavoltage CT (MVCT), which is a volumetric imaging modality, is adopted with the primary purpose of more accurate target localization.2 Moreover, with this, information about inter-fractional anatomical variations has become more accessible.3 Some head and neck cancer patients undergo significant anatomical changes, and these may result in unforeseen changes in the delivered dose.3 In ideal practice, when a patient’s anatomy changes, a new adaptive plan must be developed, in accordance with the concept of adaptive radiotherapy (ART).4 These procedures include modification of the initial plan according to the changes in the target volume or normal organs; manual contouring can be used to modify deformation in order to evaluate the dosimetric effect.5 6 7 However, the process of manual contouring is time-consuming. Therefore, a deformable image registration (DIR) can be used to resolve these challenges. By registering multiple daily CTs to the planned CT, the algorithm can automatically generate deformed contours on daily CTs while creating cumulative doses by tracking the dose to the tissue voxels throughout the course of the radiation therapy.2
As for the application of MVCT in deformable dose accumulation routinely, it would require accurate structure deformation even in low contrast regions8 because accuracy of DIR may have a significant dosimetric impact on radiation treatment planning.9 Nowadays, various deformable image registration algorithms have been developed3 and the accuracy of the deformable image registration naturally depends on the deformation model.10 Therefore, the choice of the deformation algorithms and the transformation in the MVCT image application are of great importance in the registration process as it entails important compromise between computational efficiency and richness of description for more accurate results.10
Deformable image registration and adaptive radiotherapy (DIRART)11 is a software suite for DIR plus ART. DIRART is a large set of programs developed using MATLAB. Four DIR methods by two algorithms, Horn & Schunck optical flow and demon with the two transformation frameworks, asymmetric and symmetric transformation, have been considered. In this study, weekly MVCT images from helical tomotherpy were used to generate cumulative doses for nasopharyngeal cancer (NPC) patients. Different DIR methods from DIRART were used. The weekly cumulative doses were analysed to assess the dosimetric impact of the DIR methods on dose accumulation. The dosimetric variations from the initial plan were reported, and correlations of these variations with anatomic changes and DIR methods were explored.
Patients and methods
Patient characteristics
The study population consisted of five NPC patients treated using a helical tomotherapy unit (Tomo-Therapy, Inc., Madison, Wisconsin). All the patients underwent intensity modulated radiotherapy (IMRT) with a planned dose of 70 Gy delivered to the gross disease at 2.12 Gy/fraction for a total of 33 fractions with a simultaneous integrated boost technique (SIB) according to the RTOG 022512, keeping the mean parotid dose as low as could be possibly achieved and respecting the tissue tolerance of other normal structures. Patient positioning was ensured by appropriate headrest and a personalized HN and shoulder mask. This study received ethics approval, granted by the institutional research committee.
Image acquisition
The planned kVCT images were acquired on a computerized tomography unit (Somatom, SIEMENS, Germany) by using a matrix of 512 × 512 with voxel dimension of 0.976 × 0.976 × 3mm3 for the treatment planning process. The 1st day MVCT images were also acquired on the helical tomotherapy unit as source images for deformable investigation on the same day of acquisition of the planned kVCT images.
When the radiotherapy treatment was started, the daily MVCT images were acquired on the helical tomotherapy unit prior to each treatment fraction used for patient alignment by using a matrix of 512 x512 with voxel dimension of 0.763 × 0.763 × 4 mm3. Typically, the MVCT scan range covers the entirety of the gross tumour volume (GTV), the clinical target volume (CTV), and the parotid glands bilaterally. The weekly MVCT images on the 1st, 6th, 11th, 16th, 21st, 26th, and 31st fractions were used as the target images to assess the dose accumulation in this study.
Target localization
The regions of interest (ROIs) including the target and the organ at risks (OARs) were defined by the radiation oncologist on planned kVCT images for the treatment planning processes. The ROIs on the planned kVCT images were transferred to the first-day MVCT images as the source images for each image set. The same oncologist who localized the target and the OAR for the HT treatment planning process also contoured the GTV, CTV, the bilateral parotid glands, and the spinal cord on the weekly MVCT images as the reference images. These contours were compared to the automatic deformed structure generated by the deformable image registration software.
DIR
The popular software suite for DIR and ART, DIRART version 1a developed by Yang (2009) 11, was used to create automatic deformed contours and dose accumulation from the MVCT images of NPC patients. DIRART works complementarily with computational environment for radiotherapy research (CERR) to offer more functions9 and provide the capability of selecting various deformation algorithms, transformation frameworks, and mapping directions for providing the deformation vector field (DVF) in deformable registration procedures.
In this study, the algorithms used were Horn and Schunck (HS) optical flow and demons (DM) combined with the asymmetric (Asy) and symmetric (Sym) transformation framework. Therefore, the study was carried out with four DIR methods, including the asymmetric transformation with the Horn and Schunck optical flow (AsyHS), the asymmetric transformation with the demon algorithm (AsyDM), the symmetric transformation with the Horn and Schunck optical flow (SymHS), and the symmetric transformation with the demon algorithm (SymDM).
A multi-resolution technique was used in this study. For the optimum DIR performance for each algorithm, various parameters were systematically adjusted: four multigrids were used (n = 1, 2, 3, and 4) with 10n to 40n iterations per pass.9 The number of passes for the optical flow algorithm was 6 and the number of passes for the demon algorithm was between 2 and 6. Coarser stages were typically run with a greater number of passes to improve the agreement with the target image prior to resampling at finer resolutions.9
Validation of DIR
The objective of the validation technique was to evaluate the accuracy of the automatic deformed contour by four DIR methods on weekly MVCT images, including the terms of the volume-based criterion and the deformation field analysis.
Regarding the volume-based criterion, the most common overlap metric is the Dice similarity coefficient (DSC).13 DSC is the metric that computes the number of pixels that overlaps between two volumes. If the images have no overlap, then the DSC is 0, and as the contours become identical, the DSC approaches the value of 1.13 Zimring et al.14 suggested that the satisfactory volume matching should be 70% (DSC of 0.7) or more for adaptive radiotherapy application.
Inverse consistency error (ICE) was used to ensure that the transformations were physically invertible for the deformation field analysis. Optimal transformation is found when the ICE minimizes the distance error.15
Assessment of impact of DIR methods on dose accumulation
The dose accumulation process relied on six steps, as illustrated in Figure 1. Firstly, the ROIs from the planned kVCT images were transferred to the 1st day MVCT as source images for registration. The four DIR methods were performed between the 1st day MVCT and the weekly MVCTs (step 2). The deformation vector field was applied for creating the automatic deformed structure of the ROIs and for propagation to the weekly MVCT images (step 3), and the weekly dose distribution was deformed (step 4). The weekly dose deformation values were summed to the accumulated dose (step 5) and compared to the initial planned dose distribution (step 6).
Figure 1.
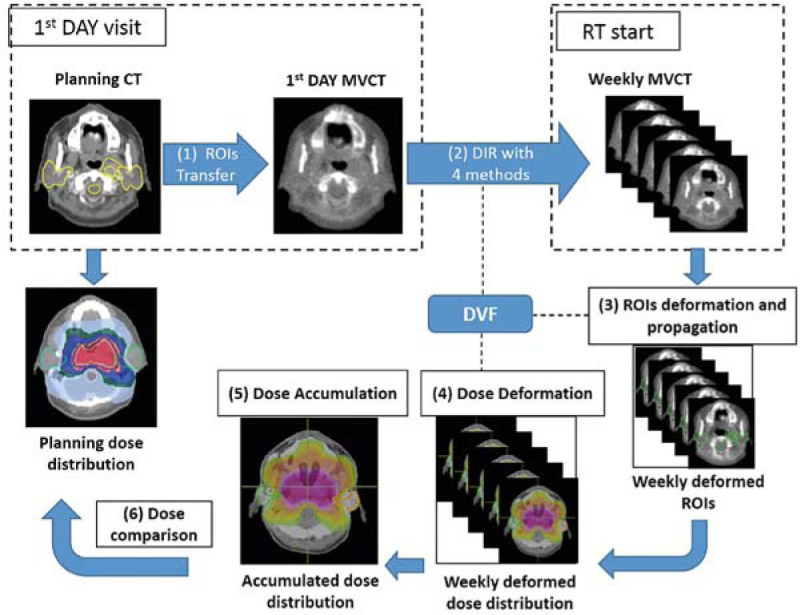
The diagram of the study workflow for dose accumulation and dose comparison.
To evaluate the effect of dose accumulation from DIR errors, the reference accumulated dose on ROIs was computed by summing the weekly doses corresponding to the weekly MVCTs defined by the radiation oncologist. Moreover, to ensure that the weekly dose summation from the DIRART software was accurate, dose accumulation from independent software, Planned Adaptive software (TomoTherapy Inc., Madison, WI) was used to compare.
Regarding the evaluation of accumulated dose, for the target volume, the median absorbed dose (D50%), the near-minimum (D98%) absorbed dose, and the near-maximum (D2%) absorbed dose values from each DIR method were assessed, the mean absorbed dose (Dmean) of the bilateral parotid glands and D2% of the spinal cord were compared to the original planned dose for the OARs investigation. The one-way analysis of variance (ANOVA) test and the paired sample t-test were carried out on each set of comparison metrics to determine the statistical significance, with a threshold of p < 0.05; SPSS statistical software version 17 was used to compare and assess the impact of each of the DIR methods.
Results
ROI volume variations
Regarding volume variations during the radiotherapy, the percent ratio to the volume at the initial treatment planning of five NPC patients is illustrated in Figure 2. The averages of the NPC patients for the volume variation in the initial plan were significantly different from the averages after the treatment in 3 weeks for GTV, with p-value = 0.025, as demonstrated in Figure 2A, and for CTV, with p-value = 0.020, as demonstrated in Figure 2B. The volume was observed to have decreased by an average of 29.8% (GTV) and 21.0% (CTV) at the end of the treatment course.
Figure 2.
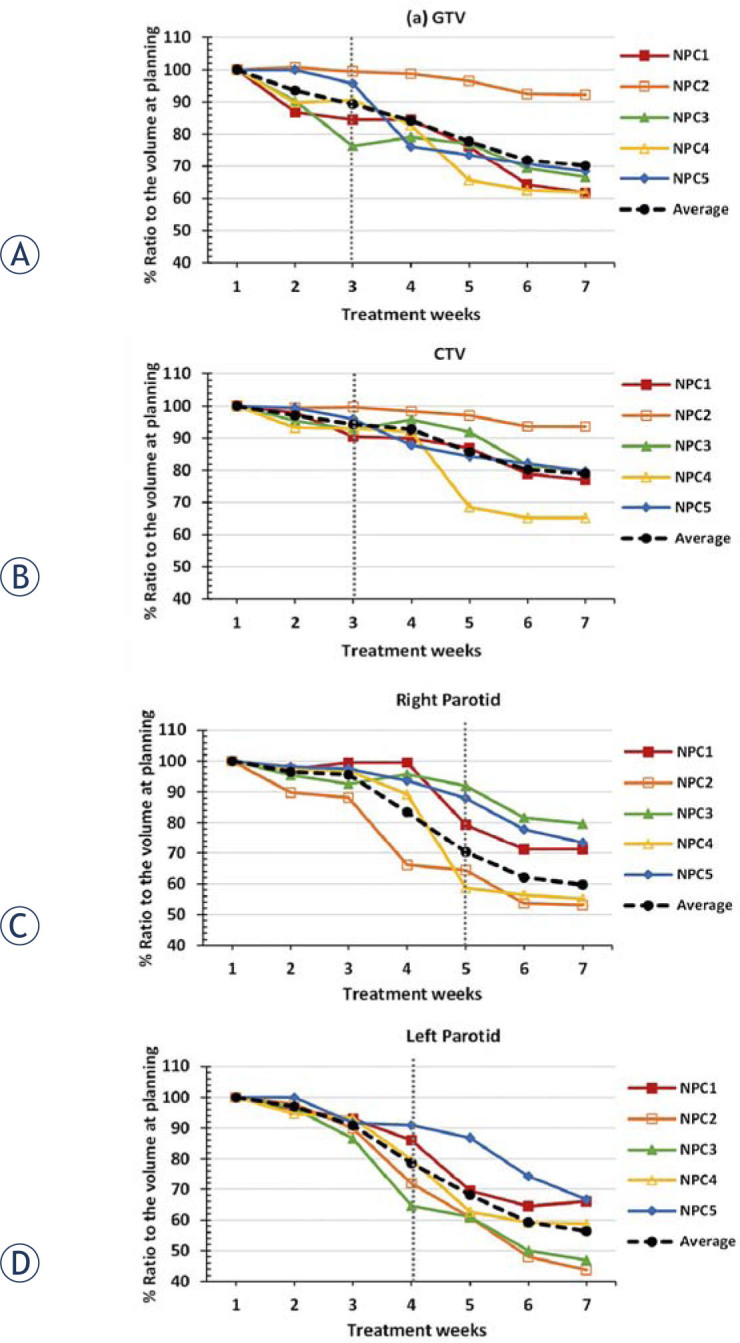
The percent ratio to the volume at the initial treatment planning of (A) gross target volume GTV, (B) clinical target volume CTV, (C) right parotid gland, and (D) left parotid gland.
As regards the OARs, the right and the left parotid volume variations were significantly different from those of the initial plan after 5 weeks and 4 weeks of treatment, with p-values of 0.017 and 0.026, respectively. The average volume decreased by 40.3% (right) and 43.6% (left) at the end of the treatment.
DIR validation
The results of DIR accuracy were consistent between the volume-based criterion, DSC, and the deformation field analysis, ICE. Figure 3A demonstrates the histogram of the DSC values for all of the ROIs by four DIR methods. The SymDM methods showed significant difference from other methods by the one-way ANOVA analysis, with p-value = 0.00, with the worst performance in terms of volume-based criterion by mean values of DSC = 0.50 ± 0.30, 0.56 ± 0.34, 0.67 ± 0.19, 0.65 ± 0.28, and 0.69 ± 0.19 for GTV, CTV, right parotid, left parotid, and spinal cord, respectively. The average of DSC value was less than 0.7 for all the ROIs, that represented unsatisfactory volume matching for adaptive radiotherapy application.14 Regarding the ICE analysis, the results were consistent with DSC value. Figure 3B illustrates the histogram of the ICE values for all of the ROIs by the four DIR methods. The SymDM method also showed the maximum error in the deformation field analysis in terms of ICE for all of the ROIs throughout the treatment. The one-way ANOVA analysis showed that the SymDM method was significantly different from other methods, with p-value = 0.00. Therefore, the SymDM failed to adequately register on weekly MVCT for the dose accumulation application in this study. This paper focuses on the three highest performing algorithms in the AsyHS, AsyDM, and SymHS methods for dose accumulation.
Figure 3.
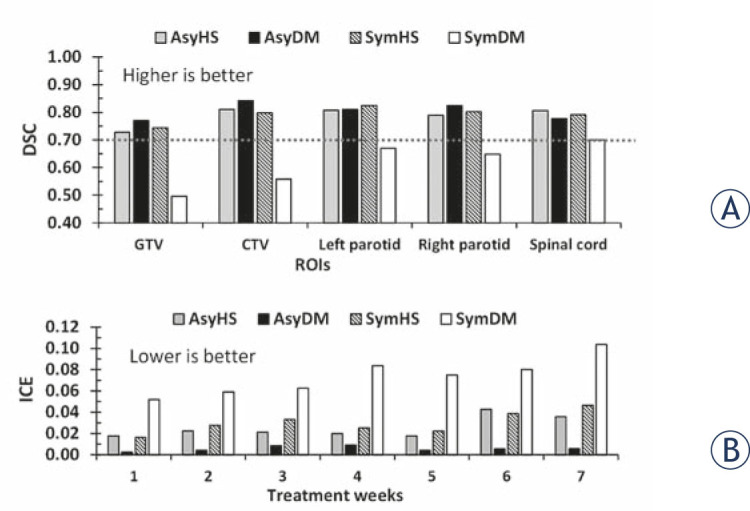
Histogram of (A) the dice similarity coefficients (DSC) for all of the targets and organs at risk and (B) the inverse consistency error (ICE) in each treatment week and by asymmetric Horn and Schunck (AsyHS), asymmetric demon (AsyDM), symmetric Horn and Schunck (SymHS), and symmetric demon (SymDM) deformable image registration (DIR) methods.
Accumulated dose variation from initial planned dose
As regards target dose variation, the median GTV and CTV doses received at the end of treatment were slightly different from those in the initial plan. They were 0.11% (range: 0–0.29%) lower than the initial planned dose. The median dose variations of the GTV and CTV were significantly different from the initial planned dose after 6 weeks of treatment, with p-value = 0.016. Regarding the minimum and the maximum doses, they are represented by near-minimum dose (D98%) and near-maximum dose (D2%), respectively. As for the D98%, they received slightly higher doses than the initial plan deals, with an average variation less than 0.5% (range: 0.29–1.60%). However, the dose at the end of treatment received slightly decreased doses of 0.45% (GTV) and 0.28% (CTV) from the initial doses planned for the D2.
Regarding organ dose variation, the dose differences tended to increase as the treatment progressed. For the bilateral parotid gland, the discrepancy between the delivered and the planned mean doses was found to have increased by 6.8% (range: 2.2 to 10.9%) for the right parotid and by 15.2% (range: -1.7 to 36.3%) for the left parotid. The average mean parotid dose increased in the ranges of 2.24 ± 0.97 Gy (right) and 5.70 ± 4.12 Gy (left) at the end of the treatment. The mean parotid dose variations were significantly different from the initial plan after 6 weeks (right) and 5 weeks (left) of the treatment, with p-value = 0.049 (right) and p-value = 0.010 (left). The spinal cord dose received increased by 6.4% (range: -1.6 to 13.2%) from the initial plan, with the average near-maximum dose increasing in the range of 1.83 ± 1.5 Gy at the end of the treatment.
Impact of DIR methods on weekly dose accumulation
For each patient, the running cumulative doses were calculated using the CERR software through the three deformable image registration methods carried out by the DIRART software. Figure 4 demonstrates the 1st day MVCT image with original bilateral parotid gland (A) and the MVCT image at the 31st fraction with the automatic deformed contour obtained using the AsyDM method (B). The initial planned dose distribution on the 1st day MVCT image, as illustrated in Figure 4C, was used for comparison with the accumulated dose distribution at the end of the treatment, as demonstrated in Figure 4D. The variations in the cumulative doses between the delivered dose and the initial planned dose are illustrated in Figure 5 and Figure 6.
Figure 4.
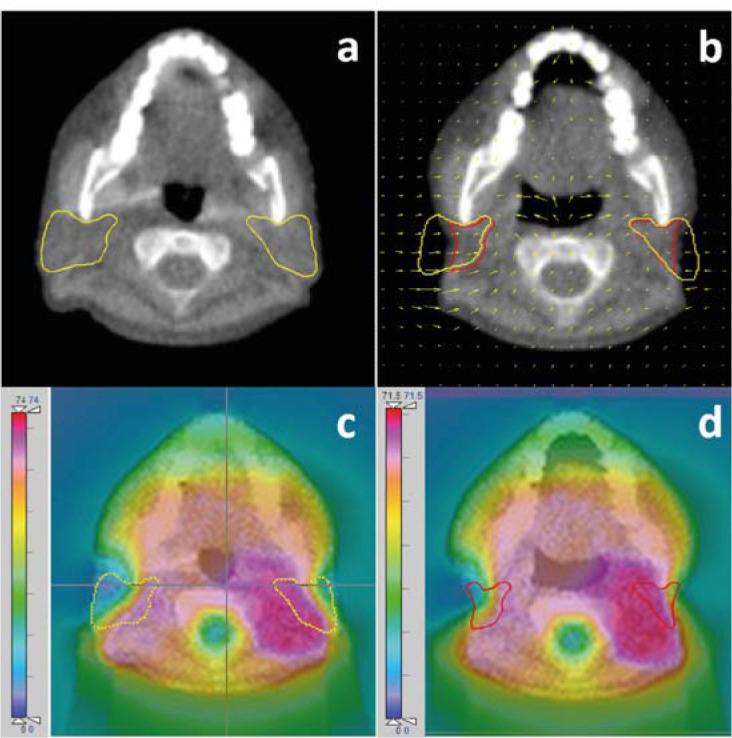
The 1st day MVCT image showing the original bilateral parotid gland (A) and the MVCT image at the 31st fraction showing the automatic deformed contour (B) from the AsyDM method. The initial planned dose distribution on the 1st day MVCT image (C) which was used to compare with the accumulated dose distribution at the end of the treatment (D).
Figure 5.
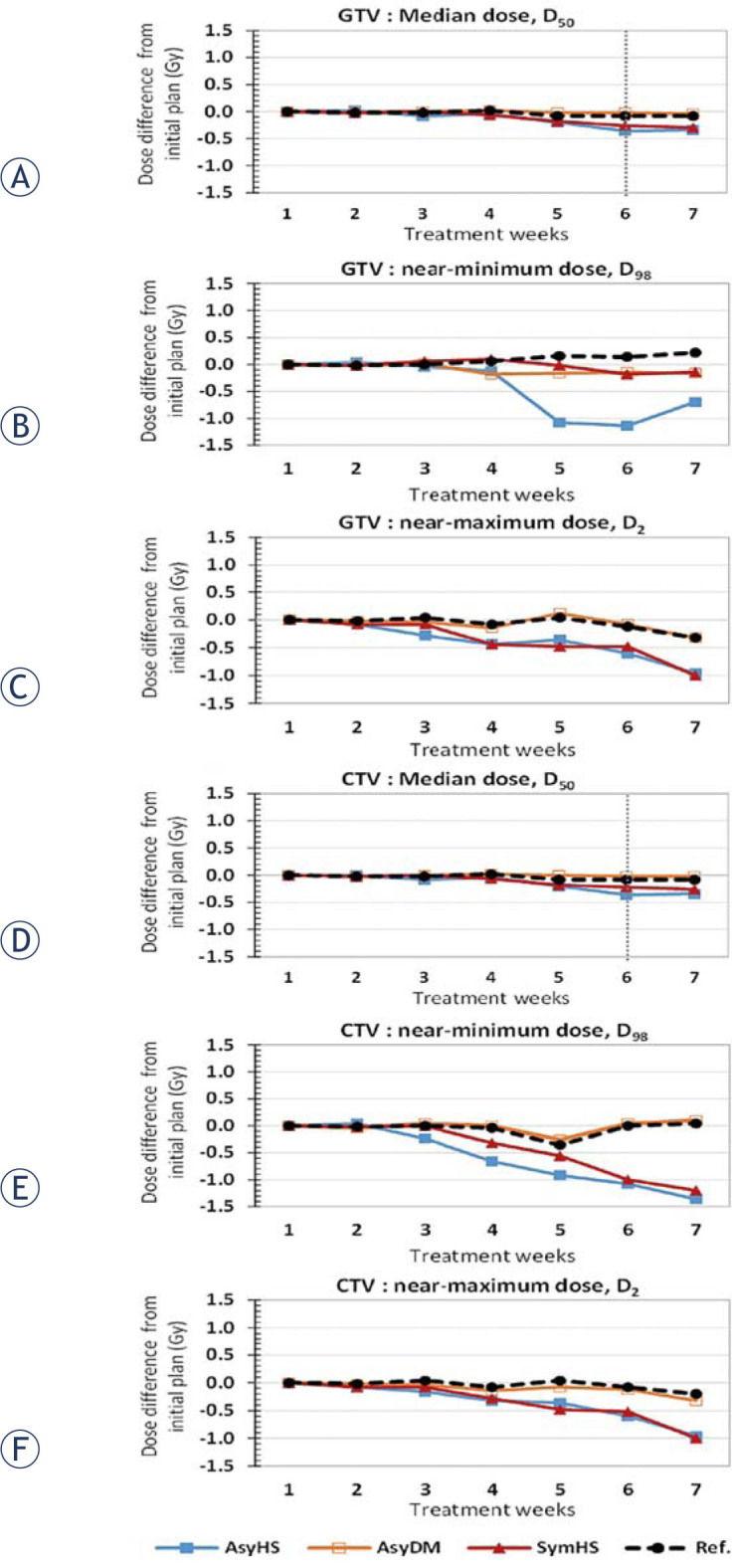
Cumulative dose comparison, calculated by the asymmetric Horn and Schunck (AsyHS), asymmetric demon (AsyDM), symmetric Horn and Schunck (SymHS), and symmetric demon (SymDM) deformable registration methods of gross tumour volume (GTV) for (A) median dose, D50 (B) near-minimum dose, D98 and (C) near-maximum dose, D2 and clinical tumour volume (CTV) for (D) median dose, D50 (E) near-minimum dose, D98, and (F) near-maximum dose, D2. The reference (Ref) accumulated dose was computed by summing the weekly doses corresponding to the weekly MVCTs defined by the radiation oncologist.
Figure 6.
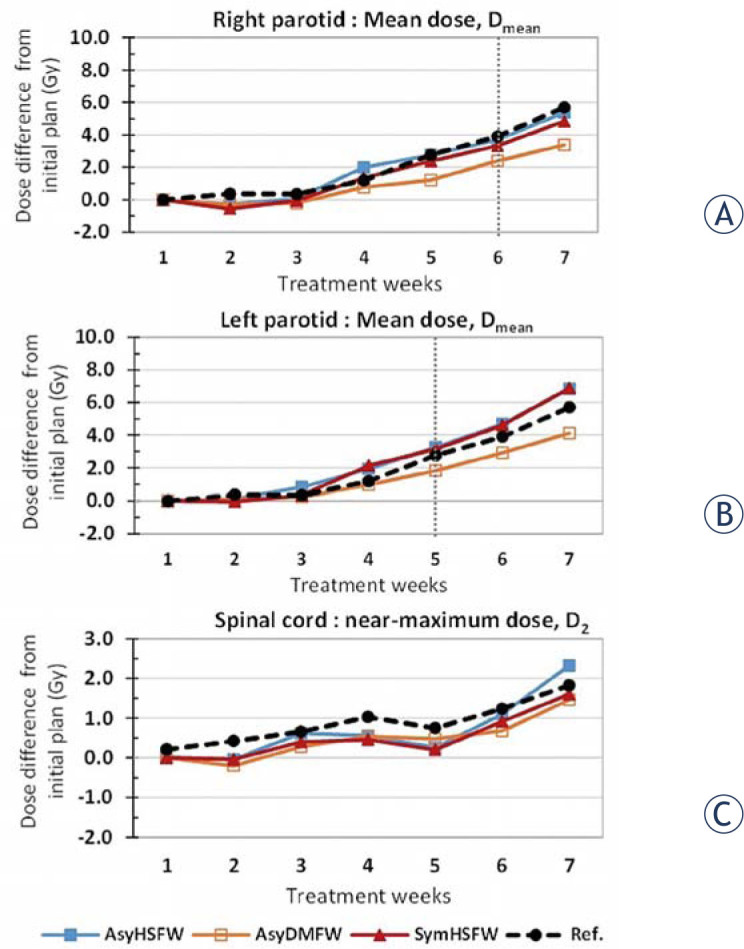
Cumulative dose comparison, calculated from the asymmetric Horn and Schunck (AsyHS), asymmetric demon (AsyDM), symmetric Horn and Schunck (SymHS), and symmetric demon (SymDM) deformable registration methods in mean dose, Dmean, of (A) right parotid gland and (B) left parotid gland, and near-maximum dose, D2, of (C) spinal cord.
Figure 5A illustrates the weekly GTV dose variation from the initial plan with three DIR methods. The average of the median dose difference for all methods at the end of the treatment was lower than that in the initial plan, with 0.34 Gy (0.5%), 0.04 Gy (0.1%), and 0.30 Gy (0.4%) for the AsyHS, AsyDm, and SymHS DIR methods, respectively. However, the reference dose of GTV was found to have decreased by 0.11%, with the accumulated GTV dose at 70.12 Gy (range: 69.9–70.4 Gy), at the end of treatment. The median dose variations of the GTV was significantly different from the initial planned dose after 6 weeks of treatment, with p-value = 0.016.
Regarding the near-minimum dose and the near-maximum dose, the D98% of GTV in three DIR methods were found to be lower than that in the initial plan, as illustrated in Figure 5B; the average discrepancy of the three DIR methods between the planned dose and the delivered dose was 0.33 Gy (0.5%) at the end of the treatment with the high differences of 1.0% after 5 week for AsyHS method. However, the reference near-minimum dose was found to have increased by 0.3% of the initial planned dose, with 69.2 Gy (range: 68.7–70 Gy). As for the maximum GTV dose consideration, the three DIR methods of D2% are presented in Figure 5C with gradually decrease in dose difference from initial plan for all methods when the time increase. The average D2% from the three methods was lower than the initial D2%, with 0.76 Gy (1.1%), at the end of the treatment. The reference D2% was found to have decreased by 0.45% of the initial planned dose, with 71.5 Gy (range: 70.9–72.5 Gy).
As regards the median CTV dose, the dose variations tended to be similar to the dose variations of GTV. Figure 5D illustrates the very small median CTV differences of various deformable registration methods from the initial planned dose. The discrepancy at the end of the treatment was lower than that in the initial planned dose, by 0.34 Gy (0.4%), 0.02 Gy (0%), and 0.26 Gy (0.4%) for AsyHS, AsyDm, and SymHS DIR methods, respectively. However, the reference median dose was found to have decreased by 0.11%, with 70.12 Gy (range: 69.9–70.4 Gy) at the end of the treatment. The median dose variations of the CTV was significantly different from the initial planned dose after 6 weeks of treatment, with p-value = 0.016.
Regarding the D98 dose analysis of CTV, the discrepancy between the initial and the delivered dose of the three DIR methods are shown in Figure 5E. The average of the D98% variations from the initial D98% in the three DIR methods was 0.89 Gy (1.2%). Figure 5F shows the D2% of CTV; the average variation from the initial D2% of the three DIR methods was 0.76 Gy (1.1%).
As regards organ dose accumulation in the three DIR methods, Figure 6 illustrates the weekly dose difference from the initial plan in the three DIR methods for the bilateral parotid gland and the spinal cord. Overall, the dose differences tended to increase as the treatment progressed. Figure 6A shows the mean right parotid dose (Dmean) to be higher than the initial planned dose, by 5.38 Gy (16.0%), 3.38 Gy (10.1%), and 4.84 Gy (14.4%) for the AsyHS, AsyDm, and SymHS methods, respectively. However, the reference mean dose was found to have increased by 2.24 Gy (range: 0.8–3.7 Gy), at 6.82%, at the end of the treatment.
For the left parotid mean dose, as illustrated in Figure 6B, these variations were higher after treatment than those for the initial planned dose, and the discrepancy was by 6.88 Gy (18.3%), 4.12 Gy (11.0%), and 6.82 Gy (18.1%) for the AsyHS, AsyDm, and SymHS DIR methods, respectively. However, the reference mean dose was found to have increased by 5.7 Gy (range: -0.6 to12.4 Gy), at 15.2% at the end of the treatment. The mean parotid dose variations were significantly different from the initial plan after 6 weeks (right parotid) and 5 weeks (left parotid) of the treatment, with p-value of 0.049 and 0.010, respectively.
As regards spinal cord weekly dose accumulation, the variations tended to increase in all the three DIR methods, by 2.33 Gy (7.9%), 1.46 Gy (4.9%) and 0.60 Gy (5.4%) for AsyHS, AsyDm, and SymHS, respectively. However, the reference cord dose variation was found to have increased by 1.83 Gy (range: -0.5 to 4.0 Gy), at 6.37% at the end of the treatment.
DIRART and planned adaptive software for dose accumulation
To ensure dose summation on the DIRART software, the independent planned adaptive software on helical tomotherapy treatment planning was used to compare the dose accumulation values. The same data set of all the reference ROIs on weekly MVCTs defined by the radiation oncologist was transferred to the planned adaptive software. The comparison of the weekly accumulated doses between DIRART and planned adaptive is illustrated in Figure 7. The variations in the accumulated median parotid doses of DIRART were not significantly different according to the planned adaptive software, with p-value = 0.972 for the right parotid gland, as shown in Figure 7A, and p-value = 0.958 for the left parotid gland, as shown in Figure 7B. The consistency in the dose variations between the two independent types of software demonstrates that the dose accumulation of the DIRART software can be applied for use in dose accumulation studies.
Figure 7.
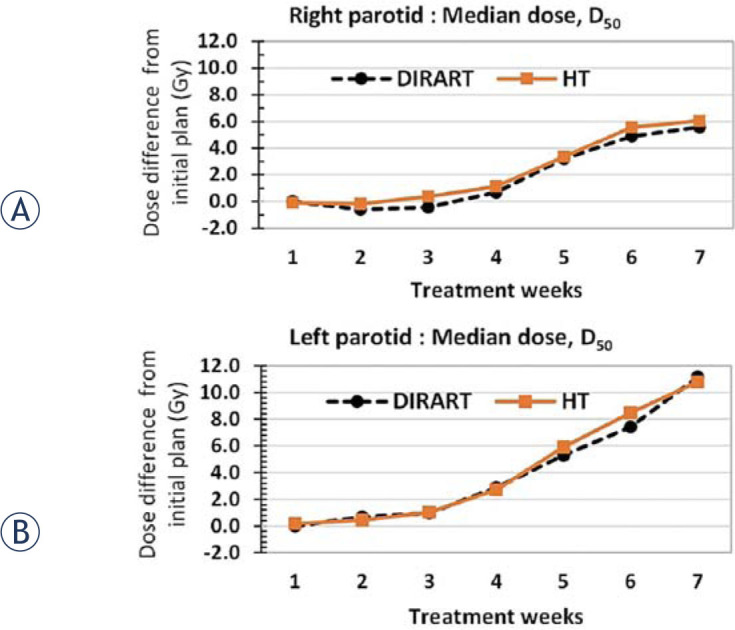
Cumulative dose comparison, derived from helical tomotherapy planned adaptive software (HT) and DIRART software in median dose, D50, of (A) right parotid gland and (B) left parotid gland.
Discussion
The validation of DIR was consistent in terms of the volume-based criterion, DSC, and the deformation field analysis, ICE. Accuracy in terms of DSC analysis tended to decrease as the treatment progressed as a result of organs with large-scale deformation causing reduction in the DIR accuracy.16 The AsyDM method showed the best performance with the highest average DSC value of all ROIs, the mean value of DSC = 0.804 ± 0.07, and also enabled the minimization of the inverse consistency error by the lowest mean value of ICE = 0.006 ± 0.002, as demonstrated in Figure 3.
As regards the weekly dose variation from the initial plan, the results demonstrated that the median, the near-minimum dose, and the near-maximum dose of the target slightly varied, by less than 0.5% of the initial plan. However, with regard to organ doses like the bilateral parotid gland, the discrepancy between the planned and the delivered mean doses was 6.8% (right) and 15.8% (left) higher than the initial plan. The accumulated mean parotid dose increased to be in the range of -0.6 to 12.4 Gy. Lee et al.2 analysed the changes in the parotid gland dose with reference to the anatomic changes throughout the course of radiotherapy. The daily parotid mean dose of the 10 patients differed from the planned dose by an average of 15%. At the end of the treatment, 3 of the 10 patients were estimated to have received greater than 10% higher mean parotid dose than in the original plan (range: 13–42%), whereas the remaining 7 patients received doses that differed by less than 10% (range: 6–8%).
Regarding the correlation between dose accumulation and DIR accuracy, there was consistency between the accuracy of ROI deformation and discrepancy of dose accumulation. The DIR method that yielded the highest DSC value was considered the best method for dose accumulation with the lowest variation from the reference dose. The AsyDM method demonstrated the best performance for target deformation with the highest mean DSC value of 0.802 ± 0.08, as presented in Figure 3, and showed the best agreement for target dose accumulation with the lowest average variation of 0.01% with the reference dose, as presented in Figure 5. This method also gave the highest mean DSC value of 0.824 ± 0.05 for the right parotid gland and the lowest mean parotid dose variation with 3.2% of the reference deformed dose, as demonstrated in Figure 6A. However, the SymHS method showed the best performance for left parotid deformation, as demonstrated in Figure 3A, with the highest mean value of DSC = 0.824 ± 0.06, and also showed the lowest mean left parotid dose variation with 2.9% of the reference deformed dose, as illustrated in Figure 6B. For the spinal cord, the AsyHS method gave the best performance, as presented in Figure 3, with the highest mean value of DSC = 0.806 ± 0.05, and also showed the lowest dose variation with 1.5% of the reference deformed dose, as presented in Figure 6C.
Regarding the uncertainty of dose accumulation, the three DIR methods demonstrated satisfactory volume matching for accumulated dose application with DSC values more than 0.7 for all the methods. Moreover, the one-way ANOVA analysis demonstrated that there was no significant difference between the three DIR methods as regards ROI deformation and dose accumulation. However, when uncertainty was considered (difference between the maximum dose and the minimum dose) in the estimation of the accumulated dose for all the DIR methods, the average of the DSC value of all the targets by the three DIR methods was 0.782 ± 0.04. The mean uncertainty for estimating the target dose was 0.21 ± 0.11 Gy (range: 0.06–0.32 Gy). Regarding the uncertainty of the parotid dose, the averages of the DSC values by the three DIR methods were 0.805 ± 0.01 (right) and 0.814 ± 0.01 (left). This shows that the mean uncertainty values for estimating the parotid dose were 1.99 ± 0.76 Gy (range: 0.01–3.14 Gy) for the right parotid and 1.19 ± 0.24 Gy (range: 0.01–1.58 Gy) for the left parotid. For the spinal cord, the average of the DSC values was 0.791 ± 0.01 Gy, while the mean uncertainty value for the estimated dose was 0.41 ± 0.04 Gy (range: 0.04–0.57 Gy). The results in this study were lower that Rigaud et al.17 who showed the mean uncertainty (difference between the maximum dose and the minimum dose, considering all the 10 DIR methods) to estimate the cumulated mean dose for the parotid gland (PG) was 4.03 Gy (SD = 2.27 Gy, range: 1.06–8.91 Gy).
Further investigation would involve applying this methodology to other treatment areas to identify patients who may benefit from adaptive treatment. DIR on megavoltage computed tomography imaging makes it possible to calculate daily and accumulated doses. Significant dose variations were observed as a result of inter-fractional anatomic changes, which is information that would benefit adaptive treatment strategies.
Acknowledgments
The author offers many thanks to the staff of the Division of Therapeutic Radiology and Oncology, Faculty of Medicine, Chiang Mai University, for supporting this study.
Footnotes
Disclosure: No potential conflicts of interest were disclosed.
References
- 1.Hardcastle N, Tome W, Cannon D, Brouwer C, Wittendorp P, Dogan N. et al. A multi-institution evaluation of deformable image registration algorithms for automatic organ delineation in adaptive head and neck radiotherapy. Radiat Oncol J. 2012;7:1–7. doi: 10.1186/1748-717X-7-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee C, Langen KM, Lu W, Haimerl J, Schnarr E, Ruchala KJ. et al. Assessment of parotid gland dose changes during head and neck cancer radiotherapy using daily megavoltage computed tomography and deformable image registration. Int J Radiat Oncol Biol Phys. 2008;71:1563–71. doi: 10.1016/j.ijrobp.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 3.Lee C, Langen KM, Lu W, Haimerl J, Schnarr E, Ruchala KJ. et al. Evaluation of geometric changes of parotid glands during head and neck cancer radiotherapy using daily MVCT and automatic deformable registration. Radiother Oncol. 2008;89:81–8. doi: 10.1016/j.radonc.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 4.Castelli J, Simon A, Louvel G, Henry O, Chajon E, Nassef M. et al. Impact of head and neck cancer adaptive radiotherapy to spare the parotid glands and decrease the risk of xerostomia. Radiat Oncol J. 2015;10:1–10. doi: 10.1186/s13014-014-0318-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kupelian PA, Langen KM, Zeidan OA, Meeks SL, Willoughby TR, Wagner TH. et al. Daily variations in delivered doses in patients treated with radiotherapy for localized prostate cancer. Int J Radiat Oncol Biol Phys. 2006;66:876–82. doi: 10.1016/j.ijrobp.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 6.Langen KM, Meeks SL, Poole DO, Wagner TH, Willoughby TR, Kupelian PA. et al. The use of megavoltage CT (MVCT) images for dose recomputations. Phys Med Biol. 2005;50:4259–76. doi: 10.1088/0031-9155/50/18/002. [DOI] [PubMed] [Google Scholar]
- 7.Morin O, Chen J, Aubin M, Gillis A, Aubry JF, Bose S. et al. Dose calculation using megavoltage cone-beam CT. Int J Radiat Oncol Biol Phys. 2007;67:1201–10. doi: 10.1016/j.ijrobp.2006.10.048. [DOI] [PubMed] [Google Scholar]
- 8.Yeo U, Supple J, Taylor M, Smith R, Kron T, Franich R.. Performance of 12 DIR algorithms in low-contrast regions for mass and density conserving deformation. Med Phys. 2013;40:1–12. doi: 10.1118/1.4819945. [DOI] [PubMed] [Google Scholar]
- 9.Nie K, Chuang C, Kirby N, Braunstein S, Pouliot J.. Site-specific deformable imaging registration algorithm selection using patient-based simulated deformations. Med Phys. 2013;40:041911. doi: 10.1118/1.4793723. [DOI] [PubMed] [Google Scholar]
- 10.Hardcastle N, Elmpt W, Cannon D, Ruysscher D, Bzdusek K, Tomé W.. Accuracy of deformable image registration for contour propagation in adaptive lung radiotherapy. Radiat Oncol J. 2013;8:243–50. doi: 10.1186/1748-717X-8-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang D, Brame S, Naqa E.. Technical Note: DIRART–A software suite for deformable image registration and adaptive radiotherapy research. Med Phys. 2011;38:71–7. doi: 10.1118/1.3521468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee N, Harris J, Garden AS, Straube W, Glisson B, Xia P. et al. Intensity-modulated radiation therapy with or without chemotherapy for nasopharyngeal carcinoma: radiation therapy oncology group phase II trial 0225. J Clin Oncol. 2009;27:3684–90. doi: 10.1200/JCO.2008.19.9109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kristy KB. Image processing in radiation therapy. New York: Taylor & Francis Group; 2013. [Google Scholar]
- 14.Zimring D, Talos F, Bhagwat G, Haker J, Black P, Zou K.. Statistical validation of brain tumor shape approximation via spherical harmonics for image-guided neurosurgery. Acad Radiol. 2005;12:459–66. doi: 10.1016/j.acra.2004.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Varadhan R, Karangelis G, Krishnan K, Hui S.. A framework for deformable image registration validation in radiotherapy clinical applications. J Appl Clin Med Phys. 2013;14:4066. doi: 10.1120/jacmp.v14i1.4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luiza B, Mischa SH, Eliana M, Vasquez O, Ben JM.. A symmetric nonrigid registration method to handle large organ deformations in cervical cancer patients. Med Phys. 2010;37:37–60. doi: 10.1118/1.3443436. [DOI] [PubMed] [Google Scholar]
- 17.Rigaud B, Antoine S, Castelli J, Gobeli M, Arango JO, Cazoulat G. et al. Evaluation of deformable image registration methods for dose monitoring in head and neck radiotherapy. Biomed Res Int. 2015:1–16. doi: 10.1155/2015/726268. [DOI] [PMC free article] [PubMed] [Google Scholar]


