Abstract
Background
The aim of this retrospective single institution study was to analyse long term results of vulvar cancer treatment with conventional 2D radiotherapy in Slovenia between years 1997–2004.
Patients and methods
Fifty-six patients, median age 74.4 years +/- 9.7 years, mainly stage T2 or T3, were included in the study. All patients were treated with radiotherapy, which was combined with surgery (group A), used as the primary treatment (group B) or at the time of relapse (group C). Chemotherapy was added in some patients. Histology, grade, lymph node status, details of surgery, radiation dose to the primary tumour, inguinofemoral and pelvic area as well as local control (LC) and survival were evaluated.
Results
Overall survival (OS), disease specific survival (DSS) and LC rates at 10-years for all patients were as follows: 22.7%, 34.5% and 41.1%, respectively. The best 10-years results of the treatment were achieved in the primary operated patients treated with adjuvant radiotherapy +/-chemotherapy (OS 31.9%, DSS 40.6% and LC 47.6%). Positive lymph nodes had a strong influence on LC. In case of positive nodes LC decreased by 60% (p = 0.03) and survival decreased by 50% (p = 0.2). There was a trend to a better LC with higher doses ≥ 54.0 Gy (p = 0.05).
Conclusions
The best treatment option for patients with advanced vulvar cancer is combined treatment with surgery and radiotherapy +/- chemotherapy, if feasible. Radiotherapy with the dose of ≥ 54.0 Gy should be considered to achieve better LC if positive adverse factors are present.
Key words: vulvar cancer, radiotherapy, surgery, local control, survival
Introduction
Vulvar cancer is a rare gynaecological cancer and it accounts for approximately 3–5% of all gynaecological malignancies. The incidence in Slovenia between years 1997–2004 was 33 to 57 (average 40) patients per year, the highest incidence occurred in women over 60 years of age.1 Surgery is the most important modality in the treatment of early vulvar cancer, while (chemo)radiotherapy +/- surgery is the most important modality in the treatment of advanced vulvar cancer. Radiotherapy can be used preoperatively, postoperatively, or as the only treatment in locally advanced vulvar cancer with bulky unresectable primary tumour or groin disease. In operated patients positive margins and positive lymph nodes are the most important indications for adjuvant radiotherapy. Other prognostic factors such as large primary tumour lesion, deep tumour invasion and in recent years also lymphovascular space involvement (LVI) are the factors that are used to determine adjuvant radiotherapy as well.2,3 The majority of the patients with vulvar cancer are over 70 years of age, with many additional comorbidities, which also favour radiotherapy as the best treatment option. Recent data are showing improvement of the treatment by adding adjuvant chemotherapy.4-7 In the past different cytotoxic agents were utilized, but nowadays the preferred is weekly cisplatin, an option which was extrapolated from treatment of advanced cervical squamous cell carcinoma (SCC).
Surgical treatment
Classical surgical approach is radical vulvectomy. More conservative surgery which reduced morbidity without compromising prognosis is used in early stage vulvar SCC. Wide local excision of the tumour with sentinel lymph node biopsy (SNB) with or without inguinofemoral lymphadenectomy is an attractive alternative.8-12 The most suitable candidates for SNB are patients with tumour diameter < 4 cm and with no palpable groin nodes.9,13,14Patients with positive sentinel-node metastases are candidates for additional treatment.8,9 The lymph node status and particularly inguinofemoral lymph node status is the most important prognostic factor for survival, while the tumour free margin is the most important prognostic factor for local recurrence.3, 15-17
Postoperative radiotherapy
Radiotherapy in postoperative adjuvant treatment is used to additionally treat the primary site, groins and/or pelvis. Radiotherapy to the primary site is indicated in case of positive or close margin (≤ 8 mm) and large primary tumour (>4 cm).2,3,18 OS of patients with close/positive margins who received adjuvant radiotherapy was similar to those with negative margins.18 Radiotherapy to the groins is indicated in case of two or more microscopically positive groin nodes, in case of one or more macroscopically positive node, or in case of extracapsular extension (ECE). When only one microscopically positive groin node is identified, adjuvant radiotherapy can be omitted, although recent recommendations show improvement in disease free survival (DFS) when adding radiotherapy also in a single microscopic node positive disease.5, 19-22 Lymph node involvement is the strongest negative predictive factor for survival. The 3-years PFS and OS for node positive disease was 35.2% and 56.2% compared to 75.2% and 90.2% for node negative disease.5,11,23 Despite adjuvant radiotherapy in node positive disease and clinical benefit in PFS, OS remains poor (57.7% with adjuvant radiotherapy vs 51.4% without radiotherapy at 3-years, p = 0.17).5
Locally advanced vulvar cancer
Radiotherapy is the treatment option for bulky primary site and/or extensive groin disease. The addition of chemotherapy to radiotherapy improves survival in several SCC.6,7,24,25 As high as 60% of patients with locally advanced disease can present with nodal metastases, the reason that urge for more aggressive treatment which combines chemotherapy and radiotherapy in locally advanced disease. After primary chemoradiotherapy surgery can be performed in selected cases. There are only a few studies, mainly single institution or phase II studies that report results of such approach.4,6,25-28
In this retrospective single institution study the long term results of vulvar cancer treatment in Slovenia between 1997 and 2004 with radiotherapy alone or combined with surgery or chemotherapy were analysed.
Patients and methods
Patients and tumours
69 patients with vulvar cancer treated with radiotherapy from January 1997 till December 2004 were retrospectively analysed. Thirteen patients were excluded from the study, because the palliative dose was used to treat the cancer. At the end 56 patients with histologically confirmed vulvar cancer treated with curative intent with radiotherapy, +/- surgery, +/- chemotherapy were enrolled and retrospectively analysed.
Mean patient age was 74.4 +/- 9.7 years. All patients were staged according to the FIGO 1988 staging system (13). FIGO stage distribution, available in 94.6% of the patients, was as follows: stage I 5 (8.9%), stage II 4 (7.1%), stage III 33 (58.9%) and stage IV 11 (19.6%) and not specified in 3 patients.
Histological type of the tumour was SCC in all patients included in the study. Other histological types ranging from malignant melanoma (2 patients), adenocarcinoma of the Bartholin’s gland (1 patient) and Paget’s disease (1 patient) were rare and were excluded from further analysis. The diagnostic work-up before the treatment consisted of clinical examination with biopsy and chest radiography in operated patients, while imaging studies such as abdominal and groin ultrasound or chest/ abdominal computer tomography (CT) were only rarely performed.
Patients, tumour and treatment characteristics are listed in Table 1.
Table 1.
Patients, tumor and treatment characteristcs
| characteristics | All | Primary operated | Primary irradiated | Relapse group |
|---|---|---|---|---|
| group A | group B | C | ||
| Number of patients (%) | 56 (100.0%) | 31 (55.4%) | 11 (19.6%) | 14 (25%) |
| Mean age (years) (+/-SD) | 74.4 (+/-9.7) | 74.9 ( +/- 9.8) | 67.9 (+/-10.8) | 87.2 (+/- 6.1) |
| Median follow-up time (months) | 22.5 (2-203) | 27 (8-203) | 9 (2-72) | 44 (5-134) |
| FIGO stage | ||||
| i | 5 (8.9%) | 0 | 0 | 5 (35.7%) |
| ii | 4 (7.1%) | 0 | 0 | 4 (28.6%) |
| iii | 33 (58.9%) | 23 (74.2%) | 6 (54.5%) | 4 (28.6%) |
| IV | 11 (19.6%) | 7 (22.6%) | 4 (36.4%) | 0 |
| not specified | 3 (5.4%) | 1 (3.2%) | 1 (9.1%) | 1 (7.1%) |
| histopathologic grade | ||||
| G1 | 21 (37.5%) | 11 (35.5%) | 3 (27.3%) | 7 (50.0%) |
| G2 | 25 (44.6%) | 15 (48.4) | 7 (63.6%) | 3 (21.4%) |
| G3 | 5 (8.9%) | 3 (9.7%) | 1 (9.1%) | 1 (7.1%) |
| not specified | 5 (8.9%) | 2 (6.5%) | 0 | 3 (21.4%) |
| T stage | ||||
| T1 | 5 (8.9%) | 2 (6.5%) | 0 | 3 (21.4%) |
| T2 | 26 (46.4%) | 18 (58.1%) | 1 (9.1%) | 7 (50.0%) |
| T3 | 16 (28.6%) | 9 (29.0%) | 7 (63.6%) | 0 |
| T4 | 3 (5.4%) | 1 (3.2%) | 2 (18.2%) | 0 |
| not specified | 6 (10.7%) | 1 (3.2%) | 1 (9.1%) | 4 (28.6%) |
| Diameter of primary tumor | ||||
| ≤ 2 cm | 10 (17.9%) | 7 (22.6%) | 0 | 3 (21.4%) |
| > 2 and ≤ 4 cm | 23 (41.1%) | 16 (51.6%) | 3 (27.3%) | 4 (28.6%) |
| > 4 cm | 12 (21.5%) | 5 (16.2%) | 6 (54.5%) | 1 (7.1%) |
| not specified | 11 (19.6%) | 3 (9.7%) | 2 (18.2%) | 6 (42.9%) |
| Tumor invasion | ||||
| lower urethra | 9 (16.1%) | 3 (9.7%) | 6 (54.5%) | 0 |
| vagina | 15 (26.8%) | 8 (25.8%) | 7 (63.6%) | 0 |
| anus | 1 (1.8%) | 0 | 1 (9.1%) | 0 |
| bladder wall | 0 | 0 | 1 (9.1%) | 0 |
| rectal wall | 1 (1.8%) | 0 | 1 (9.1%) | 0 |
| pelvic bone | 2 (7.1%) | 1 (3.2%) | 1 (9.1%) | 0 |
| Tumor location | ||||
| clitoris | 6 (10.7%) | 4 (12.9%) | 0 | 2 (14.3%) |
| labium major | 10 (17.9%) | 5 (16.1%) | 1 (9.1%) | 4 (28.6%) |
| labium minor | 8 (14.3%) | 4 (12.9%) | 0 | 4 (28.6%) |
| comissura/more than one location | 28 (50.0%) | 16 (51.6%) | 10 (90.9%) | 2 (14.3%) |
| not specified | 4 (7.1%) | 2 (6.5%) | 0 | 2 (14.3%) |
| Nodal status | ||||
| N0 | 17 (30.3%) | 4 (12.9%) | 5 (45.5%) | 8 (57.1%) |
| N1 | 25 (44.6%) | 19 (61.3%) | 2 (18.2%) | 4 (28.6%) |
| N2 | 10 (17.9%) | 7 (22.6%) | 3 (27.3%) | 0 |
| not specified | 4 (7.1%) | 1 (3.2%) | 1 (9.1%) | 2 (14.3%) |
| Nodal metastases | ||||
| micrometastases | 9 (16.1%) | 7 (22.6%) | not specified | 2 (14.3%) |
| macrometastases | 25 (44.6%) | 18 (58.1%) | 5 (45.5%) | 2 (14.3%) |
| Exstracapsular tumor spread | ||||
| positive | 10 (17.9%) | 8 (25.8%) | not specified | 2 (14.3%) |
| Residual tumor after surgery | 20 (35.7%) | 16 (51.6%) | not specified | 4 (28.6%) |
| primary tumor site | 15 (26.8%) | 13 (41.9%) | not specified | 2 (14.3%) |
| nodal site | 7 (12.5%) | 4 (12.9%) | not specified | 3 (21.4%) |
| Mean dose to the tumor (Gy) (+/-SD) | 47.4 (+/-7.9) | 47.1 (+/-8.2) | 46.7 (+/-8.7) | 46.0 (+/-5.2) |
| Mean dose to the inguinofemoral area (Gy) (+/-SD) | ||||
| ipsilateral | 47.5 (+/-8.8) | 50.7 (+/-7.9) | 46.9 (+/-8.4) | 40.1 (+/-6.7) |
| contralateral | 49.7 (+/-9.7) | 53.4 (+/-7.8) | 44.9 (+/-9.8) | 37.4 (+/-6.9) |
| Mean dose to the pelvic area (Gy) (+/-SD) | ||||
| ipsilateral | 46.2 (+/-6.2) | 47.0 (+/-6.8) | 45.0 (+/-4.9) | 43.5 (+/-6.2) |
| contralateral | 44.6 (+/-10.5) | 44.7 (+/-12.5) | 45.0 (+/-4.9) | 39.1 (+/-4.9) |
The study was approved by the Protocol Review Board at the Institute of Oncology Ljubljana (ERID-KESOPKR-14). The investigation followed recommendations of the Helsinki Declaration (1964, with later amendments) and of the European Council Convention on Protection of Human Rights in Bio-Medicine (Oviedo 1997).
Treatment
In this retrospective study radiotherapy was used as the part of the treatment in all patients and it was most frequently combined with surgery in 31 patients (55.4%). Chemoradiotherapy was used in 11 patients (17.9%). At that time the most commonly used cytotoxic agent in adjuvant setting was bleomycin or cisplatin. Radiotherapy was also used at the time of relapse in 14 patients (25%): local relapse at the site of primary tumour was detected in 6 patients, and nodal relapse in 10 patients. The majority of relapses developed within two years after the primary treatment.
In order to evaluate LC and survival among different treatment modalities, three treatment groups were formed (Table 1). 31 patients were included in the primary operated group (group A), where the primary treatment modality was surgery with radiotherapy +/- chemotherapy as an adjuvant treatment. This was the largest group, reflecting that operation therapy +/- radiotherapy is the most important treatment modality in the management of advanced vulvar cancer. Chemoradiotherapy was used only in 2/31 patients, respectively. In the primary irradiated group (group B) 11 patients were included. Radiotherapy was used as the only treatment in 4/11 patients, combined with chemotherapy in 7/11 patients, used preoperatively in 1 or pre- and postoperatively in 1 patient, respectively. In the relapse group (group C) radiotherapy alone or combined with surgery was used at the time of relapse in 9/14 patients and 5/14 patients, respectively. Chemoradiotherapy was used in 2/14 patients.
Surgical treatment
Surgery is the cornerstone in the treatment of vulvar cancer. The most frequently used type of operation was radical vulvectomy in 29 and wide local excision in 16 patients. SNB was performed only in 6 patients. Vulvar cancer patients were diagnosed in different gynaecological centres in Slovenia and were mainly operated in the three main centres: University Medical Centre Ljubljana (33.9%), Institute of Oncology Ljubljana (37.5%) and University Medical Centre Maribor (5.4%). Unilateral and bilateral inguinofemoral lymph node dissection was performed in 26.8% and 33.9%, respectively. The mean number of removed lymph nodes was 7 (range 1–19). Among all operated patients adjuvant radiotherapy was used in 65.1%, radiochemotherapy in 6.9% and radiotherapy at the time of relapse in 27.9%.
Radiotherapy
Radiotherapy is an important modality of the treatment in adjuvant setting, as a radical treatment in locally advanced cancer or as a treatment of choice at the time of relapse. External beam radiotherapy was delivered with 5–15 MV linear accelerator or Cobalt-60 machine. In addition, electrons were sometimes applied to boost inguinal areas or primary tumour. Mostly supine position has been used, but “frogleg” position has been used as well, particularly in fat patients. Various two-dimensional (2D) techniques were used to radiate primary tumour and/or inguinal nodes at that time.
First technique used wide anteroposterior (AP) field that included the pelvic and inguinal area, with a narrow posteroanterior (PA) field covering pelvis only. The fields were weighted equally 1:1. The inguinal nodes were boosted to a certain depth determined by a clinician, with a separate anterior field. Bolus material on the primary tumour or inguinal areas was used according to the physician’s decision. The physician’s determined depth of the inguinal nodes was usually 3 to 4 cm. The second technique used AP/PA field of the same dimension. An alternative technique consisted of a wide AP field and narrow PA field with a partial transmission block at the central portion of the AP field. The dose at a specified depth to the inguinal areas was delivered through the AP field.
The two opposite fields technique was applied in majority of the patients. Other techniques, such as four field (box)technique was used as well (16.2%). There were many variations in the prescribed dose to the inguinal nodes among patients due to different inguinal depth chosen by a physician and different radiation techniques that were used. The daily dose was usually 1.8–2.0 Gy per fraction, a daily dose of 2.5 –3.0 Gy per fraction was seldom used as well, with a total dose mainly in the range between 45–60 Gy. In order to enable comparison of the dose to the inguinal area, the depth dose was calculated for all patients at the same depth. The so called “inguinal depth” was determined on CT scans of the fifty women treated with pelvic radiotherapy at the time of collecting data for this study. The mean depth of the inguinal nodes was 5.5 cm (range 2–18 cm), which was more than arbitrary by a physician determined depth.
Chemotherapy
Today chemotherapy combined with radiotherapy is the fundamental strategy in the treatment of advanced vulvar cancer. At the time of this study it was not so widely used, altogether 11 patients (19.6%) got some kind of chemotherapy. Platinum-based chemotherapy is the treatment of choice in squamous cell carcinomas. In our study cisplatin was used alone or in combination with other cytotoxic drugs such as bleomycin or 5-fluorouracil. Rarely, in case of renal dysfunction, carboplatin was used instead of cisplatin.
Follow up
First follow-up was usually performed 3 months after the treatment. Consecutive follow-ups were usually performed alternately by gynaecologist or radiation oncologist at 3 to 6 month intervals for the first 5 years and once per year later on.
Statistical analysis
The data were analysed using IBM SPSS statistical software package version 23.0 (SPSS Inc., Chicago, IL, USA), p values of < 0.05 were considered statistically significant.
Kaplan-Meier method was applied to calculate actuarial survival and loco-regional control rates. The endpoint for OS was death from any cause, for DSS the disease related death and for LC any evidence of loco-regional recurrent disease. Patients without relapse were censored at the time of the last follow-up, visit or death. Surviving patients were censored at the time of the last follow-up. No effort was made to compare results between different treatment groups due to small number of patients in each group and consequently a low power of the test.
The influence of positive lymph nodes, ECE, size of primary tumour and completeness of surgical resection on survival and LC were analysed. Arbitrary cut off points for positive lymph nodes and ECE (negative versus positive), size of primary tumour (up to 4 cm versus ≥ 4 cm) and completeness of surgical resection (complete R0 versus incomplete R1/R2) were used.
The data regarding the side effects of the radiotherapy treatment or surgery were not systematically recorded and were in general missing in the patient’s record, therefore the analysis of side effects of the treatment was omitted in this study.
Results
At the time of the last follow up 21 patients (37.5%) proved to be disease free. Median follow-up for all patients was 22.5 months (range 2-203) and for surviving patients 101 months (range 72–157). For 4 surviving patients follow-up was lower than 10-years due to lost to follow-up, but according to the national Cancer Registry they were still alive at the close-out time of the study. The pattern of failure at the last follow-up is represented in Table 2. Half of the patients with distant metastases had also locoregional recurrence. The skin was the most common site of metastatic disease, other possible sites were pelvic and paraaortic lymph nodes, lungs and bones.
Table 2.
Pattern of failure at the time of last follow-up
| All | Primary operated | Primary irradiated | Relapse group | |
|---|---|---|---|---|
| group A | group B | C | ||
| Local failure | 9 | 5 | 4 | 0 |
| Inguinal failure | 6 | 3 | 1 | 2 |
| Distant failure | 5 | 3 | 1 | 1 |
| Local and inguinal failure | 10 | 5 | 3 | 2 |
| Local/inguinal and distant failure | 5 | 2 | 1 | 2 |
| Overall failure | 35 | 18 | 10 | 7 |
| No evidence of disease | 21 | 13 | 1 | 7 |
Overall survival (OS), disease specific survival (DSS) and local control (LC) for all vulvar cancer patients at 10-years were 22.7%, 34.5% and 41.1%, for primary operated group 31.9%, 40.6% and 47.6% respectively, in primary irradiated group there were no survivals at 10-years and for the relapse group survivals and LC were as follows 14.3%, 33.9% and 41.5% (Figure 1 and 2). Ten years LC for node negative disease was 57.1% for T1–2 and 55.6% for T3–4. For node positive disease T1–4N1 and T1–4N2 it was 38.4% and 29.2%, respectively. The difference was not statistically significant (p = 0.5).
Figure 1.
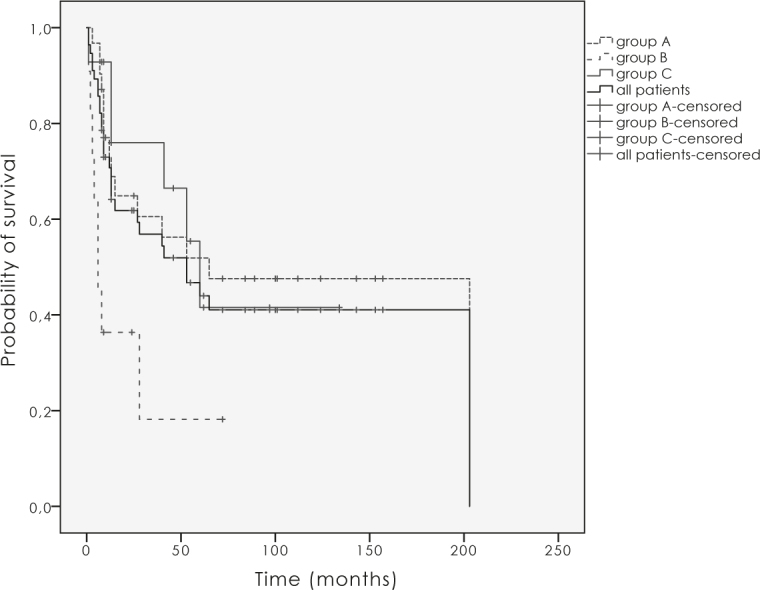
Local control for the group A (primary operated patients), B (primary irradiated patients), C (patients with relapse) and all patients.
Figure 2.
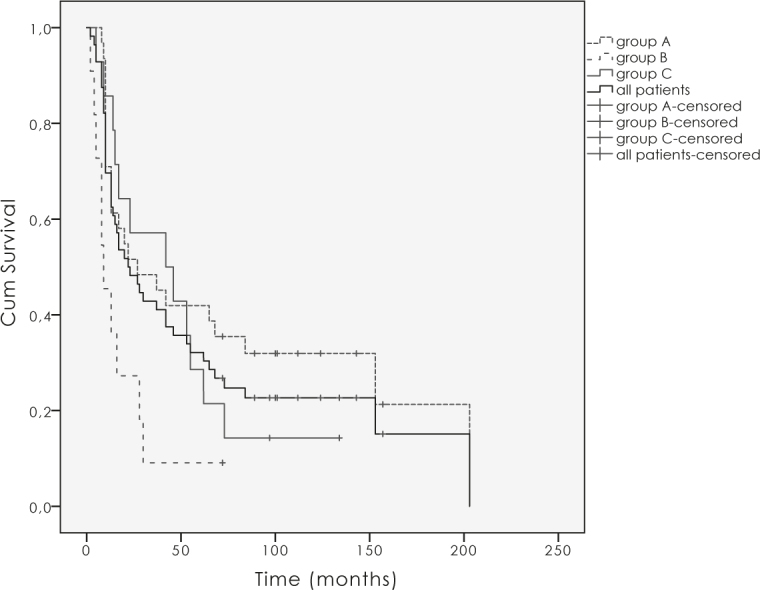
Overall survival for the group A, (primary operated patients), B (primary irradiated patients), C (patients with relapse) and all patients.
OS, DSS and LC at 10-years for stage III were as follows 30.3%, 35.3% and 44%, and for stage IV 18.2%, 18.2% and 24.9%. Data for stage I and II are not reported, because only 2 patients were alive at 5 years and only 1 patient (FIGO stage II) was alive at 10 years (134 months). Factors that contribute to lower outcome in stage I and II were higher age (mean age 79.9 +/-6.5, p = 0.04) and treatment of the relapsed disease in all stage I and II patients (local relapse 3, inguinal 4, local and inguinal 2 patients).
The mean equivalent dose in 2 Gy per daily fraction (EQD2) applied to the primary tumour was 47.4 Gy +/- 7.9 Gy. The mean equivalent dose to the inguinofemoral area was 47.5 +/- 8.8 Gy and 49.7 +/- 9.7 Gy for the ipsi- and contralateral side. The mean equivalent dose to the primary tumour and to the inguinal area for different treatment groups are listed in Table 1. Significantly higher dose was applied to the macroscopic positive lymph nodes as to the negative or microscopic lymph nodes (54.0 Gy against 40.0 Gy, p = 0.04). There was no statistically significant difference between the dose applied to operated or non-operated patients. Despite positive adverse factors, such as positive lymph nodes, ECE or large primary tumour, the dose applied to the primary site or groins was rather low. Only 16% of patients received the dose higher than 54.0 Gy (Figure 3).
Figure 3.
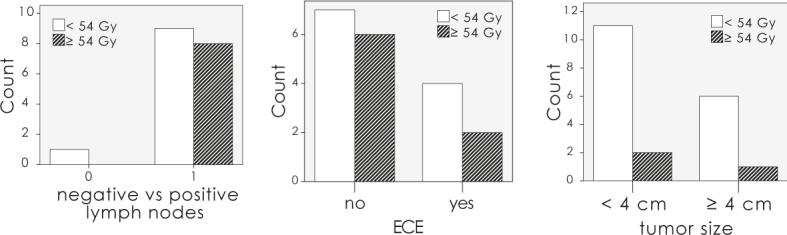
Number of patients with the dose applied to the positive lymph nodes, nodes with extracapsular lymph node extension (ECE) and large tumours.
ECE had a negative impact on LC (p = 0.02; Figure 4). Positive lymph nodes had a strong influence on LC (Figure 5). In case of positive nodes LC decreased by 60% (p = 0.03), and OS as well as DSS decreased by 50% (p = 0.2). The completeness of surgical resection had an impact on local and regional control in the first two years after the treatment, which did not prove to be statistically significant (p = 0.6). There was a trend to a better LC with higher doses over 54.0 Gy (p = 0.05).
Figure 4.
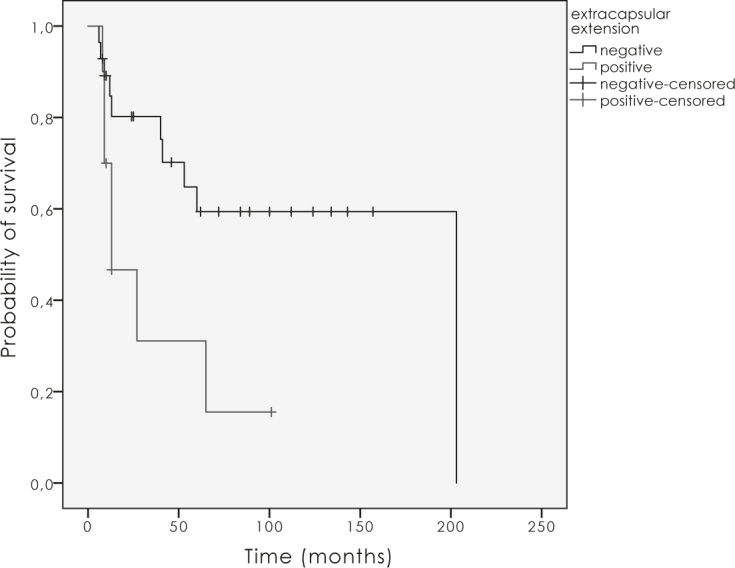
The influence of extracapsular nodal extension on local control (p = 0.02).
Figure 5.
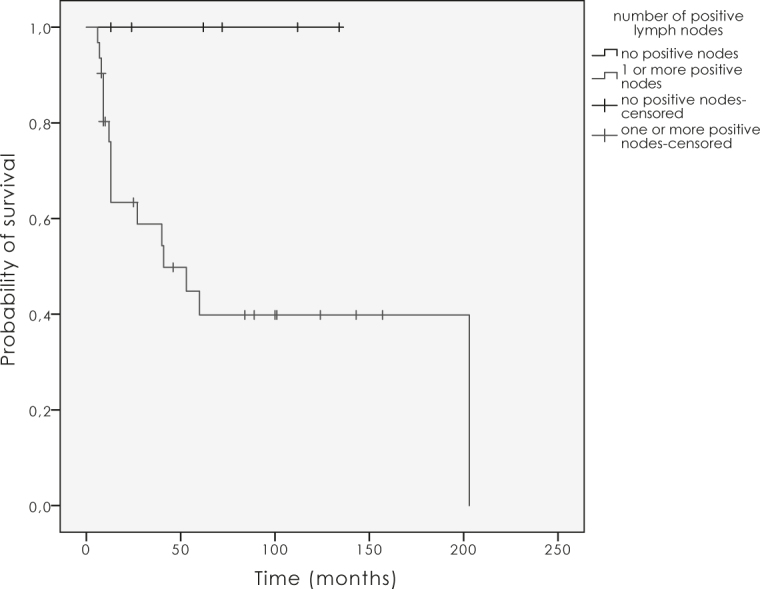
The influence of positive nodes on local control (p = 0.03).
Vulvar and groin morbidity are important factors which effect quality of life of the patient. It is compromised due to surgical treatment and radiotherapy. In this study acute and late side effects were not systematically recorded and these results were not evaluated.
Discussion
The best treatment option for early stage vulvar cancer is surgery, with adjuvant radiotherapy indicated in high risk disease with positive adverse factors such as large primary tumour size, close or positive surgical margins, positive lymph node/s or ECE. For patients, who are not candidates for primary surgery due to more extensive disease or comorbidities, radiotherapy combined with chemotherapy if feasible, is the preferred treatment option. The distribution of the patients between different treatment groups reflects the treatment strategy which was used most frequently - surgery combined with adjuvant radiotherapy. SNB in patients with early stage disease and negative groin exam as an alternative to inguinofemoral lymphadenectomy, was performed only in 10.7%, reflecting early beginnings of using this method in Slovenia. Radiotherapy as the primary treatment was used less frequently; concomitant chemotherapy was used in 63.3% (7/11) of patients. The 2D treatment technique that was used frequently at that time, with dose prescription to the groins, usually at 3 cm depth, resulted in under-dosing of the inguinal nodes. Many studies, as well as ours, showed that the inguinal lymph nodes depth is in general more than 3 cm.1,29 With the use of 3D and other novel techniques such as intensity modulated radiotherapy (IMRT) or volumetric modulated arc therapy (VMAT), the appropriate higher dose can be delivered to the groins with the dose reduction to the uninvolved tissues.4 In the study there was no significant difference between the dose applied to the patients with different risk factors and the patients in different treatment groups. The radiation dose applied after surgery is different after complete tumour removal, it ranges between 45.0 to 50.0 Gy, than after incomplete tumour removal with remaining microscopic or macroscopic disease, where the applied dose should be higher (≥ 60.0 Gy); although recent report showed that for vulvar squamous cell carcinoma with positive margins an adequate dose lies between 54.0 to 59.9 Gy.30 The results from our study showed that rather low dose was applied to the primary irradiated patients group B and relapse group C, which was, besides the existing comorbidity factors, higher age and advanced tumours, the possible reason for lower LC and survival. The risk factors for the worse outcome that were recognized in other studies were proved in this study as well, despite the small number of patients. Positive lymph nodes status proved to be one of the strongest predictor for lower disease control and survival, despite the use of adjuvant therapy.7,5,31
Despite the fact of a low quality data of the retrospective analysis with scarce diagnostic work-up performed before the treatment, the historical 2D radiotherapy technique used in majority of the patients and lacking morbidity data of the treatment, this is up to now the first and the only study of SCC of the vulva treated with radiotherapy in Slovenia.These results can be used as the basis for further analysis and improvement of the treatment of vulvar cancer patients with modern radiotherapy techniques.
Conclusions
The best treatment option for patients with squamous cell vulvar cancer is surgery with adjuvant radiotherapy +/- chemotherapy in case of positive adverse factors. The dose over 54.0 Gy is associated with better LC. More aggressive treatment with higher doses to the lymph node/s and adjuvant chemotherapy should be offered to the patients with positive lymph node/s. For the patients who are not candidates for primary surgery chemoradiotherapy is the treatment of choice. 2D radiotherapy technique with the dose prescription to the inguinal nodes at certain depth and consequently sometimes inadequate dose distribution to the inguinofemoral region can be bypassed by modern radiotherapy techniques, such as IMRT and VMAT. With the use of these techniques and more conservative surgery the side effects and cosmetic results of the treatment can be further improved.
Footnotes
Disclosure: No potential conflicts of interest were disclosed.
References
- 1.Zadnik V, Primic Zakelj M, Lokar K, Jarm K, Ivanus U, Zagar T.. Cancer burden in Slovenia with the time trends analysis. Radiol Oncol. 2017;51:47–55. doi: 10.1515/raon-2017-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jolly S, Soni P, Gaffney DK, Biagioli M, Elshaikh MA, Jhingran A. et al. ACR Appropriateness Criteria(R) Adjuvant Therapy in Vulvar Cancer. Oncology. 2015;29:867–75. [PubMed] [Google Scholar]
- 3.Heaps JM, Fu YS, Montz FJ, Hacker NF, Berek JS.. Surgical-pathologic variables predictive of local recurrence in squamous cell carcinoma of the vulva. Gynecol Oncol. 1990;38:309–14. doi: 10.1016/0090-8258(90)90064-r. [DOI] [PubMed] [Google Scholar]
- 4.Beriwal S, Coon D, Heron DE, Kelley JL, Edwards RP, Sukumvanich P. et al. Preoperative intensity-modulated radiotherapy and chemotherapy for locally advanced vulvar carcinoma. Gynecol Oncol. 2008;109:291–5. doi: 10.1016/j.ygyno.2007.10.026. [DOI] [PubMed] [Google Scholar]
- 5.Mahner S, Jueckstock J, Hilpert F, Neuser P, Harter P, de Gregorio N. et al. Adjuvant therapy in lymph node-positive vulvar cancer: the AGO-CaRE-1 study. J Natl Cancer Inst. 2015;107:1–12. doi: 10.1093/jnci/dju426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Montana GS, Thomas GM, Moore DH, Saxer A, Mangan CE, Lentz SS. et al. Preoperative chemo-radiation for carcinoma of the vulva with N2/N3 nodes: a gynecologic oncology group study. Int J Radiat Oncol Biol Phys. 2000;48:1007–13. doi: 10.1016/S0360-3016(00)00762-8. [DOI] [PubMed] [Google Scholar]
- 7.Gill BS, Bernard ME, Lin JF, Balasubramani GK, Rajagopalan MS, Sukumvanich P. et al. Impact of adjuvant chemotherapy with radiation for node-positive vulvar cancer: a National Cancer Data Base (NCDB) analysis. Gynecol Oncol. 2015;137:365–72. doi: 10.1016/j.ygyno.2015.03.056. [DOI] [PubMed] [Google Scholar]
- 8.Te Grootenhuis NC, van der Zee AG, van Doorn HC, van der Velden J, Vergote I, Zanagnolo V. et al. Sentinel nodes in vulvar cancer: long-term follow-up of the GROningen INternational Study on Sentinel nodes in Vulvar cancer (GROINSS-V) I. Gynecol Oncol. 2016;140:8–14. doi: 10.1016/j.ygyno.2015.09.077. [DOI] [PubMed] [Google Scholar]
- 9.Oonk MH, van Hemel BM, Hollema H, de Hullu JA, Ansink AC, Vergote I. et al. Size of sentinel-node metastasis and chances of non-sentinel-node involvement and survival in early stage vulvar cancer: results from GROINSS-V, a multicentre observational study. Lancet Oncol. 2010;11:646–52. doi: 10.1016/S1470-2045(10)70104-2. [DOI] [PubMed] [Google Scholar]
- 10.Van Der Zee AGJ, Oonk MH, De Hullu JA, Ansink AC, Vergote I, Verheijen RH. et al. Sentinel node dissection is safe in the treatment of early-stage vulvar cancer. J Clin Oncol. 2008;26:884–9. doi: 10.1200/JC0.2007.14.0566. [DOI] [PubMed] [Google Scholar]
- 11.de Hullu JA, van der Zee AG.. Surgery and radiotherapy in vulvar cancer. Crit Rev Oncol Hematol. 2006;60:38–58. doi: 10.1016/j.critrevonc.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 12.Vakselj A, Bebar S.. The role of sentinel lymph node detection in vulvar carcinoma and the experiences at the Institute of Oncology Ljubljana. Radiol Oncol. 2007;41:167–73. doi: 10.2478/v10019-007-0027-4. [DOI] [Google Scholar]
- 13.Benedet JL, Bender H, Jones H, Ngan H, Pecorelli S. Staging classifications and clinical practice guidelines of gynaecologic cancers. Int J Gynaecol Obstet. 2000;70:207–312. doi: 10.1016/S0020-7292(00)90001-8. [DOI] [PubMed] [Google Scholar]
- 14.Levenback CF, Ali S, Coleman RL, Gold MA, Fowler JM, Judson PL. et al. Lymphatic mapping and sentinel lymph node biopsy in women with squamous cell carcinoma of the vulva: a Gynecologic Oncology Group study. J Clin Oncol. 2012;30:3786–91. doi: 10.1200/JCO.2011.41.2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Homesley HD, Bundy BN, Sedlis A, Adcock L.. Radiation therapy versus pelvic node resection for carcinoma of the vulva with positive groin nodes. Obstet Gynecol. 1986;68:733–40. [PubMed] [Google Scholar]
- 16.Homesley HD, Bundy BN, Sedlis A, Yordan E, Berek JS, Jahshan A. et al. Assessment of current International Federation of Gynecology and Obstetrics staging of vulvar carcinoma relative to prognostic factors for survival (a Gynecologic Oncology Group study) Am J Obstet Gynecol. 1991;164:997–1003. doi: 10.1016/0002-9378(91)90573-a. [DOI] [PubMed] [Google Scholar]
- 17.De Hullu JA, Hollema H, Lolkema S, Boezen M, Boonstra H, Burger MPM. et al. Vulvar carcinoma. The price of less radical surgery. Cancer. 2002;95:2331–8. doi: 10.1002/cncr.10969. [DOI] [PubMed] [Google Scholar]
- 18.Ignatov T, Eggemann H, Burger E, Costa SD, Ignatov A.. Adjuvant radiotherapy for vulvar cancer with close or positive surgical margins. J Cancer Res Clin Oncol. 2016;142:489–95. doi: 10.1007/s00432-015-2060-9. [DOI] [PubMed] [Google Scholar]
- 19.Fons G, Groenen SMA, Oonk MHM, Ansink AC, van der Zee AGJ, Burger MPM. et al. Adjuvant radiotherapy in patients with vulvar cancer and one intra capsular lymph node metastasis is not beneficial. Gynecol Oncol. 2009;114:343–5. doi: 10.1016/j.ygyno.2009.05.017. [DOI] [PubMed] [Google Scholar]
- 20.Viswanathan AN, Pinto AP, Schultz D, Berkowitz R, Crum CP.. Relationship of margin status and radiation dose to recurrence in post-operative vulvar carcinoma. Gynecol Oncol. 2013;130:545–9. doi: 10.1016/j.ygyno.2013.05.036. [DOI] [PubMed] [Google Scholar]
- 21.Parthasarathy A, Cheung MK, Osann K, Husain A, Teng NN, Berek JS. et al. The benefit of adjuvant radiation therapy in single-node-positive squamous cell vulvar carcinoma. Gynecol Oncol. 2006;103:1095–9. doi: 10.1016/j.ygyno.2006.06.030. [DOI] [PubMed] [Google Scholar]
- 22.Woelber L, Eulenburg C, Choschzick M, Kruell A, Petersen C, Gieseking F. et al. Prognostic role of lymph node metastases in vulvar cancer and implications for adjuvant treatment. Int J Gynecol Cancer. 2012;22:503–8. doi: 10.1097/IGC.0b013e31823eed4c. [DOI] [PubMed] [Google Scholar]
- 23.Farias-Eisner R, Cirisano FD, Grouse D, Leuchter RS, Karlan BY, Lagasse LD. et al. Conservative and individualized surgery for early squamous carcinoma of the vulva: the treatment of choice for Stage I and II (T1-2 N0-1 M0) Disease. Gynecol Oncol. 1994;53:55–8. doi: 10.1006/gyno.1994.1087. [DOI] [PubMed] [Google Scholar]
- 24.Green JA, Kirwan JM, Tierney JF, Symonds P, Fresco L, Collingwood M. et al. Survival and recurrence after concomitant chemotherapy and radiotherapy for cancer of the uterine cervix: a systematic review and meta-analysis. Lancet. 2001;358:781–6. doi: 10.1016/S0140-6736(01)05965-7. [DOI] [PubMed] [Google Scholar]
- 25.Eifel P.. Prolonged continuous infusion cisplatin and 5-fluorouracil with radiation for locally advanced carcinoma of the vulva. Gynecol Oncol. 1995;59:51–6. doi: 10.1006/gyno.1995.1267. [DOI] [PubMed] [Google Scholar]
- 26.Moore DH, Thomas GM, Montana GS, Saxer A, Gallup DG, Olt G.. Preoperative chemoradiation for advanced vulvar cancer: a phase II study of the Gynecologic Oncology Group. Int J Radiat Oncol Biol Phys. 1998;42:79–85. doi: 10.1016/s0360-3016(98)00193-x. [DOI] [PubMed] [Google Scholar]
- 27.Geisler JP, Manahan KJ, Buller RE.. Neoadjuvant chemotherapy in vulvar cancer: avoiding primary exenteration. Gynecol Oncol. 2006;100:53–7. doi: 10.1016/j.ygyno.2005.06.068. [DOI] [PubMed] [Google Scholar]
- 28.Gerszten K, Selvaraj RN, Kelley J, Faul C.. Preoperative chemoradiation for locally advanced carcinoma of the vulva. Gynecol Oncol. 2005;99:640–4. doi: 10.1016/j.ygyno.2005.07.126. [DOI] [PubMed] [Google Scholar]
- 29.Koh WJ, Chiu M, Stelzer KJ, Greer BE, Mastras D, Comsia N, Russell KJ GT.. Femoral vessel depth and the implications for groin node radiation. Int J Radiat Oncol Biol Phys. 1993;15:969–74. doi: 10.1016/0360-3016(93)90476-c. [DOI] [PubMed] [Google Scholar]
- 30.Chapman B V, Gill BS, Viswanathan AN, Balasubramani GK, Sukumvanich P, Beriwal S.. Adjuvant radiation therapy for margin-positive vulvar squamous cell carcinoma: defining the ideal dose-response using the national cancer data base. Int J Radiat Oncol Biol Phys. 2016;97:1–11. doi: 10.1016/j.ijrobp.2016.09.023. [DOI] [PubMed] [Google Scholar]
- 31.Chan JK, Sugiyama V, Pham H, Gu M, Rutgers J, Osann K. et al. Margin distance and other clinico-pathologic prognostic factors in vulvar carcinoma: a multivariate analysis. Gynecol Oncol. 2007;104:636–41. doi: 10.1016/j.ygyno.2006.10.004. [DOI] [PubMed] [Google Scholar]


