Abstract
Rapid and highly efficient mating-type switching of Saccharomyces cerevisiae enables a wide variety of genetic manipulations, such as the construction of strains, for instance, isogenic haploid pairs of both mating-types, diploids and polyploids. We used the CRISPR/Cas9 system to generate a double-strand break at the MAT locus and, in a single cotransformation, both haploid and diploid cells were switched to the specified mating-type at ∼80% efficiency. The mating-type of strains carrying either rod or ring chromosome III were switched, including those lacking HMLα and HMRa cryptic mating loci. Furthermore, we transplanted the synthetic yeast chromosome V to build a haploid polysynthetic chromosome strain by using this method together with an endoreduplication intercross strategy. The CRISPR/Cas9 mating-type switching method will be useful in building the complete synthetic yeast (Sc2.0) genome. Importantly, it is a generally useful method to build polyploids of a defined genotype and generally expedites strain construction, for example, in the construction of fully a/a/α/α isogenic tetraploids.
Keywords: Saccharomyces cerevisiae, mating-type switching, CRISPR/Cas9, ring chromosome, polyploidy
The sex of Saccharomyces cerevisiae is determined by its mating-type locus, MATa or MATα, typically manifesting as either a MATa or MATα haploid or a MATa/α diploid. Strains of the opposite mating-type can mate to produce MATa/α diploids, which are incapable of mating (Hawthorne 1963). Mating-type switching is an important model for cell lineage, gene silencing, and genomic rearrangement analysis, and is also of great practical importance to construct an isogenic set (a, α, and a/α) of a given strain (Herskowitz and Jensen 1991; Haber 2012). Sc2.0 (the synthetic yeast genome project) aims to produce the first functional synthetic eukaryotic cell with redesigned chromosomes (Richardson et al. 2017). To efficiently consolidate all 16 individually synthesized chromosomes in a final functional cell, a number of sequential steps of mating-type switching and intercross will be needed. Thus, a rapid, simple, and highly efficient method of mating-type switching is critically needed to complete genome synthesis. More broadly, switching the mating-type of a strain is a standard but inefficient general method frequently used in strain construction, and enhancements in this method will be widely useful.
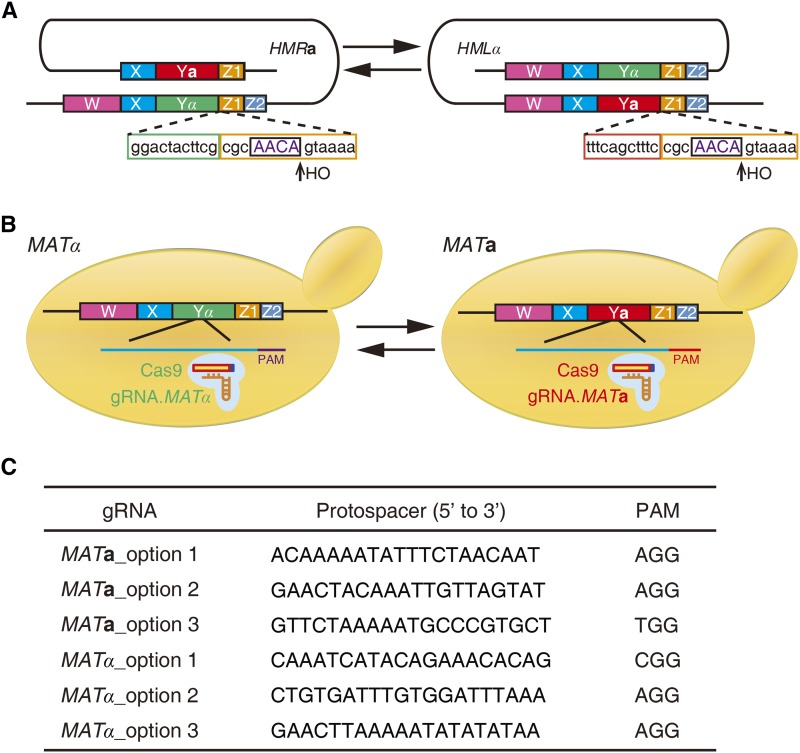
The HO gene (homothallic switching endonuclease gene) encodes a site-specific endonuclease that directs mating-type switching. The endonuclease generates a double-strand break (DSB) at the Z1 region of the MAT cassette in late G1 of the haploid cell cycle. Although the cleavage site is also encoded at the HMLα and HMRa loci, cleavage by HO endonuclease in the silent cassettes is prevented by the action of the SIR gene products (Strathern et al. 1982; Kostriken et al. 1983). Mating-type switching occurs by gene conversion of copies of silent mating-type cassettes from inactive loci, HMLα and HMRa, to the active mating-type locus, MAT (Rine and Herskowitz 1987; Haber 1998) (Figure 1A). Laboratory strains are mating-type stable and heterothallic by virtue of deletion or mutation of the HO gene. To isolate a strain of the opposite mating-type in the laboratory, a plasmid carrying the cloned HO gene can be transformed into the haploid strain to promote mating-type switching. The typical outcome of such an experiment is the subsequent mating within the population of interconverted and noninterconverted cells to yield isogenic MATa/α diploids. As such, to isolate the desired haploid cell of the opposite mating-type usually requires sporulation and tetrad dissection (Takahashi et al. 1958; Russell et al. 1986). HO gene expression can be placed under the control of a galactose promoter to allow for inducible mating-type interconversion (Herskowitz and Jensen 1991), although the efficiency of mating-type conversion is not reliably high (Nickoloff et al. 1986; Hou et al. 2013). Finally, if the HMLα and HMRa loci are deleted from a given strain, the mating-type cannot be switched by interconversion via expression of the HO gene (Klar et al. 1984). The published version of synthetic yeast chromosome III, synIII (Annaluru et al. 2014), lacks the silent cassettes; thus, every time it is consolidated with another synthetic chromosome it is necessary to switch the mating-type.
Figure 1.
Schematic of the mating-type switching. (A) Mating-type interconversion between MATa and MATα by HO-induced DSB and templated HR. (B) Schematic outlining the CRISPR/Cas9-mediated mating-type switching. DSBs are generated by Cas9 specifically targeted to the Ya or Yα region. (C) Protospacer and PAM sequences for MATa and MATα mating-type switching.
Here we demonstrate a CRISPR/Cas9-mediated method to enable efficient mating-type switching in S. cerevisiae. We show isolation of wild-type haploid or diploid cells of the desired mating-type with high efficiency via a single yeast transformation of plasmids expressing Cas9 and optimal guide RNA (gRNA) sequences together with the appropriate donor MAT locus. We also successfully switch the mating-type of cells carrying synIII, which lack the HMLα and HMRa cryptic mating loci. Finally, we couple mating-type switching of a strain carrying synthetic chromosome V, synV (Xie et al. 2017), with an endoreduplication intercross to build a polysynthetic Sc2.0 strain encoding synV and synthetic chromosome X, synX (Wu et al. 2017). The CRISPR/Cas9 mating-type switching method is generally useful for construction of isogenic strains and polyploid strains of defined genotype.
Materials and Methods
Strains and plasmids
All S. cerevisiae strains used in this study are listed in Table 1. The strain yLM422 carries a synIII chromosome with mating-type α (Annaluru et al. 2014), and lacks HMLα and HMRa. The MATa derivative synIII strain yXZX621 and the MATα derivative synV strain yXZX543 were constructed using the method described here.
Table 1. Yeast strains used in this study.
| Strain | Description | Parent | Source |
|---|---|---|---|
| 14a | MATa his1 | Trueheart et al. (1987) | |
| 17α | MATα his1 | Trueheart (et al. 1987) | |
| BY4741 | MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 LYS2 | Brachmann (et al. 1998) | |
| BY4742 | MATα his3Δ1 leu2Δ0 lys2Δ0 ura3Δ0 MET15 | Brachmann (et al. 1998) | |
| BY4743 | MATa/α his3Δ1 leu2Δ0 lys2Δ0/LYS2 MET15/met15Δ0 ura3Δ0 | Brachmann (et al. 1998) | |
| yLM422 | MATα his3Δ1 leu2Δ0 lys2Δ0 ura3Δ0 MET15 synIII HO::synSUP61 hmlΔ hmrΔ | Annaluru (et al. 2014) | |
| yXZX621 | MATa his3Δ1 leu2Δ0 lys2Δ0 ura3Δ0 MET15 synIII HO::synSUP61 hmlΔ hmrΔ | yLM422 | This study |
| yXZX716 | MATa/α his3Δ1 leu2Δ0 lys2Δ0 ura3Δ0 MET15 synIII-272123bp/synIII-272123bp HO::synSUP61/HO::synSUP61 | yLM422 x yXZX621 | This study |
| yXZX880 | MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 LYS2 ring_wtIII ring_wtIX | BY4741 | This study |
| yXZX958 | MATα his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 LYS2 ring_wtIII ring_wtIX | yXZX880 | This study |
| yXZX959 | MATa/α his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 LYS2 ring_wtIII/ring_wtIII ring_wtIX/ring_wtIX | yXZX880 x yXZX958 | This study |
| yXZX512 | MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 LYS2 synV | Xie (et al. 2017) | |
| yXZX543 | MATα his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 LYS2 synV | Xie (et al. 2017) | |
| yXZX547 | MATα his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 LYS2 synV pGAL1-CEN10::Kl.URA3 | yXZX543 | This study |
| yYW0117 | MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 LYS2 synX HO::tR(CCU)J | Wu (et al. 2017) | |
| yYW0119 | MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 LYS2 synX pGAL1-CEN5::Kl.URA3 HO::tR(CCU)J | yYW0117 | This study |
| yXZX558 | MATa/α his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 LYS2 synV/wtV synX/wtX syn-CEN5/pGAL1-CEN5::Kl.URA3 syn-CEN10/pGA1L-CEN10::Kl.URA3 HO::tR(CCU)J/HO | yXZX547 x yYW0119 | This study |
| yXZX572 | MATa/α his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 LYS2 synV synX HO::tR(CCU)J/HO | yXZX558 | This study |
| yXZX573 | MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 LYS2 synV synX HO::tR(CCU)J | yXZX572 | This study |
| yXZX625 | MATα his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 LYS2 synV synX HO::tR(CCU)J | yXZX573 | This study |
| yXZX973 | MATa/a his3Δ1 leu2Δ0 lys2Δ0/LYS2 MET15/met15Δ0 ura3Δ0 | BY4743 | This study |
| yXZX974 | MATα/α his3Δ1 leu2Δ0 lys2Δ0/LYS2 MET15/met15Δ0 ura3Δ0 | BY4743 | This study |
| yXZX975 | MATa/a his3Δ1 leu2Δ0 lys2Δ0 ura3Δ0 MET15 synIII/synIII HO::synSUP61/HO::synSUP61 | yXZX716 | This study |
| yXZX976 | MATα/α his3Δ1 leu2Δ0 lys2Δ0 ura3Δ0 MET15 synIII/synIII HO::synSUP61/HO::synSUP61 | yXZX716 | This study |
| yXZX979 | MATa/a/α/α his3Δ1 leu2Δ0 lys2Δ0/LYS2 MET15/met15Δ0 ura3Δ0 | yXZX973 x yXZX974 | This study |
| yXZX980 | MATa/a/α/α his3Δ1 leu2Δ0 lys2Δ0 ura3Δ0 MET15 synIII/synIII/synIII/synIII HO::synSUP61/HO::synSUP61 | yXZX975 x yXZX976 | This study |
A plasmid encoding the Cas9 gene, pNA0306 (pRS415-TEF1p-Cas9), was modified from Addgene plasmid #43802. The plasmid expressing one gRNA, pNA0304 (pRS426-SNR52p-gRNA-SUP4t), was modified from Addgene plasmid #43803. The plasmid expressing two gRNAs simultaneously was pNA0308 (p426-SNR52p-gRNA-SUP4t-pRPR1-gRNA-RPR1t) (Table 2). Phusion DNA polymerase and restriction enzymes were purchased from New England Biolabs.
Table 2. Plasmids used in this study.
| Plasmid No. | Plasmid Name | Description | Yeast Marker | Escherichia Marker | RE* | Source | Addgene ID |
|---|---|---|---|---|---|---|---|
| YCplac33-GAL2p-HO-KanMX4 | pGAL2-HO plasmid | KanMX4, URA3 | Amp | N/A | Hou (et al. 2013) | ||
| pRS426 | Yeast episomal vector | URA3 | Amp | N/A | Christianson (et al. 1992) | ||
| pXZX010 | pRS426-GAL2p-HO-KanMX4 | pGAL2-HO plasmid | KanMX4, URA3 | Amp | N/A | This study | |
| pNA0306 | pRS415-TEF1p-Cas9-CYC1t | Cas9 expression plasmid | LEU2 | Amp | N/A | DiCarlo (et al. 2013) | #43802 |
| pNA0304 | pRS426-SNR52p-gRNA-SUP4t | Backbone plasmid for gRNA insertion | URA3 | Amp | N/A | DiCarlo (et al. 2013) | #43803 |
| pNA0308 | pRS426-SNR52p-gRNA-SUP4t-RPR1p-gRNA-RPR1t | Double gRNA backbone plasmid | URA3 | Amp | N/A | This study | |
| pXZX339 | pRS426-SNR52p-gRNA.MATa_option1-SUP4t | 20-bp gRNA sequence targeting MATa cassette | URA3 | Amp | N/A | This study | |
| pXZX388 | pRS426-SNR52p-gRNA.MATa_option2-SUP4t | 20-bp gRNA sequence targeting MATa cassette | URA3 | Amp | N/A | This study | |
| pXZX501 | pRS426-SNR52p-gRNA.MATa_option3-SUP4t | 20-bp gRNA sequence targeting MATa cassette | URA3 | Amp | N/A | This study | |
| pXZX351 | pRS426-SNR52p-gRNA.MATα_option1-SUP4t | 20-bp gRNA sequence targeting MATα cassette | URA3 | Amp | N/A | This study | |
| pXZX503 | pRS426-SNR52p-gRNA.MATα_option2-SUP4t | 20-bp gRNA sequence targeting MATα cassette | URA3 | Amp | N/A | This study | |
| pXZX504 | pRS426-SNR52p-gRNA.MATα_option3-SUP4t | 20-bp gRNA sequences targeting MATα cassette | URA3 | Amp | N/A | This study | |
| pXZX352 | pTOPO-MATa | MATa fragment in pTOPO vector | — | Kan | NsiI | This study | |
| pXZX353 | pTOPO-MATα | MATα fragment in pTOPO vector | — | Kan | NsiI | This study | |
| pXZX448 | pRS426-SNR52p-gRNA.proto_chr3_L-SUP4t-RPR1p-gRNA-RPR1t | 20-bp gRNA sequence targeting left end of chromosome III | URA3 | Amp | N/A | This study | |
| pXZX406 | pRS426-SNR52p-gRNA.proto_chr3_L-SUP4t-RPR1p-gRNA.proto_chr3_R-RPR1t | 20-bp gRNA sequences targeting both left and right ends of chromosome III | URA3 | Amp | N/A | This study | |
| pLM182 | pGAL1-CEN5-Kl.URA3 | Kl.URA3 | Amp | Not I | Reid (et al. 2008) | ||
| pLM187 | pGAL1-CEN10-Kl.URA3 | Kl.URA3 | Amp | Not I | Reid (et al. 2008) |
RE, restriction enzyme
The GAL2p-HO-KanMX4 cassette was polymerase chain reaction (PCR) amplified from plasmid YCplac33-GHK with primers oXZX011 and oXZX012, which had 40-bp overlaps identical to the ends of EcoRI-digested plasmid pRS426 (Hou et al. 2013; Lin et al. 2015). pXZX010 was assembled in yeast by cotransforming gel-purified EcoRI-digested pRS426 and the GAL2p-HO-KanMX4 PCR amplicon, followed by selection on synthetic complete medium lacking uracil (SC–Ura) (Table 2 and Table 3).
Table 3. Primers used in this study.
| Number | Sequence |
|---|---|
| oXZX011 | GCCCCCCCTCGAGGTCGACGGTATCGATAAGCTTGATATCTATTACATTTACTGAGCATAACGGGC |
| oXZX012 | TGGCGGCCGCTCTAGAACTAGTGGATCCCCCGGGCTGCAGGCAGTATCGATCGACAGCAGT |
| oXZX292 | ACGATAACTGGTTGGAAAGCGTAA |
| oXZX293 | AGACTTGTGGCGAAGATGAATAGT |
| oXZX673 | CTCACCCGAAAGAGATGCTG |
| oXZX674 | GTTCTGTATAGTACATCCGCGCACATTCTTCCTAGCGGAAAGTCTTTACCGACGCTTGTG |
| oXZX675 | TGTGTCGTACTTTTTATTTTCACAAGCGTCGGTAAAGACTTTCCGCTAGGAAGAATGTGC |
| oXZX676 | TGCTATTGCCTATGTTCGCTC |
| oXZX677 | TATGGACACAAACCATGCCTAACC |
| oXZX678 | GCGCCTCCGAACAAACAGATG |
| oLM651 | CGCCTCCGCAACTTCCTG |
| oLM178 | TCAGCAATTTCGATGCAACCGG |
| oXZX356 | TTTGTAGTTTGCGTCTTGTTCCG |
Construction of donor DNA containing plasmids
Oligonucleotides oXZX292 and oXZX293 were used to amplify a 3293-bp MATa cassette donor DNA fragment from BY4741 genomic DNA and a 3398-bp MATα cassette donor DNA fragment from BY4742 genomic DNA (Table 3). The PCR products, after gel purification, were subcloned into a pCR-Blunt II-TOPO vector (45-0245; Invitrogen/Life Technologies, Carlsbad, CA) to generate the donor DNA carrying plasmids of pXZX352 (pTOPO-MATa) and pXZX353 (pTOPO-MATα). These two plasmids can be transformed into the yeast for mating-type switching directly upon NsiI digestion, which excises the inserts.
Construction of gRNA expression plasmids
The 20-bp protospacers were designed in the Ya or Yα regions that precede the requisite protospacer-adjacent motif (PAM) to generate a specific DSB at MATa or MATα (Figure 1B). The gRNA expression plasmid pNA0304 was digested by NotI and gel purified. Subsequently, the 20-bp gRNA cassettes of MAT locus, produced by annealing two single-strand oligonucleotides containing a 20-bp gRNA fragment and flanked by 20-bp overlaps identical to the backbone plasmid at the cut site, were assembled to the linearized backbone plasmid by using NEB Gibson Assembly Master Mix (E2611; New England BioLabs, Beverly, MA) (Figure 1C and Table 2) (Gibson 2011). All oligonucleotides were purchased from Integrated DNA Technologies (Coralville, IA) and Genewiz (Beijing, China).
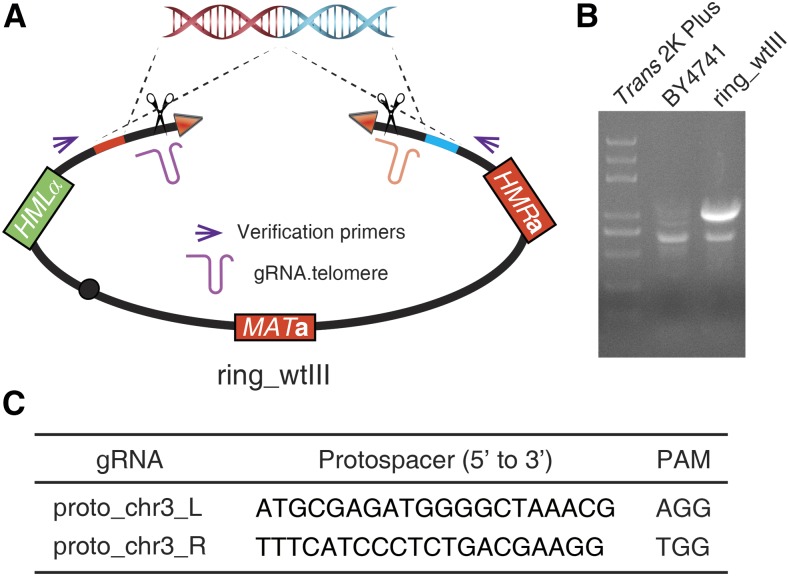
Cleavage at the ends of chromosome III was generated by a double-gRNA expression plasmid, which was constructed by inserting two 20-bp protospacers into the double-gRNA backbone plasmid pNA0308 in two steps. First, the protospacer sequence “proto_chr3_L” was Gibson assembled into NotI-digested pNA0308 to obtain the intermediate plasmid pXZX448. Second, the protospacer sequence “proto_chr3_R” was Gibson assembled into the HindIII-digested pXZX448 to result in the double-gRNA expression plasmid pXZX406.
Ring_wtIII chromosome construction
A MATa ring chromosome III derivative, ring_wtIII (yXZX880), was cyclized using a homologous recombination (HR) strategy by deleting the telomere ends of native chromosome III, wtIII, and DSBs were generated by cleaving the two subtelomeric regions with CRISPR/Cas9 (Figure 2A and Table 1). Ring_wtIII encodes all genes present on parental wtIII, including HMLα and HMRa, except for deletions of 6275 bp on the left end and 1285 bp on the right end of wtIII, which was confirmed by the appearance of a PCR amplicon of oXZX677 and oXZX678, spanning the extreme chromosome termini (Figure 2, B and C and Table 3). The strain carrying MATα ring_wtIII (yXZX958) was obtained by switching the mating-type of yXZX880 using the method described here (Table 1). Thus, this “designer” ring chromosome is distinct from previously isolated ring III chromosomes, which had many deleted genes (Haber et al. 1980).
Figure 2.
Construction and verification of ring_wtIII chromosome. (A) Circularization of wtIII yielded a designer version of ring_wtIII (yXZX880) without removal of HMLα and HMRa. (B) PCR verification of ring_wtIII. (C) Protospacer and PAM sequences for wtIII cyclization. Note that the ring_wtIII structure constructed here is distinct in sequence from ring chromosomes isolated by classical methods by Haber et al. (1980).
The donor DNA fragment for chromosome III cyclization via HR was obtained by an overlapping-extension PCR strategy. First, the left homologous arm was PCR amplified from genomic DNA of BY4741 using primers oXZX673 and oXZX674, and the right homology arm was amplified from genomic DNA of BY4741 using primers oXZX675 and oXZX676. There was an 80-bp overlap between the two homologous arms. Second, left and right homologous fragments, after gel purification, were extended and amplified by using primers oXZX673 and oXZX676 (Table 3).
Endoreduplication intercross
As described elsewhere, the “endoreduplication intercross” strategy is a powerful means to consolidate chromosomes and build polysynthetic yeast strains (Mitchell et al. 2017; Richardson et al. 2017). In such a cross, native chromosomes are modified by inserting a GAL1 promoter, capable of destabilizing an adjacent centromere, together with a URA3 gene, which can be selected against using 5-fluoroorotic acid (5-FOA). After the strains are crossed, induction of the GAL1 promoter leads to destabilization of the native chromosomes and yields hemizygotes for the corresponding synthetic chromosomes, which are capable of endoreduplication to generate a 2n state and can be sporulated. We utilized the rapid mating-type switching method described here combined with an endoreduplication intercross to consolidate synV and synX in a single cell.
First, haploid yeast strains with conditional centromeres were constructed by integrating the CEN-conditional DNA segment, gel-purified NotI-digested pLM182 (pGAL1-CEN5) or pLM187 (pGAL1-CEN10) (Reid et al. 2008), into the corresponding native chromosome. The integration of CEN-conditional V was identified by primers oLM651 and oLM178, and the integration of CEN-conditional X was identified by primers oXZX356 and oLM178. Second, the synV strain carrying CEN-conditional X and the synX strain carrying CEN-conditional V were mated to generate a heterozygous diploid synthetic strain (synV/wtV synX/wtX), which was induced with galactose to destabilize the conditional native chromosomes. Third, the loss of CEN-conditional chromosomes was selected on 5-FOA medium and confirmed with PCRTag analysis. Fourth, sporulation and dissection were carried out to build the haploid MATa synthetic strain (synV/synX, yXZX573). Subsequently, the derived MATα synV/synX strain yXZX625 was produced using the method described here.
Yeast transformation of plasmids with donor DNA
Before transformation, 1 µg of MAT cassette carrying plasmid (pTOPO-MATa or pTOPO-MATα) was digested with NsiI in a final volume of 10 μl, and the whole digestion product was used directly for yeast transformation along with ∼200 ng of Cas9 expression plasmid (pNA0306) and ∼200 ng of gRNA expression plasmid (pXZX352 or pXZX353). Yeast transformation was carried out using a modified lithium acetate transformation method as described elsewhere (Mitchell et al. 2015).
To switch MATa to MATα, the digestion product of pXZX353, gRNA expression plasmid pRS426-SNR52p-gRNA.MATa-SUP4t and Cas9 expression plasmid pNA0306 were cotransformed, while the digestion product of pXZX352, gRNA expression plasmid pRS426-SNR52p-gRNA.MATα-SUP4t and Cas9 expression plasmid pNA0306 were cotransformed to switch MATα to MATa. For each transformation, following a primary selection on SC–Ura–Leu plates for 2 d at 30°, transformants with the opposite mating-type were verified by replica plating to lawns of the tester strains.
During evaluation of mating-type switching efficiency, we first transformed the Cas9 expression plasmid into the strain and subsequently transformed the gRNA expression plasmid and donor MAT locus into the Cas9-expressing cells.
Mating-type switching by pRS426-GHK
Galactose-induced HO plasmid pXZX010 (pRS426-GAL2p-HO-KanMX4) was used to test the mating-type switching efficiency by modifying the described method (Herskowitz and Jensen 1991). One single colony of pXZX010 was picked from a SC–Ura plate and inoculated into 3 ml of SC–Ura medium at 30° for 24 hr. Subsequently, cells were harvested and washed three times with sterile water, followed by inoculation in 3 ml SC–Ura + 2% glycerol medium dextrose glycerol overnight at 30°. The pellets were spun down, washed three times with sterile water, and resuspended in 3 ml SC–Ura 2% galactose medium to an A600 = 0.6–1.0 for 5 hr at 30°. A total of 200 µl of culture was removed after growth for 2, 3, 4, and 5 hr and samples were stored on ice. Aliquots were combined after 5 hr. After washing with sterile water, cultures were diluted and plated on yeast extract peptone dextrose (YPD) to obtain 100–200 colonies per plate and grown at 30° for 2 d.
Mating-type test
Yeast mating-type determination was carried out by crossing the transformants with tester strains 17a and 14α, both carrying the his1 auxotrophic marker. Lawns of tester strains were prepared by spreading ∼500 μl overnight YPD culture of tester strains on YPD agar plates and incubating at 30° for 2 d. The lawns and transformant colony plates were replica plated onto a fresh YPD plate at the same time. After incubating at 30° overnight, a second replica plating onto synthetic dextrose (SD) medium was performed and the plates were incubated at 30° to reflect mating response and scored the next day. Haploid strains and MATa/a or MATα/α diploid strains should form a patch on just one of the two tester strains if the tester strain is of the opposite mating-type. MATa/α strains are nonmaters and will not form a prototrophic patch with either tester strain. Transformants able to mate with both an a lawn and an α lawn were scored as “bimater”.
Plasmid loss
A single colony with verified mating-type was inoculated into 5 ml of YPD medium at 30° overnight to saturation. Then, 100 μl of cultures with 10−5 dilutions were plated on a SC + 5-FOA plate and incubated at 30° for 2 d, following replica plating onto a SC–Leu plate. The plasmid-free colonies can grow on SC + 5-FOA plates but not on SC–Leu plates.
Data availability
The authors state that all data necessary for confirming the conclusions presented in the article are represented fully within the article.
Results and Discussion
Cas9-gRNA expression leads to highly efficient mating-type switching
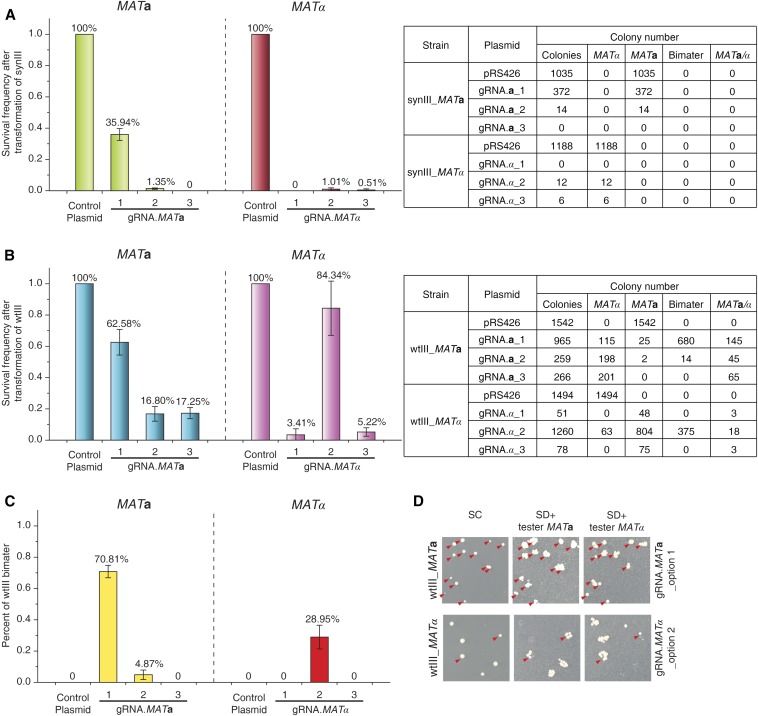
When a DSB is introduced at MAT in cells lacking the donor sequences HMLα and HMRa, for instance, by endogenous HO endonuclease or by CRISPR/Cas9, in principle all cells should die because the DSB cannot be repaired by HR (Klar et al. 1984). SynIII strains lack the silent cassettes (Annaluru et al. 2014) such that cleavage at MAT cannot be repaired by HMLα or HMRa, and mating-type switching absolutely requires a donor molecule. An optimal gRNA to direct mating-type switching should generate a DSB at MAT that promotes HR with minimal off-target effects. A strain carrying synIII represents the ideal genetic background to identify optimal gRNAs, since following formation of a DSB all cells should die; the surviving colonies could result from mutant sites refractive to DSB or perhaps off-target HR effects. To identify an optimal gRNA for mating-type switching, in synIII cells we tested three independent gRNA sequences that directed Cas9-mediated breaks to different positions within MATa or MATα, specifically within the Ya or Yα regions (Figure 1, B and C). We counted the number of surviving colonies relative to a strain transformed with a plasmid lacking the gRNA, reporting a “survival frequency.” We also tested the mating-type of surviving colonies by replica-plating, using specific tester strains of known mating-type and carrying a specific auxotrophic marker complemented by mating (Trueheart et al. 1987). We observed a very low survival frequency of ∼2% following transformation of gRNA.MATa_option 2 or 3 into MATa derivative synIII cells. On the other hand, we observed a nearly 30% survival frequency for gRNA.MATa_option 1. The mating-type of the surviving cells for all three gRNAs was a. In the synIII strain encoding MATα and using gRNA.MATα_option 2 or 3, we recovered ∼1% the number of colonies relative to an empty vector lacking gRNA. For gRNA.MATα_option 1, no colonies survived. The mating-type of the surviving colonies was all α (Figure 3A).
Figure 3.
Selection of optimal gRNAs for efficient mating-type switching. (A) Left bar graph shows survival frequency of synIII strains (yXZX621 and yLM422) after transformation of gRNA plasmids without donor DNA. Table indicates analysis of colony types. MATa/α genotype is deduced from nonmater phenotype. Survival frequency is defined as the percentage of surviving colonies recovered following transformation of a gRNA relative to an empty plasmid. (B) Same as (A) but with wtIII strains (BY4741 and BY4742). (C) Percentage of wtIII bimaters after transformation of gRNA plasmid without donor DNA. (D) Bimating phenotype of CRISPR/Cas9-mediated mating-type switching. Red triangle indicates bimater. Control plasmid is pRS426. Values are averages from three experiments, and error bars denote standard deviation SC, synthetic complete medium.
We also carried out this experiment in a wtIII strain, expecting that gene conversion templated by HMLα or HMRa would yield viable colonies and that the survival frequency would be high. Notably, after MAT locus conversion, the PAM sequence targeted by either gRNA is absent, preventing ongoing Cas9 cleavage at MAT. We counted the number of surviving colonies and evaluated their mating type. In the wtIII strain of MATa and using gRNA.MATa_option 2 or 3, we observed a survivor frequency of 17%. Interestingly, we recovered a relatively high survival frequency in the MATa derivative wtIII strain using gRNA.MATa_option 1 (62.58%). Among the surviving cells of gRNA.MATa_option 1, ∼70% were bimaters and only ∼12% were switched to α. On the other hand, for gRNA.MATα_option 1 or 3, frequency of colonies was ∼5% relative to an empty vector lacking gRNA. The mating-type of these colonies was all α. In the wtIII strain encoding MATα, we recovered ∼84% the number of colonies relative to an empty vector lacking gRNA, after transformation of gRNA.MATα_option 2, in which ∼29% of surviving colonies were bimaters and ∼64% were switched to MATa (Figure 3, B–D).
In summary, based on the best combination of low survival frequency in the synIII background, lack of bimaters in a wtIII background, and a high switching efficiency, we selected gRNA.MATa_option 3 for mating-type switching from a to α, and gRNA.MATα_option 1 for switching from α to a for all future work. The nature of the bimater phenotype observed in the wild-type background with less effective gRNAs is not clear to us at this time, but we note that (a) this phenomenon only occurs in the presence of silent cassettes and thus may result from low-level cutting at the HM loci, and (b) bimater formation is correlated with high levels of survival, suggesting relatively inefficient cutting by these gRNAs. By focusing on the two above optimal gRNAs, this class of transformants is minimized.
Enhancing mating-type switching efficiency of haploid strains
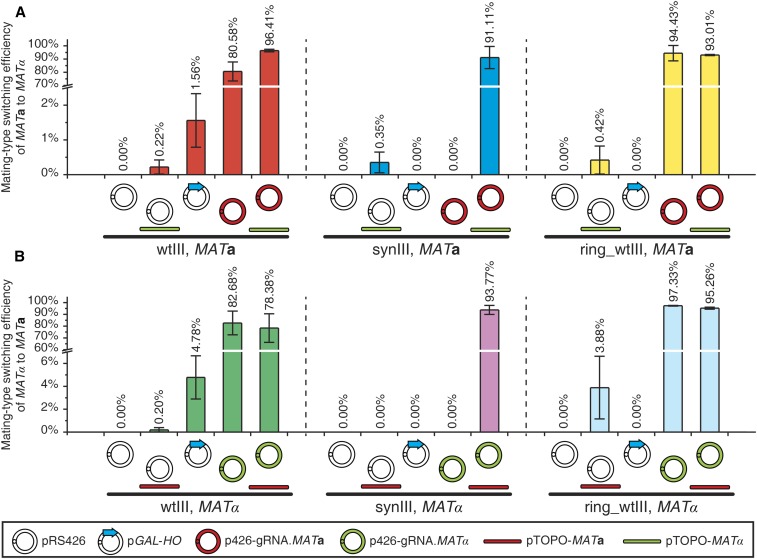
To evaluate mating-type switching efficiency in haploids using the optimal gRNAs, three different types of haploid strains were selected, wtIII, synIII, and ring_wtIII. As described before, haploid strains carrying synIII lack HMLα and HMRa cassettes, and no transformants with the opposite mating-type are recovered in the absence of a cotransformed donor DNA segment. Thus, the donor DNA segment is essential for mating-type switching in synIII strains. When the donor DNA and gRNA plasmid were cotransformed into synIII strains, ∼91% of the surviving colonies were switched to the mating-type of interest regardless of the initial mating type, whereas only a small percentage of switched colonies were recovered by inducing the GAL2p-HO plasmid. Notably, mating-type switched colonies via the GAL2p-HO system were recovered about five times more efficiently when switching from α to a than from a to α, a further practical limitation of the GAL-based system. For the haploid strains carrying wtIII and ring_wtIII, each encoding HMLα and HMRa, ∼80–98% of viable colonies were switched in the absence of donor DNA, providing gRNA and Cas9 expression plasmid only. To test whether providing donor DNA for HR would improve the mating-type switching efficiency in strains encoding the HMLα and HMRa cryptic mating loci, we cotransformed donor DNA with Cas9 and gRNA to strains carrying wtIII or ring_wtIII; no significant improvement was observed (Figure 4, A and B).
Figure 4.
Efficiency of CRISPR/Cas9-mediated mating-type switching. (A) Mating-type switching efficiency of haploid strains from MATa to MATα. (B) Mating-type switching efficiency of haploid strains from MATα to MATa. Error bars are as in Figure 3.
Building the haploid yeast with polysynthetic chromosomes
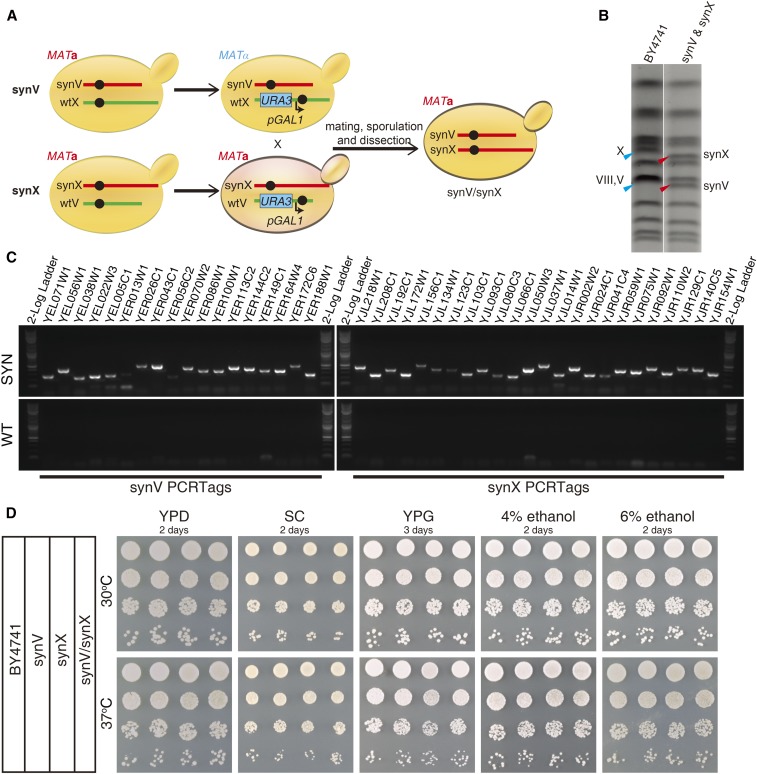
synV and synX are two of the large synthetic yeast chromosomes, with a total length of ∼1.2 Mb, corresponding to 10% of the Sc2.0 genome (Richardson et al. 2017; Wu et al. 2017; Xie et al. 2017). We transplanted and consolidated the two chromosomes into a haploid polysynthetic strain, synV/synX (yXZX573), by coupling the CRISPR/Cas9-mediated mating-type switching method with the endoreduplication intercross strategy (Figure 5A). CEN-conditional V and CEN-conditional X native chromosomes were successfully destabilized to generate a 2n − 2 diploid.
Figure 5.
Construction and characterization of polysynthetic haploid strain synV/synX. (A) The mating-type of the synV strain was switched to MATα with the CRISPR/Cas9-mediated strategy. Next, the pGAL1-CEN10-Kl.URA3 construct was integrated into the wtX chromosome of synV strain, the pGAL1-CEN5-Kl.URA3 construct was integrated into the wtV chromosome of synX strain, and the two strains were mated. The diploid strain was induced in galactose to destabilize the native chromosomes and confirmed by selection on FOA medium followed by PCRTag analysis. The final haploid polysynthetic chromosome strain synV/synX was generated by sporulation and dissection. Red indicates synthetic chromosome and green indicates native chromosome. (B) PFGE of synV and synX chromosomes. Red triangle indicates synthetic chromosome and blue triangle indicates wild-type chromosome. (C) PCRTag analysis (one PCRTag per ∼30 kb) of the polysynthetic haploid strain synV/synX. (D) Phenotypic analysis of synV, synX, synV/synX, and native (BY4741) strains under different conditions. SC, synthetic complete medium; SYN, synthetic PCRTags; YPD, yeast extract peptone dextrose; YPG, yeast extract peptone glycerol; WT, wild-type PCRTags.
No significant sporulation defect was observed in the synV/synX diploid, suggesting that the combination of the two synthetic chromosomes was sufficient to direct meiosis. Both four viable spore and two viable spore tetrads were observed. The four viable spores were consistent with the endoreduplication of two synthetic chromosomes prior to sporulation, and the two viable spore tetrads suggested the two synthetic chromosomes had not yet endoreduplicated (Mitchell et al. 2017; Richardson et al. 2017). Since tR(CCU)J, a single copy tRNA gene, was relocated from synX to the HO locus on chromosome IV in the synX strain (Wu et al. 2017), the expected ratio between the slow growth (sick) phenotype and normal growth phenotype is 2:2 in the four viable spore tetrads. On average it was 1:1 in the two viable spore tetrads; both results are as expected.
The size alterations of synV and synX were demonstrated by means of pulsed-field gel electrophoresis (PFGE) (Figure 5B). The genotype of synV/synX was characterized by PCR using both synthetic (SYN) and wild-type (WT) PCRTag primer pairs. The presence of SYN amplicons and absence of WT amplicons revealed the successful isolation of the polysynthetic haploid strain (Figure 5C). To evaluate the fitness of the polysynthetic strain, we examined the phenotype of synV, synX, synV/synX and the native strain (BY4741) under various conditions. No detectable differences were found in growth between the polysynthetic haploid strain and the native strain under most conditions (Figure 5D). The MATα derivative synV/synX strain yXZX625 was produced using the method described here.
Mating-type switching of diploids and construction of tetraploids
Polyploid and aneuploid strains are widely used in industrial manufacturing of chemicals, biofuels, and vaccines (Bidenne et al. 1992; Pfau and Amon 2012). To provide an efficient strategy to make polyploid strains of defined genotype, we evaluated mating-type switching efficiency in diploids and used CRISPR-Cas9 to construct tetraploids.
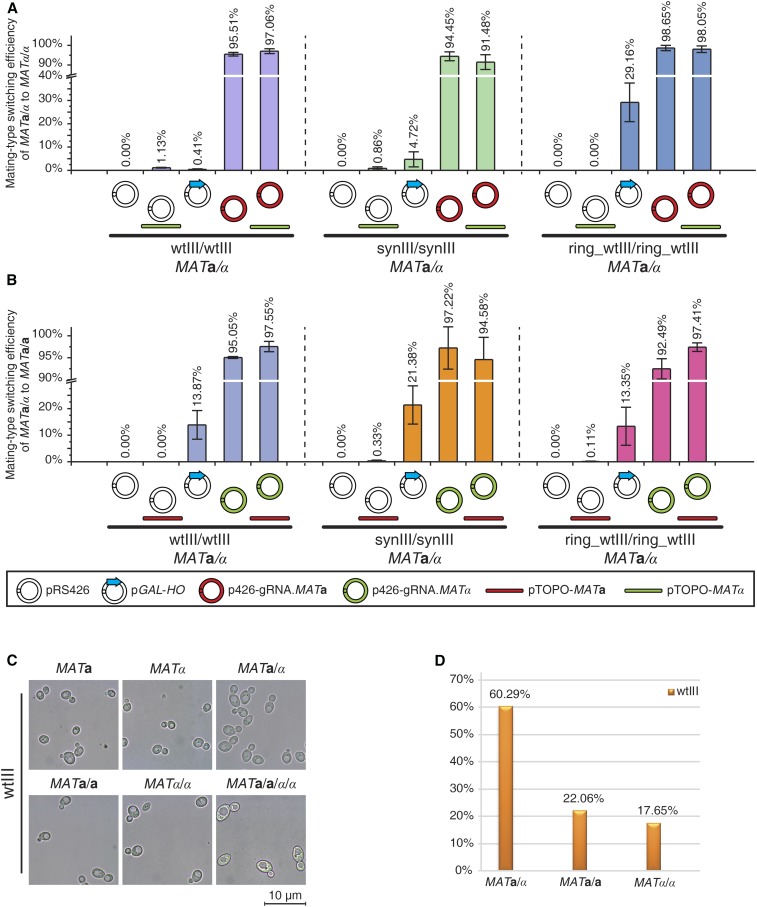
Since the two gRNAs, gRNA.MATa and gRNA.MATα, specifically target the MATa or MATα locus, respectively, the CRISPR/Cas9-mediated mating-type switching method should work even in MATa/α diploid strains, a situation in which native HO is inactive. We attempted to switch the mating-type of MATa/α diploid strains to MATa/a and MATα/α and measure switching efficiency in three kinds of MATa/α strains: wtIII/wtIII, synIII/synIII, and ring_wtIII/ring_ wtIII. Since the MAT locus on the second homolog serves as a donor for HR, >90% of surviving cells were switched to MATa/a or MATα/α, without transforming donor DNA. Similar to the case of haploid strains, donor DNA cotransformation did not improve the mating-type efficiency notably (Figure 6, A and B).
Figure 6.
Mating-type switching of diploids and construction of tetraploid strains. (A) Mating-type switching efficiency of diploid strains from MATa/α to MATα/α. (B) Mating-type switching efficiency of diploid strains from MATa/α to MATa/a. (C) Morphology analysis of haploid, diploid, and tetraploid strains constructed with the method described here. (D) Tetrad analysis of tetraploids. The results are from 17 tetrads with four viable spores. Error bars are as in Figure 3.
Mating of diploids with MATa/a and MATα/α produced isogenic tetraploids (yXZX979 and yXZX980); as expected, these demonstrated clearly larger cell size than the parental diploids (Figure 6C). After sporulation, we dissected 20 tetrads from yXZX979, and 17 of them produced four viable spores. When the mating-types of the resulting spores from the 17 tetrads was tested, 60% were nonmaters, 22% showed a mating-type a, and 18% showed a mating-type α, consistent with expectations (Figure 6D). The nonmating spores were inferred to be MATa/α, because they were capable of sporulation and produced tetrads with two MATa and two MATα spores.
Conclusions
Given the site-specificity and high efficiency of the CRISPR/Cas9 system, we describe a rapid, time-saving and highly efficient method to switch the mating-type of S. cerevisiae that is independent of both HMLα/HMRa and HO. We evaluated the gRNAs by minimizing the bimating phenotype and used this and the survival frequency to identify optimal gRNAs for this purpose. By using the CRISPR/Cas9-mediated mating-type switching method, a haploid strain of the opposite mating-type was isolated after a single cotransformation without the need for induction of HO expression, or downstream sporulation and tetrad dissection, although an isogenic diploid strain MATa/α may also be isolated directly. We constructed a polysynthetic haploid strain carrying synV and synX chromosomes, and built an isogenic set of strains carrying synIII, synV, and synV/synX using the method described here. This method will help promote completion of the synthetic genome of S. cerevisiae. Finally, MATa/α diploid strains were also switched to MATa/a and MATα/α diploids with this method, and were further used to construct isogenic tetraploids. By repeating this process, construction of an isogenic polyploid series is possible.
Acknowledgments
We thank Rodney Rothstein for the generous contribution of the set of centromere destabilizing constructs, originally developed in his laboratory, to the Sc2.0 consortium. We dedicate this paper to the memory of Amar Klar. The work in China was supported by the Ministry of Science and Technology of China (grants 2014CB745100 and 2015DFA00960), and the National Natural Science Foundation of China (grants 21390203 and 21621004). The work in the United States was supported by National Science Foundation grants MCB-1026068 and MCB-1158201 to J.D.B.
Footnotes
Communicating editor: J. Rine
Literature Cited
- Annaluru N., Muller H., Mitchell L. A., Ramalingam S., Stracquadanio G., et al. , 2014. Total synthesis of a functional designer eukaryotic chromosome. Science 344: 55–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidenne C., Blondin B., Dequin S., Vezinhet F., 1992. Analysis of the chromosomal DNA polymorphism of wine strains of Saccharomyces cerevisiae. Curr. Genet. 22: 1–7. [DOI] [PubMed] [Google Scholar]
- Brachmann C. B., Davies A., Cost G. J., Caputo E., Li J., et al. , 1998. Designer deletion strains derived from Saccharomyces cerevisiae S288C: a useful set of strains and plasmids for PCR-mediated gene disruption and other applications. Yeast 14: 115–132. [DOI] [PubMed] [Google Scholar]
- Christianson T. W., Sikorski R. S., Dante M., Shero J. H., Hieter P., 1992. Multifunctional yeast high-copy-number shuttle vectors. Gene 110: 119–122. [DOI] [PubMed] [Google Scholar]
- DiCarlo J. E., Norville J. E., Mali P., Rios X., Aach J., et al. , 2013. Genome engineering in Saccharomyces cerevisiae using CRISPR-Cas systems. Nucleic Acids Res. 41: 4336–4343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson D. G., 2011. Enzymatic assembly of overlapping DNA fragments. Methods Enzymol. 498: 349–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber J. E., 1998. Mating-type gene switching in Saccharomyces cerevisiae. Annu. Rev. Genet. 32: 561–599. [DOI] [PubMed] [Google Scholar]
- Haber J. E., 2012. Mating-type genes and MAT switching in Saccharomyces cerevisiae. Genetics 191: 33–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber J. E., Rogers D. T., McCusker J. H., 1980. Homothallic conversions of yeast mating-type genes occur by intrachromosomal recombination. Cell 22: 277–289. [DOI] [PubMed] [Google Scholar]
- Hawthorne D. C., 1963. A deletion in yeast and its bearing on the structure of the mating type locus. Genetics 48: 1727–1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herskowitz I., Jensen R. E., 1991. Putting the HO gene to work: practical uses for mating-type switching. Methods Enzymol. 194: 132–146. [DOI] [PubMed] [Google Scholar]
- Hou L., Li X., Wang C., Cao X., Wang H., 2013. Construction of ploidy series of Saccharomyces cerevisiae by the plasmid YCplac33-GHK. J. Ind. Microbiol. Biotechnol. 40: 393–397. [DOI] [PubMed] [Google Scholar]
- Klar A. J., Strathern J. N., Abraham J. A., 1984. Involvement of double-strand chromosomal breaks for mating-type switching in Saccharomyces cerevisiae. Cold Spring Harb. Symp. Quant. Biol. 49: 77–88. [DOI] [PubMed] [Google Scholar]
- Kostriken R., Strathern J. N., Klar A. J., Hicks J. B., Heffron F., 1983. A site-specific endonuclease essential for mating-type switching in Saccharomyces cerevisiae. Cell 35: 167–174. [DOI] [PubMed] [Google Scholar]
- Lin Q., Jia B., Mitchell L. A., Luo J., Yang K., et al. , 2015. RADOM, an efficient in vivo method for assembling designed DNA fragments up to 10 kb long in Saccharomyces cerevisiae. ACS Synth. Biol. 4: 213–220. [DOI] [PubMed] [Google Scholar]
- Mitchell L. A., Chuang J., Agmon N., Khunsriraksakul C., Phillips N. A., et al. , 2015. Versatile genetic assembly system (VEGAS) to assemble pathways for expression in S. cerevisiae. Nucleic Acids Res. 43: 6620–6630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell L. A., Wang A., Stracquadanio G., Kuang Z., Wang X., et al. , 2017. Synthesis, debugging, and effects of synthetic chromosome consolidation: synVI and beyond. Science 355: eaaf4831. [DOI] [PubMed] [Google Scholar]
- Nickoloff J. A., Chen E. Y., Heffron F., 1986. A 24-base-pair DNA sequence from the MAT locus stimulates intergenic recombination in yeast. Proc. Natl. Acad. Sci. USA 83: 7831–7835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfau S. J., Amon A., 2012. Chromosomal instability and aneuploidy in cancer: from yeast to man. EMBO Rep. 13: 515–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid R. J., Sunjevaric I., Voth W. P., Ciccone S., Du W., et al. , 2008. Chromosome-scale genetic mapping using a set of 16 conditionally stable Saccharomyces cerevisiae chromosomes. Genetics 180: 1799–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson S. M., Mitchell L. A., Stracquadanio G., Yang K., Dymond J. S., et al. , 2017. Design of a synthetic yeast genome. Science 355: 1040–1044. [DOI] [PubMed] [Google Scholar]
- Rine J., Herskowitz I., 1987. Four genes responsible for a position effect on expression from HML and HMR in Saccharomyces cerevisiae. Genetics 116: 9–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell D. W., Jensen R., Zoller M. J., Burke J., Errede B., et al. , 1986. Structure of the Saccharomyces cerevisiae HO gene and analysis of its upstream regulatory region. Mol. Cell. Biol. 6: 4281–4294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strathern J. N., Klar A. J. S., Hicks J. B., Abraham J. A., Ivy J. M., et al. , 1982. Homothallic switching of yeast mating type cassettes is initiated by a double-stranded cut in the MAT locus. Cell 31: 183–192. [DOI] [PubMed] [Google Scholar]
- Takahashi T., Saito H., Ikeda Y., 1958. Heterothallic behavior of a homothallic strain in Saccharomyces yeast. Genetics 43: 249–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trueheart J., Boeke J. D., Fink G. R., 1987. Two genes required for cell fusion during yeast conjugation: evidence for a pheromone-induced surface protein. Mol. Cell. Biol. 7: 2316–2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Li B. Z., Zhao M., Mitchell L. A., Xie Z. X., et al. , 2017. Bug mapping and fitness testing of chemically synthesized chromosome X. Science 355: eaaf4706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Z. X., Li B. Z., Mitchell L. A., Wu Y., Qi X., et al. , 2017. “Perfect” designer chromosome V and behavior of a ring derivative. Science 355: eaaf4704. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors state that all data necessary for confirming the conclusions presented in the article are represented fully within the article.