Abstract
Metabolic homeostasis is coordinately controlled by diverse inputs. Understanding these regulatory networks is vital to combating metabolic disorders. The nematode Caenorhabditis elegans has emerged as a powerful, genetically tractable model system for the discovery of lipid regulatory mechanisms. Here we introduce DBL-1, the C. elegans homolog of bone morphogenetic protein 2/4 (BMP2/4), as a significant regulator of lipid homeostasis. We used neutral lipid staining and a lipid droplet marker to demonstrate that both increases and decreases in DBL-1/BMP signaling result in reduced lipid stores and lipid droplet count. We find that lipid droplet size, however, correlates positively with the level of DBL-1/BMP signaling. Regulation of lipid accumulation in the intestine occurs through non-cell-autonomous signaling, since expression of SMA-3, a Smad signal transducer, in the epidermis (hypodermis) is sufficient to rescue the loss of lipid accumulation. Finally, genetic evidence indicates that DBL-1/BMP functions upstream of Insulin/IGF-1 Signaling in lipid metabolism. We conclude that BMP signaling regulates lipid metabolism in C. elegans through interorgan signaling to the Insulin pathway, shedding light on a less well-studied regulatory mechanism for metabolic homeostasis.
Keywords: BMP, insulin, lipid, homeostasis, Caenorhabditis elegans
Metabolic homeostasis in animals relies on integrating input from numerous sources to coordinately regulate energy intake and expenditure across multiple organs. Nutrient sensing pathways relay signals in the absence or presence of food. Simultaneously, feedback loops are triggered in response to the lack or abundance of cellular resources. Satiety responses are produced to slow the intake of energy when sufficient resources have been obtained. The balance of these pathways provides the basis of metabolic homeostasis. Metabolic disorders, such as type II diabetes, are the result of an imbalance in the signaling web that is homeostasis. The last few decades have provided numerous insights into this regulatory network, but new regulatory interactions continue to be discovered.
The Transforming Growth Factor β (TGFβ) superfamily is a major group of peptide ligands conserved across animal phyla. The superfamily includes the founding TGFβs, as well as Bone Morphogenetic Proteins (BMPs), growth and differentiation factors, Activin, Nodal, and others. These peptides signal through conserved signal transduction pathways responsible for development, growth, and differentiation (Shi and Massague 2003; Wu and Hill 2009). Intriguingly, emerging evidence from correlative studies in humans, as well as in vivo studies in mice, implicate several TGFβ ligands in lipid metabolism and homeostasis (Wang et al. 2004; Fain et al. 2005; Sjoholm et al. 2006; Bottcher et al. 2009; Shen et al. 2009).
BMPs are a group of TGFβ-related signals with key regulatory roles in development and differentiation. Mammalian BMP2 and BMP4 play important roles in early development and cell differentiation, as well as being critical for bone and cartilage development (Chen et al. 2004). In Drosophila, the BMP ligand DPP is required for dorsoventral patterning of the early embryo as well as later patterning of the imaginal discs (Spencer et al. 1982; Padgett et al. 1987). However, the homeostatic roles of BMP ligands are less well studied. We have used molecular genetic and imaging tools available in the nematode Caenorhabditis elegans to gain insight into the homeostatic roles of BMP ligands.
C. elegans has a conserved BMP signaling pathway that includes founding members of the Smad family of signal transducers, SMA-2, SMA-3, and SMA-4 (Savage et al. 1996). DBL-1, the C. elegans BMP2/4 homolog, plays a major role in body size regulation, male-tail development, and mesodermal patterning (Suzuki et al. 1999; Foehr et al. 2006). Initial evidence of a role for DBL-1 in metabolism came from our microarray analysis of genes regulated by the DBL-1 pathway. This analysis identified several genes related to fat metabolism including genes encoding fatty acid desaturases and genes involved in β-oxidation (Liang et al. 2007). C. elegans is a prominent model system for the study of lipid homeostasis, and is particularly suitable for the identification of cell and tissue interactions that mediate homeostasis (Ashrafi 2007). Although nematodes do not possess dedicated adipocytes, they store triglycerides in lipid droplets in the intestine and in epidermal tissue (the hypodermis) via biochemical mechanisms that are evolutionarily conserved.
In this study, we show that DBL-1/BMP signaling plays an important role in regulating lipid stores in C. elegans. Alterations to DBL-1 signaling levels result in a loss of lipids and changes in lipid droplet morphology. DBL-1 signaling acts non-cell-autonomously in the hypodermis to regulate lipid storage in the intestine. Finally, we show that the lipid phenotype of dbl-1 mutants is reliant on Insulin/IGF-1-like Signaling (IIS), a well-known regulator of fat metabolism (Kimura et al. 1997).
Materials and Methods
Nematode strains and growth conditions
C. elegans were maintained on Escherichia coli (DA837) at 15, 20, and 25° as specified. The wild-type strain used in this study was N2. The strains used in this study are as follows: LT121 dbl-1(wk70), CB678 lon-2(e678), CS24 sma-3(wk30), CS152 sma-3(wk30);qcIs6 [sma-3p::gfp::sma-3+rol-6], CS619 sma-3(wk30);qcIs59 [vha-6p::gfp::sma-3+rol-6], CS628 sma-3(wk30);qcIs55 [vha-7p::gfp::sma-3+rol-6], CS630 sma-3(wk30);qcIs53 [myo-3p::gfp::sma-3+rol-6], BW1490 ctIs40 [dbl-1(OE)+sur-5::gfp], CB1370 daf-2(e1370), LIU1 ldrIs1 [dhs-3p::dhs-3::gfp+unc-76(+)], BX106 fat-6(tm331), BX153 fat-7(wa36), BX156 fat-6(tm331);fat-7(wa36). Crosses were used to obtain: daf-2(e1370);lon-2(e678), daf-2(e1370);sma-3(wk30), dbl-1(wk70);ldrIs1, ctIs40;ldrIs1, daf-2(e1370);ldrIs1, daf-2(e1370);dbl-1(wk70);ldrIs1.
Oil red O staining
The protocol was adapted from O’Rourke et al. (2009). Animals were collected at the L4 stage in PCR tube caps and washed three times in PBS. Worms were then fixed for 1 hr in 60% isopropanol while rocking at room temperature. The isopropanol was removed and worms were stained overnight with 60% Oil Red O solution while rocking at room temperature. Oil Red O was removed and the worms were washed once with PBS w/0.01% Triton and left in PBS. Worms were mounted and imaged using an AxioCam MRc camera with AxioVision software. Images were taken using a 40× objective. Oil Red O stock solution was made with 0.25 g Oil Red O in 50 ml isopropanol. Intensity of the postpharyngeal intestine was determined using ImageJ software. Unless otherwise noted, three regions were measured (anterior, midbody, and posterior) using equivalently sized windows. Pixel intensity was measured in the green color channel of the images. Three measurements using a 50 px by 50 px area were taken at each region with background intensity subtracted for each individual picture. Statistical comparisons (two-way ANOVA, one-way ANOVA with post-hoc Tukey’s multiple comparisons test, and unpaired t-test) were performed using GraphPad Prism 7 software. For each experiment, n > 20 per strain was repeated in triplicate.
Body size
Animals were collected at the L4 stage and anesthetized with sodium azide. The worms were then imaged using a QImaging Retiga EXi camera with QCapture software. The midline of each worm was then measured in ImagePro. Statistical comparisons (one-way ANOVA with post hoc Tukey’s multiple comparisons test, and two-way ANOVA, and unpaired t-test) were performed using GraphPad Prism 7 software. For each experiment, n > 30 per strain was repeated in triplicate.
Electron microscopy
Animals were fixed and embedded by standard methods (Hall 1995). Fixation and microscopy were performed by the David H. Hall laboratory. Animals were well-fed adults grown at 20°. They were fixed 2 d post egg laying. The number of animals (n) analyzed for each strain was: N2 n = 4, dbl-1 n = 2, sma-3 n = 5, sma-9 n = 3.
Lipid droplet morphology
Animals were collected at the L4 stage and anesthetized with sodium azide. The worms were then imaged using a Zeiss ApoTome with AxioVision software. Images were taken using a 100× objective. The tail region of the worm was imaged and the diameter and count of all visible lipid droplets in a 400-µm2 area were measured using AxioVision software. Statistical comparisons (one-way ANOVA with post hoc Tukey’s multiple comparisons test, two-way ANOVA with post hoc Holm-Sidak’s multiple comparisons test, and unpaired t-test with Welch’s correction) were performed using GraphPad Prism 7 software. For each experiment, n > 15 per strain was repeated in triplicate.
Pharyngeal pumping rate
The pumping rate was counted by the number of contractions of the pharyngeal bulb per 20 sec. Two counts were made per worm and averaged. For each experiment, n > 10 per strain was repeated in triplicate. Statistical comparisons (unpaired t-test with Welch’s correction) were performed using GraphPad Prism 7 software.
Data availability
All data necessary to support the conclusions of this work are presented in the article. Strains are available upon request.
Results
DBL-1/BMP signaling is required for lipid accumulation
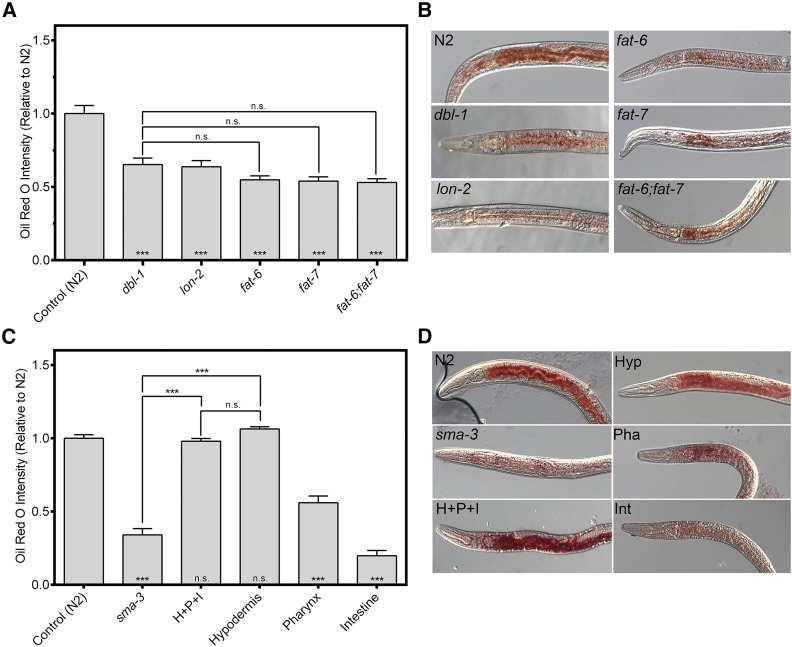
Our previous data identified numerous lipid metabolism genes as downstream targets of DBL-1/BMP signaling via microarray analysis (Liang et al. 2007). These genes include fat-6 and fat-7, which encode Δ9 fatty acid desaturases homologous to stearoyl coA desaturase. Mutations in either gene result in an overall decrease in neutral lipids, in addition to other phenotypes (Watts and Browse 2002; Ntambi et al. 2004). To determine if DBL-1 signaling is important for lipid metabolism, we measured stored lipid levels in animals with altered DBL-1 function as well as those with mutations in fat-6, fat-7, or both. Animals were grown at the standard temperature of 20° and stained with Oil Red O, at the fourth larval stage (L4), to quantify overall neutral lipid content, including lipid storage in the intestine and in the hypodermis. Consistent with previous reports, we observed a significant decrease in Oil Red O staining in fat-6, fat-7, and fat-6;fat-7 mutant animals by ∼45% compared to wild type. Similarly, we observed a decrease in dbl-1 mutants by ∼35% compared to wild type. This decrease was not significantly different from that observed in the fat mutants (P > 0.38). We also analyzed a loss-of-function mutation in lon-2, which encodes a negative regulator of DBL-1 signaling (Gumienny et al. 2007). Interestingly, lon-2 mutants also had a reduction in staining, at levels similar to that of dbl-1 mutants (P = 0.99) (Figure 1, A and B). A possible cause for the decrease in lipid storage is a reduction in food uptake, as seen in eat-2 mutants (Raizen et al. 1995). However, dbl-1 mutants had a very slight increase in the rate of pharyngeal contractions, while sma-3 animals had no significant change from wild type (Supplemental Material, Figure S1), and thus a decrease in food uptake is not likely to explain the decrease in lipid storage in dbl-1 or sma-3 mutants.
Figure 1.
Functions and tissue-specificity of DBL-1 signaling in lipid storage. (A) Both dbl-1 and lon-2 mutants show a decrease in lipid levels via Oil Red O staining, similar to that of fat mutants. Animals were grown at 20° and stained at the L4 larval stage. Quantification was done for equivalent regions of the intestine just posterior to the pharynx. (B) Images of L4 animals stained with Oil Red O taken at 400×. (C) Loss of sma-3 results in decreased lipid levels. Expression of sma-3 in the hypodermis is sufficient to rescue the lipid phenotype. Expression in either the pharynx or the intestine cannot rescue lipids to wild-type levels. Animals were grown at 20° and stained at the L4 larval stage. Quantification was done for equivalent regions of the intestine just posterior to the pharynx. (D) Images of L4 animals stained with Oil Red O taken at 400×. Anterior is to the left. For all graphs, asterisks across the bottom denote significance compared to control: n.s., not significant; *** P value < 0.001. Error bars denote SEM.
Hypodermal expression of Smads is required for lipid accumulation
This lipid storage phenotype led us to question in which tissues DBL-1 signaling is necessary to regulate fat metabolism. C. elegans do not have dedicated adipocytes, so fat is stored in the intestine and in the hypodermis. DBL-1 signaling components (receptors and Smads) are expressed in the pharynx, hypodermis, and intestine. We have previously shown that expression of sma-3/Smad in the hypodermis is sufficient and necessary to rescue the small body size phenotype of sma-3 mutants (Wang et al. 2002). We took the same approach of directing expression of sma-3(+) to specific tissues, in an otherwise sma-3 mutant background, and measured fat accumulation using Oil Red O.
Surprisingly, our results showed that sma-3 expression in the hypodermis is also sufficient for rescue of the low-fat phenotype in sma-3 mutants (Figure 1, C and D). Although sma-3 expression in the pharynx resulted in a slight increase in Oil Red O staining, intestinal sma-3 expression in sma-3 mutants resulted in a further decrease in staining, compared to sma-3 mutant animals. Thus, expression in either pharyngeal tissue or intestine was not sufficient to rescue the lipid stores in sma-3 mutants to wild-type levels. However, when sma-3 was expressed in either the hypodermis or all three tissues, Oil Red O intensity was rescued to wild-type levels with no significant difference observed (P > 0.78) (Figure 1, C and D). These data indicate that Smad activity functions nonautonomously in the hypodermis to regulate fat storage in the intestine.
DBL-1/BMP functions upstream of IIS to regulate lipid accumulation
Our tissue-specific rescue experiments suggest that SMA-3/Smad activity in the hypodermis may regulate the expression of a secreted ligand that signals to the intestine. One potential mechanism by which this signaling may occur is through the regulation of insulin ligands. C. elegans uses multiple insulin-like ligands that act through a single insulin receptor, DAF-2/InsR (Pierce et al. 2001). The IIS pathway in C. elegans was first identified for its role in regulating dauer development. A temperature-sensitive mutation in daf-2, when exposed to the restrictive temperature of 25°, results in the development of daf-2/InsR mutants into dauers, an alternate L3 stage utilized for survival in high-stress environments. In addition, daf-2 mutants exhibit other phenotypes, including increased lifespan and stress tolerance (Gottlieb and Ruvkun 1994; Tissenbaum and Ruvkun 1998). Moreover, the involvement of Insulin/IGF-1-like Signaling (IIS) in fat metabolism is well documented in C. elegans (Kimura et al. 1997). Notably, our microarray analysis revealed ins-4 as a transcriptional target of the DBL-1 pathway; expression was increased in both dbl-1 and sma-9 mutant backgrounds. INS-4 is an insulin-like ligand expressed in the hypodermis, in addition to neurons (Ritter et al. 2013).
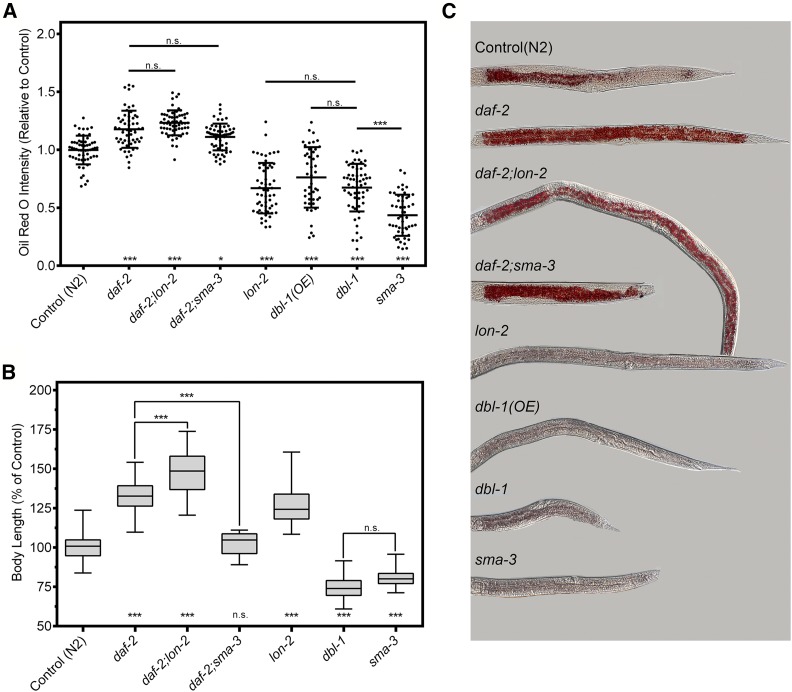
We therefore constructed strains containing mutations in both BMP and IIS pathways to test interactions between these pathways via genetic epistasis. To bypass dauer arrest in daf-2 mutant animals, these experiments were conducted by raising animals at the permissive temperature of 15° followed by a shift to 25°. Our findings confirmed the high-fat phenotype of daf-2 mutants, exhibiting an average increase of 18% over wild type. Similarly, daf-2;sma-3 double mutants showed an average increase in Oil Red O intensity of 11% (Figure 2A), which is not significantly different from daf-2 (P = 0.71). We concluded that the daf-2 high-fat phenotype is epistatic to the dbl-1 low-fat phenotype, suggesting that daf-2 acts downstream of dbl-1 with regard to fat storage regulation.
Figure 2.
DBL-1 signaling functions upstream of IIS to regulate lipid storage but not body size. (A) daf-2;lon-2 and daf-2;dbl-1 display the high-fat phenotype of daf-2 animals using Oil Red O. Graphs show combined data from three equivalent regions of each worm. See Figure S2 for separated data from each region. Epistasis analysis places DBL-1 signaling upstream of daf-2/InsR. dbl-1(OE) animals show a similar decrease in lipid levels as dbl-1 and lon-2 mutants. Animals were grown at 15° until the L2/L3 molt and then shifted to 25° and stained at the L4 larval stage. The center line denotes mean and the error bars denote SD. (B) Animals containing mutations in both DBL-1 pathway components and daf-2 exhibit additive body sizes. Both pathways regulate body size independently. Animals were grown at 15° until the L2/L3 molt and then shifted to 25° and measured at the L4 larval stage. Boxes denote the second and third quartiles, whiskers denote min and max values. (C) Images of L4 animals stained with Oil Red O taken at 200×. Anterior is to the left. For all graphs, asterisks across the bottom denote significance compared to control: n.s., not significant; * P value < 0.05; *** P value < 0.001.
We also assessed mutants in other components of the DBL-1 pathway. sma-3 mutants had an average reduction in Oil Red O intensity of >50%. To examine increased DBL-1 activity, we used both a dbl-1 overexpression strain and lon-2 mutants, which are deficient in a negative regulator of DBL-1. Consistent with the observation from lon-2 mutants, a dbl-1 overexpression strain [dbl-1(OE)] also exhibited a reduction in lipid stores (∼25% less) (Figure 2, A and C). This finding indicates precise regulation of DBL-1 signaling is required to maintain wild-type levels of lipids. It also indicates that the lipid and body size phenotypes of the DBL-1 pathway are independent, as both long and short animals exhibited decreased lipids. daf-2;lon-2 double mutants, like daf-2;dbl-1 mutants, have an increase in fat accumulation indistinguishable from that of daf-2 single mutants (P > 0.71). This epistatic relationship between the two pathways suggests that IIS functions downstream of DBL-1 signaling in the regulation of fat accumulation.
DBL-1/BMP and IIS pathways independently contribute to body size
We also tested whether these two pathways interact to regulate body size. Small body size was the first identified phenotype of the DBL-1 pathway (Savage et al. 1996; Suzuki et al. 1999). We measured animals at the L4 stage, benchmarked against wild-type controls: dbl-1 mutant animals showed an average body length reduction of 25%; sma-3 body length was reduced by 19%. Conversely, an increase in DBL-1 signaling resulted in an increase in body size, as lon-2 mutants exhibited an average increase of 26%. Interestingly, a downregulation of DAF-2/InsR showed a significant increase in body size by an average of 32% compared to wild type. When animals with mutations in both pathways were measured, an additive effect was observed. The daf-2;lon-2 mutant animals had an average increase in length of 48.0% compared to wild type, while the daf-2;sma-3 mutant animals were comparable to the length of wild type (Figure 2, B and C). These data are consistent with an independent study by the Baumeister laboratory (Qi et al. 2017) and suggests that the DBL-1 and IIS pathways work independently to regulate the overall body size of C. elegans.
Changes to DBL-1/BMP signaling levels alter lipid droplet morphology
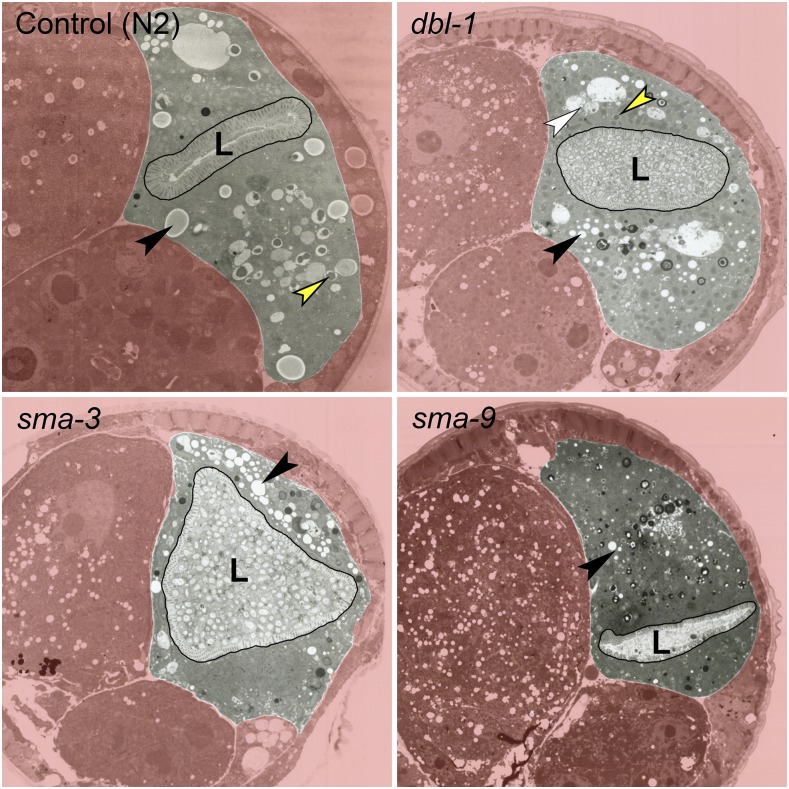
To determine how the DBL-1 pathway regulates lipid stores at a subcellular level, we analyzed lipid droplet morphology. Lipid droplets are vital for the utilization and storage of energy at the cellular level and are highly regulated. The morphology of lipid droplets can be an indicator of the function and well-being of a cell, or an organism. In humans, the function of white adipose tissue and brown adipose tissue differ significantly; these differences primarily reflect how they regulate lipid droplets (Meex et al. 2009; Yu et al. 2015; Luo and Liu 2016). We first examined lipid droplet morphology via electron microscopy. Images of dbl-1, sma-3, and sma-9 mutants depict worms with much smaller lipid droplets in the intestine, compared to that of wild-type animals (Figure 3).
Figure 3.
DBL-1 pathway mutants exhibit a decrease in lipid droplet size via electron microscopy. dbl-1, sma-3, and sma-9 mutants midbody sections display smaller lipid droplets compared to those seen in wild-type. Black arrowheads depict representative lipid droplets, white arrowhead depicts lysosome-like organelle, and yellow arrowheads depict possible yolk droplets. L marks the intestinal lumen, which tends to be enlarged and filled with bacteria in DBL-1 pathway mutants. Animals were grown at 20° and imaged at adulthood 2 d post egg lay. Total magnification is 22,000×.
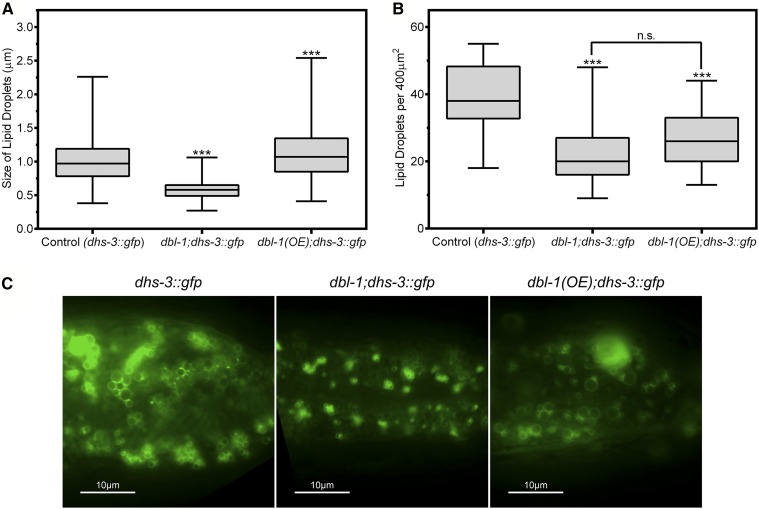
To visualize lipid droplets in a larger sample size, we used transgenic animals expressing DHS-3::GFP (Figure 4). DHS-3 is a short-chain dehydrogenase shown to bind to the surface of lipid droplets (Zhang et al. 2010). DHS-3::GFP is expressed specifically in the intestine and not in the hypodermis. Animals were grown and analyzed at the standard growth temperature of 20°. We measured the diameters of lipid droplets labeled with DHS-3::GFP. Our data indicate that lipid droplet size is positively correlated with DBL-1 signaling levels. Wild-type animals with DHS-3::GFP have an average lipid droplet diameter of 1.02 μm, with a maximum diameter of 2.26 μm (Figure 4, A and C). The dbl-1 mutants show a decrease in average droplet diameter to 0.58 μm (P < 0.001), with a maximum diameter of only 1.06 µm, a decrease of ∼53%. This reduction in lipid droplet size is consistent with the reduced lipid droplet size seen by electron microscopy. The overexpression strain, on the other hand, shows an increase to 1.13 μm on average (P < 0.001), with a maximum diameter of 2.54 µm, an increase of over 12%. The dbl-1 mutants displayed the lowest level of variance indicating that a decrease in dbl-1 expression confines the size of lipid droplets to a smaller range compared to wild type. The dbl-1(OE) mutants displayed a slightly larger level of variance compared to wild type, which indicates an increase in the maximum size of lipid droplets, but does not restrict the minimum size compared to wild type.
Figure 4.
DBL-1 pathway regulates lipid droplet morphology. (A) Lack of dbl-1 results in significantly smaller lipid droplets, while overexpression of dbl-1 increases the average diameter of lipid droplets. dhs-3::gfp was used to visualize lipid droplets. Animals were grown at 20° and imaged at the L4 larval stage. (B) Either a loss or an increase in DBL-1 signaling results in a reduction in the overall number of lipid droplets in the animals. dhs-3::gfp was used to visualize lipid droplets. Animals were grown at 20° and imaged at the L4 larval stage. (C) Images of L4 animals with altered levels of DBL-1. Images depict the posterior end of the intestine taken at 1000×. For all graphs: n.s., not significant; *** P value < 0.001; boxes denote second and third quartiles; whiskers denote min and max values.
We next determined the density of lipid droplets per unit area by counting the total number of lipid droplets per 400 μm2. Wild-type animals had an average of 40 droplets, while both dbl-1 and dbl-1(OE) showed a decrease, 24 (P < 0.001) and 27 (P < 0.001) per unit area, respectively (Figure 4, B and C). The decreased numbers of lipid droplets are concordant with the decreased intensity of Oil Red O staining in both loss-of-function and gain-of-function backgrounds. The dbl-1(OE) mutants displayed a lower level of variance compared to the wild-type and dbl-1 strains, which may help account for their overall decrease in lipids observed via Oil Red O staining (Figure 2A). Thus, while both decreasing and increasing DBL-1 signaling resulted in a reduction in total fat accumulation, the mechanisms at the level of lipid droplet size are different.
DBL-1/BMP regulation of lipid droplet morphology is partially dependent on IIS
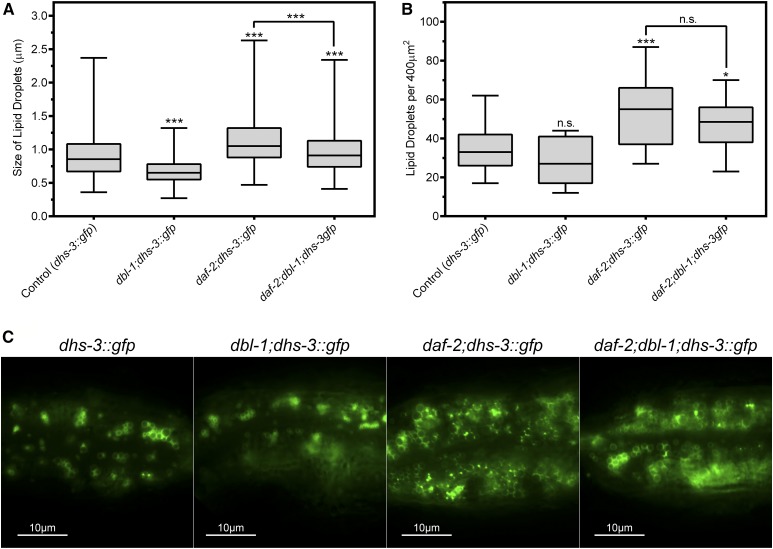
Next, we wanted to determine if IIS is required for the lipid droplet phenotypes of DBL-1 signaling mutants. To bypass dauer arrest in daf-2 mutant animals, these experiments were conducted by raising animals at the permissive temperature of 15° followed by a shift to 25°. In the daf-2 mutant background, the average diameter of lipid droplets was 1.12 μm with a maximum diameter of 2.63 μm. This was significantly larger than those of the wild type with an average of 0.90 μm and a maximum diameter of 2.37 μm (P < 0.001) (Figure 5, A and C). The daf-2 mutants also had the greatest level of variance, indicative of having a greater range of diameters than any of the other strains. The daf-2;dbl-1 double mutants had an average diameter of 0.97 μm and a maximum diameter of 2.34 μm. This size was similar to that of daf-2 mutants, but still smaller than in daf-2 mutants (P < 0.001). Thus, for lipid droplet size, we do not observe the strict epistatic relationship seen in the Oil Red O experiments. We therefore used two-way ANOVA to test for interactions between daf-2 and dbl-1 in regulation of lipid droplet size. We found that lipid droplet diameter in the double mutants was significantly larger than would be expected if the genes acted independently (P = 0.0006).We conclude that DBL-1 signaling acts in part via the IIS pathway to regulate lipid droplet size, with the possibility of a DAF-2-independent function for DBL-1 as well. Alternatively, the deviation from epistasis may be due to our use of daf-2(e1370), a strong loss-of-function but not a null allele, which would be inviable.
Figure 5.
DBL-1 regulates lipid droplet size in part via IIS. (A) The average diameter of lipid droplets in daf-2;dbl-1 mutants is similar to but slightly distinct from that of daf-2 single mutants, suggesting a partially independent effect from the DBL-1 pathway. dhs-3::gfp was used to visualize lipid droplets. Animals were grown at 15° until the L2/L3 molt and then shifted to 25° and imaged at the L4 larval stage. (B) daf-2 mutants exhibit an increase in lipid droplet count compared to both wild-type and dbl-1. At 25°, dbl-1 does not have a significant effect on the number of lipid droplets. dhs-3::gfp was used to visualize lipid droplets. Animals were grown at 15° until the L2/L3 molt and then shifted to 25° and imaged at the L4 larval stage. (C) Images of L4 animals containing mutations in either daf-2, dbl-1, or both. Images depict the posterior end of the intestine taken at 1000×. For all graphs: n.s., not significant; * P value < 0.05; *** P value < 0.001; boxes denote second and third quartiles; whiskers denote min and max values.
Next, we examined the average number of lipid droplets in the animals. daf-2 exhibited a significant increase compared to wild type, 55 and 34 (P < 0.001), respectively. The daf-2;dbl-1 animals were similarly increased over wild type, at 46 droplets (P = 0.039). The increased count of daf-2 and daf-2;dbl-1 were similar to each other and not significantly different (P = 0.276). Interestingly, the dbl-1 mutant did not exhibit a significant decrease in lipid droplet count compared to wild type, 27 vs. 34 (P = 0.471); the variance between the two samples was also very similar, suggesting no change between the two strains. These data suggest that DBL-1 regulation of lipid droplet count may be temperature sensitive (Figure 5, B and C).
Discussion
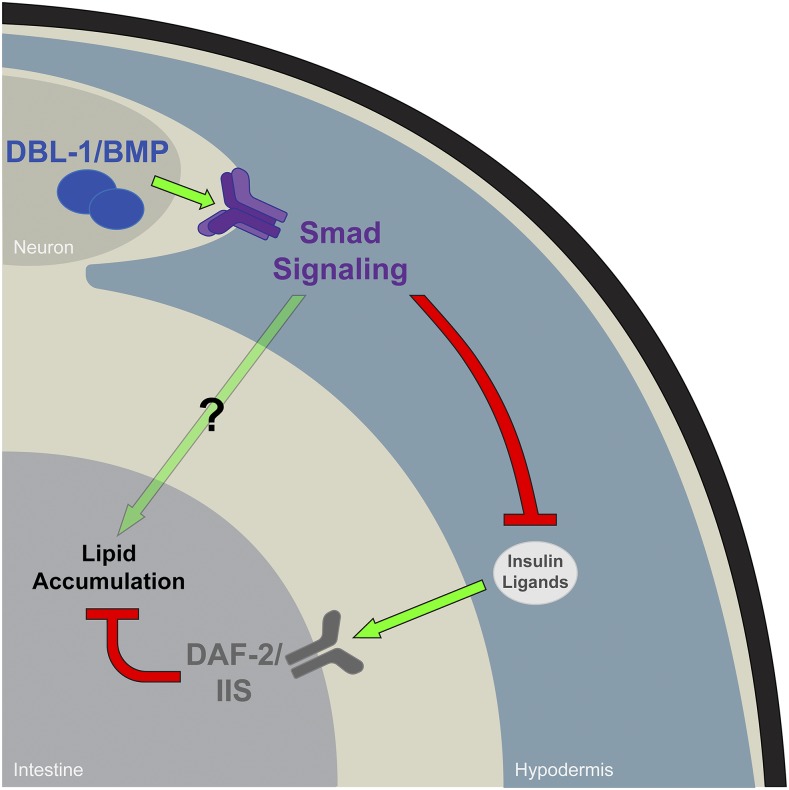
In this study, we present evidence that DBL-1/BMP signaling plays an important role in lipid metabolism, as DBL-1 pathway mutants, as well as dbl-1 overexpression, have a distinct decrease in lipid stores via Oil Red O staining. A similar conclusion was reached independently by Yu et al. (2017). In addition, we show that lipid droplet morphology of DBL-1 pathway mutants is significantly changed from wild type. We have identified a nonautonomous mechanism for this regulation, since DBL-1 signaling activity in the hypodermis is sufficient to maintain proper lipid levels in the intestine. The epistasis of daf-2/InsR in daf-2;dbl-1 double mutants indicates that DBL-1 regulates lipid levels by modulation of DAF-2/InsR activity. These data implicate a BMP–IIS signaling axis as a major player in the regulation of lipid metabolism. Our model is illustrated in Figure 6. DBL-1 is expressed primarily in neurons, from which it signals to the Smad pathway in the hypodermis, leading to inhibition of the IIS pathway and stimulation of lipid storage. Observed differences in the lipid droplet morphology between daf-2;dbl-1 double mutants and daf-2 single mutants suggest that there may also be DAF-2-independent regulation of lipids by DBL-1 (arrow with question mark).
Figure 6.
Non-cell-autonomous interaction of DBL-1 and IIS to regulate lipids. Our data suggest that Smad activation in the hypodermis is sufficient to maintain proper lipid stores. This effect may occur through controlled expression of insulin-like ligands, which in turn regulate the DAF-2/IIS response in the intestine.
We find that the lipid and body size phenotypes of DBL-1 are separable. Additionally, DAF-2/InsR regulates body size independently of DBL-1. Interestingly, the effect of daf-2 on body size is the opposite of that seen in many other species. In Drosophila, mutations in InR/InsR and chico/IRS display reduced body size resulting from decreased cell number and size (Bohni et al. 1999). In spite of this, the direction of the lipid-related phenotypes of IIS in Drosophila and in C. elegans is the same (Bohni et al. 1999). In mice, as in Drosophila, deficiency in IGF-I or IGF-II causes stunted growth and significant decreases in body weight (Baker et al. 1993). The reason for the shift in growth-regulating function of IIS in C. elegans is unknown.
Our findings are part of an emerging body of evidence for interactions between BMP and IIS pathways. In C. elegans, DBL-1 and IIS promote germline proliferation (Qi et al. 2017). The Baumeister laboratory has shown that the IIS transcription factor DAF-16/FOXO and SMA-3/Smad interact in the hypodermis to regulate this germline function. The DBL-1 and IIS pathways have also been shown to regulate reproductive aging, but in this case they act independently (Luo et al. 2009, 2010), similar to their independent functions in body size regulation. In this study, we report the first evidence of interaction between the DBL-1 and IIS pathways that produce an effect in a somatic tissue, the intestine. It is also possible that similar interactions occur in vertebrates, since BMP signaling can influence insulin sensitivity in mice (Schulz et al. 2016; Chattopadhyay et al. 2017).
Lipid droplets are vital for the utilization and storage of energy at the cellular level and are highly regulated. Lipid droplet size is maintained by the balance of triglyceride production and β-oxidation. When peroxisomal β-oxidation is inhibited, such as in maoc-1, dhs-28, and daf-22 mutants in C. elegans, average lipid droplet size is increased significantly over wild type (Zhang et al. 2010). Conversely, when fatty acid synthesis is inhibited, lipid droplet size is decreased. Animals containing mutations in fat-6 and fat-7, genes responsible for the first desaturation step in creating polyunsaturated fatty acids, display a significant decrease in average lipid droplet size (Shi et al. 2013). Multiple nutrient sensing pathways exist to provide regulatory signals that dictate lipid droplet mobilization. Lipid droplet morphology can be closely linked to the input of these pathways as energy demands fluctuate with available food. The lipid droplet size phenotype of fat-6;fat-7 mutants was shown to be independent of the AMPK and TOR pathways. However, it was shown that fat-6 and fat-7 work in conjunction with IIS, as both are required for the large lipid droplet size phenotype of daf-2/InsR mutants (Shi et al. 2013).
Our data have introduced DBL-1 signaling as a key factor in maintaining proper lipid droplet homeostasis through IIS. Future work will examine whether DBL-1 signaling influences IIS through the transcriptional regulation of insulin-like ligand genes. As SMA-3 functions in the hypodermis, but not the intestine, it may be that DBL-1 signaling is limiting the production of insulin-like ligands in the hypodermis, reducing the level of DAF-2 signaling in the intestine (Figure 6). We will also examine whether Smads interact with transcription factors downstream of DAF-2/InsR, such as DAF-16/FoxO and SKN-1/Nrf, to regulate lipid metabolism. Finally, the question of how an overexpression of DBL-1 leads to an overall reduction in lipids, while increasing the average diameter of lipid droplets, remains. Addressing these questions promises to yield further insight into whole-body lipid homeostasis by these conserved signaling pathways.
Supplementary Material
Supplemental material is available online at www.g3journal.org/lookup/suppl/doi:10.1534/g3.117.300416/-/DC1.
Acknowledgments
We thank Vanessa Almonte, Allie Dananberg, and Mariya Fabisevich for preliminary fat staining and body size experiments. We thank Hasan Zumrut for performing pharyngeal pumping analyses. We are grateful to Ross Cagan and Alicia Meléndez for critical reading of the manuscript. This work was supported in part by National Institutes of Health (NIH) R15GM112147 and R15GM097692 to C.S.D. and by NIH OD010943 to D.H.H. Some strains were provided by the Caenorhabditis Genetics Center, which is funded by NIH Office of Research Infrastructure Programs (P40OD010440). This work was carried out in partial fulfillment of the requirements for the Ph.D. degree from the Graduate Center of City University of New York (J.F.C.).
Footnotes
Communicating editor: B. Andrews
Literature Cited
- Ashrafi K., 2007. Obesity and the regulation of fat metabolism (March 9, 2007). WormBook, ed. The C. elegans Research Community WormBook, /10.1895/wormbook.1.130.1, http://www.wormbook.org. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker J., Liu J. P., Robertson E. J., Efstratiadis A., 1993. Role of insulin-like growth factors in embryonic and postnatal growth. Cell 75: 73–82. [PubMed] [Google Scholar]
- Bohni R., Riesgo-Escovar J., Oldham S., Brogiolo W., Stocker H., et al. , 1999. Autonomous control of cell and organ size by CHICO, a Drosophila homolog of vertebrate IRS1–4. Cell 97: 865–875. [DOI] [PubMed] [Google Scholar]
- Bottcher Y., Unbehauen H., Kloting N., Ruschke K., Korner A., et al. , 2009. Adipose tissue expression and genetic variants of the bone morphogenetic protein receptor 1A gene (BMPR1A) are associated with human obesity. Diabetes 58: 2119–2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay T., Singh R. R., Gupta S., Surolia A., 2017. Bone morphogenetic protein-7 (BMP-7) augments insulin sensitivity in mice with type II diabetes mellitus by potentiating PI3K/AKT pathway. Biofactors 43: 195–209. [DOI] [PubMed] [Google Scholar]
- Chen D., Zhao M., Mundy G. R., 2004. Bone morphogenetic proteins. Growth Factors 22: 233–241. [DOI] [PubMed] [Google Scholar]
- Fain J. N., Tichansky D. S., Madan A. K., 2005. Transforming growth factor beta1 release by human adipose tissue is enhanced in obesity. Metabolism 54: 1546–1551. [DOI] [PubMed] [Google Scholar]
- Foehr M. L., Lindy A. S., Fairbank R. C., Amin N. M., Xu M., et al. , 2006. An antagonistic role for the C. elegans Schnurri homolog SMA-9 in modulating TGFβ signaling during mesodermal patterning. Development 133: 2887–2896. [DOI] [PubMed] [Google Scholar]
- Gottlieb S., Ruvkun G., 1994. daf-2, daf-16 and daf-23: genetically interacting genes controlling Dauer formation in Caenorhabditis elegans. Genetics 137: 107–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumienny T. L., MacNeil L. T., Wang H., de Bono M., Wrana J. L., et al. , 2007. Glypican LON-2 is a conserved negative regulator of BMP-like signaling in Caenorhabditis elegans. Curr. Biol. 17: 159–164. [DOI] [PubMed] [Google Scholar]
- Hall D. H., 1995. Electron microscopy and three-dimensional image reconstruction. Methods Cell Biol. 48: 395–436. [DOI] [PubMed] [Google Scholar]
- Kimura K. D., Tissenbaum H. A., Liu Y., Ruvkun G., 1997. daf-2, an insulin receptor-like gene that regulates longevity and diapause in Caenorhabditis elegans. Science 277: 942–946. [DOI] [PubMed] [Google Scholar]
- Liang J., Yu L., Yin J., Savage-Dunn C., 2007. Transcriptional repressor and activator activities of SMA-9 contribute differentially to BMP-related signaling outputs. Dev. Biol. 305: 714–725. [DOI] [PubMed] [Google Scholar]
- Luo L., Liu M., 2016. Adipose tissue in control of metabolism. J. Endocrinol. 231: R77–R99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo S., Shaw W. M., Ashraf J., Murphy C. T., 2009. TGF-β Sma/Mab signaling mutations uncouple reproductive aging from somatic aging. PLoS Genet. 5: e1000789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo S., Kleemann G. A., Ashraf J. M., Shaw W. M., Murphy C. T., 2010. TGF-β and insulin signaling regulate reproductive aging via oocyte and germline quality maintenance. Cell 143: 299–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meex R. C., Schrauwen P., Hesselink M. K., 2009. Modulation of myocellular fat stores: lipid droplet dynamics in health and disease. Am. J. Physiol. Regul. Integr. Comp. Physiol. 297: R913–R924. [DOI] [PubMed] [Google Scholar]
- Ntambi J. M., Miyazaki M., Dobrzyn A., 2004. Regulation of stearoyl-CoA desaturase expression. Lipids 39: 1061–1065. [DOI] [PubMed] [Google Scholar]
- O’Rourke E. J., Soukas A. A., Carr C. E., Ruvkun G., 2009. C. elegans major fats are stored in vesicles distinct from lysosome-related organelles. Cell Metab. 10: 430–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padgett R. W., St Johnston R. D., Gelbart W. M., 1987. A transcript from a Drosophila pattern gene predicts a protein homologous to the transforming growth factor-beta family. Nature 325: 81–84. [DOI] [PubMed] [Google Scholar]
- Pierce S. B., Costa M., Wisotzkey R., Devadhar S., Homburger S. A., et al. , 2001. Regulation of DAF-2 receptor signaling by human insulin and ins-1, a member of the unusually large and diverse C. elegans insulin gene family. Genes Dev. 15: 672–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi W., Yan Y., Pfeifer D., Donner V. G. E., Wang Y., et al. , 2017. C. elegans DAF-16/FOXO interacts with TGF-ss/BMP signaling to induce germline tumor formation via mTORC1 activation. PLoS Genet. 13: e1006801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raizen D. M., Lee R. Y., Avery L., 1995. Interacting genes required for pharyngeal excitation by motor neuron MC in Caenorhabditis elegans. Genetics 141: 1365–1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritter A. D., Shen Y., Fuxman Bass J., Jeyaraj S., Deplancke B., et al. , 2013. Complex expression dynamics and robustness in C. elegans insulin networks. Genome Res. 23: 954–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage C., Das P., Finelli A. L., Townsend S. R., Sun C. Y., et al. , 1996. Caenorhabditis elegans genes sma-2, sma-3, and sma-4 define a conserved family of transforming growth factor beta pathway components. Proc. Natl. Acad. Sci. USA 93: 790–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz T. J., Graja A., Huang T. L., Xue R., An D., et al. , 2016. Loss of BMP receptor type 1A in murine adipose tissue attenuates age-related onset of insulin resistance. Diabetologia 59: 1769–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J. J., Huang L., Li L., Jorgez C., Matzuk M. M., et al. , 2009. Deficiency of growth differentiation factor 3 protects against diet-induced obesity by selectively acting on white adipose. Mol. Endocrinol. 23: 113–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi X., Li J., Zou X., Greggain J., Rodkaer S. V., et al. , 2013. Regulation of lipid droplet size and phospholipid composition by stearoyl-CoA desaturase. J. Lipid Res. 54: 2504–2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y., Massague J., 2003. Mechanisms of TGF-β signaling from cell membrane to the nucleus. Cell 113: 685–700. [DOI] [PubMed] [Google Scholar]
- Sjoholm K., Palming J., Lystig T. C., Jennische E., Woodruff T. K., et al. , 2006. The expression of inhibin beta B is high in human adipocytes, reduced by weight loss, and correlates to factors implicated in metabolic disease. Biochem. Biophys. Res. Commun. 344: 1308–1314. [DOI] [PubMed] [Google Scholar]
- Spencer F. A., Hoffmann F. M., Gelbart W. M., 1982. Decapentaplegic: a gene complex affecting morphogenesis in Drosophila melanogaster. Cell 28: 451–461. [DOI] [PubMed] [Google Scholar]
- Suzuki Y., Yandell M. D., Roy P. J., Krishna S., Savage-Dunn C., et al. , 1999. A BMP homolog acts as a dose-dependent regulator of body size and male tail patterning in Caenorhabditis elegans. Development 126: 241–250. [DOI] [PubMed] [Google Scholar]
- Tissenbaum H. A., Ruvkun G., 1998. An insulin-like signaling pathway affects both longevity and reproduction in Caenorhabditis elegans. Genetics 148: 703–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Tokarz R., Savage-Dunn C., 2002. The expression of TGFβ signal transducers in the hypodermis regulates body size in C. elegans. Development 129: 4989–4998. [DOI] [PubMed] [Google Scholar]
- Wang W., Yang Y., Meng Y., Shi Y., 2004. GDF-3 is an adipogenic cytokine under high fat dietary condition. Biochem. Biophys. Res. Commun. 321: 1024–1031. [DOI] [PubMed] [Google Scholar]
- Watts J. L., Browse J., 2002. Genetic dissection of polyunsaturated fatty acid synthesis in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 99: 5854–5859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M. Y., Hill C. S., 2009. Tgf-beta superfamily signaling in embryonic development and homeostasis. Dev. Cell 16: 329–343. [DOI] [PubMed] [Google Scholar]
- Yu J., Zhang S., Cui L., Wang W., Na H., et al. , 2015. Lipid droplet remodeling and interaction with mitochondria in mouse brown adipose tissue during cold treatment. Biochim. Biophys. Acta 1853: 918–928. [DOI] [PubMed] [Google Scholar]
- Yu Y., Mutlu A. S., Liu H., Wang M. C., 2017. High-throughput screens using photo-highlighting discover BMP signaling in mitochondrial lipid oxidation. Nat. Commun. 8: 865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S. O., Trimble R., Guo F., Mak H. Y., 2010. Lipid droplets as ubiquitous fat storage organelles in C. elegans. BMC Cell Biol. 11: 96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data necessary to support the conclusions of this work are presented in the article. Strains are available upon request.