Summary
Background
Therapeutic drug monitoring may optimize therapy for Crohn's disease (CD).
Aim
To use a population pharmacokinetic model that accounts for the time‐varying nature of covariates to simulate certolizumab pegol (CZP) concentrations to evaluate the exposure‐response relationship for CZP in Crohn's disease.
Methods
Adults (N = 2157) with Crohn's disease were treated with CZP in nine clinical trials. Simulated CZP concentrations were compared to outcomes at weeks 6 and 26, including Crohn's disease activity index (CDAI) response (decrease from baseline ≥ 100 points), remission (CDAI ≤ 150), C‐reactive protein (CRP) ≤ 5 mg/L, faecal calprotectin (FC) ≤ 250 μg/g, and a composite endpoint of CDAI ≤ 150 and FC ≤ 250 μg/g. Multivariable analyses identified covariates associated with outcomes and receiver operating characteristic analyses determined optimal CZP concentrations.
Results
CZP concentrations at weeks 2, 4 and 6 were higher in patients with clinical response, remission, CRP ≤ 5 mg/L or FC ≤ 250 μg/g at week 6 than without. In multivariable analyses, higher CZP concentrations at week 6 were associated with the composite outcome at weeks 6 and 26 (P < .001). Although the exposure‐response relationship varied among patients, approximate CZP concentrations of at least 36.1 μg/mL (positive predictive value [PPV] 22.8% and negative predictive value [NPV] 92.7%) and at least 14.8 μg/mL (PPV 28.0% and NPV 90.4%) at weeks 6 and 12 were associated with weeks 6 and 26 outcomes.
Conclusions
An exposure‐response relationship was apparent for CZP in Crohn's disease and achieving higher CZP concentrations may increase the likelihood of attaining efficacy outcomes, but this remains to be evaluated prospectively.
1. INTRODUCTION
Crohn's disease is a chronic, relapsing‐remitting inflammation of the intestinal tract. Inhibition of tumour necrosis factor‐α (TNF) was shown to induce remission in patients with Crohn's disease.1, 2, 3, 4 Certolizumab pegol is a recombinant, humanised, Fc‐free, polyethylene glycol‐conjugated antigen‐binding fragment specific for human TNF, and is effective at reducing signs and symptoms of moderately to severely active Crohn's disease and maintaining clinical response in adult patients who have had an inadequate response to conventional therapy.2, 4, 5, 6
Not all patients with Crohn's disease respond to treatment with TNF antagonists and both the time and the magnitude of response to therapy is variable.4, 7, 8 Some variation has been associated with the development of antidrug antibodies (ADAb), which have been associated with increased clearance of drug,9 but other disease‐ and patient‐related factors also influence drug concentrations.7 Factors that influence certolizumab pegol concentrations were examined in a population pharmacokinetic model for certolizumab pegol in patients with Crohn's disease.10 The model, which used data from 2157 patients with Crohn's disease treated with certolizumab pegol in nine clinical trials, followed a conventional population pharmacokinetic model‐building approach. Only baseline covariates were used, and thus individually predicted clearance remained constant over time. A refined population pharmacokinetic model was developed subsequently,11 that takes into account the time‐varying nature of patient characteristics and reduces the inter‐patient variability of certolizumab pegol clearance estimates to < 20%, which is considered low for a biologic. The revised model also includes certolizumab pegol ADAb concentration as a continuous variable, allowing for evaluation of the concentration‐dependent effect of ADAb on certolizumab pegol clearance. Other covariates that explained inter‐patient variability in apparent drug clearance were C‐reactive protein, albumin, as well as other, less disease‐related covariates such as body weight and gender. By taking into account the time‐varying nature of these covariates, the refined population pharmacokinetic model constitutes an important improvement over the previous model, which is more reflective of patient drug exposure with sustained treatment, as patient and disease characteristics of patients with Crohn's disease are expected to change over time depending on their response to therapy.
Using the refined population pharmacokinetic model to precisely simulate drug concentrations at exact time points in individual patients, we were able to conduct a pooled analysis of nine clinical trials of certolizumab pegol in Crohn's disease, each with a seemingly different trial design, encompassing various dosing regimens and sampling time‐points. To our knowledge, this constitutes the largest combined exposure‐response analysis of any TNF antagonist on the market to date. This analysis aimed to evaluate the association between various measures of certolizumab pegol exposure and clinical and biomarker outcome measures. In addition, we evaluated the effects of covariates that may influence the exposure‐response relationship of certolizumab pegol during induction (assessed at week 6) and maintenance (assessed at week 26) therapy with certolizumab pegol, as well as the certolizumab pegol concentrations that are associated with achieving these outcomes.
2. METHODS
2.1. Data sources
This analysis was conducted using pooled data from nine clinical trials of certolizumab pegol in patients with Crohn's disease,10 including C87005,12 C87031 (NCT00152490),2 C87032 (NCT00152425),4 C87037 (NCT00291668),6 C87042 (NCT00308581),13 C87043 (NCT00297648),1 C87047 (NCT00329550),6 C87048 (NCT00329420)6 and C87085 (NCT00552058)14 (Figure S1). All study protocols and consent forms were approved by institutional review boards or ethics committees at the study sites, and studies were conducted in accordance with the principles of good clinical practice and the Declaration of Helsinki. All patients provided written informed consent before study participation.
Certolizumab pegol concentrations were measured with a sandwich enzyme‐linked immunosorbent assay (ELISA), which was validated in line with the regulatory requirements of the Food and Drug Administration and the European Medicines Agency for bioanalytical methods, as previously described, with 0.41 μg/mL as the lower limit of quantification.10 The ELISA was developed to exclusively support pharmacokinetic analyses across a range of indications (rheumatoid arthritis, axial spondyloarthritis, psoriatic arthritis and Crohn's disease) for UCB Pharma. ADAb to certolizumab pegol were measured with a drug‐sensitive double antigen ( = bridging) ELISA, as previously described, and samples were classified as ADAb positive if the concentration of antibodies was >2.4 units/mL.10
Certolizumab pegol concentration‐time data in Crohn's disease were adequately described by a population pharmacokinetic model with first‐order absorption and one‐compartment disposition with linear, time‐dependent elimination that included ADAb to certolizumab pegol as a continuous variable in the structural model and took into account the time‐varying nature of covariates, as described elsewhere.11 This model was used to simulate various certolizumab pegol exposure measures including intermediate and trough concentrations at exact time points as well as area under the concentration‐time curve, for each patient available for this analysis, given the dosing history of that patient and individual time‐varying covariate values.
2.2. Efficacy outcomes
Efficacy outcomes at weeks 6 and 26 included clinical response (defined as a Crohn's disease activity index decrease from baseline of ≥ 100 points), clinical remission (defined as a Crohn's disease activity index score of ≤ 150), biological remission (defined by C‐reactive protein ≤ 5 mg/L or faecal calprotectin ≤ 250 μg/g) and a composite endpoint (Crohn's disease activity index ≤ 150 and faecal calprotectin ≤ 250 μg/g).
2.3. Analyses
Descriptive statistics were used to summarise patient and pharmacological data. Certolizumab pegol concentrations were compared between patients achieving or not achieving the efficacy outcome using a Wilcoxon two‐sample test. Certolizumab pegol concentrations were also categorised into quartiles, and the trend of patients achieving the efficacy endpoints across quartiles was evaluated using the one‐sided Cochran‐Armitage Trend test. Multivariable logistic regression was carried out to identify covariates associated with exposure‐response relationship to certolizumab pegol therapy, including continuous variables: age, gender, weight, certolizumab pegol concentration (log‐transformed), disease duration, haematocrit, albumin, C‐reactive protein and Crohn's disease activity index and categorical variables: ADAb to certolizumab pegol, prior anti‐TNF treatment, immunosuppressant use and prior surgery. Factors not significant at the 0.05 level were removed from the model by backwards elimination. Multivariable logistic regression analyses only comprised data from two randomised, double‐blind, placebo‐controlled trials (PRECiSE12 and PRECiSE24), because these were the only trials where faecal calprotectin data were recorded. Receiver operating characteristic analyses were carried out to determine optimal certolizumab pegol concentrations associated with efficacy outcomes during induction and maintenance. To maximise the sum of the specificity and sensitivity along the receiver operating characteristic curve, the Youden index was used to determine optimal certolizumab concentration thresholds.15 Certolizumab pegol concentration thresholds with high specificity (> 90%) were also evaluated.
Dropouts were considered as treatment failures for these analyses, regardless of the reason(s) for discontinuation. All authors had access to the study data, and reviewed and approved this manuscript for publication.
3. RESULTS
3.1. Baseline characteristics and outcomes to therapy
Data were collected from 2157 patients with Crohn's disease in nine studies. Of those, 2070 subjects received at least some form of certolizumab pegol induction therapy prior to week 6 and had a baseline value available for the efficacy outcome. The median age was 35 years, 56% were female, and 92% were white (Table S1). At week 6 of treatment with certolizumab pegol, 55.3% (n = 1144/2070) achieved clinical response and 35.2% (n = 729/2070) achieved clinical remission. Of the patients with C‐reactive protein > 5 mg/L at baseline, 27.1% (n = 346/1277) achieved C‐reactive protein of ≤ 5 mg/L at week 6 and of the patients with faecal calprotectin > 250 μg/g at baseline, 13.9% (n = 73/525) achieved faecal calprotectin of ≤ 250 μg/g at week 6.
3.2. Quartile analysis
A quartile analysis of simulated certolizumab pegol concentrations at week 6 provided evidence of exposure‐response relationships with certolizumab pegol concentration and outcome measures (Figure 1A‐E). Substantial proportions of responders were present in all quartiles, however, certolizumab pegol therapy was effective for a smaller proportion of patients in the lowest quartiles compared with the highest (Figure 1A‐E). The strongest exposure‐response relationship was observed for C‐reactive protein ≤ 5 mg/L and faecal calprotectin ≤ 250 μg/g, with a ~45% increase in the proportion of patients achieving the outcome from the first to fourth quartile (Figure 1C and D). Furthermore, a quartile analysis for a composite endpoint comprised of Crohn's disease activity index ≤ 150 and faecal calprotectin ≤ 250 μg/g revealed a significant relationship between certolizumab concentration and the robust efficacy outcome (P < .001; Figure 1E).
Figure 1.
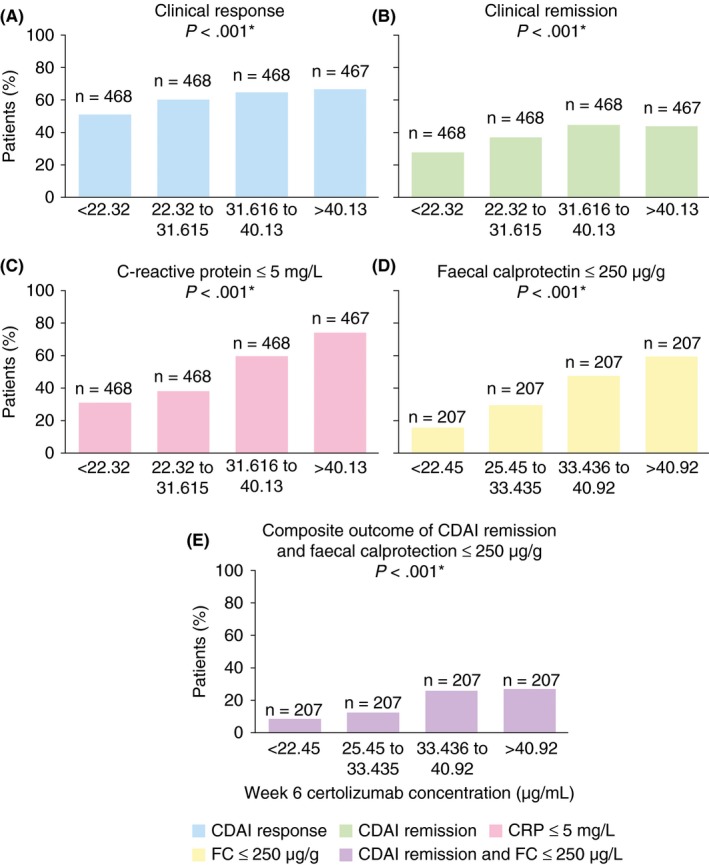
Quartile analyses of endpoints at week 6. *One‐sided Cochran‐Armitage Trend test. CDAI, Crohn's disease activity index
3.3. Certolizumab pegol exposure by efficacy outcome
Patients who achieved clinical response at week 6 had significantly higher certolizumab pegol concentrations at week 2 (20.6 vs 20.3; P = .050), week 4 (31.0 vs 28.0 μg/mL; P < .001) and week 6 (32.6 vs 29.5 μg/mL; P < .001) relative to those who did not. Similarly, patients with clinical remission at week 6 had significantly higher certolizumab pegol concentrations at week 2 (20.9 vs 20.4; P = .047), week 4 (31.3 vs 28.8 μg/mL; P < .001) and week 6 (33.7 vs 30.0 μg/mL; P < .001) relative to those who did not (Table 1).
Table 1.
Relationship between measures of certolizumab pegol exposure and various outcomes at week 6
| Week 6 endpoint | Week 6 endpoint achieved | P valuea | |||
|---|---|---|---|---|---|
| No | Yes | ||||
| n | Median (Q1, Q3) CZP concentration μg/mL | n | Median (Q1, Q3) CZP concentration μg/mL | ||
| CDAI response (decrease of ≥ 100 from baseline or CDAI ≤ 150) | |||||
| Week 2 CZP | 916 | 20.3 (15.8, 25.4) | 1144 | 20.6 (16.8, 25.6) | .050 |
| Week 4 CZP | 865 | 28.0 (18.9, 37.6) | 1143 | 31.0 (23.2, 39.8) | <.001 |
| Week 6 CZP | 743 | 29.5 (19.3, 38.3) | 1128 | 32.6 (24.4, 41.2) | <.001 |
| AUCCWeek 0‐6 | 917 | 1507.1 (1057.9, 1800.0) | 1144 | 1704.8 (1448.8, 1939.9) | <.001 |
| CDAI remission (CDAI ≤ 150) | |||||
| Week 2 CZP | 1331 | 20.4 (15.9, 25.5) | 729 | 20.9 (17.2, 25.5) | .047 |
| Week 4 CZP | 1280 | 28.8 (20.1, 38.9) | 728 | 31.3 (24.1, 39.2) | <.001 |
| Week 6 CZP | 1154 | 30.0 (20.5, 38.8) | 717 | 33.7 (25.4, 41.4) | <.001 |
| AUCCWeek 0‐6 | 1332 | 1584.3 (1205.1, 1846.0) | 729 | 1716.1 (1488.0, 1940.6) | <.001 |
| C‐reactive protein ≤ 5 mg/L | |||||
| Week 2 CZP | 1086 | 19.1 (15.0, 23.6) | 973 | 22.3 (18.4, 26.7) | <.001 |
| Week 4 CZP | 1035 | 26.8 (19.0, 35.0) | 972 | 32.9 (25.7, 41.0) | <.001 |
| Week 6 CZP | 924 | 27.2 (18.6, 34.5) | 947 | 36.0 (27.6, 44.0) | <.001 |
| AUCCWeek 0‐6 | 1087 | 1509.4 (1139.7, 1750.2) | 973 | 1774.9 (1494.7, 2003.4) | <.001 |
| Faecal calprotectin ≤ 250 μg/g | |||||
| Week 2 CZP | 583 | 19.8 (16.8, 24.6) | 314 | 22.9 (20.0, 26.8) | <.001 |
| Week 4 CZP | 563 | 28.4 (22.5, 36.9) | 314 | 34.9 (29.5, 41.7) | <.001 |
| Week 6 CZP | 514 | 30.0 (23.0, 37.0) | 314 | 38.6 (32.5, 44.5) | <.001 |
| AUCCWeek 0‐6 | 583 | 1612.5 (1398.7, 1830.3) | 314 | 1839.7 (1664.9, 2025.0) | <.001 |
| CDAI remission (CDAI ≤ 150) and faecal calprotectin ≤ 250 μg/g | |||||
| Week 2 CZP | 747 | 20.6 (17.5, 25.9) | 150 | 22.1 (19.7, 26.4) | .003 |
| Week 4 CZP | 727 | 30.3 (24.0, 39.5) | 150 | 34.2 (29.6, 41.3) | <.001 |
| Week 6 CZP | 678 | 32.0 (24.4, 39.9) | 150 | 38.0 (32.8, 43.5) | <.001 |
| AUCCWeek 0‐6 | 747 | 1668.1 (1443.7, 1880.7) | 150 | 1826.5 (1649.0, 2005.3) | <.001 |
AUCC, area under concentration‐time curve (expressed as μgd/mL); CDAI, Crohn's disease activity index; CZP, certolizumab pegol.
Wilcoxon two‐sample test.
The concentrations of certolizumab pegol were significantly higher in patients who achieved C‐reactive protein ≤ 5 mg/L at week 6 relative to those who did not (week 2: 22.3 vs 19.1 μg/mL; week 4: 32.9 vs 26.8 μg/mL; week 6: 36.0 vs 27.2; all P < .001). Similarly, certolizumab pegol concentrations were significantly higher in patients who achieved faecal calprotectin ≤ 250 μg/g at week 6 relative to those who did not (week 2: 22.9 vs 19.8 μg/mL; week 4: 34.9 vs 28.4 μg/mL; week 6: 38.6 vs 30.0 μg/mL; all P < .001). Also, for the composite endpoint of clinical remission (Crohn's disease activity index ≤ 150) and faecal calprotectin ≤ 250 μg/g at week 6, higher certolizumab pegol concentrations were observed in those who achieved the outcome vs those who did not at week 2 (22.1 vs 20.6; P = .003), week 4 (34.2 vs 30.3; P < .001) and week 6 (38.0 vs 32.0; P < .001) (Table 1).
Patients who achieved clinical response, clinical remission, C‐reactive protein ≤ 5 mg/L, or faecal calprotectin ≤ 250 μg/g had significantly higher medians for area under the concentration‐time curve for certolizumab pegol from week 0 to week 6 than those who did not (P < .001 for all; Table 1). The differences in exposure were also explored graphically by comparing certolizumab pegol concentration‐time curves (week0‐6) for the outcomes C‐reactive protein ≤ 5 mg/L and faecal calprotectin ≤ 250 μg/g at week 6 (Figure 2).
Figure 2.
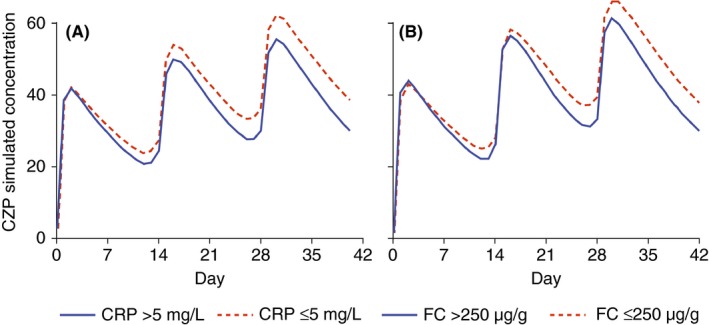
Concentration‐time curves (weeks 0‐6) for patients who achieved, or did not achieve (A) C‐reactive protein ≤ 5 mg/L or (B) faecal calprotectin ≤ 250 μg/g at week 6 of treatment with certolizumab pegol. CRP, C‐reactive protein; FC, faecal calprotectin
3.4. Logistic regression analysis
Multivariable logistic regression was carried out to identify covariates associated with a robust composite outcome of clinical remission (Crohn's disease activity index ≤ 150) and faecal calprotectin ≤ 250 μg/g to certolizumab pegol therapy. The composite outcome was achieved by 145/791 patients (18.3%) at week 6 and by 79/425 patients (18.6%) at week 26. Results of univariable associations are shown in Table S2.
The final logistic regression model for induction treatment through week 6 showed that higher certolizumab pegol concentration at week 6 (odds ratio, OR 4.86 [95% confidence interval, CI 2.20, 10.71]), no prior anti‐TNF (OR 1.89 [95% CI 1.14, 3.12]), higher baseline body weight (OR 1.03 [95% CI 1.01, 1.04]), lower baseline C‐reactive protein (OR 0.98 [95% CI 0.96, 1.00]), lower baseline Crohn's disease activity index (OR 0.99 [95% CI 0.99, 1.00]), female gender (OR 1.94 [95% CI 1.19, 3.16]) and higher baseline haematocrit (OR 1.13 [95% CI 1.06, 1.20]) were independently associated with the composite outcome at week 6 (Table 2). The model was able to discriminate responders from non‐responders with a c‐statistic of 0.80.
Table 2.
Multiple logistic regressions for composite endpoint CDAI ≤ 150 and FC ≤ 250 μg/g
| Odds ratio (95% CI) | ||
|---|---|---|
| P value | ||
| Week 6 induction | ||
| CZP concentration at week 6a | 4.86 (2.20, 10.71) | <.001 |
| No prior anti‐TNF | 1.89 (1.14, 3.12) | .013 |
| Body weight | 1.03 (1.01, 1.04) | <.001 |
| CRP | 0.98 (0.96, 1.00) | .014 |
| CDAI | 0.99 (0.99, 1.00) | <.001 |
| Gender (female) | 1.94 (1.19, 3.16) | .008 |
| Haematocrit | 1.13 (1.06, 1.20) | <.001 |
| Week 26 maintenance | ||
| CZP concentration at week 6a ‐ ADAb‐ at maintenanceb | 15.04 (3.89, 58.19) | <.001 |
| CZP concentration at week 6a ‐ ADAb+ at maintenanceb | 0.49 (0.07, 3.23) | .456 |
| CZP concentration at week 12a | 1.85 (1.28, 2.68) | .001 |
| Body weight | 1.03 (1.01, 1.05) | <.001 |
| CDAI | 0.99 (0.99, 1.00) | <.001 |
ADAb, antidrug antibody; CDAI, Crohn's disease activity index; CI, confidence interval; CRP, C‐reactive protein; CZP, certolizumab pegol; FC, faecal calprotectin.
Log‐transformed concentration used in models.
The interaction between the log‐transformed week 6 CZP and maintenance ADAb was statistically significant (P = .003).
The final logistic regression model for maintenance treatment through week 26 showed that higher certolizumab pegol concentrations at week 6 in patients who did not develop ADAb to certolizumab pegol (OR 15.04 [95% CI 3.89, 58.19]), higher certolizumab pegol concentration at week 12 (OR 1.85 [95% CI 1.28, 2.68]), higher baseline body weight (OR 1.03 [95% CI 1.01, 1.05]) and lower baseline Crohn's disease activity index (OR 0.99 [95% CI 0.99, 1.00]), were associated with the composite outcome at week 26 (Table 2). The model was able to discriminate responders from non‐responders with a c‐statistic of 0.78.
3.5. Threshold analysis
Although the relationship between certolizumab pegol exposure and response varied among patients, certolizumab pegol concentration thresholds of at least 36.1 μg/mL and 14.8 μg/mL at week 6 and 12 (as defined by the Youden index), respectively, were associated with weeks 6 and 26 efficacy outcomes in receiver operating characteristic analyses (Table 3 and Figure S2). Thresholds of 44 μg/mL and 48 μg/mL at week 6 were associated with 90% specificity of achieving C‐reactive protein ≤ 5 mg/L or faecal calprotectin ≤ 250 μg/g and with Crohn's disease activity index remission or the composite endpoint, respectively, at week 6.
Table 3.
Receiver operating characteristic analysis of weeks 6 and 12 certolizumab pegol concentrations and weeks 6 and 26 outcomes
| Outcome | Cut‐point week 6 (μg/mL) | Sensitivity (%) | Specificity (%) | Positive predictive value (%) | Negative predictive value (%) | AUC | Cut‐point week 12 (μg/mL) | Sensitivity (%) | Specificity (%) | Positive predictive value (%) | Negative predictive value (%) | AUC |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Week 6 CDAI response | 31.8*** | 53.7 | 57.6 | 65.8 | 45.1 | 0.575 | — | — | — | — | — | — |
| Week 6 CDAI remission | 31.8*** | 57.5 | 56.0 | 44.8 | 67.9 | 0.578 | — | — | — | — | — | — |
| Week 6 CRP ≤ 5 mg/L | 31.9*** | 65.1 | 68.2 | 67.7 | 64.3 | 0.690 | — | — | — | — | — | — |
| Week 6 FC ≤ 250 μg/g | 32.7*** | 73.9 | 60.3 | 53.2 | 79.1 | 0.710 | — | — | — | — | — | — |
| Week 26 FC ≤ 250 μg/g | — | — | — | — | — | — | 13.8** | 68.1 | 56.0 | 41.9 | 79.0 | 0.629 |
| Week 6 CDAI ≤ 150 and FC ≤ 250 μg/g | 34.5*** | 66.7 | 59.7 | 26.8 | 89.0 | 0.652 | — | — | — | — | — | — |
| Week 26 CDAI ≤ 150 and FC ≤ 250 μg/g | 36.1*** | 67.1 | 65.0 | 22.8 | 92.7 | 0.668 | 14.8*** | 73.2 | 57.3 | 28.0 | 90.4 | 0.669 |
AUC, area under receiver operator curve; CDAI, Crohn's disease activity index; CRP, C‐reactive protein; CZP, certolizumab pegol; FC, faecal calprotectin. **, P < .01; ***, P < .001.
4. DISCUSSION
This analysis originated from a large data set that was pooled from nine clinical trials of certolizumab pegol treatment for Crohn's disease encompassing 2157 patients with 12 926 analysable records. Our approach of using a population pharmacokinetic model that accounts for the time‐varying nature of covariates allowed for precise simulation of drug concentrations at exact time points. Using simulated data for evaluating the exposure‐response relationship constitutes an improvement over using observed drug concentration data, as this allows data to be pooled from different studies with disparate trial designs and sampling time points, and alleviates common problems such as sampling errors, protocol deviations and missing samples that may confound the results. Our data consistently show that there is a relationship between exposure to certolizumab pegol and clinically important outcomes at the end of induction (week 6) and maintenance therapy (week 26) with certolizumab pegol. Taken together, the exposure‐response data could provide an opportunity to enhance clinical outcomes for patients with Crohn's disease that are treated with certolizumab pegol.
Median certolizumab pegol concentrations were higher in patients who achieved clinical response and/or remission, as well as biologic remission defined by objective markers of inflammation like C‐reactive protein and faecal calprotectin. These findings are consistent with findings of other TNF antagonists, as previously an inverse relationship was shown between serum concentrations of infliximab and levels of C‐reactive protein.16 Furthermore, median concentrations of infliximab have also been associated with mucosal healing,17 and higher trough concentrations of adalimumab were associated with clinical response to dose escalation.18 In patients with ulcerative colitis, an exposure‐response relationship was shown for infliximab and golimumab.19, 20
Quartile analyses based on certolizumab pegol concentration showed consistently that higher certolizumab pegol concentrations are associated with clinical response, clinical remission, C‐reactive protein ≤ 5 mg/L, faecal calprotectin ≤ 250 μg/g, and a composite outcome of clinical remission and faecal calprotectin ≤ 250 μg/g. A stronger relationship (~45% increase in the proportion of patients achieving the outcome from first to fourth quartile) was observed for objective markers of inflammation such as C‐reactive protein and faecal calprotectin. These data support previous analyses showing that higher concentrations of certolizumab pegol are positively associated with endoscopic remission.1
Previous analyses examining associations between certolizumab pegol concentrations and Crohn's disease activity index had mixed results.1, 7, 14, 16, 21 Objective markers of inflammation and severity of Crohn's disease include C‐reactive protein and faecal calprotectin, which have been associated with disease activity and endoscopic healing.22, 23, 24 Specifically, faecal calprotectin values of ≤ 250 μg/g are indicative of endoscopic remission.23 In the present analyses, C‐reactive protein and faecal calprotectin were included as efficacy outcomes and these biomarkers were significantly associated with certolizumab pegol concentrations. Interestingly, sensitivity analyses showed that varying the faecal calprotectin threshold (50 μg/g vs 175 μg/g vs 250 μg/g) did not significantly change the discriminatory power of certolizumab pegol concentrations (data not shown). The robust composite outcome of Crohn's disease activity index ≤ 150 with faecal calprotectin ≤ 250 μg/g encompassed both clinical and endoscopic remission indicators, and was significantly associated with higher certolizumab pegol concentrations at both weeks 6 and 12. We recognise that a limitation of our study is that historic trials in Crohn's disease did not routinely assess endoscopic outcomes and thus these data were not available for analysis. However, a range of clinically important outcomes were evaluated, some of which are routinely used in clinical practice, and may improve the application of our results in real life.
The multivariable analysis showed that when patients were negative for ADAb to certolizumab pegol, drug concentrations at week 6 were significantly associated with the composite outcome at week 26. Previously, the presence of ADAb has been associated with increased clearance of TNF antagonists,10 increased incidence of adverse events25 and with loss of response to treatment.26 However, until now, it has been difficult to directly associate the presence or absence of ADAb with efficacy endpoints.
The receiver operating characteristic analyses showed moderate sensitivity and specificity for various certolizumab pegol concentration thresholds for the outcomes described here. Although the relationship between certolizumab pegol exposure and response varied among patients, approximate concentrations of at least 36 μg/mL and 15 μg/mL certolizumab pegol at weeks 6 and 12 were associated with weeks 6 and 26 outcomes. Previously, receiver operating characteristic analyses of adalimumab exposure and response relationships revealed that higher concentrations of adalimumab were predictive of improvements in C‐reactive protein and clinical remission, however, these relationships were not observed if the presence of ADAb was assessed.27
Measurements of ADAbs may vary from one testing method to another, presenting a challenge for interpreting the data. Ligand‐binding assays are the most common method of ADAb measurement. One such assay, ELISA, is used in these studies but it is susceptible to non‐specific binding and false‐positive findings. Several commercial assays for various anti‐TNF antibodies are available. A new assay for measuring certolizumab pegol and certolizumab pegol‐ADAb has recently been introduced, which may address some of these concerns (Miraca Life Sciences, Inc., Irving, TX, USA).28, 29 One limitation of our study was the assay used to measure ADAb, which was drug sensitive and not able to detect ADAb in samples with detectable certolizumab pegol.
It has been proposed that therapeutic drug monitoring of TNF antagonists has the potential to be useful for predicting or preventing treatment failure in patients with Crohn's disease, and it may be helpful for deciding therapeutic interventions after primary loss of efficacy,8 and to make treatment more cost‐effective.30 Knowledge of specific drug concentration thresholds that correspond with efficacy outcomes, as identified in this analysis, may inform therapeutic drug monitoring and guide decisions in clinical practice.
Overall, these results suggest that achieving higher concentrations of certolizumab pegol during induction and maintenance therapy may improve biological and clinical response. The large size of this data set and the multiple analytical approaches constitute an important improvement over previous exposure‐response analyses.14, 16, 31 These results now need to be corroborated in a prospective, interventional study including endoscopic outcomes that will test the approach of dosing based on drug exposure, by using the described population pharmacokinetic model to calculate, in individual patients, the exact dose needed to achieve the proposed certolizumab pegol concentration thresholds.
AUTHORSHIP
Guarantor of the article: N. Vande Casteele.
Author contributions: NVC, BF, SV, MY, GK and WJS contributed to the concept, design and analyses of the study. JC provided meaningful reviews of the data. All authors reviewed the paper and approved the final version of the article, including the authorship list.
Supporting information
Data S1
ACKNOWLEDGEMENTS
The authors would like to acknowledge the investigators and patients participating in the studies used for this analysis. The authors would like to acknowledge Larry Stitt for performing the statistical analyses presented in this manuscript. The authors acknowledge Patricia McChesney, PhD, CMPP, and Fiona Nitsche, PhD, CMPP, of Evidence Scientific Solutions for writing and editorial assistance, which was funded by UCB Pharma.
Declaration of personal interests: NVC received consultancy fees from Boehringer Ingelheim, UCB Pharma, Pfizer and Takeda. BF is a speakers’ bureau member for Abbott/AbbVie, Johnson & Johnson/Janssen Pharmaceuticals, Takeda, UCB and Warner Chilcott; served as a consultant and advisory board member for Abbott/AbbVie, ActoGeniX, Albireo Pharma, Amgen, AstraZeneca, Avaxia Biologics, Axcan Pharma, Baxter Healthcare, Boehringer Ingelheim, Bristol‐Myers Squibb, Calypso Biotech, Celgene, Elan/Biogen, EnGene, Ferring Pharmaceuticals, Roche/Genentech, Gicare Pharma, Gilead, Given Imaging, GlaxoSmithKline, Ironwood Pharmaceuticals, Janssen Biotech, Johnson & Johnson/Janssen Pharmaceuticals, Kyowa Hakko Kirin, Lexicon Pharmaceuticals, Lilly, Merck, Millennium, Nektar, Novo Nordisk, Pfizer, Prometheus Laboratories, Protagonist Therapeutics, Receptos, Salix, Serono, Shire, Sigmoid Pharma, Synergy, Takeda, Teva, Tillotts Pharma, Vertex, Warner Chilcott, Wyeth, Zealand Pharma and Zyngenia; and has contracted research with Abbott/AbbVie, Amgen, AstraZeneca, Bristol‐Myers Squibb, Janssen Biotech, Johnson & Johnson/Janssen Pharmaceuticals, Roche/Genentech, Millennium, Pfizer, Receptos, Sanofi, Santarus, Tillotts Pharma and UCB Pharma. SV has received financial support for research from AbbVie, Centocor, MSD, Takeda and Pfizer; has received lecture fees from AbbVie, Ferring Pharmaceuticals, Hospira, Johnson & Johnson, MSD, Takeda, Pfizer; and has served as a consultant for AbbVie, Ferring Pharmaceuticals, Galapagos, Genentech/Roche, Hospira, MSD, Mundipharma, Pfizer, Celgene, Gilead and Takeda. MY, JC and GK are employees and stockholders of UCB Pharma. WJS has served as a speaker, consultant or advisory board member for Abbott, ActoGeniX NV, AGI, Alba, Albireo, Alfa Wasserman, Amgen, AM‐Pharma BV, Anaphore, Astellas, Athersys, Atlantic Healthcare, Axcan (now Aptalis), BioBalance, Boehringer Ingelheim, Bristol‐Myers Squibb, Celek, Celgene, Cellerix SL, Cerimon, ChemoCentryx, CoMentis, Coronado Biosciences, Cosmo, Cytokine PharmaSciences, Eagle Pharmaceuticals, Eisai, Elan, Eli Lilly, enGene, EnteroMedics, Exagen Diagnostics, Ferring, Flexion, Funxional Therapeutics, Genentech (Roche), Genzyme, Gilead Sciences, Given Imaging, GlaxoSmithKline, Human Genome Sciences, Ironwood Pharmaceuticals, Janssen, KaloBios, Lexicon Pharmaceuticals, Lycera, Meda Pharmaceuticals, Merck Research Laboratories, Merck Serono, Millennium (Takeda), Nisshin Kyorin, Novo Nordisk, NPS Pharmaceuticals, Optimer Pharmaceuticals, Orexigen, PDL BioPharma, Pfizer, Procter & Gamble, Prometheus, ProtAb, PurGenesis, Receptos, Relypsa, Salient, Salix, Santarus, Schering Plough, Shire, Sigmoid Pharma, Sirtris, S. L. A. Pharma, Targacept, Teva, Therakos, Tillotts Pharma, TxCell SA, UCB Pharma, Vascular Biogenics, Viamet, Warner Chilcott and Wyeth (Pfizer); received speakers’ fees from Abbott, Bristol‐Myers Squibb and Janssen; and received financial support for research from Abbott, Bristol‐Myers Squibb, Genentech (Roche) and GlaxoSmithKline.
Declaration of funding interests: The studies and this analysis were funded in full by UCB Pharma. Writing and editorial assistance was provided by Patricia McChesney, PhD, CMPP, and Fiona Nitsche, PhD, CMPP, of Evidence Scientific Solutions (Southport, CT, USA), which was funded by UCB Pharma.
Vande Casteele N, Feagan BG, Vermeire S, et al. Exposure–response relationship of certolizumab pegol induction and maintenance therapy in patients with Crohn's disease. Aliment Pharmacol Ther. 2018;47:229‐237. https://doi.org/10.1111/apt.14421
The Handling Editor for this article was Dr. Nicholas Kennedy, and it was accepted for publication after full peer‐review.
REFERENCES
- 1. Colombel JF, Sandborn WJ, Allez M, et al. Association between plasma concentrations of certolizumab pegol and endoscopic outcomes of patients with Crohn's disease. Clin Gastroenterol Hepatol 2014;12:423‐431.e1. [DOI] [PubMed] [Google Scholar]
- 2. Sandborn WJ, Feagan BG, Stoinov S, et al. Certolizumab pegol for the treatment of Crohn's disease. N Engl J Med. 2007;357:228‐238. [DOI] [PubMed] [Google Scholar]
- 3. Sandborn WJ, Lee SD, Randall C, et al. Long‐term safety and efficacy of certolizumab pegol in the treatment of Crohn's disease: 7‐year results from the PRECiSE 3 study. Aliment Pharmacol Ther. 2014;40:903‐916. [DOI] [PubMed] [Google Scholar]
- 4. Schreiber S, Khaliq‐Kareemi M, Lawrance IC, et al. Maintenance therapy with certolizumab pegol for Crohn's disease. N Engl J Med. 2007;357:239‐250. [DOI] [PubMed] [Google Scholar]
- 5. Lichtenstein GR, Thomsen OØ, Schreiber S, et al. Continuous therapy with certolizumab pegol maintains remission of patients with Crohn's disease for up to 18 months. Clin Gastroenterol Hepatol. 2010;8:600‐609. [DOI] [PubMed] [Google Scholar]
- 6. Ogata H, Ito H, Motoya S, et al. Certolizumab pegol is effective at inducing and maintaining response and remission in Japanese patients with Crohn's disease: results from a Japanese induction and maintenance study [abstract]. Gut. 2009;58:A170. [Google Scholar]
- 7. Ordás I, Feagan BG, Sandborn WJ. Therapeutic drug monitoring of tumor necrosis factor antagonists in inflammatory bowel disease. Clin Gastroenterol Hepatol. 2012;10:1079‐1087. [DOI] [PubMed] [Google Scholar]
- 8. Papamichael K, Gils A, Rutgeerts P, et al. Role for therapeutic drug monitoring during induction therapy with TNF antagonists in IBD: evolution in the definition and management of primary nonresponse. Inflamm Bowel Dis. 2015;21:182‐197. [DOI] [PubMed] [Google Scholar]
- 9. Chaparro M, Guerra I, Munoz‐Linares P, Gisbert JP. Systematic review: antibodies and anti‐TNF‐alpha levels in inflammatory bowel disease. Aliment Pharmacol Ther. 2012;35:971‐986. [DOI] [PubMed] [Google Scholar]
- 10. Wade JR, Parker G, Kosutic G, et al. Population pharmacokinetic analysis of certolizumab pegol in patients with Crohn's disease. J Clin Pharmacol. 2015;55:866‐874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vande Casteele N, Mould DR, Coarse J, et al. Accounting for pharmacokinetic variability of certolizumab pegol in patients with Crohn's disease. Clin Pharmacokinet 2017: doi: 10.1007/s40262‐017‐0535‐3 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 12. Schreiber S, Rutgeerts P, Fedorak RN, et al. A randomized, placebo‐controlled trial of certolizumab pegol (CDP870) for treatment of Crohn's disease. Gastroenterology. 2005;129:807‐818. [DOI] [PubMed] [Google Scholar]
- 13. Sandborn WJ, Abreu MT, D'Haens G, et al. Certolizumab pegol in patients with moderate to severe Crohn's disease and secondary failure to infliximab. Clin Gastroenterol Hepatol. 2010; 8:688‐695.e2. [DOI] [PubMed] [Google Scholar]
- 14. Sandborn WJ, Schreiber S, Feagan BG, et al. Certolizumab pegol for active Crohn's disease: a placebo‐controlled, randomized trial. Clin Gastroenterol Hepatol 2011;9:670‐678.e3. [DOI] [PubMed] [Google Scholar]
- 15. Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3:32‐35. [DOI] [PubMed] [Google Scholar]
- 16. Maser EA, Villela R, Silverberg MS, Greenberg GR. Association of trough serum infliximab to clinical outcome after scheduled maintenance treatment for Crohn's disease. Clin Gastroenterol Hepatol. 2006;4:1248‐1254. [DOI] [PubMed] [Google Scholar]
- 17. Van Moerkercke W, Compernolle G, Ackaert C, et al. Mucosal healing in Crohn's disease is associated with high infliximab trough levels. J Crohns Colitis. 2010;4:30‐31. [Google Scholar]
- 18. Karmiris K, Paintaud G, Noman M, et al. Influence of trough serum levels and immunogenicity on long‐term outcome of adalimumab therapy in Crohn's disease. Gastroenterology. 2009;137:1628‐1640. [DOI] [PubMed] [Google Scholar]
- 19. Adedokun OJ, Sandborn WJ, Feagan BG, et al. Association between serum concentration of infliximab and efficacy in adult patients with ulcerative colitis. Gastroenterology. 2014;147:1296‐1307.e5. [DOI] [PubMed] [Google Scholar]
- 20. Adedokun OJ, Xu Z, Marano CW, et al. Pharmacokinetics and exposure‐response relationship of golimumab in patients with moderately‐to‐severely active ulcerative colitis: results from phase 2/3 PURSUIT induction and maintenance studies. J Crohns Colitis. 2017;11:35‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vande Casteele N, Feagan BG, Gils A, et al. Therapeutic drug monitoring in inflammatory bowel disease: current state and future perspectives. Curr Gastroenterol Rep. 2014;16:378. [DOI] [PubMed] [Google Scholar]
- 22. Magro F, Sousa P, Ministro P. C‐reactive protein in Crohn's disease: how informative is it? Expert Rev Gastroenterol Hepatol. 2014;8:393‐408. [DOI] [PubMed] [Google Scholar]
- 23. D'Haens G, Ferrante M, Vermeire S, et al. Fecal calprotectin is a surrogate marker for endoscopic lesions in inflammatory bowel disease. Inflamm Bowel Dis. 2012;18:2218‐2224. [DOI] [PubMed] [Google Scholar]
- 24. Zittan E, Kelly OB, Kirsch R, et al. Low fecal calprotectin correlates with histological remission and mucosal healing in ulcerative colitis and colonic Crohn's disease. Inflamm Bowel Dis. 2016;22:623‐630. [DOI] [PubMed] [Google Scholar]
- 25. O'Meara S, Nanda KS, Moss AC. Antibodies to infliximab and risk of infusion reactions in patients with inflammatory bowel disease: a systematic review and meta‐analysis. Inflamm Bowel Dis. 2014;20:1‐6. [DOI] [PubMed] [Google Scholar]
- 26. Nanda KS, Cheifetz AS, Moss AC. Impact of antibodies to infliximab on clinical outcomes and serum infliximab levels in patients with inflammatory bowel disease (IBD): a meta‐analysis. Am J Gastroenterol. 2013;108:40‐47;quiz 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mazor Y, Koplov U, Ben Hur D, et al. Evaluating adalimumab drug and antibody levels as predictors of clinical and laboratory response in Crohn's disease patients. J Crohns Colitis. 2013;7:S217‐S219. [Google Scholar]
- 28. Paul S, Smeraglia J, de Longueville M, et al. Comparison of two enzyme‐linked immunosorbent assays used for drug concentration monitoring in psoriatic arthritis patients treated with certolizumab pegol [abstract]. Arthritis Rheumatol. 2016;68:654.26554395 [Google Scholar]
- 29. Wolbink G, Goupille P, Sandborn W, et al. Association between plasma certolizumab pegol concentration and improvement in disease activity in rheumatoid arthritis and Crohn's disease [abstract]. Arthritis Rheumatol 2016;68:423. [Google Scholar]
- 30. Martelli L, Olivera P, Roblin X, Attar A, Peyrin‐Biroulet L. Cost‐effectiveness of drug monitoring of anti‐TNF therapy in inflammatory bowel disease and rheumatoid arthritis: a systematic review. J Gastroenterol. 2017;52:19‐25. [DOI] [PubMed] [Google Scholar]
- 31. Baert F, Noman M, Vermeire S, et al. Influence of immunogenicity on the long‐term efficacy of infliximab in Crohn's disease. N Engl J Med. 2003;348:601‐608. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1


