Abstract
Objective
To report maternal sleep practices in women who experienced a stillbirth compared with controls with ongoing live pregnancies at similar gestation.
Design
Prospective case‐control study.
Setting
Forty‐one maternity units in the United Kingdom.
Population
Women who had a stillbirth after ≥ 28 weeks’ gestation (n = 291) and women with an ongoing pregnancy at the time of interview (n = 733).
Methods
Data were collected using an interviewer‐administered questionnaire that included questions on maternal sleep practices before pregnancy, in the four weeks prior to, and on the night before the interview/stillbirth.
Main outcome measures
Maternal sleep practices during pregnancy.
Results
In multivariable analysis, supine going‐to‐sleep position the night before stillbirth had a 2.3‐fold increased risk of late stillbirth [adjusted Odds Ratio (aOR) 2.31, 95% CI 1.04–5.11] compared with the left side. In addition, women who had a stillbirth were more likely to report sleep duration less than 5.5 hours on the night before stillbirth (aOR 1.83, 95% CI 1.24–2.68), getting up to the toilet once or less (aOR 2.81, 95% CI 1.85–4.26), and a daytime nap every day (aOR 2.22, 95% CI 1.26–3.94). No interaction was detected between supine going‐to‐sleep position and a small‐for‐gestational‐age infant, maternal body mass index, or gestational age. The population‐attributable risk for supine going‐to‐sleep position was 3.7% (95% CI 0.5–9.2).
Conclusions
This study confirms that supine going‐to‐sleep position is associated with late stillbirth. Further work is required to determine whether intervention(s) can decrease the frequency of supine going‐to‐sleep position and the incidence of late stillbirth.
Tweetable abstract
Supine going‐to‐sleep position is associated with 2.3× increased risk of stillbirth after 28 weeks’ gestation.
Plain Language Summary
Stillbirth, the death of a baby before birth, is a tragedy for mothers and families. One approach to reduce stillbirths is to identify factors that are associated with stillbirth. There are few risk factors for stillbirth that can be easily changed, but this study is looking at identifying how mothers may be able to reduce their risk.
In this study, we interviewed 291 women who had a stillbirth and 733 women who had a live‐born baby from 41 maternity units throughout the UK. The mothers who had a stillbirth were interviewed as soon as practical after their baby died. Mothers who had a live birth were interviewed during their pregnancies at the same times in pregnancy as when the stillbirths occurred. We did not interview mothers who had twins or who had a baby with a major abnormality.
Mothers who went to sleep on their back had at least twice the risk of stillbirth compared with mothers who went to sleep on their left‐hand side. This study suggests that 3.7% of stillbirths after 28 weeks of pregnancy were linked with going to sleep lying on the back. This study also shows that the link between going‐to‐sleep position and late stillbirth was not affected by the duration of pregnancy after 28 weeks, the size of the baby, or the mother's weight. Women who got up to the toilet once or more at night had a reduced risk of stillbirth.
This is the largest of four similar studies that have all shown the same link between the position in which a mother goes to sleep and stillbirth after 28 weeks of pregnancy. Further studies are needed to see whether women can easily change their sleep position in late pregnancy and whether changing the position a mother goes to sleep in reduces stillbirth.
Keywords: maternal sleep position, modifiable risk factors, sleep duration, stillbirth
Short abstract
Tweetable abstract
Supine going‐to‐sleep position is associated with 2.3× increased risk of stillbirth after 28 weeks’ gestation.
 This paper includes Author Insights, a video abstract available at https://vimeo.com/rcog/authorinsights14967
This paper includes Author Insights, a video abstract available at https://vimeo.com/rcog/authorinsights14967
Introduction
Stillbirth, the death of a baby before birth, affects between 1.7 and 8.8 per 1,000 births after 28 weeks’ gestation in high‐income countries.1 The annual rate of reduction in stillbirth is 2.0% globally, but also varies widely. Within high‐income countries, there is significant disparity in stillbirth rates, with higher levels in the most economically deprived and among minority ethnic groups.2 The variations in stillbirth rates suggest that further reductions are possible. One strategy to reduce stillbirths is to identify women at increased risk; current studies have identified the importance of factors which cannot be modified in pregnancy (e.g. advanced maternal age, maternal obesity).3
An alternative approach has been to identify aspects of maternal lifestyle, personal habits, or symptoms experienced during pregnancy that are associated with stillbirth. A recent priority‐setting exercise ranked ‘Do modifiable “lifestyle” factors cause or contribute to stillbirth risk?’ in the top eleven priorities for stillbirth research.4 A series of case‐control studies has been conducted in Australia and New Zealand to investigate modifiable factors associated with late stillbirth (≥ 28 weeks’ gestation).5, 6, 7 The first of these, the Auckland Stillbirth Study, reported an association between a supine going‐to‐sleep position and late stillbirth risk [adjusted Odds Ratio (aOR) 2.54, 95% CI 1.04–6.18].5 This study also found an association between stillbirth and prolonged sleep duration (>8 hours; aOR 1.71, 95% CI 0.99–2.95) and having a regular daytime sleep (aOR 2.04, 95% CI 1.26–3.30).5 The accompanying editorial and responses to this study highlighted potential confounding factors, including fetal compromise and a small‐for‐gestational‐age fetus, and also raised the possibility of recall bias.8 Since publication of the Auckland Stillbirth Study, there have been two further studies reporting an association between supine sleep position and late stillbirth that reported aORs of 3.67 and 8.0.6, 7 However, these studies were not sufficiently large to test for interactions. To address this need and to obtain UK data, we conducted the Midlands and North of England Stillbirth Study (MiNESS) which addressed the primary hypothesis that maternal going‐to‐sleep position was associated with late stillbirth. In addition, the secondary hypotheses were that increased maternal sleep duration and sleeping during the day were associated with late stillbirth. Lastly, the study examined whether supine sleep position in conjunction with a compromised baby was associated with late stillbirth.9
Methods
The Midlands and North of England Stillbirth Study was conducted in 41 maternity units in the UK. The study was registered on www.clinicaltrials.gov (NCT02025530) and the study protocol was published.9 Ethical and research approvals were obtained (Ref 13/NW/0874). Participants were recruited between April 2014 and March 2016. Women with multiple pregnancies, a maternal age less than 16 years, or who were unable to give consent were excluded from the study. Cases were included if the stillbirth occurred at or after 28 weeks’ gestation and the fetus was not known to have a congenital anomaly. The causes for stillbirth were classified by two investigators (JB and AH) using the ReCoDe classification system.10 Stillborn babies that were found to have a lethal congenital abnormality on post‐mortem examination were excluded from the final analysis.
Controls were women with an ongoing pregnancy. Women in the control group who subsequently delivered an infant with a congenital abnormality or who had a stillborn baby were excluded from the study analysis. The number of controls selected from each participating hospital was based (in a 2:1 ratio) on the number of stillbirths in the previous four years in that hospital. The control was then randomly allocated a gestational age at which to be interviewed to ensure that the control group had a similar gestational age distribution to that for late stillbirths in that unit, again over the preceding four years. The control was then randomly selected from booking lists to identify a subject of the required EDD.
Eligible women were given a description of the study by their obstetrician or midwife. The participant information sheet stated that the goal of the study was to ‘look at medical factors associated with stillbirth but also environmental and lifestyle factors that can affect pregnancy and the wellbeing of the baby.’ Sleep position and other sleep related variables were not specifically mentioned in any of the participant information. If women did not consent to participate in the study, their age and ethnicity were recorded. Data were collected by an interviewer‐administered questionnaire which was adapted from that used in the Auckland Stillbirth Study. Participants were interviewed by research midwives at each site. For cases, the interview was conducted as close to the time of stillbirth as possible with an aim of being completed 1–6 weeks after the birth. The questionnaire (Appendix S1) included questions on demographics, maternal health, medications, smoking, drug use, fetal movements, maternal mental health, and information about sleep practices. Maternal height and weight were recorded from the antenatal booking visit. Ethnicity was recorded as per the Office of National Statistics recommendations. Education level was used as a measure of socio‐economic status and was classified as no formal education, secondary education, further education, and graduate education. Customised birthweight centiles were calculated using GROW software (Version 6.7.8, 2017, Gestation Network, www.gestation.net), a small‐for‐gestational‐age baby was defined as less than 10th centile. For cases, the gestational age at death was used to calculate the birthweight centile, and the gestational age at delivery was used for controls.
The primary outcome of interest was the odds of late stillbirth associated with self‐reported maternal going‐to‐sleep position on the night prior to stillbirth for cases and the night before the interview for controls. Going‐to‐sleep position was classified as left side, supine, right side, tummy, variable side, propped up, or unknown. The questionnaire also asked about the going‐to‐sleep position in the last four weeks before the baby died and before pregnancy. Women were also asked about the duration of sleep, how many times they got up to the toilet during the last night, and whether they had a daytime nap in the last four weeks.
Statistical analysis
The sample size was calculated based on the prevalence of a risk factor in the controls of 50% (the prevalence of non‐left sleep position in the Auckland Stillbirth Study was 57%). A sample of 291 cases and 582 controls was needed to detect an odds ratio (OR) of 1.5 with a significance level of 0.05 and power of 80%. This sample size would also be sufficient to allow an interaction with an OR of 2.5 to be detected. The number of women approached to participate in the study needed to be greater than this estimate as we anticipated that a proportion of women would not agree to participate in the study. (In the Auckland Stillbirth Study, the proportion successfully recruited was 72%.) Assuming a 30% non‐participation rate, 415 eligible cases and 830 eligible controls would need to be approached.
Statistical analyses were performed using SAS version 9.6 (SAS Institute Inc., Cary, NC, USA). We explored the relationship between continuous variables (maternal age, BMI, birthweight centile, gestation, and sleep duration) and late stillbirth using generalized additive models to determine whether variables should be analysed continuously or categorically.
We examined frequency distributions of categorical variables and normal distribution for the continuous variables. Differences between cases and controls for categorical variables were tested by chi‐square tests. Continuous variables were compared using Wilcoxon rank‐sum tests as no variables were normally distributed. Univariable logistic regression was performed to evaluate the association between sleep practices and late stillbirth risk. A multivariable logistic model was developed incorporating ethnicity and education level, variables associated with increased risk of stillbirth based on previous literature [age, BMI, parity, smoking, small‐for‐gestational‐age (SGA) status], other sleep related variables significant in univariable analysis, and variables used to select cases and controls (gestation and maternity unit). Unconditional logistic regression was used to adjust for potential confounders. No imputation was performed for missing data. The c statistic was used to assess the area under the curve for the model which describes the proportion of stillbirths that can be detected using the model.
Statistical significance was defined at the 5% level. OR and aOR with 95% confidence intervals (CI) were used to estimate risk. The population attributable risk (PAR) (the reduction in incidence that would be observed if the risk factor were absent and that the risk factor was causally related to the outcome) was calculated using the unadjusted OR for the primary outcome, for supine sleep position, and for other risk factors that remained significant in multivariable analysis.
Results
Participants
During the study period, 3490 women were identified as potentially eligible participants (660 cases and 2830 controls, Figure 1). A total of 760 women could not be contacted (77 cases and 683 controls), and 1700 women did not consent to participate in the study (287 cases and 1413 controls). Six cases were excluded after data collection (five stillbirths had previously unidentified congenital abnormalities and one control participant had a stillbirth). Cases were more likely to participate than controls (P < 0.0001), 291 cases (44.1%) and 733 controls (25.9%) participated (Figure 1). Of the women contacted, there was no difference in participation for those of white, black, or South Asian ethnic origin, but women from other ethnic groups were less likely to participate, both in cases and controls [OR for other ethnic group cases of 2.63 (95% CI 1.69–4.07) compared with cases of white ethnic origin; OR for other ethnic group controls of 2.05 (95% CI 1.58–2.65) compared with white ethnic controls]. There was no significant difference in maternal age between participants and non‐participants in the case group (30.2 versus 29.6 years, P = 0.25); control participants were significantly older than control non‐participants (30.5 versus 29.0 years, P < 0.0001).
Figure 1.
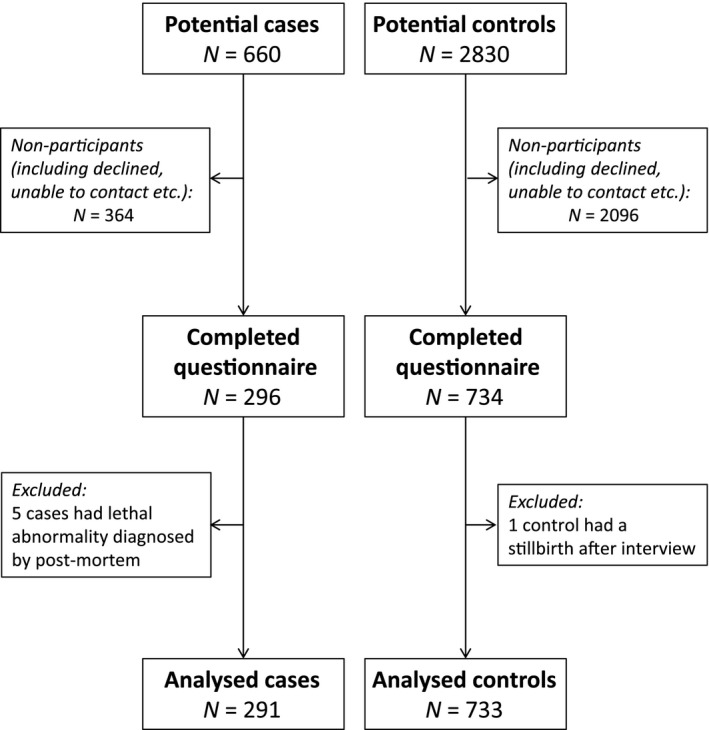
Flow diagram reporting the numbers of women eligible for the study, women who did not participate, and those included in the final analysis.
The median gestational age at interview for controls was 36 weeks 3 days [interquartile range (IQR) 32 weeks 6 days to 38 weeks 5 days]. In cases, the median gestational age at diagnosis of stillbirth was 37 weeks 4 days (IQR 33 weeks 4 days to 39 weeks 5 days, P = 0.003 compared with controls). The median time between date of diagnosing stillbirth and the presumed date of stillbirth was 0 days (IQR 0–1). The median time between the date of diagnosis of stillbirth and the interview was 25 days (IQR 17–35). The ReCoDe classification system indicated that fetal growth restriction (as defined by a baby with a birthweight < 10th centile) was the most frequent factor associated with stillbirth (45.2%, Table 1). The next most frequent causes included placental insufficiency (16.4%), placental abruption (6.5%), and acute infection (4.5%).
Table 1.
Classification of stillbirth using the ReCoDe classification system
| ReCoDe | Classification | Number of cases | Percentage of cases |
|---|---|---|---|
| A2.2 | Acute Infection | 13 | 4.5 |
| A5 | Feto‐maternal haemorrhage | 6 | 2.1 |
| A7 | Fetal growth restriction | 132 | 45.2 |
| B1 | Umbilical cord prolapse | 1 | 0.3 |
| B2 | Constricting loop or knot of cord | 10 | 3.4 |
| C1 | Placental abruption | 19 | 6.5 |
| C3 | Vasa praevia | 1 | 0.3 |
| C4 | Other placental Insufficiency (inc. histological evidence) | 48 | 16.4 |
| D1 | Chorioamnionitis | 6 | 2.1 |
| E1 | Uterine rupture | 1 | 0.3 |
| F1 | Diabetes | 9 | 3.1 |
| F6 | Obstetric cholestasis | 1 | 0.3 |
| G1 | Intrapartum asphyxia | 1 | 0.3 |
| I1 | No relevant condition identified | 42 | 14.4 |
Demographic variables
There was no difference in distribution of maternal age or ethnicity of cases and controls (Table S2). Cases were significantly more likely to be nulliparous (OR 2.37, 95% CI 1.76–3.18) or have parity ≥ 3 (OR 2.63, 95% 1.60–4.33). Univariable analysis showed cases were also more likely to have a lower educational attainment, be unmarried (cohabiting or single), and to smoke cigarettes. Stillborn babies were more likely to have a birthweight less than the 10th customised centile (OR 7.01, 95% CI 4.66–10.53).
Sleep variables
On the last night before stillbirth for the cases or the night before interview for controls, women with late stillbirth were more likely to report that they went to sleep in the supine position (OR 2.17, 95% CI 1.15–4.08, Table S2). Women with late stillbirth had an increased likelihood of not being able to recall their going‐to‐sleep position (OR 3.73, 95% CI 1.67–8.32), but were not more likely to report right‐side going‐to‐sleep position (OR 0.91, 95% CI 0.65–1.26). For the last night before stillbirth, cases reported an increased frequency of short sleep duration (≤ 5.5 hours) or long sleep (≥ 9.5 hours). There was no significant difference in sleep position in the preceding four weeks or prior to pregnancy between cases and controls. Cases were more likely to report not getting up at all or getting up only once to go to the toilet on the last night, compared with controls (OR 2.34, 95% CI 1.70–3.21). Cases were also more likely to have a daytime nap every day during the last four weeks (OR 2.07, 95% CI 1.34–3.18). 37.1% of cases and controls changed their sleeping habits during pregnancy. For the 641 women who changed their sleeping habits, the responses of 56 cases (19.2%) and 126 controls (17.3%) indicated that they paid more attention to their sleeping position; prior to pregnancy, 10.6% of respondents slept on their back, falling to 4.4.% in the four weeks prior to the date of stillbirth or interview (Table S1). The other common change was increased frequency of daytime napping reported by 72 cases (24.7%) and 156 (21.4%) controls; there was no difference in the change of sleeping habits between cases and controls (P = 0.48). A total of 50 cases and 119 controls reported receiving advice about sleep; the proportions did not differ between cases and controls. In participants who reported receiving advice about sleep habits during pregnancy, this was obtained from the internet [28 cases (56%), 50 controls (42%)], health professionals [16 cases (32%), 51 controls (42.9%)], literature [13 cases (26%), 28 controls (23.5%)] and friends and family members [7 cases (14%), 22 controls (18.5%)].
The multivariable analysis (Table S2) found significant associations between late stillbirth and supine going‐to‐sleep position on the last night (aOR 2.31, 95% CI 1.04–5.11), sleep duration ≤ 5.49 hours on the last night (aOR 1.83, 95% CI 1.24–2.68), not getting up at all or getting up only once on the last night (aOR 2.81, 95% CI 1.85–4.26), daytime nap every day (aOR 2.22, 95% CI 1.26–3.94), nulliparity (aOR 1.67, 95% CI 1.14–2.45), parity ≥ 3 (aOR 2.43, 95% CI 1.25–4.71), birthweight < 10th customised centile (aOR 6.22, 95% CI 3.79–10.23) and birthweight 10–49.9th customised centile (aOR 1.62, 95% CI 1.04–2.53). Importantly, there was no detectable interaction between the effect of supine going‐to‐sleep position and a small‐for‐gestational‐age baby (P for interaction = 0.44), maternal obesity (P for interaction = 0.36), preterm or not (P for interaction = 0.57) and maternal smoking status (P for interaction = 0.89). The c statistic for the final multivariable model is 0.827.
The PARs for late stillbirth for the modifiable factors explored in this study are shown in Table 2. The highest PAR occurred with SGA (45%, 95% CI 33.6–56.8%), followed by cigarette smoking (14.0%, 95% CI 22.3–37.1%), maternal obesity (12.0%, 95% CI 3.8–21.5%), and supine going‐to‐sleep position (3.7%, 95% CI 0.5–9.2%).
Table 2.
Population attributable risk (PAR) for stillbirth of modifiable risk factors identified in this study population
| Risk factor | Population exposed | OR | PAR | 95% CI | |
|---|---|---|---|---|---|
| Small for gestational age | 13.8% | 7.01 | 45.3% | 33.6% | 56.8% |
| Smoking during pregnancy | 17.3% | 1.94 | 14.0% | 22.3% | 37.1% |
| Obesity | 19.5% | 1.7 | 12.0% | 3.8% | 21.5% |
| Overweight | 29.5% | 1.3 | 8.1% | −1.5% | 19.5% |
| Supine going‐to‐sleep position | 3.3% | 2.17 | 3.7% | 0.5% | 9.2% |
Discussion
Main findings
The MiNESS study found that women who had a late stillbirth were 2.3 times more likely to report a supine going‐to‐sleep position on the night prior to the fetal death. Other sleep behaviours associated with late stillbirth included short and long sleep duration on the last night, getting up to the toilet only once or not at all on the last night, and daytime napping every day. These associations were independent of gestational age, the presence of an SGA fetus, obesity, education level, parity, and maternal smoking status.
Strengths and limitations
This is the largest study performed to date that has collected detailed information about maternal sleep practices and their relationship with late stillbirth. It recruited to achieve the planned study size, albeit in a longer timeframe than anticipated. As the frequency of late stillbirth in the UK is 2.9 per 1,000 live births, a case‐control design is the most appropriate method to investigate modifiable factors, as a prospective cohort study is not feasible, requiring almost 108 000 women to identify 291 late stillbirths, even more if non‐participation is considered. The most significant limitation of case‐control methodology is the potential for recall bias. With regard to recall of going‐to‐sleep position, good concordance has been shown between questionnaire responses and video‐recorded going‐to‐sleep position.11 Furthermore, maternal reporting of the presumed time of death correlates with the time of death estimated by post‐mortem.12 The potential for bias towards questions about sleep was reduced by using a structured questionnaire that contained questions about many different factors (e.g. smoking, diet, stress, sleep, and fetal movements). Sleep questions were placed towards the middle of the questionnaire. For cases, the time between the stillbirth and interview was 25 days, which might influence the accuracy of the recall but would be unlikely to create recall bias that increased the effect size.13 A further limitation of the case‐control design is that the existence of an unidentified confounding factor cannot be completely excluded.
The participation rate was lower than in the Auckland Stillbirth Study (TASS) and the Sydney Stillbirth Study (SSS) (cases: MiNESS 45% versus 72% and 67%, respectively; controls: MiNESS 26% versus 72% and 85%, respectively).5, 6 The possibility of selection bias was minimised by recruiting controls frequency‐matched to cases over the duration of the study period; this resulted in similar ages and ethnicities of cases and controls. For logistical and local ethical policy reasons we were not able to collect consistent detailed data for participants and non‐participants, but women in participant and non‐participant groups had similar profiles of maternal age and ethnicity, suggesting that the population is broadly representative of the maternity population in the study regions. The representativeness is also supported by the similar profile of causes of stillbirth by ReCoDe classification from the same geographical region.10, 14 This study used a similar questionnaire to TASS and MCSS, which facilitates comparison of the results. An individual participant data (IPD) meta‐analysis (PROSPERO registration: CRD42017047703) is planned that will further increase the statistical power to detect differences between stillbirths and live‐born controls and investigate potential interactions.15
Interpretation
Importantly, the risk factors identified from our multivariable analysis, including obesity, SGA infants, cigarette smoking, and low educational attainment,3, 16 are consistent with those reported from individual high‐quality studies and meta‐analyses of large‐scale studies in high‐income countries. The multivariable model employed here had an area under the curve of 82%, suggesting that risk factors identified in the current study have good predictive value for late stillbirth. These PARs emphasise the vital importance of measures to address the detection and optimal management of SGA infants and strategies for smoking cessation that are each key components of the ‘Saving Babies Lives Care Bundle’ produced by NHS England.17 For the population studied here, the largest PAR was for SGA, defined a priori as birthweight below the 10th percentile using customised birthweight centiles.9 Recent analysis of Scottish data suggests that there is no difference in the strength of association between SGA and adverse neonatal outcomes irrespective of whether the data were non‐customised or partially customised.18 Observational studies show that screening and management of SGA according to clinical guidelines and smoking cessation in early pregnancy are associated with a reduction in stillbirth.19, 20, 21 Therefore, these data emphasise the importance of large‐scale quality improvement and health promotion programmes to ensure that such evidence‐based practice is available to all pregnant women.
The association between supine going‐to‐sleep position and late stillbirth is consistent with the findings from three other published case control studies and one cross‐sectional study.5, 6, 7, 22 The potential importance of sleep practices in relation to stillbirth is highlighted by one study that found that 40.1% of mothers thought their baby died during the night.23 Interpretation of the association between supine going‐to‐sleep position and late stillbirth is considered in light of the Bradford Hill criteria (Table 3).24 The estimates of effect size from the four case‐control studies and the cross‐sectional study from Ghana are of a similar magnitude, which suggests that the effect is consistent across different populations, including in a low‐income country.5, 6, 7, 22 The proportion of mothers going to sleep in the supine position was also similar between these study populations.5, 6, 7, 22 The nature of the questionnaire, which explored events in late pregnancy occurring prior to stillbirth, ensures that the timing of the exposure occurred prior to fetal death.
Table 3.
Evaluation of the observations of maternal sleep position against the Bradford Hill criteria
| Criteria | Observation |
|---|---|
| Analogy | There are parallels with the relationship between infant sleeping prone and risk of SIDS. |
| Biological gradient | These case control studies do not record proportion of the night spent supine, so a biological gradient cannot be determined. |
| Coherence | Coherence between epidemiological and laboratory findings increases the likelihood of an effect. Maternal supine position results in changes indicative of fetal adaptation to hypoxic stress. |
| Consistency | In four case‐control studies showing association between supine going‐to‐sleep position and late stillbirth, the effect size appears to be similar in three different populations (Australia, UK, New Zealand) |
| Experiment | Evidence of a fall in late stillbirth in New Zealand in the last five years that may be due, at least in part, to documented changes in maternal going‐to‐sleep position (Eleventh Annual Report of the Perinatal and Maternal Mortality Review Committee. Wellington, New Zealand: Health Quality & Safety Commission). |
| Plausibility | Two biologically plausible mechanisms have been proposed, namely sleep disturbed breathing, which is more common in the supine position, and/or inferior vena caval compression and resultant fetal hypoxia. |
| Specificity | There are no data to determine whether the effect of supine‐going‐to sleep position is specific for late stillbirth. |
| Strength | The magnitude of the OR is moderate (For comparison the hazard ratio for mortality for smokers (> 10/d) versus non‐smokers is 1.8.33 |
| Temporality | Maternal sleep position occurs before the death of the baby. |
Other risk factors identified in this study including reduced interruption of sleep (not getting up to the toilet or getting up only once on the last night) suggest that undisturbed periods of sleep may be a risk factor. Although women regularly change their sleep position in the night, the going‐to‐sleep position is the one that is held for the longest and may therefore have the greatest impact on the baby.25
The association between supine going‐to‐sleep position and late stillbirth is biologically plausible. Maternal supine position is associated with compression of the inferior vena cava and reduced venous return, thereby decreasing maternal cardiac output.26, 27 A reduction in cardiac output is consistent with a reduction in uterine arterial blood flow in a supine compared with a left‐lateral position. These changes are associated with changes in fetal oxygenation as measured by reduced resistance in the middle cerebral artery antepartum and oxygen saturation assessed in labour.27, 28 Supine maternal position is recognised to be a contributor to abnormalities in fetal heart‐rate traces in labour, with guidelines from the National Institute for Health and Care Excellence recommending that a supine position be avoided and a change in maternal position be used as part of initial methods to address concerns with the fetal heart rate trace.29 Physiological studies show that the fetus more frequently adopts an F1 behavioural state (quiet sleep), which is an oxygen sparing state,30 when the mother is in the supine position.
Alternative hypotheses also need to be considered. Having an SGA baby might enable women to go to sleep supine, as they may be less uncomfortable and less likely to have symptoms of supine hypotension. In this study, however, there was no interaction between an SGA infant and supine going‐to‐sleep position. Another hypothesis is that supine position increases the risk of sleep‐disordered breathing, but TASS found no association between symptoms of sleep‐disordered breathing and the risk of late stillbirth, and the effect of supine going‐to‐sleep position is independent of maternal BMI, which predisposes to sleep‐disordered breathing. The planned IPD meta‐analysis will further increase the statistical power to detect differences between stillbirths and live‐born controls and allow more detailed exploration of potential confounding factors.15 Similarly, the IPD studies will further investigate whether right‐sided going‐to‐sleep position, which was associated with late stillbirth in TASS, but not in MiNESS, SSS, or MCSS, is independently associated with late stillbirth.
Conclusion
Maternal supine going‐to‐sleep position is associated with increased risk of late stillbirth in a UK setting. This effect has now been demonstrated consistently by five studies in populations comprising different ethnicities and geographical settings. Consideration should now be given to whether an intervention to reduce the proportion of women going to sleep in the supine position can effectively alter maternal behaviour and whether this change in behaviour would reduce the frequency of late stillbirth. This study suggests that a high proportion of women change their sleeping habits in pregnancy, suggesting that going‐to‐sleep position could be modified. This is consistent with the findings of a study of 377 women from a diverse multi‐ethnic population in New Zealand, in which 68% had received advice about sleep position and 87% felt they would have minimal difficulty changing their going‐to‐sleep position if it were recommended.31 In common with other aspects of pregnancy information,32 a high proportion of women used the internet to obtain advice about sleeping position. Such programmes should therefore include internet‐based strategies to inform women about sleep position that can be reinforced by contact with health care professionals. While an intervention study to confirm these observational data is desirable, it is not practical. This study predicts that late stillbirth in England would decrease by 3.7% if no mother went to sleep supine, but it would require an intervention study with a very large sample size – more than 1 000 000 participants in each arm – to prove it. We therefore believe that, given the consistency of findings between studies, it is timely to evaluate whether going‐to‐sleep position can be modified, and how this can best be achieved.
Disclosure of interests
None declared. Completed disclosure of interests form available to view online as supporting information.
Contribution to authorship
AH, TS, BM, DR, EM, and LM contributed to all aspects of the study design and obtained funding. AH had overall responsibility for the study. JB coordinated the running of the study. ML and JT analysed the data with input from AH, JB, RC, EM, and LM. All authors were responsible for the drafting of the manuscript. All authors gave approval for the final version of the manuscript.
Details of ethical approval
This study was reviewed by NRES Committee North West ‐ Greater Manchester Central, Reference 13/NW/0874. Approval was granted on 20 January 2014.
Supporting information
Table S1. Demographic characteristics and sleep practices in 291 women who had a late stillbirth between 2014 and 2016 compared with 733 controls that were not included in the multivariable model.
Table S2. Demographic characteristics and sleep practices in 291 women who had a late stillbirth between 2014 and 2016 compared with 733 controls. Data are presented as the number (percentage) or median (interquartile range).
Appendix S1. Participant data collection questionnaire used in the MiNESS study.
Video S1. Author insights.■
Acknowledgements
The authors thank all who participated in interviews in order to help us better understand stillbirth. The authors would also like to thank the Principal Investigators, Research Midwives, and Nurses at the following institutions for their hard work and dedication to this study: Airedale NHS Foundation Trust, Birmingham Women's NHS Trust, Blackpool Teaching Hospitals NHS Foundation Trust, Bradford Teaching Hospitals NHS Foundation Trust, Buckinghamshire Healthcare NHS Trust, Burton Hospitals NHS Foundation Trust, Calderdale and Huddersfield NHS Foundation Trust, Central Manchester Hospitals NHS Foundation Trust, Countess of Chester Hospitals NHS Foundation Trust, County Durham and Darlington NHS Foundation Trust, East Lancashire Hospitals NHS Trust, Harrogate and District NHS Foundation Trust, Heart of England NHS Foundation Trust, Hull and East Yorkshire Hospitals NHS Trust, Lancashire Teaching Hospitals NHS Foundation Trust, Leeds Teaching Hospitals NHS Trust, Liverpool Women's NHS Foundation Trust, Mid Cheshire Hospitals NHS Foundation Trust, Mid Yorkshire Hospitals NHS Trust, Northern Lincolnshire and Goole NHS Foundation Trust, Portsmouth Hospitals NHS Trust, Royal Wolverhampton Hospitals NHS Trust, Sandwell and West Birmingham NHS Trust, Sheffield Teaching Hospitals NHS Foundation Trust, Sherwood Forest Hospitals NHS Foundation Trust, St Helens and Knowsley Teaching Hospitals NHS Trust, Stockport NHS Foundation Trust, Southport and Ormskirk Hospitals NHS Trust, South Warwickshire NHS Foundation Trust, The Dudley Group NHS Foundation Trust, United Lincolnshire Hospitals NHS Trust, University Hospitals of Coventry and Warwickshire NHS Trust, University Hospitals of North Midlands NHS Trust, University of Morecambe Bay NHS Foundation Trust, Walsall Healthcare NHS Trust, Warrington and Halton Hospitals NHS Foundation Trust, Western Sussex Hospitals NHS Foundation Trust, Wirral University Teaching Hospitals NHS Foundation Trust, York Teaching Hospitals NHS Foundation Trust.
Funding
The Midland and North of England Stillbirth Study was funded by grant GN2156 from Action Medical Research, Cure Kids, and Sands. AH receives salary support from Tommy's and the National Institute of Health Research (Clinician Scientist Award CS‐13‐009). EM and JT were supported by Cure Kids.
Heazell AEP, Li M, Budd J, Thompson JMD, Stacey T, Cronin RS, Martin B, Roberts D, Mitchell EA, McCowan LME. Association between maternal sleep practices and late stillbirth – findings from a stillbirth case‐control study. BJOG 2018; 125:254–262.
 This paper includes Author Insights, a video abstract available at https://vimeo.com/rcog/authorinsights14967
This paper includes Author Insights, a video abstract available at https://vimeo.com/rcog/authorinsights14967
Linked article This article is commented on by EM McClure and RL Goldenberg, p. 264 in this issue. To view this article visit https://doi.org/10.1111/1471-0528.14985.
References
- 1. Flenady V, Wojcieszek AM, Middleton P, Ellwood D, Erwich JJ, Coory M, et al. Stillbirths: recall to action in high‐income countries. Lancet 2016;387:691–702. [DOI] [PubMed] [Google Scholar]
- 2. Manktelow BM, Smith LK, Seaton SE, Hyman‐Taylor P, Kurinczuk JJ, Field DJ, et al. MBRRACE‐UK perinatal mortality surveillance report: UK perinatal deaths for births from January to December 2014. The Infant Mortality and Morbidity Group, Department of Health Sciences, University of Leicester, Leicester, UK; 2016.
- 3. Flenady V, Koopmans L, Middleton P, Froen JF, Smith GC, Gibbons K, et al. Major risk factors for stillbirth in high‐income countries: a systematic review and meta‐analysis. Lancet 2011;377:1331–40. [DOI] [PubMed] [Google Scholar]
- 4. Heazell AE, Whitworth MK, Whitcombe J, Glover SW, Bevan C, Brewin J, et al. Research priorities for stillbirth: process overview and results from UK Stillbirth Priority Setting Partnership. Ultrasound Obstet Gynecol 2015;46:641–7. [DOI] [PubMed] [Google Scholar]
- 5. Stacey T, Thompson JM, Mitchell EA, Ekeroma AJ, Zuccollo JM, McCowan LM. Association between maternal sleep practices and risk of late stillbirth: a case‐control study. BMJ 2011;342:d3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gordon A, Raynes‐Greenow C, Bond D, Morris J, Rawlinson W, Jeffery H. Sleep position, fetal growth restriction, and late‐pregnancy stillbirth: the Sydney stillbirth study. Obstet Gynecol 2015;125:347–55. [DOI] [PubMed] [Google Scholar]
- 7. McCowan LME, Thompson JMD, Cronin RS, Li M, Stacey T, Stone PR, et al. Going to sleep in the supine position is a modifiable risk factor for late pregnancy stillbirth; Findings from the New Zealand multicentre stillbirth case‐control study. PLoS ONE 2017;12:e0179396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chappell LC, Smith GCS. Should pregnant women sleep on their left? BMJ 2011;342:d3659. [DOI] [PubMed] [Google Scholar]
- 9. Platts J, Mitchell EA, Stacey T, Martin BL, Roberts D, McCowan L, et al. The Midland and North of England Stillbirth Study (MiNESS). BMC Pregnancy Childbirth 2014;14:171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gardosi J, Kady SM, McGeown P, Francis A, Tonks A. Classification of stillbirth by relevant condition at death (ReCoDe): population based cohort study. BMJ 2005;331:1113–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. McIntyre JP, Ingham CM, Hutchinson BL, Thompson JM, McCowan LM, Stone PR, et al. A description of sleep behaviour in healthy late pregnancy, and the accuracy of self‐reports. BMC Pregnancy Childbirth 2016;16:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cronin RS, Zuccollo J, Li M, Mitchell EA, McCowan LM. Late stillbirth and post‐mortem: maternal decision‐making in a New Zealand study. J Paediatr Child Health 2017;53:21. [Google Scholar]
- 13. Sedgwick P. What is recall bias? BMJ 2012;344:e3519. [Google Scholar]
- 14. Cockerill R, Whitworth MK, Heazell AE. Do medical certificates of stillbirth provide accurate and useful information regarding the cause of death? Paediatr Perinat Epidemiol 2012;26:117–23. [DOI] [PubMed] [Google Scholar]
- 15. Li M, McCowan LM, Cronin RS, Thompson JM, Mitchell EA, Culling V, et al. Collaborative IPD analysis of maternal sleep position and late stillbirth (greater than or equal to 28 weeks of gestation). PROSPERO, York, UK: Centre for Reviews and Dissemination, University of York, York, UK;2017. p. CRD42017047703.
- 16. Stillbirth Collaborative Research Network Writing Group . Association between stillbirth and risk factors known at pregnancy confirmation. JAMA 2011;306:2469–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. O'Connor D. Saving babies’ lives: a care bundle for reducing stillbirth. NHS England 2016 [www.england.nhs.uk/wp-content/uploads/2016/03/saving-babies-lives-car-bundl.pdf]. Accessed October 14 2017.
- 18. Iliodromiti S, Mackay DF, Smith GC, Pell JP, Sattar N, Lawlor DA, et al. Customised and noncustomised birth weight centiles and prediction of stillbirth and infant mortality and morbidity: a cohort study of 979,912 term singleton pregnancies in Scotland. PLoS Med 2017;14:e1002228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gardosi J, Giddings S, Clifford S, Wood L, Francis A. Association between reduced stillbirth rates in England and regional uptake of accreditation training in customised fetal growth assessment. BMJ Open 2013;3:e003942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bjornholt SM, Leite M, Albieri V, Kjaer SK, Jensen A. Maternal smoking during pregnancy and risk of stillbirth: results from a nationwide Danish register‐based cohort study. Acta Obstet Gynecol Scand 2016;95:1305–12. [DOI] [PubMed] [Google Scholar]
- 21. Baba S, Wikstrom AK, Stephansson O, Cnattingius S. Influence of snuff and smoking habits in early pregnancy on risks for stillbirth and early neonatal mortality. Nicotine Tob Res 2014;16:78–83. [DOI] [PubMed] [Google Scholar]
- 22. Owusu JT, Anderson FJ, Coleman J, Oppong S, Seffah JD, Aikins A, et al. Association of maternal sleep practices with pre‐eclampsia, low birth weight, and stillbirth among Ghanaian women. Int J Gynaecol Obstet 2013;121:261–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ptacek I, Sebire NJ, Man JA, Brownbill P, Heazell AE. Systematic review of placental pathology reported in association with stillbirth. Placenta 2014;35:552–62. [DOI] [PubMed] [Google Scholar]
- 24. Fedak KM, Bernal A, Capshaw ZA, Gross S. Applying the Bradford Hill criteria in the 21st century: how data integration has changed causal inference in molecular epidemiology. Emerg Themes Epidemiol 2015;12:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. McIntyre JP. The Physiology of Maternal Sleep (and Sleep Position) in Healthy Late Pregnancy. Auckland, New Zealand: University of Auckland, 2017. [Google Scholar]
- 26. Jeffreys RM, Stepanchak W, Lopez B, Hardis J, Clapp JF III. Uterine blood flow during supine rest and exercise after 28 weeks of gestation. BJOG 2006;113:1239–47. [DOI] [PubMed] [Google Scholar]
- 27. Carbonne B, Benachi A, Lévèque ML, Cabrol D, Papiernik E. Maternal position during labor: effects on fetal oxygen saturation measured by pulse oximetry. Obstet Gynecol 1996;88:797–800. [DOI] [PubMed] [Google Scholar]
- 28. Khatib N, Weiner Z, Beloosesky R, Vitner D, Thaler I. The effect of maternal supine position on umbilical and cerebral blood flow indices. Eur J Obstet Gynecol Reprod Biol 2014;175:112–14. [DOI] [PubMed] [Google Scholar]
- 29. National Institute for Health and Care Excellence . Intrapartum Care for Healthy Women and Babies (CG190). London, UK: National Institute for Health and Care Excellence, 2017. [Google Scholar]
- 30. Stone PR, Burgess W, McIntyre JP, Gunn AJ, Lear CA, Bennet L, et al. Effect of maternal position on fetal behavioural state and heart rate variability in healthy late gestation pregnancy. J Physiol 2017;595:121321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cronin RS, Chelimo C, Mitchell EA, Okesene‐Gafa K, Thompson JMD, Taylor RS, et al. Survey of maternal sleep practices in late pregnancy in a multi‐ethnic sample in South Auckland, New Zealand. BMC Pregnancy Childbirth 2017;17:190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Grimes HA, Forster DA, Newton MS. Sources of information used by women during pregnancy to meet their information needs. Midwifery 2014;30:e26–33. [DOI] [PubMed] [Google Scholar]
- 33. Jacobs DR Jr, Adachi H, Mulder I, Kromhout D, Menotti A, Nissinen A, Blackburn H. Cigarette smoking and mortality risk: twenty‐five‐year follow‐up of the seven countries study. Archives of Internal Medicine 1999;159:733–40. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Demographic characteristics and sleep practices in 291 women who had a late stillbirth between 2014 and 2016 compared with 733 controls that were not included in the multivariable model.
Table S2. Demographic characteristics and sleep practices in 291 women who had a late stillbirth between 2014 and 2016 compared with 733 controls. Data are presented as the number (percentage) or median (interquartile range).
Appendix S1. Participant data collection questionnaire used in the MiNESS study.
Video S1. Author insights.■


