Abstract
Inhalation of acrolein, a highly reactive aldehyde, causes lung edema. The underlying mechanism is poorly understood and there is no effective treatment. In this study, we demonstrated that acrolein not only dose-dependently induced lung edema but also promoted LPS-induced acute lung injury. Importantly, acrolein-induced lung injury was prevented and rescued by Alda-1, an activator of mitochondrial aldehyde dehydrogenase 2. Acrolein also dose-dependently increased monolayer permeability, disrupted adherens junctions and focal adhesion complexes, and caused intercellular gap formation in primary cultured lung microvascular endothelial cells (LMVECs). These effects were attenuated by Alda-1 and the antioxidant N-acetylcysteine, but not by the NADPH inhibitor apocynin. Furthermore, acrolein inhibited AMP-activated protein kinase (AMPK) and increased mitochondrial reactive oxygen species levels in LMVECs—effects that were associated with impaired mitochondrial respiration. AMPK total protein levels were also reduced in lung tissue of mice and LMVECs exposed to acrolein. Activation of AMPK with 5-aminoimidazole-4-carboxamide-1-β-4-ribofuranoside blunted an acrolein-induced increase in endothelial monolayer permeability, but not mitochondrial oxidative stress or inhibition of mitochondrial respiration. Our results suggest that acrolein-induced mitochondrial dysfunction may not contribute to endothelial barrier dysfunction. We speculate that detoxification of acrolein by Alda-1 and activation of AMPK may be novel approaches to prevent and treat acrolein-associated acute lung injury, which may occur after smoke inhalation.
Keywords: acrolein, acute lung injury, endothelial cells, mitochondrial aldehyde dehydrogenase 2, mitochondrial respiration
Clinical Relevance
Inhalation of acrolein, a highly reactive aldehyde, causes lung edema. The underlying mechanism is poorly understood, and there is no effective treatment available. We demonstrated that acrolein-induced lung injury was prevented and rescued by Alda-1, an activator of mitochondrial aldehyde dehydrogenase 2. Acrolein-induced lung microvascular endothelial barrier dysfunction was also attenuated by Alda-1 and 5-aminoimidazole-4-carboxamide-1-β-4-ribofuranoside, an activator of AMP-activated protein kinase. We speculate that Alda-1 and AMP-activated protein kinase activators may be novel treatments for acrolein-driven lung injury.
Acrolein is a highly reactive α,β-unsaturated aldehyde with an estimated half-life of 12 hours. The safe reference concentration of acrolein for inhalation recommended by the U.S. Environmental Protection Agency is 0.02 μg/m3. The major sources of indoor acrolein are tobacco smoke and emissions from overheated oils, fireplace heating, and biomass cooking, whereas outdoor acrolein is mainly from gasoline and diesel exhaust, combustion of organic materials, forest fires, and burn pits used to dispose of waste by the U.S. military (1). As a result, tobacco smokers (2), firefighters (3, 4), restaurant workers (5), certain manufacturing workers (6), and military personnel deployed to military bases in Afghanistan and Iraq (7) can be exposed to toxic levels of acrolein. Lung tissue from mice exposed to cigarette smoke has increased levels of reactive aldehydes (8). In addition to exogenous sources, acrolein is endogenously produced via lipid peroxidation and metabolism (9).
Inhalation of low levels of acrolein causes nasal irritation, bronchial hyperreactivity, and excessive mucus production (10). Exposure to high levels of acrolein elevates blood pressure and causes quick cessation of heart beat (11). Acrolein exposure has been implicated in chronic obstructive pulmonary disease (12) and increases susceptibility to microbial infections (13). Smoke inhalation can cause acute respiratory distress syndrome (14). Acrolein is the second most common inhaled toxin, after carbon monoxide, in smoke inhalation during fires. Similarly to smoke inhalation, acrolein inhalation causes noncardiogenic pulmonary edema and respiratory distress in sheep (15, 16), dogs (17), and mice (18). In addition, acrolein was shown to increase bronchial epithelial barrier permeability (19) and alter the tight-junction protein Claudin 5 in a cultured endothelial cell line (18). However, it is not known whether acrolein directly increases lung microvascular endothelial cell (LMVEC) permeability. The mechanisms underlying acrolein-induced lung edema are not well understood.
Acrolein can be detoxified by glutathione-S-transferase alpha 4 (GSTα4), alkenal/one oxidoreductase, aldose reductase, or aldehyde dehydrogenase 2 (ALDH2) (20, 21). Excessive acrolein is subjected to Michael addition (also termed carbonylation), whereby the acrolein reacts with proteins or nucleic acids to form aldehyde adducts (1). Protein carbonylation may cause protein misfolding, cross-linking, aggregation, and degradation (22). Acrolein has been reported to deplete cellular glutathione (GSH) (23), increase reactive oxygen species (ROS) production (24, 25), suppress NF-κB–dependent gene transcription (26, 27), inhibit histone acetylation (28), and change metabolome and transcriptome profiling in mouse lungs (29).
In this study, we found that acrolein caused lung edema and promoted LPS-induced acute lung injury (ALI). Alda-1 (N-C1,3-benzodioxol-5-ylmethyl)-2,6-dichlorobenzamide), an ALDH2 activator, not only prevented but also rescued acrolein-induced lung injury. Alda-1 also attenuated acrolein-induced endothelial barrier dysfunction. AMP-activated protein kinase (AMPK) is a ubiquitously expressed major sensor of cellular bioenergetics. We found that acrolein-induced lung edema and increased LMVEC permeability were associated with inhibition of AMPK and impaired mitochondrial respiration. The AMPK activator 5-aminoimidazole-4-carboxamide-1-β-4-ribofuranoside (AICAR) abrogated acrolein-induced endothelial monolayer permeability. We speculate that Alda-1 and AMPK activators may be novel treatments for acrolein-driven lung injury.
Some of these results were presented at the American Thoracic Society International Conference, May 2015, and published in abstract form (30).
Materials and Methods
Reagents
Acrolein was purchased from Polysciences, Inc. (Warrington, PA). LPS (catalog # L5418, Escherichia coli [055:B5]), N-acetylcysteine (NAC), apocynin, and vinculin antibody were obtained from Sigma–Aldrich (St. Louis, MO). Alda-1, AICAR, and a keratinocyte chemoattractant ELISA kit were obtained from R&D Systems (Minneapolis, MN). IL-6 and TNF-α ELISA kits and β-catenin antibody were obtained from BD Biosciences (San Diego, CA). Antibodies against AMPK and phosphorylated AMPK at T172 were obtained from Cell Signaling (Danvers, MA). An IL-10 ELISA kit was obtained from Biolegend (San Diego, CA). Rat LMVECs were obtained from VEC Technologies, Inc. (Rensselaer, NY) and used between passages 2 and 7.
Mice
Male 8- to 10-week-old C57BL/6 mice were obtained from The Jackson Laboratory (Bar Harbor, ME). All animal studies were approved by the Institutional Animal Care and Use Committee of the Providence VA Medical Center. Animals were housed in standard conditions (12 h light/dark cycle, 68–72°F, and humidity of 30–70%) in ventilated racks with automatic watering systems and fed with standard chow ad libitum.
Intratracheal Administration of Acrolein and LPS
Acrolein and LPS (∼50 μl) were delivered intratracheally to mice anesthetized with 3% isoflurane as previously described (31).
Assessments of bronchoalveolar lavage (BAL) protein content, lung wet-to-dry weight ratio and inflammatory cell count were performed as previously described (32).
An ALDH2 enzymatic activity assay was performed on freshly harvested liver and lung tissue from mice treated, with acrolein in the absence or presence of Alda-1 using an ALDH2 activity assay kit (Abcam, Cambridge, MA). Optical density units of the reaction product were measured at a wavelength of 450 nm. Data are represented as means ± SE at the 1-hour time point.
An endothelial monolayer permeability assay and immunofluorescence microscopy were performed as previously described (33).
Measurement of Mitochondrial ROS
Cells were stained with Hoechst 33342 for 20 minutes and then with MitoSOX (both from ThermoFisher Scientific, Waltham, MA) for 20 minutes. The fluorescence signals of the MitoSOX and Hoechst 33342 were captured by a fluorescence spectrometer at excitations/emissions of 510/580 and 350/461, respectively. The data are presented as the ratio of MitoSOX to Hoechst 33342.
The mitochondrial oxygen consumption rate (OCR) was measured using an XF-96 Extracellular Flux Analyzer from Agilent Technologies (Santa Clara, CA) according to the manufacturer’s Mito Stress Test Kit user guide. ATP production was assessed by injection of oligomycin (1 μM). Maximal respiration capacity was measured by injection of carbonyl cyanide-4-(trifluoromethoxy)phenylhydrazone (0.5 μM). Injection of rotenone/antimycin A (0.5 μM) was used to determine nonmitochondrial respiration. All values were normalized to viable cells using the CyQUANTdirect cell assay (ThermoFisher Scientific). Each experimental condition included three to four wells that were averaged for one experimental data point.
Data Analysis
For animal studies, three to 12 mice per group for each experiment were used. All experiments using cultured cells were performed at least three independent times. Data are presented as the mean ± SE. The difference between two means was assessed using Student’s t test. The differences among three or more means were assessed using ANOVA and Fisher’s post hoc test. Differences among means are considered statistically significant when P < 0.05.
Results
Acrolein Dose-Dependently Increased Lung Edema and Inflammation
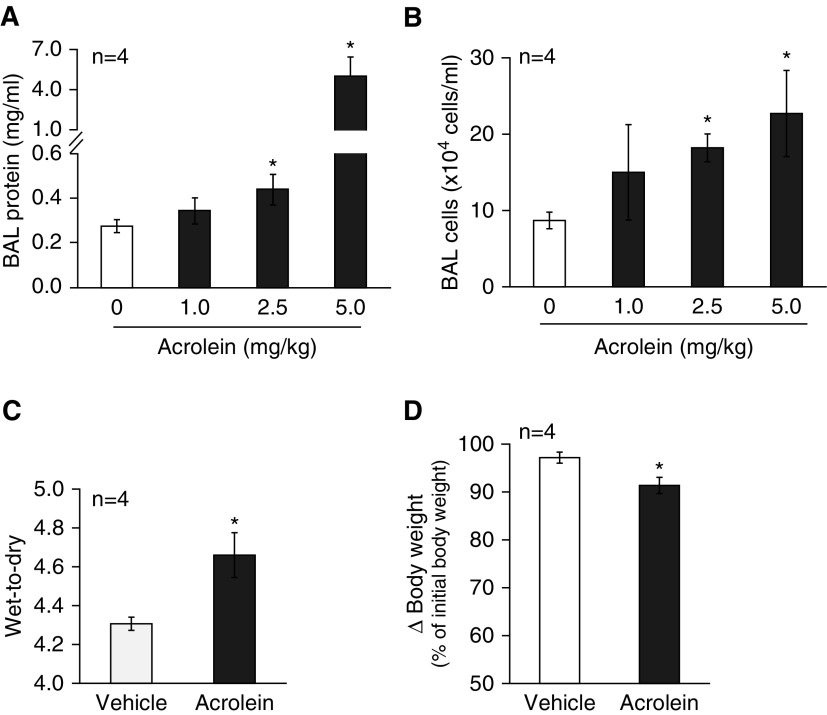
We previously showed that acute cigarette smoke exposure caused lung edema in mice (34). To determine whether acrolein has a similar effect, C57BL/6 mice were intratracheally instilled with varying doses of acrolein (0, 1, 2.5, and 5 mg/kg), and lung edema and inflammation were determined 18 hours after acrolein challenge. We found that acrolein dose-dependently increased the BAL protein content (Figure 1A) and BAL cell count (Figure 1B), with a minimal effective dose at 2.5 mg/kg. Intratracheal instillation of acrolein at 2.5 mg/kg also significantly increased the lung wet-to-dry weight ratio (Figure 1C). In addition, acrolein exposure dramatically elevated BAL cytokines, including KC, IL-6, and TNF-α (see Figures 4E–4G). These data indicate that acute acrolein exposure causes lung edema and inflammation. Additionally, mouse body weights were significantly reduced 18 hours after exposure to 2.5 mg/kg of acrolein (Figure 1D), an indication of systemic distress.
Figure 1.
Effects of acrolein on lung edema and inflammation. (A and B) Mice were intratracheally administered varying doses of acrolein (0–5 mg/kg) dissolved in sterilized saline. Bronchoalveolar lavage (BAL) fluid was collected 18 hours after instillation of acrolein. The total protein content (A) and leukocyte numbers (B) in BAL fluid were assessed. (C and D) Mice were intratracheally administered 2.5 mg/kg of acrolein or an equal volume of saline as vehicle control. The lung wet-to-dry weight ratio (C) and changes (∆) in mouse body weights (D) were determined 18 hours after instillation of acrolein. Four mice per group were used for each panel. Data are represented as means ± SE. *P < 0.05 versus mice treated with vehicle control.
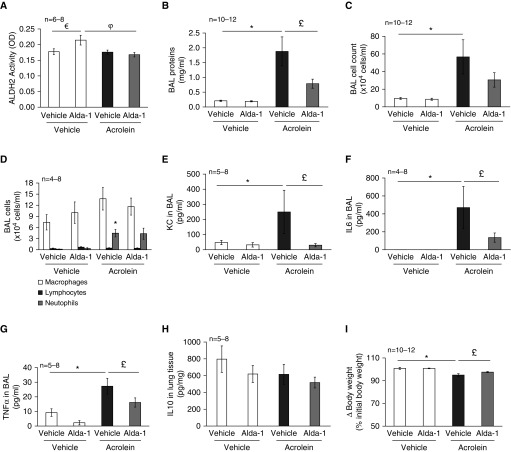
Figure 4.
Treatment effects of Alda-1 on acrolein-induced lung edema and inflammation. Mice were intratracheally administered 2.5 mg/kg of acrolein or saline. Two hours after instillation of acrolein, the mice were given Alda-1 (10 mg/kg) or an equal volume of sterilized saline via intraperitoneal injection. Liver aldehyde dehydrogenase 2 (ALDH2) activity (A), BAL protein levels (B), BAL cell count (C), BAL cell differentiation (D), BAL cytokines (E–H), and changes in mouse body weights (I) were determined 18 hours after acrolein administration. Four to 12 mice per group were used for each panel. Data are represented as means ± SE. €P < 0.05 versus mice treated with vehicle/vehicle, *P < 0.05 versus mice treated with saline control, £P < 0.05 versus mice treated with acrolein alone, φP < 0.05 versus mice treated with Alda-1 alone. KC, keratinocyte chemoattractant; OD, optical density.
Acrolein Promoted LPS-Induced ALI
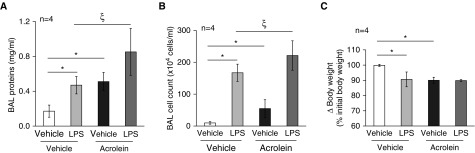
Cigarette smoking increases susceptibility to acute respiratory distress syndrome, as well as the severity of the disease, in humans (35–38) and animal models (31). Acrolein is an important component of cigarette smoke. Because other investigators and we have found that cigarette smoking increases the inflammatory response to LPS inhalation in both mice (31) and humans (38), we questioned whether preexposure to acrolein would have an effect similar to that of cigarette smoking on LPS-induced lung injury. In the current study, mice were challenged with 2.5 mg/kg of acrolein by intratracheal instillation. After 6 hours of exposure to acrolein, the mice were intratracheally administered 2.5 mg/kg of LPS. Lung injury was assessed 18 hours after instillation of LPS. We found that acrolein alone increased the BAL protein content (Figure 2A) and caused a significant loss of body weight (Figure 2C), similar to the extent observed with LPS. Acrolein alone also increased the BAL inflammatory cell count, but to a lesser extent than LPS (Figure 2B). Interestingly, mice preexposed to acrolein had a greater increase in both the BAL protein content (Figure 2A) and BAL cell count (Figure 2B) after challenge with LPS as compared with LPS alone. These results indicate that acrolein had an additive effect on LPS-induced lung injury, which suggests that populations preexposed to acrolein may experience enhanced lung injury after bacterial infections.
Figure 2.
Effects of acrolein on LPS-induced lung injury. Mice were intratracheally administered 2.5 mg/kg of acrolein or an equal volume of saline (vehicle control). Six hours after instillation of acrolein, mice were intratracheally challenged with 2.5 mg/kg of LPS or an equal volume of saline. The total BAL protein content (A), BAL cell count (B), and changes in mouse body weights (C) were determined 18 hours after instillation of LPS. Four mice per group were used for each panel. Data are represented as means ± SE. *P < 0.05 versus mice treated with vehicle, ξP < 0.05 versus mice treated with LPS alone.
Alda-1 Prevented Acrolein-Induced Lung Edema and Inflammation
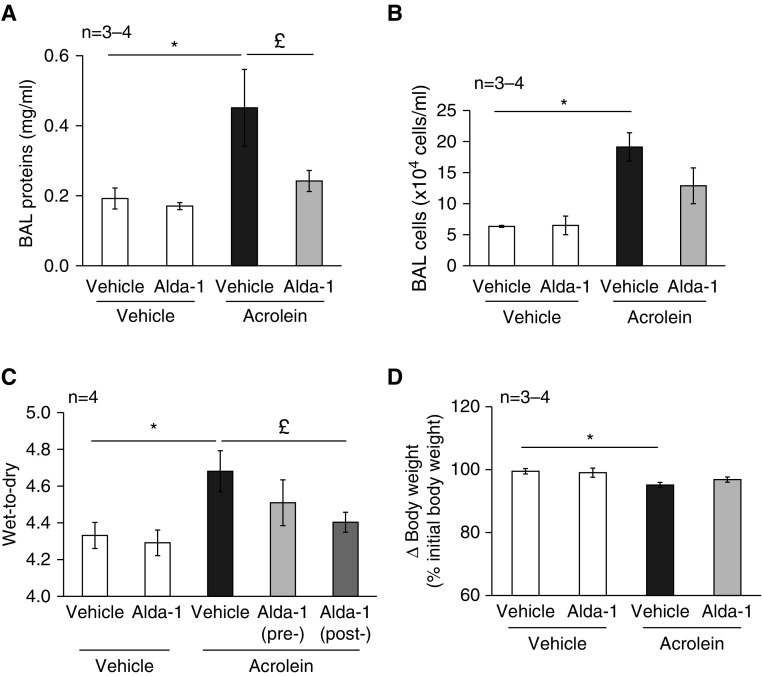
Reactive aldehydes are detoxified by ALDH2 (20, 21). Alda-1, a small-molecule activator of ALDH2, is capable of preventing the formation of cytotoxic reactive aldehydes (38). We found that pretreatment of mice with Alda-1 significantly attenuated the acrolein-induced increase in BAL protein content (Figure 3A). Alda-1 also exhibited a trend toward blunting of the acrolein-induced increase in BAL cell count (Figure 3B), lung wet-to-dry weight ratio (Figure 3C), and loss of body weight (Figure 3D).
Figure 3.
Preventive effects of Alda-1 on acrolein-induced lung edema and inflammation. Mice were administered Alda-1 (10 mg/kg) or an equal volume of sterilized saline (control) via intraperitoneal injection. After 1 hour, the mice were intratracheally administered 2.5 mg/kg of acrolein or an equal volume of sterilized saline. BAL protein levels (A), BAL cell count (B), lung wet-to-dry weight ratio (C), and changes in mouse body weights (D) were assessed 18 hours after challenge with acrolein. Some mice were treated first with acrolein for 2 hours, followed by Alda-1 (C). Three to four mice per group were used for each panel. Data are represented as means ± SE. *P < 0.05 versus mice treated with saline control, £P < 0.05 versus mice treated with acrolein alone.
Alda-1 Rescued Acrolein-Induced Lung Edema and Inflammation
To test the therapeutic effect of Alda-1 on acrolein-induced lung edema and inflammation, we administered Alda-1 to mice 2 hours after initiating lung injury by acrolein. ALDH2 activity and lung injury were assessed 18 hours after acrolein challenge. We noted that Alda-1 significantly elevated ALDH2 activity in liver tissue (Figure 4A). ALDH2 has been reported to be modestly expressed in lungs (40). However, lung ALDH2 activity was not detectable by our assay (data not shown). Although intratracheal administration of acrolein did not reduce liver ALDH2 activity, it attenuated Alda-1–induced activation of ALDH2 in livers (Figure 4A). Interestingly, Alda-1 significantly rescued the acrolein-induced increase in BAL protein content (Figure 4B), lung wet-to-dry weight ratio (see Figure 3C), and loss of body weight (Figure 4I), and showed a trend toward rescuing the acrolein-induced increase in the BAL cell count (Figure 4C). In addition, Alda-1 dramatically rescued acrolein-induced increases in proinflammatory cytokines, including KC (Figure 4E), IL-6 (Figure 4F), and TNF-α (Figure 4G). On the other hand, Alda-1 did not display a significant effect on acrolein-induced neutrophil infiltration (Figure 4D). Neither acrolein nor Alda-1 affected expression of the antiinflammatory cytokine IL-10 in lung homogenates (Figure 4H).
Acrolein Increased LMVEC Monolayer Permeability
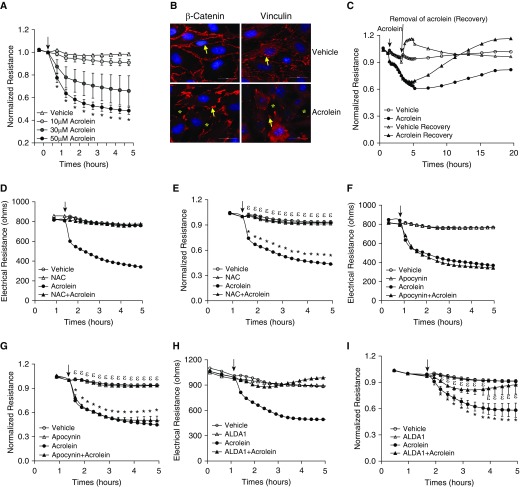
To understand the mechanism of acrolein-induced lung edema, we assessed the direct effect of acrolein on LMVEC monolayer permeability, which has not been reported previously. We found that acrolein dose-dependently decreased electrical resistance across monolayers of rat LMVECs (Figure 5A), indicating increased monolayer permeability. Similar effects were seen in human LMVECs (data not shown). Acrolein also disrupted intercellular adherens junctions (AJs), as indicated by reduced immunofluorescence staining of the AJ component β-catenin, and focal adherens complexes, as shown by loss of staining of the focal adherens complex component vinculin, and caused intercellular gap formation (Figure 5B). These data indicate that acrolein directly increases LMVEC permeability. We also found that the disruptive effect of acrolein on endothelial barrier integrity was recovered after removal of acrolein (Figure 5C), suggesting a reversible effect of acrolein on endothelial barrier function.
Figure 5.
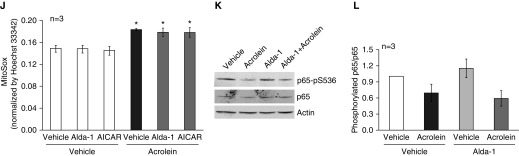
Effects of antioxidants and Alda-1 on an acrolein-induced increase in endothelial monolayer permeability. (A) Rat lung microvascular endothelial cells (LMVECs) were treated with vehicle or varying doses of acrolein for the indicated times. Monolayer permeability was assessed by measuring electrical resistance across the monolayers by electric cell-substrate impedance sensing (ECIS). Data are represented as the normalized electrical resistance of means ± SE of three independent experiments. (B) Rat LMVECs were treated with vehicle or 30 μM of acrolein for 2 hours, and β-catenin and vinculin were assessed by immunofluorescence microscopy. Arrows indicate β-catenin or vinculin, and asterisks indicate intercellular gaps. Representative images from three independent experiments are shown. Scale bars: 20 μm. (C) Rat LMVECs were treated with vehicle or 30 μM of acrolein for 2 hours. The acrolein was then removed or not removed as a control. The arrow indicates the time of acrolein removal. Monolayer permeability was assessed over time by ECIS. Representative tracings from three independent experiments are shown. (D–I) Rat LMVECs were preincubated with vehicle, 1 mM N-acetylcysteine (NAC), 10 μM apocynin, or 50 μM Alda-1 for 30 minutes and then exposed to vehicle or 30 μM acrolein in the absence or presence of NAC, apocynin, or Alda-1 for the indicated times. Monolayer permeability was assessed by ECIS. (D, F, and H) Representative tracings from three to five independent experiments. Arrows indicate the time of addition of treatments. *P < 0.05 versus vehicle-treated cells, εP < 0.05 versus cells treated with acrolein. (J–L) Rat LMVECs were preincubated with vehicle, 80 μM Alda-1, or 500 μM 5-aminoimidazole-4-carboxamide-1-β-4-ribofuranoside (AICAR) for 30 minutes and then exposed to vehicle or 50 μM acrolein in the absence or presence of Alda-1 or AICAR for 2 hours. Mitochondrial reactive oxygen species was assessed by mitoSOX staining (J). (K and L) NF-κB activity was assessed by probing p65 phosphorylation. (K) Representative immunoblots. (L) Densitometry of immunoblot signals, means ± SE of three independent experiments. *P < 0.05 versus vehicle-treated cells. Actin was used as a protein loading control.
NAC and Alda-1 Prevented an Acrolein-Induced Increase in Endothelial Monolayer Permeability
Reactive aldehydes can cause cellular oxidative stress by activating NADPH oxidases (24, 25) and/or depleting the antioxidant GSH (23). As expected, we found that NAC completely prevented an acrolein-induced increase in endothelial monolayer permeability (Figures 5D and 5E). To test whether NADPH oxidases contribute to acrolein-induced oxidative stress, leading to increased endothelial permeability, we used apocynin, which inhibits the association of p47phox with a membrane-bound heterodimer and thus acts as an NADPH oxidase assembly inhibitor (41). We found that apocynin did not have any protective effect against an acrolein-induced increase in endothelial monolayer permeability (Figures 5F and 5G), suggesting that NADPH oxidases are not important in mediating acrolein-induced lung endothelial barrier dysfunction. On the other hand, consistent with our in vivo findings, Alda-1 significantly attenuated the acrolein-induced barrier dysfunction of LMVECs (Figures 5H and 5I). To further investigate the mechanism of acrolein-induced endothelial barrier dysfunction, we assessed mitochondrial oxidative stress, because acrolein has been shown to cause mitochondrial oxidative stress in hepatocytes (42). Consistently, acrolein enhanced mitochondrial ROS levels; an effect that was not prevented by Alda-1 (Figure 5J). Acrolein has been reported to suppress NF-κB–mediated gene transcription (26, 27). Therefore, we assessed NF-κB–driven endothelial activation by probing p65 phosphorylation. We observed a trend toward a decrease in NF-κB activity by acrolein in LMVECs—an effect that was not prevented by Alda-1 (Figures 5K and 5L).
Acrolein Decreased AMPK in LMVECs In Vitro and in Lung Tissue In Vivo
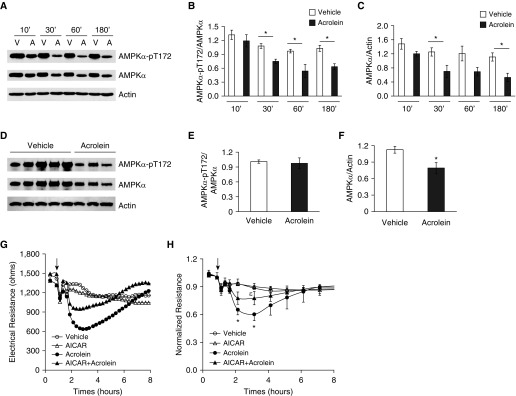
To further elucidate the mechanism by which acrolein increases endothelial cell permeability and causes lung edema, we assessed the effect of acrolein on AMPK activity by measuring AMPK phosphorylation at T172. We found that 30 μM of acrolein time-dependently reduced both AMPK phosphorylation at T172 and total AMPK protein in cultured rat LMVECs after as little as 10 minutes of exposure, peaking at 1–3 hours of exposure (Figures 6A–6C). AMPK total protein was also significantly reduced in lung homogenates of mice exposed to acrolein for 18 hours (Figures 6D–6F). These data reveal an association of decreased AMPK activity with increased endothelial monolayer permeability and lung edema upon acrolein exposure. We further demonstrated that the AMPK activator AICAR significantly attenuated the acrolein-induced increase in endothelial monolayer permeability (Figures 6G and 6H).
Figure 6.
Role of AMP-activated protein kinase (AMPK) in acrolein-induced endothelial monolayer permeability. (A–C) Rat LMVECs were treated with vehicle (V) or 30 μM acrolein (A) for the indicated times. (D–F) Mice were intratracheally administered with 2.5 mg/kg of acrolein or saline for 18 hours. AMPK activation (AMPKα-pT172) and total AMPK protein levels were assessed in cell lysates and lung tissue. Actin was used as a protein loading control. Representative immunoblots are shown in A and D. Densitometry was performed on four independent experiments (B and C) or three to five mice (E and F). The ratio of AMPKα-pT172 to AMPK (B and C) and the ratio of AMPK to actin (E and F) are presented as means ± SE. *P < 0.05 versus respective vehicle control. (G and H) Rat LMVECs were preincubated with vehicle or 500 μM AICAR for 30 minutes and then exposed to vehicle or 30 μM acrolein in the absence or presence of AICAR for the indicated times. Monolayer permeability was assessed by ECIS. (G) Representative tracing from three independent experiments. (H) Normalized electrical resistance of means ± SE of three independent experiments. Arrows indicate the time of addition of treatments. *P < 0.05 versus vehicle-treated cells. εP < 0.05 versus cells treated with acrolein.
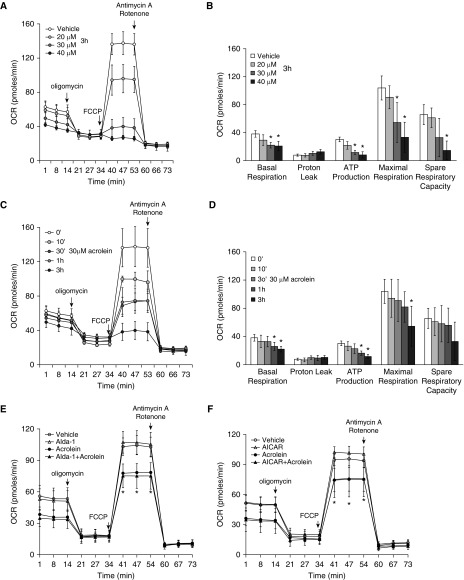
Acrolein Impaired Mitochondrial Oxygen Consumption, and the Effect Was Not Prevented by Alda-1 or AICAR
AMPK is a key signaling molecule that regulates mitochondrial oxidative phosphorylation. Because AMPK was dramatically reduced by acrolein exposure, we assessed the effect of acrolein on mitochondrial oxygen consumption and found that acrolein dose- and time-dependently reduced basal respiration, ATP production, maximal respiration, and spare respiration capacity (Figures 7A–7D).
Figure 7.
Effects of Alda-1 and AICAR on acrolein-induced impairment of mitochondrial respiration. (A–D) Rat LMVECs were treated with vehicle or the indicated doses of acrolein for the indicated times or (E and F) preincubated with vehicle, 50 μM Alda-1, or 500 μM AICAR for 30 minutes and then exposed to vehicle or 30 μM acrolein in the absence or presence of Alda-1 or AICAR for 1 hour. The mitochondrial oxygen consumption rate (OCR) was assessed with an XF Seahorse analyzer. (A and C) Representative experiments in which three to four wells were averaged for each data point. (B and D–F) Data represent the means ± SE of three to four independent experiments for each panel. *P < 0.05 versus the respective vehicle. FCCP, carbonyl cyanide-4-(trifluoromethoxy)phenylhydrazone.
Because both Alda-1 and AICAR attenuated the acrolein-induced increase in endothelial cell permeability, we assessed the effects of Alda-1 and AICAR on the acrolein-induced inhibition of mitochondrial respiration. To our surprise, neither Alda-1 nor AICAR altered acrolein-induced suppression of OCR in cultured LMVECs (Figures 7E and 7F). Our data suggest that Alda-1 and AICAR protect against acrolein-induced endothelial permeability and lung injury via a mechanism that is independent of mitochondrial dysfunction.
Discussion
Acrolein inhalation causes pulmonary edema in animals (16–18). In addition, acrolein has been reported to suppress LPS-induced T-helper cell type 1 cytokine responses (43). In this study, we demonstrated that acrolein not only caused lung edema but also worsened LPS-induced lung injury. Acrolein has been shown to increase bronchial epithelial barrier permeability (19) and to alter a tight-junction protein, Claudin 5, in an endothelial cell line (18). In this study, we showed that acrolein dose-dependently increased the permeability of primary cultured LMVECs, disrupted AJs and focal adhesion complexes in vitro, and caused lung edema in vivo. Our data suggest that the injurious effect of acrolein on LMVECs may contribute to smoke inhalation–induced lung edema. To our knowledge, this finding has not been reported previously.
Mice were intratracheally administered 2.5 mg/kg body weight of acrolein (molecular mass = 56.06 g/mole) in ∼50 μl. This is ∼1 μmole instilled in a concentration of 20 mM. Sidestream smoke is estimated to expose lungs to 25.2 mg of acrolein per pack or 450 μmoles per pack, whereas mainstream smoke exposes lungs to 1.5 mg of acrolein per pack or 188 μmoles per pack (10). Thus, the in vivo exposures to acrolein used in this study are comparable to pathophysiologic levels. Cell cultures were exposed to 10–50 μM of acrolein, which is a much smaller amount than we used in the in vivo study, but similar to those used in previous in vitro studies (19).
Kitaguchi and colleagues previously reported that acrolein caused lung septal cell apoptosis and airspace enlargement after chronic administration in rats (44). We noted that acrolein did not cause LMVEC apoptosis after 6 hours of exposure (data not shown). The difference between our results and those of Kitaguchi and colleagues may be due to the different durations (chronic versus acute) of acrolein exposure. Intranasal exposure of mice to acrolein (1–5 μmol/kg, similar to the concentrations used in this study) activates alveolar macrophages and increases proinflammatory cytokines, including TNF-α, IL-6, IL-12, and Chi3L3 (45). In the current study, we found that acrolein instillation increased lung neutrophil infiltration, with a mild effect on alveolar macrophages. Acrolein also enhanced lung proinflammatory cytokines, including TNF-α, IL-6, and KC. Although acrolein and LPS had a similar effect on BAL protein levels (0.51 mg/ml versus 0.47 mg/ml; Figure 2A), acrolein had a three times smaller effect on BAL cells than LPS (0.55 × 106 leukocytes/ml versus 1.67 × 106 leukocytes/ml; Figure 2B). In other words, acrolein was more effective in promoting endothelial cell permeability and lung edema, and had a relatively milder effect on inflammation. Acrolein has been shown to inhibit NF-κB–mediated gene transcription (26, 27). We observed a trend toward a decrease in NF-κB activity in LMVECs exposed to acrolein. Taken together, these results suggest that acrolein-induced alveolar barrier dysfunction and lung edema may be more important than proinflammatory effects in contributing to ALI.
Kasahara and colleagues demonstrated that the effect of acrolein on LPS-induced lung injury and inflammation was dependent on the timing of acrolein exposure (43). They reported that inhalation of the vapor phase of acrolein 18 hours after LPS instillation suppressed LPS-induced lung edema and proinflammatory responses (43). However, acrolein inhalation immediately after LPS instillation enhanced the LPS-induced increase in BAL protein levels and lung proinflammatory cytokines (43). In the current study, we showed that preexposure to intratracheally instilled acrolein 6 hours before LPS administration exaggerated LPS-induced lung edema and BAL inflammatory cell numbers. The difference between our results and those of Kasahara and colleagues may be due to the different doses of acrolein, the methods of exposure, and the timing of exposures relative to LPS used in the studies.
Reactive aldehydes can cause cellular oxidative stress by activating NADPH oxidase (24, 25) and/or depleting the antioxidant GSH (46) via the formation of aldehyde adducts (19, 47). Acrolein has been shown to deplete GSH and cause mitochondrial oxidative stress in hepatocytes (42). In contrast, acrolein has been shown to increase the antioxidant capacity of cells (48). In this study, we observed an increase of mitochondrial ROS in LMVECs treated with acrolein. We also found that acrolein-induced endothelial barrier dysfunction was completely prevented by the antioxidant NAC, a precursor of GSH, but not by apocynin, an NADPH oxidase inhibitor. Our results suggest that acrolein disrupts LMVEC barrier integrity, likely by depleting GSH rather than by activating NADPH oxidase.
Acrolein can be oxidized and thus detoxified by ALDH2, which is modestly expressed in lungs (40). Alda-1 is a small-molecule activator of ALDH2 (39). We confirmed that Alda-1 significantly elevated ALDH2 activity in liver. However, lung ALDH2 activity remained undetectable in our assay. Acrolein is also a potent inhibitor of both cytosolic and mitochondrial ALDH isoforms (49). We noted that liver ALDH2 activity did not decrease in mice intratracheally challenged with acrolein. This may be due to the instillation route used to administer the acrolein, and limited accumulation of acrolein in the liver, which would prevent a reduction in the baseline levels of ALDH2 in liver. However, acrolein prevented Alda-1–induced activation of ALDH2 in liver. We do not know whether acrolein inhibits basal or Alda-1–induced ALDH2 activity in lungs. We speculate that acrolein inhibits ALDH2 activity in lungs, leading to oxidative stress and lung injury. We show that Alda-1 not only prevented but also rescued acrolein-induced lung edema and lung inflammation in vivo. Alda-1 also attenuated acrolein-induced LMVEC barrier dysfunction in vitro. Although we still do not know whether Alda-1 protects against lung injury by preventing acrolein-induced inhibition of ALDH2, our data suggest that Alda-1 may be a novel strategy to treat smoke inhalation and other acrolein-associated lung injuries.
Activation of AMPK has been shown to attenuate lung inflammation and the severity of lung injuries induced by LPS (50) and sepsis (51), as well as LPS-induced lung vascular endothelial permeability (52). AMPK is activated via phosphorylation of T172. The reactive aldehyde 4-hydroxy-2-nonenal (4-HNE) has been shown to inactivate AMPK via carbonylation and thus dephosphorylation of AMPK at T172 (53). Similar to what was observed for 4-HNE, acrolein also inhibited AMPK activity, as indicated by reduced AMPK phosphorylation at T172. It is possible that acrolein causes AMPK carbonylation and thus dephosphorylation at T172. Additionally, lung AMPK expression levels were also reduced in mice exposed to acrolein—an effect that was associated with lung edema. Importantly, activation of AMPK with AICAR attenuated acrolein-induced LMVEC monolayer permeability. Our results suggest that inhibition of AMPK signaling may contribute to acrolein-induced lung injury. The mechanism by which inhibition of AMPK leads to endothelial barrier dysfunction and lung injury is not understood. High-mobility group box 1 protein (HMGB1), a component of damage-associated molecular patterns, is released during tissue injury and is one of the crucial proinflammatory cytokines that mediate the response to infection and inflammation (54). AMPK activators have been shown to prevent lethal endotoxin-induced lung inflammation and improve survival in mice by inhibiting LPS-induced HMGB1 release (55, 56). Whether HMGB1 release contributes to acrolein-induced alveolar-capillary barrier permeability and lung injury remains to be determined.
We showed that acrolein reduced total AMPK protein levels in vitro and in vivo. The underlying mechanism remains unknown. Protein carbonylation may cause protein misfolding, cross-linking, aggregation, and degradation (22). We noted that the acrolein-induced decrease in total AMPK in LMVECs was not altered by MG132 (data not shown), suggesting that the decrease in AMPK protein is independent of proteasome-mediated protein degradation. It is possible that carbonylated AMPK is degraded via the unfolded protein response, followed by protein degradation or autophagy/lysosome-associated protein degradation. It is also possible that acrolein reduced AMPK gene transcription. The exact mechanism of the acrolein-induced reduction in AMPK protein levels remains unknown and warrants further investigation.
Acrolein has been shown to impair mitochondrial respiration in alveolar type 2 epithelial cells (57). Similarly, we found that acrolein dose- and time-dependently impaired LMVEC mitochondrial respiration. Although Alda-1 and AICAR attenuated acrolein-induced LMVEC permeability, neither Alda-1 nor AICAR prevented acrolein-induced inhibition of mitochondrial respiration. It has been reported that increased monolayer permeability precedes ATP loss in acrolein-exposed Calu-3 lung adenocarcinoma cells (58). Taken together, our results suggest that acrolein increased LMVEC permeability and caused lung edema via a mechanism that is independent of mitochondrial dysfunction. Because acrolein not only reduced AMPK activity but also decreased AMPK total protein levels, the inability of the AMPK activator AICAR to prevent acrolein-induced mitochondrial dysfunction may be due to overall diminished amounts of AMPK in acrolein-treated cells. Our results also suggest that AMPK activity is essential for endothelial barrier function, whereas the amount of AMPK protein is important for mitochondrial function.
We recognize that in vivo acrolein has complex effects on multiple cell types. Alda-1 may target other cells in addition to LMVECs. Nevertheless, our results suggest that detoxification of acrolein by Alda-1 and activation of AMPK by AICAR may be novel approaches to prevent and treat acrolein-associated ALI.
Footnotes
Supported by the Providence VA Medical Center, the U.S. Department of Veterans Affairs Merit Review Program (S.R.), a Brown University Dean’s Award (S.R.), and National Institutes of Health grants P20GM103652 (S.R.; project 1, Q.L.), RO1 HL130230 (Q.L.), U54GM115677 (S.R.), R25 HL088992 (R.B. and M.O.), and HL128661 (G.C.).
Author Contributions: Conception and design: Q.L. and S.R.; data acquisition and analysis: Q.L., M.M., E.C., T.L., J.N., D.B., H.Y., R.B., and M.O.; data interpretation and drafting of the manuscript: Q.L.; revision of the manuscript: Q.L., G.C., and S.R.
Originally Published in Press as DOI: 10.1165/rcmb.2016-0342OC on August 1, 2017
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Stevens JF, Maier CS. Acrolein: sources, metabolism, and biomolecular interactions relevant to human health and disease. Mol Nutr Food Res. 2008;52:7–25. doi: 10.1002/mnfr.200700412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alwis KU, deCastro BR, Morrow JC, Blount BC. Acrolein exposure in U.S. tobacco smokers and non-tobacco users: NHANES 2005–2006. Environ Health Perspect. 2015;123:1302–1308. doi: 10.1289/ehp.1409251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Booze TF, Reinhardt TE, Quiring SJ, Ottmar RD. A screening-level assessment of the health risks of chronic smoke exposure for wildland firefighters. J Occup Environ Hyg. 2004;1:296–305. doi: 10.1080/15459620490442500. [DOI] [PubMed] [Google Scholar]
- 4.Treitman RD, Burgess WA, Gold A. Air contaminants encountered by firefighters. Am Ind Hyg Assoc J. 1980;41:796–802. doi: 10.1080/15298668091425662. [DOI] [PubMed] [Google Scholar]
- 5.Ho SS, Yu JZ, Chu KW, Yeung LL. Carbonyl emissions from commercial cooking sources in Hong Kong. J Air Waste Manag Assoc. 2006;56:1091–1098. doi: 10.1080/10473289.2006.10464532. [DOI] [PubMed] [Google Scholar]
- 6.Ho SS, Ip HS, Ho KF, Ng LP, Chan CS, Dai WT, Cao JJ. Hazardous airborne carbonyls emissions in industrial workplaces in China. J Air Waste Manag Assoc. 2013;63:864–877. doi: 10.1080/10962247.2013.797519. [DOI] [PubMed] [Google Scholar]
- 7.Blasch KW, Kolivosky JE, Heller JM. Environmental air sampling near burn pit and incinerator operations at Bagram Airfield, Afghanistan. J Occup Environ Med. 2016;58(8) Suppl 1:S38–S43. doi: 10.1097/JOM.0000000000000792. [DOI] [PubMed] [Google Scholar]
- 8.McCaskill ML, Kharbanda KK, Tuma DJ, Reynolds JD, DeVasure JM, Sisson JH, Wyatt TA. Hybrid malondialdehyde and acetaldehyde protein adducts form in the lungs of mice exposed to alcohol and cigarette smoke. Alcohol Clin Exp Res. 2011;35:1106–1113. doi: 10.1111/j.1530-0277.2011.01443.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anderson MM, Hazen SL, Hsu FF, Heinecke JW. Human neutrophils employ the myeloperoxidase-hydrogen peroxide-chloride system to convert hydroxy-amino acids into glycolaldehyde, 2-hydroxypropanal, and acrolein. A mechanism for the generation of highly reactive alpha-hydroxy and alpha,beta-unsaturated aldehydes by phagocytes at sites of inflammation. J Clin Invest. 1997;99:424–432. doi: 10.1172/JCI119176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bein K, Leikauf GD. Acrolein—a pulmonary hazard. Mol Nutr Food Res. 2011;55:1342–1360. doi: 10.1002/mnfr.201100279. [DOI] [PubMed] [Google Scholar]
- 11.Sklar JL, Anderson PG, Boor PJ. Allylamine and acrolein toxicity in perfused rat hearts. Toxicol Appl Pharmacol. 1991;107:535–544. doi: 10.1016/0041-008x(91)90316-7. [DOI] [PubMed] [Google Scholar]
- 12.Raju SV, Jackson PL, Courville CA, McNicholas CM, Sloane PA, Sabbatini G, Tidwell S, Tang LP, Liu B, Fortenberry JA, et al. Cigarette smoke induces systemic defects in cystic fibrosis transmembrane conductance regulator function. Am J Respir Crit Care Med. 2013;188:1321–1330. doi: 10.1164/rccm.201304-0733OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghilarducci DP, Tjeerdema RS. Fate and effects of acrolein. Rev Environ Contam Toxicol. 1995;144:95–146. doi: 10.1007/978-1-4612-2550-8_2. [DOI] [PubMed] [Google Scholar]
- 14.Miller AC, Elamin EM, Suffredini AF. Inhaled anticoagulation regimens for the treatment of smoke inhalation-associated acute lung injury: a systematic review. Crit Care Med. 2014;42:413–419. doi: 10.1097/CCM.0b013e3182a645e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hales CA, Musto SW, Janssens S, Jung W, Quinn DA, Witten M. Smoke aldehyde component influences pulmonary edema. J Appl Physiol (1985) 1992;72:555–561. doi: 10.1152/jappl.1992.72.2.555. [DOI] [PubMed] [Google Scholar]
- 16.Quinn DA, Robinson D, Jung W, Hales CA. Role of sulfidopeptide leukotrienes in synthetic smoke inhalation injury in sheep. J Appl Physiol. 1990;68:1962–1969. doi: 10.1152/jappl.1990.68.5.1962. [DOI] [PubMed] [Google Scholar]
- 17.Hales CA, Barkin PW, Jung W, Trautman E, Lamborghini D, Herrig N, Burke J. Synthetic smoke with acrolein but not HCl produces pulmonary edema. J Appl Physiol (1985) 1988;64:1121–1133. doi: 10.1152/jappl.1988.64.3.1121. [DOI] [PubMed] [Google Scholar]
- 18.Jang AS, Concel VJ, Bein K, Brant KA, Liu S, Pope-Varsalona H, Dopico RA, Jr, Di YP, Knoell DL, Barchowsky A, et al. Endothelial dysfunction and claudin 5 regulation during acrolein-induced lung injury. Am J Respir Cell Mol Biol. 2011;44:483–490. doi: 10.1165/rcmb.2009-0391OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grafström RC, Dypbukt JM, Willey JC, Sundqvist K, Edman C, Atzori L, Harris CC. Pathobiological effects of acrolein in cultured human bronchial epithelial cells. Cancer Res. 1988;48:1717–1721. [PubMed] [Google Scholar]
- 20.Li SY, Li Q, Shen JJ, Dong F, Sigmon VK, Liu Y, Ren J. Attenuation of acetaldehyde-induced cell injury by overexpression of aldehyde dehydrogenase-2 (ALDH2) transgene in human cardiac myocytes: role of MAP kinase signaling. J Mol Cell Cardiol. 2006;40:283–294. doi: 10.1016/j.yjmcc.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 21.Sun A, Cheng Y, Zhang Y, Zhang Q, Wang S, Tian S, Zou Y, Hu K, Ren J, Ge J. Aldehyde dehydrogenase 2 ameliorates doxorubicin-induced myocardial dysfunction through detoxification of 4-HNE and suppression of autophagy. J Mol Cell Cardiol. 2014;71:92–104. doi: 10.1016/j.yjmcc.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 22.Grimsrud PA, Xie H, Griffin TJ, Bernlohr DA. Oxidative stress and covalent modification of protein with bioactive aldehydes. J Biol Chem. 2008;283:21837–21841. doi: 10.1074/jbc.R700019200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van der Toorn M, Smit-de Vries MP, Slebos DJ, de Bruin HG, Abello N, van Oosterhout AJ, Bischoff R, Kauffman HF. Cigarette smoke irreversibly modifies glutathione in airway epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2007;293:L1156–L1162. doi: 10.1152/ajplung.00081.2007. [DOI] [PubMed] [Google Scholar]
- 24.Jaimes EA, DeMaster EG, Tian R-X, Raij L. Stable compounds of cigarette smoke induce endothelial superoxide anion production via NADPH oxidase activation. Arterioscler Thromb Vasc Biol. 2004;24:1031–1036. doi: 10.1161/01.ATV.0000127083.88549.58. [DOI] [PubMed] [Google Scholar]
- 25.Chen C-H, Sun L, Mochly-Rosen D. Mitochondrial aldehyde dehydrogenase and cardiac diseases. Cardiovasc Res. 2010;88:51–57. doi: 10.1093/cvr/cvq192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hristova M, Spiess PC, Kasahara DI, Randall MJ, Deng B, van der Vliet A. The tobacco smoke component, acrolein, suppresses innate macrophage responses by direct alkylation of c-Jun N-terminal kinase. Am J Respir Cell Mol Biol. 2012;46:23–33. doi: 10.1165/rcmb.2011-0134OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lambert C, Li J, Jonscher K, Yang TC, Reigan P, Quintana M, Harvey J, Freed BM. Acrolein inhibits cytokine gene expression by alkylating cysteine and arginine residues in the NF-kappaB1 DNA binding domain. J Biol Chem. 2007;282:19666–19675. doi: 10.1074/jbc.M611527200. [DOI] [PubMed] [Google Scholar]
- 28.Chen D, Fang L, Li H, Tang MS, Jin C. Cigarette smoke component acrolein modulates chromatin assembly by inhibiting histone acetylation. J Biol Chem. 2013;288:21678–21687. doi: 10.1074/jbc.M113.476630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fabisiak JP, Medvedovic M, Alexander DC, McDunn JE, Concel VJ, Bein K, Jang AS, Berndt A, Vuga LJ, Brant KA, et al. Integrative metabolome and transcriptome profiling reveals discordant energetic stress between mouse strains with differential sensitivity to acrolein-induced acute lung injury. Mol Nutr Food Res. 2011;55:1423–1434. doi: 10.1002/mnfr.201100291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lu Q, Lange T, Borgas D, Newton J, Sakhatskyy P, Choudhary G, Basak R, Rounds SIS. Acrolein mediates cigarette smoke-induced increase in lung endothelial cell permeability and susceptibility to acute lung injury. Am J Respir Crit Care Med. 2015;191:A5939. [Google Scholar]
- 31.Sakhatskyy P, Wang Z, Borgas D, Lomas-Neira J, Chen Y, Ayala A, Rounds S, Lu Q. Double-hit mouse model of cigarette smoke priming for acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2017;312:L56–L67. doi: 10.1152/ajplung.00436.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lu Q, Harrington EO, Newton J, Casserly B, Radin G, Warburton R, Zhou Y, Blackburn MR, Rounds S. Adenosine protected against pulmonary edema through transporter- and receptor A2-mediated endothelial barrier enhancement. Am J Physiol Lung Cell Mol Physiol. 2010;298:L755–L767. doi: 10.1152/ajplung.00330.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lu Q, Harrington EO, Hai C-M, Newton J, Garber M, Hirase T, Rounds S. Isoprenylcysteine carboxyl methyltransferase modulates endothelial monolayer permeability: involvement of RhoA carboxyl methylation. Circ Res. 2004;94:306–315. doi: 10.1161/01.RES.0000113923.85084.C1. [DOI] [PubMed] [Google Scholar]
- 34.Lu Q, Sakhatskyy P, Grinnell K, Newton J, Ortiz M, Wang Y, Sanchez-Esteban J, Harrington EO, Rounds S. Cigarette smoke causes lung vascular barrier dysfunction via oxidative stress-mediated inhibition of RhoA and focal adhesion kinase. Am J Physiol Lung Cell Mol Physiol. 2011;301:L847–L857. doi: 10.1152/ajplung.00178.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Calfee CS, Matthay MA, Eisner MD, Benowitz N, Call M, Pittet JF, Cohen MJ. Active and passive cigarette smoking and acute lung injury after severe blunt trauma. Am J Respir Crit Care Med. 2011;183:1660–1665. doi: 10.1164/rccm.201011-1802OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ho KM, Hart G, Austin D, Hunter M, Botha J, Chavan S. Dose-related effect of smoking on mortality in critically ill patients: a multicentre cohort study. Intensive Care Med. 2011;37:981–989. doi: 10.1007/s00134-011-2184-6. [DOI] [PubMed] [Google Scholar]
- 37.Hsieh SJ, Zhuo H, Benowitz NL, Thompson BT, Liu KD, Matthay MA, Calfee CS National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome Network; National Heart Lung and Blood Institute Acute Respiratory Distress Syndrome Network. Prevalence and impact of active and passive cigarette smoking in acute respiratory distress syndrome. Crit Care Med. 2014;42:2058–2068. doi: 10.1097/CCM.0000000000000418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moazed F, Burnham EL, Vandivier RW, O’Kane CM, Shyamsundar M, Hamid U, Abbott J, Thickett DR, Matthay MA, McAuley DF, et al. Cigarette smokers have exaggerated alveolar barrier disruption in response to lipopolysaccharide inhalation. Thorax. 2016;71:1130–1136. doi: 10.1136/thoraxjnl-2015-207886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen CH, Budas GR, Churchill EN, Disatnik MH, Hurley TD, Mochly-Rosen D. Activation of aldehyde dehydrogenase-2 reduces ischemic damage to the heart. Science. 2008;321:1493–1495. doi: 10.1126/science.1158554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dipple KM, Crabb DW. The mitochondrial aldehyde dehydrogenase gene resides in an HTF island but is expressed in a tissue-specific manner. Biochem Biophys Res Commun. 1993;193:420–427. doi: 10.1006/bbrc.1993.1640. [DOI] [PubMed] [Google Scholar]
- 41.Drummond GR, Selemidis S, Griendling KK, Sobey CG. Combating oxidative stress in vascular disease: NADPH oxidases as therapeutic targets. Nat Rev Drug Discov. 2011;10:453–471. doi: 10.1038/nrd3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mohammad MK, Avila D, Zhang J, Barve S, Arteel G, McClain C, Joshi-Barve S. Acrolein cytotoxicity in hepatocytes involves endoplasmic reticulum stress, mitochondrial dysfunction and oxidative stress. Toxicol Appl Pharmacol. 2012;265:73–82. doi: 10.1016/j.taap.2012.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kasahara DI, Poynter ME, Othman Z, Hemenway D, van der Vliet A. Acrolein inhalation suppresses lipopolysaccharide-induced inflammatory cytokine production but does not affect acute airways neutrophilia. J Immunol. 2008;181:736–745. doi: 10.4049/jimmunol.181.1.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kitaguchi Y, Taraseviciene-Stewart L, Hanaoka M, Natarajan R, Kraskauskas D, Voelkel NF. Acrolein induces endoplasmic reticulum stress and causes airspace enlargement. PLoS One. 2012;7:e38038. doi: 10.1371/journal.pone.0038038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sun Y, Ito S, Nishio N, Tanaka Y, Chen N, Isobe K. Acrolein induced both pulmonary inflammation and the death of lung epithelial cells. Toxicol Lett. 2014;229:384–392. doi: 10.1016/j.toxlet.2014.06.021. [DOI] [PubMed] [Google Scholar]
- 46.Kehrer JP, Biswal SS. The molecular effects of acrolein. Toxicol Sci. 2000;57:6–15. doi: 10.1093/toxsci/57.1.6. [DOI] [PubMed] [Google Scholar]
- 47.Sthijns MM, Randall MJ, Bast A, Haenen GR. Adaptation to acrolein through upregulating the protection by glutathione in human bronchial epithelial cells: the materialization of the hormesis concept. Biochem Biophys Res Commun. 2014;446:1029–1034. doi: 10.1016/j.bbrc.2014.03.081. [DOI] [PubMed] [Google Scholar]
- 48.Patel JM, Block ER. Cyclophosphamide-induced depression of the antioxidant defense mechanisms of the lung. Exp Lung Res. 1985;8:153–165. doi: 10.3109/01902148509057519. [DOI] [PubMed] [Google Scholar]
- 49.Mitchell DY, Petersen DR. Inhibition of rat liver aldehyde dehydrogenases by acrolein. Drug Metab Dispos. 1988;16:37–42. [PubMed] [Google Scholar]
- 50.Zhao X, Zmijewski JW, Lorne E, Liu G, Park YJ, Tsuruta Y, Abraham E. Activation of AMPK attenuates neutrophil proinflammatory activity and decreases the severity of acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2008;295:L497–L504. doi: 10.1152/ajplung.90210.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mulchandani N, Yang WL, Khan MM, Zhang F, Marambaud P, Nicastro J, Coppa GF, Wang P. Stimulation of brain amp-activated protein kinase attenuates inflammation and acute lung injury in sepsis. Mol Med. 2015;21:637–644. doi: 10.2119/molmed.2015.00179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jian MY, Alexeyev MF, Wolkowicz PE, Zmijewski JW, Creighton JR. Metformin-stimulated AMPK-α1 promotes microvascular repair in acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2013;305:L844–L855. doi: 10.1152/ajplung.00173.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shearn CT, Backos DS, Orlicky DJ, Smathers-McCullough RL, Petersen DR. Identification of 5′ AMP-activated kinase as a target of reactive aldehydes during chronic ingestion of high concentrations of ethanol. J Biol Chem. 2014;289:15449–15462. doi: 10.1074/jbc.M113.543942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lotze MT, Tracey KJ. High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat Rev Immunol. 2005;5:331–342. doi: 10.1038/nri1594. [DOI] [PubMed] [Google Scholar]
- 55.Kim JM, Han HJ, Hur YH, Quan H, Kwak SH, Choi JI, Bae HB. Stearoyl lysophosphatidylcholine prevents lipopolysaccharide-induced extracellular release of high mobility group box-1 through AMP-activated protein kinase activation. Int Immunopharmacol. 2015;28:540–545. doi: 10.1016/j.intimp.2015.07.010. [DOI] [PubMed] [Google Scholar]
- 56.Tsoyi K, Jang HJ, Nizamutdinova IT, Kim YM, Lee YS, Kim HJ, Seo HG, Lee JH, Chang KC. Metformin inhibits HMGB1 release in LPS-treated RAW 264.7 cells and increases survival rate of endotoxaemic mice. Br J Pharmacol. 2011;162:1498–1508. doi: 10.1111/j.1476-5381.2010.01126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Agarwal AR, Yin F, Cadenas E. Metabolic shift in lung alveolar cell mitochondria following acrolein exposure. Am J Physiol Lung Cell Mol Physiol. 2013;305:L764–L773. doi: 10.1152/ajplung.00165.2013. [DOI] [PubMed] [Google Scholar]
- 58.Burcham PC, Raso A, Henry PJ. Airborne acrolein induces keratin-8 (Ser-73) hyperphosphorylation and intermediate filament ubiquitination in bronchiolar lung cell monolayers. Toxicology. 2014;319:44–52. doi: 10.1016/j.tox.2014.02.010. [DOI] [PubMed] [Google Scholar]