Abstract
Airway smooth muscle cells (ASMCs) are phenotypically regulated to exist in either a proliferative or a contractile state. However, the influence of other airway structural cell types on ASMC phenotype is largely unknown. Although epithelial cells are known to drive ASM proliferation, their effects on the contractile phenotype are uncertain. In the current study, we tested the hypothesis that epithelial cells reduce the contractile phenotype of ASMCs. To do so, we measured force production by traction microscopy, gene and protein expression, as well as calcium release by Fura-2 ratiometric imaging. ASMCs incubated with epithelial-derived medium produced less force after histamine stimulation. We observed reduced expression of myocardin, α-smooth muscle actin, and calponin within ASMCs after coculture with epithelial cells. Peak calcium release in response to histamine was diminished, and depended on the synthesis of cyclo-oxygenase-1 products by ASM and on prostaglandin E receptors 2 and 4. Together, these in vitro results demonstrate that epithelial cells have the capacity to coordinately reduce ASM contraction by functional antagonism and by reduction of the expression of certain contractile proteins.
Keywords: epithelial cells, airway smooth muscle cells, coculture, calcium, excitability
Clinical Relevance
Asthma pathogenesis remains poorly understood. This article sheds new light on the regulation of the contractile phenotype by airway epithelial cells on airway smooth muscle cells.
Excessive airway narrowing is a key feature of asthma, and there are several potential mechanisms that may account for this phenomenon. A frequent finding that could account for excessive bronchoconstriction is airway wall remodeling (1). In particular, an increased mass of airway smooth muscle (ASM) is associated with asthma (2), and its contraction leads to airway narrowing. Whether the contractile properties of ASM are altered in asthma has been an elusive question, and recent publications still provide contradictory evidence (3–5). Nevertheless, the contractile phenotype of the human ASM cell (ASMC) could also be modulated by other tissues in vivo. The airway epithelium has been implicated as an important driving force of asthma pathogenesis (6) through the secretion of many proremodeling factors, such as chemokines (7) and growth factors (8). ASM proliferation may be stimulated by mediators derived from the nearby epithelial tissue (9).
Maturation and differentiation of ASMCs is marked by expression of contractile apparatus proteins (10) and associated nuclear translocation of the cotranscription factor, myocardin, which acts in concert with serum response factor (SRF) (11). Myocardin competes for SRF binding with the proproliferative cotranscription factors, ETS domain-containing protein Elk-1 and Kruppel-like factor 4 (12, 13). Smooth muscle cells that are actively proliferating down-regulate proteins of the contractile apparatus, such as α-smooth muscle actin (α-SMA) and calponin (14). Logically, in driving proliferation, epithelial cells might also down-regulate the contractile phenotype, as reflected in the expression of contractile proteins and in transcription factors controlling these same proteins.
Epithelial influence on ASM contractile phenotype is largely unexplored, and, given the importance of these tissues in mediating asthma pathogenesis, a better understanding of their interactions may lead to novel insights regarding this complex disease. Here, we used coculture techniques or treatment of ASMCs with epithelial conditioned medium to explore epithelial regulation of ASM contractile properties. Given the critical role of calcium ions in the initiation of cross-bridge cycling, we explored the effects of epithelial coculture on calcium release within ASMCs triggered by histamine, a biogenic amine of relevance to asthma exacerbations (15, 16). Intracellular calcium concentration is tightly regulated, and its release upon histamine stimulation depends on proteins regulating histamine receptor signal transduction (17), calcium handling proteins, and second messenger signaling mechanisms, such as cyclic adenosine monophosphate (cAMP) generation within the cell (18). prostaglandin (PG) E2 is a known airway relaxant (19) secreted by the epithelium (20), and it stimulates cAMP within ASMCs (21). As such, we considered it a possible candidate for any observed changes in contractility of ASM. We addressed whether the effects of epithelial cells on ASMCs involved the synthesis of factors associated with relaxation by ASM itself and acting in an autocrine manner.
Materials and Methods
Reagents
Histamine dihydrochloride (1 μM), collagenase type IV from Clostridium histolyticum, general cyclo-oxygenase (COX) inhibitor, indomethacin (3 μM), and the COX-2–specific inhibitor, celecoxib (100 nM), were obtained from Sigma-Aldrich (St. Louis, MO). The specific COX-1 inhibitor, 5-(4-chlorophenyl)-1-(4-methoxyphenyl)-3-trifluoromethyl pyrazole (100 nM), was purchased from Cayman Chemical (Ann Arbor, MI). Specific prostaglandin E receptor-2 receptor antagonist, 1-(4-fluorobenzoyl)-3-[[(6-methoxy-2-naphthalenyl)oxy]methyl]-3-azetidinecarboxylic acid 04418948 (1 μM), and EP4 receptor antagonist, 4-cyano-2-[[2-(4-fluoro-1-naphthalenyl)-1-oxopropyl]amino]-benzenebutanoic acid AE3 208 (1 μM), were obtained from Tocris (Avonmouth, Bristol, UK). Fura 2-AM (10 μM), Pluronic F-127 (0.02%), and quantitative PCR (qPCR) primers were obtained from Thermo Fisher Scientific (Waltham, MA).
Cell Culture
Primary human ASMCs and normal human bronchial epithelial (NHBE) cells were obtained from central airways (lower trachea or proximal bronchi) with institutional ethics approval. BEAS-2B cells were obtained from ATCC (Manassas, VA). Cells were placed in coculture, or conditioned medium from epithelial cells was used to stimulate ASMCs, as described in the supplemental Materials and Methods.
RT-qPCR
mRNA was extracted from ASMCs (Qiagen, Valencia, CA). Reverse transcription was performed on 100 ng of total RNA with AffinityScript qPCR cDNA synthesis kit (Agilent Technologies, Santa Clara, CA). qPCR was performed using iTaq SYBR green supermix (Bio-Rad Laboratories, Hercules, CA). Primer sequences are reported in Table 1. Amplification of cDNA was performed using a StepOnePlus RealTime PCR system (Applied Biosystems, Foster City, CA). Relative mRNA expression was calculated using the ΔΔCt method, and all gene expression was normalized to S9.
Table 1.
Quantitative PCR Primer Sequences
| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| S9 | CTGCTGACGCTTGATGAGAA | CAGCTTCATCTTGCCCTCA |
| CNN1 | AGCAGGAGCTGAGAGAGTGG | AAAGCCAGGAGGGTGGACTG |
| ACTA2 | TCATGATGCTGTTGTAGGTGGT | CTCTTCCAGCCATCCTTCAT |
| MYOCD | TCAGCAATTTCAGAGGTAACACA | TGACTCCGGGTCATTTGC |
| MYLK | TGGGGCTCTTATGACCTACAGT | CCTGAAGTTGCTCTGAACTGC |
| ITPR1 | CCTTTTCCGTTTCAAGCATC | AGGCATTCTTCCTCAAAGTCAG |
| H1R | AAGTCACCATCCCAAACCCCCAAG | TCAGGCCCTGCTCATCTGTCTTGA |
| SERCA | TCGAACCCTTGCCAGTAAGT | CACACAGGGAAGACGTCTCA |
| CD38 | CAGCAACAACCCTGTTTCAGT | CCATTGAGCATCACATGGAC |
| PLC-β | TCCAAGAAGAAGTGGCCAAG | ATGCATCCCTGGACATGTTT |
| PTGS | CTTCACGCATCAGTTTTTC | TCACCGTAAATATGATTTAAGTCCAC |
| PTGES | CGCTGCTGGTCATCAAGA | TCCGTGTCTCAGGGATC |
| SRF | ATCCCTGTTTCAGCAGTTCAGCTC | ATCATTCACTCTTGGTGCTGTGGG |
| ELK1 | GCTTCCTACGCATACATTGACC | GGTGCTCCAGAAGTGAATGC |
| KLF4 | CTGTGTGTTTGCGGTAGTGCC | GAAATTCGCCCGCTCCGATGA |
Bromo-Deoxyuridine Proliferation Assay
Bromo-deoxyuridine (BrdU) was added to BEAS-2B:ASMC coculture 18 hours before harvesting and staining for BrdU positivity by flow cytometry, according to manufacturer’s protocol (BD Biosciences, Franklin Lakes, NJ).
cAMP Assay
ASM intracellular cAMP concentration was measured as per manufacturer’s protocol (Cyclic AMP XP Assay Kit; Cell Signaling Technology, Danvers, MA). To prevent the degradation of cAMP, cells were incubated with 0.5 mM 3-isobutyl-1-methylxanthine. Lysis buffer was supplemented with 0.5 mM 3-isobutyl-1-methylxanthine.
Western Blot
Primary antibodies included anti–α-SMA (1A4, 1:1,000; Sigma-Aldrich) and anti–glyceraldehyde 3-phosphate dehydrogenase (6C5, 1:3,000; EMD Millipore, Billerica, MA). Membranes were incubated with secondary antibodies for 1 hour at room temperature before development using chemiluminescence (Bio-Rad Laboratories) and imaging. Image J (National Institutes of Health, Bethesda, MD) was used to quantify band density.
Measurement of Intracellular Calcium
ASM intracellular calcium responses to 1 μM histamine were measured as previously described (22) (see supplemental Material and Methods). Fluorescence intensity ratios (340:380) were converted to calcium concentrations, as previously described, using Grynkiewicz’s equation (23).
where Kd = 225, Rmin = 0.187, Rmax = 5.513, and β = 5.23.
Traction Microscopy
ASMC traction force responses to 1 μM histamine were measured as previously described (24) (see supplemental Materials and Methods). Data are presented as a fold change in root mean square traction between baseline and posthistamine treatment, as previously described (24).
Statistical Analysis
See supplemental Materials and Methods.
Results
Epithelial Cells Reduce Agonist-induced Calcium Release in ASMCs
Intracellular calcium release is an important step in the initiation of cross-bridge cycling in ASMCs. We therefore examined the effect of epithelial coculture on histamine-induced calcium release to determine whether epithelial cells had the ability to diminish agonist-induced excitability. After 24 hours of coculture with either BEAS-2B (Figure 1A) or NHBE cells (Figure 1B), peak calcium responses to stimulation with 1 μM histamine were diminished. Because both primary and BEAS-2B cells reduced the excitability of ASMCs, we performed future experiments with BEAS-2B cells.
Figure 1.
Coculture reduces agonist-induced calcium release. Airway smooth muscle cells were cultured with BEAS-2B cells (A) or normal bronchial epithelial cells (NHBE) (B) for 24 hours before Fura 2-AM calcium imaging. Peak calcium release after 1 μM histamine stimulation is reported. Data are presented as medians (±maxima and minima). (A) Control, 72 cells from 5 independent experiments; coculture, 128 cells from 5 individual experiments. (B) Control, 34 cells from 4 independent experiments; coculture, 36 cells from 4 independent experiments. Paired t test was used to compare samples with P values reported above the bars.
Calcium Release Is Not Transcriptionally Regulated
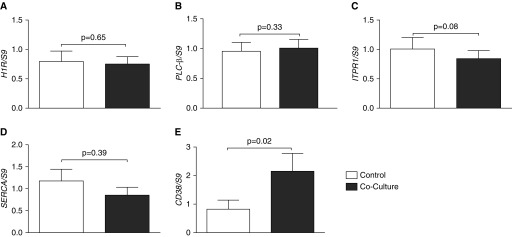
Given the observation that epithelial cells reduce ASMC excitability, we explored how this reduction is regulated. Calcium release may be regulated transcriptionally via alterations in proteins affecting calcium release or uptake intracellularly. However, we observed no reduction in the expression of mRNA of the histamine receptor (Figure 2A), in the proteins, phospholipase C-β (Figure 2B), inositol trisphosphate receptor (Figure 2C), or an increase in the expression of the calcium-reducing sarcoplasmic reticulum Ca2+–ATPase pump (Figure 2D). There was an increase in cyclic ADP ribose hydrolase (Figure 2E) mRNA after coculture, a finding that cannot explain the reduction in intracellular calcium release, as cyclic ADP ribose hydrolase is associated with increased activation of the calcium-releasing ryanodine receptor (25). These data indicate that the reduction in histamine-stimulated calcium release is not transcriptionally regulated by calcium handling proteins.
Figure 2.
Calcium release is not transcriptionally regulated. Airway smooth muscle cells (ASMCs) were cocultured with BEAS-2B cells for 24 hours. mRNA was extracted to perform RT–quantitative PCR (qPCR) examining histamine receptor (H1R) (A), phospholipase C-β (PLCβ) (B), inositol trisphosphate receptor 1 (ITPR1) (C), sarco/endoplasmic reticulum Ca2+–ATPase (SERCA) (D), and cyclic ADP ribose hydrolase (CD38) (E). Open bars, control ASMCs; solid bars, cocultured ASMCs. Data are presented as means (+SE). Paired t test was used to compare samples with P values reported above the bars.
ASMC Phenotype Is Altered after Coculture with Epithelial Cells
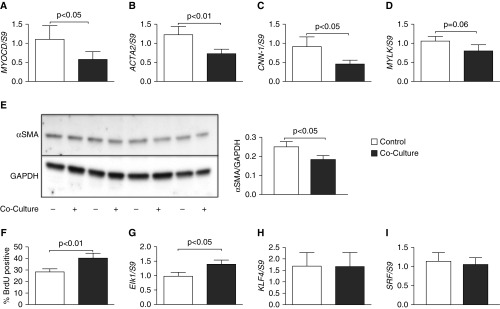
Because we observed reduced excitability of ASMCs after coculture with epithelial cells, we wished to investigate the role of transcriptional regulation in the reduction of the contractile phenotype after coculture with epithelial cells. To do this, we explored a variety of proteins associated with the contractile phenotype. Myocardin, the master regulator of contractile apparatus proteins (26), had reduced transcript expression after coculture with BEAS-2B cells (Figure 3A). We examined downstream targets of myocardin, observing a reduction in mRNA of α-SMA (Figure 3B) and calponin (Figure 3C), and a downward trend of myosin light-chain kinase (Figure 3D). Primer sequences used to probe gene expression are presented in Table 1. Furthermore, α-SMA protein was reduced after 96 hours of coculture with BEAS-2B cells (Figure 3E).
Figure 3.
ASMC phenotype is altered after coculture with epithelial cells. ASMCs were cocultured with BEAS-2B cells for 24 hours. mRNA was extracted to perform RT-qPCR, examining myocardin (MYOCD) (A), α-smooth muscle actin (ACTA2) (B), calponin (CNN-1) (C), and myosin light-chain kinase (MYLK) (D). Open bars, control ASMCs; solid bars, cocultured ASMCs. Protein lysate was separated in a polyacrylamide gel before transfer to PVDF membrane and blotted for α-smooth muscle actin (α-SMA) protein. Control ASMCs: lanes 1, 3, 5, and 7; BEAS-2B cocultured ASMCs: lanes 2, 4, 6, 8 (E). Densitometry of α-SMA blotting normalized to GAPDH is shown. Rate of proliferation was measured by bromo-deoxyuridine (BrdU) incorporation for 18 hours before the end of 24-hour coculture with BEAS-2B cells (F). mRNA for ETS domain-containing protein Elk-1 (Elk1) (G), Kruppel-like factor 4 (KLF4) (H), and serum response factor (SRF) (I) was measured within ASMCs after 24 hours of coculture with BEAS-2B cells. Data are presented as means (+SE). Paired t test was used to compare samples with P values reported above the bars.
Because airway epithelial cells have been previously reported to induce a proliferative phenotype of ASMCs (9), we examined if this also occurred in our system. To test the rate of proliferation after coculture, we incorporated BrdU into the cultures 18 hours before the end of the 24-hour coculture with BEAS-2B cells, then measured BrdU-positive cells by flow cytometry. Indeed, we observed an increased proliferative phenotype (Figure 3F). Furthermore, we observed an increase in mRNA for ETS domain-containing protein Elk-1 within ASMCs after 24-hour coculture with BEAS-2B cells (Figure 3G). We observed no difference in the expression of Kruppel-like factor 4 (Figure 3H) or SRF (Figure 3I). These results further confirm a phenotypic change of ASMCs after epithelial coculture.
Airway Epithelial Cells Reduce Histamine-induced Contraction
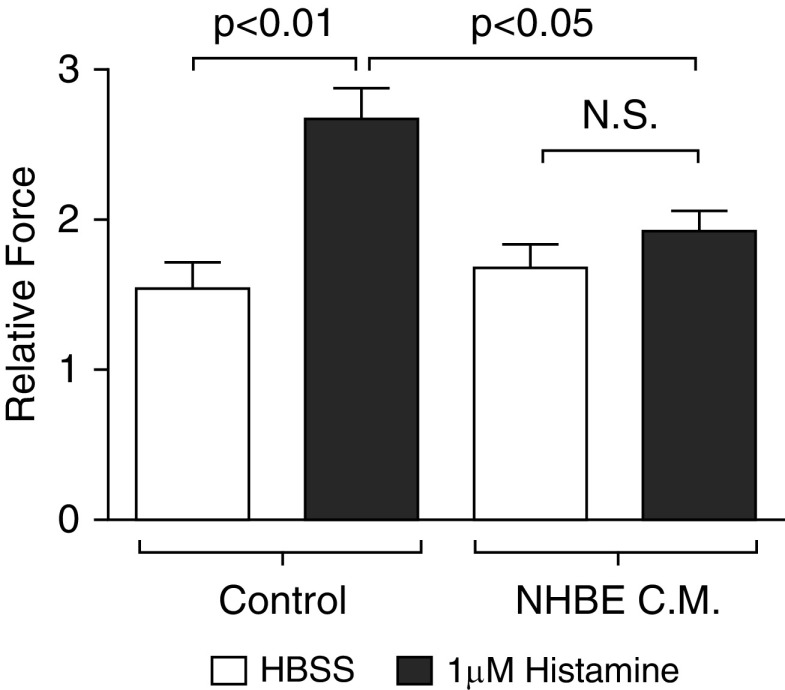
Given the observed reduction in both excitability and gene expression of contractile apparatus proteins within ASMCs after coculture with epithelial cells, we probed for a functional consequence by measuring contractile force in vitro by traction force microscopy. Cells were stimulated to contract with 1 μM histamine. ASMC cells that had been treated with conditioned medium derived from NHBE cells for 24 hours demonstrated less force generation than those that had been incubated with control starvation medium (Figure 4). This reduced force generation confirmed the reduction in the contractile phenotype observed in ASMCs.
Figure 4.
Airway epithelial cells reduce histamine-induced contraction. ASMCs were stimulated to contract with 1 μM histamine or vehicle (Hanks’ balanced salt solution [HBSS]). Control cells were incubated for 24 hours with 50:50 medium that had not been conditioned with epithelial cells. Conditioned medium (C.M.) of NHBE cells was used to deliver epithelial-derived mediators to the ASMCs. Average contractile forces of confluent ASMCs were measured using traction microscopy. Relative forces were defined as the fold change between baseline and after histamine treatment. Data are presented as means (+SE). ANOVA with Tukey’s post hoc pairwise comparisons were conducted and P values are reported. N.S., not significant.
PGE2 Diminishes Agonist-induced Calcium Release in ASMCs
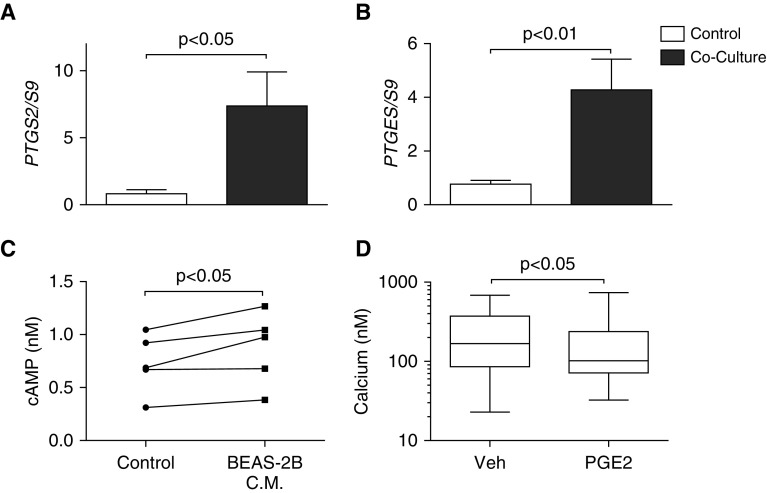
Because we did not detect diminished gene expression of calcium handling proteins, we examined an alternative pathway implicated in calcium regulation. We explored the role of arachidonic acid metabolites as possible functional antagonists of calcium release. Several prostanoids stimulate the synthesis of cAMP within ASMCs that can reduce calcium transients and diminish tone. RT-qPCR data indicated an increase in the PGE2-producing enzymes, COX-2 (Figure 5A) and membrane-associated PGE synthase-1 (Figure 5B) within the ASMCs after coculture with BEAS-2B cells. Incubation of ASMCs for 24 hours with BEAS-2B conditioned medium increased the concentration of intracellular cAMP within the ASMCs (Figure 5C). To examine the plausibility of a role for PGE2 in regulating ASMC calcium responses to histamine stimulation, we pretreated the cells with 10 μM PGE2 before stimulation with histamine. Pretreatment of ASMCs with PGE2 diminished the agonist-induced peak calcium concentration (Figure 5D). These data indicate that epithelial cells induce the up-regulation of enzymes associated with PGE2 synthesis in ASMCs as well as the downstream signaling molecule, cAMP.
Figure 5.
Prostaglandin (PG) E2 diminishes agonist-induced calcium release in ASMCs. ASMCs were cocultured with BEAS-2B cells for 24 hours. RT-qPCR was conducted for cyclo-oxygenase (COX)-2 (PTGS-2) (A) and membrane-associated PGE synthase (mPGES)-1 (PTGES) (B). ASMCs were treated with BEAS-2B conditioned medium for 24 hours before measurement of intracellular cAMP (C). ASMCs were incubated with 10 μM PGE2 or vehicle (Veh; 0.1% DMSO) for 15 minutes before stimulation with 1 μM histamine to induce intracellular calcium release (D). Data are presented as means (+SE). Paired t test was used to compare samples, and P values are reported above the bars. Calcium data are presented as medians, with whiskers showing group maxima and minima, and the Mann–Whitney test was used. Calcium was measured within 79–126 cells from 7–8 individual experiments.
Reduction in Calcium Release by Epithelial Cells Is Dependent on ASMC COX-1
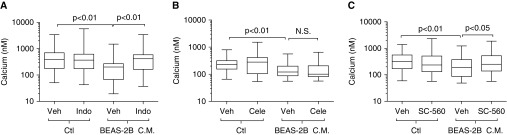
We next examined whether the inhibition of COXs restored epithelial-reduced ASMC excitability, as these enzymes determine PGE2 synthesis from arachidonic acid. ASMCs were pretreated for 24 hours with the nonselective COX inhibitor, indomethacin. COX inhibition restored the reduction in agonist-induced peak calcium release caused by BEAS-2B cell conditioned medium, indicating the role of a COX metabolite in reducing ASMC excitability (Figure 6A). To prevent confounding by inhibition of COX within the epithelium, we used conditioned medium from epithelial cells rather than coculture. This allowed the drug treatment to inhibit its target within the ASMCs alone. Furthermore, the BEAS-2B conditioned medium mimicked the data observed in the BEAS-2B coculture model (Figure 1A). Next, we examined the inducible COX isoform, COX-2, by inhibition with the selective COX-2 inhibitor, celecoxib. Pretreatment of ASMCs with celecoxib did not restore the excitability after incubation with BEAS-2B conditioned medium (Figure 6B). Because indomethacin restored excitability after incubation with conditioned medium, we reasoned that this effect must be mediated by COX-1. Treatment with the COX-1–specific inhibitor, SC560, restored ASMC excitability after treatment with conditioned medium of BEAS-2B cells (Figure 6C). These data indicate that airway epithelial cell–dependent reductions in ASMC calcium release after agonist stimulation are mediated by the synthesis of COX-1 products by ASMCs.
Figure 6.
Reduction in calcium release by epithelial cells is dependent on ASMC COX-1. ASMCs were pretreated for 24 hours with vehicle (0.1% DMSO) or inhibitors indomethacin (Indo) (A), celecoxib (Cele) (B), or 5-(4-Chlorophenyl)-1-(4-methoxyphenyl)-3-trifluoromethyl pyrazole (SC-560) (C). Cells were then treated with conditioned medium from BEAS-2B cells, with respective drug or vehicle present for 24 hours. Cells were loaded with 10 μM Fura 2-AM, and peak intracellular calcium release in response to 1 μM histamine was measured. Data are presented as box plots showing medians, with whiskers as group maxima and minima; comparisons were made with the Kruskall Wallis and Dunn’s post hoc test, with P values reported. Calcium was measured within 70–157 cells per group across 4–10 individual experiments. Ctl, control.
Epithelial-Induced Reduction in Calcium Release by ASMCs Is Prevented by EP2 and EP4 Receptor Antagonism
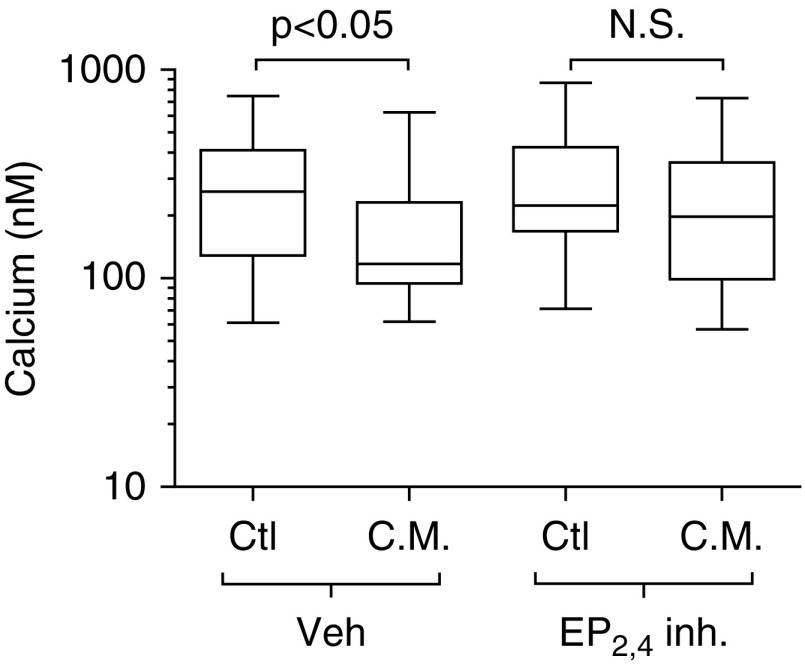
Given the possible role of PGE2 in the inhibition of calcium release, ASMCs were pretreated for 24 hours with combined EP2 and EP4 receptor antagonists, 1-(4-fluorobenzoyl)-3-[[(6-methoxy-2-naphthalenyl)oxy]methyl]-3-azetidinecarboxylic acid 04418948 and 4-cyano-2-[[2-(4-fluoro-1-naphthalenyl)-1-oxopropyl]amino]-benzenebutanoic acid AE3 208, respectively. Receptor antagonists were present during incubation for 24 hours with BEAS-2B conditioned medium. Combined inhibition of EP2 and EP4 receptors prevented the reduction in peak calcium release upon 1 μM histamine stimulation (Figure 7). Given the results indicating the requirement of ASMC COX-1 activity and EP2,4 receptor activity, we conclude that autocrine PGE2 signaling mediates the reduced excitability of ASMCs.
Figure 7.
Epithelial-induced reduction in calcium release by ASMCs is prevented by prostaglandin E receptor 2 (EP2) and EP4 receptor antagonism. ASMCs were pretreated for 24 hours with vehicle (0.1% DMSO) or a combination of 1-(4-fluorobenzoyl)-3-[[(6-methoxy-2-naphthalenyl)oxy]methyl]-3-azetidinecarboxylic acid 04418948 and 4-cyano-2-[[2-(4-fluoro-1-naphthalenyl)-1-oxopropyl]amino]-benzenebutanoic acid AE3 (EP2,4 inh.). Cells were then treated with C.M. from BEAS-2B cells, or control starvation medium (Ctl) with the combined drug (EP2,4 inh.) or vehicle (Veh) present for 24 hours. Cells were loaded with 10 μM Fura 2-AM, and peak intracellular calcium release in response to 1 μM histamine was measured. Inhibitors or vehicle was present during the Fura 2-AM washout and stimulation with histamine. Data are presented as medians, with whiskers showing group maxima and minima; comparisons were made with the Kruskall Wallis and Dunn’s post hoc test, with P values reported. Calcium was measured within 49–72 cells per group from five individual experiments
Discussion
In the current study, we examined the role of airway epithelial cells in modulating the ASMC contractile phenotype. Our results demonstrate a potent down-regulation of calcium signals in ASMCs stimulated with histamine when cocultured with epithelium or when treated with conditioned medium from cultured epithelial cells. Furthermore, we identified COX-1 activity in ASM as a regulator of the loss of ASMC excitability after coculture with epithelial cells. The reduced contractile response appears to be driven by autocrine actions of PGE2, as we observed diminished levels of cAMP within the ASMCs after incubation with epithelial-conditioned culture medium and reversal of the reduced calcium signals by EP2 and EP4 antagonism. The alterations in contractile responsiveness to histamine did not involve the down-regulation of proteins involved in the cascade of events leading to calcium release. These results confirm that epithelial cells may reduce the contractile phenotype in vitro.
The idea of an epithelial-derived relaxing factor is not a novel concept. Others have shown that tissue preparations contract more when the airway is denuded of its epithelial layers (27). Much of this work followed the discovery of endothelial-derived nitric oxide and its role in vascular tone (28). To examine the effect of epithelial mediators on ASMC contractions, we used traction microscopy, an assay based on measuring the displacement of the elastic substratum by cellular contractile force, as previously described (24). The effect of NHBE conditioned medium on baseline traction was negligible in our studies, but there was a demonstrable reduction in histamine-induced force generation by the ASMCs when treated with medium derived from primary NHBE cells. Although others have explored the concept of a direct effect of an epithelial-derived relaxing factor on ASM, we wished to further determine whether the relaxant effects on ASM could be accounted for by functional antagonism of contraction, or whether the contractile phenotype of ASM was per se affected. To do this, we examined the expression of contractile apparatus genes. Among the genes that were significantly diminished in expression after coculture with epithelial cells was myocardin. Myocardin is a cotranscription factor and driver of the contractile phenotype in ASMCs (26). Although it is currently unknown if it is altered in human asthmatic ASM, myocardin nuclear staining in ASMCs was associated with equine asthma (29). In addition to decreased expression of myocardin, we also observed a reduction in transcripts and protein for the myocardin-dependent genes, α-SMA and calponin. It is likely, therefore, that the reduced expression of these genes plays a role in the attenuated force production upon histamine stimulation.
We observed also that both BEAS-2B and NHBE cells reduced the excitability of ASMCs, as reflected in peak calcium release, when stimulated by histamine. Although the modulation of force can be mediated through a variety of pathways (30, 31), the concentration of intracellular calcium is coupled to the magnitude of force production in smooth muscle cells (32). Due to reduced intracellular calcium release by agonist stimulation after coculture, we anticipated a possible reduction in calcium handling proteins, which we hypothesized would have mediated this effect. However, we found no transcriptional change in several key enzymes, leading us to explore alternative mechanisms of smooth muscle cell relaxation. A transcriptomic analysis demonstrated an increase in expression of the eicosanoid-producing enzymes, COX-2 and membrane-associated PGE synthase-1. We therefore focused on the potential role of eicosanoids as causes of functional antagonism of calcium release of ASMCs. Human ASMCs have been shown to express EP2,4 (33), and it is established that PGE2 can induce relaxation of this tissue (34). Furthermore, EP2 has been demonstrated to mediate PGE2-induced relaxation of ASM (35), and histamine-induced calcium release in aortic smooth muscle is sensitive to blockade of this receptor (36). Our data demonstrate that ASMC EP2,4 receptor signaling is required for epithelial cells to reduce the excitability of the ASM. This adds further evidence that PGE2 mediates this phenomenon. PGE2 was recently shown to inhibit the transcription and protein expression of α-SMA during myofibroblast differentiation through a reduction in SRF expression (37), which provides further evidence supporting the relaxant effect of this prostanoid. PGE2 also increases cAMP production within these cells (38), a molecule (cAMP) that has been described to target the inositol trisphosphate receptor in ASMCs, thereby causing a reduction in calcium mobilization (39). β-agonist treatment for asthma exacerbation relies, in part, on G-protein coupled receptor generation of cAMP-induced calcium inhibition. cAMP can also activate protein kinase A in these cells, and may further reduce the contractile phenotype through altered calcium sensitization of the contractile apparatus (40). A recent study demonstrated that epithelial-induced ASMC migration was diminished when cells were treated with cAMP-elevating agents (41), adding further evidence of the importance of this second messenger in mediating epithelial–ASMC interactions.
Given the observed induction of COX-2 by conditioned medium, we expected that its inhibition might account for the restoration of the calcium responses to histamine by the nonspecific COX inhibitor, indomethacin. However, this was not the case, and, rather, treatment with a specific COX-1 inhibitor mimicked the effect of indomethacin. COX-1, a constitutively expressed protein, has been implicated in generating PGE2 in human ASMCs (42) and, recently, it was demonstrated that COX-1 products mediate the relaxing effect of glucagon on tracheal tissue preparations (43). Similarly, selective COX-1 inhibition prevented tracheal ring relaxation of murine airways by proteinase-activated receptor-2 agonism (44). Furthermore, the selective inhibitor of COX-1, SC560, augmented histamine-triggered tracheal ring contractions (45). Our data provide further evidence that SC560 may increase the responsiveness of ASM to a contractile agonist. Although the inducible isoform of COX-2 is often described to modulate inflammatory processes, others have provided evidence supporting a role of COX-1 in driving asthmatic responses (46). Besides exploring the molecular physiology of PGE2-mediated effects on ASMCs, there have been clinical investigations examining the efficacy of this prostanoid as a therapeutic agent. The fall in forced expiratory volume in 1 second by allergen challenge in patients with asthma was prevented by inhalation of PGE2 before challenge (47). Another study demonstrated that inhalation of this prostanoid can cause an initial bronchoconstriction, followed by potent dilation in healthy control subjects (48). In the rat, PGE2 has been shown to reduce cysteinyl-leukotriene production and Th2 activation after allergen challenge, demonstrating a role of this molecule as an immunomodulatory agent for the treatment of allergic asthma (49).
In conclusion, this study shows that the epithelium modulates ASMCs away from the contractile phenotype, a phenomenon that likely is linked to the previously described increase in the proliferative phenotype (9). The reduced excitability of these cells is not transcriptionally regulated, but, rather, depends on the stimulation of the synthesis of PGE2 by COX-1. There was also a coordinate reduction in contractile gene expression, as we observed a diminution in myocardin, the master regulator of transcriptional apparatus proteins, and reduced transcripts of contractile apparatus genes, as well as reduced α-SMA protein. The exploration of this epithelial–ASM interaction will be important to extend to the examination of cells derived from subjects with asthma, as these cells retain significant differences in properties ex vivo (50). Future work exploring the secretome of airway epithelial cells will be necessary to determine the soluble factor(s) responsible for the diminished ASMC contractility described here. Possible candidates include epithelial-derived nitric oxide, PGE2, proinflammatory cytokines, or neurotransmitters, and the detailed exploration of these mediators will further clarify the interaction of these cell types.
Acknowledgments
Acknowledgments
The authors thank Rebecca Hirsch (Harvard T. H. Chan School of Public Health, Harvard University, Boston, MA) for her technical assistance with the traction force microscopy assays.
Footnotes
This work was supported by Canadian Institutes of Health Research grant MOP-93747, Fonds de la Recherche en santé du Quebec (FRSQ), the Richard and Edith Strauss Foundation, and National Heart, Lung, and Blood Institute grants PO1HL120839 and R01HL107561; M.J.O’S. was the recipient of Canadian Respiratory Research Network and FRSQ Respiratory Training Program studentships.
Author Contributions: Conception and design—M.J.O’S., E.G., A.P., C.Y.P., G.I., J.J.F., A.-M.L., and J.G.M.; conducted experiments—M.J.O’S., E.G., A.P., and C.Y.P.; drafted and critically revised the manuscript—M.J.O’S., E.G., A.P., C.Y.P., G.I., J.J.F., A.-M.L., and J.G.M.; final approval of submitted manuscript—M.J.O’S., E.G., A.P., C.Y.P., G.I., J.J.F., A.-M.L., and J.G.M.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2016-0427OC on July 14, 2017
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Vignola AM, Mirabella F, Costanzo G, Di Giorgi R, Gjomarkaj M, Bellia V, Bonsignore G. Airway remodeling in asthma. Chest. 2003;123(3 s) uppl:417S–422S. doi: 10.1378/chest.123.3_suppl.417s. [DOI] [PubMed] [Google Scholar]
- 2.Johnson PR, Roth M, Tamm M, Hughes M, Ge Q, King G, Burgess JK, Black JL. Airway smooth muscle cell proliferation is increased in asthma. Am J Respir Crit Care Med. 2001;164:474–477. doi: 10.1164/ajrccm.164.3.2010109. [DOI] [PubMed] [Google Scholar]
- 3.An SS, Mitzner W, Tang WY, Ahn K, Yoon AR, Huang J, Kilic O, Yong HM, Fahey JW, Kumar S, et al. An inflammation-independent contraction mechanophenotype of airway smooth muscle in asthma. J Allergy Clin Immunol. 2016;138:294–297.e4. doi: 10.1016/j.jaci.2015.12.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ijpma G, Kachmar L, Matusovsky OS, Bates JH, Benedetti A, Martin JG, Lauzon AM. Human trachealis and main bronchi smooth muscle are normoresponsive in asthma. Am J Respir Crit Care Med. 2015;191:884–893. doi: 10.1164/rccm.201407-1296OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matusovsky OS, Kachmar L, Ijpma G, Bates G, Zitouni N, Benedetti A, Lavoie JP, Lauzon AM. Peripheral airway smooth muscle, but not the trachealis, is hypercontractile in an equine model of asthma. Am J Respir Cell Mol Biol. 2016;54:718–727. doi: 10.1165/rcmb.2015-0180OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holgate ST. The sentinel role of the airway epithelium in asthma pathogenesis. Immunol Rev. 2011;242:205–219. doi: 10.1111/j.1600-065X.2011.01030.x. [DOI] [PubMed] [Google Scholar]
- 7.Marini M, Vittori E, Hollemborg J, Mattoli S. Expression of the potent inflammatory cytokines, granulocyte-macrophage-colony-stimulating factor and interleukin-6 and interleukin-8, in bronchial epithelial cells of patients with asthma. J Allergy Clin Immunol. 1992;89:1001–1009. doi: 10.1016/0091-6749(92)90223-o. [DOI] [PubMed] [Google Scholar]
- 8.Hirota N, Risse PA, Novali M, McGovern T, Al-Alwan L, McCuaig S, Proud D, Hayden P, Hamid Q, Martin JG. Histamine may induce airway remodeling through release of epidermal growth factor receptor ligands from bronchial epithelial cells. FASEB J. 2012;26:1704–1716. doi: 10.1096/fj.11-197061. [DOI] [PubMed] [Google Scholar]
- 9.Malavia NK, Raub CB, Mahon SB, Brenner M, Panettieri RA, Jr, George SC. Airway epithelium stimulates smooth muscle proliferation. Am J Respir Cell Mol Biol. 2009;41:297–304. doi: 10.1165/rcmb.2008-0358OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mack CP. Signaling mechanisms that regulate smooth muscle cell differentiation. Arterioscler Thromb Vasc Biol. 2011;31:1495–1505. doi: 10.1161/ATVBAHA.110.221135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Du KL, Ip HS, Li J, Chen M, Dandre F, Yu W, Lu MM, Owens GK, Parmacek MS. Myocardin is a critical serum response factor cofactor in the transcriptional program regulating smooth muscle cell differentiation. Mol Cell Biol. 2003;23:2425–2437. doi: 10.1128/MCB.23.7.2425-2437.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu Y, Sinha S, McDonald OG, Shang Y, Hoofnagle MH, Owens GK. Kruppel-like factor 4 abrogates myocardin-induced activation of smooth muscle gene expression. J Biol Chem. 2005;280:9719–9727. doi: 10.1074/jbc.M412862200. [DOI] [PubMed] [Google Scholar]
- 13.Wang Z, Wang DZ, Hockemeyer D, McAnally J, Nordheim A, Olson EN. Myocardin and ternary complex factors compete for SRF to control smooth muscle gene expression. Nature. 2004;428:185–189. doi: 10.1038/nature02382. [DOI] [PubMed] [Google Scholar]
- 14.Shanahan CM, Weissberg PL, Metcalfe JC. Isolation of gene markers of differentiated and proliferating vascular smooth muscle cells. Circ Res. 1993;73:193–204. doi: 10.1161/01.res.73.1.193. [DOI] [PubMed] [Google Scholar]
- 15.Bruce C, Weatherstone R, Seaton A, Taylor WH. Histamine levels in plasma, blood, and urine in severe asthma, and the effect of corticosteroid treatment. Thorax. 1976;31:724–729. doi: 10.1136/thx.31.6.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cockcroft DW, Killian DN, Mellon JJ, Hargreave FE. Bronchial reactivity to inhaled histamine: a method and clinical survey. Clin Allergy. 1977;7:235–243. doi: 10.1111/j.1365-2222.1977.tb01448.x. [DOI] [PubMed] [Google Scholar]
- 17.Kotlikoff MI, Murray RK, Reynolds EE. Histamine-induced calcium release and phorbol antagonism in cultured airway smooth muscle cells. Am J Physiol. 1987;253:C561–C566. doi: 10.1152/ajpcell.1987.253.4.C561. [DOI] [PubMed] [Google Scholar]
- 18.Hoiting BH, Meurs H, Schuiling M, Kuipers R, Elzinga CR, Zaagsma J. Modulation of agonist-induced phosphoinositide metabolism, Ca2+ signalling and contraction of airway smooth muscle by cyclic AMP–dependent mechanisms. Br J Pharmacol. 1996;117:419–426. doi: 10.1111/j.1476-5381.1996.tb15207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Knight DA, Stewart GA, Thompson PJ. Prostaglandin E2, but not prostacyclin inhibits histamine-induced contraction of human bronchial smooth muscle. Eur J Pharmacol. 1995;272:13–19. doi: 10.1016/0014-2999(94)00602-4. [DOI] [PubMed] [Google Scholar]
- 20.Braunstein G, Labat C, Brunelleschi S, Benveniste J, Marsac J, Brink C. Evidence that the histamine sensitivity and responsiveness of guinea-pig isolated trachea are modulated by epithelial prostaglandin E2 production. Br J Pharmacol. 1988;95:300–308. doi: 10.1111/j.1476-5381.1988.tb16577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mori A, Ito S, Morioka M, Aso H, Kondo M, Sokabe M, Hasegawa Y. Effects of specific prostanoid EP receptor agonists on cell proliferation and intracellular Ca(2+) concentrations in human airway smooth muscle cells. Eur J Pharmacol. 2011;659:72–78. doi: 10.1016/j.ejphar.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 22.Risse PA, Jo T, Suarez F, Hirota N, Tolloczko B, Ferraro P, Grutter P, Martin JG. Interleukin-13 inhibits proliferation and enhances contractility of human airway smooth muscle cells without change in contractile phenotype. Am J Physiol Lung Cell Mol Physiol. 2011;300:L958–L966. doi: 10.1152/ajplung.00247.2010. [DOI] [PubMed] [Google Scholar]
- 23.Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- 24.Park CY, Zhou EH, Tambe D, Chen B, Lavoie T, Dowell M, Simeonov A, Maloney DJ, Marinkovic A, Tschumperlin DJ, et al. High-throughput screening for modulators of cellular contractile force. Integr Biol (Camb) 2015;7:1318–1324. doi: 10.1039/c5ib00054h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prakash YS, Kannan MS, Walseth TF, Sieck GC. Role of cyclic ADP-ribose in the regulation of [Ca2+]i in porcine tracheal smooth muscle. Am J Physiol. 1998;274:C1653–C1660. doi: 10.1152/ajpcell.1998.274.6.C1653. [DOI] [PubMed] [Google Scholar]
- 26.Wang Z, Wang DZ, Pipes GC, Olson EN. Myocardin is a master regulator of smooth muscle gene expression. Proc Natl Acad Sci USA. 2003;100:7129–7134. doi: 10.1073/pnas.1232341100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Flavahan NA, Aarhus LL, Rimele TJ, Vanhoutte PM. Respiratory epithelium inhibits bronchial smooth muscle tone. J Appl Physiol (1985) 1985;58:834–838. doi: 10.1152/jappl.1985.58.3.834. [DOI] [PubMed] [Google Scholar]
- 28.Palmer RM, Ferrige AG, Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987;327:524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- 29.Chevigny M, Guérin-Montpetit K, Vargas A, Lefebvre-Lavoie J, Lavoie JP. Contribution of SRF, Elk-1, and myocardin to airway smooth muscle remodeling in heaves, an asthma-like disease of horses. Am J Physiol Lung Cell Mol Physiol. 2015;309:L37–L45. doi: 10.1152/ajplung.00050.2015. [DOI] [PubMed] [Google Scholar]
- 30.Uehata M, Ishizaki T, Satoh H, Ono T, Kawahara T, Morishita T, Tamakawa H, Yamagami K, Inui J, Maekawa M, et al. Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature. 1997;389:990–994. doi: 10.1038/40187. [DOI] [PubMed] [Google Scholar]
- 31.Perez JF, Sanderson MJ. The frequency of calcium oscillations induced by 5-HT, ACH, and KCl determine the contraction of smooth muscle cells of intrapulmonary bronchioles. J Gen Physiol. 2005;125:535–553. doi: 10.1085/jgp.200409216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yagi S, Becker PL, Fay FS. Relationship between force and Ca2+ concentration in smooth muscle as revealed by measurements on single cells. Proc Natl Acad Sci USA. 1988;85:4109–4113. doi: 10.1073/pnas.85.11.4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clarke DL, Belvisi MG, Smith SJ, Hardaker E, Yacoub MH, Meja KK, Newton R, Slater DM, Giembycz MA. Prostanoid receptor expression by human airway smooth muscle cells and regulation of the secretion of granulocyte colony–stimulating factor. Am J Physiol Lung Cell Mol Physiol. 2005;288:L238–L250. doi: 10.1152/ajplung.00313.2004. [DOI] [PubMed] [Google Scholar]
- 34.Hakonarson H, Herrick DJ, Grunstein MM. Mechanism of impaired beta-adrenoceptor responsiveness in atopic sensitized airway smooth muscle. Am J Physiol. 1995;269:L645–L652. doi: 10.1152/ajplung.1995.269.5.L645. [DOI] [PubMed] [Google Scholar]
- 35.Fortner CN, Breyer RM, Paul RJ. EP2 receptors mediate airway relaxation to substance P, ATP, and PGE2. Am J Physiol Lung Cell Mol Physiol. 2001;281:L469–L474. doi: 10.1152/ajplung.2001.281.2.L469. [DOI] [PubMed] [Google Scholar]
- 36.Pantazaka E, Taylor EJ, Bernard WG, Taylor CW. Ca(2+) signals evoked by histamine H1 receptors are attenuated by activation of prostaglandin EP2 and EP4 receptors in human aortic smooth muscle cells. Br J Pharmacol. 2013;169:1624–1634. doi: 10.1111/bph.12239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Penke LR, Huang SK, White ES, Peters-Golden M. Prostaglandin E2 inhibits α-smooth muscle actin transcription during myofibroblast differentiation via distinct mechanisms of modulation of serum response factor and myocardin-related transcription factor-A. J Biol Chem. 2014;289:17151–17162. doi: 10.1074/jbc.M114.558130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Florio C, Martin JG, Styhler A, Heisler S. Antiproliferative effect of prostaglandin E2 in cultured guinea pig tracheal smooth muscle cells. Am J Physiol. 1994;266:L131–L137. doi: 10.1152/ajplung.1994.266.2.L131. [DOI] [PubMed] [Google Scholar]
- 39.Bai Y, Sanderson MJ. Airway smooth muscle relaxation results from a reduction in the frequency of Ca2+ oscillations induced by a cAMP-mediated inhibition of the IP3 receptor. Respir Res. 2006;7:34. doi: 10.1186/1465-9921-7-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Endou K, Iizuka K, Yoshii A, Tsukagoshi H, Ishizuka T, Dobashi K, Nakazawa T, Mori M. 8-Bromo-cAMP decreases the Ca2+ sensitivity of airway smooth muscle contraction through a mechanism distinct from inhibition of Rho-kinase. Am J Physiol Lung Cell Mol Physiol. 2004;287:L641–L648. doi: 10.1152/ajplung.00287.2003. [DOI] [PubMed] [Google Scholar]
- 41.Shariff S, Shelfoon C, Holden NS, Traves SL, Wiehler S, Kooi C, Proud D, Leigh R. Human rhinovirus infection of epithelial cells modulates airway smooth muscle migration. Am J Respir Cell Mol Biol. 2017;56:796–803. doi: 10.1165/rcmb.2016-0252OC. [DOI] [PubMed] [Google Scholar]
- 42.Pang L, Knox AJ. PGE2 release by bradykinin in human airway smooth muscle cells: involvement of cyclooxygenase-2 induction. Am J Physiol. 1997;273:L1132–L1140. doi: 10.1152/ajplung.1997.273.6.L1132. [DOI] [PubMed] [Google Scholar]
- 43.Insuela DB, Daleprane JB, Coelho LP, Silva AR, e Silva PM, Martins MA, Carvalho VF. Glucagon induces airway smooth muscle relaxation by nitric oxide and prostaglandin E2. J Endocrinol. 2015;225:205–217. doi: 10.1530/JOE-14-0648. [DOI] [PubMed] [Google Scholar]
- 44.Nichols HL, Saffeddine M, Theriot BS, Hegde A, Polley D, El-Mays T, Vliagoftis H, Hollenberg MD, Wilson EH, Walker JK, et al. β-Arrestin-2 mediates the proinflammatory effects of proteinase-activated receptor-2 in the airway. Proc Natl Acad Sci USA. 2012;109:16660–16665. doi: 10.1073/pnas.1208881109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nieri P, Martinelli C, Blandizzi C, Bernardini N, Greco R, Ippolito C, Del Tacca M, Breschi MC. Role of cyclooxygenase isoforms and nitric-oxide synthase in the modulation of tracheal motor responsiveness in normal and antigen-sensitized guinea pigs. J Pharmacol Exp Ther. 2006;319:648–656. doi: 10.1124/jpet.106.102475. [DOI] [PubMed] [Google Scholar]
- 46.Harrington LS, Lucas R, McMaster SK, Moreno L, Scadding G, Warner TD, Mitchell JA. COX-1, and not COX-2 activity, regulates airway function: relevance to aspirin-sensitive asthma. FASEB J. 2008;22:4005–4010. doi: 10.1096/fj.08-107979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gauvreau GM, Watson RM, O’Byrne PM. Protective effects of inhaled PGE2 on allergen-induced airway responses and airway inflammation. Am J Respir Crit Care Med. 1999;159:31–36. doi: 10.1164/ajrccm.159.1.9804030. [DOI] [PubMed] [Google Scholar]
- 48.Walters EH, Davies BH. Dual effect of prostaglandin E2 on normal airways smooth muscle in vivo. Thorax. 1982;37:918–922. doi: 10.1136/thx.37.12.918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Martin JG, Suzuki M, Maghni K, Pantano R, Ramos-Barbón D, Ihaku D, Nantel F, Denis D, Hamid Q, Powell WS. The immunomodulatory actions of prostaglandin E2 on allergic airway responses in the rat. J Immunol. 2002;169:3963–3969. doi: 10.4049/jimmunol.169.7.3963. [DOI] [PubMed] [Google Scholar]
- 50.Kicic A, Sutanto EN, Stevens PT, Knight DA, Stick SM. Intrinsic biochemical and functional differences in bronchial epithelial cells of children with asthma. Am J Respir Crit Care Med. 2006;174:1110–1118. doi: 10.1164/rccm.200603-392OC. [DOI] [PubMed] [Google Scholar]