Abstract
Type 2–associated goblet cell hyperplasia and mucus hypersecretion are well known features of asthma. 15-Lipoxygenase-1 (15LO1) is induced by the type 2 cytokine IL-13 in human airway epithelial cells (HAECs) in vitro and is increased in fresh asthmatic HAECs ex vivo. 15LO1 generates a variety of products, including 15-hydroxyeicosatetraenoic acid (15-HETE), 15-HETE-phosphatidylethanolamine (15-HETE-PE), and 13-hydroxyoctadecadienoic acid (13-HODE). In this study, we investigated the 15LO1 metabolite profile at baseline and after IL-13 treatment, as well as its influence on goblet cell differentiation in HAECs. Primary HAECs obtained from bronchial brushings of asthmatic and healthy subjects were cultured under air–liquid interface culture supplemented with arachidonic acid and linoleic acid (10 μM each) and exposed to IL-13 for 7 days. Short interfering RNA transfection and 15LO1 inhibition were applied to suppress 15LO1 expression and activity. IL-13 stimulation induced expression of 15LO1 and preferentially generated 15-HETE-PE in vitro, both of which persisted after removal of IL-13. 15LO1 inhibition (by short interfering RNA and chemical inhibitor) decreased IL-13–induced forkhead box protein A3 (FOXA3) expression and enhanced FOXA2 expression. These changes were associated with reductions in both mucin 5AC and periostin. Exogenous 15-HETE-PE stimulation (alone) recapitulated IL-13–induced FOXA3, mucin 5AC, and periostin expression. The results of this study confirm the central importance of 15LO1 and its primary product, 15-HETE-PE, for epithelial cell remodeling in HAECs.
Keywords: asthma, eicosanoid, 15-lipoxygenase-1, mucus hypersecretion, phospholipid
Clinical Relevance
In the presence of type-2 inflammation, human airway epithelial 15-lipoxygenase-1 and its exclusive product 15-HETE-phosphatidylethanolamine regulate goblet cell differentiation and mucin secretion, which are critically relevant to asthma. The results of this study suggest that pharmacological interventions targeting the 15-lipoxygenase-1 pathway could restore a more “normal” epithelium and thereby improve outcomes in asthma.
Goblet cell hyperplasia and associated mucus hypersecretion are well known features of asthma that contribute to its morbidity and mortality. They are particularly seen in patients who show evidence of type 2 (IL-4/-13)–associated inflammation (1–3). This type 2 process is believed to contribute to the differentiation of basal epithelial cells into a goblet cell/mucus-producing epithelium. Previous studies showed that mucin 5AC (MUC5AC) is the major mucin that is increased in human airway epithelial cells (HAECs) in response to type 2/IL-13 stimulation in vitro (4–10). Goblet cell differentiation appears to be tightly regulated by forkhead box (FOX) protein transcription factors, including forkhead box protein A3 (FOXA3) (for goblet cell differentiation) (11) and FOXA2 (associated with ciliated cell differentiation). FOXA3 is strongly upregulated by IL-13 (11, 12). However, the pathways by which IL-13 stimulates these downstream events are not known.
Type 2 immunity impacts additional epithelial factors, including 15-lipoxygenases (15LOXs). As the only lipoxygenase class expressed by HAECs (13), 15LOXs catalyze the oxygenation of polyunsaturated fatty acids, inserting molecular oxygen at the C15 position of arachidonic acid (AA) or the C13 position on linoleic acid (LA) to produce 15S-hydroxyeicosatetraenoic acid (15(S)-HETE [AA]), or 13S-hydroxyoctadecadienoic acid (13(S)-HODE [LA]). Humans have two distinct subtypes of 15LOX—15LO1 (ALOX15) and 15LO2 (ALOX15B)—with different tissue and cellular distributions (14–16), and possible differences in substrate preferences. Although 15LO2 exclusively oxygenates AA to generate 15(S)-HETE, with poor catalytic activity on LA (17), human reticulocyte 15LO1 has been reported to prefer LA in a cell-free system (18). 15LO1 and its product 15-HETE are known to be elevated in asthmatic lungs and upregulated by IL-4 and IL-13 in vitro (10, 19, 20), whereas 15LO2 is not (14, 19, 21). Our studies also showed that IL-4 and IL-13 induce 15LO1 expression associated with the generation of 15-HETE conjugated with phosphatidylethanolamine (15-HETE-PE) in both monocytes and macrophages, and epithelial cells (10, 22). This predisposition to 15-HETE-PE (as opposed to free 15-HETE) generation is seen in the presence of interactions with phosphatidylethanolamine binding protein 1 (PEBP1), mitogen-activated protein kinases/extracellular signal-regulated kinases (MAPK/ERK) activation, and MUC5AC expression (10, 23). However, the balance of the LA and AA products (13-HODE and 15-HETE, or their esterified forms, including 15-HETE-PE), as well as their contribution to cell differentiation and mucus hypersecretion in HAECs, has not been evaluated.
Finally, IL-13 is known to upregulate additional factors that associate with remodeling in the airway epithelium, including periostin, which has been identified as a type 2 biomarker in both HAECs and serum (24, 25). It is associated with matrix deposition and is likely part of a wound-repair process similar to mucin generation.
We therefore hypothesized that 15LO1 would preferentially metabolize AA to generate phospholipid-conjugated 15-HETE-PE, as opposed to free 15-HETE (or 13-HODE), in response to IL-13, which would regulate IL-13–induced goblet cell differentiation. To test this hypothesis, we evaluated cultured HAECs that were stimulated with IL-13 and supplemented with AA/LA for free and conjugated lipid products, using liquid chromatography/mass spectrometry (LC/MS). We evaluated the stability of 15LO1 expression and activity, as well as the effects of 15LO1 and its product 15-HETE-PE on goblet cell differentiation and periostin expression (24, 25).
Materials and Methods
Reagents, Antibodies, and Primers
ALOX15 Dicer-substrate short interfering RNA (DsiRNA) was purchased from IDT (Coralville, IA). Antibodies against FOXA3 (goat IgG) and periostin (rabbit IgG1) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA), and anti-glyceraldehyde 3-phosphate dehydrogenase antibody was obtained from Novus Biologicals (Littleton, CO). Anti-MUC5AC antibody was obtained from Neomarkers (Fremont, CA). 15LO1 antibody was a gift from Dr. Doug Conrad (University of California, San Diego) (26). BLX2477, a highly specific inhibitor of 15LO1, was a kind gift from H.-E.C. (27). All other antibodies and reagents used in this work are described in the online supplement.
Sources of HAECs
HAECs were obtained by bronchoscopic brushing of asthmatic and healthy control (HC) airways as previously described (28). See details in the online supplement.
Primary HAEC Culture at the Air–Liquid Interface, DsiRNA Transfection, and Exogenous 15-HETE-PE Stimulation
HAECs were cultured at the air–liquid interface (ALI) as previously described (23, 29), and DsiRNA transfection was performed using Lipofectamine transfection reagent (see the online supplement). LA/AA supplementation and exogenous 15-HETE-PE stimulation were performed as described in the online supplement.
15LO1 Enzymatic Inhibition with BLX2477
A selective enzymatic inhibitor of 15LO1 (BLX2477) was applied to IL-13–treated or untreated HAECs (27). BLX2477 (2 μM) was added acutely for 1 hour or up to 5 days before harvest (27). For longer-duration studies, the culture medium and BLX2477 were changed every 24 hours.
LC/MS Analysis
Cells were kept in PBS buffer containing diethylenetriaminepenta-acetic acid (100 μM) and butylated hydroxytoluene (100 μM). Free and esterified HETEs were analyzed by LC/MS as previously described (30, 31).
Western Blotting
Cell lysates were run on 4–12% SDS-PAGE gels under reducing conditions, and protein was detected as previously described (32).
Real-Time PCR
Real-time PCR was performed on the ABI Prism 7700 sequence detection system (Applied Biosystems, Foster City, CA) using primers and probes from Applied Biosystems, with β-glucuronidase as the internal control. An identical threshold cycle (Ct) was applied for each gene of interest. Relative mRNA expression levels were calculated using the ΔCt method.
Semiquantitative MUC5AC ELISA
MUC5AC protein was measured from apical culture supernatants using a semiquantitative sandwich ELISA with two different MUC5AC antibodies. All results are given in relative arbitrary units (AU)/ml and are reported as the fold change relative to the corresponding control condition. See additional details in the online supplement.
Statistical Analysis
Statistical analysis was performed using JMP software (SAS Institute, Cary, NC). Data that were normally distributed are represented as means ± SEM, and “n” indicates the number of biologically replicated experiments from different donors. For each donor condition, technical replicates were generally run in triplicate, except for western blots, which were done in singlets due to the limited amount of protein samples. Cells from asthmatic donors are identified by dashed lines in the figures, and HC donors are identified by solid lines. All comparisons of cells from specific donors, under two different conditions (scramble and ALOX15 siRNA [siALOX15]), were done using a paired t test. P values of <0.05 were considered statistically significant.
Results
Demographics of the Research Participants Who Provided HAECs for Culture
Fresh HAECs for ALI culture were obtained from a total of 44 subjects (Table E1 in the online supplement). Due to the limited cell numbers and longer-term development of the experimental models, the donor sources for each experiment varied. However, as reported previously, studies of this pathway in vitro have not identified differences in response by subject group (asthma versus HC) (10, 22). Thus, cells from different subject groups were used interchangeably with the subject group of donor cells identified on the figures (asthmatics and HCs are indicated by dashed and solid lines, respectively). There were no differences in response by age.
15LO1 Expression and Specific Enzymatic Activity in HAECs
IL-13 stimulation induces 15LO1 expression and preferentially increases 15-HETE-PE in the presence of equal LA/AA supplementation in vitro
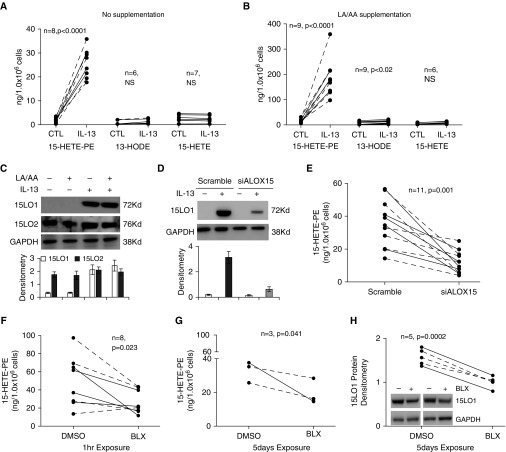
15LO1 has been reported to directly oxygenate endogenous PE to produce esterified 15-HETE-PE in response to IL-13 stimulation. To determine whether 15LO1 generates additional lipid products in HAECs under IL-13 conditions, we stimulated HAECs with IL-13 for 7 days. Intracellular (cell lysates) and extracellular (medium) levels of 15-HETE-PE, 13-HODE-PE, 13-HODE, and 15-HETE were measured. To confirm that product-specific generation was not dependent on intracellular depletion of LA and/or AA, the culture media were supplemented with equal amounts (10 μM) of exogenous LA and AA under both basal and IL-13 conditions. As expected, IL-13 induced high levels of intracellular 15-HETE-PE (25.7 ± 3.6 ng/million cells, 17.5 ± 1.4 fold change over baseline, n = 8, P < 0.0001), with only modest increases in 13-HODE or free 15-HETE in cells under unsupplemented basal culture conditions (Figure 1A). Exogenous LA/AA supplementation under basal conditions only modestly increased the overall levels of 15-HETE-PE (23.3 ± 4.1 ng/million cells), 15-HETE (7.4 ± 3.8 ng/million cells), and 13-HODE (8.9 ± 4.3 ng/million cells) without alteration in their ratios to each other as compared with the unsupplemented basal condition ratios. In contrast, exogenous LA/AA supplementation of IL-13–stimulated cells generated marked increases in 15-HETE-PE (186.1 ± 25 ng/million cells, 14.5 ± 2.0 fold change over baseline, n = 9, P < 0.0001), with only modest increases in intracellular 13-HODE (9.8 ± 2.3 ng/million cells, 1.9 ± 0.3 fold change, n = 9, P < 0.02) and 15-HETE (8.7 ± 1.6 ng/million cells, 1.6 ± 0.2 fold change, n = 6, NS; Figure 1B). There were no differences in response by subject group under any conditions. Extracellular (media) 13-HODE and 15-HETE levels were both low at baseline and increased modestly in response to IL-13 (Figure E1). As previously reported, no significant level of 15-HETE-PE was detected extracellularly with or without LA/AA supplementation (10, 22). Additionally, 13-HODE-PE was undetectable either extracellularly or intracellularly in any condition, with or without LA/AA supplementation.
Figure 1.
IL-13 stimulation induces 15-lipoxygenase-1 (15LO1) expression and preferentially increases 15-hydroxyeicosatetraenoic acid-phosphatidylethanolamine (15-HETE-PE) in the presence of equal linoleic acid/arachidonic acid (LA/AA) supplementation in vitro, and is inhibited by Dicer-substrate short interfering RNA (DsiRNA) transfection and chemical inhibition of 15LO1. Human airway epithelial cells (HAECs) were stimulated with IL-13 for 7 days with/without LA/AA supplements, and cell lysates were collected for 15-HETE-PE, 13-hydroxyoctadecadienoic acid-phosphatidylethanolamine (13-HODE-PE), free 13-HODE, and 15-HETE measurement by liquid chromatography/mass spectrometry (LC/MS) and 15LO1/2 protein detection by western blot. Solid lines: healthy controls; dashed lines: asthmatics. (A) IL-13 induced high levels of intracellular 15-HETE-PE, with only modest increases in 13-HODE and free 15-HETE under basal conditions without exogenous LA/AA supplementation. (B) Supplementation with LA/AA further increased IL-13–induced generation of 15-HETE-PE, with only modest increases in 13-HODE and 15-HETE. No 13-HODE-PE was detected. (C) Representative western blot and densitometry (n = 3) show that IL-13 induced 15LO1 expression but had no effect on 15LO2, and LA/AA supplementation did not impact 15LO1 or 15LO2 protein levels. DsiRNA knockdown of 15LO1 inhibited (D) 15LO1 protein expression (n = 4) and (E) 15-HETE-PE generation. The selective 15LO1 enzymatic inhibitor N-(2-chloro-4-fluorophenyl)triazole-4-carboxamide (BLX2477) suppressed 15-HETE-PE generation at (F) 1 hour and (G) 5 days of treatment, and (H) BLX2477 suppressed 15LO1 protein expression at 5 days of treatment. CTL, control; NS, no significance; siALOX15, scramble and ALOX15.
To determine whether the increase in 15-HETE-PE after IL-13 administration was associated with increased activity of 15LO1 or 15LO2, the levels of each protein were measured in IL-13–stimulated HAECs. As shown in Figure 1C, IL-13 markedly upregulated 15LO1, but had no effect on 15LO2. LA/AA supplementation did not impact 15LO1 or 15LO2 protein levels. 15LO1 protein was then knocked down by DsiRNA transfection (siALOX15), and 15-HETE-PE was measured. Knockdown of 15LO1 dramatically inhibited 15LO1 protein expression (n = 4; Figure 1D) and 15-HETE-PE generation (n = 11, P = 0.001; Figure 1E).
To confirm the importance of 15LO1, the effect of a selective 15LO1 enzymatic inhibitor BLX2477 was also studied. Primary HAECs were dosed with BLX2477 (2 μM) or vehicle control (DMSO) for 1 hour for acute studies or every 24 hours for up to 5 days to observe chronic effects. BLX2477 decreased 15-HETE-PE generation as soon as 1 hour after treatment (n = 8, P = 0.023; Figure 1F). Generation of 15-HETE-PE continued to be suppressed for up to 5 days of BLX2477 treatment (n = 3, P = 0.041; Figure 1G). Interestingly, 15LO1 protein (but not mRNA) was also suppressed by BLX2477 as compared with DMSO after 5 days of treatment (n = 5, P = 0.0002; Figure 1H), suggesting a positive-feedback mechanism via 15LO1 metabolites, but possibly also due to off-target effects of BLX2477 (27).
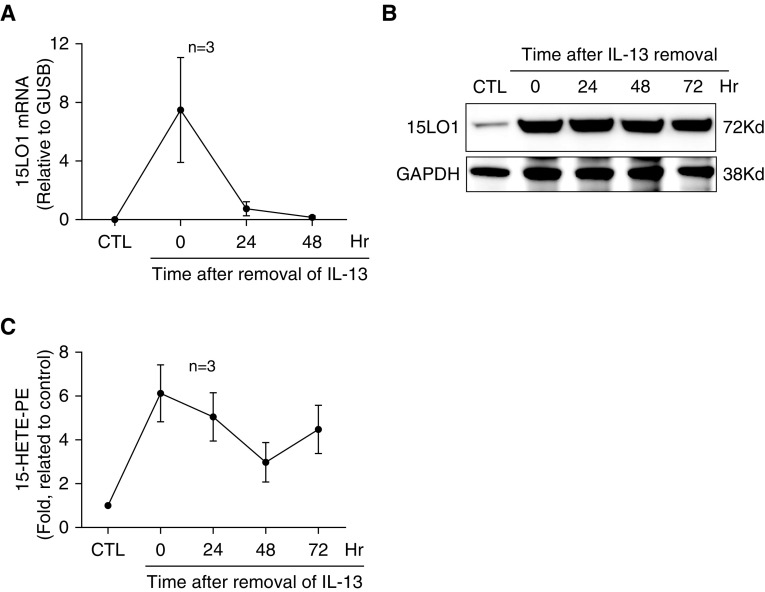
15LO1 protein levels and activity persist in the absence of IL-13
To determine the stability of the IL-13–induced 15LO1 enzyme and its activity, cells were stimulated with IL-13 for 7 days. IL-13 was then removed for the remaining culture period (up to 72 h). As shown in Figure 2A, IL-13 induced 15LO1 mRNA expression, which rapidly returned to basal levels after removal of IL-13. In contrast, 15LO1 protein levels remained high for at least 72 hours after removal of IL-13 (Figure 2B). Additionally, intracellular 15-HETE-PE levels remained elevated over baseline after IL-13 removal (Figure 2C). These results suggest the sustained presence of an active enzyme in the absence of type 2 stimulation for extended periods of time.
Figure 2.
IL-13–induced 15LO1 and 15-HETE-PE remain stable after the removal of IL-13. HAECs were stimulated with IL-13 for 7 days, except under the control condition. IL-13 was removed for the remaining culture period (up to 72 hours). Cell lysates were collected for measurement of 15-HETE-PE by LC/MS, and 15LO1 expression was determined by real-time PCR and western blot. (A) IL-13–induced 15LO1 mRNA levels rapidly returned to basal levels 24 hours after removal of IL-13. (B) 15LO1 protein levels remained high for at least 72 hours after removal of IL-13. (C) Intracellular 15-HETE-PE levels remained elevated over baseline after IL-13 removal. GUSB, β-glucuronidase.
15LO1 Pathway and Epithelial Remodeling
The 15LO1 pathway regulates IL-13 effects on FOXA3 and FOXA2
To determine whether 15LO1 expression and activity are upstream of the Forkhead-box proteins FOXA3 and FOXA2, and play a central role in goblet cell differentiation, DsiRNA 15LO1 knockdown was performed and the expression of FOXA3, FOXA2, and MUC5AC was analyzed. Confirming previous work, FOXA3 protein expression increased in a time-dependent manner in ALI culture (Figure E2A). IL-13 induced high levels of FOXA3 mRNA and protein, which paralleled the increase of 15LO1 (Figures E2A and E2B). In contrast, FOXA2 mRNA was suppressed by IL-13 stimulation (Figure E2C). 15LO1 knockdown significantly decreased FOXA3 mRNA and protein expression (Figures 3A and 3B). As a marker of goblet cell differentiation, IL-13–induced MUC5AC expression was also suppressed by 15LO1 knockdown (Figures 3C and 3D). In contrast, 15LO1 knockdown upregulated FOXA2 mRNA expression (Figure 3E). These results were confirmed by means of the selective 15LO1 enzymatic inhibitor BLX2477 (Figure 4). Similar to the results obtained with 15LO1 knockdown, BLX2477 treatment significantly inhibited IL-13–induced FOXA3 mRNA (Figure 4A) and protein (Figure 4B). IL-13–induced MUC5AC mRNA (Figure 4C) and MUC5AC protein secretion (Figure 4D) were also inhibited by BLX2477 treatment. Similar to what was observed with 15LO1 knockdown, FOXA2 mRNA was upregulated by BLX2477 (Figure 4E).
Figure 3.
15LO1 knockdown suppresses IL-13–induced forkhead box protein A3 (FOXA3) and mucin 5AC (MUC5AC), and increases FOXA2 expression. HAECs with and without siALOX15 transfection were stimulated with IL-13 for 7 days, and cell lysates were collected for protein and mRNA analysis. 15LO1 knockdown suppressed IL-13–induced FOXA3 (A) mRNA and (B) protein expression (representative western blot and densitometry, n = 4). 15LO1 knockdown suppressed MUC5AC (C) mRNA and (D) protein expression induced by IL-13 (measured by ELISA, shown as the fold change relative to the scramble control). (E) 15LO1 knockdown upregulated FOXA2 mRNA.
Figure 4.
BLX2477 treatment inhibits IL-13–induced FOXA3 and MUC5AC, and increases FOXA2 expression. HAECs stimulated with IL-13 for 7 days were treated with BLX2477 at 2 μM for 5 days with DMSO as the vehicle control, and cell lysates were collected for mRNA and protein analysis. BLX2477 decreased FOXA3 (A) mRNA and (B) protein expression induced by IL-13. BLX2477 suppressed MUC5AC (C) mRNA and (D) protein expression (measured by ELISA, shown as the fold change relative to the DMSO control) induced by IL-13. (E) BLX2477 increased FOXA2 mRNA in response to IL-13 stimulation.
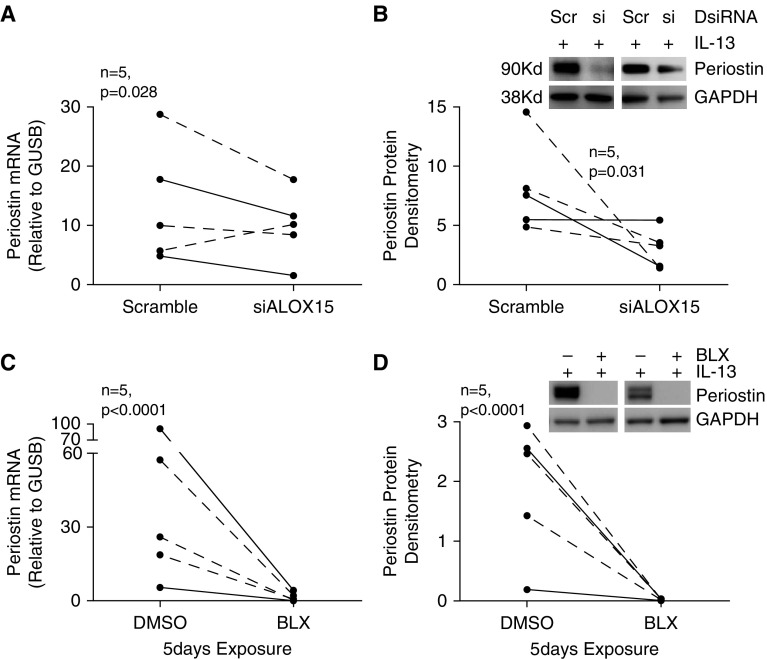
15LO1 pathway inhibition decreases periostin expression and secretion
To determine whether 15LO1 expression and activity may be involved in other known type 2 pathways in HAECs, the impact of 15LO1 pathway inhibition on periostin was investigated. As expected, IL-13 induced both periostin mRNA (Figure E3A) and protein (Figure E3B) expression, as well as secretion (Figure E3C). 15LO1 DsiRNA knockdown inhibited IL-13–induced expression of periostin mRNA (Figure 5A), as well as intracellular (Figure 5B) and secreted periostin protein (Figure E4A). Similarly, BLX2477 (2 μM) treatment decreased periostin mRNA (Figure 5C) and intracellular (Figure 5D) and secreted (Figure E4B) protein after 5 days of exposure. Thus, the 15LO1 pathway regulates a range of downstream type 2 pathways beyond those related to goblet cell differentiation.
Figure 5.
Suppression of 15LO1 levels and activity decreases IL-13–induced periostin expression. HAECs stimulated with IL-13 were transfected with DsiRNA or treated with BLX2477, and cell lysates were collected for mRNA and protein analysis. 15LO1 knockdown (A and B) and BLX2477 treatment (C and D) both suppressed IL-13–induced periostin mRNA and protein expression.
Exogenous 15-HETE-PE Stimulation Induces FOXA3, MUC5AC, and Periostin Expression in HAECs in the Absence of IL-13
To determine whether products of an activated 15LO1 pathway, in the absence of IL-13, were sufficient to induce goblet cell differentiation and periostin expression, 1 μM 15-HETE-PE (or dimyristoyl-phosphatidylethanolamine (DMPE) control) was added to HAECs in ALI every 48 hours for up to 5 days. As shown in Figure 6, exogenous 15-HETE-PE induced expression of FOXA3 (Figures 6A and 6B), as well as MUC5AC mRNA and protein (Figures 6C and 6D). Exogenous 15-HETE-PE stimulation also induced periostin mRNA and protein expression (Figures 6B and 6E). In contrast, there was no significant effect on FOXA2 (Figure 6F). These data directly support the notion that 15-HETE-PE is both necessary and sufficient for regulation of goblet cell differentiation and periostin expression. However, under the specific conditions studied, the effects of exogenous 15-HETE-PE were substantially more modest than those of IL-13 stimulation.
Figure 6.
Exogenous 15-HETE-PE stimulation induces FOXA3, MUC5AC, and periostin expression in HAECs in the absence of IL-13. HAECs under air–liquid interface culture were stimulated with 1 μM 15-HETE-PE for 5 days, with dimyristoyl-phosphatidylethanolamine (DMPE) applied as vehicle control. 15-HETE-PE induced FOXA3 (A and B), MUC5AC (C and D), and periostin (B and E) but had no effect on FOXA2 (F). Data are presented as means ± SEM, analyzed by paired t test.
Discussion
The results from this study confirm the critical importance of 15LO1 and its primary product, 15-HETE-PE, for the epithelial cell remodeling associated with asthmatic airways. 15LO1 is the primary lipoxygenase that is induced in response to the type 2 cytokine IL-13, and 15-HETE-PE is the primary product. Interestingly, this pathway remains present and active for extended periods of time despite the removal of IL-13. Using knockdown, a selective enzyme inhibitor, and exogenous addition approaches, we confirmed that the 15LO1 pathway regulates goblet cell differentiation, not only by increasing the expression of FOXA3, which is critical for goblet cell differentiation, but also by preventing the expression of pathways that are critical for ciliated cell formation (11, 33). Finally, 15LO1 also impacts a broad range of type 2/remodeling associated gene expression including a profound effect on periostin expression as well.
Lipoxygenase enzymes can act on a variety of fatty acid substrates, commonly including AA and LA, to produce a range of oxygenated lipids. In a previous study using a competitive substrate capture method in a cell-free system, human 15LO1, the most abundant lipoxygenase present in HAECs under T-helper cell type 2 conditions, was reported to prefer LA over AA to generate 13-HODEs (18). In HAECs, however, IL-4 and IL-13 preferentially increase a phospholipid (PE)-conjugated form, 15-HETE-PE, in both monocytes and macrophages (14, 19, 21), and epithelial cells (10, 22). Our previous studies confirmed that IL-13–stimulated 15LO1 interacted with PEBP1 to further induce MAPK/ERK activation and MUC5AC expression (10, 23). This suggests that different lipid products of the 15LO1 pathway generated by a specific substrate may play different biological roles in HAECs. However, it is unclear whether LA products (e.g., 13-HODE) could also play a role. In this study, IL-13–stimulated HAECs supplemented with equal amounts of AA and LA were evaluated for free and conjugated lipid product profiles by means of LC/MS.
Lipoxygenases traditionally oxygenate free fatty acids, with AA originating predominantly from arachidonyl phospholipids after activation of phospholipases (34, 35). In the presence of free AA, this leads to the insertion of molecular oxygen at the C15 position and production of the unstable 15-hydroperoxy-eicosatetraenoic acid (15-HpETE), which is rapidly reduced to stable 15-HETE by one of the glutathione peroxidases (e.g., glutathione peroxidase 4) (36, 37). However, unlike other lipoxygenases, 15LO1, under certain conditions, appears to alter its substrate preference from free AA to AA containing phospholipids, resulting in high levels of 15-HETEs conjugated to phospholipids, particularly 15-HETE-PE (10, 22, 30). In the present study under IL-13 conditions, 15LO1 activity preferentially increased 15-HETE-PE, with only minimal increases in free 15-HETE or 13-HODE, and no detectable 13-HODE-PE, despite previous studies suggesting that LA is a preferred substrate in a cell-free system. This difference could be accounted for by the different systems (in vitro versus cell-free) and experimental conditions used. As our previous study showed that 15-HETE-PE regulates MAPK/ERK activation and MUC5AC expression in HAECs (10, 23), the disproportionate increase in 15-HETE-PE suggests that it plays a role in the goblet cell hyperplasia and mucus hypersecretion associated with asthma. At the same time, the robust increase in 15-HETE-PE in the presence of free AA further suggests that the AA is incorporated into membrane phospholipids before it is acted on by IL-13. Alternatively, 15LO1 may oxygenate free or esterified AA to produce 15-HpETE that is subsequently reacylated into lyso-PE.
15LO1 has also been identified as an enzyme that is critical for generating hydroperoxy-phospholipids when 15LO1 switches from metabolizing free polyunsaturated fatty acids to esterified AA-phospholipids (38). Generation of these intracellular esterified AA-phospholipids, particularly 15-HpETE, could lead to a newly identified cell death termed “ferroptosis” (10, 30, 38). Thus, in addition to controlling cell differentiation, this switch to generation of 15-HpETE-PE (and 15-HETE-PE) could also control cell survival and death. However, the mechanisms by which 15LO1 changes its preference from free to phospholipid-conjugated fatty acids, in particular AA, require further study.
Another important finding is the stability of the 15LO1 protein and its enzyme activity. 15LO1 and its eicosanoid product 15-HETE have long been noted to be increased in human asthma in relation to severity and eosinophilic inflammation (14, 19, 39). 15LO1 is induced by type 2 cytokines in monocytes and macrophages, and in HAECs is one of the genes most strongly induced by IL-13 in vitro (20, 40, 41). Despite the very high expression of type 2 signature genes such as 15LO1, the levels of the presumed type 2 cytokines IL-4 and IL-13 (mRNA and protein) in asthmatics, especially those with more severe disease treated with corticosteroids, are low (42, 43). The reasons for this disconnect between high levels of 15LO1 and low levels of type 2 cytokines/IL-13 in vivo are not clear. However, the high, sustained 15LO1 protein levels and activity after removal of IL-13, as observed in this study, could contribute to prolonged IL-4/-13 downstream pathway activity, even in the absence of these cytokines. This long half-life appeared to be limited to protein, as mRNA levels fell rapidly. In contrast, 15LO1 protein levels remained high for at least 72 hours after removal of IL-13. More importantly, intracellular 15-HETE-PE levels also remained elevated over baseline after IL-13 removal. Given that the media were changed every 2 days, this represents newly formed 15-HETE-PE. Thus, the enzyme was functional and contributed newly formed lipid mediators long after removal of the IL-13, potentially explaining the high levels measured in vivo.
We previously reported that 15LO1 conjugated with the scaffolding protein PEBP1 is critical for IL-13–induced HAEC MUC5AC expression (23). In addition, 15LO1 activity modulated eotaxin-3/C-C motif chemokine ligand 26 and inducible nitric oxide synthase. However, its broader effects on type 2 cytokine-induced goblet cell differentiation and function are not known. Given its high levels and sustained activity, we hypothesized that it would have a broad and central role. Recent studies have suggested that goblet cell differentiation is critically dependent on a specific forkhead DNA-binding protein, FOXA3, which binds to proximal promoters of several groups of genes associated with goblet cell metaplasia (11, 33) as well as suppression of FOXA2 (11, 12, 44). Both HAEC and transgenic mice studies confirmed that FOXA3 induced goblet cell metaplasia and enhanced expression of MUC5AC. Similarly, a recent microarray study of fresh HAECs from healthy and asthmatic subjects demonstrated high FOXA3 mRNA levels in type 2–associated asthma, which correlated strongly with MUC5AC (and 15LO1) (39). In contrast, FOXA2 has been reported to suppress goblet cell metaplasia and induce ciliated cell differentiation (12). Interestingly, the results presented here, which were obtained with the use of chemical inhibitors, DsiRNA, and exogenous addition of 15-HETE-PE, clearly show that the expression and activity of the 15LO1 pathway control IL-13–induced FOXA3. At the same time, FOXA2 expression was inhibited, supporting an overall effect of 15LO1 to influence remodeling of the airway epithelium in favor of goblet cells, consistent with the microarray data (10, 39). The lack of effect of exogenous 15-HETE-PE on FOXA2 expression requires further study, but may be related to both the dose of the exogenous hydroxyl-phospholipid and its uptake into the cells. The mechanisms by which 15LO1 expression and activity control the expression of these transcription factors remain speculative, but they could include ERK/MAPK activation or interaction of membrane phospholipids with the IL-4 receptor α or its downstream signaling pathways, including mitogen-activated protein kinases/extracellular signal-regulated kinases (MAPK/ERK). Further study is needed.
To begin to determine the extent to which 15LO1 controls prominent IL-13–induced pathways, we also evaluated the impact of the 15LO1 pathway on HAEC periostin expression. Periostin is an extracellular matrix protein that can regulate the adhesion and migration of epithelial cells in airway remodeling in asthma and other diseases (45, 46). Multiple studies have reported elevated levels of periostin mRNA in freshly brushed HAECs and serum from patients with type 2 high asthma (24, 25). Our results show that IL-13 induced high levels of periostin expression and secretion in HAECs, consistent with previous studies. More importantly, our results show a marked inhibition of periostin expression in the face of 15LO1 inhibition, which parallels the reductions in goblet cell differentiation as indicated by MUC5AC expression, and mirrors the increases in periostin expression after exogenous 15-HETE-PE addition. These results suggest that periostin expression is associated with goblet cell differentiation and mucus hypersecretion in response to IL-13 stimulation in HAECs. Interestingly, in contrast to the findings reported here, periostin was recently suggested to suppress goblet cell metaplasia in a mouse model, with periostin knockout mice demonstrating increased differentiation of epithelial cells into mucus-producing goblet cells upon sensitization and challenge with ovalbumin, but no effect on allergic inflammation (47). Thus, in mice, periostin appears to have the opposite effect on goblet cell metaplasia. Although the reasons for this differences are unclear, it could be due to differences in epithelial cells between species, the knockout models used, and the challenges applied in the studies (47).
Although the studies presented here strongly support the development and testing of 15LO1 pathway inhibitors in asthma, replication of these findings in transgenic mice would add to the functional importance of the pathway. However, given the large biological differences between mouse AECs and HAECs, substantial studies to confirm the importance of this pathway in mice as compared with human airways will be required. Studies are also needed to confirm whether mouse AECs even express 15LO1 or the mouse equivalent, 12/15LO1. Previous studies in transgenic mice did not comment on epithelial expression despite the suppression of mucus (48).
Conclusions
The data presented here confirm a critical role for 15LO1 and its product 15-HETE-PE in HAEC goblet cell differentiation. Under IL-13 conditions, 15LO1 is the most abundant 15LOX in cultured HAECs, where it remains at high levels and active for prolonged periods of time even in the absence of IL-13. IL-13–induced 15LO1 strongly favors the metabolism of AA over free fatty acids to generate phospholipid-conjugated 15-HETE-PE, a process that appears to be both necessary and sufficient to impact FOXA3 and periostin expression and goblet cell differentiation in vitro. Further studies are needed to elucidate the mechanisms by which this pathway controls these epithelial changes, as well as the functional implications of this pathway for human diseases like asthma.
Footnotes
Supported by National Institutes of Health grants AI-40600-15 (S.W.) and CTSI UL1-RR024153 (S.W.), and American Lung Association grant RG-231468-N (J.Z.).
Author Contributions: J.Z. and S.W. designed research. J.Z., Y.M., E.E., J.M.C., S.N.L., V.T., M.A., and S.W. performed research. V.O’D., H.-E.C., and V.K. contributed new reagents or analytic tools. J.Z., Y.M., V.O’D., H.-E.C., V.K., and S.W. analyzed the data. J.Z., Y.M., and S.W. wrote the manuscript.
This article has a data supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2017-0031OC on July 19, 2017
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Morcillo EJ, Cortijo J. Mucus and MUC in asthma. Curr Opin Pulm Med. 2006;12:1–6. doi: 10.1097/01.mcp.0000198064.27586.37. [DOI] [PubMed] [Google Scholar]
- 2.Fahy JV. Goblet cell and mucin gene abnormalities in asthma. Chest. 2002;122(6) Suppl:320S–326S. doi: 10.1378/chest.122.6_suppl.320s. [DOI] [PubMed] [Google Scholar]
- 3.Thornton DJ, Rousseau K, McGuckin MA. Structure and function of the polymeric mucins in airways mucus. Annu Rev Physiol. 2008;70:459–486. doi: 10.1146/annurev.physiol.70.113006.100702. [DOI] [PubMed] [Google Scholar]
- 4.Zhu Z, Homer RJ, Wang Z, Chen Q, Geba GP, Wang J, Zhang Y, Elias JA. Pulmonary expression of interleukin-13 causes inflammation, mucus hypersecretion, subepithelial fibrosis, physiologic abnormalities, and eotaxin production. J Clin Invest. 1999;103:779–788. doi: 10.1172/JCI5909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fulkerson PC, Fischetti CA, Hassman LM, Nikolaidis NM, Rothenberg ME. Persistent effects induced by IL-13 in the lung. Am J Respir Cell Mol Biol. 2006;35:337–346. doi: 10.1165/rcmb.2005-0474OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fujisawa T, Ide K, Holtzman MJ, Suda T, Suzuki K, Kuroishi S, Chida K, Nakamura H. Involvement of the p38 MAPK pathway in IL-13-induced mucous cell metaplasia in mouse tracheal epithelial cells. Respirology. 2008;13:191–202. doi: 10.1111/j.1440-1843.2008.01237.x. [DOI] [PubMed] [Google Scholar]
- 7.Atherton HC, Jones G, Danahay H. IL-13-induced changes in the goblet cell density of human bronchial epithelial cell cultures: MAP kinase and phosphatidylinositol 3-kinase regulation. Am J Physiol Lung Cell Mol Physiol. 2003;285:L730–L739. doi: 10.1152/ajplung.00089.2003. [DOI] [PubMed] [Google Scholar]
- 8.Yasuo M, Fujimoto K, Tanabe T, Yaegashi H, Tsushima K, Takasuna K, Koike T, Yamaya M, Nikaido T. Relationship between calcium-activated chloride channel 1 and MUC5AC in goblet cell hyperplasia induced by interleukin-13 in human bronchial epithelial cells. Respiration. 2006;73:347–359. doi: 10.1159/000091391. [DOI] [PubMed] [Google Scholar]
- 9.Tanabe T, Fujimoto K, Yasuo M, Tsushima K, Yoshida K, Ise H, Yamaya M. Modulation of mucus production by interleukin-13 receptor α2 in the human airway epithelium. Clin Exp Allergy. 2008;38:122–134. doi: 10.1111/j.1365-2222.2007.02871.x. [DOI] [PubMed] [Google Scholar]
- 10.Zhao J, Maskrey B, Balzar S, Chibana K, Mustovich A, Hu H, Trudeau JB, O’Donnell V, Wenzel SE. Interleukin-13-induced MUC5AC is regulated by 15-lipoxygenase 1 pathway in human bronchial epithelial cells. Am J Respir Crit Care Med. 2009;179:782–790. doi: 10.1164/rccm.200811-1744OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen G, Korfhagen TR, Karp CL, Impey S, Xu Y, Randell SH, Kitzmiller J, Maeda Y, Haitchi HM, Sridharan A, et al. Foxa3 induces goblet cell metaplasia and inhibits innate antiviral immunity. Am J Respir Crit Care Med. 2014;189:301–313. doi: 10.1164/rccm.201306-1181OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wan H, Kaestner KH, Ang SL, Ikegami M, Finkelman FD, Stahlman MT, Fulkerson PC, Rothenberg ME, Whitsett JA. Foxa2 regulates alveolarization and goblet cell hyperplasia. Development. 2004;131:953–964. doi: 10.1242/dev.00966. [DOI] [PubMed] [Google Scholar]
- 13.Kühn H, O’Donnell VB. Inflammation and immune regulation by 12/15-lipoxygenases. Prog Lipid Res. 2006;45:334–356. doi: 10.1016/j.plipres.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 14.Chu HW, Balzar S, Westcott JY, Trudeau JB, Sun Y, Conrad DJ, Wenzel SE. Expression and activation of 15-lipoxygenase pathway in severe asthma: relationship to eosinophilic phenotype and collagen deposition. Clin Exp Allergy. 2002;32:1558–1565. doi: 10.1046/j.1365-2222.2002.01477.x. [DOI] [PubMed] [Google Scholar]
- 15.Gulliksson M, Brunnstrom A, Johannesson M, Backman L, Nilsson G, Harvima I, Dahlen B, Kumlin M, Claesson HE. Expression of 15-lipoxygenase type-1 in human mast cells. Biochim Biophys Acta. 2007;1771:1156–1165. doi: 10.1016/j.bbalip.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 16.Shappell SB, Boeglin WE, Olson SJ, Kasper S, Brash AR. 15-lipoxygenase-2 (15-LOX-2) is expressed in benign prostatic epithelium and reduced in prostate adenocarcinoma. Am J Pathol. 1999;155:235–245. doi: 10.1016/S0002-9440(10)65117-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brash AR, Jisaka M, Boeglin WE, Chang MS, Keeney DS, Nanney LB, Kasper S, Matusik RJ, Olson SJ, Shappell SB. Investigation of a second 15S-lipoxygenase in humans and its expression in epithelial tissues. Adv Exp Med Biol. 1999;469:83–89. doi: 10.1007/978-1-4615-4793-8_13. [DOI] [PubMed] [Google Scholar]
- 18.Wecksler AT, Kenyon V, Deschamps JD, Holman TR. Substrate specificity changes for human reticulocyte and epithelial 15-lipoxygenases reveal allosteric product regulation. Biochemistry. 2008;47:7364–7375. doi: 10.1021/bi800550n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Profita M, Sala A, Riccobono L, Paternò A, Mirabella A, Bonanno A, Guerrera D, Pace E, Bonsignore G, Bousquet J, et al. 15-Lipoxygenase expression and 15(S)-hydroxyeicoisatetraenoic acid release and reincorporation in induced sputum of asthmatic subjects. J Allergy Clin Immunol. 2000;105:711–716. doi: 10.1067/mai.2000.105122. [DOI] [PubMed] [Google Scholar]
- 20.Jayawickreme SP, Gray T, Nettesheim P, Eling T. Regulation of 15-lipoxygenase expression and mucus secretion by IL-4 in human bronchial epithelial cells. Am J Physiol. 1999;276:L596–L603. doi: 10.1152/ajplung.1999.276.4.L596. [DOI] [PubMed] [Google Scholar]
- 21.Bradding P, Redington AE, Djukanovic R, Conrad DJ, Holgate ST. 15-lipoxygenase immunoreactivity in normal and in asthmatic airways. Am J Respir Crit Care Med. 1995;151:1201–1204. doi: 10.1164/ajrccm/151.4.1201. [DOI] [PubMed] [Google Scholar]
- 22.Profita M, Vignola AM, Sala A, Mirabella A, Siena L, Pace E, Folco G, Bonsignore G. Interleukin-4 enhances 15-lipoxygenase activity and incorporation of 15(S)-HETE into cellular phospholipids in cultured pulmonary epithelial cells. Am J Respir Cell Mol Biol. 1999;20:61–68. doi: 10.1165/ajrcmb.20.1.3151. [DOI] [PubMed] [Google Scholar]
- 23.Zhao J, O’Donnell VB, Balzar S, St Croix CM, Trudeau JB, Wenzel SE. 15-Lipoxygenase 1 interacts with phosphatidylethanolamine-binding protein to regulate MAPK signaling in human airway epithelial cells. Proc Natl Acad Sci USA. 2011;108:14246–14251. doi: 10.1073/pnas.1018075108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Corren J, Lemanske RF, Hanania NA, Korenblat PE, Parsey MV, Arron JR, Harris JM, Scheerens H, Wu LC, Su Z, et al. Lebrikizumab treatment in adults with asthma. N Engl J Med. 2011;365:1088–1098. doi: 10.1056/NEJMoa1106469. [DOI] [PubMed] [Google Scholar]
- 25.Li W, Gao P, Zhi Y, Xu W, Wu Y, Yin J, Zhang J. Periostin: its role in asthma and its potential as a diagnostic or therapeutic target. Respir Res. 2015;16:57. doi: 10.1186/s12931-015-0218-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Conrad DJ, Lu M. Regulation of human 12/15-lipoxygenase by Stat6-dependent transcription. Am J Respir Cell Mol Biol. 2000;22:226–234. doi: 10.1165/ajrcmb.22.2.3786. [DOI] [PubMed] [Google Scholar]
- 27.Pelcman B, Sanin A, Nilsson P, No K, Schaal W, Öhrman S, Krog-Jensen C, Forsell P, Hallberg A, Larhed M, et al. 3-Substituted pyrazoles and 4-substituted triazoles as inhibitors of human 15-lipoxygenase-1. Bioorg Med Chem Lett. 2015;25:3024–3029. doi: 10.1016/j.bmcl.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 28.Chu HW, Balzar S, Seedorf GJ, Westcott JY, Trudeau JB, Silkoff P, Wenzel SE. Transforming growth factor-β2 induces bronchial epithelial mucin expression in asthma. Am J Pathol. 2004;165:1097–1106. doi: 10.1016/s0002-9440(10)63371-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Albano GD, Zhao J, Etling EB, Park SY, Hu H, Trudeau JB, Profita M, Wenzel SE. IL-13 desensitizes β2-adrenergic receptors in human airway epithelial cells through a 15-lipoxygenase/G protein receptor kinase 2 mechanism. J Allergy Clin Immunol. 2015;135:1144–1153e9. doi: 10.1016/j.jaci.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maskrey BH, Bermúdez-Fajardo A, Morgan AH, Stewart-Jones E, Dioszeghy V, Taylor GW, Baker PR, Coles B, Coffey MJ, Kühn H, et al. Activated platelets and monocytes generate four hydroxyphosphatidylethanolamines via lipoxygenase. J Biol Chem. 2007;282:20151–20163. doi: 10.1074/jbc.M611776200. [DOI] [PubMed] [Google Scholar]
- 31.Maskrey BH, O’Donnell VB. Analysis of eicosanoids and related lipid mediators using mass spectrometry. Biochem Soc Trans. 2008;36:1055–1059. doi: 10.1042/BST0361055. [DOI] [PubMed] [Google Scholar]
- 32.Trudeau J, Hu H, Chibana K, Chu HW, Westcott JY, Wenzel SE. Selective downregulation of prostaglandin E2-related pathways by the Th2 cytokine IL-13. J Allergy Clin Immunol. 2006;117:1446–1454. doi: 10.1016/j.jaci.2006.01.049. [DOI] [PubMed] [Google Scholar]
- 33.Rajavelu P, Chen G, Xu Y, Kitzmiller JA, Korfhagen TR, Whitsett JA. Airway epithelial SPDEF integrates goblet cell differentiation and pulmonary Th2 inflammation. J Clin Invest. 2015;125:2021–2031. doi: 10.1172/JCI79422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ford-Hutchinson AW. Arachidonate 15-lipoxygenase; characteristics and potential biological significance. Eicosanoids. 1991;4:65–74. [PubMed] [Google Scholar]
- 35.Kuhn H, Walther M, Kuban RJ. Mammalian arachidonate 15-lipoxygenases structure, function, and biological implications. Prostaglandins Other Lipid Mediat. 2002;68-69:263–290. doi: 10.1016/s0090-6980(02)00035-7. [DOI] [PubMed] [Google Scholar]
- 36.Ursini F, Maiorino M, Brigelius-Flohé R, Aumann KD, Roveri A, Schomburg D, Flohé L. Diversity of glutathione peroxidases. Methods Enzymol. 1995;252:38–53. doi: 10.1016/0076-6879(95)52007-4. [DOI] [PubMed] [Google Scholar]
- 37.Ursini F, Bindoli A. The role of selenium peroxidases in the protection against oxidative damage of membranes. Chem Phys Lipids. 1987;44:255–276. doi: 10.1016/0009-3084(87)90053-3. [DOI] [PubMed] [Google Scholar]
- 38.Kagan VE, Mao G, Qu F, Angeli JP, Doll S, Croix CS, Dar HH, Liu B, Tyurin VA, Ritov VB, et al. Oxidized arachidonic and adrenic PEs navigate cells to ferroptosis. Nat Chem Biol. 2017;13:81–90. doi: 10.1038/nchembio.2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Modena BD, Tedrow JR, Milosevic J, Bleecker ER, Meyers DA, Wu W, Bar-Joseph Z, Erzurum SC, Gaston BM, Busse WW, et al. Gene expression in relation to exhaled nitric oxide identifies novel asthma phenotypes with unique biomolecular pathways. Am J Respir Crit Care Med. 2014;190:1363–1372. doi: 10.1164/rccm.201406-1099OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brinckmann R, Topp MS, Zalán I, Heydeck D, Ludwig P, Kühn H, Berdel WE, Habenicht JR. Regulation of 15-lipoxygenase expression in lung epithelial cells by interleukin-4. Biochem J. 1996;318:305–312. doi: 10.1042/bj3180305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nassar GM, Morrow JD, Roberts LJ, II, Lakkis FG, Badr KF. Induction of 15-lipoxygenase by interleukin-13 in human blood monocytes. J Biol Chem. 1994;269:27631–27634. [PubMed] [Google Scholar]
- 42.Woodruff PG, Modrek B, Choy DF, Jia G, Abbas AR, Ellwanger A, Koth LL, Arron JR, Fahy JV.T-helper type 2-driven inflammation defines major subphenotypes of asthma Am J Respir Crit Care Med 2009180796[Published erratum appears in Am J Respir Crit Care Med 180:796.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bhakta NR, Woodruff PG. Human asthma phenotypes: from the clinic, to cytokines, and back again. Immunol Rev. 2011;242:220–232. doi: 10.1111/j.1600-065X.2011.01032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Park KS, Wells JM, Zorn AM, Wert SE, Laubach VE, Fernandez LG, Whitsett JA. Transdifferentiation of ciliated cells during repair of the respiratory epithelium. Am J Respir Cell Mol Biol. 2006;34:151–157. doi: 10.1165/rcmb.2005-0332OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Johansson MW, Annis DS, Mosher DF. α(M)β(2) integrin-mediated adhesion and motility of IL-5-stimulated eosinophils on periostin. Am J Respir Cell Mol Biol. 2013;48:503–510. doi: 10.1165/rcmb.2012-0150OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sidhu SS, Yuan S, Innes AL, Kerr S, Woodruff PG, Hou L, Muller SJ, Fahy JV. Roles of epithelial cell-derived periostin in TGF-β activation, collagen production, and collagen gel elasticity in asthma. Proc Natl Acad Sci USA. 2010;107:14170–14175. doi: 10.1073/pnas.1009426107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sehra S, Yao W, Nguyen ET, Ahyi AN, Tuana FM, Ahlfeld SK, Snider P, Tepper RS, Petrache I, Conway SJ, et al. Periostin regulates goblet cell metaplasia in a model of allergic airway inflammation. J Immunol. 2011;186:4959–4966. doi: 10.4049/jimmunol.1002359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Andersson CK, Claesson HE, Rydell-Törmänen K, Swedmark S, Hällgren A, Erjefält JS. Mice lacking 12/15-lipoxygenase have attenuated airway allergic inflammation and remodeling. Am J Respir Cell Mol Biol. 2008;39:648–656. doi: 10.1165/rcmb.2007-0443OC. [DOI] [PubMed] [Google Scholar]