Abstract
Hyaluronan (HA), a major component of the extracellular matrix, is secreted by airway structural cells. Airway fibroblasts in allergic asthma secrete elevated levels of HA in association with increased HA synthase 2 (HAS2) expression. Thus, we hypothesized that HA accumulation in the airway wall may contribute to airway remodeling and hyperresponsiveness in allergic airways disease. To examine this hypothesis, transgenic mice in which the α-smooth muscle actin (α-SMA) promoter drives HAS2 expression were generated. Mixed male and female α-SMA–HAS2 mice (HAS2+ mice, n = 16; HAS2− mice, n = 13) were sensitized via intraperitoneal injection and then chronically challenged with aerosolized ovalbumin (OVA) for 6 weeks. To test airway responsiveness, increasing doses of methacholine were delivered intravenously and airway resistance was measured using the forced oscillation technique. HA, cytokines, and cell types were analyzed in bronchoalveolar lavage fluid, serum, and whole lung homogenates. Lung sections were stained using antibodies specific for HA-binding protein (HABP) and α-SMA, as well as Masson’s trichrome stain. Staining of lung tissue demonstrated significantly increased peribronchial HA, α-SMA, and collagen deposition in OVA-challenged α-SMA–HAS2+ mice compared with α-SMA–HAS2− mice. Unexpectedly, OVA-challenged α-SMA–HAS2+ mice displayed significantly reduced airway responsiveness to methacholine compared with similarly treated α-SMA–HAS2− mice. The total numbers of inflammatory cell types in the bronchoalveolar lavage fluid did not differ significantly between OVA-challenged α-SMA–HAS2+ mice and α-SMA–HAS2− mice. We conclude that allergen-challenged mice that overexpress HAS2 in myofibroblasts and smooth muscle cells develop increased airway fibrosis, which lessens airway hyperresponsiveness to bronchoconstrictors.
Keywords: airway hyperresponsiveness, asthma, HAS2, hyaluronan
Clinical Relevance
This work demonstrates that in a model of chronic allergen challenge, mice that overexpress hyaluronan synthase 2 in myofibroblasts and smooth muscle cells exhibit increased airway remodeling, including peribronchial hyaluronan deposition and fibrosis with lessened airway hyperresponsiveness. Our data suggest that elevated expression of hyaluronan synthase 2 by airway fibroblasts, as has been shown in allergic asthma, may promote airway remodeling and impact airway responsiveness.
Chronic airway inflammation, hyperresponsiveness, and remodeling are key features of allergic asthma. These three processes are intertwined and contribute to the overall pathobiology of the disease (1, 2). The inflammatory cells that participate in the ongoing, unresolved airway inflammation in allergic asthma, such as eosinophils, macrophages, and T lymphocytes, are believed to contribute to airway remodeling by releasing increased levels of cytokines and growth factors that influence the functions of structural cells in the airway (3). These inflammatory cells direct airway mucous overproduction by epithelial cells (4), invasion of the submucosa and increased secretion of extracellular matrix within and around the airway wall by airway fibroblasts (5–7), and smooth muscle cell hyperplasia/hypertrophy, resulting in thickening of the airway wall (5) in asthma. Structural changes to the airway in allergic asthma result in fixed airway obstruction and diminished lung function over time (8, 9). Similarly, airway remodeling promotes persistent airway hyperresponsiveness (AHR) in allergic asthma by contributing to airway narrowing and concomitant increased airway resistance and responsiveness to bronchoconstrictors (10).
Hyaluronan (HA) is a large, nonsulfated glycosaminoglycan that is widely produced by fibroblasts, smooth muscle cells, and fibroblast-like cells in connective tissues (11, 12). It is a major component of the extracellular matrix, providing compressive strength, lubrication, and hydration to tissues (12, 13). Although macromolecular HA mediates many normal physiological responses, such as embryonic development (14), tissue repair and homeostasis (15), and ovulation and fertilization (16), HA degradation and the resultant accumulation of low-molecular-weight HA fragments are characteristic of diseases that involve tissue fibrosis (17, 18). In the lung, HA contributes to the maintenance and repair of injured tissue, and elevated HA synthesis is associated with inflammatory cell infiltration after both acute and chronic injuries (19). In asthma, the concentrations of HA measured in bronchoalveolar lavage (BAL) fluid are significantly correlated with severity of disease (20). Furthermore, airway fibroblasts isolated from asthma patients produce significantly greater concentrations of low-molecular-weight HA compared with cells from nonasthmatic patients (21). In murine models of allergic airways disease, deposition of HA and secretion into the airways is significantly increased after ovalbumin (OVA) or cockroach allergen challenge, and HA colocalizes with peribronchial inflammation and collagen deposition (22, 23).
HA is synthesized by three HA synthases (HASs): HAS1, HAS2, and HAS3 (24). HAS2 is the predominant isoform expressed in the human lung (25). Human airway fibroblasts in asthma were found to express increased levels of HAS2 compared with normal controls (21). In mice, HAS2 mRNA levels were shown to increase within 2 hours of allergen sensitization and challenge, and remained elevated throughout the chronic challenge (22, 23). Furthermore, mice with targeted overexpression of mesenchymal cell HAS2 were shown to develop severe lung fibrosis with increased myofibroblast invasiveness after bleomycin-induced injury (18).
We hypothesized that peribronchial HA accumulation contributes to airway inflammation, remodeling, and hyperresponsiveness in asthma. Airway myofibroblasts and smooth muscle cells are key cells that direct extracellular matrix production and airway narrowing during the pathobiology of airway fibrosis and hyperresponsiveness in asthma (5). Thus, we investigated our hypothesis by challenging mice with targeted overexpression of HAS2 in cells expressing the α−smooth muscle actin (α-SMA) promoter (myofibroblasts and smooth muscle cells) in a chronic model of allergic airways disease. We measured lung mechanics, airway inflammation, HA accumulation, and airway fibrosis in these mice after a 6-week allergen challenge.
Materials and Methods
Animals
All animal care and experimental protocols were reviewed and approved by the Institutional Animal Care and Use Committee at Duke University Medical Center and were performed in accordance with the U.S. Animal Welfare Acts. α-SMA–human HAS2 transgene-positive mice (α-SMA–HAS2+) were described previously (26). The control mice were non–transgene-expressing littermates of the transgene-positive mice (transgene-negative [α-SMA–HAS2−]). All mice were housed in pathogen-free facilities at Duke University.
OVA Model
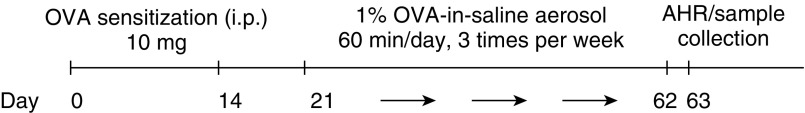
Mice (α-SMA–HAS2+, n = 16; α-SMA–HAS2−, n = 13) were sensitized and challenged with OVA in a chronic model of allergic airways disease as described previously (27) (Figure 1). Naive α-SMA–HAS2+ mice (n = 8) and α-SMA–HAS2− mice (n = 6) that had not undergone the OVA challenge were used as controls to determine whether there were intrinsic differences in α-SMA–HAS2+ mice.
Figure 1.
Schematic illustration of the ovalbumin (OVA) challenge protocol. α-SMA-HAS2+ (n = 16) and α-SMA−HAS2− littermates (n = 13) were sensitized to OVA by intraperitoneal injection on Days 0 and 14. On Day 21, the mice were challenged with aerosolized OVA three times per week for 6 weeks. Lung-mechanics measurements and lung tissues were collected 24 hours after the final exposure. α-SMA, α-smooth muscle actin; AHR, airway hyperresponsiveness; HAS2, hyaluronan synthase 2.
Lung-Mechanics Measurements
To test airway responsiveness, increasing doses of methacholine were delivered intravenously, and lung impedance was measured by forced oscillometry (Flexivent; SCIREQ, Montreal, Canada) as described previously (27). The Newtonian resistance (Rn) was calculated from the impedance signal.
Measurements of HA and Cytokines
Measurements of HA in serum, BAL fluid, and whole lung homogenates were performed using an ELISA-like assay and biotinylated HA-binding protein (HABP; R&D Systems, Minneapolis, MN). IL-13, IL-17, and transforming growth factor β1 (TGF-β1) protein levels in BAL fluid and whole lung homogenates were measured by ELISA (R&D Systems).
Airway Tissue Staining
Lungs inflated to 25 cm H2O were fixed in 4% paraformaldehyde and embedded in paraffin. Sections were stained with Masson’s trichrome stain to visualize collagen as previously described (27). Other sections were stained with biotinylated HABP, anti–α-SMA, or 4′6-diamidino-2-phenylindole (DAPI) as previously described (28). Images of 10 stained airway tissue cross-sections per mouse were imported into ImageJ software (National Institutes of Health, Bethesda, MD). The relative quantities of Masson’s trichrome, HABP, or α-SMA staining were determined by calculating the percentage of peribronchial staining within the total tissue area, which included the lumen of the airway. Perivascular staining was not included in the analyses.
Statistical Analyses
Analyses were performed using JMP (SAS, Cary, NC) and Prism (GraphPad, La Jolla, CA) statistical software. Data were analyzed using Student’s t test or one-way ANOVA. For most analyses, the data are expressed as means ± SEM, as they were normally distributed, and significance is denoted by P < 0.05. For data that were not normally distributed, data were expressed as medians (interquartile range) and compared using a two-tailed Wilcoxon rank-sum test. Two-way repeated-measures ANOVAs were conducted to evaluate lung mechanics in response to doses of methacholine at 25, 50, 100, 200, and 400 μg/kg. Data points that were more than 2 SDs from the mean were excluded from the data set. Significance is denoted by P < 0.05.
Results
HA and TGF-β1 Are Significantly Increased in Whole Lung Tissue of OVA-Challenged α-SMA–HAS2+ Mice
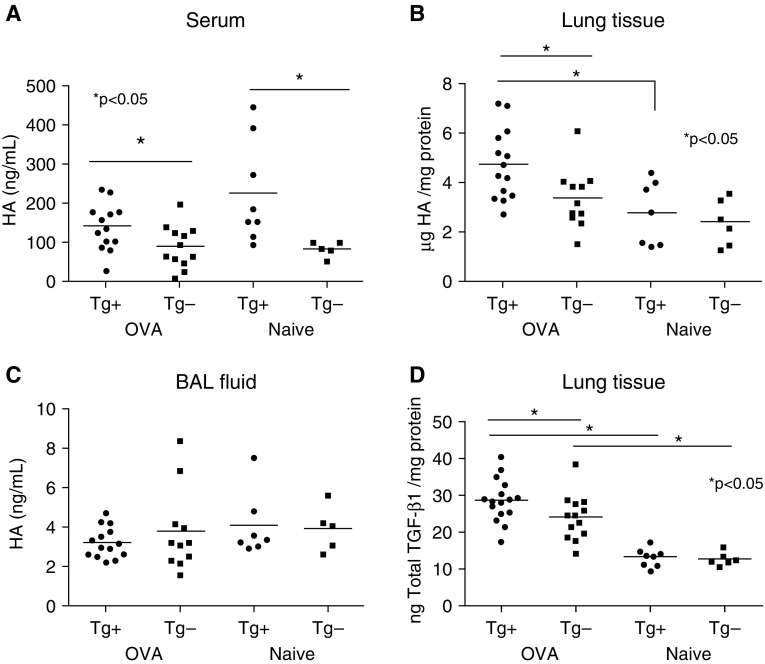
Chronic OVA challenge in mice stimulates airway fibrosis and smooth muscle cell hyperplasia, suggesting that this model recapitulates aspects of airway remodeling in human asthma (29). To evaluate the role of HAS2 expression by myofibroblasts and smooth muscle cells in the pathogenesis of asthma, we challenged transgenic mice with targeted human HAS2 expression in α-SMA–expressing cells (18, 26) with OVA for 41 days (Figure 1). α-SMA–HAS2+ mice developed normally and exhibited no abnormal phenotype when unchallenged. At baseline, unchallenged α-SMA–HAS2+ mice exhibited significantly elevated levels of HA in serum compared with α-SMA–HAS2− mice (Figure 2A). In addition, HA levels in both serum and whole lung tissue homogenates were significantly increased in OVA-challenged α-SMA–HAS2+ mice compared with OVA-challenged α-SMA–HAS2− mice (Figures 2A and 2B). Furthermore, OVA significantly augmented the levels of HA in whole lung tissue in α-SMA–HAS2+ mice compared with unchallenged α-SMA–HAS2+ mice. The levels of HA in BAL fluid of unchallenged α-SMA–HAS2+ mice were low and not significantly different from those in α-SMA–HAS2− mice (Figure 2C) (18). In OVA-challenged α-SMA–HAS2+ and α-SMA–HAS2− mice, HA levels in BAL fluid were also not significantly different between groups (Figure 2C). However, total TGF-β1 levels in whole lung homogenates were significantly increased in OVA-challenged α-SMA–HAS2+ mice compared with OVA-challenged α-SMA–HAS2− mice as well as naive α-SMA–HAS2+ mice (Figure 2D).
Figure 2.
Production of hyaluronan (HA) and TGF-β1 is increased in OVA-challenged α-SMA−HAS2+ mice. Baseline mean HA levels, as measured by ELISA, were significantly elevated in serum of naive α-SMA−HAS2+ (n = 8) mice compared with naive α-SMA−HAS2− (n = 6) mice (A); *P < 0.05. Furthermore, mean HA levels were significantly increased in serum (A) and whole lung homogenates (B) from OVA-challenged α-SMA−HAS2+ mice compared with OVA-challenged α-SMA−HAS2− mice; *P < 0.05. (C) No significant differences were observed in the mean levels of HA as measured by ELISA in BAL fluid obtained from naive or OVA-challenged α-SMA−HAS2+ mice compared with α-SMA−HAS2− mice; P > 0.05. (D) Mean total TGF-β1 levels, as measured by ELISA, were significantly increased in whole lung homogenates from OVA-challenged α-SMA-HAS2+ mice compared with OVA-challenged α-SMA−HAS2− mice, and in OVA-challenged mice compared with naive mice; *P < 0.05. Measurements of HA and TGF-β1 were normalized to total protein in whole lung homogenates. BAL, bronchoalveolar lavage; Tg, transgene; TGF-β1, transforming growth factor β1.
Peribronchial HABP and α-SMA Staining Are Significantly Increased in OVA-Challenged α-SMA–HAS2+ Mice
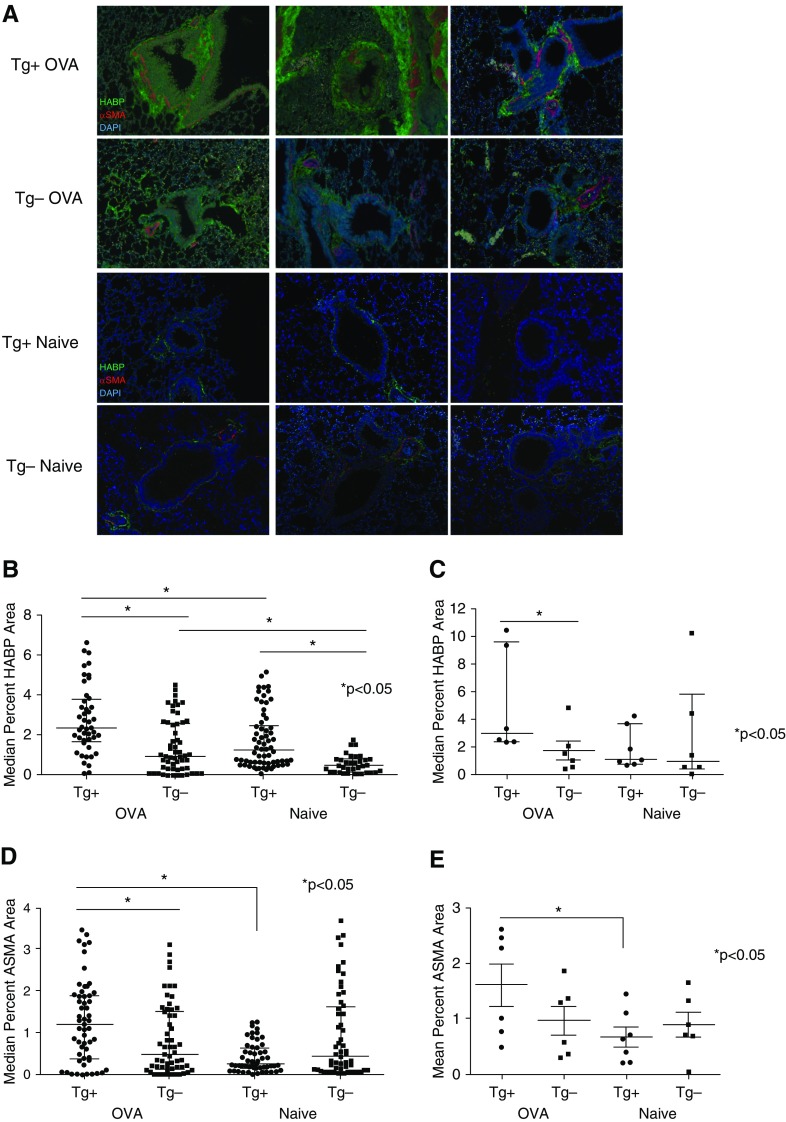
To determine the effectiveness of α-SMA–HAS2 transgene expression in facilitating airway deposition of HA in OVA-challenged α-SMA–HAS2+ mice compared with transgene-negative and -naive mice, sections of whole lung from naive and OVA-challenged α-SMA–HAS2+ and α-SMA–HAS2− mice were immunohistochemically stained with HABP, α-SMA–specific antibody, or DAPI (to visualize cell nuclei). Representative images of airways from three different mice in each group are shown in Figure 3A. Peribronchial green HABP staining was significantly enhanced in unchallenged α-SMA–HAS2+ mice compared with naive α-SMA–HAS2− mice (Figure 3B). Staining of both peribronchial HABP and α-SMA was augmented in OVA-challenged α-SMA–HAS2+ mice compared with OVA-challenged α-SMA–HAS2− mice and naive α-SMA–HAS2+ mice (Figures 3B–3E). The percent area of staining was quantified using ImageJ.
Figure 3.
Immunofluorescence staining for HA-binding protein (HABP) and α-SMA in naive and OVA-challenged α-SMA−HAS2+ and α-SMA−HAS2− mice. (A) Representative ×100 images of airway sections from three different naive (Tg+ Naive) and OVA-challenged α-SMA−HAS2+ mice (Tg+ OVA) and three different naive (Tg− Naive) and OVA-challenged α-SMA−HAS2− mice (Tg− OVA). The airways were stained for HABP (green), α-SMA (red), or DAPI (blue). ImageJ was used to quantify the median percent of green HABP (B) or red α-SMA (D) peribronchial staining per total area in 10 airways per mouse. The median percent HABP (C) or mean percent α-SMA (E) peribronchial staining per mouse is depicted, with each point representing the mean percent staining area for an individual mouse. At baseline, peribronchial HABP staining in naive α-SMA−HAS2+ (n = 7) mice was significantly increased compared with staining in naive α-SMA−HAS2− mice (n = 6); *P < 0.05. Staining of both HABP and α-SMA surrounding the airways was significantly increased in OVA-challenged α-SMA−HAS2+ mice (n = 6) compared with OVA-challenged α-SMA−HAS2− mice (n = 6) and naive α-SMA−HAS2+ (n = 7) mice; *P < 0.05.
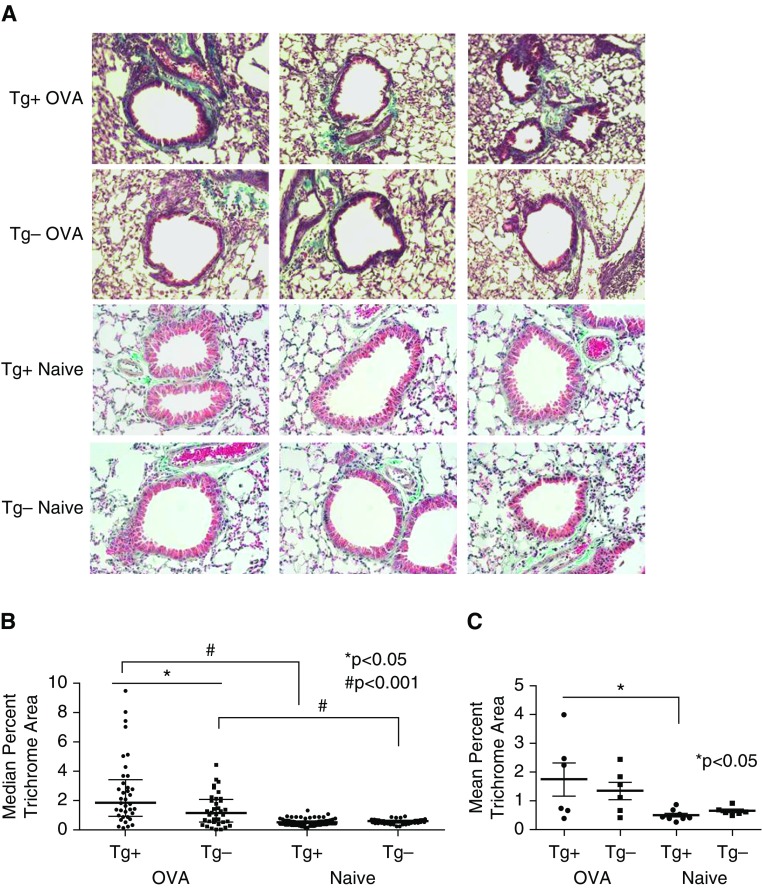
Peribronchial Trichrome Staining Is Significantly Increased in OVA-Challenged α-SMA–HAS2+ Mice
Sections of whole lung from OVA-challenged α-SMA–HAS2+ and α-SMA–HAS2− mice were stained with Masson’s trichrome stain to visualize collagen deposition around the airways. Representative images of airways from three different mice in each group are shown in Figure 4A. At baseline, unchallenged α-SMA–HAS2+ mice did not exhibit significant differences in peribronchial Masson’s trichrome staining or lung hydroxyproline content compared with α-SMA–HAS2− mice (Figures 4A–4C) (18). As expected, OVA-challenged α-SMA–HAS2− mice exhibited significantly increased peribronchial trichrome staining relative to unchallenged α-SMA–HAS2− mice (Figure 4B). Furthermore, peribronchial trichrome staining was significantly augmented in OVA-challenged α-SMA–HAS2+ mice compared with OVA-challenged α-SMA–HAS2− mice and naive α-SMA–HAS2+ mice (Figures 4B and 4C), suggesting that the chronic OVA challenge model induced airway fibrosis, as expected, but that the deposition of collagen around the airways was augmented in mice with HAS2 overexpression in myofibroblasts.
Figure 4.
Masson’s trichrome staining in naive and OVA-challenged α-SMA−HAS2+ and α-SMA−HAS2− mice. (A) Representative ×10 images of Masson’s trichrome-stained airway sections (blue staining) from three different naive (Tg+ Naive) and OVA-challenged α-SMA−HAS2+ mice (Tg+ OVA) and three different naive (Tg− Naive) and OVA-challenged α-SMA−HAS2− mice (Tg− OVA). (B) ImageJ was used to quantify the median percent of Masson’s trichrome staining per total area in 10 airways per mouse. Peribronchial trichrome staining was significantly increased in OVA-challenged α-SMA−HAS2+ mice (n = 6) compared with OVA-challenged α-SMA−HAS2− mice (n = 6; *P < 0.05) and naive α-SMA−HAS2+ mice (n = 7; #P < 0.001). Peribronchial trichrome staining was also significantly increased in OVA-challenged α-SMA−HAS2− mice (n = 6) compared with naive α-S-MA-HAS2− mice (n = 7); #P < 0.001. (C) Masson’s trichrome staining area per mouse is depicted, with each point representing the mean percent trichrome staining area for an individual mouse. When the means are compared, peribronchial trichrome staining is significantly increased in OVA-challenged α-SMA−HAS2+ mice (n = 6) compared with naive α-SMA−HAS2+ mice (n = 7); *P < 0.05.
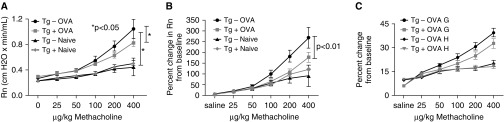
Airway Resistance Is Significantly Reduced in OVA-Challenged α-SMA–HAS2+ Mice
Chronic OVA challenge has been shown to induce significant changes in airway responsiveness to methacholine (27). We investigated whether airway Rn, tissue damping (G), tissue elastance (H), and lung compliance (C) were altered in naive or OVA-challenged α-SMA–HAS2+ mice compared with naive or OVA-challenged α-SMA–HAS2− mice. Methacholine-induced airway Rn was significantly elevated in OVA-challenged α-SMA–HAS2+ and α-SMA–HAS2− mice compared with naive α-SMA–HAS2+ and α-SMA–HAS2− mice, respectively (Figure 5A). However, the methacholine-induced elevation in Rn in OVA-challenged α-SMA–HAS2+ mice was significantly less than that observed in OVA-challenged α-SMA–HAS2− mice. Baseline Rn values were similar in all four groups, as shown in Figure 5A. The percent change from baseline Rn across all methacholine doses was significantly reduced in OVA-challenged α-SMA–HAS2+ mice compared with OVA-challenged α-SMA–HAS2− mice (Figure 5B). A trend toward the same pattern was observed for tissue damping (G) (Figure 5C). No significant difference between OVA-challenged α-SMA–HAS2+ and α-SMA–HAS2− mice was observed in the absolute or percent change from baseline for tissue elastance (H) (Figure 5C) or lung compliance (C) (data not shown).
Figure 5.
Responsiveness to methacholine in naive and OVA-challenged α-SMA−HAS2+ and α-SMA−HAS2− mice. (A) Methacholine-induced airway (Newtonian) resistance (Rn) was significantly reduced in OVA-challenged α-SMA−HAS2+ mice (n = 16) compared with OVA-challenged α-SMA−HAS2− mice (n = 13); *P < 0.05. Furthermore, methacholine-induced airway Rn was significantly elevated in OVA-challenged α-SMA−HAS2+ and α-SMA−HAS2− mice compared with naive α-SMA−HAS2+ and α-SMA−HAS2− mice; *P < 0.05. (B) The percent change from baseline in methacholine-induced airway Rn was significantly reduced in OVA-challenged α-SMA−HAS2+ mice compared with OVA-challenged HAS2− mice; P < 0.01. (C) No significant difference was observed in the percent change from baseline in methacholine-induced elastance (H) between OVA-challenged α-SMA−HAS2+ mice and OVA-challenged α-SMA−HAS2− mice; P > 0.05. The percent change from baseline in methacholine-induced tissue damping (G) was greater in OVA-challenged α-SMA−HAS2− mice compared with OVA-challenged α-SMA−HAS2+ mice; however, the change did not reach significance (P > 0.05). Data are expressed as mean ± SEM of the average peak values for each mouse at each dose of methacholine.
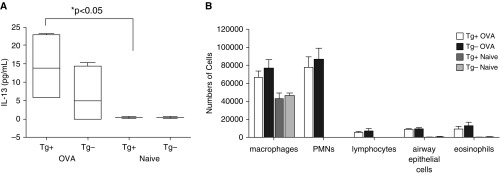
Parameters of Airway Inflammation Are Not Significantly Different between OVA-Challenged α-SMA–HAS2+ and α-SMA–HAS2− Mice
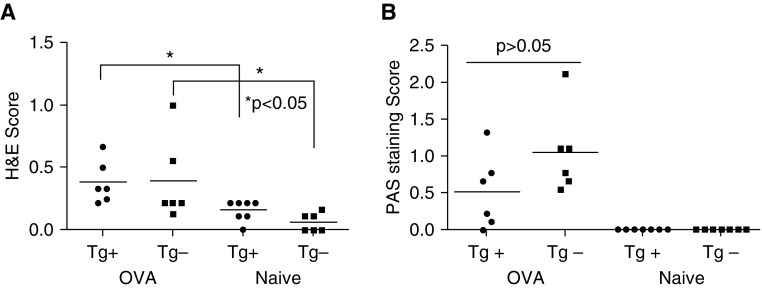
As airway inflammation and mucus production can affect responsiveness to methacholine and airway resistance, we evaluated both groups of mice for parameters of airway inflammation. First, we collected BAL fluid and measured IL-13 and IL-17 and determined the differential cell counts. Mean IL-13 levels in BAL fluid were not significantly different between OVA-challenged α-SMA–HAS2+ and α-SMA–HAS2− mice (Figure 6A). Baseline levels of IL-13 in BAL fluid of unchallenged α-SMA–HAS2+ mice were very low and significantly reduced compared with OVA-challenged α-SMA–HAS2+ mice, but they were not significantly different from those of unchallenged α-SMA–HAS2− mice (Figure 6A). In addition, baseline levels of IL-17 in BAL fluid or lung tissue homogenates were not significantly different between unchallenged α-SMA–HAS2+ and unchallenged α-SMA–HAS2− mice (Figure E1 in the online supplement). Genotype had no impact on the mean differential cell counts in BAL fluid for airway macrophages, polymorphonuclear cells, lymphocytes, airway epithelial cells, or eosinophils for either the naive or OVA-challenged condition. However, OVA treatment did significantly increase the number of each BAL cell type (except for macrophages, which were reduced) compared with BAL fluid cells from naive mice (P > 0.05 for each cell type; Figure 6B). In addition, peribronchial inflammation (visualized by hematoxylin and eosin [H&E] staining, and quantified as a score that reflects the depth and circumference of inflammatory cells associated with the airways) was significantly elevated in OVA-challenged α-SMA–HAS2+ and α-SMA–HAS2− mice compared with naive α-SMA–HAS2+ and α-SMA–HAS2− mice. However, the H&E scores were not significantly different between naive α-SMA–HAS2+ and α-SMA–HAS2− mice or OVA-challenged α-SMA–HAS2+ and α-SMA–HAS2− mice (P > 0.05; Figure 7A). In addition, airway mucin was visualized by Periodic Acid-Schiff (PAS) staining and quantified as a score that reflects the percent of the circumference that was PAS positive. Although the PAS scores were elevated in OVA-challenged α-SMA–HAS2− mice compared with OVA-challenged α-SMA–HAS2+ mice, these scores were not significantly different (P = 0.11; Figure 7B). No PAS staining was observed in the airways of naive α-SMA–HAS2+ and α-SMA–HAS2− mice.
Figure 6.
Airway IL-13 secretion and cell types in naive and OVA-challenged α-SMA−HAS2+ and α-SMA−HAS2− mice. (A) No significant differences were observed in the mean levels of IL-13 as measured by ELISA in BAL fluid obtained from naive (n = 8) or OVA-challenged α-SMA−HAS2+ mice (n = 6) compared with α-SMA−HAS2− mice (n = 6 for both naive and OVA-challenged; P > 0.05). IL-13 was significantly elevated in OVA-challenged α-SMA−HAS2+ mice compared with naive α-SMA−HAS2+ mice (*P < 0.05). (B) The mean numbers of cell types in BAL fluid from naive or OVA-challenged α-SMA−HAS2+ mice (naive, n = 8; OVA, n = 16) were not significantly different from those observed for α-SMA−HAS2− mice (naive, n = 6; OVA, n = 13); P > 0.05. PMNs, polymorphonuclear leukocyte.
Figure 7.
Airway inflammation and mucin production in naive and OVA-challenged α-SMA−HAS2+ and α-SMA−HAS2− mice. (A) No significant difference in H&E scoring of peribronchial inflammation was observed in airways of naive (n = 7) or OVA-challenged (n = 6) α-SMA−HAS2+ mice compared with α-SMA−HAS2− mice (naive, n = 6; OVA, n = 6); P > 0.05. Airway inflammation scores were significantly elevated in both groups of OVA-challenged mice compared with naive mice (*P < 0.05). (B) No significant difference in the scores of PAS staining for mucin was observed in the airways of OVA-challenged α-SMA−HAS2+ compared with α-SMA−HAS2− mice (P = 0.11), and no PAS staining was apparent in the naive mice of both groups. H&E, hematoxylin and eosin; PAS, periodic acid–Schiff.
Discussion
Airway remodeling, a hallmark of human allergic asthma, is characterized by airway wall thickening due to increased myofibroblast and smooth muscle cell proliferation (α-SMA), subepithelial fibrosis (increased collagen deposited by myofibroblasts), and goblet cell hyperplasia (mucus) (4, 5). These remodeling features are typically associated with airway lumen narrowing and thus increased AHR (5, 30).
The chronic mouse model of allergic airways disease we employed recapitulates the aforementioned lung structural changes, including increased airway basement membrane collagen deposition and increased α-SMA area (27). Chronic allergen-challenged α-SMA–HAS2+ mice displayed significantly greater airway remodeling than similarly challenged α-SMA–HAS2− mice. Unexpectedly, airway responsiveness to bronchoconstrictor was significantly reduced in allergen-challenged α-SMA–HAS2+ mice. One possible explanation for this paradox is that the enhanced airway subepithelial collagen deposition in the allergen-challenged α-SMA–HAS2+ mice may actually reduce airway responsiveness.
McParland and colleagues suggested that in airway remodeling, excessive collagen deposition stiffens the airway wall and reduces airway compliance and compressibility, and thus is protective against airway narrowing due to bronchoconstriction (30). Supporting this idea are chronic allergen exposure animal studies (31, 32) in which airway subepithelial extracellular matrix deposition was accompanied by a decrease in airway responsiveness to bronchoconstrictor.
Myofibroblasts (as well as fibroblasts and airway smooth muscle cells) release HA (33, 34), which can promote collagen synthesis and deposition (18, 35). Thus, the significantly elevated peribronchial HA production observed in the allergen-challenged α-SMA–HAS2+ mice may be driving, in part, the increased airway collagen deposition, which in turn results in stiffer airways. This airway stiffening may underlie the reduced resistance that was measured in the allergen-challenged α-SMA–HAS2+ mice in response to the bronchoconstrictor methacholine.
The effect of targeted HAS2 expression in contractile cells of the murine lung to suppress airways responsiveness does not appear to be a result of differences in allergic airway inflammation, since the numbers of inflammatory cells, tissue inflammation, and airway mucin staining, as well as HA, IL-13, and IL-17 secretion in BAL fluid, were not significantly different between the two genotypes. Increased neutrophilia and secretion of IL-17 in the airways has been associated with severe asthma (36), but in our model, neutrophils were not significantly elevated in the OVA-challenged transgene-positive mice compared with similarly treated transgene-negative mice, and at baseline, the levels of IL-17 in lung tissue were not significantly different in unchallenged α-SMA–HAS2+ mice compared with αSMA–HAS2− mice. Although the concentration of HA in BAL fluid of human asthmatics was previously reported to be significantly associated with asthma severity (20), this association was based only on annual asthma symptoms and exacerbations, and not on lung function parameters or airway responsiveness measurements. Our transgenic-mice data indicate that peribronchial HA deposition may temper the increase in airway responsiveness induced by allergen sensitization and challenge. Increased HA production in the airway tissue likely reduces the response to bronchoconstrictors through the known effect of HAS2 to promote fibrogenesis (18). In their investigation of α-SMA–HAS2+ mice, Chai and colleagues reported that overproduction of HA in the aorta leads to thinning of the elastic lamellae and increased vessel stiffness, promoting the development of atherosclerosis in the aorta (26). Similarly, Li and colleagues found that HAS2 overexpression promotes increased HA and α-SMA production by lung myofibroblasts, and is required for activation of a genetic profile that stimulates lung myofibroblast invasion, contributing to persistent lung fibrosis (18). Related to the current study, airway fibroblasts in allergic asthma have also been shown to exhibit an invasive phenotype (7) and to express increased HAS2 with increased HA production relative to cells from healthy control subjects (21). Our results support the notion that overexpression of HAS2 creates a profibrotic mesenchymal phenotype, which reveals itself in different segments of the lung depending on the injury site. Thus, in parenchymal damage by bleomycin, HAS2 overexpression by airway fibroblasts leads to parenchymal fibrosis (18). By contrast, in our allergic airway disease model, there is predominant peribronchial fibrosis leading to stiffening of the proximal (Rn) airways, whereas the parenchyma (compliance [C] and tissue elastance [H]) is unaffected.
The effect of HAS2 overexpression on airway fibrosis is clearly injury dependent, as unchallenged α-SMA–HAS2+ mice did not exhibit significantly increased peribronchial trichrome staining or lung hydroxyproline content compared with α-SMA–HAS2− mice (this study and Reference 18), even though peribronchial HA staining in these unchallenged mice was significantly increased compared with naive α-SMA–HAS2− mice. In addition, we observed that production of both peribronchial HA and α-SMA was significantly elevated in the OVA-challenged α-SMA–HAS2+ mice compared with the unchallenged α-SMA–HAS2+ mice, suggesting that allergen injury augments HA deposition surrounding the airways, which leads to increased airway fibrosis. The role of HA in promoting fibroblast differentiation and collagen deposition is complex, with contradictory reports suggesting that HA both mediates and protects against TGF-β1–induced fibrosis in specific tissue types (37–39). HA deposition by myofibroblasts is believed to occur downstream of TGF-β1 signaling (18); however, evidence from several studies indicates that HAS2-dependent production of HA mediates fibroblast-to-myofibroblast differentiation and myofibroblast phenotypic persistence (α-SMA positivity) by promoting autocrine TGF-β1 signaling (40, 41). Therefore, based on our data from the OVA-challenged α-SMA–HAS2+ mice, we conclude that increased allergen-induced peribronchial fibrosis may be mediated by a positive-feedback loop consisting of paracrine-stimulated HA production in mesenchymal cells that mediates myofibroblast autocrine TGF-β1 production and α-SMA expression, which in turn contributes to cellular invasion and excessive matrix deposition surrounding the airways. Taken together, our findings and published literature suggest that HAS2 expression and HA production by airway fibroblasts may play a role in airway remodeling and lessen elevations in airway responsiveness in allergic asthma. This effect of HAS2 expression in this model of allergic airways disease is somewhat of a double-edged sword, as the beneficial reduction in AHR follows a potentially detrimental increase in airway fibrosis. The long-term effects of airway fibrosis on lung function in this model are unknown; however, in human asthma, airway fibrosis contributes to diminished lung function over time (8, 9). Therefore, chronically high levels of HAS2 expression in the airways may be ultimately detrimental in human asthma.
HA is a key component of the extracellular matrix, which exists as a high-molecular-weight polymer in healthy lung tissue. Much research has shown that low-molecular-weight fragments of HA, generated through HA degradation by hyaluronidases or oxidative stress in the lung, are proinflammatory and profibrotic (35, 42–44). We have not yet determined the molecular size of the HA in tissues or secretions of OVA-challenged α-SMA–HAS2+ mice or the effect of allergen challenge on HA degradation. Previous studies with α-SMA–HAS2+ mice indicated that the molecular weight of HA produced by tissues in transgene-positive mice is similar to that observed in α-SMA–HAS2− mice (26), and the data herein support this conclusion because we observed no difference in our assessments of airway inflammation. However, we do not yet know whether OVA challenge causes degradation of HA in α-SMA–HAS2+ mice, or whether allergen-induced HA fragmentation would affect airway responsiveness. Indeed, it is difficult to postulate a scenario in which increased proinflammatory low-molecular-weight HA in α-SMA–HAS2+ mice would reduce AHR. OVA challenge is associated with a shift from high-molecular-weight to low-molecular-weight moieties in whole lung homogenates, but more work needs to be done to determine the actual sizes of HA in allergen-challenge models (23).
In conclusion, we have shown that in a murine model of allergic airways disease, transgenic targeted HAS2 overexpression in myofibroblasts and smooth muscle cells produces increased lung HA and TGF-β1, and is associated with airway remodeling characterized by increased peribronchial HA and collagen deposition, as well as partial protection from AHR. Elevated expression of HAS2 by airway fibroblasts, as has been shown in allergic asthma (21), may regulate both airway remodeling and responsiveness in asthma and serve as a potential biomarker or therapeutic target for allergic asthma.
Footnotes
Supported by the National Heart, Lung, and Blood Institute, National Institutes of Health (HL-05-009), and in part by funding from the Division of Intramural Research, National Institute of Environmental Health Sciences.
Author Contributions: Conception and design of experiments: J.K.L.W., D.J., P.W.N., and J.L.I. Acquisition, analysis, and interpretation of data: J.K.L.W., B.S.T., M.G., C.S.T., J.E.W., V.L.M., J.L., and J.L.I. Drafting of the manuscript for important intellectual content: J.K.L.W., S.G., M.K., and J.L.I.
This article has a data supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2017-0095OC on August 8, 2017
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Kudo M, Ishigatsubo Y, Aoki I. Pathology of asthma. Front Microbiol. 2013;4:263. doi: 10.3389/fmicb.2013.00263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Trejo Bittar HE, Yousem SA, Wenzel SE. Pathobiology of severe asthma. Annu Rev Pathol. 2015;10:511–545. doi: 10.1146/annurev-pathol-012414-040343. [DOI] [PubMed] [Google Scholar]
- 3.Berair R, Brightling CE. Asthma therapy and its effect on airway remodelling. Drugs. 2014;74:1345–1369. doi: 10.1007/s40265-014-0250-4. [DOI] [PubMed] [Google Scholar]
- 4.Gras D, Chanez P, Vachier I, Petit A, Bourdin A. Bronchial epithelium as a target for innovative treatments in asthma. Pharmacol Ther. 2013;140:290–305. doi: 10.1016/j.pharmthera.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 5.Bento AM, Hershenson MB. Airway remodeling: potential contributions of subepithelial fibrosis and airway smooth muscle hypertrophy/hyperplasia to airway narrowing in asthma. Allergy Asthma Proc. 1998;19:353–358. doi: 10.2500/108854198778612672. [DOI] [PubMed] [Google Scholar]
- 6.Firszt R, Francisco D, Church TD, Thomas JM, Ingram JL, Kraft M. Interleukin-13 induces collagen type-1 expression through matrix metalloproteinase-2 and transforming growth factor-β1 in airway fibroblasts in asthma. Eur Respir J. 2014;43:464–473. doi: 10.1183/09031936.00068712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ingram JL, Huggins MJ, Church TD, Li Y, Francisco DC, Degan S, Firszt R, Beaver DM, Lugogo NL, Wang Y, et al. Airway fibroblasts in asthma manifest an invasive phenotype. Am J Respir Crit Care Med. 2011;183:1625–1632. doi: 10.1164/rccm.201009-1452OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lange P, Parner J, Vestbo J, Schnohr P, Jensen G. A 15-year follow-up study of ventilatory function in adults with asthma. N Engl J Med. 1998;339:1194–1200. doi: 10.1056/NEJM199810223391703. [DOI] [PubMed] [Google Scholar]
- 9.Ward C, Johns DP, Bish R, Pais M, Reid DW, Ingram C, Feltis B, Walters EH. Reduced airway distensibility, fixed airflow limitation, and airway wall remodeling in asthma. Am J Respir Crit Care Med. 2001;164:1718–1721. doi: 10.1164/ajrccm.164.9.2102039. [DOI] [PubMed] [Google Scholar]
- 10.Busse WW. The relationship of airway hyperresponsiveness and airway inflammation: airway hyperresponsiveness in asthma: its measurement and clinical significance. Chest. 2010;138(2) Suppl:4S–10S. doi: 10.1378/chest.10-0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fraser JR, Laurent TC, Laurent UB. Hyaluronan: its nature, distribution, functions and turnover. J Intern Med. 1997;242:27–33. doi: 10.1046/j.1365-2796.1997.00170.x. [DOI] [PubMed] [Google Scholar]
- 12.Jiang D, Liang J, Noble PW. Hyaluronan as an immune regulator in human diseases. Physiol Rev. 2011;91:221–264. doi: 10.1152/physrev.00052.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Monslow J, Govindaraju P, Puré E. Hyaluronan—a functional and structural sweet spot in the tissue microenvironment. Front Immunol. 2015;6:231. doi: 10.3389/fimmu.2015.00231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Galloway JL, Jones SJ, Mossey PA, Ellis IR. The control and importance of hyaluronan synthase expression in palatogenesis. Front Physiol. 2013;4:10. doi: 10.3389/fphys.2013.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.D’Agostino A, Stellavato A, Busico T, Papa A, Tirino V, Papaccio G, La Gatta A, De Rosa M, Schiraldi C. In vitro analysis of the effects on wound healing of high- and low-molecular weight chains of hyaluronan and their hybrid H-HA/L-HA complexes. BMC Cell Biol. 2015;16:19. doi: 10.1186/s12860-015-0064-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shimada M, Yanai Y, Okazaki T, Noma N, Kawashima I, Mori T, Richards JS. Hyaluronan fragments generated by sperm-secreted hyaluronidase stimulate cytokine/chemokine production via the TLR2 and TLR4 pathway in cumulus cells of ovulated COCs, which may enhance fertilization. Development. 2008;135:2001–2011. doi: 10.1242/dev.020461. [DOI] [PubMed] [Google Scholar]
- 17.Colombaro V, Jadot I, Declèves AE, Voisin V, Giordano L, Habsch I, Malaisse J, Flamion B, Caron N. Lack of hyaluronidases exacerbates renal post-ischemic injury, inflammation, and fibrosis. Kidney Int. 2015;88:61–71. doi: 10.1038/ki.2015.53. [DOI] [PubMed] [Google Scholar]
- 18.Li Y, Jiang D, Liang J, Meltzer EB, Gray A, Miura R, Wogensen L, Yamaguchi Y, Noble PW. Severe lung fibrosis requires an invasive fibroblast phenotype regulated by hyaluronan and CD44. J Exp Med. 2011;208:1459–1471. doi: 10.1084/jem.20102510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lauer ME, Dweik RA, Garantziotis S, Aronica MA. The rise and fall of hyaluronan in respiratory diseases. Int J Cell Biol. 2015;2015:712507. doi: 10.1155/2015/712507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bousquet J, Chanez P, Lacoste JY, Enander I, Venge P, Peterson C, Ahlstedt S, Michel FB, Godard P. Indirect evidence of bronchial inflammation assessed by titration of inflammatory mediators in BAL fluid of patients with asthma. J Allergy Clin Immunol. 1991;88:649–660. doi: 10.1016/0091-6749(91)90159-l. [DOI] [PubMed] [Google Scholar]
- 21.Liang J, Jiang D, Jung Y, Xie T, Ingram J, Church T, Degan S, Leonard M, Kraft M, Noble PW. Role of hyaluronan and hyaluronan-binding proteins in human asthma. J Allergy Clin Immunol. 2011;128:403–411.e3. doi: 10.1016/j.jaci.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheng G, Swaidani S, Sharma M, Lauer ME, Hascall VC, Aronica MA. Hyaluronan deposition and correlation with inflammation in a murine ovalbumin model of asthma. Matrix Biol. 2011;30:126–134. doi: 10.1016/j.matbio.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheng G, Swaidani S, Sharma M, Lauer ME, Hascall VC, Aronica MA. Correlation of hyaluronan deposition with infiltration of eosinophils and lymphocytes in a cockroach-induced murine model of asthma. Glycobiology. 2013;23:43–58. doi: 10.1093/glycob/cws122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weigel PH, Hascall VC, Tammi M. Hyaluronan synthases. J Biol Chem. 1997;272:13997–14000. doi: 10.1074/jbc.272.22.13997. [DOI] [PubMed] [Google Scholar]
- 25.Jacobson A, Brinck J, Briskin MJ, Spicer AP, Heldin P. Expression of human hyaluronan synthases in response to external stimuli. Biochem J. 2000;348:29–35. [PMC free article] [PubMed] [Google Scholar]
- 26.Chai S, Chai Q, Danielsen CC, Hjorth P, Nyengaard JR, Ledet T, Yamaguchi Y, Rasmussen LM, Wogensen L. Overexpression of hyaluronan in the tunica media promotes the development of atherosclerosis. Circ Res. 2005;96:583–591. doi: 10.1161/01.RES.0000158963.37132.8b. [DOI] [PubMed] [Google Scholar]
- 27.Lin R, Degan S, Theriot BS, Fischer BM, Strachan RT, Liang J, Pierce RA, Sunday ME, Noble PW, Kraft M, et al. Chronic treatment in vivo with β-adrenoceptor agonists induces dysfunction of airway β(2) -adrenoceptors and exacerbates lung inflammation in mice. Br J Pharmacol. 2012;165:2365–2377. doi: 10.1111/j.1476-5381.2011.01725.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lazrak A, Creighton J, Yu Z, Komarova S, Doran SF, Aggarwal S, Emala CW, Sr, Stober VP, Trempus CS, Garantziotis S, et al. Hyaluronan mediates airway hyperresponsiveness in oxidative lung injury. Am J Physiol Lung Cell Mol Physiol. 2015;308:L891–L903. doi: 10.1152/ajplung.00377.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pitchford SC, Riffo-Vasquez Y, Sousa A, Momi S, Gresele P, Spina D, Page CP. Platelets are necessary for airway wall remodeling in a murine model of chronic allergic inflammation. Blood. 2004;103:639–647. doi: 10.1182/blood-2003-05-1707. [DOI] [PubMed] [Google Scholar]
- 30.McParland BE, Macklem PT, Pare PD. Airway wall remodeling: friend or foe? J Appl Physiol (1985) 2003;95:426–434. doi: 10.1152/japplphysiol.00159.2003. [DOI] [PubMed] [Google Scholar]
- 31.Palmans E, Kips JC, Pauwels RA. Prolonged allergen exposure induces structural airway changes in sensitized rats. Am J Respir Crit Care Med. 2000;161:627–635. doi: 10.1164/ajrccm.161.2.9902094. [DOI] [PubMed] [Google Scholar]
- 32.Vanacker NJ, Palmans E, Pauwels RA, Kips JC. Fluticasone inhibits the progression of allergen-induced structural airway changes. Clin Exp Allergy. 2002;32:914–920. doi: 10.1046/j.1365-2222.2002.01394.x. [DOI] [PubMed] [Google Scholar]
- 33.Klagas I, Goulet S, Karakiulakis G, Zhong J, Baraket M, Black JL, Papakonstantinou E, Roth M. Decreased hyaluronan in airway smooth muscle cells from patients with asthma and COPD. Eur Respir J. 2009;34:616–628. doi: 10.1183/09031936.00070808. [DOI] [PubMed] [Google Scholar]
- 34.Wilkinson TS, Potter-Perigo S, Tsoi C, Altman LC, Wight TN. Pro- and anti-inflammatory factors cooperate to control hyaluronan synthesis in lung fibroblasts. Am J Respir Cell Mol Biol. 2004;31:92–99. doi: 10.1165/rcmb.2003-0380OC. [DOI] [PubMed] [Google Scholar]
- 35.Li Y, Rahmanian M, Widström C, Lepperdinger G, Frost GI, Heldin P. Irradiation-induced expression of hyaluronan (HA) synthase 2 and hyaluronidase 2 genes in rat lung tissue accompanies active turnover of HA and induction of types I and III collagen gene expression. Am J Respir Cell Mol Biol. 2000;23:411–418. doi: 10.1165/ajrcmb.23.3.4102. [DOI] [PubMed] [Google Scholar]
- 36.Al-Ramli W, Préfontaine D, Chouiali F, Martin JG, Olivenstein R, Lemière C, Hamid Q. T(H)17-associated cytokines (IL-17A and IL-17F) in severe asthma. J Allergy Clin Immunol. 2009;123:1185–1187. doi: 10.1016/j.jaci.2009.02.024. [DOI] [PubMed] [Google Scholar]
- 37.Albeiroti S, Soroosh A, de la Motte CA. Hyaluronan’s role in fibrosis: a pathogenic factor or a passive player? BioMed Res Int. 2015;2015:790203. doi: 10.1155/2015/790203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meran S, Luo DD, Simpson R, Martin J, Wells A, Steadman R, Phillips AO. Hyaluronan facilitates transforming growth factor-β1-dependent proliferation via CD44 and epidermal growth factor receptor interaction. J Biol Chem. 2011;286:17618–17630. doi: 10.1074/jbc.M111.226563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Evanko SP, Potter-Perigo S, Petty LJ, Workman GA, Wight TN. Hyaluronan controls the deposition of fibronectin and collagen and modulates TGF-β1 induction of lung myofibroblasts. Matrix Biol. 2015;42:74–92. doi: 10.1016/j.matbio.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Midgley AC, Rogers M, Hallett MB, Clayton A, Bowen T, Phillips AO, Steadman R. Transforming growth factor-β1 (TGF-β1)-stimulated fibroblast to myofibroblast differentiation is mediated by hyaluronan (HA)-facilitated epidermal growth factor receptor (EGFR) and CD44 co-localization in lipid rafts. J Biol Chem. 2013;288:14824–14838. doi: 10.1074/jbc.M113.451336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Webber J, Meran S, Steadman R, Phillips A. Hyaluronan orchestrates transforming growth factor-β1-dependent maintenance of myofibroblast phenotype. J Biol Chem. 2009;284:9083–9092. doi: 10.1074/jbc.M806989200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Black KE, Collins SL, Hagan RS, Hamblin MJ, Chan-Li Y, Hallowell RW, Powell JD, Horton MR. Hyaluronan fragments induce IFNβ via a novel TLR4-TRIF-TBK1-IRF3-dependent pathway. J Inflamm (Lond) 2013;10:23. doi: 10.1186/1476-9255-10-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ghosh S, Hoselton SA, Wanjara SB, Carlson J, McCarthy JB, Dorsam GP, Schuh JM. Hyaluronan stimulates ex vivo B lymphocyte chemotaxis and cytokine production in a murine model of fungal allergic asthma. Immunobiology. 2015;220:899–909. doi: 10.1016/j.imbio.2015.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ormiston ML, Slaughter GR, Deng Y, Stewart DJ, Courtman DW. The enzymatic degradation of hyaluronan is associated with disease progression in experimental pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2010;298:L148–L157. doi: 10.1152/ajplung.00097.2009. [DOI] [PubMed] [Google Scholar]