Abstract
Successful repair and renewal of alveolar epithelial cells (AECs) are critical in prohibiting the accumulation of myofibroblasts in pulmonary fibrogenesis. MicroRNAs (miRNAs) are multifocal regulators involved in lung injury and repair. However, the contribution of miRNAs to AEC2 renewal and apoptosis is incompletely understood. We report that miRNA-29c (miR-29c) expression is lower in AEC2s of individuals with idiopathic pulmonary fibrosis than in healthy lungs. Epithelial cells overexpressing miR-29c show higher proliferative rates and viability. miR-29c protects epithelial cells from apoptosis by targeting forkhead box O3a (Foxo3a). Both overexpression of miR-29c conventionally and AEC2s specifically lead to less fibrosis and better recovery in vivo. Furthermore, deficiency of miR-29c in AEC2s results in higher apoptosis and reduced epithelial renewal. Interestingly, a gene network including a subset of apoptotic genes was coregulated by both Toll-like receptor 4 and miR-29c. Taken together, miR-29c maintains epithelial integrity and promotes recovery from lung injury, thereby attenuating lung fibrosis in mice.
Keywords: pulmonary fibrosis, microRNA-29c, alveolar epithelial type II cell renewal, alveolar epithelial type II cell apoptosis
Clinical Relevance
We documented that human alveolar epithelial type II cells (AEC2s) can express microRNA (miR)-29c, and demonstrated that miR-29c can protect AEC2 apoptosis, therefore alleviating fibrosis. This knowledge advances the field by showing that miR-29 not only is an important fibro-microRNA in fibroblasts but also maintains epithelial integrity and promotes epithelial renewal, thereby attenuating lung fibrosis.
A large number of diseases have a fibrotic pathology, and fibrosis can affect multiple organs and tissues (1). Idiopathic pulmonary fibrosis (IPF) is a devastating disease, with limited U.S. Food and Drug Administration–approved drugs that only slow disease progression (2, 3). Epithelial defects caused by chronic and repetitive microinjury are prominently recognized as the key mechanism for severe fibrosis in individuals with IPF (4). Activation, migration, and invasion of myofibroblasts, together with deposition of extracellular matrix (ECM), were proposed as subsequent results in this self-sustaining and potentially inflammation-independent disease (2). Focal epithelial destruction and occurrence of type II alveolar epithelial cell (AEC) apoptosis are acknowledged as critical hallmarks of IPF, independent of abnormal proliferation and accumulation of fibroblasts, variegated fibrotic foci or patches, and substantial deposition of ECM components (4, 5). The complex molecular network of proliferation, differentiation, spreading, apoptosis, senescence, genetic susceptibility, epigenetic alteration, mechanical damage, innate immune, ECM, environmental exposure, and the aging process in epithelial cells, as well as epithelial interplay with other cell types, could be shaped by the regulation of microRNAs (miRNAs) (6–9). It is well known that miRNAs are a type of conserved, multifocal regulator proven to be potential therapeutic interventions.
miRNAs are 22- to 25-nucleotide-long, noncoding RNAs that mediate mRNA cleavage, translational repression or mRNA destabilization. Its biogenesis begins with Drosha, RNase III endonucleases, cleaving primary (pri)-miRNAs to pre-miRNAs in the nucleus. Subsequently, pre-miRNAs are catalyzed by another RNase III, Dicer, to from mature miRNAs in the cytoplasm (10, 11). Aberrant miRNA expression profiles have been identified in interstitial lung diseases (12), in the serum of individuals with IPF (13), and in different stages of pulmonary fibrosis in bleomycin-treated mouse models (14). miRNAs have been found to regulate the activation, proliferation, differentiation, migration, apoptosis, senescence, and mesenchymal properties of myofibroblasts or fibroblasts in the lung (15–18). They target or interplay with multiple pathways, including transforming growth factor (TGF)-β and mothers against decapentaplegic homolog 3 (SMAD3) (19), wingless-type (Wnt), and β-catenin (20), and many more in lung fibroblasts. The investigation of miRNAs in lung epithelial biology is still in its infancy.
The miRNA-29 (miR-29) family includes miR-29a, miR-29b, and miR-29c. Among them, miR-29b2 and miR29c are on chromosome 1q32.2, and they are transcribed together. These miR-29s are regarded as important regulators in several diseases and organs (21, 22), specifically fibrotic diseases. miR-29 family members are perceived as “master fibro-miRNA” regulators (23, 24). It has been reported that expressions of miR-29b and -c were decreased in bleomycin-treated mouse lungs and TGF-β1–treated lung fibroblasts. miR-29 represses collagen genes and mediates ECM-associated and fibrogenesis genes in fibroblast cell lines (24, 25). There are reports on the role of miR-29 in lung fibroblasts, showing that miR-29 can mediate TGF-β1–induced ECM synthesis through activation of the phosphoinositide 3-kinase (PI3K)–protein kinase B (AKT) pathway, and increases apoptosis sensitivity via modulation of the cell-surface death receptor, Fas (15, 26). An aberrant protein phosphatase 2A/histone deacetylase C4/miR-29 signal axis can regulate collagen I expression in IPF fibroblasts (27). In regard to lung epithelial cells, miR-29 family members were found to be expressed in fetal lung AEC2s, and are thyroid transcription factor (TTF)-1–driven mediators of surfactant protein (SP)-A expression and AEC2 differentiation through repression of TGF-β signaling (28). However, the role of miR-29 in AEC2s in the adult lung has not been explored.
To determine the role of miR-29 in lung epithelial cells, we first demonstrated that miR-29c expression was down-regulated during the fibrotic phase in the mouse bleomycin model of pulmonary fibrosis and in AEC2s of individuals with IPF. We provide in vitro and in vivo proofs that miR-29c promotes the proliferation and viability of AEC2s, and inhibits the apoptosis of epithelial cells with various injuries. We further generated new mouse lines and performed gain- and loss-of-function studies to ascertain the roles of miR-29c in epithelial protection and in attenuation of lung fibrosis. Together, these data demonstrate a critical role of epithelial miR-29c in preventing fibrosis by targeting the alveolar epithelial compartment.
Materials and Methods
Mice
All animal experiments were permitted by the Institutional Animal Care and Use Committee at Duke University (Durham, NC) and Cedars-Sinai Medical Center (Los Angeles, CA) (protocols IACUC004722 and IACUC004751, respectively).
Generation of miR-29c-Overexpression-Stable Cell Line
The miR-29c overexpression mouse epithelial cell line was generated based on a previously described strategy (29, 30).
Generation of miR-29c-Overexpression-Transgenic Mice
Lentivirus vector harboring pre-mmu-miR-29c (pSico-miR-29c) was linearized and was injected to C57Bl/6J mouse embryos to generate conditional miR-29c–overexpression–transgenic (miR-29cTg) mice. These mice express green fluorescent protein (GFP) in all cell types, as confirmed by immunofluorescence staining shown in Figure E4 in the online supplement. Upon crossing with cre recombinase (Cre) mouse lines, miR-29c starts to overexpress.
Human Lung Samples
All experiments using human lung samples were approved by the Cedars-Sinai Medical Center Institutional Review Board and were in agreement with the instructions defined by the Board (IRB: Pro00035396). All subjects gave written informed consent.
Mouse Lung Fibrosis Model
Bleomycin instillation was described previously (2). Under anesthesia, 2.5 U/kg bleomycin (Hospira, Lake Forest, IL) in saline was injected into the mouse trachea with a 25-guage needle inserted between the cartilaginous rings of the trachea. Control animals received saline alone. The tracheostomy site was closed by wound clip, and the animals were allowed to recover. Mouse lungs were collected at different time points for experiments.
Statistical Analysis
Data are presented as the mean (±SEM). Student’s t tests (two-tailed) or Wilcoxon’s rank-sum test was used for nonparametric two comparisons. One-way ANOVA with Bonferroni test or Kruskal-Wallis test was performed for multiple comparisons. Ordinary two-way ANOVA with Sidak’s multiple comparisons test was conducted for grouped data sets. Log-rank tests were performed to compare the survival differences. Results were considered statistically significant at P less than or equal to 0.05. Statistical analysis was done with GraphPad Prism software (GraphPad Software Inc., La Jolla, CA).
Results
miR-29c Expression Is Detected in AEC2s and Is Lower in Fibrotic Lung Tissues
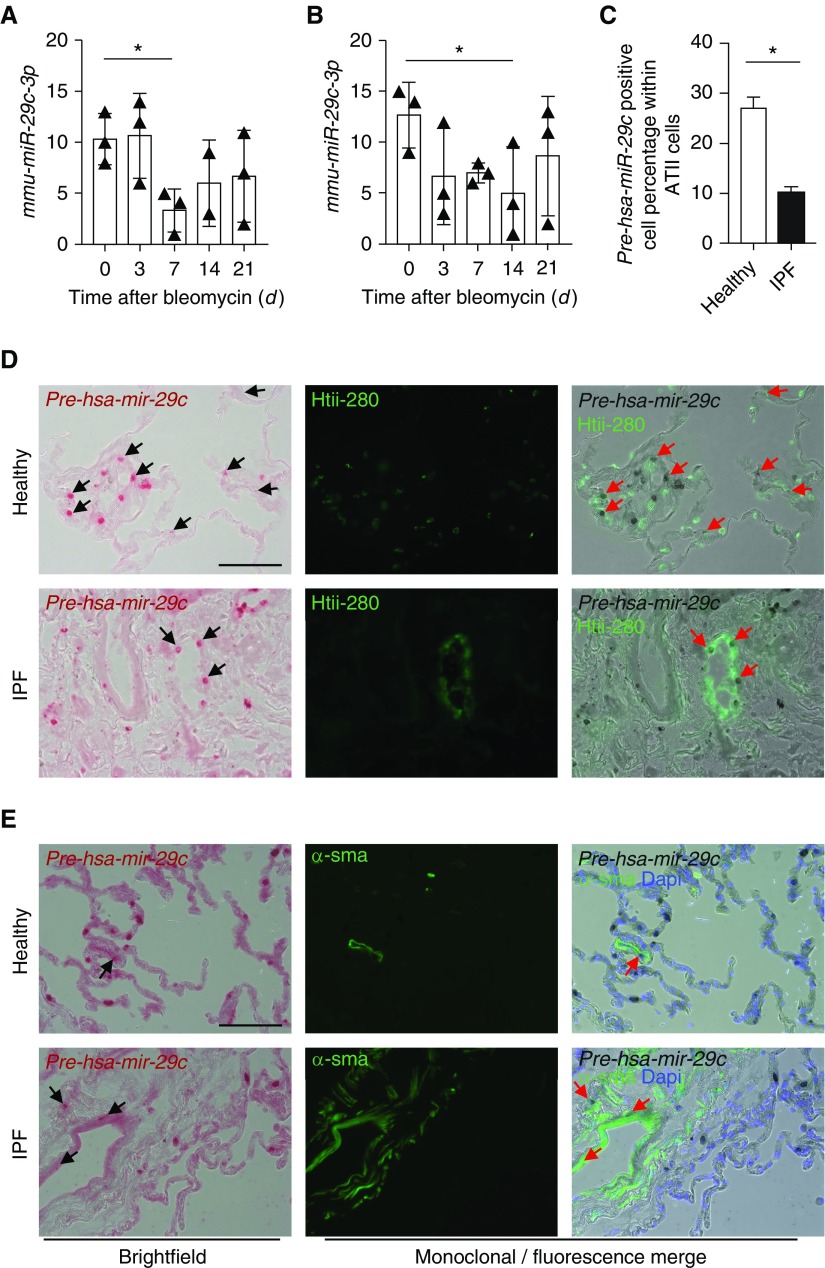
To explore the expression pattern of mature miR-29c, we performed miRNA array analysis on mouse lung tissue after bleomycin-induced injury. Expression of miR-29c-3p was decreased starting from Day 7 after injury when compared with untreated mice (Figure 1A). Confirmation of miR-29c-3p expression by quantitative RT-PCR revealed that its expression was down-regulated from Day 7 after injury and remained at low levels during the fibrotic phase (Figure 1B). The decreased expression of miR-29c-3p in fibrotic lung tissue found in our studies is consistent with others (24, 31). To gain more insight on the expression and location of miR-29c in human lung, we detected miR-29c precursor expression by using the Basescope in situ hybridization assay (Advanced Cell Diagnostics, Inc., Newark, CA). miR-29c precursors were found in human type II (HTII)-280+ AEC2s from explant lung tissues of healthy individuals or those with IPF. The percentage of dual pre-miR-29c + HTII-280+ AEC2s within total HTII-280+ AEC2s were significantly fewer in fibrotic areas of lung tissue from individuals with IPF (Figures 1C and 1D). The percentages of pre-miR-29c+ AEC2s are similar between nonfibrotic and fibrotic region in individuals with IPF (Figures E1A and E1B). The colocalization of miR-29c precursor and α-smooth muscle actin+ were found in both non-IPF and IPF lung tissue (Figure 1E), which is consistent with other studies (32). Thus, the fibrotic phenotype may be dependent on miR-29c deficiency in AEC2s.
Figure 1.
microRNA (miRNA)-29 (miR-29) c expression in mouse and human lungs. (A) mmu-miR-29c-3p expression in the lungs of untreated and bleomycin-treated wild-type (WT) mice at Days 3, 7, 14, and 21 after bleomycin treatment, as examined by miRNA array (n = 3 mice per group for Day [d] 3, d7, and d21, and n = 2 in the d14 group). (B) mmu-miR-29c-3p expression in the lungs of bleomycin-treated WT mouse lung at indicated time points by quantitative RT-PCR (n = 3 per group). (C) pre-has-miR-29c expression in alveolar epithelial cells (AECs) type 2 from normal (n = 4) and idiopathic pulmonary fibrosis (IPF) lungs (n = 7). (D) Representative images of colocalization of pre-has-miR-29c and AEC2s (human type II [HTII]-280+) in lung sections from normal (n = 4) and IPF (n = 7) by Basescope and immunofluorescence analysis. (E) Representative images of colocalization of pre-has-miR-29c and myofibroblasts (α-smooth muscle actin [α-SMA+]) from normal (n = 4) and IPF (n = 7) lungs by Basescope and immunofluorescence analysis. Arrows show coexpressing cells. *P ≤ 0.05, by one-way ANOVA (A and B); Student’s t test (C); rank values with mean (±SEM) (A and B); mean (±SEM) (C). Scale bars: 100 μm.
miR-29c Promotes Epithelial Cell Proliferation
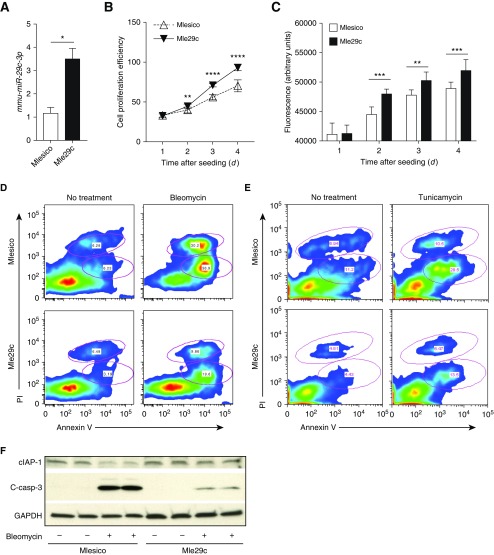
To decipher the function of miR-29c on epithelial proliferation in vitro, we first established a mouse epithelial cell line overexpressing miR-29c using the same strategy as described previously (29). Briefly, miR-29c–overexpressing epithelial cell line was generated by transducing lentivirus harboring pre-mmu-miR-29c (pSico-miR-29c) or empty lentivirus (pSico) to mouse lung epithelial cell line (MLE12) cells. One copy of pre-mmu-miR-29c gene was inserted following the cytomegalovirus (CMV)-GFP cassette in a pSico vector (30). The presence of Cre enables the removal of the CMV-GFP cassette and triggers the expression of miR-29c (Figure E2A). The confirmation of Cre-activated GFP removal was analyzed by PCR, as shown by the bands indicating the recombination and unrecombination of DNA (Figure E2B). Elevated levels of miR-29c were confirmed in cells overexpressing miR-29c (Figure 2A). To test the assumption that the gain of miR-29c function would increase epithelial proliferation and viability, we performed the proliferation and viability assays. As compared with mouse lung epithelial control cells (Mlesico), mouse lung epithelial pre-mmu-miR29c over-expressing cells (Mle29c) demonstrated significantly enhanced cellular growth (Figure 2B) and higher cellular viability (Figure 2C). These in vitro data demonstrate that miR-29c promotes the growth and survival of lung epithelial cells.
Figure 2.
miR-29c overexpression promotes epithelial cell proliferation and inhibits apoptosis. (A) Verification of miR-29c overexpression in stable mouse epithelial cell line tested by quantitative RT (qRT)-PCR. (B) Cell proliferation assay for mouse lung epithelial control cells (Mlesico) and mouse lung epithelial pre-mmu-miR29c over-expressing cells (Mle29c) in medium containing 10% FBS. (C) Viability of Mlesico and Mle29c cells after 4 days of growth in 10% FBS medium. (D) Representative flow cytometry analysis of apoptotic and necrotic cells gated from Mlesico and Mle29c epithelial cells with or without bleomycin treatment (250 ng/ml). (E) Representative flow cytometry analysis of apoptotic and necrotic cells from Mlesico and Mle29c cells with or without tunicamycin (5 ng/ml) treatment. (F) Representative Western blot analysis for cellular inhibitor of apoptosis 1 (cIAP1) and cleaved-caspase (c-casp)-3 in Mlesico and Mle29c cells with or without bleomycin (125 ng/ml) treatment. Representative of three independent experiments (n = 5). *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, ****P ≤ 0.0001, by Student’s t test (A) or two-way ANOVA (B and C). Data presented are means (±SEM). PI, propidium iodide.
miR-29c Inhibits Bleomycin-induced Epithelial Apoptosis
We further investigated the apoptotic response of miR-29c overexpression in epithelial cells to bleomycin treatment in vitro. Treatment with bleomycin induced a higher proportion of apoptotic cells (annexin V+) and necrotic cells (propidium iodide [PI]+). However, these effects were dampened by miR-29c overexpression (Figure 2D, Figures E3A and E3B). Cellular Inhibitor of apoptosis 1 (cIAP1) can inhibit apoptosis and regulates caspases. Expression of cIAP1 was decreased after bleomycin treatment, and we found that miR-29c overexpression in Mle29c cells led to restoration of cIAP1. Cleaved-caspase (c-casp)-3 plays a central role in activating a cascade of caspases in the execution of apoptosis. Notably, bleomycin significantly induced the c-casp-3 expression, and miR-29c overexpression dampened the induction of c-casp-3 levels by bleomycin (Figure 2F, Figures E3C and E3D). These data indicate that miR-29c functions to dampen the damage-induced epithelial apoptosis.
miR-29c Inhibits Endoplasmic Reticulum Stress–induced Epithelial Apoptosis
We further evaluated how miR-29c functions in endoplasmic reticulum (ER) stress–induced apoptosis in lung epithelial cells. Tunicamycin is an antibiotic derived from Streptomyces lysosuperificus, and contains glucosamine. It can impair the glycosylation during protein synthesis and interrupt protein folding in the ER or inhibit the secretion of some proteins from the cell, thus inducing ER stress. Tunicamycin-stimulated ER stress can trigger cell apoptosis both in vivo and in vitro (33, 34). To investigate the role of miR-29c in ER stress–induced cell apoptosis and death, we examined survival of Mlesico or Mle29c epithelial cells against tunicamycin. Epithelial cells overexpressing miR-29c showed significant resistance to ER stress–induced cell apoptosis compared with control cells, as determined by flow cytometry analysis of annexin V expression (Figure 2E, Figure E3E). There was a slight decrease in the percentage of necrotic cells in Mle29c cells over Mlesico cells (Figure E3F). These data demonstrate that miR-29c protects against ER stress–induced epithelial cell apoptosis.
Conventional Overexpression of miR-29c Reduces Pulmonary Fibrosis
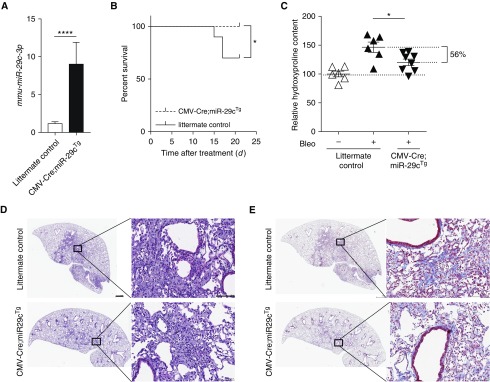
We further sought to determine whether miR-29c could protect mice from bleomycin-induced pulmonary fibrosis in vivo. Conditional miR-29c overexpression mice, termed miR-29cTg, were generated in the laboratory, as confirmed by immunofluorescence analysis of GFP expression (Figure E4). To conventionally overexpress miR-29c, we crossed miR-29cTg mice with CMV-Cre mice. We confirmed that miR-29c was overexpressed in the lungs of CMV-Cre;miR-29cTg mice when compared with the lungs of littermate control mice (Figure 3A). Notably, miR-29c mice showed a marked increase in mortality after bleomycin-induced lung injury as compared with littermate control mice (Figure 3B), and they showed a decreased fibrotic response to the same doses of bleomycin, as indicated by lower hydroxyproline content in lung tissues 21 days after bleomycin exposure (Figure 3C). Less severe fibrosis (56%) in miR-29cTg mice was also illustrated by less distorted lung structure and less collagen staining (Figures 3D and 3E). The diminished fibrotic response in conventional miR-29c–overexpressing mice led us to further investigate the cell-specific effect of miR-29c in AEC2s.
Figure 3.
Conventional miR-29c overexpression mice are less susceptible to experimental fibrosis. (A) qRT-PCR shows mmu-miR-29c-3p overexpression in miR-29c-overexpression-transgenic (cytomegalovirus [CMV]-cre recombinase (Cre);miR-29cTg) mouse lung tissue. (B) Survival curves of CMV-Cre;miR-29cTg (n = 10) and littermate control (n = 12) after bleomycin treatment. (C) Hydroxyproline contents in the lungs of CMV-Cre;miR-29cTg (n = 6) and littermate control mice with (n = 8) or without (n = 6) bleomycin treatment. (D–E) Representative images (n = 8 images per group, and 24 images in total) of hematoxylin and eosin (H&E) (D) and trichrome staining (E) of lung sections from CMV-Cre;miR-29cTg (n = 8) and littermate control (n = 8) mice harvested on Day 21 after bleomycin treatment (2.5 U/kg). Scale bars: 100 μm. *P ≤ 0.05, ****P < 0.0001 by Student’s t test (A), log-rank test (B), or one-way ANOVA (C). (D and E) Scale bars: 1 mm, 100 μm (insets). Data presented are means (±SEM).
Specific Overexpression of miR-29c in AEC2s Reduces Pulmonary Fibrosis
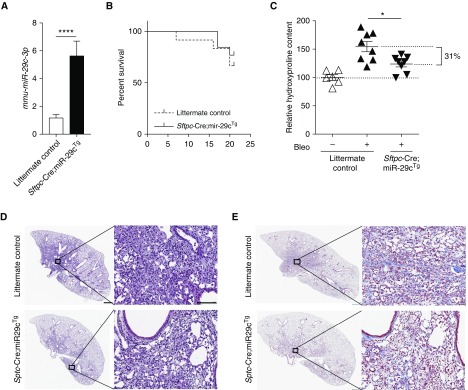
To determine whether overexpression of miR-29c in AEC2s affects the outcome of bleomycin-induced pulmonary fibrosis, we crossed miR-29cTg with Sftpc-Cre mice to generate Sftpc-Cre;miR-29cTg mice. The mature miR-29c expression in lung tissue of uninjured Sftpc-Cre;miR-29cTg mice was higher than those in the lungs of their littermates (Figure 4A). We hypothesized that higher miR-29c in AEC2s would lead to diminished susceptibility to bleomycin injury. Indeed, Sftpc-Cre;miR-29cTg mice showed 31% less lung fibrosis at Day 21 after bleomycin treatment as compared with injured littermate controls, although Sftpc-Cre;miR-29cTg mice only showed marginally better survival (Figures 4B and 4C). Sftpc-Cre;miR-29cTg mice demonstrated a less fibrodestructive response and less accumulated abundance of collagen (Figures 4D and 4E).
Figure 4.
AEC2-specific miR-29c overexpression reduces lung fibrosis. (A) qRT-PCR shows mmu-miR-29c-3p overexpression in Sftpc-Cre;miR-29cTg mouse lung tissue. (B) Survival curves of Sftpc-Cre;miR-29cTg (n = 13) and littermate control (n = 12) after bleomycin treatment. (C) Hydroxyproline contents in the lungs of Sftpc-Cre;miR-29cTg (n = 8) and littermate control mice (n = 8). (D–E) Representative images (n = 8 images per group, and 24 images in total) of H&E (D) and trichrome staining (E) of lung sections from Sftpc-Cre;miR-29cTg (n = 8) and littermate control (n = 8) mice harvested on Day 21 after bleomycin treatment (2.5 U/kg). Scale bars: 100 μm. *P ≤ 0.05, ****P ≤ 0.0001, by Student’s t test (A) or one-way ANOVA (C). (D and E) Scale bars: 1 mm, 100 μm (insets). Data presented are means (±SEM).
miR-29c Prohibits Apoptosis of AEC2s by Targeting Foxo3a
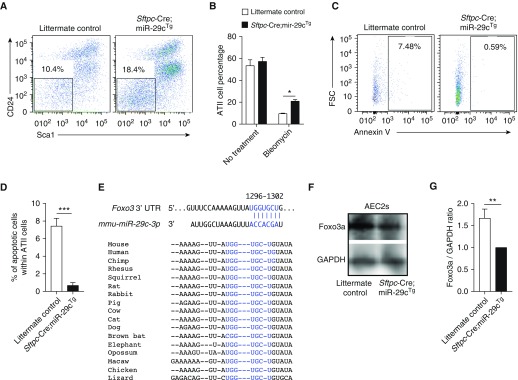
Successful recovery from lung injury requires the repair and renewal of AECs (4). We analyzed the role of miR-29c in the epithelial repair and renewal process. We employed an approach specifically designed to dissociate and fractionate lung tissue to produce and enrich AEC2 fraction, as indicated previously here. The AEC2 fraction was isolated as CD24−Sca1− subset (4, 35). The higher percentage of CD24−Sca1− AEC2s in Sftpc-Cre;miR-29cTg mice was observed at Day 14 after bleomycin injury (Figures 5A and 5B). Furthermore, we observed significantly lower levels of apoptotic CD24−Sca1− AEC2s in bleomycin-injured lungs from Sftpc-Cre;miR-29cTg mice versus WT mice (Figures 5C and 5D).
Figure 5.
miR-29c overexpression protects AEC2s from apoptosis by targeting forkhead box o (Foxo3a). (A) Representative flow cytometry analysis of AEC2s (7-Aminoactinomycin D [7AAD−]epithelial cell adhesion molecule [Epcam+]CD31/34/45−CD24−ScaI−) from Sftpc-Cre;miR-29cTg (n = 3) and littermate control (n = 3) mice 14 days after bleomycin treatment (2.5 U/kg). (B) Percentage of AEC2s from lungs of bleomycin-treated Sftpc-Cre;miR-29cTg (n = 3) and littermate control (n = 3) mice at Day 14. (C) Representative flow cytometry analysis of apoptotic AEC2s from Sftpc-Cre;miR-29cTg (n = 4) and littermate control (n = 4) mice 14 days after bleomycin treatment (2.5 U/kg). (D) Percentage of AEC2s from C. (E) Conservation of the miR-29c-3p targeting sites in the Foxo3a 3′ untranslated region (UTR). (F) Representative Western blot of Foxo3a expression in AEC2s of Sftpc-Cre;miR-29cTg (n = 3) and littermate control (n = 3) mice without challenge. GAPDH bands were from the same gels used to detect the corresponding Foxo3a bands. (G) Foxo3a/GAPDH densitometry ratios were calculated. Representative of three independent experiments. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, by Student’s t test (D and G) or two-way ANOVA (B). Data presented are means (±SEM). FSC, forward scatter.
Forkhead box o (Foxo3a) is a transcription factor that belongs to the O subclass of the forkhead family. It functions as a trigger for apoptosis through up-regulation of genes necessary for cell death and down-regulation of antiapoptotic proteins (36). TargetScan predicts that Foxo3a is a potential target for miR-29c-3p (Figure 5E). We compared AEC2s isolated from Sftpc-Cre;miR-29cTg and littermate control mice without bleomycin challenge. The AEC2s from Sftpc-Cre;miR-29cTg demonstrated decreased Foxo3a protein levels (Figures 5F and 5G). Foxo3a overexpression reduced the cell proliferation in both Mlesico and Mle29c epithelial cells (Figures E5A–E5C). The overexpression of Foxo3a also resulted in an increase in apoptosis upon bleomycin treatment in vitro (Figure E5D and E5E). Thus, Foxo3a overexpression is contrary to miR-29c overexpression phenotype in vitro in AEC2 cell line. The Foxo3a targeting effect by miR-29c is consistent with the previous report in human lung myofibroblast cells (32). Taken together, our gain-of-function transgenic mouse strategy supported an underlying role for miR-29c in the maintenance of AEC2s.
Loss of miR-29c Increases AEC2 Apoptosis and Retards AEC2 Renewal
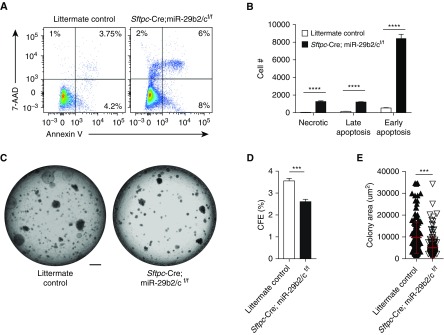
To ascertain the role of miR-29c in AEC2 function, we sought to determine whether loss of miR-29c could affect the regenerative ability of lung AEC2s. By adopting miR-29b2/cf/f mice and crossing them with Sftpc-Cre mice, we generated mouse lung epithelial–specific miR-29b2/c knockout mice (Sftpc-Cre;miR-29b2/cf/f). miR-29b2/c knockout was associated with a higher percentage of different stages of apoptotic CD24−Sca-1− AEC2s within the lung epithelial cell fraction by FACS analysis (Figure 6A) and an increased percentage of c-casp-3+ cells within Sftpc+ AEC2s (Figures E6A and E6B). There were also larger numbers of CD24−Sca-1− AEC2s at different stages of apoptosis from dissociated lung tissue of miR-29b2/c knockout mice (Figure 6B). To evaluate the role of miR-29c in AEC2 organoid growth, flow-sorted CD24−Sca-1− AEC2s were cocultured with mouse lung fibroblasts (MLg2908) embedded in matrigel medium mix for the generation of clonally derived three-dimensional organoids (Figure 6C). AEC2s isolated from Sftpc-Cre;miR-29b2/cf/f mice failed to augment in terms of organoid number (Figure 6D), and yielded substantially smaller organoids (Figure 6E). Collectively, these loss-of-function experiments suggested a protective role of miR-29c in the apoptosis and renewal of AEC2s.
Figure 6.
Loss of miR-29c in AEC2s promotes apoptosis and reduces cell renewal. (A) Representative flow cytometry analysis of apoptotic and necrotic AEC2s from nontreated Sftpc-Cre;miR-29b2/cflox/flox and littermate control mice. (B) Numbers of early apoptosis, late apoptosis, and necrotic CD24−Sca-1− AEC2s recovered from the lungs of normal Sftpc-Cre;miR-29b2/cf/f and littermate control mice. (C) Representative images of organoid culture from AEC2s of normal Sftpc-Cre;miR-29b2/cf/f and littermate control mice. (D) Colony formation efficiencies (CEFs) of CD24−Sca-1− AEC2s of normal Sftpc-Cre;miR-29b2/cf/f and littermate control mice. (E) Colony sizes of CD24−Sca-1− AEC2s from Sftpc-Cre;miR-29b2/cf/f (n = 175) and littermate control mice (n = 156). Representative of three independent experiments were performed; n = 3 in each group. ***P ≤ 0.001, ****P ≤ 0.0001, by Student’s t test (D and E) or two-way ANOVA (B). Scale bar: 1 mm. Data presented are means (±SEM).
miR-29c and Toll-Like Receptor 4 Modulates a Set of Apoptosis-Related Gene Network in Lung Epithelial Cells
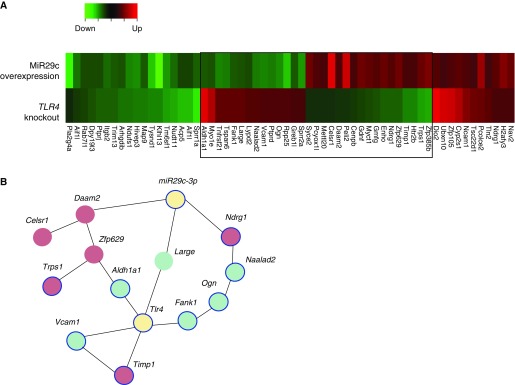
Previous studies have shown that Toll-like receptor (TLR) 4 on lung epithelial cells is critical for epithelial renewal and inhibition of apoptosis (4, 37). We therefore investigated the converging and independent genes modulated by miR-29c and TLR4. We assembled all genes that showed regulation either by miR-29c or TLR4 and analyzed for similarity (fold change of >1.5). We generated four easily distinguishable compartments of genes, elevated or decreased either by miR-29c or by TLR4 (Figure 7A). We used network analysis to determine whether miR-29c and TLR4 coordinately regulated genes interacted. The interactions among miR-29c– and TLR4-modulated genes indicate that several genes may coordinately function together. In addition, 8 out of 12 genes within the network are associated with apoptosis (Figure 7B). This regulation included both the elevated and decreased genes.
Figure 7.
Converging and independent gene regulation of miR-29c and Toll-like receptor (Tlr) 4 in lung epithelial cells. (A) Heat map shows the overlap of a differentially expressed gene set from miR-29c–overexpressing and Tlr4 knockout mouse lung epithelial cells. Blue box outlines the genes modulated by both miR-29c and Tlr4. (B) Apoptosis-associated interaction network for miR-29c and Tlr4 coordinately modulated genes. Red nodes, up-regulated genes; green nodes, down-regulated genes; blue outline, apoptosis-associated genes.
Discussion
We have explored the role of miR-29c in regulating AEC2 renewal and apoptosis after noninfectious injury–induced lung fibrosis. Our newly acquired Basescope pre-miRNA technology detected a previously unrecognized expression pattern of pre–miR-29c in human healthy and IPF type II cells. Overexpression of miR-29c increased epithelial cell proliferation and viability, and protected bleomycin and ER stress–induced epithelial apoptosis. In vivo studies further confirmed that AEC2 miR-29c–overexpressing mice showed a decrease in fibrosis upon injury, and these mice demonstrated enhanced restoration of AEC2s and inhibited epithelial apoptosis that is regulated by Foxo3a, a miR-29c target. In addition, loss of miR-29c in AEC2s revealed an elevated apoptotic response and mitigated a regenerative phenotype. Finally, miR-29c is associated with innate immune receptor TLR4 interaction network in modulating AEC2 apoptosis.
Most of the previous miR-29 studies performed in lung tissue were focused on mesenchymal cell types, especially fibroblasts (25–27, 32). Fibroblasts show reduced expression of miR-29 when growing on ECM derived from decellularized IPF lung, and miR-29 can target ECM proteins associated with IPF, suggesting that miR-29 is engaged in the vicious signaling circle orchestrated by the IPF ECM and fibroblasts (25). In this setting, polymerized type I collagen, an ECM component, interacted with IPF fibroblasts. This resulted in an abnormal protein phosphatase 2 (PP2A) function, leading to histone deacetylase 4 (HDAC4) phosphorylation and reduced nuclear translocation of HDAC4. This axis suppresses miR-29, activating the positive feedback loop, thus inducing an increase in type I collagen expression (27). In addition, miR-29 can be reduced by TGF-β1. miR-29 is also involved in prosurvival phosphoinositide 3-kinase (PI3K)–protein kinase B (Akt) signaling pathway by TGF-β1 in lung fibroblast cell lines (26). Furthermore, miR-29 expression is also detected in another mesenchymal cell type, smooth muscle cells, as it is colocalized with α-smooth muscle actin+ cells of blood vessel walls in Embryonic Day 18.5 mouse lung and of distal pulmonary artery in human lung (32). These identified cell types are consistent with studies done in abdominal aortic aneurysms (38), systemic sclerosis (39), and cardiac fibrosis (40). Aside from that, the role of miR-29 in other cell types has been explored (41–43). For example, miR-29 expression can be detected in mouse and human fetal lung AEC2s (28). The miR-29 family was found to be up-regulated in AEC2s from human fetal lung during cAMP-induced differentiation and significantly increased in adult mouse lungs compared with neonatal mouse lungs (44). Whether miR-29 is expressed in the epithelial cells of human adult lung was not yet discovered, and the role of miR-29 in epithelial cells was unknown. In our current study, we discovered that miR-29c was expressed on AEC2s in healthy and IPF human lungs. We further demonstrated that miR-29c functions as a promoter of epithelial cell renewal and inhibitor for epithelial apoptosis by gain- and loss-of-function studies in vitro and in vivo.
Of note, the abnormal ECM in fibrotic lung can alter the behavior of epithelial and mesenchymal cells, and drive progressive fibrogenesis without any further initiating trigger. Although the feedback loop of IPF ECM and miR-29 have been examined in fibroblasts (25), the contribution of ECM signaling and miRNA to the modulation of homeostasis and renewal in epithelial cells has not been well studied until now. It is plausible that, during fibrogenesis, increased ECM reduces miR-29 in lung epithelial cells. Alternatively, perturbation of the stiff environment in the fibrotic lung could diminish miR-29, thereby activating apoptosis of pulmonary epithelial cells. In addition, the regulation of miR-29c–mediated epithelial cell renewal or apoptosis by different components of the ECM, such as collagen and hyaluronic acid, needs to be investigated in the future.
Lung epithelial cell renewal and repair is essential for host survival and subsequent fibrogenesis in lung injury. Timely repair of AEC2s is critical for restoration of lung function and quality of life. Inappropriate repair can result in disrupted barrier function and promote fibrogenesis, thus leading to potentially life-threatening outcomes. miRNAs can suppress protein expression by cleavage of mRNA or translational suppression. miRNAs are major players in regulating various biological processes ranging from development to homeostasis and disease. Recent studies have identified several miRNAs that play a major role in lung epithelial injury and repair. Loss of miR34a in aged mice is beneficial in experimental lung fibrosis, and miR34a can induce cellular senescence, apoptosis, and mitochondrial aberrations in AECs (18). Indeed, expression levels of miR34 family members were higher in AEC2s from individuals with IPF, and their expression was associated with AEC2 senescence (45). miR-130b-3p was down-regulated in IPF lungs, and it can inhibit the epithelium-induced collagen 1 expression and enhance proliferation and migration ability of fibroblasts in coculture systems through insulin like growth factor 1 (IGF-1) (46). Furthermore, miR-323a-3p is down-regulated in the epithelial cells of lungs with bronchiolitis obliterans syndrome after lung transplantation, IPF, and experimental fibrotic models. It can also modulate fibrotic pathways by directly targeting TGF-α and Smad2 in TGF-β signaling and lower casp-3 expression in the epithelium. Induction of epithelial miR-323a-3p can inhibit fibroblast activation, thus revealing a protective role of this miRNA (47). Our current data showed a protective effect of miR-29c, which is essential in AEC2 renewal and apoptosis. We found that mice with miR-29c overexpression showed increased survival and decreased fibrosis. Indeed, AEC2-specific miR-29c overexpression has a similar protective effect by targeting the expression of apoptosis trigger, Foxo3a. In addition, AEC2s lacking miR-29c exhibit elevated apoptosis and deficiency of renewal. These data are, to our knowledge, the first to suggest a role for miR-29c in maintaining AEC2 function in the context of tissue fibrosis.
We defined an apoptosis associated gene network coordinately regulated by miR-29c and TLR4 that is specific for lung epithelial cells. This gene network includes Aldh1a1, Celsr1, Daam2, Fank1, Large, Ndrg1, Naalad2, Ogn, Timp1, Trps1, Vcam1, and Zfp629. Within the 12 genes, 8 genes (Trps1, Aldh1a1, Vcam1, Timp1, Fank1, Ogn, Naalad2, and Ndrg1) are previously reported to be correlated with apoptosis. Tlr4 is directly associated with Aldh1a1, Vcam1, Timp1, and Fank1, whereas miR29c is directly associated with Daam2, Large, and Ndrg2. This network includes genes both positively and negatively coexpressed, which implicate an miR-29c– and TLR4-regulated apoptosis response as a comprehensive regulatory module.
It is intriguing how miR-29 is regulated. miRNA can be regulated both transcriptionally (changes in gene expression and promoter hypermethylation) and post-transcriptionally (changes in miRNA processing), as well as by endogenous (hormones, cytokines) and exogenous (xenobiotics) compounds. miR-29 is negatively regulated by TGF-β–Smad3 signaling via inhibiting myogenic differentiation 1 (MyoD) binding and enhancing Yin and Yang 1 (YY1)-recruited Polycomb association in a muscle cell line (48). In addition, miR-29 exists in a feedback loop involving NF-κB and YY1. miR-29 is repressed by NF-κB and acts through YY1 and the Polycomb in myoblasts. During myogenesis, miR-29 is triggered to promote myogenesis by abrogating the regulatory circuit on YY1 (49). It has been shown that activation of NF-κB activated by hyaluronan and TLR2/4 in epithelial cells can induce apoptosis (37). This implies that there might be connections between hyaluronan, TLR2/4, NF-κB, and miR-29c. It will be very interesting to explore the role of miR-29c within the signaling cascade under hyraluronan- and TLR2/4-induced NF-κB activation in epithelial cells. Although this exceeds the range of this study, the dedicated process of miR-29 upstream regulation should be emphasized in future investigations.
miRNA has been reported to block pulmonary fibrosis in experimental models (31). It has previously been reported that intravenous injection of synthetic RNA duplexes can increase miR-29 levels in vivo for several days. Moreover, therapeutic delivery of miR-29 mimics during bleomycin-induced pulmonary fibrosis restored endogenous miR-29 function, decreased collagen expression, and blocked and reversed pulmonary fibrosis (50). It is conceivable that the intravenously injected miR-29 functions on multiple cell types. Our current study focused on the function of miR-29c in the epithelial cell compartment, and supports a previously unrecognized role for miR-29c in limiting the extent of the fibrosis by inhibiting AEC2 apoptosis and, potentially, by promoting AEC2 renewal.
Taken together, our results indicate that miR-29c suppresses AEC2 apoptosis and perpetuates lung epithelial renewal by potentially targeting Foxo3a, thus limiting the extent of tissue fibrosis. These studies provide new insights into the antifibrotic roles of miR-29c through its impact on AEC2s, and further support a role for this unique regulatory pathway in lung disease.
Acknowledgments
Acknowledgments
The authors thank L. Rowlette and Z. Wei (Center for Genomic and Computational Biology, Duke University, Durham, NC) for microarray analysis, and are grateful to L. Dobbs (University of California, San Francisco, CA) for providing critical reagents for the study.
Footnotes
This work was supported by National Institutes of Health grants P01-HL108793, R01-HL060539, and AI052201 (P.W.N), and R01-HL122068 (D.J.).
Author Contributions: D.J. and T.X. developed and outlined the study; T.X. conducted most of the experiments and examined the data; J.L., Y.G., N.L., A.K., and V.K. took part in mouse and cell culture experiments; T.X., D.L., N.D., Z.L., J.S., and P.C. analyzed data; T.X. wrote the manuscript; all authors discussed the data; P.W.N. and D.J. edited and agreed on the submission of the manuscript.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2017-0133OC on August 11, 2017
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Wynn TA, Ramalingam TR. Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat Med. 2012;18:1028–1040. doi: 10.1038/nm.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xie T, Liang J, Liu N, Huan C, Zhang Y, Liu W, Kumar M, Xiao R, D’Armiento J, Metzger D, et al. Transcription factor TBX4 regulates myofibroblast accumulation and lung fibrosis. J Clin Invest. 2016;126:3063–3079. doi: 10.1172/JCI85328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barkauskas CE, Cronce MJ, Rackley CR, Bowie EJ, Keene DR, Stripp BR, Randell SH, Noble PW, Hogan BL. Type 2 alveolar cells are stem cells in adult lung. J Clin Invest. 2013;123:3025–3036. doi: 10.1172/JCI68782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liang J, Zhang Y, Xie T, Liu N, Chen H, Geng Y, Kurkciyan A, Mena JM, Stripp BR, Jiang D, et al. Hyaluronan and TLR4 promote surfactant-protein-C–positive alveolar progenitor cell renewal and prevent severe pulmonary fibrosis in mice. Nat Med. 2016;22:1285–1293. doi: 10.1038/nm.4192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chambers RC, Mercer PF. Mechanisms of alveolar epithelial injury, repair, and fibrosis. Ann Am Thorac Soc. 2015;12:S16–S20. doi: 10.1513/AnnalsATS.201410-448MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Y, Frank DB, Morley MP, Zhou S, Wang X, Lu MM, Lazar MA, Morrisey EE. HDAC3-dependent epigenetic pathway controls lung alveolar epithelial cell remodeling and spreading via miR-17-92 and TGF-β signaling regulation. Dev Cell. 2016;36:303–315. doi: 10.1016/j.devcel.2015.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mathis C, Poussin C, Weisensee D, Gebel S, Hengstermann A, Sewer A, Belcastro V, Xiang Y, Ansari S, Wagner S, et al. Human bronchial epithelial cells exposed in vitro to cigarette smoke at the air–liquid interface resemble bronchial epithelium from human smokers. Am J Physiol Lung Cell Mol Physiol. 2013;304:L489–L503. doi: 10.1152/ajplung.00181.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sato F, Tsuchiya S, Meltzer SJ, Shimizu K. microRNAs and epigenetics. FEBS J. 2011;278:1598–1609. doi: 10.1111/j.1742-4658.2011.08089.x. [DOI] [PubMed] [Google Scholar]
- 9.Huang Y, Crawford M, Higuita-Castro N, Nana-Sinkam P, Ghadiali SN. miR-146a regulates mechanotransduction and pressure-induced inflammation in small airway epithelium. FASEB J. 2012;26:3351–3364. doi: 10.1096/fj.11-199240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bartel DP, Chen CZ. Micromanagers of gene expression: the potentially widespread influence of metazoan microRNAs. Nat Rev Genet. 2004;5:396–400. doi: 10.1038/nrg1328. [DOI] [PubMed] [Google Scholar]
- 11.Lee Y, Ahn C, Han J, Choi H, Kim J, Yim J, Lee J, Provost P, Rådmark O, Kim S, et al. The nuclear RNase III Drosha initiates microRNA processing. Nature. 2003;425:415–419. doi: 10.1038/nature01957. [DOI] [PubMed] [Google Scholar]
- 12.Cho JH, Gelinas R, Wang K, Etheridge A, Piper MG, Batte K, Dakhallah D, Price J, Bornman D, Zhang S, et al. Systems biology of interstitial lung diseases: integration of mRNA and microRNA expression changes. BMC Med Genomics. 2011;4:8. doi: 10.1186/1755-8794-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 13.Li P, Li J, Chen T, Wang H, Chu H, Chang J, Zang W, Wang Y, Ma Y, Du Y, et al. Expression analysis of serum microRNAs in idiopathic pulmonary fibrosis. Int J Mol Med. 2014;33:1554–1562. doi: 10.3892/ijmm.2014.1712. [DOI] [PubMed] [Google Scholar]
- 14.Xie T, Liang J, Guo R, Liu N, Noble PW, Jiang D. Comprehensive microRNA analysis in bleomycin-induced pulmonary fibrosis identifies multiple sites of molecular regulation. Physiol Genomics. 2011;43:479–487. doi: 10.1152/physiolgenomics.00222.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matsushima S, Ishiyama J. microRNA-29c regulates apoptosis sensitivity via modulation of the cell-surface death receptor, Fas, in lung fibroblasts. Am J Physiol Lung Cell Mol Physiol. 2016;311:L1050–L1061. doi: 10.1152/ajplung.00252.2016. [DOI] [PubMed] [Google Scholar]
- 16.Liu G, Friggeri A, Yang Y, Milosevic J, Ding Q, Thannickal VJ, Kaminski N, Abraham E. miR-21 mediates fibrogenic activation of pulmonary fibroblasts and lung fibrosis. J Exp Med. 2010;207:1589–1597. doi: 10.1084/jem.20100035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cui H, Ge J, Xie N, Banerjee S, Zhou Y, Antony VB, Thannickal VJ, Liu G. miR-34a inhibits lung fibrosis by inducing lung fibroblast senescence. Am J Respir Cell Mol Biol. 2017;56:168–178. doi: 10.1165/rcmb.2016-0163OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cui H, Ge J, Xie N, Banerjee S, Zhou Y, Liu RM, Thannickal VJ, Liu G. miR-34a promotes fibrosis in aged lungs by inducing alveolar epithelial dysfunctions. Am J Physiol Lung Cell Mol Physiol. 2016;312:L415–L424. doi: 10.1152/ajplung.00335.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang Y, Huang XR, Wei LH, Chung AC, Yu CM, Lan HY. miR-29b as a therapeutic agent for angiotensin II-induced cardiac fibrosis by targeting TGF-β/Smad3 signaling. Mol Ther. 2014;22:974–985. doi: 10.1038/mt.2014.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Y, Liu J, Chen J, Feng T, Guo Q. miR-29 mediates TGFβ 1–induced extracellular matrix synthesis through activation of Wnt/β-catenin pathway in human pulmonary fibroblasts. Technol Health Care. 2015;23:S119–S125. doi: 10.3233/thc-150943. [DOI] [PubMed] [Google Scholar]
- 21.Robaina MC, Mazzoccoli L, Arruda VO, Reis FR, Apa AG, de Rezende LM, Klumb CE. Deregulation of DNMT1, DNMT3B and miR-29s in Burkitt lymphoma suggests novel contribution for disease pathogenesis. Exp Mol Pathol. 2015;98:200–207. doi: 10.1016/j.yexmp.2015.03.006. [DOI] [PubMed] [Google Scholar]
- 22.Rostas JW, III, Pruitt HC, Metge BJ, Mitra A, Bailey SK, Bae S, Singh KP, Devine DJ, Dyess DL, Richards WO, et al. microRNA-29 negatively regulates EMT regulator N-myc interactor in breast cancer. Mol Cancer. 2014;13:200. doi: 10.1186/1476-4598-13-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O’Reilly S. microRNAs in fibrosis: opportunities and challenges. Arthritis Res Ther. 2016;18:11. doi: 10.1186/s13075-016-0929-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cushing L, Kuang PP, Qian J, Shao F, Wu J, Little F, Thannickal VJ, Cardoso WV, Lü J. miR-29 is a major regulator of genes associated with pulmonary fibrosis. Am J Respir Cell Mol Biol. 2011;45:287–294. doi: 10.1165/rcmb.2010-0323OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parker MW, Rossi D, Peterson M, Smith K, Sikström K, White ES, Connett JE, Henke CA, Larsson O, Bitterman PB. Fibrotic extracellular matrix activates a profibrotic positive feedback loop. J Clin Invest. 2014;124:1622–1635. doi: 10.1172/JCI71386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang T, Liang Y, Lin Q, Liu J, Luo F, Li X, Zhou H, Zhuang S, Zhang H. miR-29 mediates TGFβ1-induced extracellular matrix synthesis through activation of PI3K–AKT pathway in human lung fibroblasts. J Cell Biochem. 2013;114:1336–1342. doi: 10.1002/jcb.24474. [DOI] [PubMed] [Google Scholar]
- 27.Khalil W, Xia H, Bodempudi V, Kahm J, Hergert P, Smith K, Peterson M, Parker M, Herrera J, Bitterman PB, et al. Pathologic regulation of collagen i by an aberrant protein phosphatase 2A/histone deacetylase C4/microRNA-29 signal axis in idiopathic pulmonary fibrosis fibroblasts. Am J Respir Cell Mol Biol. 2015;53:391–399. doi: 10.1165/rcmb.2014-0150OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guo W, Benlhabib H, Mendelson CR. The microRNA 29 family promotes type II cell differentiation in developing lung. Mol Cell Biol. 2016;36:2141. doi: 10.1128/MCB.00096-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xie T, Liang J, Liu N, Wang Q, Li Y, Noble PW, Jiang D. microRNA-127 inhibits lung inflammation by targeting IgG Fcγ receptor I. J Immunol. 2012;188:2437–2444. doi: 10.4049/jimmunol.1101070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ventura A, Meissner A, Dillon CP, McManus M, Sharp PA, Van Parijs L, Jaenisch R, Jacks T. Cre-lox–regulated conditional RNA interference from transgenes. Proc Natl Acad Sci USA. 2004;101:10380–10385. doi: 10.1073/pnas.0403954101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xiao J, Meng XM, Huang XR, Chung AC, Feng YL, Hui DS, Yu CM, Sung JJ, Lan HY. miR-29 inhibits bleomycin-induced pulmonary fibrosis in mice. Mol Ther. 2012;20:1251–1260. doi: 10.1038/mt.2012.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cushing L, Costinean S, Xu W, Jiang Z, Madden L, Kuang P, Huang J, Weisman A, Hata A, Croce CM, et al. Disruption of miR-29 leads to aberrant differentiation of smooth muscle cells selectively associated with distal lung vasculature. PLoS Genet. 2015;11:e1005238. doi: 10.1371/journal.pgen.1005238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oslowski CM, Urano F. Measuring ER stress and the unfolded protein response using mammalian tissue culture system. Methods Enzymol. 2011;490:71–92. doi: 10.1016/B978-0-12-385114-7.00004-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shiraishi H, Okamoto H, Yoshimura A, Yoshida H. ER stress–induced apoptosis and caspase-12 activation occurs downstream of mitochondrial apoptosis involving Apaf-1. J Cell Sci. 2006;119:3958–3966. doi: 10.1242/jcs.03160. [DOI] [PubMed] [Google Scholar]
- 35.Chen H, Matsumoto K, Brockway BL, Rackley CR, Liang J, Lee JH, Jiang D, Noble PW, Randell SH, Kim CF, et al. Airway epithelial progenitors are region specific and show differential responses to bleomycin-induced lung injury. Stem Cells. 2012;30:1948–1960. doi: 10.1002/stem.1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fu Z, Tindall DJ. FOXOs, cancer and regulation of apoptosis. Oncogene. 2008;27:2312–2319. doi: 10.1038/onc.2008.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jiang D, Liang J, Fan J, Yu S, Chen S, Luo Y, Prestwich GD, Mascarenhas MM, Garg HG, Quinn DA, et al. Regulation of lung injury and repair by Toll-like receptors and hyaluronan. Nat Med. 2005;11:1173–1179. doi: 10.1038/nm1315. [DOI] [PubMed] [Google Scholar]
- 38.Maegdefessel L, Azuma J, Toh R, Merk DR, Deng A, Chin JT, Raaz U, Schoelmerich AM, Raiesdana A, Leeper NJ, et al. Inhibition of microRNA-29b reduces murine abdominal aortic aneurysm development. J Clin Invest. 2012;122:497–506. doi: 10.1172/JCI61598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maurer B, Stanczyk J, Jüngel A, Akhmetshina A, Trenkmann M, Brock M, Kowal-Bielecka O, Gay RE, Michel BA, Distler JH, et al. microRNA-29, a key regulator of collagen expression in systemic sclerosis. Arthritis Rheum. 2010;62:1733–1743. doi: 10.1002/art.27443. [DOI] [PubMed] [Google Scholar]
- 40.Drummond CA, Hill MC, Shi H, Fan X, Xie JX, Haller ST, Kennedy DJ, Liu J, Garrett MR, Xie Z, et al. Na/K-ATPase signaling regulates collagen synthesis through microRNA-29b-3p in cardiac fibroblasts. Physiol Genomics. 2016;48:220–229. doi: 10.1152/physiolgenomics.00116.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Adoro S, Cubillos-Ruiz JR, Chen X, Deruaz M, Vrbanac VD, Song M, Park S, Murooka TT, Dudek TE, Luster AD, et al. IL-21 induces antiviral microRNA-29 in CD4 T cells to limit HIV-1 infection. Nat Commun. 2015;6:7562. doi: 10.1038/ncomms8562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ma F, Xu S, Liu X, Zhang Q, Xu X, Liu M, Hua M, Li N, Yao H, Cao X. The microRNA miR-29 controls innate and adaptive immune responses to intracellular bacterial infection by targeting interferon-γ. Nat Immunol. 2011;12:861–869. doi: 10.1038/ni.2073. [DOI] [PubMed] [Google Scholar]
- 43.Mundy-Bosse BL, Scoville SD, Chen L, McConnell K, Mao HC, Ahmed EH, Zorko N, Harvey S, Cole J, Zhang X, et al. microRNA-29b mediates altered innate immune development in acute leukemia. J Clin Invest. 2016;126:4404–4416. doi: 10.1172/JCI85413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dong J, Jiang G, Asmann YW, Tomaszek S, Jen J, Kislinger T, Wigle DA. microRNA networks in mouse lung organogenesis. PLoS One. 2010;5:e10854. doi: 10.1371/journal.pone.0010854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Disayabutr S, Kim EK, Cha SI, Green G, Naikawadi RP, Jones KD, Golden JA, Schroeder A, Matthay MA, Kukreja J, et al. miR-34 miRNAs regulate cellular senescence in type II alveolar epithelial cells of patients with idiopathic pulmonary fibrosis. PLoS One. 2016;11:e0158367. doi: 10.1371/journal.pone.0158367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li S, Geng J, Xu X, Huang X, Leng D, Jiang D, Liang J, Wang C, Jiang D, Dai H. miR-130b-3p modulates epithelial–mesenchymal crosstalk in lung fibrosis by targeting IGF-1. PLoS One. 2016;11:e0150418. doi: 10.1371/journal.pone.0150418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ge L, Habiel DM, Hansbro PM, Kim RY, Gharib SA, Edelman JD, Königshoff M, Parimon T, Brauer R, Huang Y, et al. miR-323a-3p regulates lung fibrosis by targeting multiple profibrotic pathways. JCI Insight. 2016;1:e90301. doi: 10.1172/jci.insight.90301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhou L, Wang L, Lu L, Jiang P, Sun H, Wang H. Inhibition of miR-29 by TGF-β–Smad3 signaling through dual mechanisms promotes transdifferentiation of mouse myoblasts into myofibroblasts. PLoS One. 2012;7:e33766. doi: 10.1371/journal.pone.0033766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang H, Garzon R, Sun H, Ladner KJ, Singh R, Dahlman J, Cheng A, Hall BM, Qualman SJ, Chandler DS, et al. NF-κB-YY1-miR-29 regulatory circuitry in skeletal myogenesis and rhabdomyosarcoma. Cancer Cell. 2008;14:369–381. doi: 10.1016/j.ccr.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Montgomery RL, Yu G, Latimer PA, Stack C, Robinson K, Dalby CM, Kaminski N, van Rooij E. microRNA mimicry blocks pulmonary fibrosis. EMBO Mol Med. 2014;6:1347–1356. doi: 10.15252/emmm.201303604. [DOI] [PMC free article] [PubMed] [Google Scholar]