Abstract
Objective
One of the possible explanations for the antidepressant resistance is tolerance to the effect of increasing synaptic serotonin. Vortioxetine is thought to work through a combination of two pharmacological modes of action: serotonin reuptake inhibition and modification of serotonin receptor activity, in a dose-dependent manner. This mechanism of action allows for examination of the hypothesis that antidepressant non-response may be due to exposure to persistently elevated synaptic amine levels.
Methods
We hypothesized that lower doses of vortioxetine, which exclusively inhibit serotonin reuptake, would not be effective in the setting of prolonged exposure to antidepressants, but higher doses, which interact in various ways to multiple post-synaptic serotonin receptors, would be relatively more effective in the setting of prolonged, prestudy antidepressant exposure. We examined the relationship between Defined Daily Dose (DDD), which is a measure of the extent of antidepressant use in each country, and the minimal effective dose of vortioxetine.
Principal Observation
There is a significant relationship between the DDD and effective vortioxetine dose (P = 0.035).
Conclusions
In countries with high antidepressant utilization, higher doses of vortioxetine were required, and obverse was true in countries with lower antidepressant utilization. These data support the hypothesis that tolerance to serotonin reuptake inhibition drives poor antidepressant response.
Keywords: vortioxetine, antidepressant tolerance, defined daily dose
Major depressive disorder (MDD) is one of the most common debilitating illnesses.1 Today, depression is the second cause of Disability Adjusted Life Years (DALYs) in the age category 15–44 years.1 According to World Health Organization (WHO), by the year 2020, depression is projected to reach second place in the ranking of DALY calculated for all ages. By 2030, it is expected to become the leading cause of disability in industrialized countries.2
Serotonin reuptake inhibitors (SRIs), initially introduced in 1988, are the first choice for the treatment of acute MDD. However, the long-term use of these agents may be less than ideal.3 Recurrence will eventually occur in approximately 80% of patients with MDD.4–6 This may be related to the phenomenon of antidepressant tachyphylaxis.7,8 However, once antidepressant response is lost, it rarely is reachieved.6,9,10 Consequently, over the last 30 years the rate of treatment-resistant MDD has been increasing in the United States.6,11 One of the possible explanations for the antidepressant resistance is tolerance to the effect of chronic increase of synaptic serotonin. This is similar to the effect observed in people who possess the genetic variant of short form of the serotonin (5-hydroxytryptamine or 5HT) transporter (SERT).12
Vortioxetine, a bis-arylsulfanyl amine compound (1[2-(2,4-dimethyl-phenylsulfanyl)-phenyl]-piperazine-hydrobromide, Lu AA21004), is a novel multimodal antidepressant.13 It is thought to work through a combination of two pharmacological modes of action: inhibition of the presynaptic serotonin transporter and direct modulation of postsynaptic serotonin receptor activity. Specifically, vortioxetine is a 5HT)3, 5HT)7, and 5HT1D receptor antagonist, 5-HT1B receptor partial agonist, 5-HT1A receptor agonist, and an inhibitor of 5HT reuptake through inhibition of the SERT.14 Importantly, the affinities at these receptors is highest for the SERT and lowest for 5HT1D, so that progressively higher doses are required to bring about each receptor’s mechanistic benefit (Ki for SERT = 1.6 nM; 5HT)3 = 3.7 nM; 5HT1A = 15 nM; 5HT)7 = 19 nM; 5HT1B = 33 nM; and 5HT1D = 54 nM).13,14 In-vivo nonclinical studies have demonstrated that vortioxetine enhances levels of multiple neurotransmitters (5HT, noradrenaline, dopamine, acetylcholine and histamine) in specific areas of the brain.15 The efficacy of vortioxetine in reducing depressive symptoms has been demonstrated in patients with MDD at a range of different doses.16–29
The defined daily dose (DDD) is a statistical measure of drug consumption defined by the World Health Organization (WHO). It is used to standardize the comparison of drug usage between different drugs or between different health care environments. It is the assumed average maintenance dose per day for a drug, or drug class—such as antidepressants—used for its main indication in adults (WHO Collaborating Centre for Drug Statistics Methodology).30
In this study, we compared the minimal effective dose of vortioxetine dose in different countries and the DDD. We hypothesized that if tachyphylaxis, or antidepressant tolerance, occurs, then countries with higher antidepressant usage (i.e., higher DDD), would require higher vortioxetine dosage to be effective.
Methods
Sources of Data
PubMed, Medline, Clinical Trials databases were interrogated using the key words “vortioxetine”, “LuAA21004”, “major depressive Disorder” and “clinical trials.”
Publication Selection
Studies testing the efficacy of vortioxetine for the treatment of MDD were eligible for inclusion. Included studies had to be randomized clinical trials (RCTs) comparing vortioxetine with placebo and/or another antidepressant. Patients needed to meet the criteria for MDD according to the Diagnostic and Statistical Manual of Mental Disorders, 4th edition, text revision (DSM-IV-TR). Trials that recruited patients for evaluation of other outcomes were included if they also met the aforementioned criteria for MDD and included data for outcomes of MDD. Studies were required to have a Montgomery–Asberg Depression Rating Scale (MADRS)31 and/or Hamilton Anxiety Rating Scale (HAMA)32 to assess depression and improvement of depression after treatment. Depressive symptoms had to be statistically significantly improved on at least one of these scales to be accepted.
There were no requirements or restrictions regarding the severity of MDD, sex, age, number of participants, study location or inpatient versus outpatient treatment. No restrictions regarding the pharmaceutical form or dose regimen (fixed v. flexible) were applied. Studies for which no effective dose could be determined were excluded from this study, as a determined effective dose was necessary to perform statistical analysis. Ten studies met these criteria (Table 1).
Table 1. Characteristics of Specific Groups in The Ten Publications That Have Been Included in This Study.
| COUNTRY | MASKING | PATIENT SETTING | DOSAGE | DURATION | ACTIVE COMPARATOR | #PATINETS FINISHED THE STUDY | SCALE | P-VALUE | AUTHORS |
|---|---|---|---|---|---|---|---|---|---|
| 14 countries1 | Double Blind | N/A | Placebo | 8 weeks | N/A | 127 | HAM | N/A | Henigsberd, 2012 |
| 14 countries1 | Double Blind | N/A | 1 mg | 8 weeks | N/A | 127 | HAM | <0.001 | Henigsberd, 2012 |
| 14 countries1 | Double Blind | N/A | 5 mg | 8 weeks | N/A | 129 | HAM | <0.001 | Henigsberd, 2012 |
| 14 countries1 | Double Blind | N/A | 10 mg | 8 weeks | N/A | 122 | HAM | <0.001 | Henigsberd, 2012 |
| USA | Double Blind | N/A | Placebo | 8 weeks | N/A | 157 | MADRS | N/A | Jacobsen, 2015 |
| USA | Double Blind | N/A | 10 mg | 8 weeks | N/A | 155 | MADRS | 0.058 | Jacobsen, 2015 |
| USA | Double Blind | N/A | 20 mg | 8 weeks | N/A | 150 | MADRS | 0.002 | Jacobsen, 2015 |
| USA | Double Blind | N/A | Placebo | 9 weeks | Duloxetine 60 mg | 161 | MADRS | N/A | Mahableshwarkar January 2015 |
| USA | Double Blind | N/A | 15 mg | 9 weeks | Duloxetine 60 mg | 147 | MADRS | 0.224 | Mahableshwarkar January 2015 |
| USA | Double Blind | N/A | 20 mg | 9 weeks | Duloxetine 60 mg | 154 | MADRS | 0.023 | Mahableshwarkar January 2015 |
| USA | Double Blind | N/A | Duloxetine 60 mg | 9 weeks | Duloxetine 60 mg | 152 | MADRS | <0.001 | Mahableshwarkar January 2015 |
| 11 countries + USA2 | Double Blind | Inpatient and outpatient | Placebo | 8 weeks | N/A | 196 | MADRS | N/A | Mclntyre, 2014 |
| 11 countries + USA2 | Double Blind | Inpatient and outpatient | 10 mg | 8 weeks | N/A | 195 | MADRS | <0.0001 | Mclntyre, 2014 |
| 11 countries + USA2 | Double Blind | Inpatient and outpatient | 20 mg | 8 weeks | N/A | 207 | MADRS | <0.0001 | Mclntyre, 2014 |
| 13 countries3 | Double Blind | Inpatient and outpatient | Placebo | 8 weeks | Duloxetine 60 mg | 158 | MADRS | Boulenger, 2014 | |
| 13 countries3 | Double Blind | Inpatient and outpatient | 15 mg | 8 weeks | Duloxetine 60 mg | 151 | MADRS | <0.0001 | Boulenger, 2014 |
| 13 countries3 | Double Blind | Inpatient and outpatient | 20 mg | 8 weeks | Duloxetine 60 mg | 151 | MADRS | <0.0001 | Boulenger, 2014 |
| 13 countries3 | Double Blind | Inpatient and outpatient | Duloxetine 60 mg | 8 weeks | Duloxetine 60 mg | 147 | MADRS | <0.0001 | Boulenger, 2014 |
| 17 countries4 | Open Labelled + double Blind | Inpatient and outpatient | 5-10 mg | 24 weeks | N/A | 492 | MADRS | 0.0035 | Boulenger, 2012 |
| 14 countries5 | Double Blind | Inpatient and outpatient | Vortioxetine 15/20 mg | 12 weeks | Agomelatine 25/50 mg | 253 | MADRS | 0.0054 | Montgomery, 2014 |
| 14 countries5 | Double Blind | Inpatient and outpatient | Agomelatine 25/50 mg | 12 weeks | Agomelatine 25/50 mg | 242 | MADRS | 0.0054 | Montgomery, 2014 |
| USA + 6 countries6 | Double Blind | N/A | placebo | 8 weeks | Duloxetine 60 mg | 128 | HAM | N/A | Katona, 2012 |
| USA + 6 countries6 | Double Blind | N/A | Vortioxetine 5 mg | 8 weeks | Duloxetine 60 mg | 136 | HAM | P = 0.0011 | Katona, 2012 |
| USA + 6 countries6 | Double Blind | N/A | Duloxetine 60 mg | 8 weeks | Duloxetine 60 mg | 128 | HAM | <0.0001 | Katona, 2012 |
| USA | Double Blind | N/A | Placebo | 8 weeks | Duloxetine 60 mg | N/A | MADRS | N/A | Mahableshwarkar, 2014 |
| USA | Double Blind | N/A | Duloxetine 60 mg | 8 weeks | Duloxetine 60 mg | N/A | MADRS | <0.001 | Mahableshwarkar, 2014 |
| USA | Double Blind | N/A | Vortioxetine 15 mg | 8 weeks | Duloxetine 60 mg | N/A | MADRS | 0.224 | Mahableshwarkar, 2014 |
| USA | Double Blind | N/A | Vortioxetine 20 mg | 8 weeks | Duloxetine 60 mg | N/A | MADRS | 0.023 | Mahableshwarkar, 2014 |
| 11 Countries7 | Double Blind | Outpatient | Placebo | 6 weeks | Venlafaxine 225 mg | 105 | MADRS | N/A | Alvarez, 2012 |
| 11 Countries7 | Double Blind | Outpatient | 5 mg | 6 weeks | Venlafaxine 225 mg | 108 | MADRS | <0.0001 | Alvarez, 2012 |
| 11 Countries7 | Double Blind | Outpatient | 10 mg | 6 weeks | Venlafaxine 225 mg | 100 | MADRS | <0.0001 | Alvarez, 2012 |
| 11 Countries7 | Double Blind | Outpatient | Venlafaxine 225 mg | 6 weeks | Venlafaxine 225 mg | 113 | MADRS | <0.0001 | Alvarez, 2012 |
Notes: 1Croatia, Germany, Finlandia, India, Japan, Latvia, Malaysia, Philippines, Poland, Romania, Rusia, Serbia, South Korea, Ukraine.
2Australia, Canada, Finland, France, Germany, Latvia, Mexico, Serbia, Slovakia, South Africa, Ukraine, and the US.
3Belgium, Estonia, Finland, France, Germany, Latvia, Lithuania, Norway, Russia, Slovakia, South Africa, Sweden and the Ukraine.
4Australia, Austria, Belgium, Canada, Finland, France, Germany, India, the Republic of Korea, Norway, Poland, South Africa, Sweden, Taiwan, Thailand, Turkey, the United Kingdom.
5Austria, Belgium, Bulgaria, Czech Republic, Estonia, Germany, Italy, Lithuania, Poland, Romania, Russia, Spain, Sweden and the UK.
6Canada, Finland, France, Germany, Sweden, Ukraine and the USA.
7Australia, Austria, Canada, Czech Republic, Finland, France, Italy, Malaysia, Slovakia, Spain, Sweden.
Data Extraction
The collected information included the study design, patient selection criteria, medication dose, trial duration, type of comparator (active v. placebo), study location, publication status, outpatient versus inpatient treatment, post-treatment mean change scores from baseline.
Efficacy Measures
The primary efficacy measures were the mean change from baseline in total scores on the MADRS and/or HAMA, as defined by the individual study.
DDD Calculation
DDDs are available for many countries (Australia, Austria, Bulgaria, Canada, Croatia, Czech Republic, Estonia, Finland, France, Germany, Italy, Japan, Latvia, Lithuania, Norway, Poland, Romania, Slovakia, South Korea, Spain, Sweden, United Kingdom,)33 (Table 2a). For the countries that there is no available DDD (China, Hong Kong, India, Malaysia, Philippines, Russia, Taiwan, Thailand, Turkey, Ukraine, United States) (Table 2b) DDDs were estimated by reviewing available antidepressants, yearly antidepressant usage (used pill amount was available for some countries; for instance; 740 million antidepressant pills used in Turkey in 2012), per capita income, and cultural and geographical similarities with the countries that have DDDs. For example, the USA does not have available DDD but Canada (DDD: 86) and United Kingdom (DDD: 71) are geographically, culturally, economically similar to USA. We conservatively estimated DDD for the USA conservatively at 75 (though it is the opinion of the researchers that the value for the USA is likely closer to that of Canada, above 86).
Table 2. DDD Values in Countries with WHO-Calculated Values.
| Austria | 56.54 |
| Belgium | 67.76 |
| Bulgaria | 7.61 |
| Canada | 86 |
| Chile | 13 |
| Croatia | 22.9 |
| Czech Republic | 44 |
| Denmark | 77.6 |
| Estonia | 18 |
| Finland | 64.21 |
| France | 49.26 |
| Germany | 20 |
| Greece | 49.14 |
| Hungary | 27 |
| Iceland | 98.3 |
| Ireland | 55.51 |
| Italy | 42 |
| South Korea | 13 |
| Latvia | 6.14 |
| Lithuania | 15.53 |
| Luxembourg | 51 |
| Netherlands | 42 |
| Norway | 54.19 |
| Poland | 16.66 |
| Portugal | 78 |
| Romania | 6.09 |
| Slovakia | 31 |
| Slovenia | 43.2 |
| Spain | 59.06 |
| Sweden | 72.64 |
| Switzerland | 49.08 |
| UK | 71 |
| Japan | 12.3 |
| Australia | 51.5 |
Table 2B. Countries Without a WHO-Calculated DDD, and The DDD Values Estimated by The Authors.
| USA | 75.3 |
| Turkey | 27.09 |
| Malaysia | 6.8 |
| Ukraine | 20 |
| Russia | 12 |
| India | 6.8 |
| Philippines | 6.8 |
| Hong Kong | 6.8 |
| Taiwan | 6.8 |
| China | 5 |
| Thailand | 6.8 |
The average DDDs were calculated for each study based on the countries in which the studies were performed. For example, Alverez et al16 conducted their study in 11 countries. DDDs are available for 10 (Australia, Austria, Canada, Czech Republic, Finland, France, Italy, Slovakia, Spain, Sweden) of the 11 countries. Malaysia did not have an available DDD, but it was estimated at 6.3. Thus, the average DDD for the Alverez study16 was 51.2 when Malaysia was included and 55.62 when Malaysia was not included. The average DDD for each study is calculated with and without estimated DDDs (for countries that do not have caluculated DDDs). Table 3 presents the average DDDs utilizing only established DDD values and by including estimated values. The minimal effective vortioxetine dose was utilized for the linear regression analysis.
Table 3. The Average DDDs for The Studies. Small DDDs Correspond to Infrequent Antidepressant Use in The Population Studied, Large DDDs Correspond to Frequent Antidepressant Use in The Population Studied. The Minimal Effective Vortioxetine Dose Increases Directly in Proportion to The DDD.
| STUDIES | AVERAGE DDDs WITHOUT ESTIMATED VALUES | AVERAGE DDDs WITH ESTIMATED VALUES | MINIMAL EFFECTIVE VORTIOXETINE DOSE |
|---|---|---|---|
| Henigsberd, 2012 | 24.1 | 25.3 | 1 |
| Alvarez, 2012 | 55.62 | 51.2 | 5 |
| Katona, 2012 | 58.43 | 55.3 | 5 |
| Boulenger, 2012 | 49.9 | 41 | 10 |
| Mclntyre, 2014 | 39.36 | 37.4 | 10 |
| Boulenger, 2014 | 39.8 | 35.2 | 15 |
| Montgomery, 2014 | 38.2 | 36.3 | 15 |
| Mahablashwarkar, 2014 | 75 | 75 | 20 |
| Mahableshwarkar, Jan 2015 | 75 | 75 | 20 |
| Jacobsen, 2015 | 75 | 75 | 20 |
Abbreviation: DDD, Defined daily dose.
Results
A total of ten studies met the inclusion criteria. Five of them were conducted in non-USA countries,15,16,18,20,24 three of them were done in the USA19,22,25 and two were mixed (US and non-US)17,23 studies (Table 1). In one study,24 patients were given vortioxetine 10 mg or 20 mg and data was analyzed collectively. We considered mean of these two doses (15 mg) as the effective dose for this study.
In studies performed in the USA, depressive symptoms are significantly improved only with a 20 mg dose of vortioxetine; studies failed to show significant improvement with lesser doses.21,22,29 On the other hand, there is significant improvement in depressive symptoms with vortioxetine 1 mg in countries in which DDD is low (Table 3). Vortioxetine 10–15 mg was found to be very effective in some countries with low DDDs that correspond to antidepressant-naïve populations.
Statistical analysis was performed separately on data including or excluding estimated DDDs, to control for the potential that the estimated DDD are inaccurate. Results for both groups were found to be statistically significant (evaluation including the estimated DDDs: P = 0.035, r2 = 0.448; evaluation excluding the estimated DDDs: P = 0.039, r2 = 0.429) (Figure 1a–1b).
Figure 1A.
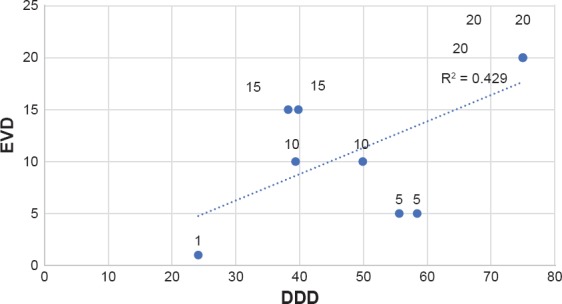
EVD-DDD Correlation*
Figure 1B.
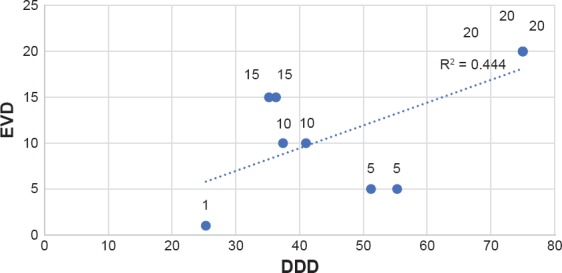
EVD-DDD Correlation**
Discussion
Vortioxetine achieves at least 63% ± 23% 5HT transporter occupancy at 10 mg/day.34 Similar levels of post-synaptic serotonin receptor occupancy will occur in a range of 15 to 30 mg daily. The only dosage that significantly improves depressive symptoms in the USA is 20 mg daily, with the exception of geriatric subjects.21,22,25,26,28,29 Five studies were performed in non-USA countries15,16,18,20,24 and 2 studies were performed in multiple countries that included the USA.17,23 In those studies, the effective dose of vortioxetine was directly related to the average rate of utilization of antidepressants in the countries where the studies were performed as reflected by the DDD. Specifically, in studies where the average DDD was lower (reflective of lower antidepressant utilization in the populations where the studies were performed), lower doses of vortioxetine were effective. As the DDD went up, higher doses of vortioxetine were required for depression to improve.
Vortioxetine is a unique molecule that has multiple mechanisms of antidepressant action as reflected by interactions with the presynaptic 5HT reuptake transporter and multiple post-synaptic and presynaptic 5HT receptors. The varying affinities at these receptors mean that there is a dose-related recruitment of different mechanisms. The highest affinity, or the receptor requiring the lowest dosage for efficacy, is with the 5HT transporter. Adequate receptor occupancy is achieved here at 10 mg/day. As the dosage of vortioxetine increases, there is a progressive recruitment of other receptors in a sequence that is determined by the respective affinities: 5HT)3, 5HT1A, 5HT)7, 5HT1B, and finally 5HT1D.35 We predicted that vortioxetine would not be effective at the minimal effective dose (10 mg) in countries with high antidepressant usage rates. We believe this to be due to presumed higher rates of antidepressant tolerance in the potential study population. Specifically, it is proposed that exposure to serotonin reuptake inhibition results in upregulation of SERT, down-regulation of post synaptic 5HT receptors, and possible elimination of serotoninergic synapses, which makes patients resistant to 5HT reuptake inhibition as a method of antidepressant action.11 In countries with high antidepressant utilization, such as the USA, the majority of study participants have had significant prior antidepressant exposure36 and consequently, will have a lower proportion of subjects that will be expected to respond to SERT inhibition. However, since few of these individuals have been exposed to the other potential mechanisms of vortioxetine (specifically binding to 5HT)3, 5HT1A, 5HT)7, 5HT1B, and 5HT1D, which require a higher dosage to get adequate receptor binding), they responded to the higher doses of this drug. Indeed, in preclinical studies in which vortioxetine is administered to mice that have been genetically modified to be non-responsive to SERT blockade, vortioxetine maintains its antidepressant effect due to these alternative mechanisms.37
Western countries with well-developed health care tend to have greater antidepressant utilization as reflected by the DDD. Iceland has the biggest DDD (98.3) for antidepressants. Canada, United Kingdom, Denmark, Portugal, Sweden also have high DDDs for antidepressants. Food and Drug Administration (FDA) has not announced the DDD of antidepressants for the USA but it is likely to be among the highest.
The major weakness of this study is the lack of DDDs for every county included in the study. While we attempted to address this weakness by estimating a conservative values for the DDDs for these countries, and analyzing the data with and without the estimated DDDs, the data would clearly be more robust with accurate values. Furthermore, many studies include both countries with high DDDs, like UK or Canada, and countries that have low DDDs like Estonia and Latvia. But the data was analyzed collectively because we could not determine the outcome of specific individuals from specific countries. Utilization of the average DDDs for whole studies would be expected to have reduced the power of the analysis, and certainly can introduce potential error. Finally, the DDD measure itself is a measure of antidepressant exposure in the source population, but not necessarily in the study participants, but we did not have information specific to the study participants. The fact that the regression analysis was highly significant increases the confidence in the finding, but does not eliminate the problem.
Conclusion
Despite these limitations, this study shows that while in countries with low antidepressant utilization (as reflected by low DDD), lower doses of vortioxetine are very effective, in countries with high antidepressant utilization, higher doses (i.e., dosage that recruit additional post-synaptic serotonin receptors) are required. We speculate this is due to antidepressant tolerance, and in particular tolerance to SRIs. Vortioxetine has multiple novel mechanisms of action that are dose-dependent, that allows for the visualization of the effects of tolerance due to SRI exposure. These findings have important implications for treatment, research, and regulation. Simplistically, clinicians should utilize novel antidepressant mechanisms in patients with presumed antidepressant tolerance. Researchers should stratify subjects based on previous SRI exposure. Additionally, performance of studies in low DDD countries may yield greater power, particularly for agents that utilize SERT inhibition. Finally, regulators may consider requiring information of efficacy of agents as a function of previous antidepressant exposure, or at the very least, country-specific data.
Footnotes
Conflicts of Interest
Dr. El-Mallakh receives research funding from the National Institute of Mental Health, the State of Kentucky, Janssen, Alexza, and Sage Pharmaceuticals. He is on the speakers’ boards of Merck, Lundbeck, Otsuka, Sunovion, and Takeda. The other authors do not have any conflicts of interest to report.
References
- 1.World Health Organization. WHO Press; Geneva: 2008. [accessed 10 September 2017]. The global burden of disease: 2004 update. http://www.who.int/healthinfo/global_burden_disease/GBD_report_2004update_full.pdf?ua=1. [Google Scholar]
- 2.Mathers CD, Loncar D. Projections of Global Mortality and Burden of Disease from 2002 to 2030. PloS Med. 2006;3(11):e442. doi: 10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.El-Mallakh RS, Briscoe B. Studies of long-term use of antidepressants: How should the data from them be Interpreted? CNS Drugs. 2012;26(2):1–13. doi: 10.2165/11599450-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 4.Bockting CL, Spinhoven P, Koeter MWJ, Wouters LF, Schene AH. Depression Evaluation Longitudinal Therapy Assessment Study Group: prediction of recurrence on recurrent depression and the influence of consecutive episodes on vulnerability for depression: a 2-year prospective study. J Clin Psychiatry. 2006;67:747–755. [PubMed] [Google Scholar]
- 5.Solomon D, Leon AC, Mueller TI, Coryell W, Teres JJ, Posternak MA, Judd LL, Endicott J, Keller MB. Tachyphalaxis in unipolar major depressive disorder. J Clin Psychiatry. 2005;66:283–290. doi: 10.4088/jcp.v66n0302. [DOI] [PubMed] [Google Scholar]
- 6.Posternak MA, Zimmerman M. Dual reuptake inhibitors incur lower rates of tachyphylaxis than selective serotonin reuptake inhibitors: a retrospective study. J Clin Psychiatry. 2005;66:705–707. doi: 10.4088/jcp.v66n0605. [DOI] [PubMed] [Google Scholar]
- 7.Lieb J, Balter A. Antidepressant tachyphylaxis. Med Hypotheses. 1984;15:279–291. doi: 10.1016/0306-9877(84)90018-5. [DOI] [PubMed] [Google Scholar]
- 8.Cohen B, Baldessarini R. Tolerance to therapeutic effects of antidepressants. Am J Psychiatry. 1985;142:489–490. doi: 10.1176/ajp.142.4.489. [DOI] [PubMed] [Google Scholar]
- 9.Reimherr FW, Amsterdam JD, Quitkin FM, Rosenbaum JF, Fava M, Zajecka J, Beasley CM, Jr, Michelson D, Roback P, Sundell K. Optimal length of continuation therapy in depression. Am J Psychiatry. 1998;155:1247–1253. doi: 10.1176/ajp.155.9.1247. [DOI] [PubMed] [Google Scholar]
- 10.Sharma V. Loss of response to antidepressants and subsequent refractoriness: diagnostic issues in a retrospective case series. J Affect Disord. 2001;64:99–106. doi: 10.1016/s0165-0327(00)00212-3. [DOI] [PubMed] [Google Scholar]
- 11.El-Mallakh RS, Gao Y, Briscoe BT, Roberts RJ. Tardive dysphoria: The role of long term antidepressant use in inducing chronic depression. Med Hypoth. 2011;80(1):57–59. doi: 10.1016/j.mehy.2011.01.020. [DOI] [PubMed] [Google Scholar]
- 12.Luddington NS, Mandadapu A, Husk M, El-Mallakh RS. Clinical implications of genetic variation of the promoter region of the serotonin transporter. Prim Care Companion J Clin Psychiatry. 2009;11(3):93–102. doi: 10.4088/pcc.08r00656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bang-Andersen B, Ruhland T, Jørgensen M, Smith G, Frederiksen K, Jensen KG, Zhong H, Nielsen SM, Hogg S, Mørk A, Stensbøl TB. Discovery of 1-[2(2,4-imethylphenylsulfanyl)phenyl] piperazine (Lu AA21004): a novel multimodal compound for the treatment of major depressive disorder. J Med Chem. 2011;54(9):3206–3221. doi: 10.1021/jm101459g. [DOI] [PubMed] [Google Scholar]
- 14.Mørk A, Pehrson A, Brennum LT, Nielsen SM, Zhong H, Lassen AB, Miller S, Westrich L, Boyle NJ, Sánchez C, Fischer CW, Liebenberg N, Wegener G, Bundgaard C, Hogg S, Bang-Andersen B, Stensbøl TB. Pharmacological effects of Lu AA21004: a novel multimodal compound for the treatment of major depressive disorder. J Pharmacol Exp Ther. 2012;340(3):666–675. doi: 10.1124/jpet.111.189068. [DOI] [PubMed] [Google Scholar]
- 15.Boulenger JP, Loft H, Florea I. A randomized clinical study of Lu AA21004 in the prevention of relapse in patients with major depressive disorder. J Psychopharmacol. 2012;26(11):1408–1416. doi: 10.1177/0269881112441866. [DOI] [PubMed] [Google Scholar]
- 16.Alvarez E, Perez V, Dragheim M, Loft H, Artigas F. A double-blind, randomized, placebo-controlled, active reference study of Lu AA21004 in patients with major depressive disorder. Int J Neuropsychopharmacol. 2012;15:589–600. doi: 10.1017/S1461145711001027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Katona C, Hansen T, Olsen CK. A randomized, double-blind, placebo-controlled, duloxetine-referenced, fixed-dose study comparing the efficacy and safety of Lu AA21004 in elderly patients with major depressive disorder. Int Clin Psychopharmacol. 2012;27:215–223. doi: 10.1097/YIC.0b013e3283542457. [DOI] [PubMed] [Google Scholar]
- 18.Henigsberg N, Mahableshwarkar AR, Jacobsen P, Chen Y, Thase ME. A Randomized, double-blind, placebo-controlled 8-Week Trial of the Efficacy and Tolerability of Multiple Doses of Lu AA21004 in Adults with Major Depressive Disorder. J Clin Psychiatry. 2012;73(7):953–959. doi: 10.4088/JCP.11m07470. [DOI] [PubMed] [Google Scholar]
- 19.Jacobsen PL, Mahableshwarkar AR, Serenko M, Chan S, Trivedi MH. A Randomized, double-blind, placebo-controlled Study of the Efficacy and Safety of Vortioxetine 10 mg and 20 mg in Adults with Major Depressive Disorder. J Clin Psychiatry. 2015;76(5):575–582. doi: 10.4088/JCP.14m09335. [DOI] [PubMed] [Google Scholar]
- 20.Boulenger JP, Loft H, Olsen CK. Efficacy and safety of vortioxetine (Lu AA21004), 15 and 20 mg/day: a randomized, double-blind, placebo-controlled, duloxetine-referenced study in the acute treatment of adult patients with major depressive disorder. Int Clin Psychopharmacol. 2014;29:138–149. doi: 10.1097/YIC.0000000000000018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jain R, Mahableshwarkar AR, Jacobsen PL, Chen Y, Thase ME. A randomized, double-blind, placebo-controlled 6-wk trial of the efficacy and tolerability of 5 mg vortioxetine in adults with major depressive disorder. Int J Neuropsychopharmacol. 2013;16:313–321. doi: 10.1017/S1461145712000727. [DOI] [PubMed] [Google Scholar]
- 22.Mahableshwarkar AR, Jacobsen PL, Chen Y. A randomized, double-blind trial of 2.5 mg and 5 mg vortioxetine (Lu AA21004) versus placebo for 8 weeks in adults with major depressive disorder. Curr Med Res Opinion. 2013;29(3):217–226. doi: 10.1185/03007995.2012.761600. [DOI] [PubMed] [Google Scholar]
- 23.McIntyre RS, Lophaven S, Olsen CK. A randomized, double-blind, placebo-controlled study of vortioxetine on cognitive function in depressed adults. Int J Neuropsychopharmacol. 2014;17:1557–1567. doi: 10.1017/S1461145714000546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Montgomery SA, Nielsen RZ, Poulsen LH, Häggström L. A randomised, double-blind study in adults with major depressive disorder with an inadequate response to a single course of selective serotonin reuptake inhibitor or serotonin-noradrenaline reuptake inhibitor treatment switched to vortioxetine or agomelatine. Hum Psychopharmacol Clin Exp. 2014;29:470–482. doi: 10.1002/hup.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mahableshwarkar AR, Jacobsen PL, Chen Y, Serenko M, Trivedi MH. A randomized, double-blind, duloxetine-referenced study comparing efficacy and tolerability of 2 fixed doses of vortioxetine in the acute treatment of adults with MDD. Psychopharmacology (Berl) 2015;232(12):2061–2070. doi: 10.1007/s00213-014-3839-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jacobsen PL, Mahableshwarkar AR, Serenko M, Chan S, Trivedi MH. A randomized, double-blind, Placebo-Controlled study of the Efficacy and Safety of Vortioxetine 10 mg and 20 mg in Adults with Major Depressive Disorder. J Clin Psychiatry. 2015;76(5):575–582. doi: 10.4088/JCP.14m09335. [DOI] [PubMed] [Google Scholar]
- 27.Wang G, Gislum M, Filippov G, Montgomery S. Comparison of vortioxetine versus venlafaxine XR in adults in Asia with major depressive disorder: a randomized, double-blind study. Curr Med Res Opinion. 2015;31(4):785–794. doi: 10.1185/03007995.2015.1014028. [DOI] [PubMed] [Google Scholar]
- 28.Mahableshwarkar AR, Jacobsen PL, Chen Y, Serenko M, Trivedi MH. A randomized, double-blind, duloxetine-referenced study comparing efficacy and tolerability of 2 fixed doses of vortioxetine in the acute treatment of adults with MDD. Psychopharmacology (Berl) 2015;232:2061–2070. doi: 10.1007/s00213-014-3839-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mahableshwarkar AR, Jacobsen PL, Serenko M, Chen Y, Trivedi MH. A randomized, double-blind, Placebo-Controlled study of the Efficacy and Safety of 2 doses of Vortioxetine in Adults with Major Depressive Disorder. J Clin Psychiatry. 2015;76(5):583–591. doi: 10.4088/JCP.14m09337. [DOI] [PubMed] [Google Scholar]
- 30.WHO Collaborating Centre for Drug Statistics Methodology. 2009. [accessed 10 September 2017]. http://www.whocc.no/ddd/definition_and_general_considera/ [Google Scholar]
- 31.Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 32.Hamilton M. The assessment of anxiety states by rating. BrJ Med Psychol. 1959;32:50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- 33.Gusmão R, Quintão S, McDaid D, Arensman E, Van Audenhove C, Coffey C, Värnik A, Värnik P, Coyne J, Hegerl U. Antidepressant Utilization and Suicide in Europe: An Ecological Multi-National Study. PLoS One. 2013;8(6):e66455. doi: 10.1371/journal.pone.0066455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Areberg J, Luntang-Jensen M, Søgaard B, Nilausen DØ. Occupancy of the Serotonin Transporter after Administration of Lu AA21004 and its Relation to Plasma Concentration in Healthy Subjects. Basic Clin Pharmacol Toxicol. 2012;110:401–440. doi: 10.1111/j.1742-7843.2011.00810.x. [DOI] [PubMed] [Google Scholar]
- 35.Bang-Andersen B, Ruhland T, Jørgensen M, Smith G, Frederiksen K, Jensen KG, Zhong H, Nielsen SM, Hogg S, Mørk A, Stensbøl TB. Discovery of 1-[2-(2,4-dimethylphenylsulfanyl)phenyl] piperazine (Lu AA21004): a novel multimodal compound for the treatment of major depressive disorder. J Med Chem. 2011;54(9):3206–3221. doi: 10.1021/jm101459g. [DOI] [PubMed] [Google Scholar]
- 36.Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, Niederehe G, Thase ME, Lavori PW, Lebowitz BD, McGrath PJ, Rosenbaum JF, Sackeim HA, Kupfer DJ, Luther J, Fava M. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163(11):1905–1917. doi: 10.1176/ajp.2006.163.11.1905. [DOI] [PubMed] [Google Scholar]
- 37.Nackenoff AG, Simmler LD, Baganz NL, Pehrson AL, Sánchez C, Blakely RD. Serotonin Transporter-Independent Actions of the Antidepressant Vortioxetine As Revealed Using the SERT Met172 Mouse. ACS Chem Neurosci. 2017;8(5):1092–1100. doi: 10.1021/acschemneuro.7b00038. [DOI] [PubMed] [Google Scholar]


