Abstract
Objective: Recent research has indicated that altered promoter methylation of oncogenes and tumor suppressor genes is an important mechanism in lung cancer development and progression. In this study, we investigated the association between promoter methylation of TMEM88, a possible inhibitor of the Wnt/β-Catenin signaling, and the survival of patients with non-small cell lung cancer (NSCLC). Methods: Twelve pairs of tumor and adjacent non-tumor samples were used for microarray analyses of DNA methylation and gene expression. For validation, more than two hundred additional samples were analyzed for methylation using bisulfite pyrosequencing and for gene expression using qRT-PCR. Then the cell function were tested by wound healing, transwell, CCK8 and cell cycle assay. Results: Our analysis of patient specimens showed that TMEM88 methylation was higher in NSCLC tumors (82.2% ± 10.3, P < 0.01) compared with the adjacent normal tissues (65.9% ± 7.2). The survival analysis revealed that patients with high TMEM88 methylation had a shorter overall survival (46 months) compared with patients with low TMEM88 methylation (>56 months;P=0.021). In addition, we found that demethylation treatment could inhibit tumor cell proliferation, migration, and invasion, which was supportive of an association between methylation and survival. Conclusions: Based on these consistent observations, we concluded that TMEM88 may play an important role in NSCLC progression and that promoter methylation of TMEM88 may serve as a biomarker for NSCLC prognosis and treatment.
Keywords: TMEM88, lung cancer, methylation, prognosis, Wnt/β-Catenin signaling
Introduction
Lung cancer is one of the most common malignant tumors in the world. According to the data from GLOBOCAN 2012, 1.8 million people are diagnosed with lung cancer every year, which represents 13% of all newly diagnosed cancer patients. Lung cancer is also the number one cause of cancer-related death, accounting for 1.6 million deaths per year, which represents 19% of all cancer deaths1. Non-small cell lung cancer (NSCLC) is the most common form of lung cancer and constitutes approximately 85% of all lung cancer cases. Studies have shown that the disruption of normal epigenetic regulation is an important mechanism in the initiation and progression of cancer2. Research has also indicated that the Wnt/β-catenin signaling pathway is critical in tumorigenesis3.
DNA methylation plays a crucial role in the epigenetic regulation of gene activity and function4-6. Substantial alterations in DNA methylation and gene expression have been observed in many types of cancer, including breast7, prostate8, and lung9 cancer. It has also been reported that the downregulation of the Wnt/β-Catenin signal inhibitors by hypermethylation is common in NSCLC10-13. Recently, Zhang et al.14 found that TMEM88 could inhibit canonical Wnt/β-Catenin signaling through interaction with the Wnt pathway factor Dishevelled (DVLS). TMEM88, located on chromosome 17p13.1, is a transmembrane protein that plays an important role in embryonic development and stem cell differentiation15. However, it remains unclear whether TMEM88 hypermethylation in lung cancer has any biological relevance to tumorigenesis and if this has any clinical implications for disease prognosis.
In this study, we investigated the role of TMEM88 methylation in NSCLC with regard to its association with the clinical and pathological features of patients with NSCLC. Using the demethylation agent DAC, we evaluated the methylation and expression of TMEM88 in lung cancer cells and its impact on cancer cell migration, invasion, proliferation, and cell cycle progression. We also analyzed the association of TMEM88 methylation with the overall survival of patients with NSCLC.
Materials and methods
Patients
Patients with NSCLC were recruited from the Tianjin Medical University Cancer Hospital (TMUCH) between May 2006 and July 2011. The fresh tumor samples and adjacent non-tumor tissues were collected from the patients during the surgical resection of tumors. Twelve pairs of tumor and adjacent non-tumor samples were used for microarray analyses of DNA methylation and gene expression. For validation, an additional 213 tumor samples and 30 matched adjacent non-tumor tissues were analyzed for methylation by using bisulfite pyrosequencing; further, the gene expression of 201 tumor samples and 66 matched adjacent non-tumor tissues was tested by using qRT-PCR; and, from these two batches, samples from 173 patients were analyzed for both the methylation and expression of TMEM88. The clinical and pathological information for each patient was extracted from hospital medical records and pathology reports. All patients were followed after surgery until August 2013. The study was approved by the Medical Ethical Review Committees at Tianjin Medical University Cancer Institute and Hospital and Shanghai Jiao Tong University School of Public Health.
RNA extraction and qRT-PCR
Total RNA was extracted from the tissue samples and cultured cancer cells by using a commercial kit. Quantitative RT-PCR assays were performed as previously described16.
DNA extraction and analysis of DNA methylation
Genomic DNA was extracted from the tissue samples and cancer cells by using the QIAamp DNA Mini Kit (Qiagen, Hilden, Germany, Cat no. 51304). The extracted DNA samples were treated with sodium bisulfite by using the EpiTect plus DNA Bisulfite Kit (Qiagen, Cat no. 59124) according to the manufacturer’s protocol. Genome-wide DNA methylation profiles were measured in 12 pairs of tissue samples by using the Illumina HumanMethylation450 BeadChip (Illumina, Cat no. WG-314-1003); the procedure for the microarray analysis of DNA methylation has been described elsewhere14. Bisulfite pyrosequencing was performed to validate our microarray results from tissue samples. For pyrosequencing, the treated DNA samples were amplified by PCR and fragmented. The processed samples were then precipitated, suspended, and genotyped in the Pyro Mark Q96 system (Qiagen, Hilden, Germany). The sequencing primers were designed to include CpG sites within 0.5 kb of the transcription start site. A methylation level of 15% or higher was considered positive for methylation and any levels equal to or lower than that were considered undistinguishable from the background.
Cell culture
The NSCLC cell lines A549 and H1299 were purchased from Cell Bank of Chinese Academy of Sciences (Cell Bank of CAS, Cat nos. TCHu150 and TCHu160) and maintained in RPMI-1640 medium at 37 °C supplemented with 10% fetal calf serum (Sigma, Cat no. F2442) in a humid atmosphere containing 5% CO2, as recommended by CAS. For the experiments that evaluated cell behavior, cells in the exponential growth phase were used.
Treatment with 5-aza-2’-deoxycytidine (DAC)
NSCLC cells in culture were treated with 10 μM/L 5-aza-2’-deoxycytidine (DAC) (Sigma-Aldrich, Cat no. A3656) for 72 h. After treatment, the cells were harvested to extract genomic DNA and total RNAs for use in gene expression analysis and DNA methylation. For the experiments involving DAC treatment, DMSO was used as the control treatment.
Assays for cell proliferation, migration, invasion, and cell cycle progression
Cell proliferation was analyzed by using Cell Counting Kit-8 (Dojindo, Cat no. CK04, Japan). After DAC treatment for 0 h, 24 h, and 48 h, the cells were treated with 10% CCK-8 in new media for 2 h at 37 °C and the absorbance at 450 nm was measured. Cell migration was measured by using a wound healing assay in 6-well plates. After the cultured cells reached full confluence, a 100-μL pipette tip was used to scrape a line in each well. The images of the cultures were taken at 72 h after DAC treatment. The invasion assay was performed by using Transwell chamber inserts (Corning, USA) with Matrigel according to the manufacturer’s protocol. Cell cycle progression was analyzed by C6 flow cytometer (BD, Cat no. 653160).
For details, please refer to the protocols described in our previous publication16.
siRNA transfection
The transfection assays of TMEM88 siRNA were performed according to the protocols described in our previous publication17.
Statistical analysis
SPSS software version 16.0 was used for the computation of statistical analyses. Student’s t-test was performed to compare changes in cell-based experiments. Chi-squared tests were used to analyze TMEM88 methylation and expression in association with clinical and pathological parameters. The Kaplan-Meier survival curves and log-rank tests were used to evaluate the association between methylation and overall survival. Cox proportional-hazards regression, in which covariates and confounding factors were adjusted, was also used for multivariate survival analysis. The overall survival time was calculated from the date of surgery to the date of death or last live contact. Pearson’s correlation coefficient was calculated to assess the correlation between the expression of TMEM88 and the genes involved in the Wnt signaling pathway by using data from TCGA (The Cancer Genome Atlas). All statistical tests were based on two-tailed analysis.
Results
TMEM88 expression and methylation in NSCLC
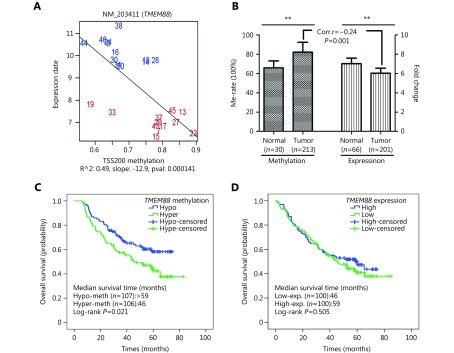
In our microarray analyses of DNA methylation and gene expression, we found that TMEM88 was hypermethylated in 12 NSCLC tumors compared with 12 matched adjacent non-tumor samples and that the methylation was inversely correlated with the expression of TMEM88 (Figure. 1A). High methylation in the TMEM88 promoter region was confirmed in additional 213 tumor samples compared with 30 adjacent non-tumor tissues by using bisulfite pyrosequencing. A low expression of TMEM88 was validated in 201 tumor samples in comparison with 66 adjacent non-tumor tissues by using qRT-PCR. The inverse correlation between methylation and expression was also validated in 173 subjects (P=0.001) (Figure. 1B), which supported the results of the microarray analyses.
1.
Correlation between TMEM88 expression and methylation, and the association TMEM88 expression and methylation with NSCLC overall survival.
TMEM88 and NSCLC characteristics
The methylation and expression of TMEM88 associated with the clinical and pathological features of NSCLC are shown in Table 1. The analysis indicated that TMEM88 methylation was associated with tumor size and histology (P=0.002 and P=0.010, respectively). However, neither TMEM88 methylation nor expression were associated with patient age at diagnosis, gender, BMI, family history of lung cancer, smoking status, and disease stage (Table 1).
1.
Associations of TMEM88 methylation and expression with patient clinical and pathological features
| Characteristics | Group | Methylation | Expression | ||||||||||
| n | Low n (%) | High n (%) | χ2 | P | n | Low n (%) | High n (%) | χ2 | P | ||||
| Age (years) | ≤ 60 | 213 | 56 (54) | 47 (46) | 1.364 | 0.243 | 201 | 50 (51) | 48 (49) | 0.046 | 0.831 | ||
| > 60 | 51 (46) | 59 (54) | 51 (50) | 52 (50) | |||||||||
| Stage | I-II | 208 | 74 (53) | 65 (47) | 0.868 | 0.351 | 197 | 58 (48) | 64 (52) | 0.943 | 0.331 | ||
| III-IV | 32 (46) | 37 (54) | 41 (55) | 34 (45) | |||||||||
| BMI | ≤ 24 | 212 | 59 (52) | 55 (48) | 0.162 | 0.687 | 199 | 48 (49) | 51 (51) | 0.126 | 0.723 | ||
| > 24 | 48 (49) | 50 (51) | 51 (51) | 49 (49) | |||||||||
| Gender | Male | 213 | 58 (47) | 66 (53) | 1.422 | 0.233 | 201 | 65 (52) | 61 (48) | 0.242 | 0.623 | ||
| Female | 49 (55) | 40 (45) | 36 (48) | 39 (52) | |||||||||
| History of lung diseases | No | 213 | 97 (50) | 98 (50) | 0.223 | 0.637 | 201 | 94 (51) | 91 (49) | 0.294 | 0.588 | ||
| Yes | 10 (56) | 8 (44) | 7 (44) | 9 (56) | |||||||||
| Family history | No | 212 | 90 (51) | 88 (49) | 0.004 | 0.952 | 200 | 81 (50) | 81 (50) | 0.085 | 0.770 | ||
| Yes | 17 (50) | 17 (50) | 20 (53) | 18 (47) | |||||||||
| Smoking status | No | 213 | 43 (57) | 33 (43) | 1.902 | 0.168 | 201 | 25 (42) | 34 (58) | 2.072 | 0.150 | ||
| Yes | 64 (47) | 73 (53) | 76 (54) | 66 (46) | |||||||||
| Histology | LUSC | 185 | 40 (42) | 55 (58) | 6.683 | 0.010 | 167 | 52 (53) | 47 (47) | 0.776 | 0.378 | ||
| LUAD | 55 (61) | 35 (39) | 31 (46) | 37 (54) | |||||||||
| Tumor size (cm) | < 3 | 212 | 46 (66) | 24 (34) | 9.713 | 0.002 | 200 | 30 (52) | 28 (48) | 0.097 | 0.755 | ||
| ≥ 3 | 61 (43) | 81 (57) | 70 (49) | 72 (51) | |||||||||
TMEM88 and NSCLC survival
To evaluate the association between TMEM88 methylation and overall survival, we performed Kaplan-Meier survival analysis on 213 patients with NSCLC. The analysis showed that patients with high TMEM88 methylation had a shorter overall survival (median survival of 46 months) than those with low TMEM88 methylation (median survival >59 months, P=0.021) (Figure. 1C and Table 2). This survival association with TMEM88 methylation in NSCLC remained significant after the adjustment for covariates and potential confounding factors (Table 2). However, no association was observed between TMEM88 expression and overall survival in the study (Table 2).
2.
Cox regression analysis of overall survival and patient characteristics
| Variable | Univariate | Multivariate | |||
| RR (95%CI) | P | RR (95%CI) | P | ||
| RR: relative risk; 95%CI: 95% confidence interval | |||||
| Age | 1.53 (1.06–2.20) | 0.023 | 1.51 (0.96–2.37) | 0.077 | |
| Gender | |||||
| Male | 1.00 | 0.672 | 1.00 | 0.484 | |
| Female | 1.08 (0.75–1.56) | 0.80 (0.44–1.48) | |||
| Smoking status | |||||
| No | 1.00 | 0.241 | 1.00 | 0.211 | |
| Yes | 0.80 (0.56–1.16) | 0.67 (0.36–1.26) | |||
| Stage | |||||
| I–II | 1.00 | < 0.001 | 1.00 | < 0.001 | |
| III–IV | 4.18 (2.86–6.10) | 3.44 (2.18–5.44) | |||
| Family history of cancer | |||||
| No | 1.00 | 0.341 | 1.00 | 0.519 | |
| Yes | 1.25 (0.79–1.97) | 1.21 (0.68–2.17) | |||
| TMEM88 methylation | |||||
| Low | 1.00 | 0.021 | 1.00 | 0.018 | |
| High | 1.57 (1.07–2.38) | 1.74 (1.10–2.74) | |||
| TMEM88 expression | |||||
| Low | 1.00 | 0.521 | 1.00 | 0.940 | |
| High | 0.88 (0.60–1.30) | 0.98 (0.63–1.53) | |||
TMEM88 expression in NSCLC cell lines after DAC treatment
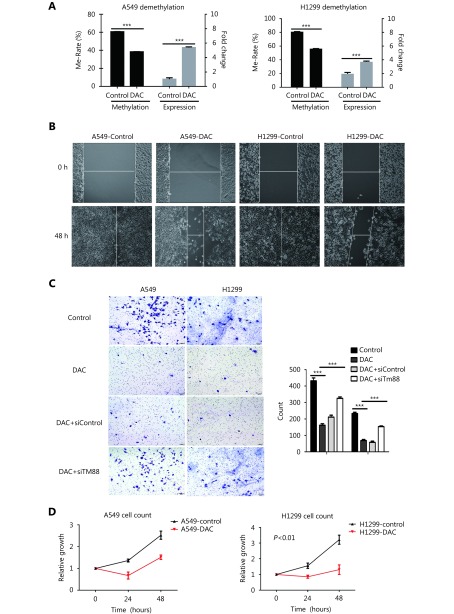
To assess the functional relevance of TMEM88 methylation in NSCLC, we analyzed the methylation changes related to TMEM88 expression after the treatment of the NSCLC cell lines A549 and H1299 with the demethylation agent, DAC. Our experiments showed that DAC treatment reduced TMEM88 methylation and resulted in elevated TMEM88 expression in both cell lines (Figure. 2A).
2.
2.
Demethylation of TMEM88 by DAC and cell migration, invasion, proliferation, and cell cycle.
DAC-induced TMEM88 expression and NSCLC cell behavior
To evaluate the effect of TMEM88 expression on NSCLC cells, we performed a number of in vitro experiments. First, we investigated cell migration and invasion after the DAC-induced upregulation of TMEM88 expression in H1299 and A549 cells that originally had a low expression and high methylation of TMEM88. The experiments showed that the demethylation of TMEM88 in H1299 and A549 significantly delayed cell migration (Figure. 2B). Moreover, the invasion ability of H1299 and A549 cells was also significantly decreased (P < 0.05 and P < 0.01, respectively) after demethylation treatment ( Figure. 2C). In order to further validate the effect of TMEM88 expression on NSCLC cells, we transfected the cells with TMEM88 siRNA after DAC treatment, which reduced TMEM88. The experiments showed that the demethylation of TMEM88 in H1299 and A549 significantly delayed cell migration (Figure. 2C), but that these effects were abolished when the cells were transfected with TMEM88 siRNA.
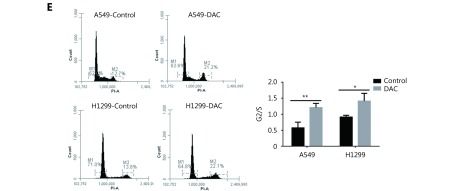
We then analyzed cell proliferation and cell cycle progression after DAC treatment. Cell proliferation in DAC-treated H1299 cells was decreased by 60% in comparison with the untreated cells (P < 0.01). Proliferation of A549 cells was also decreased, but the change was not statistically significant ( Figure. 2D). Therefore, the demethylation of TMEM88 retarded cell cycle progression at the G2/M phase in both cell lines (Figure. 2E).
Correlation between TMEM88 and molecules in the Wnt signaling pathway
TMEM88 was reported to inhibit canonical Wnt/β-Catenin signaling through interaction with the Wnt pathway factor Dishevelled (DVLS)14 As an inhibitor of the Wnt signaling, TMEM88 may interact with the molecules in the signaling pathway. To determine whether the expression of TMEM88 was correlated with the genes involved in Wnt/β-Catenin signaling, we analyzed data from TCGA. We downloaded freely available gene expression data from lung cancer samples and calculated expression correlations between TMEM88 and several components in the Wnt/β-Catenin signaling pathway, such as Wnt, Frizzled class receptor (FZD), Receptor tyrosine kinase like orphan receptor (ROR), and Dishevelled segment polarity protein (DVL). Modest correlations were found between TMEM88 expression and some of these genes, including DVL1 (r=0.21), FZD4 (r=0.27), FZD5 (r=0.39), and ROR1 (r=0.30) (Table 3).
3.
Pearson correlation coefficients between the expression of TMEM88 and the genes involved in the Wnt signal pathway in TCGA.
| Gene symbol | Description | P | Pearson χ2 |
| DVL1 | Dishevelled segment
polarity protein 1 |
< 0.001 | 0.206 |
| DVL2 | Dishevelled segment
polarity protein 2 |
< 0.050 | 0.102 |
| DVL3 | Dishevelled segment
polarity protein 3 |
< 0.050 | –0.107 |
| FZD10 | Frizzled class receptor 10 | < 0.050 | –0.101 |
| FZD2 | Frizzled class receptor 2 | < 0.010 | 0.139 |
| FZD4 | Frizzled class receptor 4 | < 0.001 | 0.266 |
| FZD5 | Frizzled class receptor 5 | < 0.001 | 0.390 |
| FZD7 | Frizzled class receptor 7 | < 0.050 | –0.124 |
| FZD8 | Frizzled class receptor 8 | < 0.010 | 0.138 |
| ROR1 | Receptor tyrosine kinase like orphan receptor 1 | < 0.001 | 0.301 |
| WNT5A | Wnt family member 5A | < 0.010 | –0.155 |
Discussion
Our study demonstrated that the TMEM88 promoter was highly methylated in NSCLC and that this hypermethylation was correlated with a low expression of TMEM88. The survival analysis showed that patients with NSCLC and methylated TMEM88 had poor overall survival compared with those without methylated TMEM88. In addition, patients with higher TMEM88 expression had a longer survival time in more than 36 months survival group (P>0.05). Furthermore, ourin vitro experiments revealed that TMEM88 expression was increased after the treatment of NSCLC cells with the demethylation agent DAC; the treatment also inhibited cell proliferation, migration, and invasion, which indicated that our observations in patients were biologically relevant. These findings suggested that promoter methylation of TMEM88 may serve as a potential biomarker for NSCLC prognosis and treatment.
TMEM88 is a transmembrane protein located on the cell membrane of Xenopus embryonic cells18. As a newly discovered gene, limited studies have been conducted on TMEM88. Although the mechanism is not fully understood, evidence has suggested that TMEM88 may play an important role in the regulation of human stem cell differentiation and embryonic development and have significant involvement in cell signaling3. However, no studies have previously shown that TMEM88 methylation is implicated in lung cancer prognosis.
Many genes have been reported to undergo aberrant DNA methylation in cancer19,20, such as the DNA repair gene O(6)-methylguanine DNA methyltransferase (MGMT) in B-cell lymphoma21, dickkopf 3 (Dkk3) gene in gastric cancer22, secreted frizzled-related protein 1 (SFRP1) in breast cancer23 and colorectal cancer24, MGMT and MLH1 in colorectal cancer25, and WIF-126 and Axin27 in lung cancer. In our study, we discovered that the hypermethylation of TMEM88 in NSCLC was associated with an unfavorable prognosis. In addition to our findings, Mullapudi et al.28 performed a genome-wide analysis of the lung cancer methylome and identified the hypermethylation of TMEM88. Lee et al.26 demonstrated that promoter methylation of Wnt inhibitory factor 1 (Wif1) was an early and frequent event and the hypermethylation of Wif1 was an indicator of the unfavorable prognosis of non-small cell lung cancer. Hypermethylated Axin was also correlated with the progression of lung cancer27. Zhang et al.23 revealed that the hypermethylation of frizzled-related protein 1 (SFRP1) was a common contributory factor in lung carcinogenesis and could serve as a potential biomarker for NSCLC in the Chinese population.
In our study, we demonstrated that TMEM88 expression level was increased after the demethylation treatment of NSCLC cells and that the treatment resulted in the inhibition of cell migration, invasion, and proliferation. These results suggested that TMEM88 may behave as a tumor suppressor in NSCLC and that the hypermethylation of TMEM88 in NSCLC may be associated with an unfavorable prognosis. Previous studies have suggested that the activation of the Wnt signaling pathway promotes tumorigenesis29; conversely, the inhibition of Wnt signaling suppresses this process3. Our analysis of online data also indicated that TMEM88 expression was correlated with some of the molecules involved in the Wnt signaling pathway. TMEM88 inhibits Wnt/β-Catenin signaling through interaction with the Wnt pathway factor Dishevelled (DVLS)14. Therefore, the hypermethylation of the TMEM88 gene may inhibit its function as a negative modulator of Wnt/β-Catenin signaling, which results in the constitutive activation of this signal pathway in NSCLC30. This may explain why the hypermethylation of TMEM88 in NSCLC is associated with an unfavorable prognosis. There is evidence to demonstrate that promoter hypermethylation inactivates various Wnt pathway inhibitors, such as WIF-126,31, sFRP-112, sFRP-532, and Dkk-333, which are also associated with poor prognosis. Therefore, we concluded that the Wnt/β-Catenin signaling pathway was important in NSCLC biology and prognosis and TMEM88 act as an inhibitor of this signal pathway. Further, the methylation of TMEM88 may serve as a potential biomarker for Wnt/β-Catenin signaling when the pathway is targeted for therapy.
Recently, Zhang et al.14 suggested that the presence of the TMEM88 protein in the cytosol of NSCLC cells could promote cell invasion and metastasis through the activation of the p38-GSK3ß-Snail signal pathway. However, our study suggested that TMEM88 had high methylation and low expression in tumors compared with normal tissues and that demethylation treatment could inhibit the invasion and migration of NSCLC cells (Figure. 1 and 2). Jang et al.34 demonstrated that mir-708 could promote the invasion of NSCLC cells through the suppression of the expression of TMEM88, which suggested that TMEM88 may be a tumor suppressor. Furthermore, the expression of TMEM88 in 58 tumor samples and matched adjacent non-tumor tissues in the TCGA database was 25.27±12.58 and 171.88±97.04, respectively (P < 0.0001), which supported the behavior of TMEM88 as a tumor suppressor. Studies have also suggested that TMEM88 may have different forms of molecules, which are located in different cellular compartments and have different functions and activities14,15. Thus, more studies are needed to clarify the role of TMEM88 in lung cancer.
In summary, we observed that the epigenetic silencing of TMEM88 by promoter methylation was associated with a poor prognosis of NSCLC and that TMEM88 could inhibit cell proliferation, migration, and invasion. These findings suggested that TMEM88 may function as a tumor suppressor and serve as a potential biomarker for NSCLC prognosis and treatment.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant No.81573231) and the Medical Professionals Crossing Project of Shanghai Jiao Tong University (Grant No. YG2015ZD01).
Conflict of interest statement
No potential conflicts of interest were disclosed.
References
- 1.Globocan 2012: Estimated Cancer Incidence. Mortality and Prevalence Worldwide in 2012. 2013. Available at http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx Αccessed May 2017.
- 2.Rusek AM, Abba M, Eljaszewicz A, Moniuszko M, Niklinski J, Allgayer H. MicroRNA modulators of epigenetic regulation, the tumor microenvironment and the immune system in lung cancer. Mol Cancer. 2015; 14: 34.
- 3.Stewart DJ. Wnt signaling pathway in non-small cell lung cancer. J Natl Cancer Inst. 2014; 106: djt356.
- 4.Doerfler W. DNA methylation and gene activity. Annu Rev Biochem. 1983;52:93–124. doi: 10.1146/annurev.bi.52.070183.000521. [DOI] [PubMed] [Google Scholar]
- 5.Busslinger M, Hurst J, Flavell RA. DNA methylation and the regulation of globin gene expression. Cell. 1983;34:197–206. doi: 10.1016/0092-8674(83)90150-2. [DOI] [PubMed] [Google Scholar]
- 6.Sepulveda H, Villagra A, Montecino M. Tet-mediated DNA demethylation is required for SWI/SNF-dependent chromatin remodeling and histone modifying activities that trigger expression of the Sp7 osteoblast master gene during mesenchymal lineage commitment. Mol Cell Biol. 2017; 37: e00177-17.
- 7.Rhee JK, Kim K, Chae H, Evans J, Yan P, Zhang BT, et al. Integrated analysis of genome-wide DNA methylation and gene expression profiles in molecular subtypes of breast cancer. Nucleic Acids Res. 2013;41:8464–74. doi: 10.1093/nar/gkt643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu YP, Ding Y, Chen R, Liao SG, Ren BG, Michalopoulos A, et al. Whole-genome methylation sequencing reveals distinct impact of differential methylations on gene transcription in prostate cancer. Am J Pathol. 2013;183:1960–70. doi: 10.1016/j.ajpath.2013.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li J, Ching T, Huang S, Garmire LX. Using epigenomics data to predict gene expression in lung cancer. BMC Bioinformatics. 16 Suppl 5: S10.
- 10.Licchesi JDF, Westra WH, Hooker CM, Machida EO, Baylin SB, Herman JG. Epigenetic alteration of Wnt pathway antagonists in progressive glandular neoplasia of the lung. Carcinogenesis. 2008;29:895–904. doi: 10.1093/carcin/bgn017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kobayashi K, Ouchida M, Tsuji T, Hanafusa H, Miyazaki M, Namba M, et al. Reduced expression of the REIC/Dkk-3 gene by promoter-hypermethylation in human tumor cells . Gene. 2002;282:151–8. doi: 10.1016/s0378-1119(01)00838-1. [DOI] [PubMed] [Google Scholar]
- 12.Zhang YW, Miao YF, Yi J, Geng J, Wang R, Chen LB. Transcriptional inactivation of secreted frizzled-related protein 1 by promoter hypermethylation as a potential biomarker for non-small cell lung cancer. Neoplasma. 2010;57:228–33. doi: 10.4149/neo_2010_03_228. [DOI] [PubMed] [Google Scholar]
- 13.Mazieres J, He B, You L, Xu ZD, Lee AY, Mikami I, et al. Wnt inhibitory factor-1 is silenced by promoter hypermethylation in human lung cancer. Cancer Res. 2004;64:4717–20. doi: 10.1158/0008-5472.CAN-04-1389. [DOI] [PubMed] [Google Scholar]
- 14.Zhang XP, Yu XM, Jiang GY, Miao Y, Wang L, Zhang Y, et al. Cytosolic TMEM88 promotes invasion and metastasis in lung cancer cells by binding DVLS . Cancer Res. 2015;75:4527–37. doi: 10.1158/0008-5472.CAN-14-3828. [DOI] [PubMed] [Google Scholar]
- 15.Palpant NJ, Pabon L, Rabinowitz JS, Hadland BK, Stoick-Cooper CL, Paige SL, et al. Transmembrane protein 88: a Wnt regulatory protein that specifies cardiomyocyte development. Development. 2013;140:3799–808. doi: 10.1242/dev.094789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu B, Qu JL, Xu FX, Guo Y, Wang Y, Yu H, et al. Mir-195 suppresses non-small cell lung cancer by targeting CHEK1. Oncotarget. 2015;6:9445–56. doi: 10.18632/oncotarget.3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feng NN, Ching T, Wang Y, Liu B, Lin HY, Shi OM, et al. Analysis of microarray data on gene expression and methylation to identify long non-coding RNAs in non-small cell lung cancer. Sci Rep. 2016; 6: 37233.
- 18.Lee HJ, Finkelstein D, Li XF, Wu DQ, Shi DL, Zheng JJ. Identification of transmembrane protein 88 (TMEM8888) as a dishevelled-binding protein . J Biol Chem. 2010;285:41549–56. doi: 10.1074/jbc.M110.193383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herman JG, Baylin SB. Gene silencing in cancer in association with promoter hypermethylation. N Engl J Med. 2003;349:2042–54. doi: 10.1056/NEJMra023075. [DOI] [PubMed] [Google Scholar]
- 20.Egger G, Liang GN, Aparicio A, Jones PA. Epigenetics in human disease and prospects for epigenetic therapy. Nature. 2004;429:457–63. doi: 10.1038/nature02625. [DOI] [PubMed] [Google Scholar]
- 21.Esteller M, Gaidano G, Goodman SN, Zagonel V, Capello D, Botto B, et al. Hypermethylation of the DNA repair gene O6-methylguanine DNA methyltransferase and survival of patients with diffuse large B-cell lymphoma . J Natl Cancer Inst. 2002;94:26–32. doi: 10.1093/jnci/94.1.26. [DOI] [PubMed] [Google Scholar]
- 22.Yu J, Tao Q, Cheng YY, Lee KY, Ng SSM, Cheung KF, et al. Promoter methylation of the Wnt/β-catenin signaling antagonist Dkk-3 is associated with poor survival in gastric cancer . Cancer. 2009;115:49–60. doi: 10.1002/cncr.23989. [DOI] [PubMed] [Google Scholar]
- 23.Veeck J, Niederacher D, An H, Klopocki E, Wiesmann F, Betz B, et al. Aberrant methylation of the Wnt antagonist SFRP1 in breast cancer is associated with unfavourable prognosis. Oncogene. 2006;25:3479–88. doi: 10.1038/sj.onc.1209386. [DOI] [PubMed] [Google Scholar]
- 24.Chen JJ, Wang AQ, Chen QQ. DNA methylation assay for colorectal carcinoma. Cancer Biol Med. 2017;14:42–9. doi: 10.20892/j.issn.2095-3941.2016.0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tariq K, Ghias K. Colorectal cancer carcinogenesis: a review of mechanisms. Cancer Biol Med. 2016;13:120–35. doi: 10.28092/j.issn.2095-3941.2015.0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee SM, Park JY, Kim DS. Wif1 hypermethylation as unfavorable prognosis of non-small cell lung cancers with EGFR mutation . Mol Cells. 2013;36:69–73. doi: 10.1007/s10059-013-0060-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang LH, Xu HT, Li QC, Jiang GY, Zhang XP, Zhao HY, et al. Abnormal hypermethylation and clinicopathological significance of Axin gene in lung cancer. Tumour Biol. 2013;34:749–57. doi: 10.1007/s13277-012-0604-z. [DOI] [PubMed] [Google Scholar]
- 28.Mullapudi N, Ye B, Suzuki M, Fazzari M, Han WG, Shi MK, et al. Genome wide methylome alterations in lung cancer. PLoS One. 2015; 10: e0143826.
- 29.Lin SY, Xia WY, Wang JC, Kwong KY, Spohn B, Wen Y, et al. β-catenin, a novel prognostic marker for breast cancer: its roles in cyclin D1 expression and cancer progression. Proc Natl Acad Sci USA. 2000;97:4262–66. doi: 10.1073/pnas.060025397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cai SP, Cheng XY, Chen PJ, Pan XY, Xu T, Huang C, et al. Transmembrane protein 88 attenuates liver fibrosis by promoting apoptosis and reversion of activated hepatic stellate cells. Mol Immunol. 2016;80:58–67. doi: 10.1016/j.molimm.2016.11.002. [DOI] [PubMed] [Google Scholar]
- 31.Suzuki M, Wada H, Yoshino M, Tian L, Shigematsu H, Suzuki H, et al. Molecular characterization of chronic obstructive pulmonary disease-related non-small cell lung cancer through aberrant methylation and alterations of EGFR signaling. Ann Surg Oncol. 2010;17:878–88. doi: 10.1245/s10434-009-0739-3. [DOI] [PubMed] [Google Scholar]
- 32.Zhu J, Wang YY, Duan JC, Bai H, Wang ZJ, Wei L, et al. DNA Methylation status of Wnt antagonist SFRP5 can predict the response to the EGFR-tyrosine kinase inhibitor therapy in non-small cell lung cancer. J Exp Clin Cancer Res. 2012; 31: 80.
- 33.Suzuki M, Shigematsu H, Nakajima T, Kubo R, Motohashi S, Sekine Y, et al. Synchronous alterations of Wnt and epidermal growth factor receptor signaling pathways through aberrant methylation and mutation in non-small cell lung cancer. Clin Cancer Res. 2007;13:6087–92. doi: 10.1158/1078-0432.CCR-07-0591. [DOI] [PubMed] [Google Scholar]
- 34.Jang JS, Jeon HS, Sun ZF, Aubry MC, Tang H, Park CH, et al. Increased miR-708 expression in NSCLC and its association with poor survival in lung adenocarcinoma from never smokers. Clin Cancer Res. 2012;18:3658–67. doi: 10.1158/1078-0432.CCR-11-2857. [DOI] [PMC free article] [PubMed] [Google Scholar]