Abstract
Multiple endocrine neoplasia type 1 (MEN1) is a familial cancer syndrome with neuroendocrine tumorigenesis of the parathyroid glands, pituitary gland, and pancreatic islet cells. The MEN1 gene codes for the canonical tumor suppressor protein, menin. Its protein structure has recently been crystallized, and it has been investigated in a multitude of other tissues. In this review, we summarize recent advancements in understanding the structure of the menin protein and its function as a scaffold protein in histone modification and epigenetic gene regulation. Furthermore, we explore its role in hepatobiliary autoimmune diseases, cancers, and metabolic diseases. In particular, we discuss how menin expression and function are regulated by extracellular signaling factors and nuclear receptor activation in various hepatic cell types. How the many signaling pathways and tissue types affect menin’s diverse functions is not fully understood. We show that small-molecule inhibitors affecting menin function can shed light on menin’s broad role in pathophysiology and elucidate distinct menin-dependent processes. This review reveals menin’s often dichotomous function through analysis of its role in multiple disease processes and could potentially lead to novel small-molecule therapies in the treatment of cholangiocarcinoma or biliary autoimmune diseases.
Key words: Biliary epithelium, Fibrosis, Transforming growth factor-β (TGF-β), Mixed lineage leukemia (MLL), Histone deacetylase, Cholangiocarcinoma (CCA), Hepatocellular carcinoma (HCC), JunD, Pancreas, Immunology, Metabolic disease
INTRODUCTION
The protein menin was originally studied within the context of multiple endocrine neoplasia type 1 (MEN1), a rare hereditary autosomal dominant tumor syndrome1. MEN1 syndrome penetrance and expressivity are variable, even among patients from the same family tree. The most common manifestation (∼100% penetrance by age 50) is primary hyperparathyroidism (HPT), followed by the development of anterior pituitary adenomas (∼10%–60% penetrance)2. Pancreatic neuroendocrine tumors (PNETs), thymic or bronchial carcinoid tumors, and adrenocortical tumors account for a significant remainder of the morbidity and mortality seen in MEN1 syndrome3. Additionally, nonendocrine tumors such as angiofibromas, collagenomas, lipomas, and melanomas are common manifestations2. Emerging evidence points to more clinically subtle complications of MEN1 syndrome, possibly due to hormonal imbalances as a result of neuroendocrine growths. Patients often suffer from kidney and bone disease, premature cardiovascular disease, insulin resistance, and glucose intolerance4.
MENIN STRUCTURE AND FUNCTION
Menin is a 67-kDa nuclear protein (Fig. 1) coded by 10 exons spanning 9 kb of genomic sequence (Fig. 2) and expressed ubiquitously. Patients with MEN1 syndrome inherit a mutated MEN1 allele (11q13) from one of their parents. Tissue-specific somatic mutation in the remaining functional allele, or “second hit,” results in complete loss of menin expression and organ tumorigenesis. Diagnosis of MEN1 syndrome is validated by the identification of a mutation in the exon-coding region or intron–exon junctions of the MEN1 gene locus3. One genomic analysis of >1,300 MEN1 patients revealed that over 70% of mutations lead to truncated or nonexistent forms of menin5. Inhibition of the proteasome–ubiquitin pathway can restore protein expression in some cases6. It should be noted that not all MEN1 families have a mutation in the MEN1 coding region, indicating a need to understand the regulatory elements surrounding menin expression and function. Thus, it appears that other genetic and environmental factors play a role in the MEN1 syndrome disease process.
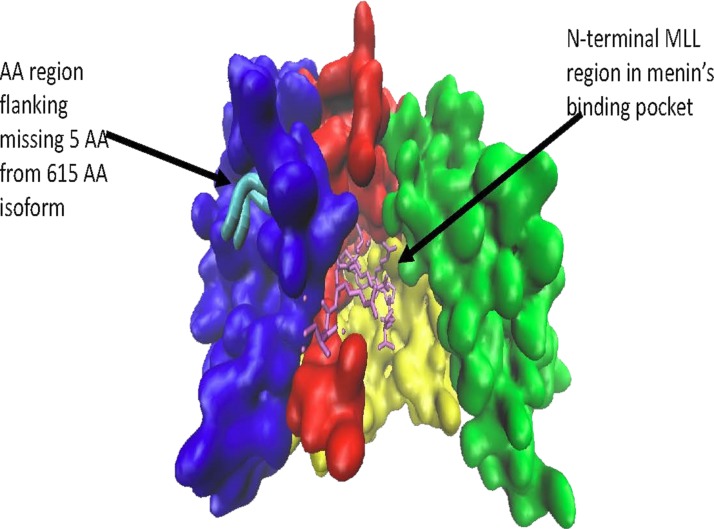
Figure 1.
A 610-amino acid menin isoform (type 2). Note how overall binding groove appears intact and the missing five amino acids are located on the surface away from menin’s binding pocket. This image was rendered from the online RCSB Protein Data Bank code 4GQ6 using VMD 1.9.3 Graphics (University of Illinois at Urbana-Champaign).
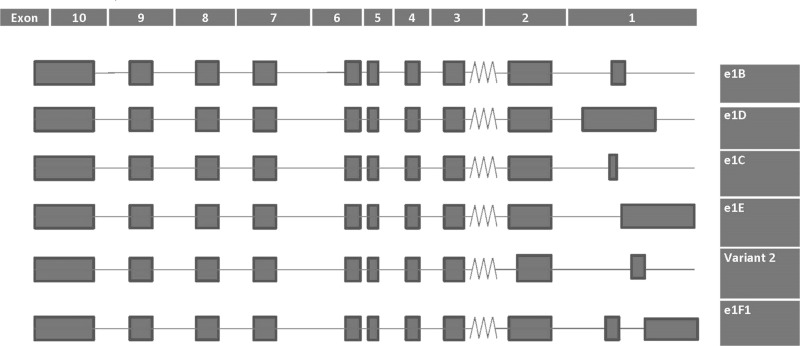
Figure 2.
Graphical depiction of multiple endocrine neoplasia type 1 (MEN1) transcript variants taken from UCSC Genome Browser (1/25/17). Variant 2 codes for the shortened menin isoform 2 (610 amino acids). Note how the five amino acids appear to be lost from the C-terminal end of exon 2.
Phylogenetically, menin is evolutionarily conserved among vertebrates and Drosophila; however, its amino acid sequence does not share homology with other known proteins7. Mounting evidence points to its role as a scaffold protein with many direct and indirect binding partners7. For example, menin associates with many hundreds of loci on the mammalian genome despite its lack of a known DNA-binding sequence8. It is generally agreed that menin’s primary role is to regulate chromatin architecture through histone modifications resulting in altered gene transcription or even DNA repair7. Menin has been shown to directly bind to JunD, an AP-1 transcription factor, to facilitate histone deacetylation and turn off transcription of JunD targets. It has also been shown to enable transcription of mixed-lineage leukemia (MLL) protein target genes by modifying histones with activating trimethylation marks9,10.
While absolute loss of menin expression is required for MEN1 syndrome tumorigenesis, relative menin expression has since been implicated in cellular proliferation of the lung, stomach, liver, blood, breast, and prostate11–14. However, its function is tissue and pathway specific. For instance, it is oncogenic in certain leukemias and prostate cancers, but it acts as a tumor suppressor in canonical MEN1 syndrome organ sites. Furthermore, menin can act through distinct signaling pathways, such as nuclear factor-κB (NF-κB) and transforming growth factor-β (TGF-β), and affect diverse functional outcomes such as apoptosis, cell cycle, gene transcription, and DNA repair7,13,15. It remains unclear why tumors arise only in neuroendocrine organs and not in other tissue types in patients with MEN1 syndrome. Furthermore, the surrounding regulatory elements that allow variable expressivity and penetrance seen in MEN1 syndrome are not yet understood.
MEN1 ISOFORMS
One explanation for menin’s broad and variable role is the 5′ heterogeneity of the MEN1 mRNA transcript10. Northern blot, 5′ RACE, and positional deletion studies revealed that the MEN1 transcripts come in two sizes: a ubiquitously expressed 2.8-kb transcript and a larger 4.2-kb transcript with expression restricted to the pancreas and thymus16. The 2.8-kb transcript codes for two isoforms of 615- and 610-amino acid length (NP_000235.2 and NP_570711.1, respectively). There are additional transcript variants based on alternative splicing of exon 1 (UCSC genome browser, 1/25/17) (Fig. 2). MEN1’s promoter is located upstream of exon 2 with multiple transcriptional start sites (TSSs) located along with corresponding initiator elements (Inr) directing transcription of individualized exon 1 splice variants. These matching TSS/Inr sequences and splice variants, along with specific upstream and downstream cis-regulatory sequences, regulate menin expression on a cell-by-cell basis, suggesting a cell-specific regulation of MEN1 transcription17.
Four MEN1 transcript variants were detected using RNA-Sequence data obtained from human cholangiocarcinoma (CCA) and hepatocellular carcinoma (HCC) from The Cancer Genome Atlas (http://cancergenome.nih.gov) using Python version 2.7 (http://www.python.org). Example coding can be found at https://github.com/ehrl1ch/RNA-Seq. There is a small shift in menin variant expression between CCA and HCC tumor samples and matched normal tissues (Fig. 3). As noted in Figure 2, menin’s various isoforms are quite similar and vary only in their TSS and exon splicing. The difference between the primary 615-amino acid isoform and the 610-amino acid isoform is the loss of Trp–Ser–Pro–Val–Gly at the 149 position (see Fig. 1 for details). While no functional differences between MEN1’s various isoforms have been characterized to date, it is possible that the expression of two different isoforms in the same tissue represents distinct pathways or at least an artifact of functional dysregulation. The cause and effect of such dysregulation are unknown.
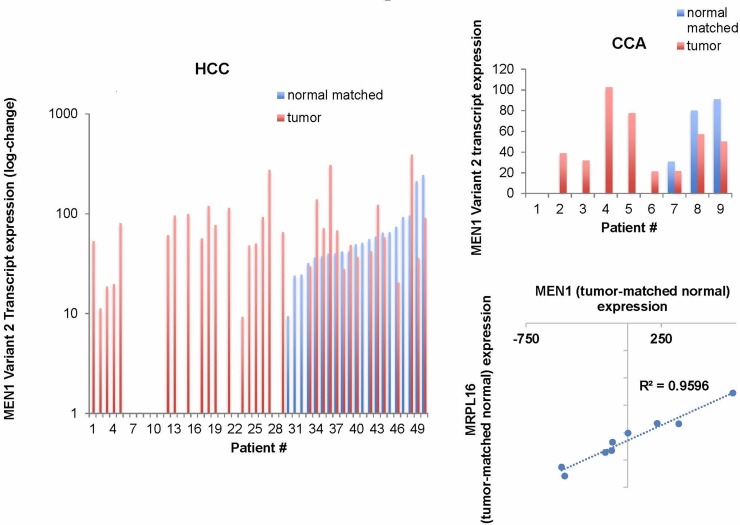
Figure 3.
MEN1 transcript variant 2 is aberrantly expressed in human hepatocellular carcinoma (HCC) and cholangiocarcinoma (CCA) tissues compared to matched normal tissue. MRPL16, a nuclear-coded mitochondrial ribosome, shows significant positive correlation with MEN1 expression in CCA using the Pearson correlation test. MRPL16 is located on chromosome 11 only a few hundred thousand base pairs away from MEN1. It is of potential interest given the amount of mutation in mitochondrial pathways in CCA. p < 0.1.
FIBROSIS
TGF-β signaling is well characterized in the liver as well18. For instance, TGF-β inhibits activation of quiescent hepatic stellate cells (HSCs) via Smad7 activity but stimulates fibrotic reaction in activated HSCs or differentiated myofibroblasts via Smad3 activity18. TGF-β is antiproliferative and proapoptotic in hepatocytes18. Loss of hepatic TGF-β signaling in dominant negative TGF-β receptor type II (dnTGFβrII) mice is a model for primary biliary cirrhosis (PBC) featuring anti-mitochondrial antibodies, lymphocytic infiltration, periportal fibrosis, and increased incidence of HCC19. One study showed that dnTGFβrII mice displayed increased proinflammatory Tregs compared to wild type (WT)20, indicating that TGF-β can affect nonparenchymal cell types.
The relationship between menin and TGF-β in HSC-driven fibrosis was further explored using a bioinformatics approach21. This study examined HCC arising in patients with cirrhosis and discovered upregulated MEN1 gene expression correlated with an increase in TGF-β pathway signaling. Menin expression was associated with HSC activation and positively correlated with profibrotic collagen and metallopeptidase inhibitor gene expression, both TGF-β/Smad transcription targets. Furthermore, TGF-β increases menin expression in both hepatocytes and HSCs21,22. Interestingly, one study showed that menin drives HCC formation through epigenetic upregulation of Yes-associated protein (YAP), which itself has a diverse array of functions including antagonism of Smad-associated TGF-β pathway23. However, this contradicts previous studies confirming menin’s tumor suppressor role in HCC13. Further studies may shed light on menin’s role in balancing proliferation and fibrosis during HCC development and progression.
IMMUNOLOGY
As a ubiquitously expressed protein, menin plays a role in immune cells. Recently, menin has been implicated in CD4+ T-cell immunosenescence and senescence-associated secretory phenotype24. Senescent CD4+ T cells exhibit irregular homeostasis and cytokine production that can contribute to cancer, infectious disease, and autoimmune disease24. Menin binds to the BACH2 locus and suppresses its expression through histone deacetylation. Decreased BACH2 expression increases cellular senescence. The role of menin in immune cells and hepatobiliary diseases such as primary sclerosing cholangitis (PSC), PBC, and CCA has not been explored.
Th17+ T-cell differentiation is menin dependent as well. In naive CD4+ T cells, menin is recruited to the IL17 locus to drive its expression. Furthermore, menin binds the RORC locus to drive the expression of RORγδ in order to maintain Th17+ T-cell differentiation and function25. IL-17 secretion by Th17+ T cells can trigger HSC activation, collagen deposition, and subsequent hepatic fibrosis26. Peripheral blood mononuclear cells from patients with PSC showed significantly higher proportion of Th17+ cells compared to healthy patients27. In MDR2−/− mice, the prevalence of IL-17+CD4+ T cells is thought to come at the expense of anti-inflammatory Foxp3+ regulatory T cells28–30. Interestingly, Foxp3 interacts with EZH2, a Polycomb Group protein and menin-binding partner involved in chromatin silencing to suppress gene expression through DNA and histone methylation. Ezh2−/− T cells failed to adequately differentiate into Foxp3+ regulatory T cells nor function properly in vivo31. Thus, targeting menin expression in Th17+ or naive CD4+ T cells could ameliorate fibrotic reaction in cholestatic liver disease.
Inhibiting menin expression also seems to decrease effector antigen-specific CD8+ T cells, which were shown to be profibrotic in a bile duct ligated mouse model of cholestasis29,32. Yamada et al. infected CD8+ T-cell-specific menin knockout mice with Listeria monocytogenes ovalbumin-expressing bacteria in order to assess menin’s role in antigen-specific CD8+ T-cell response. CD8+ T cell’s lack of menin expression reduced its own proliferation and survival upon infection via induction of cell cycle inhibitors, proapoptotic genes, and transcription factors associated with effector CD8+ T-cell death32. Thus, theoretically overexpressing menin could drive proliferation and survival of effector CD8+ T cells necessary to clear infection but provoke hepatic fibrosis as a consequence.
While menin expression is directly implicated in CD4+ T-cell senescence, Th17+ T-cell differentiation and maintenance, and CD8+ effector T-cell survival, it is indirectly implicated in CD4+ Th2 cell development through the MLL complex33. MLL+/− cells were able to differentiate into Th2 memory cells but were not able to maintain H3K4 methylation and H3K9 acetylation at the Th2 and Gata loci nor the expression of Th2 cytokines IL-4, IL-5, and IL-1333. Later studies coimmunoprecipitated menin and MLL from the c-Myb/GATA-2 complex necessary for Th2 memory cell formation34. Interestingly, menin was more prevalent at the GATA-3 promoter site under Th2-promoting conditions, while MLL was more prevalent among CD4+ effector/memory cells. Enhanced Th2 activity has been implicated in IG4-related cholangitis35. However, overall measurements of Th1/Th2 ratios in PSC/PBC populations have been inconsistent36.
Chromatin-modifying factors are implicated in the macrophage differentiation between the proinflammatory M1 phenotype and the alternative M2 phenotype37. MLL-dependent H3K4me3 and H3K27 demethylation characterize M1 polarization, while M2 polarization is characterized by DNA methyltransferases and histone deacetylases (HDACs)37. M1 macrophages treated with a menin–MLL inhibitor exhibited decreased expression of proinflammatory cytokine CXCL1037. Interestingly, the M2 macrophage phenotype is more prevalent in CCA tissue and is associated with secretion of factors that suppress immunity and facilitate CCA angiogenesis and epithelial-to-mesenchymal transition (EMT) activity38. However, miR-24 activity was shown to suppress cytokine production and M1-type proinflammatory differentiation from macrophage cells39. In our CCA xenograft models, miR-24 inhibition increases menin protein expression in CCA tumor cells and leads to increased mononuclear cell tumor infiltration, increased fibrosis, and decreased angiogenesis and proliferation40. The role of menin and miR-24 in macrophage polarization remains to be fully understood.
CANCERS
MLL
Of great interest to menin-related pathologies is the potential use of the menin–MLL inhibitor. Originally, it was investigated in the context of a type of leukemia called “mixed-lineage leukemia,” from which the MLL protein is named. In MLL, chromosomal translocations at the MLL locus yield a myriad of MLL fusion proteins that result in either constitutive activity and/or alternate gene targets for histone methyltransferase (HMT) activity. MLL fusion proteins still require the menin protein in order to traffic to specific genomic locations and trimethylate histone tails. Thus, blocking the menin–MLL interaction with a specific small-molecule inhibitor has the potential to decrease hematopoietic proliferation in MLLs41.
MLL proteins play a role in bile acid physiology through interaction with nuclear receptors. MLL3 interacts with Farnesoid X receptor (FXR) and retinoic acid receptor (RAR) to positively regulate expression of genes (BSEP, MRP2, and NTCP) involved in hepatic bile acid export42. Genes involved in FXR/RAR activation and hepatic cholestasis (ABCC2, ABCC3, FABP6, ASB2, and IL-18) and subunits of cAMP-mediated signaling were enriched following gene expression analysis of WT versus MLL2−/− mice43. It is important to note that MLL1/2 interacts with menin, whereas MLL3/4 does not44. Recent exome sequencing of CCA tumors revealed mutations in genes coding for chromatin-modifying complexes, notably ARID1A and BAP1. Mutations in genes encoding MLL family proteins have also been identified45. It is not yet known if MLL fusion proteins exist in nonleukemic cancers. Our lab is currently investigating the effects of menin–MLL inhibitor on proliferation, angiogenesis, and fibrosis in cholestatic and CCA models.
Another possible link between menin, MLL, and nuclear receptors involves the vitamin D receptor (VDR). Treatment with vitamin D, and unsurprisingly retinoic acid, was able to force differentiation of an MLL cell line in vitro46. Menin was shown to drive expression of VDR transcription targets; however, overall levels of H3K4me3 (an MLL-dependent molecular signature) were unchanged44. Hepatic VDR expression is mostly localized to cholangiocytes, Kupffer cells, and HSCs and is negatively associated with cholestasis and liver damage47,48. Vitamin D treatment decreased HSC activation in vitro and decreased liver damage in MDR2−/− mice through reduced TNF-R1 expression and inflammatory macrophage cells48.
TGF-β
TGF-β signaling and menin are well characterized in MEN1 syndrome. TGF-β binds TGF-β receptors to recruit SMAD proteins that translocate to the nucleus and activate transcription. It is shown in both the pituitary and parathyroid glands that menin physically interacts with Smad3 and drives expression of target genes15. Loss of menin expression leads to uninhibited proliferation and, ultimately, carcinogenesis as seen in MEN1 syndrome. TGF-β signaling plays a prominent role in pancreatic physiology, albeit its connection with menin is less understood. TGF-β signaling decreases proliferation in a number of pancreatic cell types: β-cells49, pancreatic stellate cells50, and pancreatic ductal adenocarcinoma (PDAC) cells51. Interestingly, a pancreatic-specific dnTGFβrII transgenic mouse exhibited less pancreatic fibrosis in response to injury52. Thus, exploring the relationship between TGF-β and menin may help elucidate nuances between cellular proliferation and organ fibrosis.
JunD
The Jun family transcription factors (JunD, c-Jun, and JunB) form both hetero- and homodimers to regulate transcription. Menin binds the N terminus of JunD, but not c-Jun or JunB, in an mSin3A binding-specific manner and recruits HDAC complexes53,54. The complex includes Sap18, Sap30, SDS3, and RbAP4855. The menin–JunD traffics the mSin3A–HDAC complex to JunD target promoters to effectively turn off transcription. Additionally, menin inhibits phosphorylation of JunD, ELK-1, and c-Jun by both the c-Jun N-terminal kinase (JNK) and ERK–MEK pathways54,56. Loss of menin abolishes JunD repression of RAS-mediated proliferation and of transcription of JunD target genes57,58. Agarwal et al. showed that JunD, in the presence of menin, decreases cyclin D1 expression in order to halt cell growth57. Alternative splicing of the JunD mRNA transcript can yield a truncated isoform lacking several crucial amino acids at its N terminus necessary to bind menin. The truncated JunD isoform facilitates transcription at JunD target sites even in the presence of menin59. Many of the menin–JunD effects can also be reversed by HDAC inhibitor treatments such as trichostatin A9 (Fig. 4).
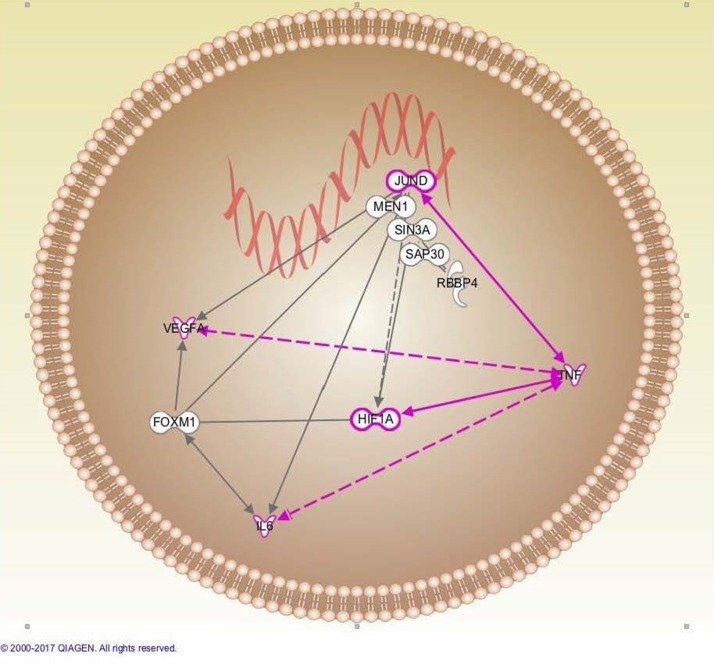
Figure 4.
Network analysis depicting menin in complex with JunD, Sin3A, Sap30, and RBBP4 mediating histone deacetylase 1/2 (HDAC1/2)-dependent histone deacetylation and suppression of angiogenesis (VEGF-A) and inflammation (IL-6). Jun–TNF-α drives survival in a HDAC1/2-independent manner by antagonizing menin–JunD–HDAC1/2 activity. QIAGEN’s Ingenuity® Pathway Analysis (IPA®; www.qiagen.com/ingenuity; QIAGEN, Redwood City, CA, USA).
JunD’s effects are paradoxical: it halts RAS-mediated cellular transformation but can also prevent premature senescence and apoptosis. JunD−/− cells showed nuclear accumulation of p53 and p27 that resulted in early cell death. JunD responds to TNF-α signaling to drive antiapoptotic cell survival. TNF-α increased JunD and NF-κB binding to target gene promoter sites, and JunD overexpression drastically increased NF-κB-driven gene expression in rat hepatocytes58 (Fig. 4). JunD−/− mice were protected from carbon tetrachloride-induced liver damage and fibrosis. JunD was shown to drive expression of TIMP-1, IL-6, and MMP-9 in both hepatocytes and activated HSCs in a JNK-independent fashion60. JunD, like menin, appears to serve dichotomous functions in order to maintain the cell in a quiescent state.
Both JunD and menin appear to play roles in hepatic oxidative stress; however, the physical link between the two has yet to be established. Gerald et al. showed that JunD antagonizes RAS-mediated transformation by upregulating antioxidant genes and downregulating ROS-producing genes. Consequently, decreased ROS results in a reduction of HIF-1α levels and represses vascular endothelial growth factor (VEGF-A) transcription and tumor angiogenesis. JunD−/− cells exhibited increased ROS damage, VEGF-A expression, and tumor angiogenesis61. In an ischemia/reperfusion (I/R) injury model, characterized by an abnormal level of ROS production, JunD was found to play a protective role by antagonizing expression of other AP-1 transcription factors. JunD reduced expression of cyclin D1 and decreased early phase proliferation that ultimately decreased ROS production and liver damage. Loss of JunD enhances proliferation and caspase-mediated liver damage following I/R injury62.
One group found that menin expression is required for proper functioning of heat shock proteins in Drosophila melanogaster. Loss of menin expression leads to increased hypoxia and oxidative stress63. Loss of menin expression was shown to increase vascularity and VEGF-A expression in PNETs64. We have previously shown that menin overexpression decreases VEGF-A expression in CCA cell lines, potentially through an upregulation of the JunD–Menin interaction40.
miR-24
Recently, menin was shown to exist in a negative regulatory feedback loop with miR-2465. miR-24 binds to the 3′-untranslated region of the MEN1 mRNA transcript to negatively regulate its protein expression. Menin, in turn, can positively regulate expression of miR-24 via MLL-driven histone trimethylation at both of the miR-24 chromosomal locations, chromosomes 9 and 1910. Overexpression of menin represses MEN1 promoter activity, indicating negative self-regulation17. Increase in miR-24 expression is thought to be a possible mechanism driving MEN1 syndrome, and thus targeting miR-24 expression is an attractive therapeutic possibility.
miR-24 activity is documented to drive both pancreatic and liver cancers, although its role in other tissues remains controversial66–68. miR-24 is well documented to negatively regulate menin expression in the endocrine pancreas and drive tumorigenesis65. There is strong evidence for the oncogenic role of miR-24 in PDAC as well. miR-24 overexpression was shown to decrease FZD5 (WNT receptor), HNF1B (HOX transcription factor), and TMEM92 to drive PDAC EMT. A separate study showed that miR-24 regulated the BIM pathway to drive PDAC angiogenesis67,68. Melatonin negatively regulates miR-24 expression in several cancers to inhibit tumor proliferation and migration, and lower melatonin signaling in cholangiocytes leads to increased VEGF expression69. miR-24 expression is increased in HCC, and its inhibition reduces proliferation, migration, and invasion70.
We have shown that miR-24 possesses oncogenic properties in the setting of CCA. Inhibiting its expression increases menin protein expression and decreases CCA proliferation and angiogenesis as demonstrated by both in vitro and mouse xenograft models40. miR-24 inhibition in MDR2−/− mice, a model for PSC, actually increased hepatic fibrosis and proliferation while increasing menin expression (unpublished). miR-24 has been shown to negatively regulate SMAD protein expression in vascular endothelial cells71. However, its role in TGF-β signaling and hepatic fibrosis has not yet been explored.
Pancreas
While it is well established that patients with MEN1 syndrome develop PNET, the molecular mechanisms underpinning this phenomenon have recently been elucidated72–76. The menin–MLL interaction, primarily known for its oncogenic effect in MLL77, plays a critical tumor suppressor role in islet cells by driving expression of cell cycle inhibitors p27 and p18 with chromatin-activing histone H3 lysine 4 trimethylation (H3K4me3) marks72. Agarwal and Jothi further demonstrated menin-dependent H3K4me3 marks within the imprinted Dlk1–Meg3 locus and within all four HOX loci73, which in turn suppresses islet proliferation74. It is important to note that the genetic landscape of HOX loci is functionally diverse, and only certain homeobox genes contained within these clusters may be activated by menin–MLL-driven H3K4me3. Furthermore, epigenetic regulation is a dynamic process where loss of H3K4me3 can functionally enrich H3K27me3 chromatin-silencing marks78.
Menin is necessary for the antiproliferative effects of Wnt signaling in pancreatic islet cells as well75. Menin physically interacts with Wnt pathway proteins such as β-catenin and TCFs, and menin expression positively correlates with downstream target AXIN2 expression and H3K4me3 marks on the AXIN2 promoter site. Wnt-dependent H3K4me3 is mediated through MLL activity in other tissues79, but this connection has not yet been established in pancreatic islets. We described above how miR-24 expression decreases FZD5 expression (a WNT signaling receptor) to help drive PDAC growth, potentially through a downregulation of menin67.
Dreijerink et al. postulate that menin–MLL activity is necessary for nuclear receptor-mediated transcription in MEN1 syndrome80. Specifically, they show that menin and estrogen receptors promote H3K4me3 and expression of the TFF1 gene80, a canonical tumor suppressor critical for the maintenance of epithelial barrier and mucosal protection in both pancreatic and biliary ducts81,82. Interestingly, estrogen signaling downregulates TNF by decreasing JNK activity and subsequent phosphorylation of JunD and c-Jun, which is itself a menin-dependent process83. This kind of regulation would imply that menin operates under distinct pathways that are often overlapping and either redundant and/or synergistic.
Menin can signal through other pathways in the islet pancreas as well. It binds with protein arginine methyltransferase 5 (PRMT5) to promote H4R3 dimethylation and suppress GAS1 gene expression and proproliferative hedgehog signaling84. HOX gene HOXHB9, also known as HLXB9, is downregulated upon menin overexpression85. Menin can participate in posttranslational modifications within the β-cell cytoplasm as well. Menin was shown to bind to IQGAP1, a cytoplasmic scaffold protein, and aggregate E-cadherin and β-catenin at cell-to-cell contact sites to increase adhesion and mitigate Rac1 signaling to decrease migration86. Wang et al. documented that menin physically interacts with AKT in the cytoplasm and suppresses its downstream phosphorylation activity87.
Menin’s role in PDAC is less characterized. Recessive homozygosity and promoter methylation at the MEN1 locus were observed in a small subset of PDAC88. Cheng et al. demonstrated that menin interacts with DNA methyltransferase (DNMT1) and observed menin downregulation associated with PDAC development89. They showed that menin regulates p27 and p18 expression through DNMT1-dependent promoter methylation. Interestingly, in vitro modulation of menin positively regulated p16, HOXA9, HDAC5, and CBX4 while downregulating CDK2, CDK4, ESM1, IGFBP7, and GAS1 (a member of the Hedgehog signaling family), indicating other molecular mechanisms of action distinct from CpG island methylation. This study reveals how menin can act through different mechanisms in a tissue- and pathway-specific manner.
Liver
Literature regarding menin’s role in HCC is controversial. Gang et al. reported a decrease in menin expression in primary human HCC tumor tissues and cell lines and were able to regulate cell proliferation through the modulation of menin expression13. They demonstrated that menin physically interacts with Sirt1 to deacetylate p65 and repress NF-κβ-mediated transcription. However, Zindy et al. reported an increase in menin expression in human HCC samples from patients with underlying cirrhosis21. In cirrhotic patients, menin expression positively correlated with tumor size and changed uniformly among tumor, matched normal, parenchymal, and nonparenchymal cells. Furthermore, menin expression positively correlated with levels of hepatic fibrosis and expression of fibrotic markers, likely through the association of HSC activation.
We have demonstrated in our lab that menin acts as a tumor suppressor in biliary epithelium and is negatively regulated by miR-2440. In vitro modulation of menin inversely regulates proliferation, angiogenesis, migration, and invasion. Inhibition of miR-24 in xenograft CCA models increases menin expression and decreases proliferation and angiogenesis.
METABOLIC DISEASE
Pancreas
Menin plays a role in certain metabolic diseases in addition to cancer. For example, Karnik et al. demonstrated decreased menin expression levels following pregnancy-induced expansion of pancreatic islets in mice90. β-Cell-specific menin overexpression reduced islet hyperplasia, induced hyperglycemia, and impaired glucose tolerance, features similar to gestational diabetes. Steroids, such as progesterone, increased menin expression, while prolactin decreased menin expression. It should be noted that insulin levels and insulin gene-related expression were unchanged in this model. Recent evidence suggests that, in addition to menin repression, FOXM1 expression and serotonin signaling are important to β-cell expansion in response to prolactin/lactogen signaling91. How menin interacts with these other players remains to be elucidated. It is interesting to note that loss of histone acetylation and trimethylation are known epigenetic features of type 2 diabetes92.
Neurohormonal stimulation (i.e., prolactin or progesterone stimulation) is one of many mechanisms maintaining islet cell homeostasis. Zhang et al. demonstrated that glucose stimulates islet cell proliferation through decreased menin expression levels via PI3K/Akt/Foxo1 signaling93. High glucose stimulation-dependent islet cell hyperplasia both in vitro and in vivo also correlated with reduced menin expression93. Growth was reversed with menin overexpression or PI3K/Akt pathway inhibitors.
Duodenum
The duodenum is an important regulatory source for the hepatobiliary system. S cells of duodenum release secretin, which works in conjugation with somatostatin to regulate the bile and pancreatic duct physiology94. Somatostatin signaling increases menin expression in the duodenum7, and our lab has preliminary data showing that secretin decreases menin expression in cholangiocytes (unpublished). Furthermore, menin expression negatively regulates gastric inhibitory polypeptide via PI3K/AKT signaling, which counteracts the effects of secretin95.
Liver
Wuescher et al. studied a liver-specific hemizygous deletion of menin mouse model. Consistent with other data, these mice showed decreased tolerance to high-fat diet with increased weight gain; decreased glucose tolerance; increased serum insulin, glucose, and glucagon; and increased liver triglycerides95. Menin expression itself was tied to fasting, refeeding, and insulin levels. Wuescher et al. demonstrated that insulin signaling via the PKB/AKT pathway downregulated menin protein levels but not mRNA levels and that menin is required to suppress FOXO1 target gene transcription96.
Menin interacts with nuclear receptors in the liver as well. Cheng et al. showed that menin expression binds with PPARα to activate transcription of genes involved in fatty acid oxidation. Loss of hepatic menin expression leads to liver steatosis, hepatic triglyceride accumulation, and increased serum levels of ketone bodies97. miR-24 expression, a negative regulator of menin expression, was significantly increased in high-fat diet-fed mice. Inhibiting miR-24 leads to upregulation of insulin-induced gene 1 and decreased hepatic fat accumulation39. It is interesting to note that MRPL16, a nuclear-coded mitochondrial ribosomal protein located adjacent to MEN1 loci, significantly correlates with MEN1 expression in CCA, indicating menin may regulate promoter activity on the nearby loci (TCGA, 4/7/16) (Fig. 3).
Hepatic menin expression is decreased in aging and diabetic mice, and further downregulation leads to liver steatosis98. While Cheng et al. tied hepatic menin expression to activation of genes involved in fatty acid oxidation, Cao et al. associated loss of menin expression to activation of genes involved in fat deposition97,98. Menin and SIRT1, a nuclear NAD+-dependent HDAC, bind at the promoter sites of several genes involved in fat deposition, including CD36, facilitating histone deacetylation and loss of expression98. Sirtuin1 is a regulator of FXR-dependent bile acid signaling99. We described above how MLL facilitates transcription of FXR bile acid export target genes in HMT-dependent mechanism42, and how menin and Sirt1 act together to antagonize p65-mediated transcription of NF-κβ target genes13. It is unclear which signaling pathway is at work here.
CONCLUSION/FUTURE PERSPECTIVES
This review focuses on the emerging role of menin in noncanonical MEN1 disease states (Table 1). Since the discovery of its crystal structure in 2011, more detail has emerged surrounding its mechanism56. It binds directly with JunD and MLL and indirectly with a host of other binding partners, presumably based on the protein complex it recruits. This review highlights the dichotomous function menin can play in disease. Loss of menin appears detrimental in both hepatobiliary cancers and metabolic disease, whereas menin overexpression appears detrimental in fibrotic autoimmune diseases. Specifically, it appears that loss of menin in parenchymal cells is harmful, whereas menin overexpression in stromal cells (stellate and immune cells) is also harmful. How signaling pathways such as TGF-β, NF-κβ, or nuclear receptor activation affect menin’s role as a histone writer remains to be fully elucidated. Of particular interest is understanding how small-molecule inhibitors of menin–MLL, HDAC, or miR-24 activity may be used to further understand menin’s regulation and function and to combat menin-related pathologies.
Table 1.
Summary of the Various Signaling Pathways Involving Menin From Upstream Extracellular Signals to Menin-Binding Partners, Mechanism of Action, and Functional Outcome as a Result
| Organ | Disease | Signaling | Menin Binding Partners | Mechanism | Outcome |
|---|---|---|---|---|---|
| Parathyroid/pituitary | Normal | TGF-β | Smad3 | ? | Inhibit proliferation |
| Liver | HCC/cirrhosis | TGF-β | Smad3 | ? | COL1α2 expression, fibrosis, activated HSCs |
| Liver | HCC | ? | ? | H3K4me3 | YAP transcription |
| Liver/fibroblast | Normal | ? | JunD | HDAC | Cyclin D1 repression |
| Liver | Normal | TNF-α | JunD/NF-κb subunits | HDAC | Cell survival: increased TIMP, IL-6, and MMP-9 expression. Decreased p53/p27 expression |
| Liver/stellate cells | MDR2−/− | Vitamin D | VDR | ? | Decreased fibrosis/HSC activation |
| Liver | HCC | NF-κβ | P65 | ? | Decreased transcription/growth |
| Liver | Steatosis | ? | PPARα | ? | Fatty acid oxidation |
| Liver | Steatosis | ? | Sirt1 | HDAC | Loss of CD36 (fatty acid oxidation) |
| Pancreas islets | Diabetes | Progesterone/prolactin | ? | ? | Decreased growth/increased growth |
| Pancreas | PNET | ? | MLL | H3K4 | p27/18, HOX loci expression. Decreased islet proliferation |
| Pancreas | Normal | Wnt | β-Catenin/TCF | H3K4me3 | AXIN2 expression/inhibit proliferation |
| Pancreas | MEN1 syndrome | Estrogen | ER/menin | H3K4me3 | TFF1 expression/survival |
The left two columns specify the organ and disease state. ? indicates that particular signaling aspect in relation to menin pathways remains to be elucidated.
ACKNOWLEDGMENTS
This work was supported in part by the Dr. Nicholas C. Hightower Centennial Chair of Gastroenterology from Scott & White, a VA Research Career Scientist Award, a VA Merit award to Dr. Alpini (5I01BX000574), a VA Merit Award (5I01BX002192) to Dr. Glaser, a VA Merit Award (1I01BX001724) to Dr. Meng from the US Department of Veterans Affairs Biomedical Laboratory Research, and NIH grants DK058411, DK076898, DK107310, DK062975, and DK110035 to Drs. Alpini, Meng, and Glaser. This material is the result of work supported by resources at the Central Texas Veterans Health Care System. The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs. The authors declare no conflicts of interest.
REFERENCES
- 1. Chandrasekharappa SC, Guru SC, Manickam P, Olufemi S-E, Collins FS, Emmert-Buck MR, Debelenko LV, Zhuang Z, Lubensky IA, Liotta LA, Crabtree JS, Wang Y, Roe BA, Weisemann J, Boguski MS, Agarwal SK, Kester MB, Kim YS, Heppner C, Dong Q, Spiegel AM, Burns AL, Marx SJ. Positional cloning of the gene for multiple endocrine neoplasia-type 1. Science 1997;276(5311):404–7. [DOI] [PubMed] [Google Scholar]
- 2. Norton JA, Krampitz G, Jensen RT. Multiple endocrine neoplasia: Genetics and clinical management. Surg Oncol Clin N Am. 2015;24(4):795–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Marini F, Giusti F, Brandi ML. Genetic test in multiple endocrine neoplasia type 1 syndrome: An evolving story. World J Exp Med. 2015;5(2):124–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. McCallum RW, Parameswaran V, Burgess JR. Multiple endocrine neoplasia type 1 (MEN 1) is associated with an increased prevalence of diabetes mellitus and impaired fasting glucose. Clin Endocrinol. (Oxf) 2006;65(2):163–8. [DOI] [PubMed] [Google Scholar]
- 5. Lemos MC, Thakker RV. Multiple endocrine neoplasia type 1 (MEN1): Analysis of 1336 mutations reported in the first decade following identification of the gene. Hum Mutat. 2008;29(1):22–32. [DOI] [PubMed] [Google Scholar]
- 6. Canaff L, Vanbellinghen JF, Kanazawa I, Kwak H, Garfield N, Vautour L, Hendy GN. Menin missense mutants encoded by the MEN1 gene that are targeted to the proteasome: Restoration of expression and activity by CHIP siRNA. J Clin Endocrinol Metab. 2012;97(2):E282–91. [DOI] [PubMed] [Google Scholar]
- 7. Matkar S, Thiel A, Hua X. Menin: A scaffold protein that controls gene expression and cell signaling. Trends Biochem Sci. 2013;38(8):394–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Agarwal SK, Impey S, McWeeney S, Scacheri PC, Collins FS, Goodman RH, Spiegel AM, Marx SJ. Distribution of menin-occupied regions in chromatin specifies a broad role of menin in transcriptional regulation. Neoplasia 2007;9(2):101–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gobl AE, Berg M, Lopez-Egido JR, Oberg K, Skogseid B, Westin G. Menin represses JunD-activated transcription by a histone deacetylase-dependent mechanism. Biochim Biophys Acta 1999;1447(1):51–6. [DOI] [PubMed] [Google Scholar]
- 10. Vijayaraghavan J, Maggi EC, Crabtree JS. miR-24 regulates menin in the endocrine pancreas. Am J Physiol Endocrinol Metab. 2014;307(1):E84–92. [DOI] [PubMed] [Google Scholar]
- 11. Wu Y, Feng ZJ, Gao SB, Matkar S, Xu B, Duan HB, Lin X, Li SH, Hua X, Jin GH. Interplay between menin and K-Ras in regulating lung adenocarcinoma. J Biol Chem. 2012;287(47):40003–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Veniaminova NA, Hayes MM, Varney JM, Merchant JL. Conditional deletion of menin results in antral G cell hyperplasia and hypergastrinemia. Am J Physiol Gastrointest Liver Physiol. 2012;303(6):G752–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gang D, Hongwei H, Hedai L, Ming Z, Qian H, Zhijun L. The tumor suppressor protein menin inhibits NF-kappaB-mediated transactivation through recruitment of Sirt1 in hepatocellular carcinoma. Mol Biol Rep. 2013;40(3):2461–6. [DOI] [PubMed] [Google Scholar]
- 14. Yokoyama A, Somervaille TCP, Smith KS, Rozenblatt-Rosen O, Meyerson M, Cleary ML. The menin tumor suppressor protein is an essential oncogenic cofactor for MLL-associated leukemogenesis. Cell 2005;123(2):207–18. [DOI] [PubMed] [Google Scholar]
- 15. Hendy GN, Kaji H, Sowa H, Lebrun JJ, Canaff L. Menin and TGF-beta superfamily member signaling via the Smad pathway in pituitary, parathyroid and osteoblast. Horm Metab Res. 2005;37(6):375–9. [DOI] [PubMed] [Google Scholar]
- 16. Khodaei-O’Brien S, Zablewska B, Fromaget M, Bylund L, Weber G, Gaudray P. Heterogeneity at the 5′-end of MEN1 transcripts. Biochem Biophys Res Commun. 2000;276(2):508–14. [DOI] [PubMed] [Google Scholar]
- 17. Fromaget M, Vercherat C, Zhang CX, Zablewska B, Gaudray P, Chayvialle JA, Calender A, Cordier-Bussat M. Functional characterization of a promoter region in the human MEN1 tumor suppressor gene. J Mol Biol. 2003;333(1):87–102. [DOI] [PubMed] [Google Scholar]
- 18. Dooley S, ten Dijke P. TGF-β in progression of liver disease. Cell Tissue Res. 2012;347(1):245–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Oertelt S, Lian ZX, Cheng CM, Chuang YH, Padgett KA, He XS, Ridgway WM, Ansari AA, Coppel RL, Li MO, Flavell RA, Kronenberg M, Mackay IR, Gershwin ME. Anti-mitochondrial antibodies and primary biliary cirrhosis in TGF-beta receptor II dominant-negative mice. J Immunol. 2006;177(3):1655–60. [DOI] [PubMed] [Google Scholar]
- 20. Wang YH, Yang W, Yang JB, Jia YJ, Tang W, Gershwin ME, Ridgway WM, Lian ZX. Systems biologic analysis of T regulatory cells genetic pathways in murine primary biliary cirrhosis. J Autoimmun. 2015;59:26–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zindy PJ, L’Helgoualc’h A, Bonnier D, Le Bechec A, Bourd-Boitin K, Zhang CX, Musso O, Glaise D, Troadec MB, Loreal O, Turlin B, Léger J, Clément B, Théret N. Upregulation of the tumor suppressor gene menin in hepatocellular carcinomas and its significance in fibrogenesis. Hepatology 2006;44(5):1296–307. [DOI] [PubMed] [Google Scholar]
- 22. Kaji H, Canaff L, Lebrun JJ, Goltzman D, Hendy GN. Inactivation of menin, a Smad3-interacting protein, blocks transforming growth factor type beta signaling. Proc Natl Acad Sci USA 2001;98(7):3837–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nguyen Q, Anders RA, Alpini G, Bai H. Yes-associated protein in the liver: Regulation of hepatic development, repair, cell fate determination and tumorigenesis. Dig Liver Dis. 2015;47(10):826–35. [DOI] [PubMed] [Google Scholar]
- 24. Kuwahara M, Suzuki J, Tofukuji S, Yamada T, Kanoh M, Matsumoto A, Maruyama S, Kometani K, Kurosaki T, Ohara O, Nakayama T, Yamashita M. The Menin-Bach2 axis is critical for regulating CD4 T-cell senescence and cytokine homeostasis. Nat Commun. 2014;5:3555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Watanabe Y, Onodera A, Kanai U, Ichikawa T, Obata-Ninomiya K, Wada T, Kiuchi M, Iwamura C, Tumes DJ, Shinoda K, Yagi R, Motohashi S, Hirahara K, Nakayama T. Trithorax complex component Menin controls differentiation and maintenance of T helper 17 cells. Proc Natl Acad Sci USA 2014;111(35):12829–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tan Z, Qian X, Jiang R, Liu Q, Wang Y, Chen C, Wang X, Ryffel B, Sun B. IL-17A plays a critical role in the pathogenesis of liver fibrosis through hepatic stellate cell activation. J Immunol. 2013;19(4):1835–44. [DOI] [PubMed] [Google Scholar]
- 27. Katt J, Schwinge D, Schoknecht T, Quaas A, Sobottka I, Burandt E, Becker C, Neurath MF, Lohse AW, Herkel J, Schramm C. Increased T helper type 17 response to pathogen stimulation in patients with primary sclerosing cholangitis. Hepatology 2013;58(3):1084–93. [DOI] [PubMed] [Google Scholar]
- 28. Tanner SM, Staley EM, Lorenz RG. Altered generation of induced regulatory T cells In the FVB.mdr1a(−/−) mouse model of colitis. Mucosal Immunol. 2013;6(2):309–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Roh YS, Park S, Lim CW, Kim B. Depletion of Foxp3+ regulatory T cells promotes profibrogenic milieu of cholestasis-induced liver injury. Dig Dis Sci. 2015;60(7):2009–18. [DOI] [PubMed] [Google Scholar]
- 30. Schwinge D, von Haxthausen F, Quaas A, Carambia A, Otto B, Glaser F, Höh B, Thiele N, Schoknecht T, Huber S, Steffens N, Lohse AW, Herkel J, Schramm C. Dysfunction of hepatic regulatory T cells in experimental sclerosing cholangitis is related to IL-12 signaling. J Hepatol. 2017;66(4):798–805. [DOI] [PubMed] [Google Scholar]
- 31. Yang X-P, Jiang K, Hirahara K, Vahedi G, Afzali B, Sciume G, Bonelli M, Sun H-W, Jankovic D, Kanno Y, Sartorelli V, O’Shea JJ, Laurence A. EZH2 is crucial for both differentiation of regulatory T cells and T effector cell expansion. Sci Rep. 2015;5:10643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yamada T, Kanoh M, Nabe S, Yasuoka T, Suzuki J, Matsumoto A, Kuwahara M, Maruyama S, Fujimoto T, Sakisuka R, Yasukawa M, Yamashita M. Menin plays a critical role in the regulation of the antigen-specific CD8+ T cell response upon Listeria infection. J Immunol. 2016;197(10):4079–89. [DOI] [PubMed] [Google Scholar]
- 33. Yamashita M, Hirahara K, Shinnakasu R, Hosokawa H, Norikane S, Kimura MY, Hasegawa A, Nakayama T. Crucial role of MLL for the maintenance of memory T helper type 2 cell responses. Immunity 2006;24(5):611–22. [DOI] [PubMed] [Google Scholar]
- 34. Nakata Y, Brignier AC, Jin S, Shen Y, Rudnick SI, Sugita M, Gewirtz AM. c-Myb, Menin, GATA-3, and MLL form a dynamic transcription complex that plays a pivotal role in human T helper type 2 cell development. Blood 2010;116(8):1280–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zen Y, Fujii T, Harada K, Kawano M, Yamada K, Takahira M, Nakanuma Y. Th2 and regulatory immune reactions are increased in immunoglobin G4-related sclerosing pancreatitis and cholangitis. Hepatology 2007;45(6):1538–46. [DOI] [PubMed] [Google Scholar]
- 36. Pollheimer MJ, Halilbasic E, Fickert P, Trauner M. Pathogenesis of primary sclerosing cholangitis. Best Pract Res Clin Gastroenterol. 2011;25(6):727–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kittan NA, Allen RM, Dhaliwal A, Cavassani KA, Schaller M, Gallagher KA, Carson WFIV, Mukherjee S, Grembecka J, Cierpicki T, Jarai G, Westwick J, Kunkel SL, Hogaboam CM. Cytokine induced phenotypic and epigenetic signatures are key to establishing specific macrophage phenotypes. PLoS One 2013;8(10):e78045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Raggi C, Invernizzi P, Andersen JB. Impact of microenvironment and stem-like plasticity in cholangiocarcinoma: Molecular networks and biological concepts. J Hepatol. 2015;62(1):198–207. [DOI] [PubMed] [Google Scholar]
- 39. Fordham JB, Naqvi AR, Nares S. Regulation of miR-24, miR-30b, and miR-142-3p during macrophage and dendritic cell differentiation potentiates innate immunity. J Leukoc Biol. 2015;98(2):195–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ehrlich L, Hall C, Venter J, Dostal D, Bernuzzi F, Invernizzi P, Meng F, Trzeciakowski JP, Zhou T, Standeford H, Alpini G, Lairmore TC, Glaser S. miR-24 inhibition increases menin expression and decreases cholangiocarcinoma proliferation. Am J Pathol. 2017;187(3):570–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Krivtsov AV, Armstrong SA. MLL translocations, histone modifications and leukaemia stem-cell development. Nat Rev Cancer 2007;7(11):823–33. [DOI] [PubMed] [Google Scholar]
- 42. Ananthanarayanan M, Li Y, Surapureddi S, Balasubramaniyan N, Ahn J, Goldstein JA, Suchy FJ. Histone H3K4 trimethylation by MLL3 as part of ASCOM complex is critical for NR activation of bile acid transporter genes and is downregulated in cholestasis. Am J Physiol Gastrointest Liver Physiol. 2011;300(5):G771–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Guo C, Chang CC, Wortham M, Chen LH, Kernagis DN, Qin X, Cho YW, Chi JT, Grant GA, McLendon RE, Yan H, Ge K, Papadopoulos N, Bigner DD, He Y. Global identification of MLL2-targeted loci reveals MLL2’s role in diverse signaling pathways. Proc Natl Acad Sci USA 2012;109(43):17603–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Dreijerink KM, Varier RA, van Nuland R, Broekhuizen R, Valk GD, van der Wal JE, Lips CJ, Kummer JA, Timmers HT. Regulation of vitamin D receptor function in MEN1-related parathyroid adenomas. Mol Cell Endocrinol. 2009;313(1–2):1–8. [DOI] [PubMed] [Google Scholar]
- 45. Zou S, Li J, Zhou H, Frech C, Jiang X, Chu JS, Zhao X, Li Y, Li Q, Wang H, Hu J, Kong G, Wu M, Ding C, Chen N, Hu H. Mutational landscape of intrahepatic cholangiocarcinoma. Nat Commun. 2014;5:5696. [DOI] [PubMed] [Google Scholar]
- 46. Liedtke M, Cleary ML. Therapeutic targeting of MLL. Blood 2009;113(24):6061–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gonzalez-Sanchez E, Firrincieli D, Housset C, Chignard N. Nuclear receptors in acute and chronic cholestasis. Dig Dis. 2015;33(3):357–66. [DOI] [PubMed] [Google Scholar]
- 48. Reiter FP, Hohenester S, Nagel JM, Wimmer R, Artmann R, Wottke L, Makeschin MC, Mayr D, Rust C, Trauner M, Denk GU. 1,25-(OH)(2)-vitamin D(3) prevents activation of hepatic stellate cells in vitro and ameliorates inflammatory liver damage but not fibrosis in the Abcb4(-/-) model. Biochem Biophys Res Commun. 2015;459(2):227–33. [DOI] [PubMed] [Google Scholar]
- 49. Dhawan S, Dirice E, Kulkarni RN, Bhushan A. Inhibition of TGF-beta signaling promotes human pancreatic beta-cell replication. Diabetes 2016;65(5):1208–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Qian ZY, Peng Q, Zhang ZW, Zhou LA, Miao Y. Roles of Smad3 and Smad7 in rat pancreatic stellate cells activated by transforming growth factor-beta 1. Hepatobiliary Pancreat Dis Int. 2010;9(5):531–6. [PubMed] [Google Scholar]
- 51. Ungefroren H, Groth S, Sebens S, Lehnert H, Gieseler F, Fandrich F. Differential roles of Smad2 and Smad3 in the regulation of TGF-beta1-mediated growth inhibition and cell migration in pancreatic ductal adenocarcinoma cells: Control by Rac1. Mol Cancer 2011;10:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Yoo BM, Yeo M, Oh TY, Choi JH, Kim WW, Kim JH, Cho SW, Kim SJ, Hahm KB. Amelioration of pancreatic fibrosis in mice with defective TGF-beta signaling. Pancreas 2005;30(3):e71–9. [DOI] [PubMed] [Google Scholar]
- 53. Kim H, Lee JE, Cho EJ, Liu JO, Youn HD. Menin, a tumor suppressor, represses JunD-mediated transcriptional activity by association with an mSin3A-histone deacetylase complex. Cancer Res. 2003;63(19):6135–9. [PubMed] [Google Scholar]
- 54. Gallo A, Cuozzo C, Esposito I, Maggiolini M, Bonofiglio D, Vivacqua A, Garramone M, Weiss C, Bohmann D, Musti AM. Menin uncouples Elk-1, JunD and c-Jun phosphorylation from MAP kinase activation. Oncogene 2002;21(42):6434–45. [DOI] [PubMed] [Google Scholar]
- 55. Laherty CD, Yang WM, Sun JM, Davie JR, Seto E, Eisenman RN. Histone deacetylases associated with the mSin3 corepressor mediate mad transcriptional repression. Cell 1997;89(3):349–56. [DOI] [PubMed] [Google Scholar]
- 56. Huang J, Gurung B, Wan B, Wan K, Hua X, Lei M. The same pocket in menin binds both MLL and JunD, but oppositely regulates transcription. Nature 2012;482(7386):542–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Agarwal SK, Novotny EA, Crabtree JS, Weitzman JB, Yaniv M, Burns AL, Chandrasekharappa SC, Collins FS, Spiegel AM, Marx SJ. Transcription factor JunD, deprived of menin, switches from growth suppressor to growth promoter. Proc Natl Acad Sci USA 2003;100(19):10770–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Mechta-Grigoriou F, Gerald D, Yaniv M. The mammalian Jun proteins: Redundancy and specificity. Oncogene 2001;20(19):2378–89. [DOI] [PubMed] [Google Scholar]
- 59. Yazgan O, Pfarr CM. Differential binding of the Menin tumor suppressor protein to JunD isoforms. Cancer Res. 2001;61(3):916–20. [PubMed] [Google Scholar]
- 60. Smart DE, Green K, Oakley F, Weitzman JB, Yaniv M, Reynolds G, Mann J, Millward-Sadler H, Mann DA. JunD is a profibrogenic transcription factor regulated by Jun N-terminal kinase-independent phosphorylation. Hepatology 2006;44(6):1432–40. [DOI] [PubMed] [Google Scholar]
- 61. Gerald D, Berra E, Frapart YM, Chan DA, Giaccia AJ, Mansuy D, Pouysségur J, Yaniv M, Mechta-Grigoriou F. JunD reduces tumor angiogenesis by protecting cells from oxidative stress. Cell 2004;118(6):781–94. [DOI] [PubMed] [Google Scholar]
- 62. Marden JJ, Zhang Y, Oakley FD, Zhou W, Luo M, Jia HP, McCray PB, Yaniv M, Weitzman JB, Engelhardt JF. JunD protects the liver from ischemia/reperfusion injury by dampening AP-1 transcriptional activation. J Biol Chem. 2008;283(11):6687–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Angevine KR. Menin regulates oxidative stress through heme oxygenase-1 and p38 MAPK pathway [dissertation]. The University of Toledo; 2012. 254 p. Available from http://utdr.utoledo.edu/cgi/viewcontent.cgi?article=1275&context=theses-dissertations [Google Scholar]
- 64. Perigny M, Hammel P, Corcos O, Larochelle O, Giraud S, Richard S, Sauvanet A, Belghiti J, Ruszniewski P, Bedossa P, Couvelard A. Pancreatic endocrine microadenomatosis in patients with vonHippel-Lindau disease: Characterization by VHL/HIF pathway proteins expression. Am J Surg Pathol. 2009;33(5):739–48. [DOI] [PubMed] [Google Scholar]
- 65. Luzi E, Marini F, Giusti F, Galli G, Cavalli L, Brandi ML. The negative feedback-loop between the oncomir Mir-24-1 and menin modulates the Men1 tumorigenesis by mimicking the “Knudson’s second hit”. PLoS One 2012;7(6):e39767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Liu R, Zhang H, Wang X, Zhou L, Li H, Deng T, Qu Y, Duan J, Bai M, Ge S, Ning T, Zhang L, Huang D, Ba Y. The miR-24-Bim pathway promotes tumor growth and angiogenesis in pancreatic carcinoma. Oncotarget 2015;6(41):43831–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Liu Y-X, Long X-D, Xi Z-F, Ma Y, Huang X-Y, Yao J-G, Wang C, Xing T-Y, Xia Q. MicroRNA-24 modulates aflatoxin B1-related hepatocellular carcinoma prognosis and tumorigenesis. BioMed Res Int. 2014;2014:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Listing H, Mardin WA, Wohlfromm S, Mees ST, Haier J. MiR-23a/-24-induced gene silencing results in mesothelial cell integration of pancreatic cancer. Br J Cancer 2015;112(1):131–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Mori F, Ferraiuolo M, Santoro R, Sacconi A, Goeman F, Pallocca M, Pulito C, Korita E, Fanciulli M, Muti P, Blandino G, Strano S. Multitargeting activity of miR-24 inhibits long-term melatonin anticancer effects. Oncotarget 2016;7(15):20532–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Ma Y, She XG, Ming YZ, Wan QQ. miR-24 promotes the proliferation and invasion of HCC cells by targeting SOX7. Tumour Biol. 2014;35(11):10731–6. [DOI] [PubMed] [Google Scholar]
- 71. Chan MC, Hilyard AC, Wu C, Davis BN, Hill NS, Lal A, Lieberman J, Lagna G, Hata A. Molecular basis for antagonism between PDGF and the TGFbeta family of signalling pathways by control of miR-24 expression. EMBO J. 2010;29(3):559–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Karnik SK, Hughes CM, Gu X, Rozenblatt-Rosen O, McLean GW, Xiong Y, Meyerson M, Kim SK. Menin regulates pancreatic islet growth by promoting histone methylation and expression of genes encoding p27Kip1 and p18INK4c. Proc Natl Acad Sci USA 2005;102(41):14659–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Agarwal SK, Jothi R. Genome-wide characterization of menin-dependent H3K4me3 reveals a specific role for menin in the regulation of genes implicated in MEN1-like tumors. PLoS One 2012;7(5):e37952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Modali SD, Parekh VI, Kebebew E, Agarwal SK. Epigenetic regulation of the lncRNA MEG3 and its target c-MET in pancreatic neuroendocrine tumors. Mol Endocrinol. 2015;29(2):224–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Chen G, A J, Wang M, Farley S, Lee LY, Lee LC, Sawicki MP. Menin promotes the Wnt signaling pathway in pancreatic endocrine cells. Mol Cancer Res. 2008;6(12):1894–907. [DOI] [PubMed] [Google Scholar]
- 76. Dreijerink KMA, Mulder KW, Winkler GS, Höppener JWM, Lips CJM, Timmers HTM. Menin links estrogen receptor activation to histone H3K4 trimethylation. Cancer Res. 2006;66(9):4929–35. [DOI] [PubMed] [Google Scholar]
- 77. Thiel AT, Feng Z, Pant DK, Chodosh LA, Hua X. The trithorax protein partner menin acts in tandem with EZH2 to suppress C/EBPalpha and differentiation in MLL-AF9 leukemia. Haematologica 2013;98(6):918–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Lin W, Watanabe H, Peng S, Francis JM, Kaplan N, Pedamallu CS, Ramachandran A, Agoston A, Bass AJ, Meyerson M. Dynamic epigenetic regulation by menin during pancreatic islet tumor formation. Mol Cancer Res. 2015;13(4):689–98. [DOI] [PubMed] [Google Scholar]
- 79. Wend P, Fang L, Zhu Q, Schipper JH, Loddenkemper C, Kosel F, Brinkmann V, Eckert K, Hindersin S, Holland JD, Lehr S, Kahn M, Ziebold U, Birchmeier W. Wnt/β-catenin signalling induces MLL to create epigenetic changes in salivary gland tumours. EMBO J. 2013;32(14):1977–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Dreijerink KM, Mulder KW, Winkler GS, Hoppener JW, Lips CJ, Timmers HT. Menin links estrogen receptor activation to histone H3K4 trimethylation. Cancer Res. 2006;66(9):4929–35. [DOI] [PubMed] [Google Scholar]
- 81. Xiao P, Ling H, Lan G, Liu J, Hu H, Yang R. Trefoil factors: Gastrointestinal-specific proteins associated with gastric cancer. Clin Chim Acta 2015;450:127–34. [DOI] [PubMed] [Google Scholar]
- 82. Sasaki M, Tsuneyama K, Nakanuma Y. Aberrant expression of trefoil factor family 1 in biliary epithelium in hepatolithiasis and cholangiocarcinoma. Lab Invest. 2003;83(10):1403–13. [DOI] [PubMed] [Google Scholar]
- 83. Srivastava S, Weitzmann MN, Cenci S, Ross FP, Adler S, Pacifici R. Estrogen decreases TNF gene expression by blocking JNK activity and the resulting production of c-Jun and JunD. J Clin Invest. 1999;104(4):503–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Gurung B, Feng Z, Iwamoto DV, Thiel A, Jin G, Fan CM, Ng JM, Curran T, Hua X. Menin epigenetically represses Hedgehog signaling in MEN1 tumor syndrome. Cancer Res. 2013;73(8):2650–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Shi K, Parekh VI, Roy S, Desai SS, Agarwal SK. The embryonic transcription factor Hlxb9 is a menin interacting partner that controls pancreatic beta-cell proliferation and the expression of insulin regulators. Endocr Relat Cancer 2013;20(1):111–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Yan J, Yang Y, Zhang H, King C, Kan H-M, Cai Y, Yuan C-X, Bloom GS, Hua X. Menin interacts with IQGAP1 to enhance intercellular adhesion of β cells. Oncogene 2009;28(7):973–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Wang Y, Ozawa A, Zaman S, Prasad NB, Chandrasekharappa SC, Agarwal SK, Marx SJ. The tumor suppressor protein menin inhibits AKT activation by regulating its cellular localization. Cancer Res. 2011;71(2):371–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Cavallari I, Silic-Benussi M, Rende F, Martines A, Fogar P, Basso D, Vella MD, Pedrazzoli S, Herman JG, Chieco-Bianchi L and others. Decreased expression and promoter methylation of the menin tumor suppressor in pancreatic ductal adenocarcinoma. Genes Chromosomes Cancer 2009;48(5):383–96. [DOI] [PubMed] [Google Scholar]
- 89. Cheng P, Wang YF, Li G, Yang SS, Liu C, Hu H, Jin G, Hu XG. Interplay between menin and Dnmt1 reversibly regulates pancreatic cancer cell growth downstream of the Hedgehog signaling pathway. Cancer Lett. 2016;370(1):136–44. [DOI] [PubMed] [Google Scholar]
- 90. Karnik SK, Chen H, McLean GW, Heit JJ, Gu X, Zhang AY, Fontaine M, Yen MH, Kim SK. Menin controls growth of pancreatic beta-cells in pregnant mice and promotes gestational diabetes mellitus. Science 2007;318(5851):806–9. [DOI] [PubMed] [Google Scholar]
- 91. Georgia S, Bhushan A. Pregnancy hormones boost beta cells via serotonin. Nat Med. 2010;16(7):756–57. [DOI] [PubMed] [Google Scholar]
- 92. Pinney SE, Simmons RA. Epigenetic mechanisms in the development of type 2 diabetes. Trends Endocrinol Metab. 2010;21(4):223–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Zhang H, Li W, Wang Q, Wang X, Li F, Zhang C, Wu L, Long H, Liu Y, Li X, Luo M, Li G, Ning G. Glucose-mediated repression of menin promotes pancreatic beta-cell proliferation. Endocrinology 2012;153(2):602–11. [DOI] [PubMed] [Google Scholar]
- 94. Rao JN WJ, editor. Regulation of gastrointestinal mucosal growth. San Rafael (CA): Claypool Life Sciences; 2010. [PubMed] [Google Scholar]
- 95. Wuescher L, Angevine K, Patel PR, Mensah-Osman E. Menin liver-specific hemizygous mice challenged with high fat diet show increased weight gain and markers of metabolic impairment. Nutr Diabetes 2012;2:e34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Wuescher L, Angevine K, Hinds T, Ramakrishnan S, Najjar SM, Mensah-Osman EJ. Insulin regulates menin expression, cytoplasmic localization, and interaction with FOXO1. Am J Physiol Endocrinol Metab. 2011;301(3):E474–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Cheng Peng YSS, Hu Xian Gui, Zhou Xu Yu, Zhang Yi Jie, Jin Gang, Zhou Ying Qi. Menin prevents liver steatosis through co-activation of peroxisome proliferator-activated receptor alpha. FEBS Lett. 2011;585. [DOI] [PubMed] [Google Scholar]
- 98. Cao Y, Xue Y, Xue L, Jiang X, Wang X, Zhang Z, Yang J, Lu J, Zhang C, Wang W, Ning G. Hepatic menin recruits SIRT1 to control liver steatosis through histone deacetylation. J Hepatol. 2013;59(6):1299–306. [DOI] [PubMed] [Google Scholar]
- 99. Kulkarni SR, Soroka CJ, Hagey LR, Boyer JL. Sirtuin 1 activation alleviates cholestatic liver injury in a cholic acid-fed mouse model of cholestasis. Hepatology 2016;64(6):2151–64. [DOI] [PMC free article] [PubMed] [Google Scholar]