Abstract
Despite the current standard of multimodal management, glioblastoma (GBM) inevitably recurs and effective therapy is not available for recurrent disease. A subset of tumor cells with stem-like properties, termed GBM stem-like cells (GSCs), are considered to play a role in tumor relapse. Although oncolytic herpes simplex virus (oHSV) is a promising therapeutic for GBM, its efficacy against recurrent GBM is incompletely characterized. Transforming growth factor beta (TGF-β) plays vital roles in maintaining GSC stemness and GBM pathogenesis. We hypothesized that oHSV and TGF-β inhibitors would synergistically exert anti-tumor effects for recurrent GBM. Here we established a panel of patient-derived recurrent tumor models from GBMs that relapsed after post-surgical radiation and chemotherapy, based on GSC-enriched tumor sphere cultures. These GSCs are resistant to the standard-of-care temozolomide but susceptible to oHSVs G47Δ and MG18L. Inhibition of TGF-β receptor kinase with selective targeted small molecules reduced clonogenic sphere formation in all tested recurrent GSCs. The combination of oHSV and TGF-βR inhibitor was synergistic in killing recurrent GSCs through, in part, an inhibitor-induced JNK-MAPK blockade and increase in oHSV replication. In vivo, systemic treatment with TGF-βR inhibitor greatly enhanced the anti-tumor effects of single intratumoral oHSV injections, resulting in cures in 60% of mice bearing orthotopic recurrent GBM. These results reveal a novel synergistic interaction of oHSV therapy and TGF-β signaling blockade, and warrant further investigations aimed at clinical translation of this combination strategy for GBM patients.
Keywords: Oncolytic HSV, recurrent glioblastoma, TGF-β, JNK
Introduction
Glioblastoma (GBM) is the most common primary malignant brain tumor in adults1. Despite the current standard management including surgery followed by radiotherapy and temozolomide chemotherapy, GBM remains lethal with median survival of only 14.6 months2. The refractory nature of GBM is characterized by its inevitable recurrence after intensive multimodality therapy. Once GBM recurs, median survival is only 4–7 months3, 4. Thus far, exploratory clinical investigations of a variety of molecular targeted and anti-angiogenic agents have failed to achieve enduring effects or overall survival benefit5. The culprit may be a subpopulation of GBM cells termed GBM stem-like or initiating cells (GSCs) possessing stem-like properties and sustaining tumor growth6. GSCs may survive cytotoxic therapy due to their inherent resistant phenotype and propagate recurrent disease. Thus GSCs represent a critical therapeutic target. In addition, patient-derived GSCs maintain patient-specific genetic and phenotypic alterations, and provide clinically representative GBM models suitable for therapeutic development7, 8. However, there is a paucity of the preclinical models of recurrent GBM that are based on patient-derived GSCs.
Oncolytic herpes simplex virus (oHSV) is a genetically engineered HSV that selectively replicates and kills cancer cells, while leaving non-neoplastic cells unharmed9. oHSVs have been extensively studied in both preclinical and clinical settings as a potential therapeutic for GBM. Early clinical trials that assessed oHSVs for malignant gliomas demonstrated the safety and feasibility of inoculating oHSV into the human brain, but their efficacy remains anecdotal9. Maximizing the therapeutic potential of oHSV will likely require its use in conjunction with other anti-cancer modalities such as chemotherapeutics or molecular targeted agents10. In fact, our group has reported beneficial or synergistic anti-GBM effects by oHSV combinations with cytotoxic chemotherapeutics11, 12, PI3kinase inhibitors13 and anti-angiogenic agents14, 15, via distinct mechanisms of action in orthotopic GSC GBM models. Although in the past recurrent GBM tended to be the primary target for oHSV clinical trials, the activity of oHSV against recurrent GBM has not been preclinically validated in detail. Selective targeting of signaling pathways critical for GSC maintenance or resistance might provide a means for novel combination strategies with oHSV to enhance efficacy in the treatment of refractory, recurrent GBM.
The transforming growth factor beta (TGF-β) family of cytokines, TGF-1, 2, and 3, have diverse functions in the pathogenesis of cancer including GBM16–18. TGF-β1 and 2 are highly expressed in malignant gliomas and association with poor prognosis has been reported19, 20. TGF-β interacts with its cognate cell surface receptor tyrosine kinase TGF-βR2, which forms heterodimers with TGF-βR1 (also termed ALK5) and activates both canonical signaling pathways through phosphorylating the signaling transducer Smads and non-canonical pathways such as MAP kinase pathways. TGF-β signaling drives a variety of malignant phenotypes of GBM, including invasion/migration, angiogenesis, drug/radiation resistance and immune-suppression17, 18. Importantly, accumulating evidence indicates that TGF-β signaling plays a vital role in the maintenance of GSC stemness and promotes GBM oncogenesis21, 22. These observations support the TGF-β signaling pathway as a promising therapeutic target in GBM and its GSC subpopulation. As such, a number of small molecule inhibitors of TGF-βR kinases (hereafter abbreviated as TβR) are in preclinical and clinical development. TβR inhibition by the selective TβRI/II dual small molecule inhibitor LY2109761 radiosensitized GSCs and combination treatment of radiation and LY2109761 extended survival in orthotopic GSC-derived GBM in mice23. A first-in-human dose study of the TβR1 kinase inhibitor galunisertib (LY2157299) revealed its safety24, and early phase clinical trials evaluating galunisertib for GBM are ongoing25.
The current study was devised to address our hypothesis that oHSV and TβR inhibitors would synergistically exert anti-tumor effects for recurrent GBM. Using a panel of patient-derived recurrent GBM models based on GSC-enriched tumor sphere cultures isolated from recurrent GBM resections, we show anti-tumor effects of targeted TβR inhibition and oHSV. Furthermore, combination treatment of TβR inhibitor and oHSV is synergistic in vitro, and mediates markedly enhanced therapeutic effects for recurrent GSC GBM models in vivo.
Materials and methods
Cells and xenograft models
Eight recurrent GSC lines (MGG24R, MGG31, MGG45, MGG50, MGG85, MGG91. MGG111R, MGG123) were established from GBMs that recurred after standard adjuvant therapy including surgical resection, radiotherapy, and temozolomide chemotherapy, and these are referred to as recGSCs. Primary GSCs (MGG4, MGG8, and MGG64) were established from newly diagnosed GBMs7, 8, and termed as newly diagnosed GSCs. Tissue collection was approved by the Institutional Review Board at Massachusetts General Hospital, and the method to initiate culture has been previously described7. All GSCs were cultured as neurospheres in defined serum free media supplemented with EGF and FGF-27. Vero cells (African green monkey kidney cells) were obtained from the American Type Culture Collection, and cultured in Dulbecco’s Modified Eagle’s Medium (DMEM; Mediatech) supplemented with 10% calf serum. GSCs were intracerebrally implanted in SCID mice (NCI) as described7, 8 and mice were euthanized when they developed significant neurological deficits or general condition deterioration (e.g. >15% body weight loss) for brain collection and pathological studies. EGFR copy number was determined by FISH as described26, 27. Targeted sequencing using SNapShot genotyping was used to detect hotspot mutations in TP53 and PTEN27. EGFRVIII mutant was detected by RT-PCR following the method described in28.
Viruses
oHSV G47Δ contains deletions of both copies of γ34.5, α47, and a LacZ insertion inactivating ICP629. G47Δ-mCherry and G47Δ-US11fluc are recombinant HSVs derived from G47Δ, and express mCherry driven by the HSV IE4/5 immediate-early promoter and firefly luciferase driven by the HSV US11 true late promoter of HSV-1, respectively30. oHSV MG18L contains a deletion of US3 and a LacZ insertion inactivating ICP613. All viruses were grown, purified, and titered on Vero cells.
Reagents
SB431542 (Sigma-Aldrich), LY2109761 (Selleckchem, Houston, TX), and galunisertib (LY2157299, Selleckchem) are selective and reversible inhibitors of the TGF-β receptor type I kinase. Selective and reversible JNK inhibitor SP600125 and the alkylating agent temozolomide were from Sigma-Aldrich. All the regents were dissolved in dimethyl sulfoxide (DMSO; Sigma-Aldrich). Recombinant human TGF-β1 (Peprotech) was dissolved in 0.1 M citric acid.
Immunohistochemistry
Immunohistochemistry (IHC) for CD44 (Anti-CD44 from Cell Signaling Technology), human nestin (Santa Cruz) and YKL-40 (Quidel) was performed on formalin-fixed paraffin embedded sections using the protocol described previously7 with slight modifications. For antigen retrieval, 0.1% saponin (Sigma) treatment for 5 minutes was used for CD44 and YKL-40, and microwave in citrate buffer for human nestin.
Western blot
Immunoblot was performed as described previously12. Briefly, lysates were separated by 4–15% SDS-PAGE, and electro-blotted to PVDF membranes. After blocking with 5% non-fat dry milk, membranes were incubated with primary antibodies, followed by appropriate peroxidase-conjugated secondary antibodies. Primary antibodies used were: anti-Smad 2/3, phosphor-Smad2/3, JNK, phospho-JNK (all from Cell Signaling Technology), and Vinculin (Thermo).
RT-PCR
Total RNA was isolated from GSCs using Trizol reagent (Invitrogen) and first strand DNA was synthesized using Superscript II (Invitrogen). Real time PCR was conducted using SYBR green master mix (Applied Biosystems) in a StepOnePlus Real-time PCR System (Applied Biosystems). PCR primer sequences are: TGFB1 (TGF-β1, forward: GGCTACCATGCCAACTTCTG, reverse: CCGGGTTATGCTGGTTGTA), TGFB2 (TGF-β2, forward: TTCAGACACTCAGCACAGCA, reverse: TTGGGTGTTTTGCCAATGTA), TGFBR2 (TGF-β receptor II, forward: TGTGTCGAAAGCATGAAGGA, reverse: GGTCCCAGCACTCAGTCAAC) and GAPDH (forward: CAATGACCCCTTCATTGACC, reverse: GACAAGCTTCCCGTTCTCAG).
Sphere formation assay
To measure clonogenicity of GSCs, sphere formation assay was performed as described previously7. Briefly, 1 or 5 cells / well were seeded into 96 well plates with TβR inhibitor or DMSO control, and spheres (> 80 µm in diameter) were counted 12–14 days later.
Cell viability and cell count assay
Dissociated GSCs were plated in triplicate in 96-well plates and treated with drug or virus the next day and incubated at 37°C for 5 days. Cell viability was measured by incubating with MTS (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium) (Sigma) for 2–4 hours and absorbance measurement at 490nm on a plate reader. For cell count assay, GSCs were plated in 24-well plates and treated with TβR inhibitor and virus for 5 days. Viable cells that exclude trypan blue were counted on a hemocytometer.
Virus spread and yield assay
Dissociated GSCs were plated with 4 × 104 cells/well in 24-well plates. The next day, cells were treated with indicated doses of TβR inhibitors and/or MG18L or G47Δ at MOI=1 in triplicate. Cells and media were harvested at indicated time points, and virus released by 3 cycles of freeze/thaw was titrated by plaque assay on Vero cells. Fluorescent and phase contrast microscope pictures were captured on Day 1, 3 and 5 post-infection of GSCs to assess spread of G47Δ-mCherry (MOI=0.1).
Luciferase assay
GSCs were plated into 96-well plates in triplicate and next day treated with drug and G47Δ-Us11fluc as indicated. Twenty four hours post infection, D-Luciferin (Gold Biotechnology) was added to cells to 1 mM and luminescence was immediately measured on a BioTek Synergy HT multipurpose plate reader.
Chou-Talalay synergy analysis
The interaction of TβR inhibitors and virus on GSCs in vitro was determined with Chou-Talalay analysis31 as described in12, 32.
Caspase 3/7 activity assay
GSCs were plated at 5000 cells per well into 96-well plates and treated with drug and virus. Twenty four hours later, activation of the execution caspases 3/7 was measured by the Caspase-Glo 3/7 assay kit (Promega), following the manufacturer’s instructions.
Treatment studies in orthotopic GSC tumor models
One hundred thousand MGG31 GSCs (in 3 µl) were stereotactically implanted into the right cerebrum of anesthetized 7–8-week-old female SCID mice (2.3 mm lateral of bregma, 2.5 mm deep from dural surface). Mice were randomized and assigned to 4 treatment arms (N=6 / group): 1) control (vehicle and PBS), 2) vehicle and MG18L, 3) LY2157299 and PBS, and 4) combination of MG18L and galunisertib. Galunisertib (100 mg/kg) or vehicle was given by oral gavage daily from day 7 to 16. MG18L (1×106 pfu/3 µl) or PBS was injected stereotactically into the tumors on day 9. Mice were followed for health status with body weight measurement 2–3 times a week. Mice were euthanized when they developed significant neurological deficits or general condition deterioration (>15% body weight loss). Survival was analyzed by using the Kaplan-Meier method. All the animal procedures were approved by the IACUC at Massachusetts General Hospital.
Statistical analysis
Student t test (2-sided) was used to compare 2 groups. Statistical differences of animal survival were determined by log-rank test. P values <0.05 were considered significant. Statistical analyses were done with Excel and Graph Pad Prism.
Results
Establishment of recurrent GSCs based orthotopic xenograft models
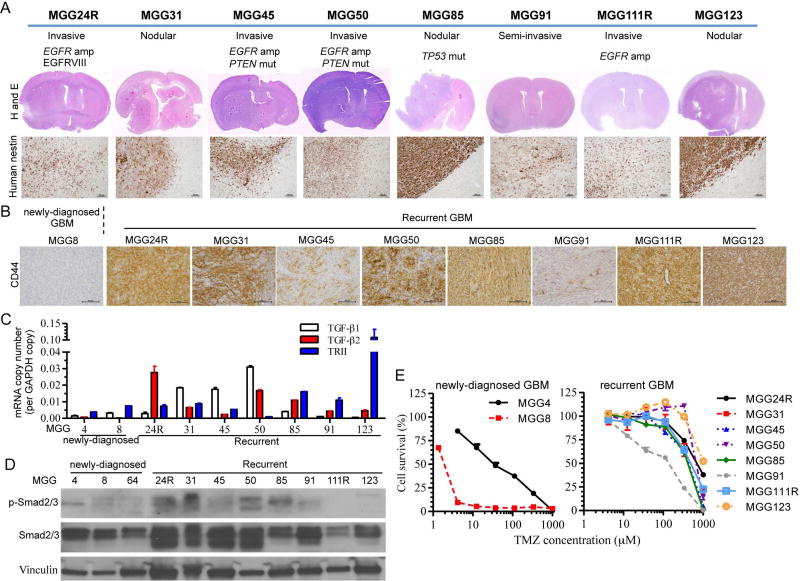
To develop therapeutic strategies for recurrent GBM, we first established a panel of patient-derived GSC-based xenograft models of recurrent GBM that are based on neurosphere-forming stem-like GBM cells. By applying the stem cell enriching culture methodology that enabled us to establish a large panel of primary GSC lines from newly-diagnosed GBM7, we were able to establish 8 orthotopic recurrent GBM (recGBM) PDX models (Fig. 1A). The recurrent GBM models demonstrated highly variable in vivo phenotypes: human specific nestin staining revealed that 4 lines (MGG24R, 45, 50, and 111R) exhibited extensive tumor invasiveness/migration and infiltration to brain parenchyma, 1 line (MGG91) showed semi-invasiveness, while the other 3 lines (MGG31, 85, and 123) showed a nodular phenotype with relatively discrete tumor-brain borders (Fig. 1A). One of the nodular recGSCs, MGG85, displayed a characteristic sarcomatous histology (Supplementary Fig. S1). MGG123 was previously described and contained pseudopalisading necrosis with a hypoxic microenvironment33. Genetically, MGG24R, 45, 50 and 111R had EGFR amplification, with MGG24R having the EGFRvIII mutant. All of these EGFR-amplified lines were invasive in vivo, in line with the reported function of EGFR in promoting invasiveness of GBM34, 35. MGG45 and 50 had PTEN mutations, and MGG85 gliosarcoma had a TP53 mutation (p.Arg248Trp).
Figure 1. Generation of recurrent GSCs-derived orthotopic xenografts and characterization of their mesenchymal phenotype.
(A) Histopathological and molecular characterization of orthotopic xenografts generated from recurrent GSCs established from 8 patients. Molecular (EGFR amplification, P53 and PTEN status) and phenotypic features (invasive vs nodular)(upper). Hematoxylin and eosin (H&E) stain of coronal sections of xenografts (middle) and microscopic images of human nestin immunohistochemistry (lower, original magnification, 100×, scale bars: 100 µm). (B) CD44 immunohistochemistry of GSC xenografts. All the xenografts generated from recurrent GBM expressed CD44 (brown, original magnification, 200×, scale bars: 100 µm). Newly diagnosed GBM-derived model, MGG8, served as negative control. (C) Quantitative RT-PCR analysis of TGF-β1, TGF-β2 and TGF-β receptor type II (TRII) mRNA levels in newly diagnosed and recurrent GSCs. GAPDH was used as reference. Data are shown as mean ± SD. (D) Western blot analysis of TGF-β receptor signal transducer Smad2/3 and its phosphorylation in newly diagnosed and recurrent GSCs. Vinculin is loading control. (E) Cell viability assay showing temozolomide (TMZ) dose response curves of newly diagnosed (left) and recurrent GSCs (right) in vitro. Cells were exposed to TMZ for 5 days. Data are shown as mean ± SD.
Mesenchymal-like phenotype and activated TGF-β pathway in recurrent GSCs
CD44 has been shown to be a representative marker of the mesenchymal subtype of GBM36–38. We therefore used CD44 IHC as a surrogate of a mesenchymal phenotype of GBM to characterize our tumor models. Tumor cells were strongly immuno-positive for CD44 in all the recGBM models tested, while the newly diagnosed GSC xenograft of the proneural subtype (MGG8) was negative (Fig. 1B). Another mesenchymal marker YKL-40 was immuno-positive in the majority of the recGBM models, although staining was variable and typically patchy (Supplementary Fig. S1). TGF-β signaling pathway has been implicated in epithelial-to-mesenchymal transition (EMT) in cancer16, 39, 40 and mesenchymal GBM38. RT-PCR analysis of TGF-β1, 2, and TβRII showed a tendency toward increased expression of these components of the TGF-β signaling pathway in our recGSC lines compared with newly diagnosed GSCs in vitro (Fig. 1C, Supplementary Fig. S2A). Furthermore, most of the recGSC lines had activation of the canonical TGF-β signaling in vitro as shown by phosphorylated forms of the TβR downstream signaling molecules Smad2 and Smad3 (p-Smad2/3) in western blots (Fig. 1D).
Since these recGSCs were isolated from recurrent GBM having failed the standard of care radiation and the alkylating agent temozolomide (TMZ), they were expected to be resistant to temozolomide in vitro. Indeed, in vitro cell viability assays of the recGSCs demonstrated resistance to TMZ, with IC50 values typically >400 µM, as opposed to some of our previously characterized TMZ-sensitive primary GSC lines (MGG4 and MGG8) (Fig. 1E, Supplementary Table). Thus we established a set of orthotopic GBM models generated from recurrent GSCs that display a mesenchymal phenotype and have an activated TGF-β pathway.
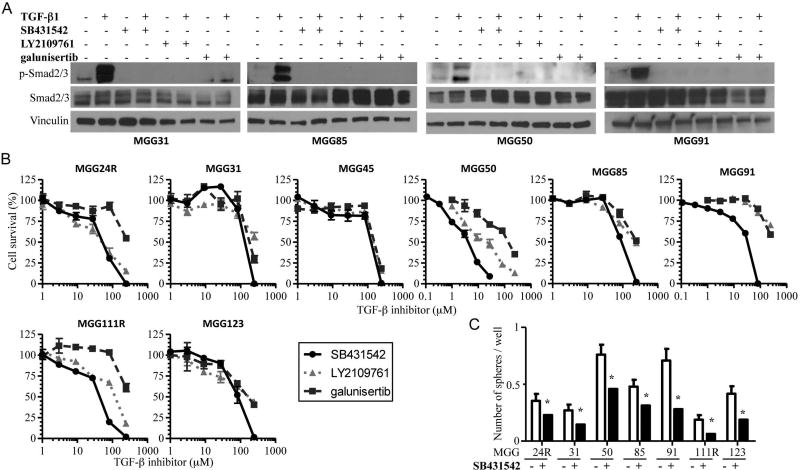
Suppression of TGF-β pathway decreased clonogenicity and viability of recurrent GSCs
The TGF-β signaling pathway has been shown to play a role in the maintenance of GSC21, 22, however its role in recGSCs is unknown. We therefore tested small molecule inhibitors that selectively target TβRI kinase for their impact on recGSC phenotypes in vitro. Supplementation of recombinant TGF-β1 to stem cell culture media potently activated p-Smad2/3 in recGSCs (Fig. 2A). Exposure of recGSCs to TβR inhibitors, SB431542, LY2109761, and galunisertib, almost completely shut down p-Smad2/3 in the presence or absence of TGF-β1 (Fig. 2A). TβR inhibitors reduced cell viability of recGSCs typically at relatively high doses (> 50µM), with SB431542 generally being the most potent (Fig. 2B, Supplementary Table). Clonogenic assays showed that SB431542 at non-cytotoxic doses consistently inhibited sphere formation of recGSCs (Fig. 2C, Supplementary Fig. S2B), supporting its activity on self-renewal of recGSCs. Thus, we show that TGF-β signaling promotes clonogenicity and viability of recGSCs.
Figure 2. Suppression of TGF-β receptor signaling decreases clonogenicity and viability of recurrent GSCs.
(A) Western blot analysis of p-Smad2/3 and Smad2/3 in recurrent GSCs after treatment with TGF-β1 (1 nM) and TGF-β receptor inhibitors, SB431542 (1 µM for MGG50, 10µM for the others), LY2109761 (1 µM for MGG50, 10µM for the others), and galunisertib (10 µM) for 24 hours. Vinculin is loading control. (B) Cell viability assay showing TGF-β receptor inhibitors, SB431542, LY2109761, and galunisertib, dose response curves in a cohort of recurrent GSCs in vitro. Cells were exposed to inhibitors for 5 days. Data are shown as mean ± SD. (C) In vitro sphere formation assay showing the inhibitory effect of TGF-β receptor inhibitor SB431542 (0.5 µM for MGG50, 10µM for the others) in a cohort of recurrent GSCs. Experiments were done with 1 cell per well. Data are shown as mean ± SD. *, p<0.05.
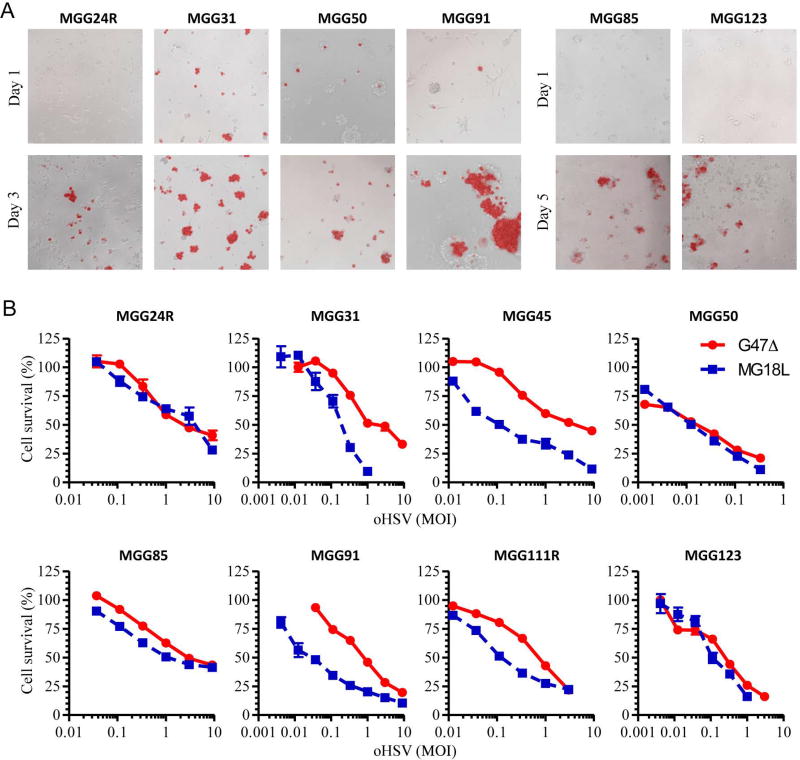
oHSV infects, spreads, and kills recurrent GSCs in vitro
We next investigated oHSV as a therapeutic for recGSCs. Infection of 6 recGSC lines with G47Δ-mCherry at low MOI resulted in the spread of oHSV within the spheres over 3–5 days post infection (Fig. 3A). We then determined the cytotoxicity of two clinically translatable oHSVs, G47Δ and MG18L, in recGSCs. Cell viability assays revealed that all 8 recGSC lines tested were susceptible to both oHSVs, with MG18L more efficacious in 4 lines (MGG31, MGG45, MGG91 and MGG111R) (Fig. 3B, Supplementary Table).
Figure 3. Oncolytic activity of oHSVs against recurrent GSCs in vitro.
(A) Merged phase contrast and fluorescence microscopy images showing spread of G47Δ-mCherry within the spheres of recurrent GSCs. Images captured on Day 1 (upper), 3 or 5 (lower) post-infection. (B) Cell viability assay showing dose response curves of oHSVs, G47Δ and MG18L, in a cohort of recurrent GSCs in vitro. Assays were done 5 days post infection. Data are shown as mean ± SD.
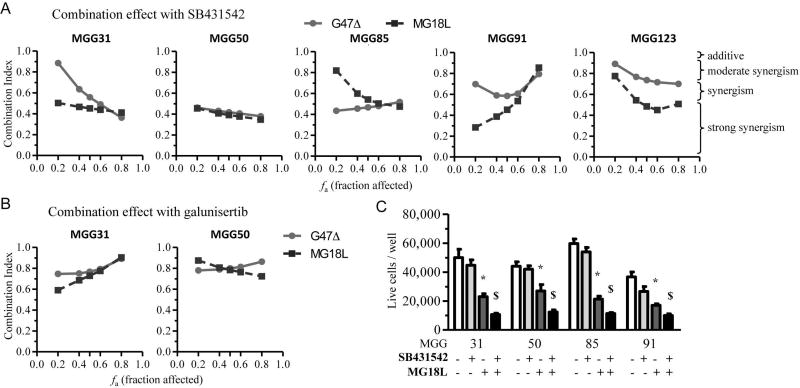
Combination of oHSV and TGF-β receptor inhibitors synergistically kills recurrent GSCs
We then combined oHSV and TβR inhibitors and studied whether the two agents acted synergistically in killing recGSCs. Synergy was measured using the median-effect method of Chou-Talalay12, 31, 32. Across the different recGSC lines, the interaction of oHSV (G47Δ and MG18L) and TβR inhibitors (SB431542 and galunisertib) was synergistic at most fraction affected doses (Fig. 4AB, Supplementary Fig. S3). The beneficial impact of combination therapy was confirmed using viable cell count assays, where the oHSV/TβR inhibitor combination was superior to either monotherapy in mediating recGSC death (Fig. 4C).
Figure 4. TGF-β receptor inhibitors synergize with oHSVs in the treatment of recurrent GSC models in vitro.
(A and B) Chou-Talalay analysis to examine the interaction between oHSVs, G47Δ or MG18L, and TGF-β inhibitor, SB431542 (A) or galunisertib (B), in the treatment of recurrent GSCs. Cells were treated for 5 days. Data are plotted as fraction affected (Fa) versus combination index (CI). (C) Viable cell count assay in recurrent GSCs treated with TGF-β inhibitor SB431542 (1 µM for MGG50, 10 µM for the others) and MG18L (MOI=0.4) for 5 days. Data are shown as mean ± SD. *, P< 0.05; $, P<0.01.
TGF-β receptor inhibition potently synergizes with oHSV in an orthotopic recurrent GSC xenograft model in vivo
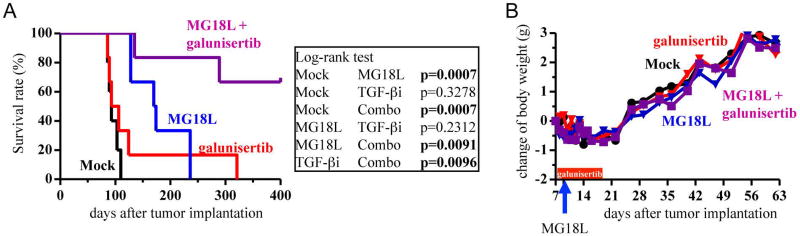
We next asked whether combination therapy with oHSV and TβR inhibitor improves therapeutic efficacy in orthotopic GBM models generated from recGSCs. We chose galunisertib as the TβR inhibitor to use as this is currently being investigated in clinical trials for recurrent GBM (NCT01582269, NCT02423343, NCT01220271). The MGG31 model was selected because of this line’s consistent tumorigenic ability and small variation in survival times. Systemic treatment of galunisertib alone had no effect on survival of mice bearing orthotopic MGG31 xenografts, although 1 animal lived an additional 200 days (Fig. 5A). Single intratumoral injection of MG18L significantly extended animal survival (approximately 75% prolongation of median survival over control), but all animals succumbed to tumor progression eventually. In contrast, combination treatment of systemic LY2157299 and local MG18L greatly extended survival, with 60% of the treated mice surviving long-term with no evidence of tumor at autopsy on day 400. We did not observe noticeable adverse events in any treatment group as evidenced by the lack of changes in body weight (Fig. 5B). Thus, TβR inhibition dramatically potentiated the therapeutic efficacy of oHSV in an orthotopic recurrent GSC GBM model in vivo.
Figure 5. TGF-β receptor inhibitor augments therapeutic efficacy of oHSV, prolonging survival in a recurrent GSC orthotopic xenograft model.
(A) Kaplan-Meier survival analysis of mice bearing MGG31 GSC brain tumors treated with mock, MG18L (on day 9), galunisertib (TGF-βi, day 7 through 16) and the combination. (B) Body weight of the mice during the course of treatment. No difference was observed between the groups.
TGF-β receptor inhibition increases oHSV replication
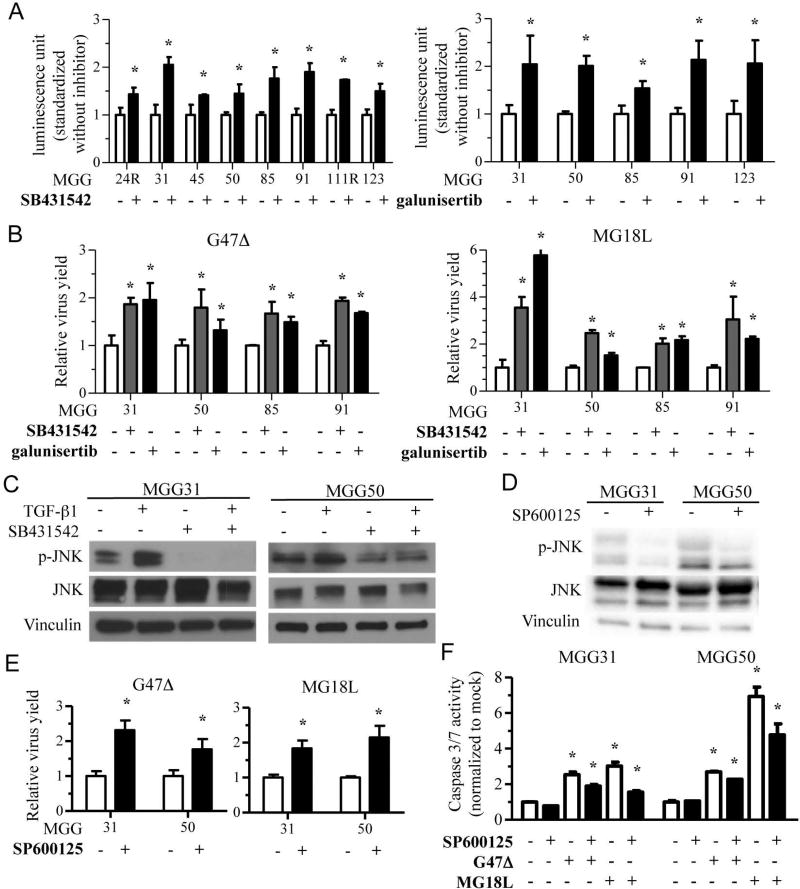
We next asked whether increases in oHSV replication played a role in the benefit of combination therapy. We first used G47Δ-US11fluc since the activity of its reporter, firefly luciferase, reflects late gene expression, which serves as a surrogate for virus replication30. There was a consistent increase in G47Δ-US11fluc-induced luciferase bioluminescence when infected recGSC lines were treated with TβR inhibitors SB431542 or galunisertib (Fig. 6A). We next determined virus yields after infecting recGSC lines with G47Δ or MG18L with or without TβR inhibitors. At 48 hours post infection, there was about a 1.5–2-fold and 2–6-fold increase in oHSV yields with G47Δ and MG18L, respectively, when co-treated with SB431542 or galunisertib (Fig. 6B, Supplementary Fig. S4). Addition of recombinant TGF-β did not alter the replication of oHSV at the doses tested (Supplementary Fig. S5). These data indicate that TGF-β signaling inhibition leads to increased oHSV replication, which may underlie the synergistic interaction between oHSV and TβR inhibitors in recGSCs.
Figure 6. TGF-β inhibition increases oHSV yields via inhibition of JNK signaling in recurrent GSCs.
(A) Measurement of firefly luciferase luminescence after infection of recurrent GSCs with oHSV G47Δ-Us11fluc (MOI=1) with and without TGF-β receptor inhibitor, SB431542 (left, 10 µM except for 1 µM for MGG50) or galunisertib (right, 10 µM). Data are normalized to the values without TGF-β inhibitor, and are shown as mean ± SD. *, P< 0.05. (B) Relative virus yields 48 hours post oHSV (G47Δ, left panel; MG18L, right panel) infection at MOI=1 of recurrent GSCs in the presence of DMSO, TGF-β inhibitor SB431542 (10 µM except for 1 µM for MGG50) or galunisertib (10 µM). Data were normalized to the values with DMSO treatment, and are shown as mean ± SD. *, P< 0.05. (C) Western blot showing inhibition of JNK phosphorylation (p-JNK, an activated form of JNK) in recurrent GSCs with or without exogenous TGF-β (1 nM) after TGF-β receptor inhibitor SB431542 (10 µM for MGG31 and 1 µM for MGG50) treatment for 24 hours. Vinculin is loading control. (D) Western blot showing JNK phosphorylation (p-JNK) in recurrent GSCs treated with JNK inhibitor SP600125 (600 nM) for 24 hours. Vinculin is loading control. (E) Virus growth assay showing relative virus yields recovered from recurrent GSCs at 48 hours after infection with oHSV G47Δ or MG18L at MOI=1 in the presence of DMSO or JNK inhibitor SP600125 (600 nM). Data is normalized to the values with DMSO treatment. Data are shown as mean ± SD. *, P< 0.05. (F) Caspase 3/7 activity assay after 24-hour treatment of recurrent GSCs with oHSV G47Δ or MG18L (MOI=1) in the presence of DMSO or JNK inhibitor SP600125 (600 nM). Data were normalized to the values with mock (control) treatment and shown as mean ± SD. *, P< 0.05.
TGF-β receptor inhibition attenuates JNK, and JNK inhibition increases oHSV replication
HSV infection activates the JNK-MAPK signaling pathway, which could regulate the survival of infected cells and induce apoptosis in some contexts41–43. Given an involvement of JNK signaling pathway in non-canonical signaling pathways triggered by TGF-β receptor activation44, we tested whether the JNK pathway played a role in the increased oHSV replication induced by TβR blockade. Exogenous recombinant TGF-β activated JNK phosphorylation in recGSCs, which was suppressed by TβR inhibitor SB431542 in vitro (Fig. 6C). This result led us to test SP600125, a small molecule compound selectively inhibiting JNK, to determine the function of the JNK pathway in oHSV therapy of recGSCs. A non-cytotoxic dose (600 nM) of SP600125 (Supplementary Fig. S6A) blocked phosphorylation of JNK (Fig. 6D), and increased luciferase expression upon G47Δ-US11fluc infection of recGSCs (Supplementary Fig. S6B). Virus yield assays confirmed that pharmacological inhibition of JNK enhanced yields of G47Δ and MG18L at 48 hours post-infection (Fig. 6E, Supplementary Fig. S5C). SP600125 attenuated oHSV-induced caspase 3/7 activation in recGSCs, to a larger extent with MG18L, suggesting that SP600125 blockade of apoptosis in infected cells may contribute to increases in oHSV yields (Fig. 6F).
Discussion
The current standard of care for newly diagnosed GBM is unable to prevent tumor recurrence, and no effective therapy exists for recurrent GBM. Recurrent GBM is characterized by resistance to therapy, dissemination to distal regions of the CNS, and aggressive behavior45. Such phenotypic traits of recurrent GBM are accompanied by molecular alterations, such as increased stemness marker expression, a hypermutator genotype caused primarily by TMZ, and clonal evolution from the primary GBM45, 46. Preclinical models that represent clinical recurrent GBM will be useful for a better understanding of its unique biology and development of novel therapeutic approaches, which are sorely needed. However, infrequent biopsy for recurrent disease and the mixed presence of treatment effects (i.e., radiation necrosis), and low viable tumor cells limit the utility of patient specimens. Herein, we characterized a set of orthotopic recurrent GBM models that are based on GSCs derived from post-standard-of-care recurrent GBM. These models are variable in both in vivo phenotypes (invasive vs nodular phenotypes) and genotypes (e.g., EGFR and p53 status), likely reflecting the diverse molecular alterations in recGBM between patients46. Despite this heterogeneity, we observed consistent expression of the mesenchymal marker CD44 in our recGBM models. While EMT is a well-documented process that is involved in malignant progression in cancer, EMT or an analogous phenomenon (mesenchymal shift) seems to occur in GBM recurrence36, 47, 48. Recently, this was confirmed by Wood et al, who, through analysis of paired primary and recurrent GBM samples, observed upregulated expression of mesenchymal proteins including CD44 and YKL-40 upon recurrence when initial tumors have lower levels49.
TGF-β has diverse functions in the pathogenesis of cancer including GBM17 and plays a pivotal role in proliferation, invasion, therapy resistance, immune suppression and GSC maintenance in GBM17, 18. In addition, the TGF-β/Smad signaling pathway has been shown to promote EMT in cancer16, 40 and TGFBR2 overexpression marks the mesenchymal subtype of GBM37. In our recurrent GBM models, TGFβ signaling was active as TGF-β1, TGF-β2 and TβR were highly expressed and phosphorylation of canonical signaling molecules Smad 2 and 3 was present. Small molecule inhibition of TβR blocked Smad2/3 phosphorylation and potently inhibited sphere formation of our recGSC, consistent with prior reports showing the critical role of TGF-β in GSC self-renewal21, 22, 50. The TβR inhibitor LY2109761 has previously been demonstrated to block both TβR1 and TβR2 and have anti-GBM effects in GSC-derived orthotopic models23, 50. In our orthotopic recGSC-derived model, MGG31, we did not observe significant survival benefits with TβR inhibitor monotherapy.
The recGSC lines we tested were permissive to oHSV, as both G47Δ and MG18L exhibited the ability to infect, spread and kill our recGSCs in vitro. MG18L was more potent than G47Δ against 4 recGSCs (i.e., MGG31, 45, 91, and 111R), and single intratumoral injection showed significant monotherapy efficacy in an intracranial recurrent GBM model. Given that our recGSCs are resistant to the standard-or-care agent TMZ, the consistent potency of oHSV is particularly meaningful, and underscores the distinct cytotoxic mechanism employed by oHSV. Importantly, combination of oHSVs (G47Δ and MG18L) and TβR inhibitors (SB431542 and galunisertib) was synergistic in the treatment of multiple recGSCs in vitro. We found that inhibition of the TGF-β signaling significantly increased virus yield in multiple recGSCs, which may have contributed to synergy. Mechanistically, we observed that TβR inhibitor-mediated blockade of the JNK-MAPK pathway, one of the SMAD-independent pathways that TGF-β activates44, mediated viral replication enhancement. JNK signaling activation in HSV-infected cells is considered to be an innate defense mechanism, leading to caspase cascade activation and apoptosis of infected cells41, 42. A selective inhibitor of JNK (SP600125) similarly blocked JNK phosphorylation and increased oHSV amplification in recGSCs, suggesting a role for non-canonical TGF-β/JNK signaling in regulating oHSV replication in GBM. However, it is possible that SMAD-mediated transcriptional activation is also involved in modulating oHSV replication.
Despite the lack of in vivo activity of TβR inhibitor monotherapy in our recurrent GBM GSC model, galunisertib greatly potentiated the anti-GBM activity of oHSV MG18L in an orthotopic recurrent GBM GSC model, resulting in >60% long-term survivors compared with none in the single treatment groups. In vivo models in the current work are orthotopically transplanted xenografts in SCID mice that lack both T and B lymphocytes. Innate immune cells, particularly NK cells, have been shown to affect the outcome of oHSV therapy in xenografts by, in part, promoting the clearance of virus-infected cells and inhibiting intratumoral virus spread (reviewed in51, 52). TGF-β regulates the function of NK cells53. Han et al reported that pre-treatment of animals with TGF-β augmented the therapeutic effects of oHSV in both xenograft (human GBM) and syngeneic GBM models in vivo, phenocopying the outcomes of innate immune NK cell depletion54. On the other hand, Ilkow et al demonstrated that TGF-β produced by tumor cells reprogrammed nearby cancer-associated fibroblasts and rendered them sensitive to oncolytic rhabdovirus infection, leading to enhanced overall virus replication55. Thus, GBM-produced TGF-β acts on different cell types and impacts the complex cellular cross-talk between GBM and stromal/immune cells within the tumor microenvironment, which likely affects oncolytic virus activity. Although these published reports and our current work seem discordant, the role of TGF-β in oHSV virotherapy may be context- and schedule-dependent. It is possible that both exogenous TGF-β and TGF-β inhibition could enhance oHSV therapy via different mechanisms.
Using clinically relevant orthotopic patient-derived GSC recurrent GBM models, we observed a potent, synergistic anti-tumor interaction between TβR inhibition and oHSV therapy that warrants further investigation as a treatment for refractory GBM. However, the limitations of our animal study include: 1) treatment at an early time-point when tumors are perhaps only initiating, and 2) the use of a nodular (poorly invasive) GBM model. Further research, such as evaluation in additional models including invasive xenografts and immune-competent GBM models, is necessary to define the role of TGF-β and optimize the use of TβR inhibitor toward maximizing oHSV therapy for recurrent GBM.
Supplementary Material
Novelty and Impact.
No effective treatment exists for glioblastoma once it recurs after standard of care. This work demonstrates that blockade of TGF-β signaling pathway enhances the therapeutic efficacy of oncolytic herpes simplex virus in patient-derived cancer stem cell-based recurrent glioblastoma models, supporting a clinical trial testing this combination approach.
Acknowledgments
These studies were supported in part by National Institutes of Health Grants R01NS032677 (R.L.M.), R01CA160762 (S.D.R.), ABTA discovery grant (H.W.) and Japan Herpesvirus Infections Forum Scholarship (S.E.).
Footnotes
Conflict of Interest: The authors declare that there is no conflict of interest to disclose.
References
- 1.Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359:492–507. doi: 10.1056/NEJMra0708126. [DOI] [PubMed] [Google Scholar]
- 2.Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, Ludwin SK, Allgeier A, Fisher B, Belanger K, Hau P, Brandes AA, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–66. doi: 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- 3.Ballman KV, Buckner JC, Brown PD, Giannini C, Flynn PJ, LaPlant BR, Jaeckle KA. The relationship between six-month progression-free survival and 12-month overall survival end points for phase II trials in patients with glioblastoma multiforme. Neuro Oncol. 2007;9:29–38. doi: 10.1215/15228517-2006-025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lamborn KR, Yung WK, Chang SM, Wen PY, Cloughesy TF, DeAngelis LM, Robins HI, Lieberman FS, Fine HA, Fink KL, Junck L, Abrey L, et al. Progression-free survival: an important end point in evaluating therapy for recurrent high-grade gliomas. Neuro Oncol. 2008;10:162–70. doi: 10.1215/15228517-2007-062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weller M, Cloughesy T, Perry JR, Wick W. Standards of care for treatment of recurrent glioblastoma--are we there yet? Neuro Oncol. 2013;15:4–27. doi: 10.1093/neuonc/nos273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lathia JD, Mack SC, Mulkearns-Hubert EE, Valentim CL, Rich JN. Cancer stem cells in glioblastoma. Genes Dev. 2015;29:1203–17. doi: 10.1101/gad.261982.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wakimoto H, Kesari S, Farrell CJ, Curry WT, Jr, Zaupa C, Aghi M, Kuroda T, Stemmer-Rachamimov A, Shah K, Liu TC, Jeyaretna DS, Debasitis J, et al. Human glioblastoma-derived cancer stem cells: establishment of invasive glioma models and treatment with oncolytic herpes simplex virus vectors. Cancer Res. 2009;69:3472–81. doi: 10.1158/0008-5472.CAN-08-3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wakimoto H, Mohapatra G, Kanai R, Curry WT, Jr, Yip S, Nitta M, Patel AP, Barnard ZR, Stemmer-Rachamimov AO, Louis DN, Martuza RL, Rabkin SD. Maintenance of primary tumor phenotype and genotype in glioblastoma stem cells. Neuro Oncol. 2012;14:132–44. doi: 10.1093/neuonc/nor195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ning J, Wakimoto H. Oncolytic herpes simplex virus-based strategies: toward a breakthrough in glioblastoma therapy. Front Microbiol. 2014;5:303. doi: 10.3389/fmicb.2014.00303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kanai R, Rabkin SD. Combinatorial strategies for oncolytic herpes simplex virus therapy of brain tumors. CNS Oncol. 2013;2:129–42. doi: 10.2217/cns.12.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheema TA, Kanai R, Kim GW, Wakimoto H, Passer B, Rabkin SD, Martuza RL. Enhanced antitumor efficacy of low-dose Etoposide with oncolytic herpes simplex virus in human glioblastoma stem cell xenografts. Clin Cancer Res. 2011;17:7383–93. doi: 10.1158/1078-0432.CCR-11-1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kanai R, Rabkin SD, Yip S, Sgubin D, Zaupa CM, Hirose Y, Louis DN, Wakimoto H, Martuza RL. Oncolytic virus-mediated manipulation of DNA damage responses: synergy with chemotherapy in killing glioblastoma stem cells. J Natl Cancer Inst. 2012;104:42–55. doi: 10.1093/jnci/djr509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kanai R, Wakimoto H, Martuza RL, Rabkin SD. A novel oncolytic herpes simplex virus that synergizes with phosphoinositide 3-kinase/Akt pathway inhibitors to target glioblastoma stem cells. Clin Cancer Res. 2011;17:3686–96. doi: 10.1158/1078-0432.CCR-10-3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang W, Fulci G, Buhrman JS, Stemmer-Rachamimov AO, Chen JW, Wojtkiewicz GR, Weissleder R, Rabkin SD, Martuza RL. Bevacizumab with angiostatin-armed oHSV increases antiangiogenesis and decreases bevacizumab-induced invasion in U87 glioma. Mol Ther. 2012;20:37–45. doi: 10.1038/mt.2011.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang W, Fulci G, Wakimoto H, Cheema TA, Buhrman JS, Jeyaretna DS, Stemmer Rachamimov AO, Rabkin SD, Martuza RL. Combination of oncolytic herpes simplex viruses armed with angiostatin and IL-12 enhances antitumor efficacy in human glioblastoma models. Neoplasia. 2013;15:591–9. doi: 10.1593/neo.13158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heldin CH, Vanlandewijck M, Moustakas A. Regulation of EMT by TGFbeta in cancer. FEBS Lett. 2012;586:1959–70. doi: 10.1016/j.febslet.2012.02.037. [DOI] [PubMed] [Google Scholar]
- 17.Caja L, Bellomo C, Moustakas A. Transforming growth factor beta and bone morphogenetic protein actions in brain tumors. FEBS Lett. 2015;589:1588–97. doi: 10.1016/j.febslet.2015.04.058. [DOI] [PubMed] [Google Scholar]
- 18.Nana AW, Yang PM, Lin HY. Overview of Transforming Growth Factor beta Superfamily Involvement in Glioblastoma Initiation and Progression. Asian Pac J Cancer Prev. 2015;16:6813–23. doi: 10.7314/apjcp.2015.16.16.6813. [DOI] [PubMed] [Google Scholar]
- 19.Kjellman C, Olofsson SP, Hansson O, Von Schantz T, Lindvall M, Nilsson I, Salford LG, Sjogren HO, Widegren B. Expression of TGF-beta isoforms, TGF-beta receptors, and SMAD molecules at different stages of human glioma. Int J Cancer. 2000;89:251–8. doi: 10.1002/1097-0215(20000520)89:3<251::aid-ijc7>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 20.Bruna A, Darken RS, Rojo F, Ocana A, Penuelas S, Arias A, Paris R, Tortosa A, Mora J, Baselga J, Seoane J. High TGFbeta-Smad activity confers poor prognosis in glioma patients and promotes cell proliferation depending on the methylation of the PDGF-B gene. Cancer Cell. 2007;11:147–60. doi: 10.1016/j.ccr.2006.11.023. [DOI] [PubMed] [Google Scholar]
- 21.Ikushima H, Todo T, Ino Y, Takahashi M, Miyazawa K, Miyazono K. Autocrine TGF-beta signaling maintains tumorigenicity of glioma-initiating cells through Sry-related HMG-box factors. Cell Stem Cell. 2009;5:504–14. doi: 10.1016/j.stem.2009.08.018. [DOI] [PubMed] [Google Scholar]
- 22.Penuelas S, Anido J, Prieto-Sanchez RM, Folch G, Barba I, Cuartas I, Garcia-Dorado D, Poca MA, Sahuquillo J, Baselga J, Seoane J. TGF-beta increases glioma-initiating cell self-renewal through the induction of LIF in human glioblastoma. Cancer Cell. 2009;15:315–27. doi: 10.1016/j.ccr.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 23.Zhang M, Kleber S, Rohrich M, Timke C, Han N, Tuettenberg J, Martin-Villalba A, Debus J, Peschke P, Wirkner U, Lahn M, Huber PE. Blockade of TGF-beta signaling by the TGFbetaR-I kinase inhibitor LY2109761 enhances radiation response and prolongs survival in glioblastoma. Cancer Res. 2011;71:7155–67. doi: 10.1158/0008-5472.CAN-11-1212. [DOI] [PubMed] [Google Scholar]
- 24.Herbertz S, Sawyer JS, Stauber AJ, Gueorguieva I, Driscoll KE, Estrem ST, Cleverly AL, Desaiah D, Guba SC, Benhadji KA, Slapak CA, Lahn MM. Clinical development of galunisertib (LY2157299 monohydrate), a small molecule inhibitor of transforming growth factor-beta signaling pathway. Drug Des Devel Ther. 2015;9:4479–99. doi: 10.2147/DDDT.S86621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brandes AA, Carpentier AF, Kesari S, Sepulveda-Sanchez JM, Wheeler HR, Chinot O, Cher L, Steinbach JP, Capper D, Specenier P, Rodon J, Cleverly A, et al. A Phase II randomized study of galunisertib monotherapy or galunisertib plus lomustine compared with lomustine monotherapy in patients with recurrent glioblastoma. Neuro Oncol. 2016;18:1146–56. doi: 10.1093/neuonc/now009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wakimoto H, Tanaka S, Curry WT, Loebel F, Zhao D, Tateishi K, Chen J, Klofas LK, Lelic N, Kim JC, Dias-Santagata D, Ellisen LW, et al. Targetable signaling pathway mutations are associated with malignant phenotype in IDH-mutant gliomas. Clin Cancer Res. 2014;20:2898–909. doi: 10.1158/1078-0432.CCR-13-3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chi AS, Batchelor TT, Dias-Santagata D, Borger D, Stiles CD, Wang DL, Curry WT, Wen PY, Ligon KL, Ellisen L, Louis DN, Iafrate AJ. Prospective, high-throughput molecular profiling of human gliomas. J Neurooncol. 2012;110:89–98. doi: 10.1007/s11060-012-0938-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nitta M, Kozono D, Kennedy R, Stommel J, Ng K, Zinn PO, Kushwaha D, Kesari S, Inda MM, Wykosky J, Furnari F, Hoadley KA, et al. Targeting EGFR induced oxidative stress by PARP1 inhibition in glioblastoma therapy. PLoS One. 2010;5:e10767. doi: 10.1371/journal.pone.0010767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Todo T, Martuza RL, Rabkin SD, Johnson PA. Oncolytic herpes simplex virus vector with enhanced MHC class I presentation and tumor cell killing. Proc Natl Acad Sci U S A. 2001;98:6396–401. doi: 10.1073/pnas.101136398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sgubin D, Wakimoto H, Kanai R, Rabkin SD, Martuza RL. Oncolytic herpes simplex virus counteracts the hypoxia-induced modulation of glioblastoma stem-like cells. Stem Cells Transl Med. 2012;1:322–32. doi: 10.5966/sctm.2011-0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 32.Aghi M, Rabkin S, Martuza RL. Effect of chemotherapy-induced DNA repair on oncolytic herpes simplex viral replication. J Natl Cancer Inst. 2006;98:38–50. doi: 10.1093/jnci/djj003. [DOI] [PubMed] [Google Scholar]
- 33.Nigim F, Cavanaugh J, Patel AP, Curry WT, Jr, Esaki S, Kasper EM, Chi AS, Louis DN, Martuza RL, Rabkin SD, Wakimoto H. Targeting Hypoxia-Inducible Factor 1alpha in a New Orthotopic Model of Glioblastoma Recapitulating the Hypoxic Tumor Microenvironment. J Neuropathol Exp Neurol. 2015;74:710–22. doi: 10.1097/NEN.0000000000000210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martens T, Laabs Y, Gunther HS, Kemming D, Zhu Z, Witte L, Hagel C, Westphal M, Lamszus K. Inhibition of glioblastoma growth in a highly invasive nude mouse model can be achieved by targeting epidermal growth factor receptor but not vascular endothelial growth factor receptor-2. Clin Cancer Res. 2008;14:5447–58. doi: 10.1158/1078-0432.CCR-08-0147. [DOI] [PubMed] [Google Scholar]
- 35.Talasila KM, Soentgerath A, Euskirchen P, Rosland GV, Wang J, Huszthy PC, Prestegarden L, Skaftnesmo KO, Sakariassen PO, Eskilsson E, Stieber D, Keunen O, et al. EGFR wild-type amplification and activation promote invasion and development of glioblastoma independent of angiogenesis. Acta Neuropathol. 2013;125:683–98. doi: 10.1007/s00401-013-1101-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Phillips HS, Kharbanda S, Chen R, Forrest WF, Soriano RH, Wu TD, Misra A, Nigro JM, Colman H, Soroceanu L, Williams PM, Modrusan Z, et al. Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell. 2006;9:157–73. doi: 10.1016/j.ccr.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 37.Verhaak RG, Hoadley KA, Purdom E, Wang V, Qi Y, Wilkerson MD, Miller CR, Ding L, Golub T, Mesirov JP, Alexe G, Lawrence M, et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell. 2010;17:98–110. doi: 10.1016/j.ccr.2009.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mooney KL, Choy W, Sidhu S, Pelargos P, Bui TT, Voth B, Barnette N, Yang I. The role of CD44 in glioblastoma multiforme. J Clin Neurosci. 2016 doi: 10.1016/j.jocn.2016.05.012. [DOI] [PubMed] [Google Scholar]
- 39.Iwatsuki M, Mimori K, Yokobori T, Ishi H, Beppu T, Nakamori S, Baba H, Mori M. Epithelial-mesenchymal transition in cancer development and its clinical significance. Cancer Sci. 2010;101:293–9. doi: 10.1111/j.1349-7006.2009.01419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wendt MK, Allington TM, Schiemann WP. Mechanisms of the epithelial-mesenchymal transition by TGF-beta. Future Oncol. 2009;5:1145–68. doi: 10.2217/fon.09.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Esaki S, Rabkin SD, Martuza RL, Wakimoto H. Transient fasting enhances replication of oncolytic herpes simplex virus in glioblastoma. Am J Cancer Res. 2016;6:300–11. [PMC free article] [PubMed] [Google Scholar]
- 42.Perkins D, Gyure KA, Pereira EF, Aurelian L. Herpes simplex virus type 1-induced encephalitis has an apoptotic component associated with activation of c-Jun N-terminal kinase. J Neurovirol. 2003;9:101–11. doi: 10.1080/13550280390173427. [DOI] [PubMed] [Google Scholar]
- 43.Tamura K, Wakimoto H, Agarwal AS, Rabkin SD, Bhere D, Martuza RL, Kuroda T, Kasmieh R, Shah K. Multimechanistic tumor targeted oncolytic virus overcomes resistance in brain tumors. Mol Ther. 2013;21:68–77. doi: 10.1038/mt.2012.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003;425:577–84. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- 45.Campos B, Olsen LR, Urup T, Poulsen HS. A comprehensive profile of recurrent glioblastoma. Oncogene. 2016;35:5819–25. doi: 10.1038/onc.2016.85. [DOI] [PubMed] [Google Scholar]
- 46.Wang J, Cazzato E, Ladewig E, Frattini V, Rosenbloom DI, Zairis S, Abate F, Liu Z, Elliott O, Shin YJ, Lee JK, Lee IH, et al. Clonal evolution of glioblastoma under therapy. Nat Genet. 2016;48:768–76. doi: 10.1038/ng.3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kahlert UD, Nikkhah G, Maciaczyk J. Epithelial-to-mesenchymal(-like) transition as a relevant molecular event in malignant gliomas. Cancer Lett. 2013;331:131–8. doi: 10.1016/j.canlet.2012.12.010. [DOI] [PubMed] [Google Scholar]
- 48.Iwadate Y. Epithelial-mesenchymal transition in glioblastoma progression. Oncol Lett. 2016;11:1615–20. doi: 10.3892/ol.2016.4113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wood MD, Reis GF, Reuss DE, Phillips JJ. Protein Analysis of Glioblastoma Primary and Posttreatment Pairs Suggests a Mesenchymal Shift at Recurrence. J Neuropathol Exp Neurol. 2016;75:925–35. doi: 10.1093/jnen/nlw068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Anido J, Saez-Borderias A, Gonzalez-Junca A, Rodon L, Folch G, Carmona MA, Prieto-Sanchez RM, Barba I, Martinez-Saez E, Prudkin L, Cuartas I, Raventos C, et al. TGF-beta Receptor Inhibitors Target the CD44(high)/Id1(high) Glioma-Initiating Cell Population in Human Glioblastoma. Cancer Cell. 2010;18:655–68. doi: 10.1016/j.ccr.2010.10.023. [DOI] [PubMed] [Google Scholar]
- 51.Bhat R, Rommelaere J. Emerging role of Natural killer cells in oncolytic virotherapy. Immunotargets Ther. 2015;4:65–77. doi: 10.2147/ITT.S55549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Braidwood L, Graham SV, Graham A, Conner J. Oncolytic herpes viruses, chemotherapeutics, and other cancer drugs. Oncolytic Virother. 2013;2:57–74. doi: 10.2147/OV.S52601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kelly A, Houston SA, Sherwood E, Casulli J, Travis MA. Regulation of Innate and Adaptive Immunity by TGFbeta. Adv Immunol. 2017;134:137–233. doi: 10.1016/bs.ai.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 54.Han J, Chen X, Chu J, Xu B, Meisen WH, Chen L, Zhang L, Zhang J, He X, Wang QE, Chiocca EA, Kaur B, et al. TGFbeta Treatment Enhances Glioblastoma Virotherapy by Inhibiting the Innate Immune Response. Cancer Res. 2015;75:5273–82. doi: 10.1158/0008-5472.CAN-15-0894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ilkow CS, Marguerie M, Batenchuk C, Mayer J, Ben Neriah D, Cousineau S, Falls T, Jennings VA, Boileau M, Bellamy D, Bastin D, de Souza CT, et al. Reciprocal cellular cross-talk within the tumor microenvironment promotes oncolytic virus activity. Nat Med. 2015;21:530–6. doi: 10.1038/nm.3848. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.