Abstract
Human aquaporin 4 (AQP4) is the primary water channel protein in brain astrocytes. Hypothermia is known to cause astrocyte swelling in culture, but the precise role of AQP4 in this process is unknown. Primary human cortical astrocytes were cultured under hypothermic (32 °C) or normothermic (37 °C) conditions. AQP4 transcript, total protein and surface‐localized protein were quantified using RT‐qPCR, sandwich ELISA with whole cell lysates or cell surface biotinylation, followed by ELISA analysis of the surface‐localized protein, respectively. Four‐hour mild hypothermic treatment increased the surface localization of AQP4 in human astrocytes to 155 ± 4% of normothermic controls, despite no change in total protein expression levels. The hypothermia‐mediated increase in AQP4 surface abundance on human astrocytes was blocked using either calmodulin antagonist (trifluoperazine, TFP); TRPV4 antagonist, HC‐067047 or calcium chelation using EGTA‐AM. The TRPV4 agonist (GSK1016790A) mimicked the effect of hypothermia compared with untreated normothermic astrocytes. Hypothermia led to an increase in surface localization of AQP4 in human astrocytes through a mechanism likely dependent on the TRPV4 calcium channel and calmodulin activation. Understanding the effects of hypothermia on astrocytic AQP4 cell surface expression may help develop new treatments for brain swelling based on an in‐depth mechanistic understanding of AQP4 translocation.
Keywords: aquaporin 4, astrocyte, calcium, calmodulin, mild therapeutic hypothermia, TRPV4
Introduction
Hypothermia is known to have specific effects on the blood–brain barrier (BBB), including changes in blood flow, metabolism, water homeostasis and excitotoxicity, as well as signalling profiles leading to changes in apoptosis and cell survival (Yenari & Han, 2012). Hypothermia was reported to be neuroprotective in stroke patients (Yenari & Hemmen, 2010), but in traumatic brain injury (TBI), a therapeutic role for hypothermia is controversial; different clinical trials have reported both positive and negative outcomes on patient recovery (Andrews et al., 2015; Lazaridis & Robertson, 2016). Cytotoxic oedema, which is a feature of stroke and TBI, occurs when water enters the CNS via astrocytes at the BBB, predominantly through the aquaporin 4 (AQP4) channel, and knockout of AQP4 is protective against cytotoxic oedema development in various animal models (Verkman et al., 2006). The specific effects of hypothermia on AQP4 expression in human astrocytes are not known. AQP4 expression in rat astrocytes has been reported to increase after 2 h in response to transarterial (cold saline) hypothermia infusion in a middle cerebral artery occlusion (MCAO) model (Kurisu et al., 2016). In a rat model of thrombin‐induced vasogenic oedema, AQP4 protein levels increased after 24 h, peaking at 48 h (Gao et al., 2015). Conversely, hypothermia‐induced reduction in cytotoxic cerebral oedema, in a porcine model of cardiac arrest/resuscitation, was concomitant with a reduction in AQP4 protein levels after 24 h (Zhao et al., 2012). Additionally, mild hypothermia was reported to cause a significant astrocyte volume increase to 107.3 ± 0.4% (mean ± SEM) of control, after 30 min at 32 °C (Plesnila et al., 2000).
Many factors have been reported to alter AQP4 abundance and subcellular localization, including vasopressin, histamine and astrocytic glutamate (Conner et al., 2013). We recently described a mechanism of short‐term AQP4 relocalization during tonicity‐driven cell swelling in rat astrocytes (Kitchen et al., 2015). To our knowledge, hypothermia has not been previously investigated as a trigger of AQP4 relocalization.
In addition to AQP4, the stretch‐activated transient receptor potential vanilloid 4 calcium channel (TRPV4) is known to be essential in cell volume regulation: a TRPV4‐mediated increase in Ca2+ influx has been suggested to have an effect on the AQP‐mediated cell volume regulation following osmotic stress and the activity and localization of TRPV4 was often dependent on aquaporin expression (Benfenati et al., 2011). They proposed that a TRPV4/AQP4 complex at the astrocyte membrane was an essential component of an osmosensory system that couples osmotic stress to downstream signalling pathways (Benfenati et al., 2011).
This study demonstrates that mild hypothermia, as used therapeutically (32 °C), can increase the localization of endogenous AQP4 to the plasma membrane of human primary cortical astrocytes and that this process can be disrupted by inhibition of TRPV4 or calmodulin and by chelation of intracellular calcium. Given the well‐established permissive role of AQP4 in cytotoxic oedema development, our data may help to explain why hypothermic intervention in conditions involving cytotoxic oedema is sometimes deleterious and suggest novel hypothermic/pharmacological combination therapies for cytotoxic oedema.
Materials and methods
Cell culture
Primary human cortical astrocytes (ScienCell) were used following the manufacturer's protocol. All cells were used at passage 4 or below. The cells were incubated in humidified 5% (v/v) CO2 in air at 37 °C or 32 °C.
RT‐qPCR
Total RNA was isolated using a Qiagen RNeasy Plus Mini Kit (Qiagen), according to the manufacturer's instructions, and multi‐target RT‐qPCR was performed using a StepOnePlus™ Real‐Time PCR System (Applied Biosystems) with purified cDNA samples generated using SuperScript™ III Reverse Transcriptase (Thermo Fisher Scientific) and QIAquick PCR Purification Kit (Qiagen) to investigate gene expression levels of AQP4 mRNA using Taqman primers (Thermo Fisher; ID: Hs00242342_m1). PPIA (assay ID: Hs99999904_m1) and CDKN1B (assay ID: Hs00153277_m1; Applied Biosystems) were used as control housekeeping genes. Results were analysed using the method and presented as relative gene expression normalized to the average cycle threshold for the two housekeeping genes.
ELISA
AQP4 and calmodulin protein levels were measured by sandwich ELISA following the manufacturer's instructions (Abcam) and as described by Salman et al. (2017), using rabbit polyclonal anti‐AQP4 antibody (Abcam, ab46182) and mouse monoclonal anti‐AQP4 antibody (Abcam, ab9512) or rabbit polyclonal anti‐calmodulin antibody (Abcam, ab38590) with mouse monoclonal anti‐calmodulin antibody (Abcam, ab2860). The secondary antibody used in both assays was chicken anti‐mouse IgG‐HRP antibody (Santa Cruz, sc‐2954).
Cell surface biotinylation (CSB)
This was performed as described by Kitchen et al. (2015). Briefly, cell surface proteins were biotinylated using a cell‐impermeable amine‐reactive biotinylation reagent (EZ‐Link Sulfo‐NHS‐SS‐Biotin; Thermo Cat. No. 21331). 96‐well Pierce™ NeutrAvidin™ coated plates (Thermo Scientific; Cat. No. 15129) were used to pull down the biotinylated proteins within the total cell lysate. After washing off unbound proteins, the plate was then incubated with anti‐AQP4 antibody (Abcam, ab128906) diluted 1 : 500 in 0.05% PBS‐tween (PBS‐T), which bound to the cell surface biotinylated AQP4 proteins attached to the avidin coated plate. Anti‐AQP4 antibody validation data are provided in Fig. S1.
Cell treatments
Hypotonic challenges were achieved by dilution of media with dH2O to 85 mOsm/kg H2O; osmolality was measured using an Osmomat 3000 freezing point depression osmometer (Gonotec). Inhibitors were used at 100× published IC50 values and were added 30 min prior to initiation and maintained for the duration of each experimental setting. TRPV4 inhibitor (HC‐067047) was used at a concentration of 4.8 μm, while calmodulin antagonist (trifluoperazine, TFP) was used at a concentration of 127 μm. The potent TRPV4 channel agonist GSK1016790A (Sigma) was used at a concentration of 2.1 μm; it was added either 30 min before the intervention or at the same time as the intervention. Intracellular calcium chelation was performed using EGTA AM (Invitrogen; E1219) at a concentration of 5 μm following the manufacturer's recommendation. In all cases, cells were at least 92% viable, assessed using CellTiter‐Glo® Luminescent Cell Viability Assay.
Statistical analysis
All data are presented as a fold change normalized to the experimental control. RT‐qPCR expression, ELISA and cell surface biotinylation data were found to be nonparametric in distribution using the Shapiro–Wilk test. A Kruskal–Wallis analysis with a Conover–Inman post hoc test was therefore used to identify significant differences (P ≤ 0.05) using statsdirect 3 software. Additionally, data have been reproduced as box‐and‐whisker plots and are provided collectively in Fig. S2.
Results
RT‐qPCR and ELISA of AQP4 following hypothermia in primary human astrocytes
There was a modest increase in AQP4 mRNA levels in cultured human primary astrocytes (measured using RT‐qPCR) following 4 h mild hypothermia (32 °C) compared with control cells grown at 37 °C (Fig. 1A): the increase was 156% ± 16%; P = 0.035 (n = 4). However, Fig. 1B shows that this small increase in transcript abundance was not translated into a change in protein levels (measured by ELISA), which were not significantly different (P > 0.05).
Figure 1.
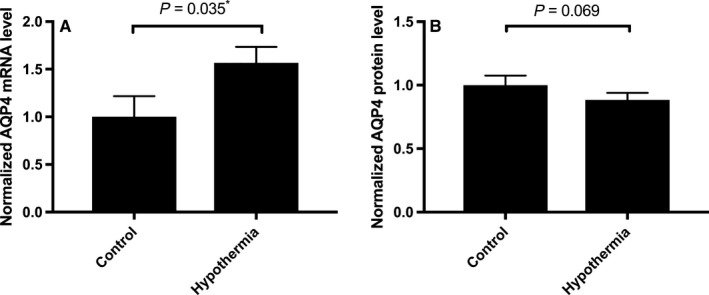
AQP4 mRNA and protein expression levels in cultured primary human astrocytes. Data are mean fold changes in expression (±SEM) of cells incubated at 32 °C for 4 h (labelled ‘Hypothermia’) compared with normothermic (‘Control’) astrocytes. Each bar represents the mean ± SEM for each of the conditions. Kruskal–Wallis with Conover–Inman post hoc analysis tests were used to identify significant differences between samples. *Represents statistical significance (P < 0.05). Panel (A) shows RT‐qPCR data (N = 5) using Taqman probes and analysis normalized to the two housekeeping genes, PPIA and CDKN1B. Panel (B) shows sandwich ELISA data (N = 4).
AQP4 plasma membrane abundance using cell surface biotinylation following hypothermia in primary human astrocytes
Cell surface AQP4 expression after 4 h mild hypothermia (32 °C) was compared to cells at 37 °C using an AQP4 biotinylation assay [as described in our previous study (Kitchen et al., 2015)]. The results show that despite there being no change in total AQP4 protein levels (Fig. 1B), AQP4 levels at the cell surface increased to 155 ± 4% (P < 0.0001) of control values following hypothermia (Fig. 2); the modest decrease in surface localization of the control membrane protein, amino acid transporter EAAT1, indicates that the hypothermia‐induced increase in AQP4 plasma membrane localization is not a global response to hypothermia associated with all membrane proteins.
Figure 2.
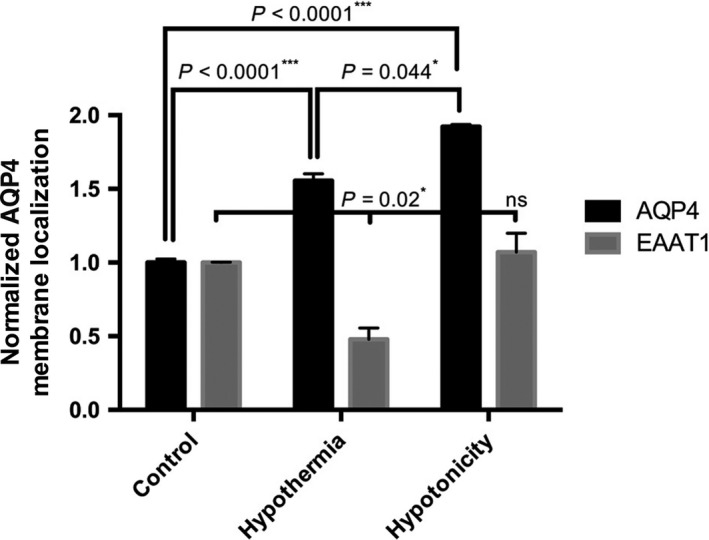
AQP4 cell surface localization in primary human astrocytes. Data are mean fold changes in expression (±SEM) of cells incubated at 32 °C for 4 h (Hypothermia) or 85 mOsm/kg H2O (Hypotonicity) compared with normothermic (Control) astrocytes. Each bar represents the mean ± SEM for each of the conditions. Kruskal–Wallis with Conover–Inman post hoc analysis tests were used to identify significant differences between samples (n = 4). *, ***Represents statistical significance (*P < 0.05 and ***P < 0.001) using statsdirect 3 software.
Hypotonicity is known to cause cellular swelling and increase in AQP4 surface localization and was used a positive control. Figure 2 shows that hypotonicity significantly increases the AQP4 levels at the cell surface to 192 ± 11% (P < 0.0001) of control values with no significant difference in EAAT1 levels (P = 0.579).
Validation of calmodulin‐ and TRPV4‐dependent mechanism in AQP4 translocation following hypothermia in primary human astrocytes
We previously identified a regulatory mechanism for AQP4 surface localization in an in vitro model using rat astrocyte cell swelling in response to reduced extracellular osmolality (Kitchen et al., 2015). Calmodulin was a key regulator of this process, and extracellular Ca2+ was essential to mediate the translocation. We therefore measured the localization of AQP4 following inhibition of either calmodulin (using TFP); TRPV4 (using HC‐067047) or calcium (using EGTA‐AM); Fig. 3 shows that all the investigated inhibitors blocked relocalization of AQP4 in human primary astrocytes following 32 °C hypothermia. We have previously shown no effect of either TFP or removal of extracellular calcium on the initial (i.e. pre‐swelling‐induced relocalization) distribution of AQP4 in rat astrocytes (Kitchen et al., 2015) suggesting TRPV4 and calmodulin inhibition are disrupting this hypothermic relocalization phenomenon.
Figure 3.
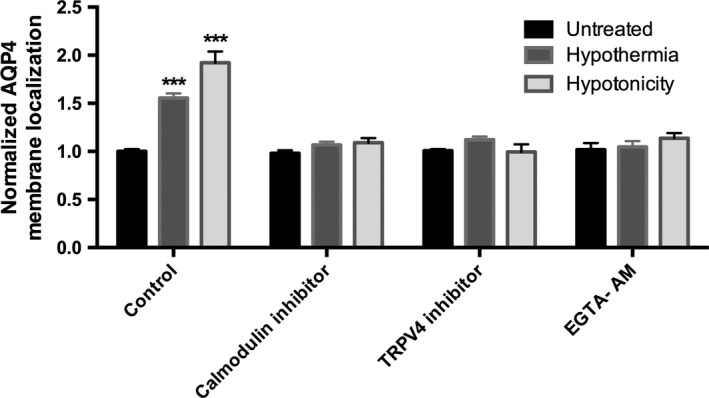
Inhibition of hypothermia‐induced increase in AQP4 cell surface localization in primary human astrocytes. Data are mean fold changes in expression (±SEM) of cells incubated at 32 °C for 4 h (Hypothermia) or 85 mOsm/kg H2O (Hypotonicity) compared with normothermic (Control) astrocytes with or without the indicated inhibitors. The calmodulin inhibitor is 127 μm TFP; the TRPV4 inhibitor is 4.8 μm HC‐067047; the intracellular calcium chelator is 5 μm EGTA AM. Kruskal–Wallis with Conover–Inman post hoc analysis tests were used to identify significant differences between samples (n = 4). ***Represents statistical significance (***P < 0.001) using statsdirect 3 software.
The data in Fig. 3 indicated that calmodulin activation is required for AQP4 relocalization. Therefore, we assessed calmodulin protein levels under the same hypothermic conditions. Our data showed that 4 h mild hypothermia (32 °C) significantly increased calmodulin expression to 158 ± 5% (P < 0.0001) compared with cells grown at 37 °C (n = 4).
The involvement of TRPV4 was investigated further using the TRPV4 agonist, GSK1016790A. Figure 4 shows that treating astrocytes with GSK1016790A causes a significant increase in AQP4 surface expression, 145 ± 10% (P = 0.0042) compared to control levels, similar to what was observed after hypothermia. Interestingly, there was no additive effect of TRPV4 agonist and hypothermia. Agonist was added 30 min before hypothermia in order to follow the same experimental protocol used for the antagonists. We speculated that this ‘pre‐treatment’ may have caused internalization of TRPV4 (which has been reported for GSK1016790A; Shibasaki et al., 2015), leading to the absence of TRPV4 at the surface for activation by hypothermia. To test this, we repeated the experiment applying the TRPV4 agonist and hypothermia simultaneously. In this case, an additive effect of TRPV4 agonism and hypothermia was seen, with AQP4 surface expression increasing to 183 ± 4% (P = 0.0003) of control, significantly higher than either TRPV4 agonist alone (P = 0.0039) or hypothermia alone (P = 0.025). The same additive effect was observed following hypotonic intervention: AQP4 surface expression was increased to 189 ± 10% (P = 0.045) with TRPV4 agonist pre‐treatment and 223 ± 5% (P = 0.0253) with simultaneous treatment.
Figure 4.
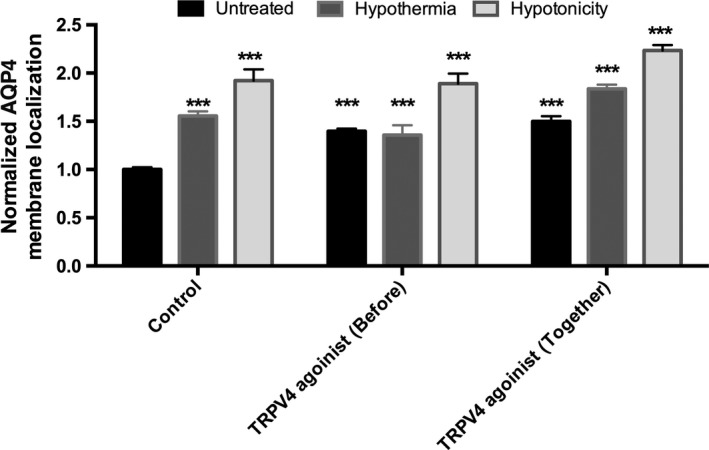
TRPV4 channel agonist increases AQP4 cell surface localization in primary human astrocytes. Data are mean fold changes in expression (±SEM) of cells incubated at 32 °C for 4 h (Hypothermia) or 85 mOsm/kg H2O (Hypotonicity) compared with normothermic (Control) astrocytes with or without the potent TRPV4 channel agonist (GSK1016790A) at 2.1 μm. The TRPV4 agonist was added either 30 min before the intervention (Before) or at the same time of the intervention (Together). Kruskal–Wallis with Conover–Inman post hoc analysis tests were used to identify significant differences between samples (n = 4). ***Represents statistical significance (***P < 0.001) using statsdirect 3 software.
There were no significant changes in EAAT1 surface expression levels following the agonist/inhibitors in Figs 3 and 4 (data not shown).
Discussion
Hypothermia affects many physiological mechanisms of the brain and has a widely recognized therapeutic potential in the reduction of early‐stage (cytotoxic) oedema (Previch et al., 2016). This oedema can form when astrocytes have reduced ion homeostasis and increased intracellular tonicity, which is followed by water influx via osmosis. This in turn is thought to activate the mechanosensitive calcium channels (mainly TRPV4) leading to an increase in calcium influx (Benfenati et al., 2011) enhancing calmodulin activity, including calmodulin‐mediated AQP4 surface relocalization (Verkman et al., 2014; Vella et al., 2015). The proposal that hypothermia influences important cellular signalling mechanisms is supported by recent work showing that hypothermia/TRPV4 mediated effects on intracellular signalling (Zou et al., 2015).
To our knowledge, this is the first demonstration of changes to AQP4 surface localization levels in response to hypothermic treatment of human primary cortical astrocytes. We have also demonstrated that inhibiting calmodulin (using either the antagonist, TFP or the TRPV4 inhibitor, HC‐067047) or intracellular calcium (using EGTA‐AM) blocks the hypothermia‐mediated increase in AQP4 surface expression in human astrocytes. Interestingly, it has been shown that TRPV4 activity is inhibited by cold (temperature < 34 °C) in mouse neurons (Shibasaki et al., 2015). It is also well‐established that TRPV4 opens in response to cell swelling, but the calcium signalling responses are different in neurons (fast, inactivating currents) and glia (slow currents, with sustained elevation; Ryskamp et al., 2014). In astrocytes, where hypothermia induces cell swelling, it is not obvious whether TRPV4 would be activated by mechanical swelling, inactivated by cold, or at intermediate activity, depending on the exact temperature and cell volume. Our data suggest that TRPV4 retains at least some activity in astrocytes at 32 °C, as the AQP4 relocalization observed in response to hypothermia is blocked by a TRPV4 antagonist and recapitulated by a TRPV4 agonist under normothermic (37 °C) conditions. The absence of any additive effect between pre‐treatment with TRPV4 agonist and its level at the time of hypothermic intervention could be due to receptor internalization, which could explain the additive effect seen when cells are exposed to the intervention and the agonist at the same time.
It is thought that AQP4 deletion/knockdown is protective and AQP4 overexpression is harmful in animal models of early brain oedema (Verkman et al., 2006). We suggest that some of the deleterious effects of hypothermic intervention in oedema that have been reported (Lazaridis & Robertson, 2016) may be mediated by increased AQP4 surface expression via a TRPV4‐/calmodulin‐dependent relocalization mechanism. Further work showing inhibition of human astrocytic swelling in the presence of calmodulin and/or TRPV4 inhibitors would further support the role of AQP4 in astrocytic swelling in stoke and traumatic injury.
Separating the mechanisms involved in the beneficial and damaging effects of hypothermic intervention may allow us to further refine the clinical value of hypothermia for oedema prevention following stroke or TBI. In the future, further, co‐treatment with putative AQP4 inhibitors targeting the subcellular relocalization pathway would allow exploitation of the neuroprotective effects of hypothermia while mitigating any harmful effects.
Conflict of interest
The authors do not have any competing interests.
Author contributions
MMS performed all laboratory work and initial data analysis, contributed to study design and helped draft the manuscript. MTC, ACC, RMB, MNW and PK conceived the study, participated in its design and coordination, assisted in data and statistical analysis and co‐wrote the manuscript with the help of JEB. All authors read and approved the final manuscript.
Data accessibility
All relevant data are within the article and its Supporting Information files were made publicly available at https://doi.org/10.6084/m9.figshare.5293672
Abbreviations
- AQP4
aquaporin 4
- EAAT1
excitatory amino acid transporter 1
- ELISA
enzyme‐linked immunosorbent assay
- TBI
transient brain injury
- TRPV4
transient receptor potential vanilloid 4
Supporting information
Acknowledgements
This work was supported by BMRC Sheffield Hallam University, RIHS University of Wolverhampton, School of Life and Health Sciences Aston University and the HCED/Iraq grant number GD‐13‐3 (M Salman).
Edited by Masahiko Watanabe
Reviewed by Masanori Tachikawa, Tohoku University, Japan; and Koji Shibasaki, Gunma University Graduate School of Medicine, Japan
The associated peer review process communications can be found in the online version of this article.
Contributor Information
Roslyn M. Bill, Email: r.m.bill@aston.ac.uk
Alex C. Conner, Email: a.c.conner@bham.ac.uk
Matthew T. Conner, Email: m.conner@wlv.ac.uk.
References
- Andrews, P.J. , Sinclair, H.L. , Rodriguez, A. , Harris, B.A. , Battison, C.G. , Rhodes, J.K. , Murray, G.D. & Eurotherm Trial, C. (2015) Hypothermia for intracranial hypertension after traumatic brain injury. New Engl. J. Med., 373, 2403–2412. [DOI] [PubMed] [Google Scholar]
- Benfenati, V. , Caprini, M. , Dovizio, M. , Mylonakou, M.N. , Ferroni, S. , Ottersen, O.P. & Amiry‐Moghaddam, M. (2011) An aquaporin‐4/transient receptor potential vanilloid 4 (AQP4/TRPV4) complex is essential for cell‐volume control in astrocytes. Proc. Natl. Acad. Sci. USA, 108, 2563–2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conner, A.C. , Bill, R.M. & Conner, M.T. (2013) An emerging consensus on aquaporin translocation as a regulatory mechanism. Mol. Membr. Biol., 30, 1–12. [DOI] [PubMed] [Google Scholar]
- Gao, D. , Ding, F. , Lei, G. , Luan, G. , Zhang, S. , Li, K. , Wang, D. , Zhang, L. et al (2015) Effects of focal mild hypothermia on thrombin‐induced brain edema formation and the expression of protease activated receptor‐1, matrix metalloproteinase‐9 and aquaporin 4 in rats. Mol. Med. Rep., 11, 3009–3014. [DOI] [PubMed] [Google Scholar]
- Kitchen, P. , Day, R.E. , Taylor, L.H. , Salman, M.M. , Bill, R.M. , Conner, M.T. & Conner, A.C. (2015) Identification and molecular mechanisms of the rapid tonicity‐induced relocalization of the aquaporin 4 channel. J. Biol. Chem., 290, 16873–16881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurisu, K. , Abumiya, T. , Nakamura, H. , Shimbo, D. , Shichinohe, H. , Nakayama, N. , Kazumata, K. , Shimizu, H. et al (2016) Transarterial regional brain hypothermia inhibits acute aquaporin‐4 surge and sequential microvascular events in ischemia/reperfusion injury. Neurosurgery, 79, 125–134. [DOI] [PubMed] [Google Scholar]
- Lazaridis, C. & Robertson, C.S. (2016) Hypothermia for increased intracranial pressure: is it dead? Curr. Neurol. Neurosci., 16, 78. [DOI] [PubMed] [Google Scholar]
- Plesnila, N. , Muller, E. , Guretzki, S. , Ringel, F. , Staub, F. & Baethmann, A. (2000) Effect of hypothermia on the volume of rat glial cells. J. Physiol., 523(Pt 1), 155–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Previch, L.E. , Ma, L. , Wright, J.C. , Singh, S. , Geng, X. & Ding, Y. (2016) Progress in AQP research and new developments in therapeutic approaches to ischemic and hemorrhagic stroke. Int. J. Mol. Sci., 17, 1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryskamp, D.A. , Jo, A.O. , Frye, A.M. , Vazquez‐Chona, F. , MacAulay, N. , Thoreson, W.B. & Križaj, D. (2014) Swelling and eicosanoid metabolites differentially gate TRPV4 channels in retinal neurons and glia. J. Neurosci., 34, 15689–15700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salman, M.M. , Sheilabi, M.A. , Bhattacharyya, D. , Kitchen, P. , Conner, A.C. , Bill, R.M. , Woodroofe, M.N. , Conner, M.T. et al (2017) Transcriptome analysis suggests a role for the differential expression of cerebral aquaporins and the MAPK signalling pathway in human temporal lobe epilepsy. Eur. J. Neurosci., 46, 2121–2132. [DOI] [PubMed] [Google Scholar]
- Shibasaki, K. , Sugio, S. , Takao, K. , Yamanaka, A. , Miyakawa, T. , Tominaga, M. & Ishizaki, Y. (2015) TRPV4 activation at the physiological temperature is a critical determinant of neuronal excitability and behavior. Pflüg. Arch. Eur. J. Phy., 467, 2495–2507. [DOI] [PubMed] [Google Scholar]
- Vella, J. , Zammit, C. , Di Giovanni, G. , Muscat, R. & Valentino, M. (2015) The central role of aquaporins in the pathophysiology of ischemic stroke. Front. Cell. Neurosci., 9, 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkman, A.S. , Binder, D.K. , Bloch, O. , Auguste, K. & Papadopoulos, M.C. (2006) Three distinct roles of aquaporin‐4 in brain function revealed by knockout mice. Biochem. Biophys. Acta., 1758, 1085–1093. [DOI] [PubMed] [Google Scholar]
- Verkman, A.S. , Anderson, M.O. & Papadopoulos, M.C. (2014) Aquaporins: important but elusive drug targets. Nat. Rev. Drug Discov., 13, 259–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yenari, M.A. & Han, H.S. (2012) Neuroprotective mechanisms of hypothermia in brain ischaemia. Nat. Rev. Neurosci., 13, 267–278. [DOI] [PubMed] [Google Scholar]
- Yenari, M.A. & Hemmen, T.M. (2010) Therapeutic hypothermia for brain ischemia. Stroke, 41, S72–S74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, H. , Li, C.‐S. , Gong, P. , Tang, Z.‐R. , Hua, R. , Mei, X. , Zhang, M.‐Y. & Cui, J. (2012) Molecular mechanisms of therapeutic hypothermia on neurological function in a swine model of cardiopulmonary resuscitation. Resuscitation, 83, 913–920. [DOI] [PubMed] [Google Scholar]
- Zou, Q. , Leung, S. & Vanhoutte, P. (2015) Transient receptor potential channel opening releases endogenous acetylcholine, which contributes to endothelium‐dependent relaxation induced by mild hypothermia in spontaneously hypertensive rat but not Wistar‐Kyoto rat arteries. J. Pharmacol. Exp. Ther., 354, 121–130. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All relevant data are within the article and its Supporting Information files were made publicly available at https://doi.org/10.6084/m9.figshare.5293672


