Abstract
Background and purpose
Cognitive rehabilitation has demonstrated efficacy in producing short‐term cognitive and brain changes in patients with Parkinson's disease (PD). To date, no study has assessed the long‐term effects of cognitive rehabilitation using neuroimaging techniques in PD. The aim was to assess the longitudinal effects of a 3‐month cognitive rehabilitation programme evaluating the cognitive, behavioural and neuroimaging changes after 18 months.
Methods
Fifteen patients with PD underwent a cognitive, behavioural and neuroimaging assessment at pre‐treatment (T0), post‐treatment (T1) and after 18 months (T2). This study examined the long‐term effects (from T0 to T2) and the maintenance of the changes (from T1 to T2). T1‐weighted, diffusion‐weighted, functional magnetic resonance imaging during both a resting‐state and a memory paradigm were acquired. Voxel‐based morphometry and tract‐based spatial statistics were used for grey and white matter analyses. A region‐of‐interest‐to‐region‐of‐interest approach was used for resting‐state functional connectivity (FC) and a model‐based approach was used for brain activation during the memory paradigm.
Results
Patients with PD showed increased cognitive performance, decreased functional disability, increased brain FC and activation at T2 compared with T0 (P < 0.05, FDR). Moreover, patients showed maintenance of the improvements in cognition and functionality, and maintenance of the increased brain FC and activation at T2 compared with T1. However, significant grey matter reduction and alterations of white matter integrity were found at T2 (P < 0.05, FWE).
Conclusions
Findings suggest that the improved cognitive performance and increased brain FC and activation after cognitive rehabilitation were significantly maintained after 18 months in patients with PD, despite the structural brain changes, consistent with a progression of neurodegenerative processes.
Keywords: brain changes, brain plasticity, cognitive rehabilitation, functional disability, longitudinal, Parkinson's disease
Short abstract
Click here for the corresponding questions to this CME article.
Introduction
Cognitive impairment in patients with Parkinson's disease (PD) is present from the early stages of the disease 1. These cognitive symptoms are related to quality‐of‐life deterioration and functional disability in patients with PD 2. Due to the relevance of these symptoms, specific therapeutic strategies are needed to treat cognitive deficits 3.
Cognitive rehabilitation programmes in PD have demonstrated their efficacy in improving a wide range of cognitive domains 4. Previous PD studies have also shown functional brain changes after cognitive rehabilitation in the short term 5, 6. Recently, in a previous study from our group, patients with PD, after attending a 3‐month cognitive rehabilitation programme, demonstrated improvements in cognition and functional disability 7, which were accompanied by increased brain functional connectivity (FC) and activation 8.
Longitudinal maintenance of cognitive improvements is one of the main goals of cognitive treatments. However, little is known about the maintenance of the cognitive improvements in patients with PD over time 9. One study showed the persistence of some cognitive improvements over 6 months after cognitive training 10. Another study in PD assessed the long‐term (12‐month) effects of cognitive rehabilitation and found that cognitive treatments could prevent cognitive decline 11. However, another study in PD found that cognitive training, only when combined with physical activity, resulted in maintained cognitive improvements after a 6‐month follow‐up period 12. To the best of our knowledge, no studies have been published using longer term periods and, to date, no study has assessed the long‐term effects of cognitive rehabilitation in PD using neuroimaging techniques. Therefore, this study aimed to investigate the longitudinal effects of a cognitive rehabilitation programme evaluating the cognitive, behavioural and neuroimaging changes after an 18‐month follow‐up period.
Methods
Participants
Patients with PD were recruited from the Department of Neurology at the Hospital of Galdakao and from the PD Biscay Association (ASPARBI). Inclusion criteria were: (i) fulfilled the UK PD Society Brain Bank diagnostic criteria; (ii) aged between 45 and 75 years; and (iii) Hoehn and Yahr disease stage ≤3 and Unified Parkinson's Disease Rating Scale (UPDRS) as evaluated by the neurologist. Exclusion criteria were: (i) presence of dementia as defined by the DSM‐IV‐R and the Movement Disorders Society (MDS) clinical criteria; (ii) presence of other neurological illness/injury; (iii) unstable psychiatric disorders; and (iv) visual hallucinations as assessed by the Neuropsychiatric Inventory Questionnaire. Patients were symptomatically stable and were tested while on their medication. Their levodopa equivalent daily dose (LEDD) was recorded 13. Sociodemographic characteristics are shown in Table 1.
Table 1.
Sociodemographic characteristics of patients with Parkinson's disease
| Pre‐treatment (T0) | Post‐treatment (T1) | Longitudinal follow‐up (T2) | Statistics | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| T0 vs. T2 | T1 vs. T2 | |||||||||
| Mean | SD | Mean | SD | Mean | SD | W | P | W | P | |
| Age (years) | 66.07 | 4.84 | 66.63 | 4.60 | 68.00 | 4.61 | – | – | – | – |
| Gender (male) | 56.25% | – | – | – | – | – | – | – | – | – |
| Education (years) | 10.80 | 4.64 | – | – | – | – | – | – | – | – |
| MMSE | 27.93 | 1.10 | 27.94 | 1.43 | 28.19 | 1.94 | −0.91 | 0.35 | −0.70 | 0.47 |
| TAP | 20.40 | 7.10 | – | – | – | – | – | – | – | – |
| Cognitive reserve | 12.40 | 4.77 | – | – | – | – | – | – | – | – |
| LEDD | 646.80 | 406.43 | 612.14 | 367.08 | 641.72 | 374.64 | −0.42 | 0.67 | −1.46 | 0.14 |
| UPDRS III | 18.13 | 8.84 | 17.12 | 8.54 | 31.08 | 12.31 | −2.79 | <0.01 | −3.18 | <0.01 |
| H&Y scale | 1.84 | 0.36 | 1.84 | 0.45 | 2.35 | 0.65 | −1.89 | 0.06 | −2.34 | 0.02 |
| Age of disease onset (years) | 60.03 | 8.92 | – | – | – | – | – | – | – | – |
H&Y, Hoehn and Yahr; LEDD, levodopa equivalent daily dose; MMSE, Mini‐Mental State Examination; TAP, pre‐morbid intelligence test; UPDRS III, Unified Parkinson's Disease Rating Scale (motor scale); W, Wilcoxon.
Procedure
Patients with PD were assessed at pre‐treatment (T0), post‐treatment (T1) and after 18 months (T2). Neuroimaging changes after cognitive rehabilitation (from T0 to T1) were reported in 30 patients with PD (15 each in the experimental and control groups) 8. The present study evaluated the long‐term effects of cognitive rehabilitation comparing T0 with T2 and maintenance of the changes comparing T1 with T2, by reassessing the 15 patients with PD in the experimental group at T2. This longitudinal follow‐up study was part of a randomized controlled trial (NCT02118480).
Cognitive and behavioural assessment
Patients with PD underwent the same neuropsychological battery at T0, T1 and T2 as previously described 7, which included the assessment of five cognitive domains: (i) processing speed (PS), including the Trail Making Test–A and Salthouse Letter Comparison Test; (ii) verbal memory (VM), using the Hopkins Verbal Learning Test (learning and recall) (version 3); (iii) visual memory (VIM), using the Brief Visual Memory Test (learning and recall) (version 5); (iv) executive functions (EFs), using the Stroop test (word‐colour and interference scores); and (v) theory of mind (ToM), using the Happé test. The following behavioural aspects were also evaluated: (i) apathy, using the Lille Apathy Rating Scale; (ii) depression, using the Geriatric Depression Scale; and (iii) functional disability, using the World Health Organization Disability Assessment Schedule II.
Neuroimage acquisition
Structural and functional imaging data were acquired using a 3‐T magnetic resonance imaging scanner (Philips Achieva TX) at OSATEK, Hospital of Galdakao. All sequences were acquired during a single session, and the same acquisition protocol was used at T0, T1 and T2. The neuroimaging acquisition parameters and memory functional magnetic resonance imaging (fMRI) paradigm description are included in Appendix S1.
Neuroimage pre‐processing
A comprehensive description of the neuroimaging pre‐processing in grey matter (GM), white matter (WM), resting‐state and memory fMRI paradigm acquisition types is included in Appendix S2.
Grey matter
Voxel‐based morphometry 14 analysis was carried out using the FMRIB Software Library tools. In addition, cortical thickness changes were analysed with FreeSurfer.
White matter
Diffusion data were also pre‐processed and analysed using FMRIB Software Library. Fractional anisotropy (FA), mean diffusivity (MD), radial diffusivity (RD) and axial diffusivity (AD) data were extracted and tract‐based spatial statistics was used for longitudinal analysis.
Resting‐state functional magnetic resonance imaging
The FC pre‐processing and analyses were performed using the Conn FC Toolbox 15. Whole‐brain analyses were performed. Region‐of‐interest (ROI) analyses were also performed to test the maintenance at T2 of the increased FC from T0 to T1 found in patients with PD after attending cognitive rehabilitation (Appendix S2). FC values between these ROIs were extracted from patients with PD at the three time points and entered into SPSS.
Memory functional magnetic resonance imaging paradigm
The fMRI data were pre‐processed and analysed using SPM8 (Statistical Parametric Mapping). Whole‐brain analyses were performed. ROI analyses were also performed to test the maintenance at T2 of the increased brain activation from T0 to T1 found in patients with PD (Appendix S2). Activation values from these ROIs at the three time points were also extracted from patients with PD and entered into SPSS.
Intervention
From T0 to T1, patients with PD attended an integrative group‐based cognitive intervention (REHACOP) over a 13‐week period (three times/week; 1 h/day). They trained different cognitive functions, in the following order: attention (4 weeks; sustained, selective, alternant, divided), memory (3 weeks; verbal and visual learning, recall, recognition), language (3 weeks; verbal fluency, synonyms/antonyms), EF (2 weeks; cognitive planning, verbal reasoning) and social cognition (1 week; moral dilemmas, ToM). PS was trained during the programme because several tasks were timed. The REHACOP programme is based on the restoration, compensation and optimization of the cognitive functions, and was described extensively in a previous study 7. Patients with PD did not receive booster sessions from T1 to T2.
Statistical analysis
Normality of data was tested using the Shapiro–Wilk test. To evaluate the evolution of the 15 patients with PD after attending cognitive rehabilitation, non‐parametric paired t‐tests were performed in cognitive, behavioural and neuroimaging data between T0 and T2 assessments and between T1 and T2 assessments. All neuroimaging analyses were performed at P < 0.05 corrected for multiple comparisons and fMRI data used LEDD as covariate 16.
Ethical statement
The study protocol was approved by the Ethics Committee at the Health Department of the Basque Mental Health System in Spain and the Ethics Committee of the University of Deusto. All subjects were volunteers and provided a written informed consent before participating in the study.
Results
Changes from T0 to T2
Cognitive and behavioural changes
Patients with PD showed increased performance in VM, VIM, EF and ToM, and decreased functional disability at T2 compared with T0 (Table 2). Moreover, there was also a statistical trend toward differences in scores on the apathy scale at T2. Post‐hoc analyses were performed to test the changes in the nine subscales of the apathy test and results revealed improvements in the ‘Everyday productivity’ (W = 2.33; P = 0.02; r = 0.60) and ‘Lack of interest’ (W = 2.44; P = 0.01; r = 0.63) subscales. Finally, patients with PD showed significant deterioration in UPDRS III (motor score) and a statistical trend towards a progression in disease on the Hoehn and Yahr scale at T2 compared with T0 (Table 1). Post‐hoc correlation analyses showed no significant relationships between the change in UPDRS III and Hoehn and Yahr scale with the cognitive and behavioural changes at T2.
Table 2.
Cognitive and behavioural changes
| Pre‐treatment (T0) | Post‐treatment (T1) | Longitudinal follow‐up (T2) | Statistics | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T0 vs. T2 | T1 vs. T2 | |||||||||||
| Mean | SD | Mean | SD | Mean | SD | W | P | Effect size (r) | W | P | Effect size (r) | |
| PS | ||||||||||||
| Composite score | – | – | – | – | – | – | 0.79 | 0.427 | – | 0.62 | 0.532 | – |
| TMTA (time) | 51.47 | 16.83 | 47.87 | 14.89 | 58.53 | 29.73 | 0.1.02 | 0.306 | – | 1.42 | 0.155 | – |
| Salthouse (hits) | 19.07 | 7.19 | 22.07 | 7.77 | 19.00 | 9.01 | 0.19 | 0.842 | – | 2.93 | 0.003 | 0.75 |
| VM | ||||||||||||
| Composite score | – | – | – | – | – | – | 2.89 | 0.004 | 0.74 | 0.17 | 0.865 | – |
| Learning (hits) | 16.87 | 5.65 | 20.07 | 5.88 | 20.33 | 6.32 | 3.27 | 0.001 | 0.84 | 0.25 | 0.801 | – |
| Recall (hits) | 4.53 | 3.56 | 7.20 | 2.80 | 6.80 | 2.72 | 2.28 | 0.023 | 0.58 | 0.56 | 0.571 | – |
| VIM | ||||||||||||
| Composite score | – | – | – | – | – | – | 2.89 | 0.004 | 0.74 | 2.32 | 0.020 | 0.59 |
| Learning (hits) | 13.13 | 8.54 | 15.93 | 7.29 | 18.80 | 7.60 | 2.92 | 0.003 | 0.75 | 2.28 | 0.023 | 0.58 |
| Recall (hits) | 5.67 | 2.94 | 6.20 | 2.78 | 7.20 | 3.46 | 2.29 | 0.022 | 0.59 | 1.83 | 0.066 | – |
| EFs | ||||||||||||
| Composite score | – | – | – | – | – | – | 3.29 | 0.001 | 0.85 | 3.35 | 0.001 | 0.86 |
|
Stroop word‐colour (hits) |
27.67 | 11.54 | 28.87 | 11.21 | 35.47 | 10.26 | 3.12 | 0.002 | 0.80 | 3.11 | 0.002 | 0.80 |
| Stroop interference | −1.03 | 8.04 | −1.37 | 8.54 | 7.24 | 8.09 | 3.35 | 0.001 | 0.86 | 3.29 | 0.001 | 0.85 |
| ToM | 5.47 | 1.35 | 6.13 | 1.35 | 6.47 | 1.64 | 3.41 | 0.001 | 0.88 | 0.81 | 0.416 | – |
| Apathy | −27.47 | 4.67 | −28.13 | 4.22 | −29.47 | 3.44 | 1.85 | 0.063 | – | 1.81 | 0.069 | – |
| GDS | 2.33 | 2.49 | 1.87 | 2.35 | 2.47 | 3.29 | 0.05 | 0.957 | – | 1.72 | 0.084 | – |
| WHO‐DAS‐II | 20.86 | 6.23 | 15.80 | 3.32 | 17.53 | 3.81 | 2.66 | 0.008 | 0.68 | 1.65 | 0.098 | – |
Bold values are significant at P < 0.05; EF, Executive Functions; GDS, Geriatric Depression Scale; PS, Processing Speed; Salthouse, Salthouse Letter Comparison Test; TMTA, Trail Making Test (part A); ToM, Theory of Mind; VIM, Visual Memory; VM, Verbal Memory; W, Wilcoxon; WHO‐DAS‐II, World Health Organization Disability Assessment Schedule.
Neuroimaging changes
Structural changes
Significant reduction in GM volume in the inferior part of the precuneus and the occipital fusiform gyrus was found at T2 compared with T0 (Table 3, Fig. 1). However, mean cortical thickness results showed no significant differences. Moreover, decreased WM FA was found mostly in the corpus callosum and corticospinal tract, and increased MD and RD in the posterior thalamic radiation and optic radiation, respectively, at T2 (Table 3, Fig. 1).
Table 3.
Structural grey matter (GM) and white matter (WM) changes from T0 to T2
| Cluster (voxels) | MNI coordinates | t | P | Cohen's d | |||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| GM volume | |||||||
| Baseline (T0) > longitudinal follow‐up (T2) | |||||||
| R precuneus/intracalcarine/lingual gyrus | 90 | 6 | −60 | 10 | 6.12 | 0.02 | 1.58 |
| L occipital fusiform gyrus | 85 | −22 | −70 | −14 | 6.26 | 0.02 | 1.61 |
| WM indices | |||||||
| Baseline (T0) > longitudinal follow‐up (T2) | |||||||
| FA | |||||||
| L callosal body | 19879 | −18 | −22 | 35 | 3.71 | 0.001 | 0.95 |
| R corticospinal tract | 649 | 32 | −13 | 46 | 4.89 | 0.03 | 1.26 |
| 98 | 26 | −18 | 12 | 1.84 | 0.04 | 0.47 | |
| Baseline (T0) < longitudinal follow‐up (T2) | |||||||
| MD | |||||||
| R posterior thalamic radiation (including optic radiation) | 5805 | 30 | −59 | 18 | 4.84 | 0.02 | 1.25 |
| RD | |||||||
| R optic radiation | 7543 | 22 | −60 | 33 | 3.51 | 0.02 | 0.90 |
| R corticospinal tract | 179 | 28 | −11 | 43 | 3.58 | 0.04 | 0.92 |
FA, Fractional Anisotropy; L, Left; MD, Mean Diffusivity; R, Right; RD, Radial Diffusivity. Cluster size denotes the extent of the cluster of significant voxels. Montreal Neurological Institute (MNI) coordinates refer to the location of the most statistically significant voxel in the cluster. Results are shown at P < 0.05, FWE corrected.
Figure 1.
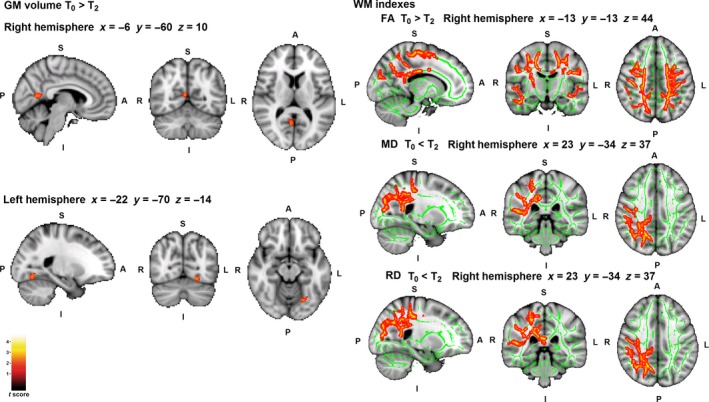
Whole‐brain structural changes from T0 to T2. Significant grey matter (GM) and white matter (WM) regions are shown in red/yellow; the WM skeleton is shown in green. A, Anterior; FA, Fractional Anisotropy; I, Inferior; L, Left; MD, Mean Diffusivity; P, Posterior; R, Right; RD, Radial Diffusivity; S, Superior. Coordinates are shown in Montreal Neurological Institute space. Results are shown at P < 0.05 corrected for multiple comparisons. [Colour figure can be viewed at wileyonlinelibrary.com]
Functional magnetic resonance imaging changes
Resting‐state fMRI analyses showed significantly increased FC in the frontotemporal network at T2 compared with T0, between the perirhinal cortex [Brodmann area (BA)35L] and dorsolateral prefrontal cortex (BA9L) (t = 4.18; P = 0.029, FDR; d = 1.08), parahippocampal gyrus (BA36L) (t = 4.24; P = 0.029, FDR; d = 1.09) and fusiform gyrus (BA37L) (t = 4.70; P = 0.029, FDR; d = 1.21) (Fig. 2). Whole‐brain analyses showed no significant differences in brain activation in the memory fMRI paradigm.
Figure 2.
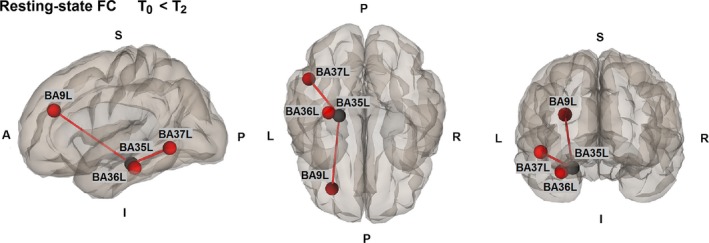
Whole‐brain functional connectivity (FC) changes from T0 to T2. Red line, increased FC at T2 compared with T0; black point, seed; red points, targets. A, Anterior; BA, Brodmann area; I, Inferior; L, Left; P, Posterior; R, Right; S, Superior. Results are shown at P < 0.05 corrected for multiple comparisons.[Colour figure can be viewed at wileyonlinelibrary.com].
In the previous study, patients with PD showed increased FC during resting‐state fMRI in the frontotemporal network and increased brain activation in the memory fMRI paradigm in the frontal and temporal areas after rehabilitation 8. Therefore, for exploratory purposes, ROI connectivity and activation values were extracted from the 15 patients with PD assessed at the three time points (T0/T1/T2). Results showed that the brain FC and activation values in the specific ROIs at T2 were higher compared with T0, although they were reduced compared with T1 (Fig. 3).
Figure 3.

Neuroimaging changes with region of interest (ROI) analyses from patients with Parkinson's disease (PD) at three time points. (a) Values represent the functional connectivity of patients with PD between the two ROIs at the three time points.  , BA20L–BA9R;
, BA20L–BA9R;  , BA20L–BA9L. (b) Values represent the brain activation of patients with PD in the ROI mask at the three time points.
, BA20L–BA9L. (b) Values represent the brain activation of patients with PD in the ROI mask at the three time points.  , learning fMRI task, ROI mask: left inferior frontal lobe;
, learning fMRI task, ROI mask: left inferior frontal lobe;  , recognition fMRI task, ROI mask: left middle temporal lobe. [Colour figure can be viewed at wileyonlinelibrary.com].
, recognition fMRI task, ROI mask: left middle temporal lobe. [Colour figure can be viewed at wileyonlinelibrary.com].
Changes from T1 to T2
Cognitive and behavioural changes
Patients with PD showed no significant changes in PS, VM, ToM, apathy, symptoms of depression or functional disability and showed increased performance in VIM and EF at T2 compared with T1 (Table 2).
Neuroimaging changes
Structural changes
We found no significant changes in GM volume or mean cortical thickness. However, WM indices showed significantly decreased FA and increased MD, RD and AD at T2 compared with T1 (Table S1, Fig. S1).
Functional magnetic resonance imaging changes
No significant changes were found during the memory fMRI paradigm from T1 to T2. During resting‐state, patients with PD showed decreased FC within the default‐mode network between BA33 and the posterior cingulate cortex (t = 5.07; P = 0.02, FDR; d = 1.31). In addition, patients with PD also showed increased FC between the somatomotor network and default‐mode network, specifically between BA5–BA28 (t = 4.62; P = 0.047, FDR; d = 1.19) and bilateral BA7–BA30 (right: t = 4.41; P = 0.01, FDR; d = 1.13; left: t = 5.22; P = 0.03, FDR; d = 1.34) (Fig. S2).
Discussion
This study aimed to investigate the long‐term (18‐month) effects of an integrative cognitive rehabilitation programme in PD, assessing cognitive, behavioural and neuroimaging changes. Results showed that the improvements found from baseline to post‐treatment in cognition and functional disability 7, and the increased FC and activation 8, were still present after an 18‐month follow‐up.
Findings revealed increased cognitive performance in most cognitive domains in the long‐term compared with baseline, which supports the long‐term effects of cognitive rehabilitation. In the previous study, after attending a 3‐month cognitive rehabilitation programme, patients with PD improved in PS, VIM, ToM and tendency to change in VM 7. In the present study, patients with PD showed maintenance of these cognitive improvements in the long term. Previous studies in PD showed maintenance of cognitive improvements after 6 months 10, 12 and 12 months 11. This study found that cognitive improvements could be maintained for a longer period of time. The REHACOP is a structured programme based on the theoretical models of the neuropsychological rehabilitation (restoration, compensation and optimization). Therefore, the combination of these three orientations as well as the combination of function training and strategy training could have helped to maintain these improvements over time. However, reduced performance in the Salthouse Letter Comparison test also been found. We hypothesized that booster sessions might have been useful for the maintenance of this specific cognitive domain.
Another important finding of the study was that functional disability and some aspects of apathetic symptomatology could be maintained after 18 months. It is important to mention that the functional disability score in the present study was decreased in the long term compared with baseline, but slightly increased compared with post‐treatment. More research is needed to evaluate the long‐term effects on functional outcome with longer periods of time and the possible benefits of booster sessions. Nevertheless, the fact that transfer effects on behavioural aspects could be maintained over time in PD is an important finding with regard to the efficacy of cognitive rehabilitation programmes.
Regarding neuroimaging results, patients with PD showed that both increased brain connectivity during resting‐state fMRI and increased brain activation during the memory fMRI paradigm found in patients with PD after rehabilitation 8 were still present at follow‐up. In the previous study, patients with PD after cognitive rehabilitation showed increased FC in the frontotemporal network (BA9L–BA20 bilateral) from baseline to post‐treatment 8, and results from this study showed increased FC in the same network in the long‐term compared with baseline. Interestingly, the same brain area in the frontal lobe (BA9L) maintained the increased FC with the temporal lobe. Moreover, after 18 months, the brain connectivity and activation values from specific ROIs were increased compared with baseline, but decreased compared with post‐treatment. We hypothesized that the reduction of FC in the long‐term compared with post‐treatment could be related to the progression of the neurodegenerative disease, which may result in a future decline of the cognitive improvements.
Furthermore, neuroimaging assessment detected structural changes consistent with a progression of neurodegenerative processes. Slight GM reduction in the occipital and parietal lobes was detected at long‐term follow‐up. Previous PD studies assessing the progression of the disease found GM reduction in the same brain areas 17, 18. Moreover, the neurodegenerative process was mostly detected in alterations of WM integrity. Patients with PD showed bilateral reduced FA and increased MD and RD mostly in the posterior part of the right hemisphere. We have to be cautious when interpreting WM indices, but literature suggests that FA, MD and RD could be biomarkers for microstructural brain tissue integrity, membrane cell density and myelin integrity respectively 19. A recent PD study also found alterations in WM integrity with disease progression, showing FA reduction and AD and RD increment after 12 months in the frontal, temporal and occipital lobes, including the corpus callosum 20. Finally, the degenerative process of patients with PD was also reflected in the deterioration of motor symptoms. The progression of motor symptoms is part of the evolution of PD 21, and this study found that, despite the motor evolution, the non‐motor symptoms of the disease can be improved.
This is the first study in PD to use neuroimaging techniques to assess the long‐term effects of cognitive rehabilitation. Neuroimaging data provide insight into the brain changes that are associated with cognitive and behavioural changes, and they show the cerebral bases of cognitive rehabilitation. Findings reinforce the efficacy of cognitive improvements after training, and support the long‐term effects of cognitive treatments.
Some limitations should be taken into account. First, this study did not include a long‐term follow‐up of the control group. Several PD studies showed that the normal course of brain activity is the decrement of FC and activation as the disease progresses, these decrements being correlated with clinical and cognitive decline 22, 23. These previous findings support the significance of the present study and suggest that, despite the absence of a control group, findings from this study are promising and important for the field of neurorehabilitation in PD. Moreover, the Stroop test was used to evaluate EF, but other tests, such as the Wisconsin Card Sorting Test, would be more representative. Furthermore, language domain was not included in the assessment. Finally, it would be interesting to evaluate the long‐term effects of cognitive rehabilitation with booster sessions.
Conclusions
Findings suggest the long‐term maintenance of the cognitive, functionality and brain changes after attending a 3‐month cognitive rehabilitation programme. These findings support the possibility that cognitive rehabilitation programmes in PD might prevent cognitive deterioration even after 18 months, and showed brain plasticity in a neurodegenerative disease, which encourages the replication of these findings.
Disclosure of conflicts of interest
N.O. and J.P. are coauthors and copyright holders of the REHACOP cognitive rehabilitation programme, published by Parima Digital, S.L. (Bilbao, Spain). M.D.C., A.C.Z., O.L.J., J.C.G.E., M.Á.G.B. and N.I.‐B. declare no financial or other conflicts of interest.
Supporting information
Figure S1. Whole‐brain white matter changes from T1 to T2.
Figure S2. Whole‐brain functional connectivity changes from T1 to T2.
Table S1. White matter indices changes from T1 to T2.
Appendix S1. Neuroimaging acquisition parameters.
Appendix S2. Neuroimaging pre‐processing.
Acknowledgements
The authors thank ASPARBI and all of the patients involved in the study. This study was supported by the Department of Health of the Basque Government (2011111117), Spanish Ministry of Economy and Competitiveness (PSI2012‐32441) and Department of Education and Science of the Basque Government (Equipo A) (IT946‐16).
This is a Continuing Medical Education article, and can be found with corresponding questions on the EAN website, LEARN section https://www.ean.org/CME.2714.0.html. Certificates for correctly answered questions will be issued by EAN directly, you simply have to be logged‐in. With positive results, EAN recommends accreditation of 1 hour of CME, which may be claimed with the national body in charge of CME accreditation.
References
- 1. Aarsland D, Bronnick K, Larsen JP, Tysnes OB, Alves G, Norwegian ParkWest Study Group . Cognitive impairment in incident, untreated Parkinson disease: the Norwegian ParkWest study. Neurology 2009; 72: 1121–1126. [DOI] [PubMed] [Google Scholar]
- 2. Leroi I, McDonald K, Pantula H, Harbishettar V. Cognitive impairment in Parkinson disease: impact on quality of life, disability, and caregiver burden. J Geriatr Psychiatry Neurol 2012; 25: 208–214. [DOI] [PubMed] [Google Scholar]
- 3. Petersen R, Caracciolo B, Brayne C, Gauthier S, Jelic V, Fratiglioni L. Mild cognitive impairment: a concept in evolution. J Intern Med 2014; 275: 214–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Leung IH. Cognitive training in Parkinson disease. Neurology 2015; 85: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cerasa A, Gioia MC, Salsone M, et al Neurofunctional correlates of attention rehabilitation in Parkinson's disease: an explorative study. Neurol Sci 2014; 35: 1173–1180. [DOI] [PubMed] [Google Scholar]
- 6. Nombela C, Bustillo PJ, Castell PF, Sanchez L, Medina V, Herrero MT. Cognitive rehabilitation in Parkinson's disease: evidence from neuroimaging. Front Neurol 2011; 2: 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pena J, Ibarretxe‐Bilbao N, Garcia‐Gorostiaga I, Gomez‐Beldarrain MA, Diez‐Cirarda M, Ojeda N. Improving functional disability and cognition in Parkinson disease: randomized controlled trial. Neurology 2014; 83: 2167–2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Díez‐Cirarda M, Ojeda N, Peña J, et al Increased brain connectivity and activation after cognitive rehabilitation in Parkinson's disease: a randomized controlled trial. Brain Imaging Behav 2016; 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Walton CC, Naismith SL, Lampit A, Mowszowski L, Lewis SJ. Cognitive training in Parkinson's disease: a theoretical perspective. Neurorehabil Neural Repair 2016; 31: 207–216. [DOI] [PubMed] [Google Scholar]
- 10. Sinforiani E, Banchieri L, Zucchella C, Pacchetti C, Sandrini G. Cognitive rehabilitation in Parkinson's disease. Arch Gerontol Geriatr 2004; 38: 387–391. [DOI] [PubMed] [Google Scholar]
- 11. Petrelli A, Kaesberg S, Barbe M, et al Cognitive training in Parkinson's disease reduces cognitive decline in the long term. Eur J Neurol 2015; 22: 640–647. [DOI] [PubMed] [Google Scholar]
- 12. Reuter I, Mehnert S, Sammer G, Oechsner M, Engelhardt M. Efficacy of a multimodal cognitive rehabilitation including psychomotor and endurance training in Parkinson's disease. J Aging Res 2012; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE. Systematic review of levodopa dose equivalency reporting in Parkinson's disease. Mov Disord 2010; 25: 2649–2653. [DOI] [PubMed] [Google Scholar]
- 14. Douaud G, Smith S, Jenkinson M, et al Anatomically related grey and white matter abnormalities in adolescent‐onset schizophrenia. Brain 2007; 130: 2375–2386. [DOI] [PubMed] [Google Scholar]
- 15. Whitfield‐Gabrieli S, Nieto‐Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect 2012; 2: 125–141. [DOI] [PubMed] [Google Scholar]
- 16. Mattay VS, Tessitore A, Callicott JH, et al Dopaminergic modulation of cortical function in patients with Parkinson's disease. Ann Neurol 2002; 51: 156–164. [DOI] [PubMed] [Google Scholar]
- 17. Ibarretxe‐Bilbao N, Junque C, Segura B, et al Progression of cortical thinning in early Parkinson's disease. Mov Disord 2012; 27: 1746–1753. [DOI] [PubMed] [Google Scholar]
- 18. Ramírez–Ruiz B, Martí MJ, Tolosa E, et al Longitudinal evaluation of cerebral morphological changes in Parkinson's disease with and without dementia. J Neurol 2005; 252: 1345–1352. [DOI] [PubMed] [Google Scholar]
- 19. Alexander AL, Lee JE, Lazar M, Field AS. Diffusion tensor imaging of the brain. Neurotherapeutics 2007; 4: 316–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhang Y, Wu I, Tosun D, Foster E, Schuff N. Progression of regional microstructural degeneration in Parkinson's disease: a multicenter diffusion tensor imaging study. PLoS ONE 2016; 11: e0165540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hoehn MM, Yahr MD. Parkinsonism: onset, progression, and mortality. 1967. Neurology 1998; 50: 318–334. [DOI] [PubMed] [Google Scholar]
- 22. Olde Dubbelink KT, Schoonheim MM, Deijen JB, Twisk JW, Barkhof F, Berendse HW. Functional connectivity and cognitive decline over 3 years in Parkinson disease. Neurology 2014; 83: 2046–2053. [DOI] [PubMed] [Google Scholar]
- 23. Segura B, Ibarretxe‐Bilbao N, Sala‐Llonch R, et al Progressive changes in a recognition memory network in Parkinson's disease. J Neurol Neurosurg Psychiatry 2013; 84: 370–378. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Whole‐brain white matter changes from T1 to T2.
Figure S2. Whole‐brain functional connectivity changes from T1 to T2.
Table S1. White matter indices changes from T1 to T2.
Appendix S1. Neuroimaging acquisition parameters.
Appendix S2. Neuroimaging pre‐processing.


