Abstract
Aims
To determine clinical outcomes and explore prognostic factors related to ulcer healing in people with a clinically infected diabetic foot ulcer.
Methods
This multicentre, prospective, observational study reviewed participants’ data at 12 months after culture of a diabetic foot ulcer requiring antibiotic therapy. From participants’ notes, we obtained information on the incidence of wound healing, ulcer recurrence, lower extremity amputation, lower extremity revascularization and death. We estimated the cumulative incidence of healing at 6 and 12 months, adjusted for lower extremity amputation and death using a competing risk analysis, and explored the relationship between baseline factors and healing incidence.
Results
In the first year after culture of the index ulcer, 45/299 participants (15.1%) had died. The ulcer had healed in 136 participants (45.5%), but recurred in 13 (9.6%). An ipsilateral lower extremity amputation was recorded in 52 (17.4%) and revascularization surgery in 18 participants (6.0%). Participants with an ulcer present for ~2 months or more had a lower incidence of healing (hazard ratio 0.55, 95% CI 0.39 to 0.77), as did those with a PEDIS (perfusion, extent, depth, infection, sensation) perfusion grade of ≥2 (hazard ratio 0.37, 95% CI 0.25 to 0.55). Participants with a single ulcer on their index foot had a higher incidence of healing than those with multiple ulcers (hazard ratio 1.90, 95% CI 1.18 to 3.06).
Conclusions
Clinical outcomes at 12 months for people with an infected diabetic foot ulcer are generally poor. Our data confirm the adverse prognostic effect of limb ischaemia, longer ulcer duration and the presence of multiple ulcers.
What's new?
This is the first prospective study to estimate the incidence of healing and prognostic factors associated with healing in people with a clinically infected diabetic foot ulcer, and specifically in the presence of competing risks of amputation and death.
Outcomes for people with a clinically infected diabetic foot ulcer are poorer than previously thought; in the first year after presentation with an infected ulcer, 15.1% of our participants had died and 17.4% underwent at least partial lower extremity amputation.
Healing incidence within 1 year was 44.5% (95% CI 38.9 to 50.1). Three key factors served as the best independent predictors of healing: PEDIS (perfusion, extent, depth, infection, sensation) perfusion grade; the absence of multiple foot ulcers; and shorter ulcer duration.
What's new?
This is the first prospective study to estimate the incidence of healing and prognostic factors associated with healing in people with a clinically infected diabetic foot ulcer, and specifically in the presence of competing risks of amputation and death.
Outcomes for people with a clinically infected diabetic foot ulcer are poorer than previously thought; in the first year after presentation with an infected ulcer, 15.1% of our participants had died and 17.4% underwent at least partial lower extremity amputation.
Healing incidence within 1 year was 44.5% (95% CI 38.9 to 50.1). Three key factors served as the best independent predictors of healing: PEDIS (perfusion, extent, depth, infection, sensation) perfusion grade; the absence of multiple foot ulcers; and shorter ulcer duration.
Introduction
One of the most common and serious complications of diabetes mellitus is the development of a foot ulcer, which occurs in up to a quarter of patients over their lifetime 1. These diabetic foot ulcers, which primarily result from peripheral nerve damage and arterial disease, are associated with substantial morbidity, often including hospitalization and lower extremity amputation, as well as increased mortality 2. These complications also lead to substantial healthcare costs and loss of productivity 3, 4, 5. Over half of diabetic foot ulcers are clinically infected at presentation 6, and these infected diabetic foot ulcers are especially associated with poor outcomes 7, 8, 9, 10, 11, 12.
Understanding factors associated with diabetic foot ulcer healing may better inform prevention and management strategies. Prospective data on clinical outcomes of infected diabetic foot ulcer are scarce. Two large inception cohort studies 5, 13, 14, 15 assessed factors associated with diabetic foot ulcer healing, but neither specifically recruited people with infected ulcers and both were limited to specialist centres. Ince et al. 13 and Jeffcoate et al. 14 found that among people attending one diabetic foot ulcer clinic for whom there was a 1‐year follow‐up, there was a relationship between time to healing and ulcer area, peripheral arterial disease, ulcer site and diabetes duration. The European Study Group on Diabetes and the Lower Extremity (Eurodiale) study 5, 15 in people with a new diabetic foot ulcer presenting to one of 14 diabetic foot ulcer clinics, and for whom there was a 1‐year follow‐up, identified independent predictors of delayed healing as: older age;, male sex; larger ulcer size; heart failure; inability to stand or walk without help; end‐stage renal disease; peripheral neuropathy; and peripheral arterial disease 15. Current guidelines for the management of infected diabetic foot ulcer 16, 17, 18, 19, 20 highlight the importance of prevention, early diagnosis and appropriate therapy delivered by a multidisciplinary team.
Our group recently completed a large, multicentre, cross‐sectional study that compared culture results from swab and tissue specimens concurrently obtained from people with a diabetic foot ulcer with suspected infection (the Concordance in Diabetic Foot Ulcer Infection [CODIFI] study) 21. This study collected comprehensive clinical details, including ulcer classification using both the PEDIS (perfusion, extent, depth, infection, sensation) 22 and the Wagner systems, and wound culture results. After gaining additional funding we obtained consent from participants to enrol them in a prospective observational study to assess the 12‐month clinical outcomes of people with a clinically infected diabetic foot ulcer and to explore prognostic factors related to the incidence of wound healing.
Methods
Study design and participants
The CODIFI study enrolled 400 participants between November 2011 and May 2013. Participants were recruited from community podiatry‐led and multi‐disciplinary diabetic foot ulcer clinics. Eligibility criteria were: diagnosis of diabetes mellitus; foot ulcer suspected of being infected based on clinical signs and symptoms using Infectious Diseases Society of America/International Working Group on the Diabetic Foot 16, 20 criteria and clinical judgement; plan to treat with antibiotics for their infected ulcer; and age ≥ 18 years with written consent provided.
Mid‐way through recruitment, we obtained ethics approval for an amendment (November 2012) to enable us to conduct a case note review at 12 months after CODIFI study enrolment. This allowed us to obtain consent prospectively from all subsequently enrolled participants for the 12‐month case note review and retrospectively from those previously enrolled in CODIFI who were still alive (by telephone using an ethically approved verbal consent script). We also obtained ethics approval to perform the case note review for enrolled participants who had since died.
Assessments/case note review
At enrolment for the CODIFI study 21 we recorded the following data: baseline demographics; diabetes history; clinical ulcer details (wound duration and site, aetiology, PEDIS and Wagner classification); type, if any, of antibiotic or antimicrobial treatment; and culture results from swab and tissue samples taken from the ulcer.
For the 12‐month outcome assessment, research nurses or podiatrists at each participating centre conducted a detailed case note review seeking information on participant status and outcomes relating to the index ulcer, including: whether or not it healed, and if so, the date of healing; index ulcer recurrence; ipsilateral lower limb revascularization procedures; ipsilateral lower extremity amputation; or death in the 12 months following enrolment.
Statistical analysis
Sample size
We expected to obtain consent from at least 200 participants. Assuming a diabetic foot ulcer healing rate of 50%, this would provide an estimate of healing with a precision (half width of the 95% CI) of ± 6.9%. It would also allow us to include a maximum of 10 parameter estimates in an exploratory prognostic model of time to healing 23.
Population
Analyses were conducted on the follow‐up population, which consisted of the sample of all registered and consented CODIFI participants for whom a subsequent case note review was completed. To explore the generalizability of our results, baseline characteristics of all CODIFI participants included vs not included in the follow‐up population were compared, with differences explored using the chi‐squared and t‐test as appropriate.
12‐month outcomes
We summarized the outcome of study participants by ulcer‐related events (wound healing, wound recurrence, lower extremity amputation, limb revascularization, and death) as well as the time to the event. Using competing risk analysis (with cumulative incidence functions) we estimated the cumulative incidence of healing at 6 and 12 months. Because either amputation or death would preclude further observation of healing of the index ulcer, for unhealed ulcers we considered death and amputation to be competing risks; thus, we provided healing estimates (with 95% CIs) and cumulative incidence curves of the time to healing, in the presence of competing risks. We censored participants who were alive and without lower extremity amputation or healing of their index ulcer at 12 months post‐baseline or the date of their case note review, if this took place before the 12‐month follow‐up.
Prognostic factors relating to healing
To explore the relationship of baseline factors with cumulative incidence (rate) of healing, we used a proportional sub‐distribution hazards model 24 for competing risks data. Using exploratory univariable analyses (single explanatory variable), we first tested the association between each prespecified baseline prognostic factor (Table 1) separately with incidence of healing. Then, we explored associations between prognostic factors found to be significantly associated with healing using the chi‐squared test. We then entered all prespecified factors in a preliminary multivariable (multiple explanatory variables) analysis to examine their independent effects on healing. Lastly, we entered factors that achieved statistical significance at the 10% level into a final multivariable model. For all analyses, we present hazard ratios (HRs) with 95% CIs and P values for prognostic factors we found significantly associated with healing at the 5% level.
Table 1.
Candidate prognostic factors for healing of infected diabetic foot ulcer
| Baseline clinical characteristics | Baseline microbiologya |
|---|---|
|
|
DCCT, Diabetes Control and Complications Trial.
As reported in either swab or tissue sample
This refers to participants who were reported as receiving antibiotic therapy, or having an antimicrobial dressing on their ulcer, at the time immediately prior to their baseline assessment and wound culture.
Missing data
We assumed that missing data for participants in the follow‐up population (e.g. date of healing, baseline covariates) were missing at random and imputed these values using multiple imputation 25. Characteristics of participants with and without missing data were compared to explore the pattern of missing data. We performed separate imputation analyses according to the participants’ healed status with an imputation model that included all factors considered in the prognostic model, centre, and other outcomes of interest (recurrence, revascularization, amputation and death). We made a total of 10 imputations using the Markov chain Monte Carlo method 26, and combined results using Rubin's rules 27.
Results
Consent and participant characteristics
We were able to include a total of 299 participants for the 12‐month follow‐up analyses in this study. Of these, investigators obtained consent from 250 participants (155 written and 95 by telephone), and for 49 participants who had died but had clinical data available. Figure 1 summarizes the participant flow for this study. The main reason for exclusion was no response to our request for consent (55/400 participants, 14%). As shown in Table 2, baseline characteristics were largely similar for those included and excluded; however, participants included in the follow‐up population were slightly older (mean age 64 vs 59 years; P=0.001), had more recurrent ulcers (30.4% vs 18.8%; P=0.0217), and had more serious infections (65.2% vs 53.5% grade 3 or 4 infection; P=0.0352).
Figure 1.
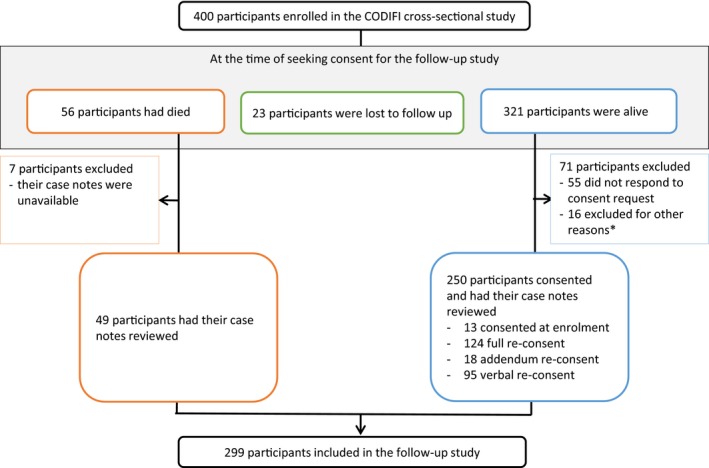
Participant flow diagram. *Other reasons for exclusion: consent was not attained for unknown reasons (11 participants); lacked capacity to consent (2 participants); provided incomplete consent (1 participant); declined to consent (1 participant); consented but case note review not completed (1 participant).
Table 2.
Baseline characteristics of participants included vs not included in the 12‐month observational study
| Participant characteristics | Included in this study, N=299 | Not included in this study, N=101 |
|---|---|---|
| Mean (sd) agea, years | 64.3 (12.8) | 59.3 (14.2) |
| Sex: male, n (%) | 233 (77.9) | 83 (82.2) |
| Type of facility, n (%) | ||
| Hospital ward | 38 (12.7) | 15 (14.9) |
| Outpatient clinic | 241 (80.6) | 78 (77.2) |
| Community clinic | 20 (6.7) | 8 (7.9) |
| Diabetes type, n (%) | ||
| Type 1 | 40 (13.4) | 18 (17.8) |
| Type 2 | 259 (86.6) | 83 (82.2) |
| Mean (sd) diabetes duration, years | 17.2 (11.1) | 15.5 (10.5) |
| Mean (sd) HbA1c | ||
| mmol/mol | 70.6 (24.51) | 75.3 (26.20) |
| % | 8.61 (2.24) | 9.04 (2.40) |
| Current diabetes treatment, n (%) | ||
| Oral hypoglycaemic | 77 (26.6) | 30 (31.3) |
| Insulin | 126 (43.6) | 42 (43.8) |
| Both insulin and oral hypoglycaemic | 85 (29.4) | 24 (25.0) |
| Other | 1 (0.3) | 0 (0.0) |
| None | 10 (3.3) | 5 (5.0) |
| Foot with index ulcer, n (%) | ||
| Right foot | 150 (50.2) | 55 (54.5) |
| Number of ulcers on index foot, n (%) | ||
| Single ulcer | 222 (74.2) | 82 (81.2) |
| Multiple ulcers | 77 (25.8) | 19 (18.8) |
| Index ulcer location, n (%) | ||
| Apex (i.e. tip of toe) | 31 (10.4) | 16 (15.8) |
| Interdigital | 18 (6.0) | 7 (6.9) |
| Plantar | 133 (44.5) | 39 (38.6) |
| Dorsum | 38 (12.7) | 18 (17.8) |
| Digital | 70 (23.4) | 20 (19.8) |
| Other | 7 (2.3) | 1 (1.0) |
| Duration of index ulcer, months | ||
| Median (IQR)Range | 1.8 (0.7 to 6.0) (0.1 to 75.0) | 1.8 (0.7 to 4.6) (0.2 to 144.0) |
| Ulcer recurrenceb, n (%) | ||
| Incident (first) | 206 (68.9) | 82 (81.2) |
| Recurrent (repeat) | 91 (30.4) | 19 (18.8) |
| Ulcer type, n (%) | ||
| Ischaemic or neuro‐ischaemic | 142 (47.5) | 54 (53.5) |
| Neuropathic only | 155 (51.8) | 47 (46.5) |
| PEDIS classification, n (%) | ||
| Perfusion | ||
| Grade 1: no symptoms/signs of PAD | 147 (49.2) | 53 (52.5) |
| Grade 2: symptoms or signs of PAD, no critical limb ischaemia | 146 (48.8) | 46 (45.5) |
| Grade 3: critical limb ischaemia | 6 (2.0) | 2 (2.0) |
| Depth/tissue loss | ||
| Grade 1: superficial full‐thickness ulcer not penetrating structures deeper than the dermis | 96 (32.1) | 35 (34.7) |
| Grade 2: ulcer penetrating below dermis to subcutaneous structures | 100 (33.4) | 34 (33.7) |
| Grade 3: all subsequent layers of foot, including bone/joint | 103 (34.4) | 32 (31.7) |
| Infection | ||
| Grade 1: no symptoms/signs of inflammation | 0 (0.0) | 2 (2.0) |
| Grade 2: inflammation of skin/subcutaneous tissue only | 104 (34.8) | 45 (44.6) |
| Grade 3: extensive erythema deeper than skin/subcutaneous tissue | 185 (61.9) | 52 (51.5) |
| Grade 4: systemic inflammatory response syndrome | 10 (3.3) | 2 (2.0) |
| Sensation | ||
| Grade 1: no loss of protective sensation | 20 (6.7) | 7 (6.9) |
| Grade 2: loss of protective sensation | 279 (93.3) | 94 (93.1) |
| Wagner classification, n (%) | ||
| Grade 1: superficial diabetic ulcer | 104 (34.8) | 32 (31.7%) |
| Grade 2: ulcer extension ligament, tendon, joint capsule or deep fascia without abscess or osteomyelitis | 93 (31.1) | 41 (40.6) |
| Grade 3: deep ulcer with abscess, osteomyelitis or joint sepsis | 96 (32.1) | 26 (25.7) |
| Grade 4: gangrene localized to portion of forefoot or heel | 5 (1.7) | 2 (2.0) |
| Grade 5: extensive gangrenous involvement of the entire foot | 1 (0.3) | 0 (0.0) |
| Antimicrobial dressing on ulcer, n (%) | 175 (58.5) | 66 (65.3) |
| Currently on antibiotic therapy, n (%) | 139 (46.5) | 48 (47.5) |
| Pathogens reported, n (%) | 263 (88.0) | 90 (89.1) |
PAD, peripheral arterial disease.
t‐test for difference in age between groups P=0.001.
Chi‐squared test for association between ulcer recurrence and group P=0.0217
Chi‐squared test for association between infection grades (Grade 1/2 vs 3/4) and group P=0.0352.
Missing data
There were minimal missing data for participants included in the follow‐up population. Date of healing was missing for 12 participants; 4% of all participants and 8.8% of healed participants. There was no clear evidence, however, of a pattern of missing data when comparing participants’ baseline characteristics. There were also missing data for the baseline covariates diabetes duration, extent of ulcer, ulcer type and recurrent ulcer for two participants (0.7%), ulcer duration for four participants (1.3%), antimicrobial dressing and HbA1c for five participants (1.7%) and previous antibiotic regimen for 15 participants (5%). Given the low level of missing data and the use of multiple imputation based on all factors considered in the prognostic model to mitigate against bias, missing data in the long‐term follow‐up population are unlikely to have biased the statistical analysis.
12‐month clinical outcomes
Table 3 summarizes the 12‐month clinical outcomes, based on whether or not the index ulcer healed. Healing of the index ulcer at 12 months occurred in 136 participants (45.5%). Among these, ulcer recurrence was reported within the 12 months of follow‐up in 13 participants (9.6%). A substantial minority of participants underwent a surgical procedure of the affected lower extremity, with 52 (17.4%) having an amputation of some part of the foot and 18 (6.0%) undergoing revascularization surgery, 10 (3.3%) of whom underwent both revascularization surgery and amputation. Furthermore, 45 participants (15.1%) had died within the 12 month follow‐up period.
Table 3.
Cross‐tabulation of index ulcer healing status against other 12‐month clinical outcomes
| Clinical outcome | Index ulcer healed, n (%) | Index ulcer not healed, n (%) | Total, n (%) |
|---|---|---|---|
| Participant died | |||
| Yes | 8 (2.7) | 37 (12.4) | 45 (15.1) |
| No | 128 (42.8) | 126 (42.1) | 254 (84.9) |
| Total | 136 (45.5) | 163 (54.5) | 299 (100.0) |
| Amputation (of/on the index foot)? | |||
| Yes | 12 (4.0)a | 40 (13.4) | 52 (17.4) |
| No | 124 (41.5) | 123 (41.1) | 247 (82.6) |
| Total | 136 (45.5) | 163 (54.5) | 299 (100.0) |
| Revascularization surgery? | |||
| Yes | 8 (2.7) | 10 (3.3) | 18 (6.0) |
| No | 128 (42.8) | 153 (51.2) | 281 (94.0) |
| Total | 136 (45.5) | 163 (54.5) | 299 (100.0) |
| Index ulcer recurred? | |||
| Yes | 13 (9.6) | NA | 13 (4.3) |
| No | 123 (90.4) | NA | 123 (90.4) |
| Total | 136 (100.0) | NA | 136 (100.0) |
For two participants amputation occurred after reported healing of the index ulcer due to another non‐index ulcer on the index foot, either present at baseline or developed subsequently. For 10 participants amputation occurred prior to healing of the index ulcer with the amputation being on the index foot but of a different site to that of the index ulcer due to another non‐index ulcer on the index foot, either present at baseline or developed subsequently.
Time to healing and other clinical outcomes
For participants whose index ulcer healed, the median (range) time to healing was 4.5 (0.5–12.9) months (n=136, missing n=12). Adverse events generally occurred relatively early in the clinical course of treatment. For those with a recurrence of the index ulcer, the median (range) time of recurrence was 1.7 (0.3–10.7) months post‐healing. For those who underwent revascularization surgery, the median (range) time to surgery was 3.0 (0.1–9.5) months. For those who underwent a lower extremity amputation, the median (range) time to amputation was 2.0 (0.0–10.6) months. Finally, for those who died during the 12‐month follow‐up, the median (range) time to death was 5.6 (0.6–11.5) months.
The estimated incidence of healing, accounting for competing events of amputation or death, was 27.5% (95% CI 22.4, 32.5) at 6 months and 44.5% (95% CI 38.9, 50.1) at 12 months. Figure 2 shows the estimated cumulative incidence curves of the time to healing and the competing risks of death and amputation.
Figure 2.

Healing estimates and cumulative incidence functions of the time to healing in the presence of competing risks of death or amputation. *This refers to the number of participants left in the ‘risk’ set consisting of those uncensored without an event (healing, death, or amputation).
Prognostic factors associated with healing
Table 4 summarizes participants’ healed status, estimated HRs (95% CI) and P values for baseline factors identified by the explorative univariable, preliminary multivariable and final multivariable analyses as significantly associated with the incidence of healing.
Table 4.
Healing status by baseline factors, univariable and multivariable analysis for factors with a significant univariable association with the incidence of healing
| Baseline factora | Healed(%) | Not healed (%) | Reference levelb | Exploratory univariable analysis HR (95%CI) | Preliminary multivariable analysis HR (95%CI) | Final multivariable analysis HR (95%CI) |
|---|---|---|---|---|---|---|
| Ulcer type | ||||||
| Ischaemic or neuro‐ischaemic | 49 (34.5) | 93 (65.5) | Neuropathic | 0.5 (0.35 to 0.71)P<0.0001 | 1.09 (0.59 to 2.02)P=0.7837 | |
| Neuropathic only | 85 (54.8) | 70 (45.2) | ||||
| Wagner grade | ||||||
| Grade 1 | 56 (53.8) | 48 (46.2) | Grade 1 | |||
| Grade 2 | 41 (44.1) | 52 (55.9) | 0.65 (0.44 to 0.98)P=0.0397 | 0.56 (0.25 to 1.23)P=0.1477 | ||
| Grade 3, 4 or 5 | 39 (38.2) | 63 (61.8) | 0.55 (0.36 to 0.82)P=0.0038 | 0.59 (0.25 to 1.37)P=0.2159 | ||
| PEDIS: perfusion | ||||||
| Grade 1 | 85 (57.8) | 62 (42.2) | Grade 1 | 0.44 (0.31 to 0.62)P<0.0001 | 0.43 (0.22 to 0.83)P=0.0113 | 0.37 (0.25 to 0.55)P<0.0001 |
| Grade ≥2 | 51 (33.6) | 101 (66.4) | ||||
| PEDIS: depth | ||||||
| Grade 1 | 51 (53.1) | 45 (46.9) | Grade 1 | |||
| Grade 2 | 47 (47.0) | 53 (53.0) | 0.70 (0.47 to 1.05)P=0.0843 | 1.61 (0.74 to 3.49)P=0.2311 | ||
| Grade 3 | 38 (36.9) | 65 (63.1) | 0.54 (0.35 to 0.83)P=0.0046 | 1.16 (0.47 to 2.91)P=0.7451 | ||
| PEDIS: infectionc | ||||||
| Grade 2 | 55 (52.9) | 49 (47.1) | Grade 2 | 0.65 (0.46 to 0.91)P=0.0135 | 0.91 (0.59 to 1.41)P=0.6831 | |
| Grade 3 | 78 (42.2) | 107 (57.8) | ||||
| Grade 4 | 3 (30.0) | 7 (70.0) | ||||
| Wound duration | ||||||
| <56 days | 77 (53.8) | 66 (46.2) | <56 days | 0.54 (0.39 to 0.76)P=0.0004 | 0.46 (0.30 to 0.70)P=0.0003 | 0.55 (0.39 to 0.77)P=0.0005 |
| ≥56 days | 56 (36.8) | 96 (63.2) | ||||
| Prior antimicrobial dressing on ulcer | ||||||
| Yes | 69 (39.4) | 106 (60.6) | No | 0.65 (0.46 to 0.91)P=0.0123 | 0.77 (0.52 to 1.14)P=0.1934 | |
| No | 64 (53.8) | 55 (46.2) | ||||
| Only one (rather than≥2) ulcer on index foot | ||||||
| Yes | 113 (50.9) | 109 (49.1) | No | 1.96 (1.25 to 3.07)P=0.0034 | 1.91 (1.15 to 3.17)P=0.0122 | 1.90 (1.18 to 3.06)P=0.0081 |
| No | 23 (29.9) | 54 (70.1) | ||||
| Coagulase‐negative Staphylococcus reported | ||||||
| Yes | 24 (63.2) | 14 (36.8) | No | 1.69 (1.11 to 2.59)P=0.0147 | 1.98 (1.08 to 3.61)P=0.0270 | 1.53 (0.98 to 2.40)P=0.0603 |
| No | 112 (42.9) | 149 (57.1) | ||||
| Methicillin‐resistant S. aureus reported | ||||||
| Yes | 8 (29.6) | 19 (70.4) | No | 0.50 (0.26 to 0.97)P=0.0419 | 0.67 (0.28 to 1.63)P=0.3802 | |
| No | 128 (47.1) | 144 (52.9) | ||||
| Age (continuous ‐ per 5 year increase)d | 1.02 (0.95 to 1.09)P=0.5887 | 1.11 (1.02 to 1.22)P=0.0169 | 1.11 (1.03 to 1.19)P=0.0081 |
HR, hazard ratio. Bold numbers indicate the direction in which the event is more likely to occur (healing or not healing).
The following factors reported in Table 1 were also included in the exploratory univariable and preliminary multivariable analysis, however, no significant associations were detected at the 10% level: diabetes duration; diabetes type: type 2 vs type 1; insulin therapy; HbA1c; ulcer extent; PEDIS sensation grade; incident or recurrent ulcer; ulcer location; previous antibiotic therapy; any reported pathogens; overall anaerobes; Gram‐positive cocci; Gram‐negative bacilli; Enterobacteriaceae; Gram‐positive bacilli; MSSA; Streptococcus; Enterococcus excluding vancomycin resistant, Corynebacterium; Pseudomonas.
The reference level refers to the level of the factor used as the reference in the HRs, i.e. participants with ischaemic ulcers compared with the reference neuropathic ulcers have a lower rate of healing with a HR of 0.5 in univariable analysis.
PEDIS infection grades 3 and 4 were combined in the analysis.
The association with age was not supported when age was explored categorically at various ‘splits’ in the data.
Univariable analyses showed that 11 of the prespecified factors were potentially associated (based on a P value <0.1) with the incidence of healing of the index ulcer. The incidence of healing was higher if there was only one ulcer (rather than multiple) on the index foot, or if coagulase‐negative staphylococci were identified from a culture of the ulcer. The incidence of healing was lower if the index ulcer: (i) had a PEDIS perfusion grade of ≥2; (ii) was classified as ischaemic; (iii) had been present for ~2 months or longer prior to CODIFI enrolment; (iv) had a Wagner grade of ≥2; (v) had a PEDIS depth grade of ≥2; (vi) had a PEDIS infection grade of ≥3; (vii) was covered with an antimicrobial dressing at baseline; or (viii) had methicillin‐resistant Staphylococcus aureus (MRSA) identified from the ulcer culture (Table 4).
When fitting all potential prognostic factors into a multivariable proportional sub‐distribution hazards model (a preliminary analysis), then into a final analysis model, only four remained statistically significantly associated with the incidence of healing (excluding age; Table 4). The incidence of healing was higher if: (i) there was only one ulcer on the index foot (HR 1.9, 95% CI 1.18 to 3.06); or (ii) coagulase‐negative staphylococci was identified from the ulcer culture (HR 1.53, 95% CI 0.98 to 2.40). The incidence of healing was lower if the index ulcer: (i) had a PEDIS perfusion grade of ≥2; (HR 0.37, 95% CI 0.25 to 0.55) or (ii) had been present for ~2 months or longer prior to CODIFI enrolment (HR 0.55, 95% CI 0.39 to 0.77).
Associations between factors
Table 5 summarizes pairwise associations between factors related to the incidence of healing. Several factors that were statistically significantly associated with healing in the univariable analysis were no longer statistically significant in the multivariable analysis (ulcer type, PEDIS depth, PEDIS infection, Wagner grade, antimicrobial dressing at baseline, and MRSA isolation).
Table 5.
Association (P values) between factors found to be statistically significant in the univariable analysis
| Ulcer type | Wagner grade | PEDIS perfusion | PEDIS depth | PEDIS infection | Single ulcer | Wound duration | Antimicrobial dressing | MRSA cultured | CoNS cultured | |
|---|---|---|---|---|---|---|---|---|---|---|
| <0.0001 | <0.0001 | <0.0001 | 0.0036 | 0.0300 | 0.0219 | 0.0012 | 0.0042 | 0.0453 | Ulcer type | |
| <0.0001 | <0.0001 | <0.0001 | Wagner grade | |||||||
| <0.0001 | 0.0002 | 0.0423 | <0.0001 | PEDIS perfusion | ||||||
| <0.0001 | PEDIS Depth | |||||||||
| PEDIS Infection | ||||||||||
| Single ulcer | ||||||||||
| 0.0201 | Wound duration | |||||||||
| Antimicrobial dressing | ||||||||||
| 0.0376 | MRSA cultured | |||||||||
| CoNS cultured |
CoNS, Coagulase‐negative Staphylococcus
Numbers are the P values of significant associations between factors.
Note that shaded cells in the top row and final column indicate factors that were no longer statistically significant in the multivariable analysis (ulcer type, Wagner grade, PEDIS dept, PEDIS infection, presence of antimicrobial dressing, MRSA cultured). Whilst unshaded cells indicate factors (PEDIS perfusion, single ulcer, would duration, CoNS cultured) that remained significant. In the body of the table, ushaded cells containing a P value indicate associations where neither of the factors remained significant in the multivariable model, whilst unshaded cells indicate associations where at least one of the two factors remained significant in the multivariable model. Cells without a P value indicate non‐significant associations at the 5% level.
Pairwise associations between various factors that met statistical significance (P<0.05; cross‐tabulations quantifying these associations are presented in Table S1) included ulcer type, and PEDIS perfusion, PEDIS depth, PEDIS infection and Wagner grades. Among these, only PEDIS perfusion grade remained statistically significant in the multivariable analysis, with a higher grade associated with ischaemic ulcers, greater depth, more severe infection and higher Wagner grades. Ischaemic ulcers were also associated with having multiple ulcers on the index foot, a longer‐duration ulcer, and a lower rate of isolation of coagulase‐negative staphylococci from the ulcer culture, all of which were statistically significant in the multivariable analysis. The presence of an antimicrobial dressing on the ulcer at baseline did not remain statistically significant in the multivariable analysis, but was associated with a higher perfusion grade and a longer ulcer duration. Isolation of MRSA from the ulcer culture was associated with ulcer type, and was more frequently reported from cultures of ischaemic ulcers. Interestingly, isolation on ulcer culture of MRSA was inversely associated with isolation of coagulase‐negative staphylococci, each being only reported in the absence of the other.
Discussion
In this large, prospective study with 12 months’ follow‐up, we found that three key factors served as the best independent predictors of healing of an infected diabetic foot ulcer: the PEDIS perfusion grade; the absence of multiple foot ulcers; and a shorter ulcer duration. A lower perfusion grade suggests the presence of peripheral arterial disease, which previous studies have shown to be an important predictor of poor outcomes (i.e. lack of healing and lower extremity amputation) in people with a diabetic foot ulcer 5, 13, 14, 15. In the present study, among the five PEDIS domains, perfusion (indicating peripheral arterial disease) was shown to be the only independent predictor of healing. Ulcer duration and presence of multiple ulcers have also been reported as significant predictors for non‐healing in other studies 28, 29.
The participants in the present study with a clinically suspected diabetic foot ulcer infection had poorer outcomes than reported in previously published studies, which enrolled people with various types of diabetic foot ulcers 5, 13, 14, 15. We found a 12‐month incidence of healing of 44.5%, while Prompers et al. 5, Ince et al. 13 and Jeffcoate et al. 14 reported healing rates at 12 months of 77% and 68.3%, respectively. In addition, we found that during 12 months of follow‐up for their infected diabetic foot ulcer, 17.4% of our participants underwent at least partial lower extremity amputation and 15.1% died, compared with 22% for amputation and 6% deaths in the study by Prompers et al. 5 and 5.9% for amputation or death (reported as combined) in the study by Ince et al. 13 These differences may have arisen because we specifically recruited people with a suspected infected diabetic foot ulcer, whereas Prompers et al. 5 included only people presenting with a new foot ulcer and excluded those treated at the participating centres for an ulcer on the ipsilateral foot during the previous 12 months and those with a life expectancy of <1 year; compared with our participants, we would expect their population to have had a better prognosis from the outset. Ince et al. 13 recruited participants from a heterogeneous clinic population. They also used conventional survival analyses to estimate healing rates, which neither accounted nor adjusted for the competing events of amputation or death; this probably overestimates the incidence of healing.
A more complex issue is the association we found between culturing coagulase‐negative staphylococci and higher incidence of healing. This is a group of relatively avirulent organisms, and the fact that their presence was inversely related to the presence of the more virulent MRSA may explain the association with healing. There were no statistically significant associations between ulcer healing and any other ulcer culture results, including whether or not the microbiology laboratory reported growth of any microorganisms. Unlike other studies, we found no significant association between the incidence of healing and prognostic factors such as glycaemic control 30, duration of diabetes 5, 13, ulcer site, sex 15, wound area or wound depth 28. Noteworthy is that poor arterial perfusion often leads to ‘punched out’ ulcers that are likely to be deeper than non‐ischaemic ulcers 23. Our analyses suggest that it is the poor perfusion (peripheral arterial disease) rather than the depth of the ulcer that leads to impaired healing.
We also found a higher incidence of ulcer healing among older participants, but this finding was not consistently supported when we explored age categorically at various ‘splits’ in the data. As there is no apparent biological basis for this finding or support from results of other studies 31, we think it is likely to be spurious.
To our knowledge, this is the first prospective study of people with infected diabetic foot ulcers to estimate methodically the cumulative incidence of healing, to report the time to healing, and to identify factors associated with the incidence of healing while adjusting for competing risks. Taking these factors into consideration, we found that conventional survival analysis would have overestimated the incidence of healing in the present study by almost 10%.
As this study recruited participants from 25 centres across England, ranging from large teaching hospitals to small primary healthcare centres, these results are likely to be generalizable to outcomes of diabetic foot ulcers across England, and perhaps in other higher‐income countries. This is also the first large study of outcomes of diabetic foot ulcers conducted since publication of the 2011 UK National Institute for Health and Care Excellence (NICE) guidelines on management of diabetic foot problems 17, which have been updated by new guidelines 19. The poor outcomes we found in the present study may suggest that either the guideline recommendations have not been widely implemented or they are not very effective. The current NICE guidelines 19 recommend placing systems across all care settings designed to prevent and manage diabetic foot problems; however, a recent UK national audit 32 found that the implementation of the NICE guidelines was not universal.
Diagnosing whether or not a diabetic foot ulcer is infected can be difficult, especially in the presence of peripheral neuropathy or limb ischaemia, but authorities generally agree that it should be based on finding signs or symptoms of inflammation, purulence and possibly other ‘secondary’ clinical findings 16, 18. Of the participants enrolled in the main CODIFI study 21, 12% had a swab or tissue sample growing no pathogens isolated, but almost half were receiving antibiotic therapy and two‐thirds were using an antimicrobial dressing. Furthermore, deficiencies in specimen collection, transport or processing may have led to false‐negative cultures.
The present study has three key limitations. First, our 12‐month case note review was an extension to the original CODIFI study 21, resulting in incomplete follow‐up for those who could not be re‐contacted, did not re‐consent for the study extension or where case notes were unavailable, leading to 25% of participants lost to follow‐up. We did not include participants lost to follow‐up because of the assumptions required in order to impute data for multiple outcomes (healing, amputation, death), and the timing of these outcomes. The characteristics of participants included and participants not included were largely similar, but participants included were slightly older, with more recurrent ulcers and more severe infections.
Second, as we did not require retrospective consent for case note review of participants who had died prior to our ethics amendment for this study, attrition bias may have contributed to the slightly older age, more severe infection and the overall inflated death rate. Among the 377 participants for whom we had follow‐up data and known survival status at the point of consent, 56 (14.9%) had died. As this includes deaths outside of the 12‐month follow‐up period, we believe the minimum death rate within the 12‐month follow‐up period was 13% (49/377) and the maximum 16.4% (49/299).
Third, we do not have 12‐month data on whether or not infection resolved. Although resolution of infection is important for ulcer healing, our study design focused on healing, and the consequences of non‐healing. Our case note review included data on antibiotics prescribed to participants prior to baseline sampling and immediately after sampling. This allowed us to assess the relationship between the various outcomes of interest and the pathogens isolated from the diabetic foot ulcer, as well as the antibiotic regimens prescribed. We think it would be useful if a future prospective study explored the relationship between healing of an infected ulcer and the appropriateness of anti‐infective therapy, determined by whether the antibiotic therapy was active against the isolated organisms.
In conclusion, the present study has confirmed that people with a clinically infected diabetic foot ulcer have a poor prognosis. Our results also confirmed that the presence of limb ischaemia, multiple foot ulcers and a longer ulcer duration were most predictive of poor 12‐month outcomes. These findings should be useful to clinicians in various care settings to help identify people most at risk of poor outcomes who may need prioritization for increased interventions or referral to specialist centres. Our findings on prognostic factors or diabetic foot ulcer healing should also be useful to inform the design and analysis of future clinical studies.
Funding sources
This paper presents independent research funded by the National Institute for Health Research (NIHR) Health Technology Assessment Programme, HTA 09/75/01. The views expressed are those of the authors and not necessarily those of the National Health Service, the NIHR or the Department of Health. The funder was not involved in the preparation of the study protocol, the running of the study or the analysis or preparation of this paper.
Competing interests
E.A.N. was a member of the HTA Commissioning Board (2011–2015). The remaining authors have no competing interests.
Supporting information
Table S1. Cross tabulations of factors with a significant association (P<0.005).
Acknowledgements
The authors wish to thank the members of the study steering committee: Prof. John Deeks, Prof. Rose Cooper, Dr Roger Gadsby, Prof. Anne‐Maree Keenan and Mrs Christine Thomas, who was the patient representative. The authors wish to thank the following researchers for their contribution in this study: Prof. Christopher Dowson who was a co‐applicant on the study grant; Dr Michael Edmonds who was a co‐applicant on the study grant; and Mr Tom Dickie who was a co‐applicant on the study grant and contributed patients to the study. The authors also wish to thank the following research collaborators across 25 hospitals, and their teams: Prof. Peter Vowden (Bradford Teaching Hospitals, Bradford), Dr Kilimangalam Narayanan (Queen Elizabeth Hospital, Gateshead), Prof. Satyan Rajbhandari (Chorley and South Ribble Hospital, Chorley), Dr Cuong Dang (North Manchester General Hospital, Manchester), Dr Deirdre Maguire (Harrogate District Hospital, Harrogate), Dr Robert Moisey (Huddersfield Royal Infirmary, Huddersfield), Dr Simon Ashwell (James Cook University Hospital, Middlesbrough), Dr Frank Bowling (Manchester Royal Infirmary, Manchester), Dr Varadarajan Baskarn (New Cross Hospital, Wolverhampton), Dr Ketan Dhatariya (Norfolk and Norwich University Hospital, Norwich), Dr Ryan D'Costa (Pinderfields General Hospital, Wakefield), Dr Mujahid Saeed (Queen Elizabeth Hospital, Birmingham), Dr Sunil Sharma (Queen Elizabeth Hospital, King's Lynn), Dr Paul Smith (Royal Lancaster Infirmary, Lancaster), Dr Deepak Bhatnagar (Royal Oldham Hospital, Oldham), Dr Khaled Dukhan (South Tyneside District Hospital, South Shields), Dr Jonathan Bodansky (St James's University Hospital, Leeds), Dr Edward Jude (Tameside General Hospital, Lancashire), Dr Harpal Randeva (University Hospital Coventry, Coventry), Dr Yashica Nathan (University Hospital Lewisham, Lewisham), Dr Fahmy Hanna (University Hospital of North Staffordshire, Stoke‐on‐Trent), Dr Sony Anthony (University Hospital of North Tees, Stockton‐on‐Tees) and Dr Parag Singhal (Weston General Hospital, Weston‐Super‐Mare).
Author contributions
All authors also contributed to the writing of this manuscript and read and approved the final manuscript. Further to this contribution, E. A. Nelson is the lead grant holder. M. Ndosi is the clinical coordinator for the 12‐month follow up study and drafted this manuscript. A. Wright‐Hughes is the trial statistician and conducted the analysis and drafted this manuscript. S. Brown is a co‐applicant on the study grant and the statistical guarantor. M. Backhouse was the clinical coordinator for the initial CODIFI study. M. Bhogal is the trial manager. C. Reynolds is the data manager. B. A. Lipsky, P. Vowden, E. Jude, and J. Nixon were co‐applicants on the study grant. All authors have fulfilled the four ICMJE authorship criteria.
Diabet. Med. 35, 78–88 (2018)
M.N. and A.W.H., and J.N. and E.A.N., contributed equally to this paper.
References
- 1. Singh N, Armstrong DG, Lipsky BA. Preventing foot ulcers in patients with diabetes. JAMA 2005; 293: 217–228. [DOI] [PubMed] [Google Scholar]
- 2. Brownrigg JRW, Davey J, Holt PJ, Davis WA, Thompson MM, Ray KK, et al The association of ulceration of the foot with cardiovascular and all‐cause mortality in patients with diabetes: a meta‐analysis. Diabetologia 2012; 55: 2906–2912. [DOI] [PubMed] [Google Scholar]
- 3. Vileikyte L, Rubin RR, Leventhal H. Psychological aspects of diabetic neuropathic foot complications: an overview. Diabetes Metab Res Rev 2004; 20(Suppl. 1): S13–18. [DOI] [PubMed] [Google Scholar]
- 4. Boulton AJ, Kirsner RS, Vileikyte L. Clinical practice. Neuropathic diabetic foot ulcers. N Engl J Med 2004; 351: 48–55. [DOI] [PubMed] [Google Scholar]
- 5. Prompers L, Huijberts M, Schaper N, Apelqvist J, Bakker K, Edmonds M, et al Resource utilisation and costs associated with the treatment of diabetic foot ulcers. Prospective data from the Eurodiale Study. Diabetologia 2008; 51: 1826–1834. [DOI] [PubMed] [Google Scholar]
- 6. Prompers L, Huijberts M, Apelqvist J, Jude E, Piaggesi A, Bakker K, et al High prevalence of ischaemia, infection and serious comorbidity in patients with diabetic foot disease in Europe. Baseline results from the Eurodiale study. Diabetologia 2007; 50: 18–25. [DOI] [PubMed] [Google Scholar]
- 7. Faglia E, Favales F, Morabito A. New ulceration, new major amputation, and survival rates in diabetic subjects hospitalized for foot ulceration from 1990 to 1993: a 6.5‐year follow‐up. Diabetes Care 2001; 24: 78–83. [DOI] [PubMed] [Google Scholar]
- 8. Moulik PK, Mtonga R, Gill GV. Amputation and mortality in new‐onset diabetic foot ulcers stratified by etiology. Diabetes Care 2003; 26: 491–494. [DOI] [PubMed] [Google Scholar]
- 9. Ghanassia E, Villon L, Thuan Dit Dieudonne JF, Boegner C, Avignon A, Sultan A. Long‐term outcome and disability of diabetic patients hospitalized for diabetic foot ulcers: a 6.5‐year follow‐up study. Diabetes Care 2008; 31: 1288–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Morbach S, Furchert H, Groblinghoff U, Hoffmeier H, Kersten K, Klauke GT, et al Long‐term prognosis of diabetic foot patients and their limbs: amputation and death over the course of a decade. Diabetes Care 2012; 35: 2021–2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Holman N, Young RJ, Jeffcoate WJ. Variation in the recorded incidence of amputation of the lower limb in England. Diabetologia 2012; 55: 1919–1925. [DOI] [PubMed] [Google Scholar]
- 12. Kerr M. Foot care for people with diabetes: the economic case for change. NHS Diabetes Kidney Care, 2012.
- 13. Ince P, Kendrick D, Game F, Jeffcoate W. The association between baseline characteristics and the outcome of foot lesions in a UK population with diabetes. Diabet Med 2007; 24: 977–981. [DOI] [PubMed] [Google Scholar]
- 14. Jeffcoate WJ, Chipchase SY, Ince P, Game FL. Assessing the outcome of the management of diabetic foot ulcers using ulcer‐related and person‐related measures. Diabetes Care 2006; 29: 1784–1787. [DOI] [PubMed] [Google Scholar]
- 15. Pickwell KM, Siersma VD, Kars M, Holstein PE, Schaper NC. Eurodiale consortium. Diabetic foot disease: impact of ulcer location on ulcer healing. Diabetes Metab Res Rev 2013; 29: 377–383. [DOI] [PubMed] [Google Scholar]
- 16. Lipsky BA, Berendt AR, Cornia PB, Pile JC, Peters EJ, Armstrong DG, et al 2012 Infectious Diseases Society of America clinical practice guideline for the diagnosis and treatment of diabetic foot infections. Clin Infect Dis 2012; 54: e132–173. [DOI] [PubMed] [Google Scholar]
- 17. NICE . Diabetic foot problems: Inpatient management of diabetic foot problems. Manchester: National Institute for Health and Clinical Excellence, 2011. [PubMed] [Google Scholar]
- 18. Lipsky BA, Aragon‐Sanchez J, Diggle M, Embil J, Kono S, Lavery L, et al IWGDF guidance on the diagnosis and management of foot infections in persons with diabetes. Diabetes Metab Res Rev 2016; 32(Suppl 1): 45–74. [DOI] [PubMed] [Google Scholar]
- 19. NICE . Diabetic foot problems: prevention and management. Nice Guideline NG19 London: National Institute for Health and Care Excellence, 2015. [Google Scholar]
- 20. Schaper NC, Van Netten JJ, Apelqvist J, Lipsky BA, Bakker K, International Working Group on the Diabetic Foot . Prevention and management of foot problems in diabetes: A Summary Guidance for Daily Practice 2015, based on the IWGDF guidance documents. Diabetes Res Clin Pract 2017; 124: 84–92. [DOI] [PubMed] [Google Scholar]
- 21. Nelson A, Backhose M, Wright‐Hughes A, Bhogal M, Brown S, Nixon J, et al Extent of disagreement and difference between tissue and swab samples from infected diabetic foot ulcers: the CODIFI Study. Diabetologia 2014; 57: S471–S472. [Google Scholar]
- 22. Schaper N. Diabetic foot ulcer classification system for research purposes: a progress report on criteria for including patients in research studies. Diabetes Metab Res Rev 2004; 20: S90–S95. [DOI] [PubMed] [Google Scholar]
- 23. Irving G, Hargreaves S. Venous and arterial leg ulceration. InnovAiT 2009; 2: 415–422. [Google Scholar]
- 24. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999; 94: 496–509. [Google Scholar]
- 25. Little RJA, Rubin DB. Statistical analysis with missing data, 2nd ed Chichester: Wiley, 2002. [Google Scholar]
- 26. Brooks S, Gelman A, Jones G, Meng X‐L. Handbook of Markov Chain. Monte Carlo: CRC Press, 2011. [Google Scholar]
- 27. Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York: John Wiley & Sons Inc, 1987. [Google Scholar]
- 28. Margolis DJ, Allen‐Taylor L, Hoffstad O, Berlin JA. Diabetic neuropathic foot ulcers: the association of wound size, wound duration, and wound grade on healing. Diabetes Care 2002; 25: 1835–1839. [DOI] [PubMed] [Google Scholar]
- 29. Fife CE, Horn SD, Smout RJ, Barrett RS, Thomson B. A Predictive Model for Diabetic Foot Ulcer Outcome: The Wound Healing Index. Adv Wound Care 2016; 5: 279–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Christman AL, Selvin E, Margolis DJ, Lazarus GS, Garza LA. Hemoglobin A1c predicts healing rate in diabetic wounds. J Invest Dermatol 2011; 131: 2121–2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Prompers L, Schaper N, Apelqvist J, Edmonds M, Jude E, Mauricio D, et al Prediction of outcome in individuals with diabetic foot ulcers: focus on the differences between individuals with and without peripheral arterial disease. The EURODIALE Study. Diabetologia 2008; 51: 747–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Health and Social Care Information Centre . National Diabetes Foot Care Audit Report 2014–2015. London: Health and Social Care Information Centre, 2016. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Cross tabulations of factors with a significant association (P<0.005).


