Abstract
Botanical dietary supplements contain multiple bioactive compounds that target numerous biological pathways. The lack of uniform standardization requirements is one reason that inconsistent clinical effects are reported frequently. The multifaceted biological interactions of active principles can be disentangled by a coupled pharmacological/phytochemical approach using specialized (“knock-out”) extracts. This is demonstrated for hops, a botanical for menopausal symptom management. Employing targeted, adsorbent-free countercurrent separation, Humulus lupulus extracts were designed for pre- and postmenopausal women by containing various amounts of the phytoestrogen 8-prenylnaringenin (8-PN) and the chemopreventive constituent xanthohumol (XH). Analysis of their estrogenic (alkaline phosphatase), chemopreventive (NAD(P)H-quinone oxidoreductase 1 [NQO1]), and cytotoxic bioactivities revealed that the estrogenicity of hops is a function of 8-PN, whereas their NQO1 induction and cytotoxic properties depend on XH levels. Antagonization of the estrogenicity of 8-PN by elevated XH concentrations provided evidence for the interdependence of the biological effects. A designed postmenopausal hop extract was prepared to balance 8-PN and XH levels for both estrogenic and chemopreventive properties. An extract designed for premenopausal women contains reduced 8-PN levels and high XH concentrations to minimize estrogenic while retaining chemopreventive properties. This study demonstrates the feasibility of modulating the concentrations of bioactive compounds in botanical extracts for potentially improved efficacy and safety.
Botanical extracts are backed by centuries of human use and are gaining in popularity and importance as products for human health.1−3 These trends result from the growing interest in preventive care and healthy living, an increasingly aging population, and the widespread perception that botanicals may be generally safer than drugs.4 However, rigorous efficacy and safety evidence for botanical dietary supplements is scarce. This knowledge gap is partly due to a lack of global standardization practices for botanical products. Importantly, this situation reflects the major scientific challenges associated with assigning biological/clinical effects to one or only a few bioactive markers for which the optimal clinical dose is not yet known. Collectively, this explains why for many given medicinal plants a large variety of herbal products with inconsistent bioactivities exist.
Botanical extracts consist of a multitude of constituents that affect a range of biological targets, leading to diverse pharmacological actions (Scheme 1). The concept that a drug or multiple compounds, in this case contained in botanical extracts, target different pharmacological targets is called “polypharmacology”.5 Polypharmacology holds the promise of being useful for the alleviation of chronic and complex ailments, such as menopausal symptoms. Botanical extracts contain an array of constituents to target multiple pharmacological pathways, and their ethnomedical selection makes them particularly valuable to remedy chronic conditions.6 The polypharmacological nature of plant extracts, as well as the assignment of the bioactive compounds to the clinically relevant pharmacological actions in these extracts, has rarely been analyzed. Knowledge of the relative contribution of the main bioactive compounds to the overall bioactivity is key for improving the efficacy and safety of botanical products and provides a rationale for the advanced botanical standardization to multiple rather than single bioactive compounds (Scheme 1).7−9 Interdisciplinary evaluation and standardization of botanical dietary supplements widely used for women’s health is the overarching goal of the UIC/NIH Center for Botanical Dietary Supplements Research.2,10 This report summarizes investigations about the feasibility of optimizing the bioactivity of botanical extracts with the model botanical hops.
Scheme 1. Specialized and DESIGNER Extracts as Tools for Phytomedical and Natural Product Research.
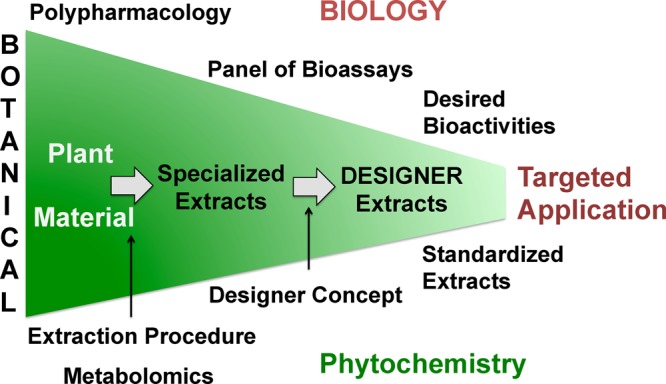
Standardized extracts optimized for desired bioactivities can be generated from crude plant material in three main steps: (1) selection of plant material, (2) extraction via a specific procedure, (3) application of the DESIGNER concept.26 The resulting DESIGNER extracts can be standardized to desired concentrations of different bioactive compounds and represent materials with potentially more targeted biological profiles.
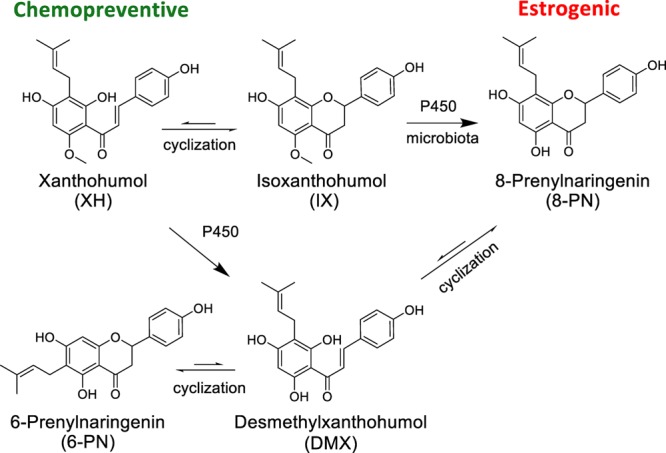
The strobili of hops (Humulus lupulus L., Cannabaceae) have a long tradition of use as a botanical remedy for mood and sleep disturbances and more recently for the relief of menopausal symptoms.11 The major bioactive compounds in hops include 8-prenylnaringenin (8-PN; estrogenic),12−14 6-prenylnaringenin (6-PN; aryl hydrocarbon receptor (AhR) agonist),15 and xanthohumol (XH; chemopreventive)16 (Figure 1). 8-PN is one of the most potent phytoestrogens known, with an EC50 in the low nanomolar range.14,17,18 The chalcone XH is the major prenylated phenol in hops and is mainly responsible for the documented chemopreventive, cytotoxic, and anti-inflammatory activities of hops.19−22 The chemopreventive mechanism likely involves induction of detoxification enzymes such as NAD(P)H-quinone oxidoreductase 1 (NQO1).21,23,24 Furthermore, XH-rich hop extracts have been recommended as cancer preventive agents.25
Figure 1.
Major bioactive phytoconstituents of spent H. lupulus.33
In this study, using a new approach, a chemical “knock-out/-down” of certain phytoconstituents,26 analogous to a gene “knock-out” concept,27 has been utilized for specialized extracts. This approach provides a new means of uncovering the contribution of single compounds to the multifaceted biological effects of a botanical extract and reveals interactions between the phytoconstituents. Once individual pharmacological effects are assigned to certain phytoconstituents and compound interactions are known, it becomes possible to design specialized extracts by removing (“knocking-out/-down”) compounds that interfere with the desired bioactivity or are responsible for (dose-dependent) adverse effects. Similarly, constituents with desired activities can be enriched (“knocked-in”) for optimal efficacy. This enables the production of optimized and standardized extracts with targeted compound-bioactivity profiles and likely enhanced efficacy and safety (Scheme 1). To achieve selective chemical “knock-out/-down” of certain phytoconstituents in a botanical extract in a loss-free manner, countercurrent separation (CCS) was applied.28 CCS utilizes immiscible liquid–liquid two-phase solvent systems as chromatographic phases. It represents a relatively high-resolution chromatography with high (preparative) loading capacity, and its selectivity allows a high rate of efficiency for the enrichment of a target compound. Adjusting/depleting the amount of a single or multiple targeted metabolites from a chemically complex mixture, i.e., a botanical extract, by means of CCS generates DESIGNER extracts [Deplete and Enrich Select Ingredients to Generate Normalized Extract Resources; DESIGNER extract = total extract ± target compound(s)].26
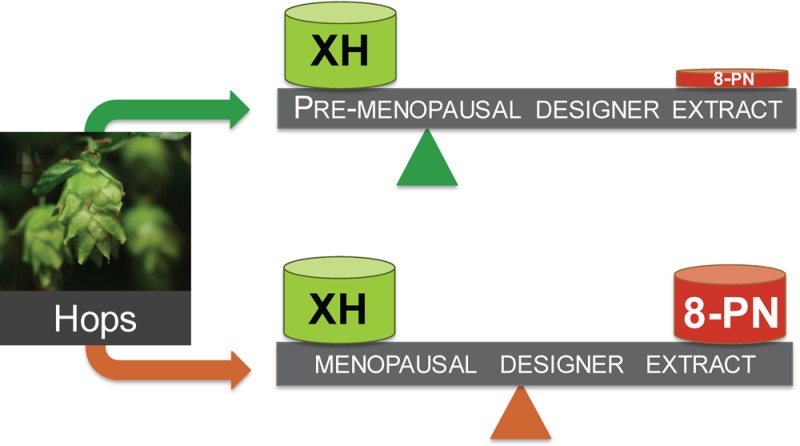
The present study shows how three mechanistically distinct bioactivities can be modulated by designing specialized H. lupulus extracts using the DESIGNER concept.26 In addition, application of CCS to “knock-down” target constituents in a clinical hop extract enabled the study of the respective contributions and possible interactions of certain bioactive compounds in the metabolomic mixture of the extract. Its relatively well established phytochemical profile,11 multiple biological activities, and popular use specifically among postmenopausal women2 made the spent hop extract a suitable model for this novel approach. The overarching goal was to show how the ratios and levels of active constituents can be modified, leading to H. lupulus extracts with more targeted efficacy, such as combined estrogenic and chemopreventive properties for postmenopausal women versus chemoprevention without estrogenic activities for premenopausal women (Scheme 2). Premenopausal women may prefer chemopreventive hop extracts without additional estrogenic properties. Analogous studies can be envisioned for other botanical extracts to potentially enhance efficacy and limit toxicity.
Scheme 2. Concept of Targeted Application.
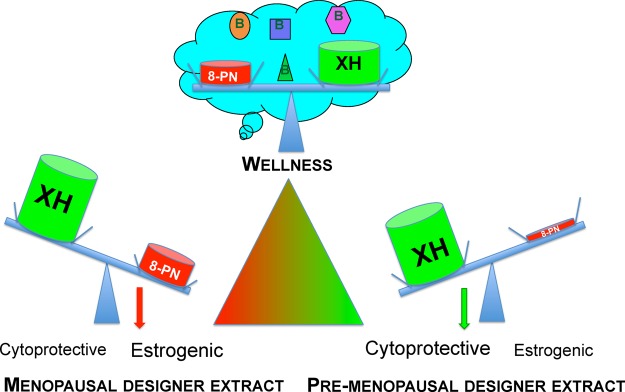
DESIGNER extracts are used to optimize the bioactivity profiles of hops by balancing two dual bioactive constituents: the potent phytoestrogen 8-PN and XH, the major cytoprotective constituent of hops. Targeted and selective depletion/enrichment of 8-PN, IX, and/or XH allows the design of extracts with various biological properties, such as menopausal, Ex3, versus pre-menopausal DESIGNER extract (Ex5+Ex7). Presented are the symbols for XH and 8-PN in the size that depicts their estimated mass %. Other hops constituents are omitted for clarity. The optimal holistic hop extract (wellness) might be standardized to multiple bioactive hop constituents (metabolome), leading to polypharmacological activities.
Results and Discussion
DESIGNER Hop Extracts
The DESIGNER extracts of H. lupulus were generated using CCS in two steps, as described previously.26 The first CCS step produced the initial level of DESIGNER extracts. A second CCS step was carried out to enhance the overall “knock-out/-down” selectivity (depletion factor). This second step utilized either a polarity-adjusted variant of the same class of biphasic mixtures (CCS solvent system family) or a chemically distinct solvent system with orthogonal separation characteristics. The residual amounts of the target metabolites in these DESIGNER extracts were determined by three different methods: UV-UHPLC, qHNMR, and LC-MS/MS (Table 1).26 It is important to recognize that the degree of “knock-out/-down” selectivity and (apparent) efficiency are intrinsically limited by both the preparative separation (i.e., CCS) and the analytical evaluation (i.e., HPLC, LC-MS, qHNMR) methods. This explains the importance of combining multistep, orthogonal CCS with multiple analytical evaluation methods, as employed in this study.
Table 1. Content of Bioactive Constituents (% w/w) Determined by LC-MS/MS of the DESIGNER Extracts That Are Based on “Knock-out” Technology26 or on Specialized Extraction.
| DESIGNER extracta | LC-MS/MSb | ||||
|---|---|---|---|---|---|
| Specialized Extracts | 8-PN% | XH% | 6-PN% | IX% | ratio XH/8-PN |
| Ex1 | 0.95 | 3.17 | 0.67 | 62.38c | 3.3 |
| Ex2 | 0.47 | 0.77 | 0.32 | 12.19c | 1.6 |
| Ex3 | 0.28 | 33.20c,d | 1.22 | 1.11 | 118.6 |
| Ex4 | 0.13 | 8.81 | 0.46 | 1.05 | 67.8 |
| “Knock-out”-Type Extracts | |||||
| Ex5 | 0.075 | 21.23c | 0.58 | 0.16 | 283.1 |
| Ex6 | 0.057 | 0.12 | 0.12 | 0.12 | 2.1 |
| Ex7 | 0.047 | 18.03c | 0.16 | 0.79 | 383.6 |
| Ex8 | 0.0016 | 0.056 | 0.12 | 0.34 | 35.0 |
Rank order based on 8-PN content with Ex1 representing the extract with the highest concentration and Ex8 that with the lowest level of 8-PN.
Authentic reference compounds were used as calibrants.
The high-concentration analytes were determined by a parallel UV-UHPLC method.
This content was confirmed independently by an orthogonal qHNMR method.
The Estrogenic Potency of DESIGNER Hop Extracts Is Mainly a Function of 8-Prenylnaringenin Content
The relatively high estrogenic activity (EC50: 7.0 nM) of the phytoestrogen 8-PN was confirmed using the estrogen-inducible alkaline phosphatase (AP) enzyme induction assay in Ishikawa endometrial cancer cells (Figure 2A, Table 2).29 In this assay, 8-PN is an agonist with an efficacy similar to that of 17β-estradiol (E2). In comparison, 6-PN and isoxanthohumol (IX) are about 100-fold weaker phytoestrogens (EC50: 0.4 and 1.4 μM, respectively), showing both lower potency and efficacy (Figure 2A, Table 2). As expected, XH did not show any estrogenic activity (Figure 2A). The different estrogenic potencies and efficacies of these four prenylated phenolic substances are in line with previous in vitro data.7,13,14,29,30 Although representing multicompound mixtures, the estrogenic potency of the hop DESIGNER extracts correlated significantly with their log 8-PN% concentration in this concentration range (Figures 2B and S1, Supporting Information; Pearson correlation: r = −0.95 with p < 0.0034, r2 = 0.91). However, two exceptions were observed: Ex5 and Ex7 demonstrated no estrogenicity, although they did not have the lowest 8-PN content of the produced DESIGNER extracts. In addition, extracts Ex1 and Ex2 demonstrated efficacy that was overproportional to their 8-PN content (Figure 2B). Collectively, this suggested that other compounds might affect the overall estrogenic activity of the H. lupulus extracts. Specifically, IX might add to their estrogenic activity, as IX is the major constituent in Ex1 and Ex2 (Table 1). To assess the reduced estrogenic activity of Ex5 and Ex7, the influence of the major hops phenol, XH, on 8-PN’s estrogenicity was analyzed.
Figure 2.
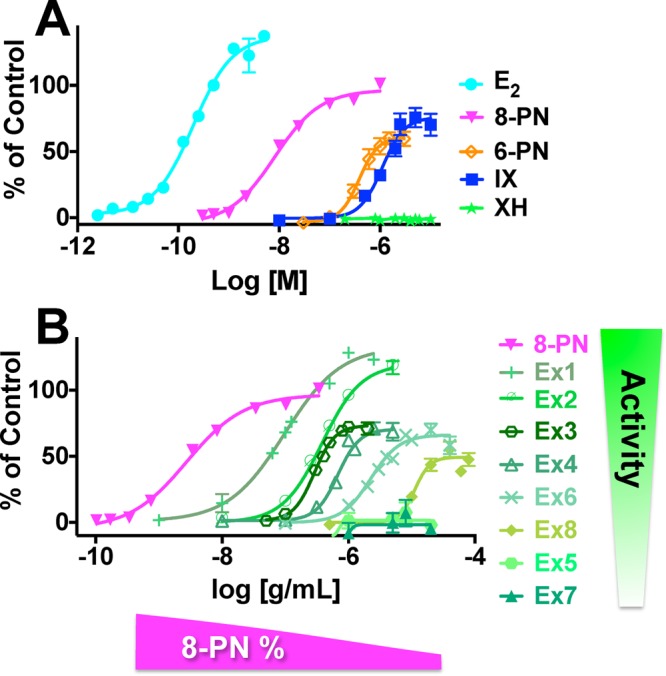
Estrogenic activity of hop DESIGNER extracts is mainly a function of 8-PN concentration. Induction of alkaline phosphatase in Ishikawa cells by (A) the major bioactive constituents in hops (8-PN, IX, XH, 6-PN) and E2; (B) 8-PN and the hop DESIGNER extracts. Results were normalized to the control (0.5 nM E2) and are shown as % of control.
Table 2. Alkaline Phosphatase (AP) Induction (Estrogenicity), Cytotoxicity, and NQO1 Induction Potency of the Hop DESIGNER Extracts and Hop Purified Constituents.
| Ishikawa
cells |
Hepa1c1c7
cells |
||||||
|---|---|---|---|---|---|---|---|
| potency | efficacy | cytotoxicity | NQO1
induction |
cytotoxicity | |||
| material | EC50 AP [μg/mL; μM]a | max AP induction | IC50 [μg/mL; μM]a | CDb [μg/mL; μM]a | slope | r2 | IC50 [μg/mL; μM]a |
| DESIGNER Extracts | |||||||
| Ex1 | 0.1 ± 0.02 | 136.4 ± 7.6 | 7.0 ± 0.7 | 6.8 ± 1.9 | 0.1 ± 0.01 | 0.80 | >20 |
| Ex2 | 0.4 ± 0.07 | 119.8 ± 11.4 | 28.1 ± 8.6 | 6.3 ± 2.1 | 0.1 ± 0.008 | 0.82 | >20 |
| Ex3 | 0.3 ± 0.008 | 74.7 ± 7.2 | 3.3 ± 0.4 | 0.8 ± 0.1 | 1.3 ± 0.03 | 0.98 | >20 |
| Ex4 | 0.7 ± 0.05 | 71.4 ± 8.2 | 10.3 ± 0.8 | 1.5 ± 0.2 | 0.6 ± 0.04 | 0.86 | 27.1 ± 2.9 |
| Ex5 | NAc | NAc | 4.8 ± 1.4 | 1.0 ± 0.1 | 0.8 ± 0.03 | 0.96 | >20 |
| Ex6 | 2.2 ± 0.6 | 66.6 ± 10.8 | >80 | 7.0 ± 0.6 | 0.1 ± 0.005 | 0.94 | >20 |
| Ex7 | NAc | NAc | 6.2 ± 0.4 | 1.8 ± 0.2 | 0.5 ± 0.03 | 0.85 | >20 |
| Ex8 | 12.0 ± 3.7 | 52.3 ± 11.2 | >80 | 11.7 ± 3.8 | 0.08 ± 0.004 | 0.89 | >20 |
| Compounds | |||||||
| 8-PN | 0.007 ± 0.004 | 97.1 ± 2.6 | 19.1 ± 4.7 | >30d | NA | >20d | |
| IX | 1.4 ± 0.4 | 80.7 ± 14.5 | 24.9 ± 3.7 | >50d | NA | 30.7 ± 2.9d | |
| XH | NAc | NAc | 4.2 ± 0.2 | 1.3 ± 0.7 | 1.3 ± 0.08 | 0.88 | 17.5 ± 2.3 |
| 6-PN | 0.4 ± 0.1 | 57.4 ± 10.2 | >5 | >30d | NA | >20d | |
| 17β-estradiol (E2)e | 0.0002 ± 0.00005 | 137.0 ± 2.5 | NA | NDf | ND | ND | |
| 4′-bromoflavone | NDf | ND | ND | 0.02 ± 0.001 | 19.6 ± 1.5 | 0.8 | >0.7 (>166)g |
Elevated Concentrations of Xanthohumol Reduce while Isoxanthohumol (IX) Increases the Estrogenic Efficacy of 8-Prenylnaringenin
The relative ability of both XH and IX to influence the estrogenic activity of 8-PN is shown in Figure 3 and displays an example of the interactions of phytoconstituents in a botanical extract. For example, Ex7 did not show any estrogenic activity in the AP assay, even though it contained an amount of 8-PN that is expected to display estrogenic activity (Figure 2B, Table 1). Indeed, pure 8-PN alone tested at a concentration equivalent to that in Ex7 (5 μg/mL) demonstrated the expected induction of AP activity by 40-fold (Figure 3A). However, addition of XH in concentrations equivalent to Ex7 reduced significantly the activity of 8-PN by about 50% (Figure 3A). As the estrogenic activity is further abolished in Ex7, other hops constituents might also reduce 8-PN’s estrogenicity (Figure 3A). The interactions between 8-PN and IX were analyzed by assaying concentrations of 8-PN and IX equivalent to 0.09 μg/mL of Ex1 individually and combined (Figure 3B). The results revealed that the weaker phytoestrogen, IX, increased the estrogenicity of 8-PN, even at low concentrations (158 nM) at which IX alone showed no estrogenicity (Figure 3B). As isoxanthohumol is reported to be a weak estrogen receptor (ER) ligand,14 the enhanced estrogenic activity caused by IX might be at least in part due to the metabolism of IX to 8-PN (Figure 1).31
Figure 3.
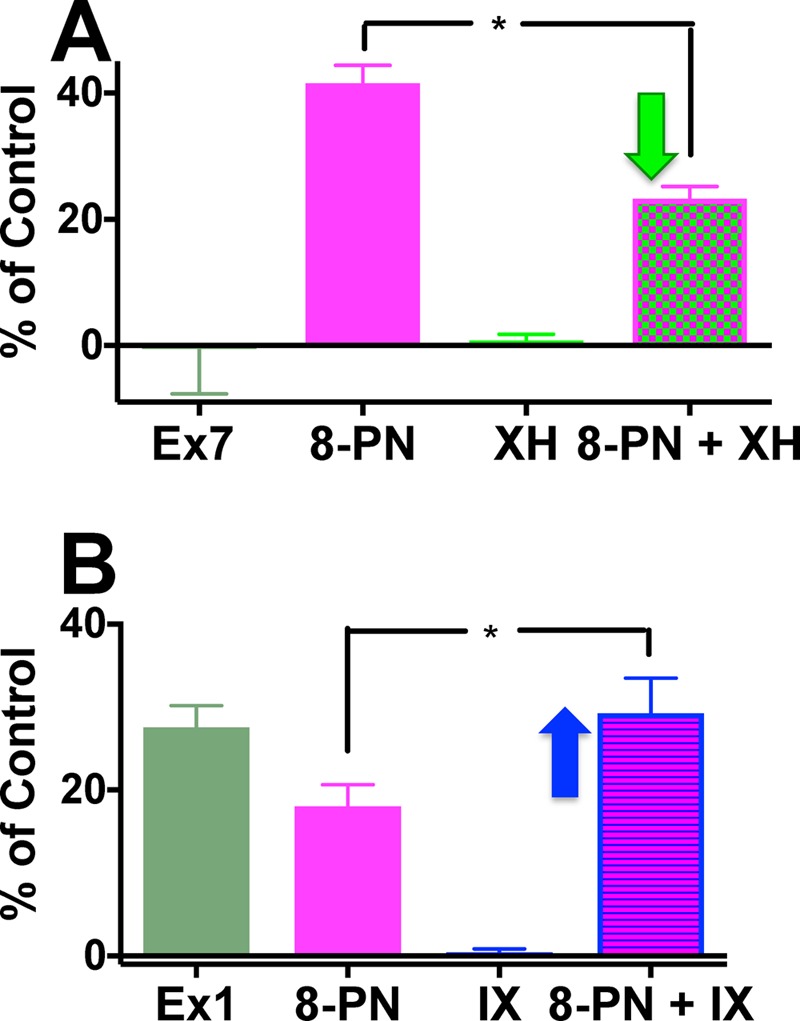
XH decreased and IX increased the estrogenic efficacy of 8-PN. (A) Equivalent concentrations of Ex7 (5 μg/mL) [8-PN (6.9 nM), XH (2.5 μM), and their combination]; (B) equivalent concentrations of 0.09 μg/mL of Ex1 [8-PN (2.5 nM), IX (158 nM), and their combination]. *Indicates significance of at least p < 0.05.
The Cytotoxicity of the DESIGNER Hop Extracts Is Mainly a Function of the Xanthohumol Concentration
In parallel, the cytotoxicity of the purified compounds and the DESIGNER extracts was studied in the sulforhodamine B (SRB) assay in Ishikawa cells. In general, the extracts were more potent in the estrogenic assay than in the SRB assay (Table 2). As expected, XH exhibited the strongest ( = lowest) IC50 value of all tested materials (IC50: 4.2 μM; Figure 4A,B, Table 2). Pure 8-PN and IX showed relatively low IC50 values of 19.1 and 24.9 μM, respectively. Importantly, the cytotoxicities (IC50 values) of the hop DESIGNER extracts correlated well with the log XH% concentration in the corresponding extracts in this concentration range (Pearson r = −0.88, p < 0.021, r2 = 0.78; Figures 4B and S2, Supporting Information). Ex1 was the only outlier in this correlation: although Ex1 contained lower XH levels than Ex4 (Table 1), it demonstrated higher cytotoxicity (Figure 4B, Table 2). As Ex1 contains an exceptional high amount of IX (62.4%, Table 1), IX was partially responsible for the observed cytotoxicities, likely because IX can undergo a reversible Michael addition32 to form XH (Figures 1 and S3A, Supporting Information). In addition, there is evidence that compounds other than IX and XH contributed to the cytotoxic effect of Ex1; for example, hops extracts contain a variety of other chalcones similar to XH.33,34
Figure 4.
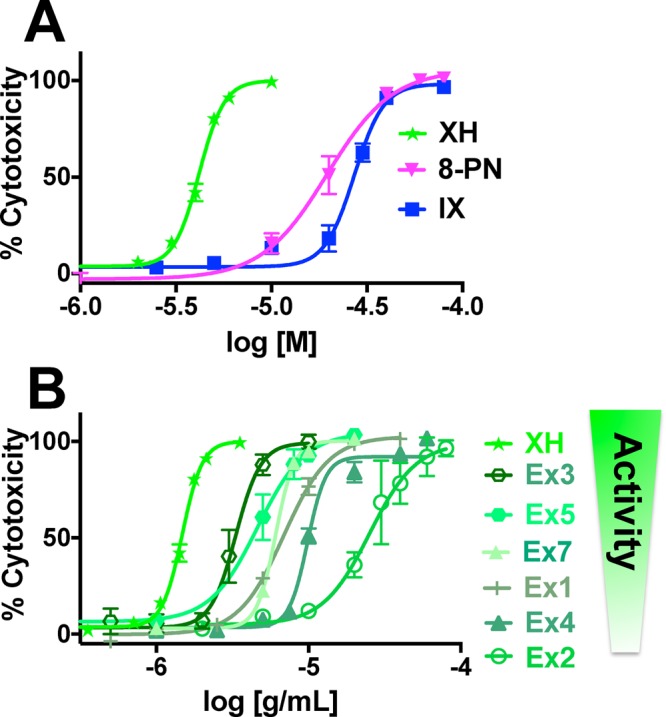
Cytotoxicity of the extracts depends mainly on the XH concentration. Cytotoxicity was performed in parallel to the AP assay with the SRB assay in Ishikawa cells. Cytotoxicity (A) of the pure compounds, 8-PN, IX, and XH; (B) of the DESIGNER hop extracts.
The Ability of the Hop DESIGNER Extracts To Induce NQO1 Activity Is Mainly a Function of the Xanthohumol Concentration
Hops and XH have previously been shown to induce the detoxification enzyme NQO1 in vitro and in vivo.16,21 NQO1 is a detoxification enzyme that can be used as a chemopreventive marker, because compounds that induce NQO1 typically also activate other chemopreventive pathways.35 The DESIGNER extracts were analyzed for their NQO1 activity in murine hepatoma cells (Hepa1c1c7) in relation to their XH content. Extracts with high XH content showed the expected higher NQO1 activity (Figure 5A, Tables 1 and 2). The CD values (concentration to double NQO1 activity) (Table 2) of the extracts showed significant correlation with the XH content (Pearson correlation: r = −0.95, p < 0.0003, r2 = 0.91) and exhibited good linear regression in this concentration range (Figure 5B). Ex3, with the highest XH concentration, showed the highest NQO1-inducing potential. In general, all extracts and XH were more active in the NQO1 induction assay compared to the cytotoxicity assays (Table 2). In comparison to cytotoxicity assays in the Ishikawa cells, the cytotoxicity of XH and of the hop extracts was lower in the Hepa1c1c7 hepatoma cell line (Table 2), likely due to higher GSH levels in liver cells.
Figure 5.
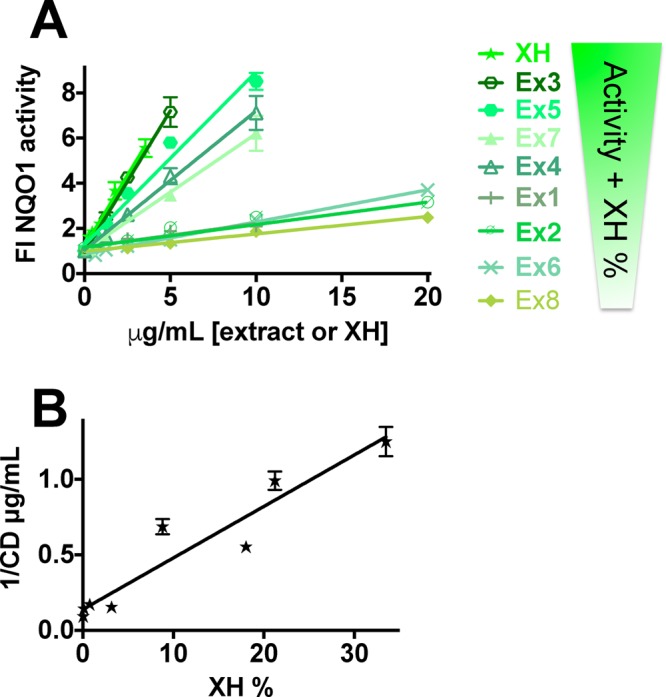
NQO1 induction was a function of XH concentration. (A) Linear regression of NQO1 induction activity in Hepa1c1c7 cells by DESIGNER hop extracts and XH. Results are shown as fold induction and are the means ± SEM of at least three independent determinations in duplicate. Linear regression was performed with Graph-Pad Prism 6. (B) Linear regression of the NQO1 induction potency, presented as 1/CD values of the hop extracts as a function of the corresponding XH% (r2 = 0.91). CD values (concentration to double NQO1 activity) were generated from three different independent evaluations in duplicate.
Modulation of Phytoconstituent Profiles for Pre- or Postmenopausal Women
Both specialized extraction techniques and the “knock-out/-down” technology can be used concurrently to optimize extracts toward desired biological activities (targeted application) and/or reduced unwanted effects (Scheme 1). For example, H. lupulus extracts containing estrogenic and chemopreventive compounds are suitable for the relief of postmenopausal symptoms, whereas extracts with only chemopreventive compounds are likely preferred for premenopausal women’s health (Scheme 2). One DESIGNER extract is Ex3 from spent hop cones, which is the hop material after extraction of bitter acids and essential oil. Ex3 has a high abundance of hop prenylated phenols and relatively high 8-PN and XH levels; thus, it exerts good estrogenic and detoxification enzyme-inducing properties (Figure 6A). Another improved extract is extract Ex5, which was reduced in both 8-PN and IX content via (semi)selective separation using countercurrent chromatography: the high XH level was retained, yielding an extract with minimal estrogenic but significant chemopreventive potential useful for premenopausal women (Figure 6B, Scheme 2). Similarly, the application profile of Ex7 involves low estrogenicity and relatively high NQO1 induction (Table 2).
Figure 6.
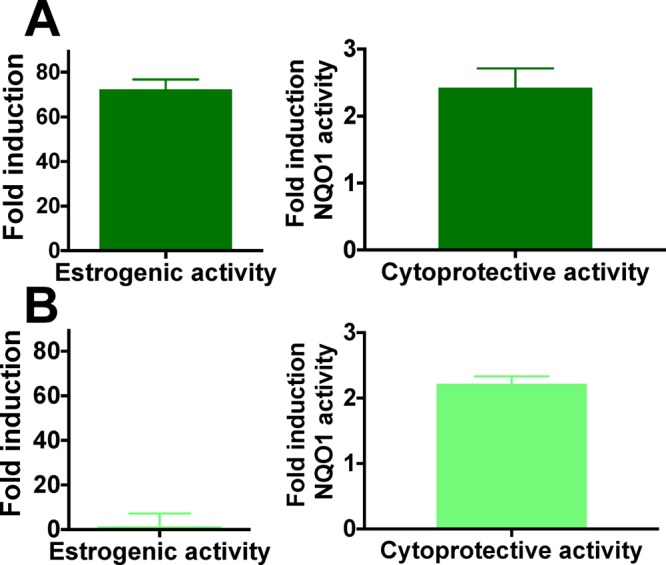
Comparison of estrogenic (AP fold induction, 1 μg/mL) and chemopreventive activity (NQO1 fold induction, 1.25 μg/mL) of (A) Ex3 (postmenopausal extract) and (B) Ex5 (premenopausal extract).
Polypharmacological Targets of Botanicals with the Example of Hops
Botanical extracts contain a wide array of bioactive constituents. There is growing evidence that their simultaneous action can be beneficial for managing and preventing complex chronic conditions, such as management of menopausal symptoms, through targeting diverse pharmacological targets (polypharmacology).36,37 However, several factors argue against herbal treatments: the concentration of bioactive constituents in crude botanical extracts is frequently low,2 the extracts contain a wide array of compounds, including constituents that might have antagonistic activities (simultaneous chemical and biological complexity), and instability of phytoconstituents is often observed, especially in biological systems (dynamic residual complexity).38 In addition, standardization is mostly performed to single and not multiple phytoconstituents.2 The present study illustrates how specialized (“designed”) standardized extracts can modulate and optimize bioactivity to a targeted application profile (Scheme 1). Hops extracts were used as an example, as their metabolomic composition and multiple bioactivities made them a suitable lead botanical when studying multifactorial chemical and biological standardization.2,39 The potent hops phytoestrogen 8-PN can, as a single entity, reduce hot flashes in vivo,17 but also leads to increased proliferation of estrogen-sensitive tissue in animal models.40,41 However, as 8-PN-containing hop extracts not been associated with uterotrophic properties, other H. lupulus constituents might counteract the proliferative activity of 8-PN.40 Hop extracts have also been associated with beneficial chemopreventive and antiproliferative effects, mainly due to the chalcone XH.16,42 Therefore, two main counteracting compounds in hops may be 8-PN, a minor flavanone constituent with nanomolar estrogenic potency,14,40 and XH, a major chalcone and micromolar chemopreventive agent.21,43 As the chalcones are in equilibrium with their isomeric flavanones via a Michael-type addition mechanism, XH isomerization can yield IX, which subsequently can be metabolized to 8-PN (Figure 1).31,44 Therefore, the biological activities of IX, XH, and 8-PN are interconnected by (bio)chemical processes. Another prenylated phenolic substance with distinct biological activity is 6-PN, the A-ring regioisomer of 8-PN (Figure 1). It demonstrated an ability to modulate the chemical estrogen carcinogenesis pathway and therefore might contribute to the chemopreventive properties of hop extracts for women.15,22 6-PN (Figure 1) favors the induction of the nongenotoxic 2-hydroxylation over the genotoxic 4-hydroxylation pathway of estradiol through binding to the AhR and preferential induction of P450 1A1, which is mainly responsible for the benign 2-hydroxylation pathway.15,45,46 P450 1A1/1B1 also metabolize the bioactivation of polycyclic aromatic hydrocarbons (PAHs) to carcinogenic metabolites.47 PAHs enhance their own bioactivation through induction of P450 1A1/1B1 through AhR. Interestingly, 6-PN has been demonstrated to be a partial AhR agonist, as it dose-dependently decreased the AhR-mediated xenobiotic response element activation of the strong AhR inducer 2,3,7,8-tetrachlorodibenzo-p-dioxin.15 Therefore, 6-PN will likely decrease PAHs-induced CYP1 activity and thereby their bioactivation; however, future experiments will need to delineate the activity of 6-PN on the bioactivation of PAHs in detail.
The DESIGNER Extract Concept
The investigated DESIGNER hop extracts26 contained different proportions and absolute amounts of the four key bioactive phenols XH, 8-PN, IX, and 6-PN. Analyzing these extracts for various biological end points provided insights into different biological targets (poly/oligo) as well as the chemical variety of hops. In order to prepare extracts with enhanced, targeted activities, various enrichment steps of certain bioactive compounds were performed (Scheme 1). An initial enrichment was achieved by using a spent hops extract, which is depleted of the lipophilic bitter acids, prenylated phloroglucinols (“resins”), and essential oils, the main H. lupulus constituents used in beer brewing.16 Further enrichment in IX, for example, was achieved through isomerization processes in alkaline solution. Additional specification of the desired bioactivity can be achieved via CCS-based “knocking-down” of target compounds employing the recently established DESIGNER method.26 The distinctive liquid-only nature of CCS enables the required loss-free separation that is not achievable with other chromatographic techniques. CCS utilizes immiscible liquid–liquid two-phase solvent systems and can achieve the targeted separation of a metabolite from a complex mixture in a loss-free manner by targeting the metabolite’s partition coefficient (K value).28 Moreover, choosing different (ideally, orthogonal) solvent systems can overcome imperfect CCS separations, characterized by the inevitable peak overlap in metabolomic mixtures.48 As part of the DESIGNER extract procedure, the extracts will be standardized to multiple bioactive compounds. “Knock-out” extracts have been generated previously using immunoaffinity chromatography.49 Advantages of the CCS-based chemical subtraction method are the absence of cross reactivity and substantially higher loading capacities, resulting in increased production efficiency.26,50
Multifactorial Impact on Estrogenic Activity
As demonstrated, the ability to design extracts selectively and with defined intercompound concentration relationships of active principles makes the DESIGNER extracts a potentially unique pharmacological tool. For example, the present study has revealed that the estrogenic properties of DESIGNER and other specialized extracts of H. lupulus clearly correlate with their respective 8-PN content (Figures 2B and S1, Supporting Information) and allowed the establishment of this correlation despite the relatively low 8-PN concentrations (Table 1). The interaction studies with IX, XH, and 8-PN revealed an antagonizing effect at high XH concentrations on the estrogenic activity of 8-PN (Ex5 and Ex7, Figure 3A). The mechanistic reason for this observation is currently unknown. Apoptotic and antiproliferative activities in different cell lines including ER (+) breast cancer cells (MCF-7) have been described for XH.34,43 However, at concentrations where XH exhibits only weak cytotoxicity, it showed antiestrogenic properties in the AP assay (Figure S4, Supporting Information). Inhibition of estradiol-induced AP activity by XH has been described earlier,30 although ER-binding properties were not observed.14 Interestingly, XH has been reported to inhibit the growth of ERα-positive breast cancer cells through reactivation of the tumor suppressor protein prohibitin 2, thus leading to suppression of E2-signaling pathways.51 In contrast to XH, IX may enhance the estrogenic efficacy of 8-PN (Figure 3B). Further studies are required to delineate the underlying mechanism of IX action. Isoxanthohumol itself is only a weak ER agonist.13,14 However, this compound is converted to 8-PN via P450 1A2 in metabolically active cells and in vivo by gut microbiota (Figure 1).31,44 The observed estrogenic activity of IX in Ishikawa cells (Figure 2A) might in part be due to such metabolism, as it has been described for liver cells.31 In the case of hops, the three tested bioactivities, estrogenicity, cytotoxicity, and chemopreventive activities, could be attributed mainly to two major compounds, 8-PN and XH, respectively. Both compounds were clearly correlated with the respective bioactivity (Figures 5B, S1, and S2, Supporting Information). However, the efficacy of herbal medicine is often seen as the combined action of multiple constituents leading to “synergistic” (nonlinear; overadditive) effects, as it has been demonstrated for the antimicrobial activity in Hydrastis canadensis.52 “Knock-out” studies with this botanical for example might lead to very different outcomes by lacking a linear dose–response relationship with one main bioactive compound. One asset of the DESIGNER extract approach is that through “knock-out/-down” of certain compounds in the otherwise complete extract matrix, synergistic, additive, or antagonistic effects would become apparent. Alternatively, as in the case of hops, major constituents can be established as the bioactive compounds.
Chemopreventive Properties of Xanthohumol
In contrast to the antiproliferative/cytotoxic activity of XH (IC50: 17.5 μM), its chemopreventive activity, such as the induction of NQO1 [CD: 1.3 μM, (Table 2)], is in the low μM range; therefore, the chemopreventive activity will likely prevail in vivo.2 Indeed, various animal studies have confirmed the NQO1 induction activity and anti-inflammatory effects of XH in vivo.16,19,53 A recent human intervention trial also confirmed DNA-protecting properties of XH.54 The chemopreventive properties of XH are mainly due to its Michael acceptor structure (Figure 1), which leads to the covalent modification of proteins, thus activating detoxification pathways, such as the Keap1/Nrf2/ARE (Kelch-like ECH-associated protein 1; nuclear factor (erythroid-derived 2)-like 2; antioxidant response element) pathway.16,30,43,55 At higher concentrations, XH may bind covalently to other enzymes, causing apoptosis and cytotoxicity. One enzyme that is regulated through the ARE is NQO1, which detoxifies quinones to the respective hydroquinone.35,56 XH has been shown to reduce menadione-induced DNA damage through upregulating NQO1, demonstrating cytoprotective activity.21
Botanical Health Products with Targeted Application for Specific Populations
The present study illustrates how specialized (“designed”) extracts can modulate and optimize bioactivity to a targeted application profile using chemical subtraction by CCS (Scheme 1). For example, at certain times in a woman’s life, estrogenic effects of botanical extracts might be preferred (menopause), while at other times estrogenic properties might be undesirable (premenopause). Extracts that exert both estrogenic and chemopreventive properties, such as Ex3, might be preparations with enhanced safety and potential relief of postmenopausal symptoms (Scheme 2, Figure 6A; postmenopausal extract).7 Ex3 has been proven to be safe in different animal studies and showed moderate activity in an osteoporosis animal model.57 In the case where certain bioactivities might be unintended, it is desirable to “knock-out/-down” the underlying bioactive compounds, thereby reducing unwanted or even adverse effects. For example, premenopausal women may seek natural chemopreventive agents, such as hop extracts, without estrogenic potential. Therefore, depletion of the major phytoestrogen 8-PN, such as in the H. lupulus extracts Ex5 and Ex7, may be desirable (Scheme 2). Simultaneous reduction of the 8-PN and IX content in both extracts was achieved via CCS of Ex3, while leaving the XH content nearly unaffected (slight reduction) and increasing the XH/8-PN ratio (Table 1). As a result, Ex5 and Ex7 showed no estrogenic activity (Figure 6B, Figure 2B), but retained the NQO1 induction activity of their precursor extract, Ex3, due to the nearly unaffected XH content (Figures 5A and 6B, premenopausal extract). It is important to mention that the actual concentrations of 8-PN achievable in vivo also depend on the level of XH and IX in the extract. IX can be metabolized to the estrogenic 8-PN, and IX’s equilibrium with XH can replenish the much less abundant pro-phytoestrogen IX (Figure 1). In fact, an in vivo and a clinical study have demonstrated that relatively more 8-PN can be detected in the serum compared to the expected amount based on the 8-PN level in the administered extract, likely as a result of metabolic formation of 8-PN from IX.16,58 A clinical pharmacokinetic study administering pure XH revealed that XH and IX conjugates were the major metabolites, indicating in vivo cyclization of XH to IX.59 However, free and 8-PN conjugates were not detected in most subjects, and only 8-PN conjugates were determined as minor metabolites in some subjects. These studies were contrary to rat studies, which showed higher 8-PN formation.60 Interindividual variability in metabolism of IX to 8-PN has been demonstrated previously.44,58,61 For example, a dietary intervention trial determined that 60% of postmenopausal women receiving a hop dietary supplement were poor “8-PN producers” and 15% strong “8-PN producers”.61 Similarly, polymorphism in the CYP1A1 gene can lead to interindividual differences in the amount of IX metabolism to 8-PN.58 On the basis of this information, it is possible that after clinical administration of a “knock-out/-down” extract that is depleted of the phytoestrogen 8-PN and its precursor, IX (Ex5), 8-PN conjugates may be detected in the serum through XH cyclization to IX and metabolism to 8-PN. However, as determined in the study by Legette et al. (2014),59 8-PN is likely a minor metabolite and might therefore likely not reach pharmacologically active concentrations. Future in vivo and clinical studies are required to determine the best relative and absolute concentrations for XH and 8-PN to optimize the balance of efficacy and safety. The optimal holistic wellness extract might have balanced biological activity from estrogenic and chemopreventive compounds, based on multiple constituents leading to polypharmacological pathways and ultimately chemoprevention (Scheme 2).
Concluding Remarks
The present study exemplifies how chemically complex botanical extracts with numerous pharmacological effects either can be transformed into pharmacological tools that reveal otherwise invisible compound/compound interactions or can be designed chemically toward a desired bioactivity profile for certain clinical purposes. One notable aspect of the present study is that it evaluated a widely used botanical extract in a panel of pharmacological parameters and for an array of marker constituents, making it multifactorial in both the biological and chemical domains. By maintaining the chemical context of an otherwise intact, metabolomic extract matrix, the applied DESIGNER methodology enabled a new level in the combined chemical and biological standardization of botanical products. The ability of specialized extracts to modulate bioactivities via phytochemical design was demonstrated for hops (H. lupulus) as prototype of a botanical that is used widely including the U.S. and Europe. The design of extracts with varying contents of the estrogen 8-PN and the chemopreventive compound XH as lead active principles yielded extracts that were different in bioactivity balances (Scheme 2). Ex3 of these, with balanced estrogenic and chemopreventive activities, was designated as a “menopausal extract” and Ex5 and Ex7, with mainly chemopreventive properties, were designated as “pre-menopausal extracts”. The concept of extract design via CCS-based chemical subtraction can be seen as a tool to advance dietary supplements and phytomedicines to more rational, botanical-derived remedies with more targeted applications (Scheme 1). This approach may also be used more widely to reduce interfering or unwanted bioactivities in botanical extracts, potentially leading to the production of natural remedies with increased efficacy and safety.
Experimental Section
General Experimental Procedures
All chemicals were purchased from Thermo Fisher Scientific (Hanover Park, IL, USA) or Sigma-Aldrich (St. Louis, MO, USA) unless otherwise indicated. All media for cell culture were purchased from Invitrogen (Carlsbad, CA, USA). Fetal bovine serum (FBS) was acquired from Gemini Bio-Products (West Sacramento, CA, USA), and 4′-bromoflavone (BF) from Toronto Research Chemicals (North York, Ontario, Canada). General procedures involved in the preparation and analysis of the DESIGNER extracts have been described previously.26
Plant Material and Specialized Extracts
Two standardized, XH-enriched (Ex3, Ex4) extracts of female inflorescences of Humulus lupulus and two IX-enriched extracts (Ex1, Ex2) of the same material were provided by Hopsteiner (Mainburg, Germany, and New York, NY, USA). The strobili were first bulk extracted with food-grade ethanol (55 °C, 1 h). After solvent evaporation, the extract was dispersed in diatomaceous earth, and the mixture was bulk-extracted with supercritical CO2 (280 bar, 50 °C, 5 h) to yield two materials: the bitter acid extract (not used in this study) and the spent hop extract dispersed on the diatomaceous earth. For the preparation of Ex4, the diatomaceous earth was removed by solubilization of the extractibles with ethanol, filtration, and evaporation to dryness in vacuo. Ex3 was a 2:1 mixture of Ex4 and a XH-enriched extract containing 82% XH, prepared according to the process documented in ref (62). The IX-enriched extracts (Ex1, Ex2) were produced by dissolving the two XH-enriched bulk extracts in 5% NaOH solution and stirring of the mixture for 1 h at ambient temperature (20 °C). The precipitate formed after acidification with sulfuric acid to pH 5 was filtered off and dried in air for 48 h to yield Ex1 and Ex2.
DESIGNER Extracts
The XH-enriched H. lupulus extract (Ex3) was used as the starting material for the DESIGNER extracts and has been deposited as specimen BC402 in the UIC Botanical Center (College of Pharmacy, UIC, Chicago, IL, USA). The specimens of Ex1, Ex2, and Ex4 were deposited under the codes BC #690–692 (S5, Supporting Information). The contents (in %) of the four markers or target compounds in these standardized extracts were determined by either UHPLC-UV, quantitative 1H NMR aided with 1H iterative full spin analysis (qHNMR-HiFSA), or LC-MS/MS (Table 1). The DESIGNER extracts were prepared as “knock-down”/“knock-out” extracts, as described previously.26 Quantitative LC-MS/MS analysis used structurally verified reference compounds as calibrants and was performed to determine the content of XH, 8-PN, 6-PN, and IX in the DESIGNER extracts (w/w% of the spent hops extract, Table 1). UHPLC-UV chromatograms of the “knock-out” extracts, Ex5–Ex8, and the enriched extract, Ex3, have been published previously.26
Purified Constituents
Racemic 8-PN was synthesized as described previously.40 Pure XH was isolated from the enriched hop extract Ex3, which was used for the preparation of DESIGNER extracts. XH was further purified with crystallization. IX was chemically converted from XH prior to further CCS purification. Racemic 6-PN was purchased from Sigma. Its planar structure was confirmed simultaneously during purity determination with a qNMR method, and the ECD spectrum of 6-PN was measured on a JASCO 815 CD instrument (Easton, MD, USA) in methanol. The purity of all compounds was determined by the 100% quantitative 1H NMR method63 and expressed as % w/w, as follows: 8-PN 95.6%, XH 98.7%, IX 97.6%, and 6-PN 98.5%.
LC-MS Analysis
The content of prenylated phenols in the DESIGNER extracts was determined using a previously published LC/MS-MS method.7,64
Cell Culture
The Ishikawa cell line was provided by Dr. R. B. Hochberg (Yale University, New Haven, CT, USA) and was maintained in Dulbecco’s modified Eagle’s medium (DMEM/F12) containing 1% sodium pyruvate, 1% nonessential amino acids, 1% Glutamax, 0.05% insulin, and 10% heat-inactivated FBS (Gemini Bioproducts).65−67 The Ishikawa cell line is a well-established ERα (+) endometrial cancer cell line for the evaluation of estrogens and antiestrogens.65,67 Two days before treating the cells, the medium was replaced with phenol-red-free DMEM/F12 medium containing charcoal/dextran-stripped FBS (Gemini Bioproducts) and supplements as mentioned above. The cell line was authenticated via determination of the short tandem repeat profile and is in accordance with the Ishikawa cell line according to the Health Protection Agency Culture Collection in the UK. Hepa1c1c7 murine hepatoma cells were supplied by Dr. J. P. Whitlock, Jr. (Stanford University, Stanford, CA, USA). Cells were maintained in α-minimum essential medium supplemented with 1% penicillin–streptomycin and 10% FBS (Gemini Bioproducts) and incubated in 5% CO2 at 37 °C. DMSO concentrations for all cell culture assays were less than 0.1%.
Induction of an Estrogen-Responsive Alkaline Phosphatase Enzyme in Ishikawa Cells
The protocol by Pisha et al. was used as described previously.67 Ishikawa cells were preincubated in estrogen-free medium for 24 h and plated in 96-well plates (3.9 × 104 cells/well). After another 24 h, the test samples were dissolved in DMSO (final concentration <0.1%), and the positive control, estradiol (0.5 nM), and the negative control, DMSO, were added. For the determination of antiestrogenic activity, 1 nM estradiol was added to the medium, and 4-hydroxytamoxifen (5 μM) was used as positive control. The plates were incubated at 37 °C for 96 h. Cells were washed with phosphate-buffered saline and lysed by adding 50 μL of 0.01% Triton X-100 in 0.1 M Tris buffer (pH 9.8), followed by one freeze and thaw cycle at −80 and 37 °C, respectively. p-Nitrophenol phosphate (phosphatase substrate; 2.69 mM) was added to each well, and the alkaline phosphatase activity was measured by reading the formation of p-nitrophenol at 405 nm every 15 s with a 10 s shake between readings for 16 readings using a Power Wave 200 microplate scanning spectrophotometer (Bio-Tek Instruments, Winooski, VT, USA). The maximum slope of the kinetic curves was calculated for each experimental well. The percent induction of alkaline phosphatase for every treatment, compared to that of the estradiol control (0.5 nM), was calculated using eq 1 as estrogenic activity. Antiestrogenic activity was calculated using eq 2 as the percent induction of alkaline phosphatase compared to background induction control. Except when XH was present at certain concentrations, none of the extracts or compounds showed antiestrogenic properties.
| 1 |
| 2 |
Sulforhodamine B Assay
In parallel to the alkaline phosphatase induction/inhibition assay in Ishikawa cells, the cellular protein content and, thus, the cytotoxicity of the hop extracts and purified compounds were determined with a sulforhodamine B (SRB) assay, as described previously.68 Briefly, Ishikawa cells, at 1.4 × 104 cells/well, were plated into 96-well plates, and, 24 h later, cells were treated with the same test samples and concentrations as used in the alkaline phosphatase induction/inhibition assay. Plates were incubated at 37 °C for 96 h. Subsequently, cells were fixed with trichloroacetic acid and then stained with 0.4% SRB dissolved in 1% acetic acid. SRB was removed, and the cells were rinsed four times with 1% acetic acid to remove unbound dye. Next, the plates were air-dried, and bound dye was solubilized with 10 mM unbuffered Tris base (pH 10.5). The optical density was determined in a Power Wave 200 microplate scanning spectrophotometer (Bio-Tek Instruments) at 490 nm.
In Vitro NQO1 Assay
Induction of NQO1 activity was assessed in Hepa1c1c7 cells. The cells were seeded in 96-well plates at a density of 1.0 × 104 cells/mL in 190 μL of medium. After 24 h of incubation, the test samples were added to each well and the cells incubated for an additional 48 h. Subsequently, the NQO1 and cytotoxicity assays were performed as previously described.21
Statistical Analysis
One-way ANOVA with Dunnett’s post test was performed using GraphPad Prism version 6.0c for Macintosh (GraphPad Software, San Diego, CA, USA). In all cases, a p value of at least <0.05 was considered to indicate significance. All experimental values are expressed as means ± SEM of at least three independent determinations in triplicate (AP and cytotoxicity assay) or duplicate (NQO1 induction assay).
Acknowledgments
We gratefully acknowledge Hopsteiner (S. S. Steiner, New York, NY, and Steiner Hopfen GmbH, Mainburg, Germany) for providing the hop extract as well as H. Schwarz for his support and helpful discussions. We are thankful to J. P. Whitlock, Jr., for providing the Hepa1c1c7 cells and to R.B. Hochberg for providing the Ishikawa cells. The authors also thank A. Minassi and G. Appendino (Università del Piemonte Orientale, Novara, Italy) for the synthesis of 8-PN reference material.40 This work was supported through grants P50 AT000155 and U41 AT 08706 by NCCIH and ODS.. The funders had no role in the study design, data collection and analysis, the decision to publish, or the preparation of the manuscript.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.jnatprod.7b00284.
Estrogenic activity of the DESIGNER hop extracts correlate with the 8-PN concentration (S1); cytotoxicity of the hop DESIGNER extracts correlates with the XH concentration (S2); cytotoxicity of the Hop DESIGNER extracts is mainly but not only dependent on the XH concentration (S3); XH depicts antiestrogenic activities before it shows cytotoxicity (S4); names and codes of DESIGNER extracts with their deposited specimen codes (S5) (PDF)
Author Present Address
§ René F. Ramos Alvarenga, School of Pharmacy, University of Wisconsin−Madison, 777 Highland Avenue, Madison, Wisconsin 53705 United States.
The authors declare the following competing financial interest(s): M.B. is director of research and development/analytics of Hopsteiner HHV GmbH. The other authors declare no competing financial interest.
Supplementary Material
References
- Smith T.; Lynch M. E.; Johnson J.; Kawa K.; Bauman H.; Blumenthal M. Herbalgram 2015, 107, 52–59. [Google Scholar]
- Dietz B. M.; Hajirahimkhan A.; Dunlap T.; Bolton J. L. Pharmacol. Rev. 2016, 68, 1026–1073. 10.1124/pr.115.010843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordell G. A. Phytochem. Lett. 2015, 11, 332–346. 10.1016/j.phytol.2014.09.003. [DOI] [Google Scholar]
- Global Industry Analysts, I. Herbal Supplements and Remedies: A Global, Strategic Business Report. http://www.strategyr.com/MarketResearch/Herbal_Supplements_and_Remedies_Market_Trends.asp (June 26, 2016).
- Reddy A. S.; Zhang S. Expert Rev. Clin. Pharmacol. 2013, 6, 41–47. 10.1586/ecp.12.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox P. A. Science 2000, 287, 44–45. 10.1126/science.287.5450.44. [DOI] [PubMed] [Google Scholar]
- Krause E. C.; Yuan Y.; Hajirahimkhan A.; Dong H.; Dietz B. M.; Nikolic D.; Pauli G. F.; Bolton J. L.; van Breemen R. B. Biomed. Chromatogr. 2014, 29, 729–734. 10.1002/bmc.3177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmler C.; Chen S. N.; Anderson J.; Lankin D. C.; Phansalkar R.; Krause E.; Dietz B. M.; Bolton J. L.; Nikolic D.; van Breemen R. B.; Pauli G. F.. HerbalEGram 2015, 12. [PMC free article] [PubMed]
- Wagner H. Fitoterapia 2011, 82, 34–37. 10.1016/j.fitote.2010.11.016. [DOI] [PubMed] [Google Scholar]
- Farnsworth N. R.; Krause E. C.; Bolton J. L.; Pauli G. F.; van Breemen R. B.; Graham J. G. Am. J. Clin. Nutr. 2008, 87, 504S–508S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadwick L. R.; Pauli G. F.; Farnsworth N. R. Phytomedicine 2006, 13, 119–131. 10.1016/j.phymed.2004.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan S.; Kalita J.; Pocock V.; Heyerick A.; De Cooman L.; Rong H.; De Keukeleire D. Reproduction 2002, 123, 235–242. 10.1530/rep.0.1230235. [DOI] [PubMed] [Google Scholar]
- Milligan S. R.; Kalita J. C.; Pocock V.; Van De Kauter V.; Stevens J. F.; Deinzer M. L.; Rong H.; De Keukeleire D. J. Clin. Endocrinol. Metab. 2000, 85, 4912–4915. 10.1210/jcem.85.12.7168. [DOI] [PubMed] [Google Scholar]
- Overk C. R.; Yao P.; Chadwick L. R.; Nikolic D.; Sun Y.; Cuendet M. A.; Deng Y.; Hedayat A. S.; Pauli G. F.; Farnsworth N. R.; van Breemen R. B.; Bolton J. L. J. Agric. Food Chem. 2005, 53, 6246–6253. 10.1021/jf050448p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S.; Dunlap T. L.; Howell C. E.; Mbachu O. C.; Rue E. A.; Chen S. N.; Pauli G. F.; Dietz B. M.; Bolton J. L. Chem. Res. Toxicol. 2016, 29, 1142–1150. 10.1021/acs.chemrestox.6b00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz B. M.; Hagos G. K.; Eskra J. N.; Wijewickrama G. T.; Anderson J. R.; Nikolic D.; Guo J.; Wright B.; Chen S. N.; Pauli G. F.; van Breemen R. B.; Bolton J. L. Mol. Nutr. Food Res. 2013, 57, 1055–1066. 10.1002/mnfr.201200534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowe J.; Li X. F.; Kinsey-Jones J.; Heyerick A.; Brain S.; Milligan S.; O’Byrne K. J. Endocrinol. 2006, 191, 399–405. 10.1677/joe.1.06919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan S. R.; Kalita J. C.; Heyerick A.; Rong H.; De Cooman L.; De Keukeleire D. J. Clin. Endocrinol. Metab. 1999, 84, 2249–2252. 10.1210/jcem.84.6.5887. [DOI] [PubMed] [Google Scholar]
- Dorn C.; Heilmann J.; Hellerbrand C. Int. J. Clin. Exp. Pathol. 2012, 5, 29–36. [PMC free article] [PubMed] [Google Scholar]
- Harikumar K. B.; Kunnumakkara A. B.; Ahn K. S.; Anand P.; Krishnan S.; Guha S.; Aggarwal B. B. Blood 2009, 113, 2003–2013. 10.1182/blood-2008-04-151944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz B. M.; Kang Y. H.; Liu G.; Eggler A. L.; Yao P.; Chadwick L. R.; Pauli G. F.; Farnsworth N. R.; Mesecar A. D.; van Breemen R. B.; Bolton J. L. Chem. Res. Toxicol. 2005, 18, 1296–1305. 10.1021/tx050058x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemachandra L. P.; Madhubhani P.; Chandrasena R.; Esala P.; Chen S. N.; Main M.; Lankin D. C.; Scism R. A.; Dietz B. M.; Pauli G. F.; Thatcher G. R.; Bolton J. L. Cancer Prev. Res. 2012, 5, 73–81. 10.1158/1940-6207.CAPR-11-0348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda C. L.; Aponso G. L.; Stevens J. F.; Deinzer M. L.; Buhler D. R. Cancer Lett. 2000, 149, 21–29. 10.1016/S0304-3835(99)00328-6. [DOI] [PubMed] [Google Scholar]
- Krajka-Kuzniak V.; Paluszczak J.; Baer-Dubowska W. Toxicol. In Vitro 2013, 27, 149–156. 10.1016/j.tiv.2012.10.008. [DOI] [PubMed] [Google Scholar]
- Magalhaes P. J.; Carvalho D. O.; Cruz J. M.; Guido L. F.; Barros A. A. Nat. Prod. Commun. 2009, 4, 591–610. [PubMed] [Google Scholar]
- Ramos Alvarenga R. F.; Friesen J. B.; Nikolic D.; Simmler C.; Napolitano J. G.; van Breemen R.; Lankin D. C.; McAlpine J. B.; Pauli G. F.; Chen S. N. J. Nat. Prod. 2014, 77, 2595–2604. 10.1021/np500376g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirma N. B.; Tekmal R. R. J. Steroid Biochem. Mol. Biol. 2012, 131, 76–82. 10.1016/j.jsbmb.2011.11.005. [DOI] [PubMed] [Google Scholar]
- Friesen J. B.; McAlpine J. B.; Chen S. N.; Pauli G. F. J. Nat. Prod. 2015, 78, 1765–1796. 10.1021/np501065h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajirahimkhan A.; Simmler C.; Yuan Y.; Anderson J. R.; Chen S. N.; Nikolic D.; Dietz B. M.; Pauli G. F.; van Breemen R. B.; Bolton J. L. PLoS One 2013, 8, e67947. 10.1371/journal.pone.0067947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhäuser C.; Alt A.; Heiss E.; Gamal-Eldeen A.; Klimo K.; Knauft J.; Neumann I.; Scherf H. R.; Frank N.; Bartsch H.; Becker H. Mol. Cancer Ther. 2002, 1, 959–969. [PubMed] [Google Scholar]
- Guo J.; Nikolic D.; Chadwick L. R.; Pauli G. F.; van Breemen R. B. Drug Metab. Dispos. 2006, 34, 1152–1159. 10.1124/dmd.105.008250. [DOI] [PubMed] [Google Scholar]
- Johansson M. H. Mini-Rev. Med. Chem. 2012, 12, 1330–1344. 10.2174/13895575112091330. [DOI] [PubMed] [Google Scholar]
- Chadwick L. R.; Nikolic D.; Burdette J. E.; Overk C. R.; Bolton J. L.; van Breemen R. B.; Froehlich R.; Fong H. H. S.; Farnsworth N. R.; Pauli G. F. J. Nat. Prod. 2004, 67, 2024–2032. 10.1021/np049783i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda C. L.; Stevens J. F.; Helmrich A.; Henderson M. C.; Rodriguez R. J.; Yang Y. H.; Deinzer M. L.; Barnes D. W.; Buhler D. R. Food Chem. Toxicol. 1999, 37, 271–285. 10.1016/S0278-6915(99)00019-8. [DOI] [PubMed] [Google Scholar]
- Cuendet M.; Oteham C. P.; Moon R. C.; Pezzuto J. M. J. Nat. Prod. 2006, 69, 460–463. 10.1021/np050362q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins A. L. Nat. Chem. Biol. 2008, 4, 682–690. 10.1038/nchembio.118. [DOI] [PubMed] [Google Scholar]
- Li S.; Zhang B.; Zhang N. BMC Syst. Biol. 2011, 5, S10. 10.1186/1752-0509-5-S1-S10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmler C.; Hajirahimkhan A.; Lankin D. C.; Bolton J. L.; Jones T.; Soejarto D. D.; Chen C.; Pauli G. F. J. Agric. Food Chem. 2013, 61, 2146–2157. 10.1021/jf304445p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyerick A.; Vervarcke S.; Depypere H.; Bracke M.; De Keukeleire D. Maturitas 2006, 54, 164–175. 10.1016/j.maturitas.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Overk C. R.; Guo J.; Chadwick L. R.; Lantvit D. D.; Minassi A.; Appendino G.; Chen S. N.; Lankin D. C.; Farnsworth N. R.; Pauli G. F.; van Breemen R. B.; Bolton J. L. Chem.-Biol. Interact. 2008, 176, 30–39. 10.1016/j.cbi.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helle J.; Kraker K.; Bader M. I.; Keiler A. M.; Zierau O.; Vollmer G.; Welsh J.; Kretzschmar G. Mol. Cell. Endocrinol. 2014, 392, 125–135. 10.1016/j.mce.2014.05.014. [DOI] [PubMed] [Google Scholar]
- Hajirahimkhan A.; Simmler C.; Dong H.; Lantvit D.; Li G.; Chen S.-N.; Nikolic D.; Pauli G. F.; van Breemen R. B.; Dietz B. M.; Bolton J. L. Chem. Res. Toxicol. 2015, 28, 2130–2141. 10.1021/acs.chemrestox.5b00310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venturelli S.; Burkard M.; Biendl M.; Lauer U. M.; Frank J.; Busch C. Nutrition 2016, 32, 1171–1178. 10.1016/j.nut.2016.03.020. [DOI] [PubMed] [Google Scholar]
- Possemiers S.; Bolca S.; Grootaert C.; Heyerick A.; Decroos K.; Dhooge W.; De Keukeleire D.; Rabot S.; Verstraete W.; Van de Wiele T. J. Nutr. 2006, 136, 1862–1867. [DOI] [PubMed] [Google Scholar]
- Zahid M.; Kohli E.; Saeed M.; Rogan E.; Cavalieri E. Chem. Res. Toxicol. 2006, 19, 164–172. 10.1021/tx050229y. [DOI] [PubMed] [Google Scholar]
- Ziegler R. G.; Fuhrman B. J.; Moore S. C.; Matthews C. E. Steroids 2015, 99, 67–75. 10.1016/j.steroids.2015.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebert D. W.; Dalton T. P.; Okey A. B.; Gonzalez F. J. J. Biol. Chem. 2004, 279, 23847–23850. 10.1074/jbc.R400004200. [DOI] [PubMed] [Google Scholar]
- Friesen J. B.; Ahmed S.; Pauli G. F. J. Chromatogr. A 2015, 1377, 55–63. 10.1016/j.chroma.2014.11.085. [DOI] [PubMed] [Google Scholar]
- Yuan C. S.; Tanaka H. Curr. Drug Discovery Technol. 2011, 8, 32–41. 10.2174/157016311794519992. [DOI] [PubMed] [Google Scholar]
- Chen S. N.; Turner A.; Jaki B. U.; Nikolic D.; van Breemen R. B.; Friesen J. B.; Pauli G. F. J. Pharm. Biomed. Anal. 2008, 46, 692–698. 10.1016/j.jpba.2007.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimaru T.; Komatsu M.; Tashiro E.; Imoto M.; Osada H.; Miyoshi Y.; Honda J.; Sasa M.; Katagiri T. Sci. Rep. 2015, 4, 7355. 10.1038/srep07355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junio H. A.; Sy-Cordero A. A.; Ettefagh K. A.; Burns J. T.; Micko K. T.; Graf T. N.; Richter S. J.; Cannon R. E.; Oberlies N. H.; Cech N. B. J. Nat. Prod. 2011, 74, 1621–1629. 10.1021/np200336g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteiro R.; Calhau C.; Silva A. O.; Pinheiro-Silva S.; Guerreiro S.; Gartner F.; Azevedo I.; Soares R. J. Cell. Biochem. 2008, 104, 1699–1707. 10.1002/jcb.21738. [DOI] [PubMed] [Google Scholar]
- Ferk F.; Misik M.; Nersesyan A.; Pichler C.; Jager W.; Szekeres T.; Marculescu R.; Poulsen H. E.; Henriksen T.; Bono R.; Romanazzi V.; Al-Serori H.; Biendl M.; Wagner K. H.; Kundi M.; Knasmuller S. Mol. Nutr. Food Res. 2016, 60, 773–786. 10.1002/mnfr.201500355. [DOI] [PubMed] [Google Scholar]
- Wakabayashi N.; Dinkova-Kostova A. T.; Holtzclaw W. D.; Kang M. I.; Kobayashi A.; Yamamoto M.; Kensler T. W.; Talalay P. Proc. Natl. Acad. Sci. U. S. A. 2004, 101, 2040–2045. 10.1073/pnas.0307301101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinkova-Kostova A. T.; Talalay P. Arch. Biochem. Biophys. 2010, 501, 116–123. 10.1016/j.abb.2010.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keiler A. M.; Helle J.; Bader M. I.; Ehrhardt T.; Nestler K.; Kretzschmar G.; Bernhardt R.; Vollmer G.; Nikolić D.; Bolton J. L.; Pauli G. F.; Chen S. N.; Dietz B. M.; van Breemen R. B.; Zierau O. Phytomedicine 2017, 34, 50–58. 10.1016/j.phymed.2017.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Breemen R. B.; Yuan Y.; Banuvar S.; Shulman L. P.; Qiu X.; Ramos Alvarenga R. F.; Chen S. N.; Dietz B. M.; Bolton J. L.; Pauli G. F.; Krause E.; Viana M.; Nikolic D.. Mol. Nutr. Food Res. 2014, 58, 1962. 10.1002/mnfr.201400245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legette L.; Karnpracha C.; Reed R. L.; Choi J.; Bobe G.; Christensen J. M.; Rodriguez-Proteau R.; Purnell J. Q.; Stevens J. F. Mol. Nutr. Food Res. 2014, 58, 248–255. 10.1002/mnfr.201300333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legette L.; Ma L.; Reed R. L.; Miranda C. L.; Christensen J. M.; Rodriguez-Proteau R.; Stevens J. F. Mol. Nutr. Food Res. 2012, 56, 466–474. 10.1002/mnfr.201100554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolca S.; Possemiers S.; Maervoet V.; Huybrechts I.; Heyerick A.; Vervarcke S.; Depypere H.; De Keukeleire D.; Bracke M.; De Henauw S.; Verstraete W.; Van de Wiele T. Br. J. Nutr. 2007, 98, 950–959. 10.1017/S0007114507749243. [DOI] [PubMed] [Google Scholar]
- Biendl M.; Becker H.; Nookandeh A. U.S. patent US 6,867,332 B1, 2005.
- Pauli G. F.; Jaki B. U.; Lankin D. C. J. Nat. Prod. 2007, 70, 589–595. 10.1021/np060535r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Y.; Qiu X.; Nikolic D.; Dahl J. H.; van Breemen R. B. J. AOAC Int. 2012, 95, 1744–1749. 10.5740/jaoacint.11-542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littlefield B. A.; Gurpide E.; Markiewicz L.; McKinley B.; Hochberg R. B. Endocrinology 1990, 127, 2757–2762. 10.1210/endo-127-6-2757. [DOI] [PubMed] [Google Scholar]
- Hata H.; Holinka C. F.; Pahuja S. L.; Hochberg R. B.; Kuramoto H.; Gurpide E. J. Steroid Biochem. 1987, 26, 699–704. 10.1016/0022-4731(87)91042-9. [DOI] [PubMed] [Google Scholar]
- Pisha E.; Pezzuto J. M. Methods Cell Sci. 1997, 19, 37–43. 10.1023/A:1009746605060. [DOI] [Google Scholar]
- Skehan P.; Storeng R.; Scudiero D.; Monks A.; McMahon J.; Vistica D.; Warren J. T.; Bokesch H.; Kenney S.; Boyd M. R. J. Natl. Cancer Inst. 1990, 82, 1107–1112. 10.1093/jnci/82.13.1107. [DOI] [PubMed] [Google Scholar]
- Song L. L.; Kosmeder J. W. II.; Lee S. K.; Gerhäuser C.; Lantvit D.; Moon R. C.; Moriarty R. M.; Pezzuto J. M. Cancer Res. 1999, 59, 578–585. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.