Abstract
Objective
Insulin resistance is a major risk factor for type 2 diabetes. ApolipoproteinJ (ApoJ) has been implicated in altered pathophysiologic states including cardiovascular and Alzheimer’s disease. However, the function of ApoJ in regulation of glucose homeostasis remains unclear. This study sought to determine whether serum ApoJ levels are associated with insulin resistance in human subjects and if they change after interventions that improve insulin sensitivity.
Methods
Serum ApoJ levels and insulin resistance status were assessed in nondiabetic (ND) and type 2 diabetic (T2D) subjects. The impacts of rosiglitazone or metformin therapy on serum ApoJ levels and glucose disposal rate (GDR) during a hyperinsulinemic/euglycemic clamp were evaluated in a separate cohort of T2D subjects. Total ApoJ protein or that associated with the HDL and LDL fractions was measured by immunoblotting or ELISA.
Results
Fasting serum ApoJ levels were greatly elevated in T2D subjects (ND vs T2D; 100±8.3 vs. 150.6±8.5 AU, P <0.0001). Circulating ApoJ levels strongly correlated with fasting glucose, fasting insulin, HOMA-IR, and BMI. ApoJ levels were significantly and independently associated with HOMA-IR, even after adjustment for age, sex, and BMI. Rosiglitazone treatment in T2D subjects resulted in a reduction in serum ApoJ levels (before vs. after treatment; 100±13.9 vs. 77±15.2 AU, P=0.015), whereas metformin had no effect on ApoJ levels. The change in ApoJ levels during treatment was inversely associated with the change in GDR. Interestingly, ApoJ content in the LDL fraction was inversely associated with HOMA-IR.
Conclusion
Serum ApoJ levels are closely correlated with the magnitude of insulin resistance regardless of obesity, and decrease along with improvement of insulin resistance in response only to rosiglitazone in type 2 diabetes.
Keywords: apolipoproteinJ, insulin resistance, type 2 diabetes
1. Introduction
Type 2 diabetes is a major global health problem that affects millions of people worldwide and is associated with serious comorbidities, including cardiovascular diseases [1]. Insulin resistance, accompanied by failure of the pancreatic β-cells to compensate sufficiently by increased insulin secretion, leads to the development of type 2 diabetes [2]. A major challenge in the field of metabolic diseases has been identifying molecular markers linked to insulin resistance [3], which may contribute to a better understanding of the pathogenesis of type 2 diabetes.
Apolipoprotein J (ApoJ, also called clusterin) is a secreted sulfated glycoprotein that is widely distributed in various tissues, including the liver, brain, ovary, testis, heart and blood vessels [4]. Secreted ApoJ binds a broad network of metabolic mediators, including the anorexigenic hormone leptin, growth hormone, paroxonase, and proinflammatory cytokines, as well as lipid-carrying lipoprotein particles (HDL, LDL) in the circulation [5–9]. In addition to the ApoJ found in serum, nuclear-localized and cytoplasmic isoforms of ApoJ have been described, each with distinct functions [10]. Emerging data suggests that ApoJ is induced by stress and has a role as a cytoprotective extracellular chaperone [11, 12]. However, the functions of secreted ApoJ on glucose metabolism in human subjects have not been addressed.
ApoJ has been implicated in various human diseases, including atherosclerosis [13], Alzheimer’s disease [14], and cancer [15]. It has been reported that circulating ApoJ levels are elevated in humans with type 2 diabetes, obesity and systemic inflammation, all of which are pathogenically featured by insulin resistance [16–18], as is Alzheimer’s [19]. In addition, a human ApoJ gene polymorphism was found to be associated with type 2 diabetes [20, 21]. Interestingly, a recent study reported that ApoJ in HDL is correlated with insulin sensitivity, while ApoJ in LDL/VLDL is associated with insulin resistance [9], suggesting a potential link between ApoJ and insulin action. However, no data are available about the relationship between circulating ApoJ levels and direct measures of insulin resistance in humans.
In this study, we assessed whether serum ApoJ levels are correlated with the magnitude of insulin resistance, and whether therapeutic interventions that improve insulin sensitivity would be associated with changes in serum ApoJ levels in human subjects with type 2 diabetes.
2. Methods
2.1. Subjects and study design
Study 1
For the comparison of circulating ApoJ levels between nondiabetic (ND) and type 2 diabetic (T2D) subjects and for the evaluation of possible associations of serum ApoJ levels with insulin resistance, 27 ND subjects and 26 T2D subjects participated in our study (n=53). All ND subjects had normal glucose tolerance as defined by 2-h glucose level from a standard 75 gm oral glucose tolerance test (OGTT) <140mg/dL [22]. The ND subjects were healthy, weight stable, and were not taking any medications known to influence glucose metabolism. Treatment of diabetic subjects was as follows: metformin alone, n=11; metformin plus insulin, n=1; metformin + glipizide, n=3; glipizide alone, n=1; metformin + pioglitazone, n=1; metformin + liraglutide, n=1; metformin + insulin + liraglutide, n=2; metformin + canagliflozin, n=1; canagliflozin alone, n=1; metformin + canagliflozin + glipizide + saxaglipin, n=1; lifestyle alone, n=3. Diabetic subjects remained on their medications up to the time of sample collection.
Study 2
To evaluate the effect of rosiglitazone and metformin treatment on serum ApoJ levels, an additional set of T2D subjects (n=17) were enrolled in this study. Aspects of this study have been published previously [23, 24]. Following enrollment those participants on an anti-diabetic medication were asked to discontinue therapy for a 6-week washout period. All subjects were then randomized to one of 2 treatment arms: (1) high dose rosiglitazone (4 mg bid, n=8), or (2) high dose metformin (1000 mg bid, n=9). Medications were started below target doses then titrated up over the initial 2 weeks to minimize potential side effects. Both subjects and investigators were blinded to treatment. Participants were placed on a standardized weight-maintaining diet and followed by a dietician monthly for adjustments in caloric intake. The same sets of evaluations were performed at baseline and 4 months, including: blood collection for serum evaluation, 75 gm OGTT, and euglycemic hyperinsulinemic clamps. The experimental protocol was approved by the Committee on Human Investigation of the University of California, San Diego. Written informed consent was obtained after explanation of the protocol.
Study 3
To compare the relationship between HDL and LDL associated ApoJ contents and insulin resistance index, nondiabetic (ND) (n=5) and type 2 diabetic (T2D) (n=4) subjects were enrolled. Fasting blood was drawn by venipuncture by a registered nurse in the Clinical Research Center (CRC) at Beth Israel Deaconess Medical Center (BIDMC). Samples were immediately processed for serum isolation according to standard operating procedures and stored at −80°C until analysis as previously described [25]. All subjects provided written informed consent to participate in this study, approved by the BIDMC institutional review board.
2.2. Anthropometric measurements and blood tests
Height and body weight were measured in participants wearing light clothing using standard methods. The body mass index (BMI) was calculated as weight divided by height squared (kg/m2). All procedures and laboratory evaluations were performed after a 10–12 h overnight fast. The plasma concentrations of glucose were measured enzymatically using a glucose/lactate analyzer (YSI, Yellow Springs, OH). Fasting HDL-cholesterol, LDL-cholesterol and triglyceride levels were measured enzymatically (ARUP Laboratories). The glycosylated hemoglobin (HbA1c) level was measured by high-performance liquid chromatography/boronate affinity (ARUP Labs). Fasting plasma insulin concentrations were determined using a chemiluminescent immunoassay (ARUP Labs). Insulin resistance was calculated using the homeostasis model of assessment of insulin resistance (HOMA-IR), calculated as fasting plasma glucose (mmol/L) × fasting insulin (μU/mL) / 22.5 [26]. The insulin secretion index was determined using HOMA-β (%) [27]. Area under the curve for glucose during the OGTT (AUGg) was calculated using the trapezoidal rule [28].
2.3. Measurement of serum ApoJ concentrations using quantitative immunoblotting analysis
Fasting serum ApoJ levels were measured in triplicate using western blot analysis as described previously [29]. Briefly, sera were diluted 1:100 in SDS-PAGE buffer (without DTT) and boiled for 7 min. Five μl of diluted sera were subjected to 10% Tris-glycine SDS-PAGE gels (Criterion brand; BioRad) and transferred to nitrocellulose membranes. The membranes were incubated with a polyclonal primary antibody against ApoJ (Santa Cruz Biotechnology) diluted 1:1000. The membranes were then washed and incubated with horseradish peroxidase secondary antibodies (1:2000 dilution; GE Healthcare Life Sciences, Pittsburgh, PA). The bands were visualized with enhanced chemiluminescence and quantified by densitometry [30]. In a non-reducing condition, a single band for ApoJ, migrating at about 70 kDa, was observed. This condition produced consistent and reproducible results of ApoJ protein levels. To avoid irregularities due to gel quality or protein transfer, two reference serum samples were placed in the same position on each gel, and the four outermost lanes were not used for quantification.
2.4 Measurement of ApoJ contents in the fraction of HDL and LDL
Lipoproteins (HDL and LDL) in plasma were fractionated by sixty percent (w/v) iodixanol solution (Optiprep™, Sigma-Aldrich), followed by ultracentrifugation. Briefly, 1 ml of serum was mixed with 60% iodixanol and then transferred to a Quick-Seal® polypropylene tube to fit a TLA120 rotor. The tubes were then ultracentrifuged at 350,000 g for 3hr at 16°C (TL-100 Ultracentrifuge, Beckman). Fractions of HDL and LDL were collected by tube puncture. Each obtained fractions were directly analyzed by Sudan Black contained agarose gel [31]. The same amount of protein from the HDL and LDL fraction was subjected to SDS-PAGE to detect ApoJ level as described in above (2.3)
2.5 Measurement of serum ApoJ by enzyme-linked immunosorbent assay (ELISA)
Total ApoJ protein was measured in duplicate by ELISA kit (Human Clusterin ELISA Kit, ab174447, Abcam, Cambridge, MA) with 1:300 dilution of serum samples, according to manufacture’s instructions. Inter- and intra-assay coefficients of varibility were 1.63% and 5.01%, respectively. The range of detectable concentratin of ApoJ was 38–2400 ng/ml.
2.6. Hyperinsulinemic/euglycemic clamp
The subjects in Study 2 were admitted to the Special Diagnostic and Treatment Unit at the Veterans Administration Medical Center, San Diego before and after 4-months treatment with rosiglitazone or metformin. In vivo insulin action was determined by performing a 2-step 5-hour hyperinsulinemic (60 mU/m2/min for 3 h, then 120 mU/m2/min for 2 h) euglycemic (5 mM) clamp as described previously [32, 33]. The glucose disposal rates (GDR) in each patient were determined from the values obtained during the steady-state periods for each insulin infusion, i.e. the values between the 140th and 180th minute of the low dose clamp and the 260th and 300th minute of the high dose clamp [34]. Insulin levels attained during the low-dose infusion, 149 ± 4 μU/mL, are sub-maximal and would provide information about insulin sensitivity. Insulin levels reached at steady state during the high dose infusion, 319 ± 12 μU/mL, should be at or near maximally stimulating concentrations, revealing insulin responsiveness for stimulation of whole body glucose disposal. Clamp insulin levels did not differ with treatment.
2.7. Statistical Analysis
The demographic characteristics of the study subjects were expressed as means ± SEM, or numbers and percentages, or median and interquartile ranges if their distributions were skewed. Differences between the mean values of T2D and ND subjects (Study 1) were examined using the Student’s t-test or chi-squared test. Using Pearson’s correlation analyses, the relationship between serum ApoJ levels and each covariate was evaluated. The independent association of ApoJ with fasting glucose, fasting insulin, HOMA-IR, and HOMA-β (%) was estimated using multiple regression models adjusted for age, sex and BMI. For Study 2 subjects, a paired t-test was used to compare baseline serum Apo J levels with those after a 4-month treatment with rosiglitazone or metformin, respectively. We performed analyses on the correlations between the change in ApoJ (ΔApoJ) and the change in GDR (ΔGDR) and AUGg (ΔAUGg) during treatment using Spearman partial correlation analysis. Statistical analyses were conducted using SAS version 9.1 for Windows (SAS Institute Inc., Cary, NC, USA). All reported p values were two-tailed. P-values less than 0.05 were considered statistically significant.
3. Results
3.1. Clinical and metabolic characteristics of study subjects (Study 1)
The ND and T2D subjects were similar in age (Table 1). BMI was significantly increased in T2D subjects compared with ND subjects. Fasting serum glucose, fasting plasma insulin and HOMA-IR were all markedly elevated in T2D subjects compared with ND subjects. Neither blood pressure nor HOMA-β differed between ND and T2D subjects.
Table 1.
Clinical and metabolic characteristics of nondiabetic and type 2 diabetic subjects (Study 1 and Study 3) and type 2 diabetic subjects during rosiglitazone or metformin treatment (Study 2).
| Study 1 | Study 2 | Study 3 | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Nondiabetes | Type 2 diabetes | p-value | Rosiglitazone | Metformin | Nondiabetes | Type 2 diabetes | |||
| Pre-treatment | Post-treatment | Pre-treatment | Post-treatment | ||||||
| N | 27 | 26 | 8 | 9 | 5 | 4 | |||
| Sex, M/F | 24/3 | 18/8 | 0.078 | 7/1 | 8/1 | 3/2 | 3/1 | ||
| Age (years) | 54.2±2.3 | 57.4±1.5 | 0.253 | 54.5±4.3 | 56.9±2.8 | 51.8±6.0 | 55.3±3.4 | ||
| Body mass index (kg/m2) | 29.6±1.0 | 33.6±1.1 | 0.012 | 33.8±2.1 | 39.8±4.2 | 35.8±1.6 | 34.0±1.6† | 34.9±2.2 | 35.1±3.7 |
| Systolic blood pressure (mmHg) | 122.3±2.9 | 126.1±2.9 | 0.367 | 119.9±3.1 | 125.2±7.3 | 124.8±4.5 | 120.9±4.0 | 132.8±2.5 | 134.7±6.0 |
| Diastolic blood pressure (mmHg) | 80.2±2.3 | 79.6±2.4 | 0.859 | 69.9±2.4 | 68.3±2.8† | 70.8±2.9 | 69.2±2.9 | 82.2±2.0 | 77.7±7.2 |
| Fasting glucose (mmol/L) | 5.3±0.1 | 9.7±0.8 | <.0001 | 8.1±0.7 | 6.0±0.3† | 7.3±0.6 | 6.3±0.4† | 5.3±0.2 | 6.2±0.6 |
| Fasting insulin (IU/L)* | 6.5 (3.6–12.2) | 14.0 (9.0–20.1) | 0.0008 | 13 (10–17) | 9 (9–11) | 30 (9–38) | 25 (12–29) | 13 (10–17) | 17 (11–29) |
| HOMA-IR* | 1.4 (0.8–3.3) | 6.1 (4.5–8.0) | <.0001 | 4.8 (4.2–5.9) | 3.1 (2.4–3.1) † | 7.0 (2.9–11.9) | 6.2 (4.0–7.2) | 3.83±1.1 | 4.5±0.82 |
| HOMA-β (%)* | 70.7 (55.9–121.8) | 66.6 (33.0–90.2) | 0.223 | - | - | - | - | - | - |
| HbA1c (%) | - | - | 7.1±0.4 | 6.5±0.3† | 6.7±0.2 | 6.2±0.2† | 5.7±0.1 | 7.7±0.7 | |
| LDL-cholesterol (mmol/L) | - | - | 2.7±0.3 | 2.9±0.1 | 2.5±0.2 | 2.5±0.3 | 2.7±0.3 | 1.9±0.2 | |
| HDL-cholesterol (mmol/L) | - | - | 0.89±0.06 | 0.98±0.07† | 0.87±0.06 | 0.93±0.07 | 1.25±0.05 | 1.09±0.17 | |
| Triglyceride (mmol/L) | - | - | 2.1±0.4 | 2.1±0.2 | 2.3±0.5 | 2.3±0.5 | 1.2±0.4 | 1.3±0.4 | |
| GDR60 (mg/kg/min) | - | - | 2.7±0.3 | 4.3±0.5† | 2.7±0.6 | 3.0±0.5 | - | - | |
| GDR120 (mg/kg/min) | - | - | 5.3±0.6 | 7.4±0.7† | 5.0±0.6 | 5.4±0.4 | - | - | |
| AUGg | - | - | 26623±2089 | 23338±1401† | 26870±1996 | 24248±2137 | - | - | |
Data are expressed as means ± SEM.
Data are expressed as median (interquartile range), p-value from log transformation.
For Study1: P-values are from the Student’s t-test or chi-squared test.
For Study2: P-values are from the paired t-test.
P<0.05 vs. pre-treatment in each group.
Abbreviations: HOMA-IR, homeostasis model assessment-insulin resistance; HOMA-β (%), homeostasis model assessment-β (%); GDR, glucose disposal rate; AUGg, area under the curve for glucose during the OGTT.
3.2. Serum Apo J levels and their correlations with metabolic parameters (Study 1)
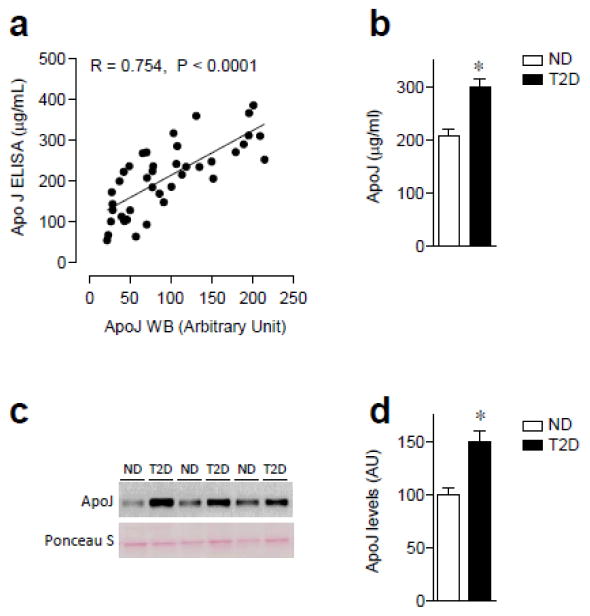
ELISA and quantitative western blotting analysis were performed to measure serum ApoJ levels from healthy subjects and those with type 2 diabetes. A stong correlationship between western blotting analysis and results from the ELISA (r = 0.7542, p < 0.0001) was found in (Fig. 1a). Fasting serum ApoJ levels determined by both ELISA and immunoblotting analysis were greatly elevated, by ~50%, in T2D subjects compared to ND subjects (ELISA: ND vs. T2D; 207.1±13.3 vs. 298.5±15.5 μg/ml, P < 0.0002, Western: ND vs. T2D; 100.4±8.3 vs. 150.6±8.5 AU, P < 0.0001, Fig. 1b–d). When we classified ND subjects into tertiles according to HOMA-IR levels, subjects in the higher tertiles of HOMA-IR had higher levels of ApoJ than subjects in the lower tertiles of HOMA-IR (ApoJ levels in the 1st, 2nd and 3rd tertiles of HOMA-IR; 80.0±11.6, 94.6±11.3 and 116.6±11.3 AU, P for trend=0.038).
Figure 1. Serum levels of ApoJ in nondiabetic (ND) and type 2 diabetic (T2D) subjects.
(a) Correlation of ApoJ levels between western blotting analysis and ELISA in ND and T2D subjects. (b) Fasting serum ApoJ concentration in ND and T2D subjects. Serum ApoJ protein was measured by ELISA with 1:300 dilution of serum samples. Results are mean ± SEM for 13–27 subjects per group. *P<0.001 vs. ND. (c) Serum ApoJ protein in ND and T2D subjects. Proteins in sera were separated by SDS/PAGE and transferred to nitrocellulose membranes. ApoJ proteins were visualized by immunoblotting in a non-reducing condition, which shows ~ 70 kDa as a single band. Ponceau S staining indicates that even amount of protein in each lane was loaded on the gel. Each lane in the blot represents a different subject. The immunoblots shown are representative of three blots. (d) Bars show densitometric quantitation of serum ApoJ from ND and T2D subjects. Results are means ± SEM for 26–27 subjects per group. *P<0.001 vs. ND.
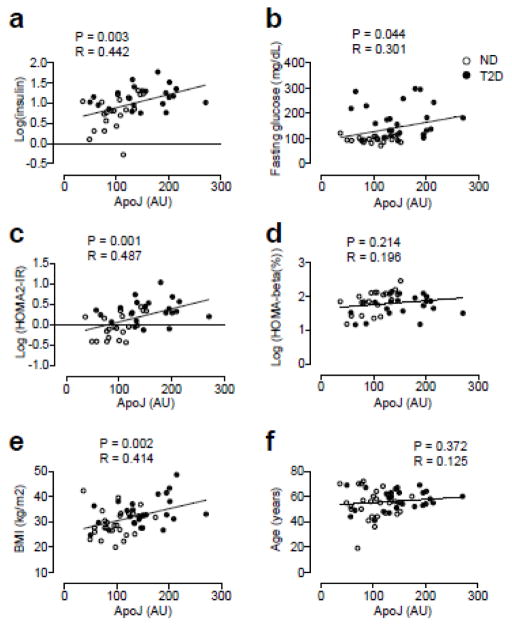
Importantly, circulating ApoJ levels in all subjects were strongly correlated with several metabolic parameters, including fasting glucose, fasting insulin, HOMA-IR, and BMI, but not with age or HOMA-β (%) (Fig. 2a–f). In multiple regression analysis, ApoJ levels were a significant independent association factor for fasting insulin and HOMA-IR after adjusting for age, sex and BMI (Table 2). However, when we segregated the subjects according to the presence of diabetes, these associations were maintained in ND subjects but not in T2D subjects.
Figure 2. Correlations of ApoJ levels and risk factors for insulin resistance in nondiabetic (ND) and type 2 diabetic (T2D) subjects.
Relationship of serum ApoJ with (a) fasting insulin levels, (b) fasting glucose levels, (c) HOMA-IR, (d) HOMA-β (%), (e) BMI, and (f) age.
Table 2.
Multiple regression models with serum ApoJ level as an independent variable and logarithmic transformed fasting insulin, HOMA-IR, and fasting glucose as a dependent variable respectively.
| Dependent variable | Model | All (n=53) | Nondiabetes (n=27) | Type 2 diabetes (n=26) | |||
|---|---|---|---|---|---|---|---|
|
| |||||||
| standardized β | P | standardized β | p | standardized β | p | ||
| Log(fasting insulin) | 1 | 0.517 | 0.0004 | 0.488 | 0.023 | 0.297 | 0.226 |
| 2 | 0.368 | 0.010 | 0.489 | 0.005 | 0.434 | 0.081 | |
|
| |||||||
| Log(HOMA-IR) | 1 | 0.521 | 0.007 | 0.450 | 0.038 | 0.094 | 0.712 |
| 2 | 0.388 | 0.007 | 0.443 | 0.008 | 0.249 | 0.376 | |
|
| |||||||
| Log(fasting glucose)o | 1 | 0.356 | 0.020 | −0.009 | 0.969 | −0.199 | 0.402 |
| 2 | 0.272 | 0.105 | −0.051 | 0.792 | −0.241 | 0.448 | |
Model 1 : no adjustment
Model 2 : adjustment for age, sex and body mass index
Analyses were performed in the combined data of subjects with nondiabetes and type 2 diabetes (Study 1, All), in subjects with nondiabetes, and in subjects with type 2 diabetes using separate regression models.
3.3. Clinical and metabolic characteristics of type 2 diabetic subjects treated with rosiglitazone or metformin (Study 2)
The type 2 diabetic subjects in the rosiglitazone and metformin groups were matched for age and obesity (Table 1). Just as was the case for the T2D subjects in Study 1, there was no association between baseline ApoJ levels and HOMA-IR (r=0.108, P=0.752). The same was true for ApoJ levels and baseline GDR60 and GDR120 (r=−0.171, P=0.512 and r=−0.188, P=0.470, respectively). After 4 months of rosiglitazone treatment, BMI was modestly increased, though the difference did not reach statistical significance, compared with before treatment. Treatment of T2D subjects with metformin for 4 months led to a slight decrease in BMI. Rosiglitzone treatment induced an increase of HDL-cholesterol and decrease of diastolic blood pressure. Both rosiglitazone and metformin therapy significantly reduced HbA1c and fasting glucose. However, only rosiglitazone decreased HOMA-IR significantly. At baseline insulin-stimulated whole body glucose disposal (GDR) was similar in both groups, at either insulin infusion rate. However, while rosiglitazone treatment increased the GDR at both the lower and higher insulin infusion rates, by 59% and 39%, respectively, metformin treatment did not result in significant changes in GDR (Table 1), Modest effects of metformin on GDR, compared to those induced by thiazolidinedione treatment, have been noted previously [35, 36].
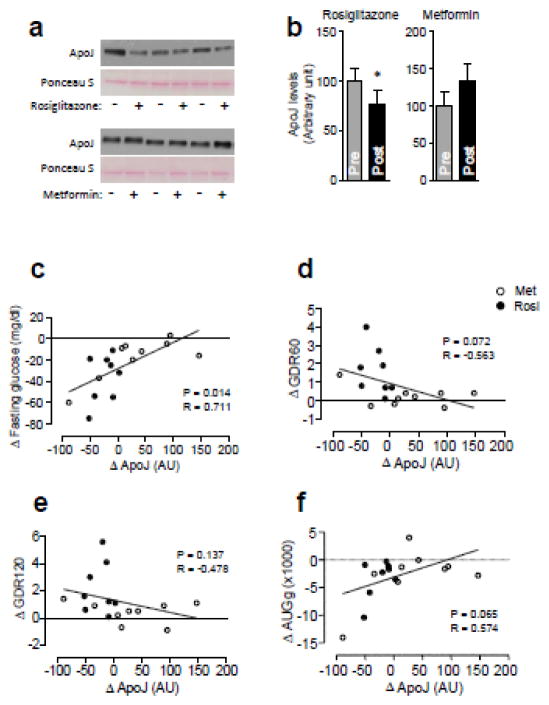
3.4. Changes in serum Apo J levels after rosiglitazone or metformin treatment inversely correlate with GDR (Study 2)
Rosiglitazone treatment of subjects with T2D resulted in a reduction in serum ApoJ levels (before vs. after treatment; 100±13.9 vs. 77±15.2 AU, P=0.015), while metformin had no effect on ApoJ levels (before vs. after treatment; 100.0±18.9 vs. 133.2±22.8 AU, P=0.199) (Fig. 3). When we performed correlation analyses between the change in ApoJ (ΔApoJ) and the changes in insulin action after treatment, ΔApoJ was strongly associated with Δfasting glucose and inversely associated with both ΔGDR60 (low dose) and ΔGDR120 (high dose) (Fig. 3). Perhaps due to the smaller sample size, the correlations between ΔApoJ and ΔGDR60 and ΔGDR120 were lost when the treatments were considered individually. No correlations were found between ΔApoJ and Δlipids (HDL-cholesterol, LDL-cholesterol, and triglyceride, all P > 0.9)
Figure 3. Effects of rosiglitazone and metformin treatment on serum ApoJ levels in subjects with type 2 diabetes.
All subjects (n=17) underwent a 2-step 5-hour hyperinsulinemic-euglycemic clamp and blood was drawn before and at the end of the clamp. (a) Serum ApoJ protein in T2D subjects treated with rosiglitazone or metformin. Each lane in the blot represents a different subject. The immunoblots shown are representative of three blots. (b) Bars show densitometric quantitation of serum ApoJ from T2D subjects with rosiglitazone or metformin. Results are mean ± SEM for 8–9 subjects per group. *P=0.015 vs. pre-treatment of rosiglitazone.
Relationship of serum ΔApoJ with (c) Δfasting glucose levels, (d) ΔGDR60, (e) ΔGDR120, and (f) ΔAUGg. Serum ΔApoJ levels were calculated by subtracting pre-treatment ApoJ levels from posttreatment ApoJ levels.
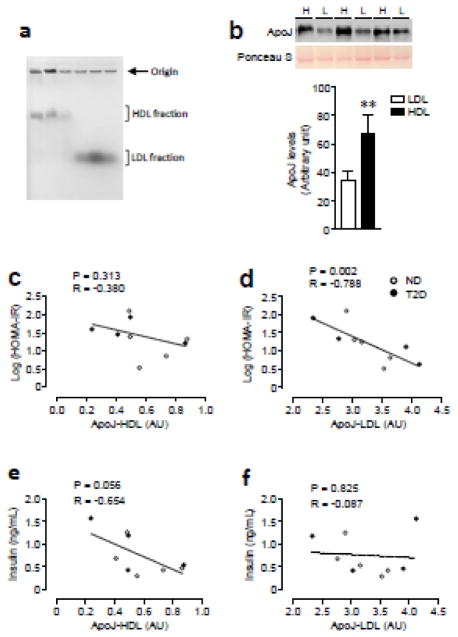
3.5. ApoJ content in HDL and LDL fraction (Study 3)
The non-diabetic and Type 2 diabetic subjects were matched for age and obesity (Table 1). The electrophoretic mobility of the HDL and LDL fraction is shown in Fig. 4a. As expected [31], the LDL fraction migrated further into the gel than the HDL fraction (Fig. 4a). ApoJ levels of the HDL-containing fraction were elevated by ~2 fold compared with that of the LDL-containing fraction (Fig. 4b). While the levels of ApoJ bound to LDL were inversely correlated with HOMA-IR (Fig. 4c), no correlation between ApoJ content in HDL and HOMA-IR was observed (Fig. 4d). Serum insulin inversely correlated with ApoJ in the HDL-containing fraction but not in the LDL-containing fraction (Fig. 4e & f).
Figure 4. Correlations of ApoJ contents in HDL and LDL fraction and risk factor for insulin resistance in human subjects.
(a) Serum lipoproteins (HDL and LDL) in iodixanol gradients were separated by Sudan Block containing agarose gel. (b) ApoJ proteins in HDL and LDL fraction were separated by SDS/PAGE and transferred to nitrocellulose membranes. Each lane in the blot represents a different subject. The immunoblots shown are representative of three blots (H: HDL, L: LDL). Bars show densitometric quantitation of ApoJ from HDL and LDL fractions. Results are mean ± SEM for 9 subjects per group. *P<0.0001 vs. ApoJ from LDL.
(c–f) Relationship of ApoJ bound to HDL or LDL with HOMA-IR and fasting insulin levels.
4. Discussion
The current study provides crucial evidence of a close relationship between serum ApoJ levels and measures describing insulin resistance, including after an intervention that improves insulin action. Particularly, we show that the level of ApoJ in the serum correlates with a measure of insulin resistance (HOMA-IR), independently of obesity, especially in ND subjects. In T2D subjects, who had ~50% higher levels of serum ApoJ than ND subjects, we found that treatment with the insulin sensitizer rosiglitazone induced a decrease in serum ApoJ levels. Importantly, a correlation between the extent of reduction in serum ApoJ levels and the degree of improvement of insulin resistance was detected after treatment.
ApoJ is present in plasma as a soluble protein, as a component of a lipid-poor subclass of HDLs, or bound to LDLs and VLDLs in a small portion [9, 37]. Circulating ApoJ has been implicated in various cardiometabolic abnormalities, including dyslipidemia, atherosclerotic vascular diseases, and metabolic syndrome, as well as Alzheimer’s disease, all of which are characterized by insulin resistance [16, 18, 19, 38]. Furthermore, circulating ApoJ is closely correlated with total cholesterol or LDL cholesterol [16, 38, 39] and is elevated in people with coronary heart disease [16]. During the progression of vascular damage, ApoJ was found to accumulate in the aortic wall, but not in the normal aorta [40]. However, administration of ApoE null mice with oral ApoJ peptide dramatically reduced atherosclerosis [41], suggesting that ApoJ plays a protective role in the development of atherosclerosis and that elevated ApoJ concentrations in vascular disease may be secondary, adaptive effects. In line with these findings, plasma ApoJ levels were shown to increase in humans with obesity, metabolic syndrome, and/or systemic inflammation, while they were reduced after weight loss [18, 42]. These observations are extended by the current results that serum ApoJ levels in T2D subjects are greatly increased, by ~50%, compared with ND subjects. What the current report adds is more detailed quantitative information about the relationships between ApoJ levels and insulin resistance, as well as the effects of therapeutic interventions. Together, these data raise the possibility that up-regulation of ApoJ levels in serum could be one feature of metabolically dysregulated states such as insulin resistance in human subjects.
Data demonstrating the importance of ApoJ content in lipoproteins for insulin action include the fact that ApoJ level in HDL was negatively associated with insulin resistance in a variety group of human subjects, including lean insulin-sensitive, lean insulin-resistant, obese insulin-resistant subjects, healthy and diabetic subjects [9]. While we did not observe such a relationship, we did find that ApoJ in the LDL fraction was inversely correlated with insulin resistance in normal and T2D subjects, a point not considered by those investigators. This descrepancy could be due to either differences in the methodologies for ApoJ measuments, or metabolic characteristics, as all of the subjects in Study 3 were obese, or another yet to be identified factor. Future studies will need to clarify the exact role of ApoJ associated with HDL and LDL in the development of insulin resistance.
How might ApoJ be playing a role in metabolic regulation? Our recent work identified ApoJ as an important regulator of the hypothalamic leptin signaling pathway that is essential for the regulation of energy balance [29]. Furthermore, we have preliminary data in animals indicating that ApoJ is required for optimal insulin action [43]. Considering this animal data, together with our human data, where increasing levels are associated with insulin resistance, it appears that the ApoJ/insulin signaling axis is under tight control. A ~50% increase in ApoJ levels compared to healthy individuals is a feature of T2D (Fig. 1), while a modest 30% reduction with rosiglitazone treatment is associated with improved insulin resistance.
Again, it is important to note that serum ApoJ levels were independently correlated with the insulin resistance index in the combined data from both ND and T2D subjects but the correlation was maintained only in ND subjects when evaluated in separate regression models. This could be explained by several possibilities, including uncontrolled confounding. First, circulating ApoJ may be involved not only in insulin resistance, but also control of insulin secretion. Experimental evidence revealed that an ApoJ gene polymorphism is associated with both the insulin resistance index (HOMA-IR) and insulin secretion index (HOMA-β (%)) [20]. Interestingly, in a separate regression model adjusted for age, sex, and BMI, we found a positive correlation of ApoJ with HOMA-β in ND subjects (p=0.002), but not in T2D subjects (p=0.138), supporting involvement of ApoJ in regulation of pancreatic β-cell function. In this regard, some studies have demonstrated that ApoJ plays a role in the regulation of pancreatic β-cell neogenesis as a growth factor-like molecule [44, 45]. Considering that β-cell dysfunction is a major pathogenic mechanism of T2D and is aggravated with disease progression, it is conceivable that each individual with T2D could have a variable extent of β-cell dysfunction. Thus, the mixed effects of insulin resistance and insulin secretion on ApoJ levels may confound the correlations in subjects with T2D. Second, the index for insulin resistance we used for the correlation analysis in the cross-sectional study (Study 1), HOMA-IR, may not be sufficiently sensitive, as it is considered more suitable for epidemiological surveys involving a larger number of subjects [46]. In addition, HOMA-IR is more informative about insulin action in the liver [47]. Meanwhile, GDR assessed by the clamp is a more reliable means of evaluating insulin sensitivity and glucose homeostasis in peripheral tissues, primarily skeletal muscle and adipose tissue [32]. However, in the T2D subjects where insulin sensitivity was evaluated by the clamp (Study 2), the lack of correlation between ApoJ levels and insulin sensitivity was also seen for GDR at both insulin infusion rates, as well as with HOMA-IR.
It is interesting to note that while both thiazolidinediones, such as rosiglitazone, and metformin can improve glucose intolerance and insulin action, they have been shown to do so through actions on different tissues and through activating different mechanisms [35, 36, 48]. We found significantly increased serum adiponectin levels with rosiglitazone treatment (before vs. after treatment, 9.3±1.5 vs. 22.5±5.1ug/mL, P=0.02) but not with metformin treatment (before vs. after treatment, 8.7±3.0 vs. 9.1±1.4 ug/mL, P=0.83) in this study (n=6 in each group, Study 2). Congruent with the current results, thiazolidinediones and metformin have divergent effects on circulating adiponectin and ApoJ levels in T2D subjects [49].
Limitations of the current investigation include the following: We could not evaluate causal relationships between serum ApoJ and insulin resistance due to the cross-sectional design of Study 1. The variety of anti-diabetic medications taken by the T2D subjects in Study 1 could also represent a source of variability. Yet the T2D subjects in Study 2 underwent a prolonged washout period and displayed the same behavior of ApoJ levels vs HOMA-IR as subjects who remained on therapy (Study 1). Furthermore, the number of females in the study groups was small, making it difficult to determine gender influences on the relationships and responses we observed. Only a portion of the subjects had insulin sensitivity determined by the more robust clamp procedure, yet the lack of correlation with ApoJ in T2D subjects was similar for HOMA-IR and GDR, validating the use of HOMA-IR in Study 1. In addition, while the numbers of subjects in the rosiglitazone and metformin treatment groups were small, they were, when combined, sufficient to show statistically significant effects for some outcomes. Nevertheless, we found strong correlations between circulating ApoJ levels and risk factors for insulin resistance in ND subjects, and improvement of insulin action together with falls in ApoJ levels with rosiglitazone treatment in T2D subjects, indicating that, within a limited range, serum ApoJ levels can be tightly linked to glucose metabolism and insulin sensitivity.
In conclusion, our data demonstrates that serum ApoJ levels are closely correlated with the magnitude of insulin resistance independent of obesity in ND subjects and in T2D subjects are elevated and reduced after treatment with the insulin-sensitizer rosiglitazone but not metformin. Further studies are neede to explore the role of circulating ApoJ as a novel metabolic marker of insulin resistance and to study whether it may be playing a pivotal role in the maintenance of normal insulin sensitivity in human subjects.
Acknowledgments
The authors thank the subject volunteers, whose time and effort are critical to the success of this work. The authors are thankful to Hyunjoo Cho who helped statistical analysis.
Funding
This work was supported by grants from the American Diabetes Association (7-12-BS-094 to YBK) and the National Institutes of Health (R01DK111529 and R01DK106076 to YBK), the Medical Research Service, and the General Clinical Research Branch, Division of Research Resources, a grant from the Korean Diabetes Association (2017S-2 to JAS), and a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare Republic of Korea (grant number: HI14C1277). This material is the result of work supported with resources and the use of facilities at the VA San Diego Medical Center.
Abbreviations
- ApoJ
apolipoprotein J
- HOMA-IR
homeostasis model assessment-insulin resistance
- GDR
glucose disposal rate
- BMI
body mass index
- HOMA-β (%)
homeostasis model assessment-β (%)
- OGTT
oral glucose tolerance test
Footnotes
Disclosure Statement: The authors have nothing to disclose.
Author contributions
Y.-B.K., T.P.C., and R.R.H designed the study. J.A.S. M.C., and S.S.K. performed most of immunoblotting analysis for Study 1 and 2. W.B.H. and D.M.L carried out lipoprotein fractions and immunoblotting analysis for Study 3. K.S.P. provided conceptual advice and contributed the data analysis. All of the authors interpreted experimental data, and wrote experimental methods and results. R.R.H., C.C., and T.P.C conducted the clinical study (Study 1 and 2), collected data and contributed to the editing of the manuscript. O.F. and C.M. conducted Study 3. The contents do not represent the views of the U.S. department of Veterans Affairs or the United States Government. J.A.S. and Y.-B.K. wrote the manuscript. Y.-B.K. is the guarantor of this work and, as such, had full access to all the data in this work and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.IDF Diabetes Atlas. 6. Brussels, Belgium: International Diabetes Federation; 2014. pp. 32–9. [Google Scholar]
- 2.Kahn BB. Type 2 diabetes: when insulin secretion fails to compensate for insulin resistance. Cell. 1998;92:593–6. doi: 10.1016/s0092-8674(00)81125-3. [DOI] [PubMed] [Google Scholar]
- 3.Nowak C, Sundstrom J, Gustafsson S, Giedraitis V, Lind L, Ingelsson E, et al. Protein Biomarkers for Insulin Resistance and Type 2 Diabetes Risk in Two Large Community Cohorts. Diabetes. 2016;65:276–84. doi: 10.2337/db15-0881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosenberg ME, Silkensen J. Clusterin: physiologic and pathophysiologic considerations. Int J Biochem Cell Biol. 1995;27:633–45. doi: 10.1016/1357-2725(95)00027-m. [DOI] [PubMed] [Google Scholar]
- 5.Bajari TM, Strasser V, Nimpf J, Schneider WJ. A model for modulation of leptin activity by association with clusterin. FASEB J. 2003;17:1505–7. doi: 10.1096/fj.02-1106fje. [DOI] [PubMed] [Google Scholar]
- 6.Zeng W, Lu YH, Lee J, Friedman JM. Reanalysis of parabiosis of obesity mutants in the age of leptin. Proc Natl Acad Sci U S A. 2015;112:E3874–82. doi: 10.1073/pnas.1510378112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Falgarone G, Chiocchia G. Chapter 8: Clusterin: A multifacet protein at the crossroad of inflammation and autoimmunity. Adv Cancer Res. 2009;104:139–70. doi: 10.1016/S0065-230X(09)04008-1. [DOI] [PubMed] [Google Scholar]
- 8.Kelso GJ, Stuart WD, Richter RJ, Furlong CE, Jordan-Starck TC, Harmony JA. Apolipoprotein J is associated with paraoxonase in human plasma. Biochemistry. 1994;33:832–9. doi: 10.1021/bi00169a026. [DOI] [PubMed] [Google Scholar]
- 9.Hoofnagle AN, Wu M, Gosmanova AK, Becker JO, Wijsman EM, Brunzell JD, et al. Low clusterin levels in high-density lipoprotein associate with insulin resistance, obesity, and dyslipoproteinemia. Arterioscler Thromb Vasc Biol. 2010;30:2528–34. doi: 10.1161/ATVBAHA.110.212894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jones SE, Jomary C. Clusterin. Int J Biochem Cell Biol. 2002;34:427–31. doi: 10.1016/s1357-2725(01)00155-8. [DOI] [PubMed] [Google Scholar]
- 11.Zoubeidi A, Chi K, Gleave M. Targeting the cytoprotective chaperone, clusterin, for treatment of advanced cancer. Clin Cancer Res. 2010;16:1088–93. doi: 10.1158/1078-0432.CCR-09-2917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Humphreys DT, Carver JA, Easterbrook-Smith SB, Wilson MR. Clusterin has chaperone-like activity similar to that of small heat shock proteins. J Biol Chem. 1999;274:6875–81. doi: 10.1074/jbc.274.11.6875. [DOI] [PubMed] [Google Scholar]
- 13.Miwa Y, Takiuchi S, Kamide K, Yoshii M, Horio T, Tanaka C, et al. Insertion/deletion polymorphism in clusterin gene influences serum lipid levels and carotid intima-media thickness in hypertensive Japanese females. Biochem Biophys Res Commun. 2005;331:1587–93. doi: 10.1016/j.bbrc.2005.04.069. [DOI] [PubMed] [Google Scholar]
- 14.Thambisetty M, Simmons A, Velayudhan L, Hye A, Campbell J, Zhang Y, et al. Association of plasma clusterin concentration with severity, pathology, and progression in Alzheimer disease. Arch Gen Psychiatry. 2010;67:739–48. doi: 10.1001/archgenpsychiatry.2010.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bi J, Guo AL, Lai YR, Li B, Zhong JM, Wu HQ, et al. Overexpression of clusterin correlates with tumor progression, metastasis in gastric cancer: a study on tissue microarrays. Neoplasma. 2010;57:191–7. doi: 10.4149/neo_2010_03_191. [DOI] [PubMed] [Google Scholar]
- 16.Trougakos IP, Poulakou M, Stathatos M, Chalikia A, Melidonis A, Gonos ES. Serum levels of the senescence biomarker clusterin/apolipoprotein J increase significantly in diabetes type II and during development of coronary heart disease or at myocardial infarction. Exp Gerontol. 2002;37:1175–87. doi: 10.1016/s0531-5565(02)00139-0. [DOI] [PubMed] [Google Scholar]
- 17.Kujiraoka T, Hattori H, Miwa Y, Ishihara M, Ueno T, Ishii J, et al. Serum apolipoprotein j in health, coronary heart disease and type 2 diabetes mellitus. J Atheroscler Thromb. 2006;13:314–22. doi: 10.5551/jat.13.314. [DOI] [PubMed] [Google Scholar]
- 18.Won JC, Park CY, Oh SW, Lee ES, Youn BS, Kim MS. Plasma clusterin (ApoJ) levels are associated with adiposity and systemic inflammation. PLoS One. 2014;9:e103351. doi: 10.1371/journal.pone.0103351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Felice FG, Lourenco MV, Ferreira ST. How does brain insulin resistance develop in Alzheimer’s disease? Alzheimers Dement. 2014;10:S26–32. doi: 10.1016/j.jalz.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 20.Daimon M, Oizumi T, Karasawa S, Kaino W, Takase K, Tada K, et al. Association of the clusterin gene polymorphisms with type 2 diabetes mellitus. Metabolism. 2011;60:815–22. doi: 10.1016/j.metabol.2010.07.033. [DOI] [PubMed] [Google Scholar]
- 21.Aronis KN, Kim YB, Mantzoros CS. Clusterin (apolipoprotein J): wither link with diabetes and cardiometabolic risk? Metabolism. 2011;60:747–8. doi: 10.1016/j.metabol.2010.12.013. [DOI] [PubMed] [Google Scholar]
- 22.Feldman LJ, Himbert D, Juliard JM, Karrillon GJ, Benamer H, Aubry P, et al. Reperfusion syndrome: relationship of coronary blood flow reserve to left ventricular function and infarct size. J Am Coll Cardiol. 2000;35:1162–9. doi: 10.1016/s0735-1097(00)00523-4. [DOI] [PubMed] [Google Scholar]
- 23.Phillips SA, Kung J, Ciaraldi TP, Choe C, Christiansen L, Mudaliar S, et al. Selective regulation of cellular and secreted multimeric adiponectin by antidiabetic therapies in humans. Am J Physiol Endocrinol Metab. 2009;297:E767–73. doi: 10.1152/ajpendo.00378.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang J, Ciaraldi TP, Samad F. Tissue factor expression in obese type 2 diabetic subjects and its regulation by antidiabetic agents. J Obes. 2015;2015:291209. doi: 10.1155/2015/291209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Farr OM, Tsoukas MA, Triantafyllou G, Dincer F, Filippaios A, Ko BJ, et al. Short-term administration of the GLP-1 analog liraglutide decreases circulating leptin and increases GIP levels and these changes are associated with alterations in CNS responses to food cues: A randomized, placebo-controlled, crossover study. Metabolism. 2016;65:945–53. doi: 10.1016/j.metabol.2016.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 27.Albareda M, Rodriguez-Espinosa J, Murugo M, de Leiva A, Corcoy R. Assessment of insulin sensitivity and beta-cell function from measurements in the fasting state and during an oral glucose tolerance test. Diabetologia. 2000;43:1507–11. doi: 10.1007/s001250051561. [DOI] [PubMed] [Google Scholar]
- 28.Lee DH, Shi J, Jeoung NH, Kim MS, Zabolotny JM, Lee SW, et al. Targeted disruption of ROCK1 causes insulin resistance in vivo. J Biol Chem. 2009;284:11776–80. doi: 10.1074/jbc.C900014200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gil SY, Youn BS, Byun K, Huang H, Namkoong C, Jang PG, et al. Clusterin and LRP2 are critical components of the hypothalamic feeding regulatory pathway. Nat Commun. 2013;4:1862. doi: 10.1038/ncomms2896. [DOI] [PubMed] [Google Scholar]
- 30.Kim YB, Shulman GI, Kahn BB. Fatty acid infusion selectively impairs insulin action on Akt1 and protein kinase C lambda/zeta but not on glycogen synthase kinase-3. J Biol Chem. 2002;277:32915–22. doi: 10.1074/jbc.M204710200. [DOI] [PubMed] [Google Scholar]
- 31.Graham JM, Higgins JA, Gillott T, Taylor T, Wilkinson J, Ford T, et al. A novel method for the rapid separation of plasma lipoproteins using self-generating gradients of iodixanol. Atherosclerosis. 1996;124:125–35. doi: 10.1016/0021-9150(96)05797-8. [DOI] [PubMed] [Google Scholar]
- 32.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979;237:E214–23. doi: 10.1152/ajpendo.1979.237.3.E214. [DOI] [PubMed] [Google Scholar]
- 33.Sowell M, Mukhopadhyay N, Cavazzoni P, Carlson C, Mudaliar S, Chinnapongse S, et al. Evaluation of insulin sensitivity in healthy volunteers treated with olanzapine, risperidone, or placebo: a prospective, randomized study using the two-step hyperinsulinemic, euglycemic clamp. J Clin Endocrinol Metab. 2003;88:5875–80. doi: 10.1210/jc.2002-021884. [DOI] [PubMed] [Google Scholar]
- 34.Hardy TA, Henry RR, Forrester TD, Kryzhanovskaya LA, Campbell GM, Marks DM, et al. Impact of olanzapine or risperidone treatment on insulin sensitivity in schizophrenia or schizoaffective disorder. Diabetes Obes Metab. 2011;13:726–35. doi: 10.1111/j.1463-1326.2011.01398.x. [DOI] [PubMed] [Google Scholar]
- 35.Inzucchi SE, Maggs DG, Spollett GR, Page SL, Rife FS, Walton V, et al. Efficacy and metabolic effects of metformin and troglitazone in type II diabetes mellitus. N Engl J Med. 1998;338:867–72. doi: 10.1056/NEJM199803263381303. [DOI] [PubMed] [Google Scholar]
- 36.Kim YB, Ciaraldi TP, Kong A, Kim D, Chu N, Mohideen P, et al. Troglitazone but not metformin restores insulin-stimulated phosphoinositide 3-kinase activity and increases p110beta protein levels in skeletal muscle of type 2 diabetic subjects. Diabetes. 2002;51:443–8. doi: 10.2337/diabetes.51.2.443. [DOI] [PubMed] [Google Scholar]
- 37.Banfi C, Brioschi M, Barcella S, Wait R, Begum S, Galli S, et al. Proteomic analysis of human low-density lipoprotein reveals the presence of prenylcysteine lyase, a hydrogen peroxide-generating enzyme. Proteomics. 2009;9:1344–52. doi: 10.1002/pmic.200800566. [DOI] [PubMed] [Google Scholar]
- 38.Baralla A, Sotgiu E, Deiana M, Pasella S, Pinna S, Mannu A, et al. Plasma Clusterin and Lipid Profile: A Link with Aging and Cardiovascular Diseases in a Population with a Consistent Number of Centenarians. PLoS One. 2015;10:e0128029. doi: 10.1371/journal.pone.0128029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aronis KN, Vamvini MT, Chamberland JP, Mantzoros CS. Circulating clusterin (apolipoprotein J) levels do not have any day/night variability and are positively associated with total and LDL cholesterol levels in young healthy individuals. J Clin Endocrinol Metab. 2011;96:E1871–5. doi: 10.1210/jc.2011-1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mackness B, Hunt R, Durrington PN, Mackness MI. Increased immunolocalization of paraoxonase, clusterin, and apolipoprotein A-I in the human artery wall with the progression of atherosclerosis. Arterioscler Thromb Vasc Biol. 1997;17:1233–8. doi: 10.1161/01.atv.17.7.1233. [DOI] [PubMed] [Google Scholar]
- 41.Navab M, Anantharamaiah GM, Reddy ST, Van Lenten BJ, Wagner AC, Hama S, et al. An oral apoJ peptide renders HDL antiinflammatory in mice and monkeys and dramatically reduces atherosclerosis in apolipoprotein E-null mice. Arterioscler Thromb Vasc Biol. 2005;25:1932–7. doi: 10.1161/01.ATV.0000174589.70190.e2. [DOI] [PubMed] [Google Scholar]
- 42.Arnold T, Brandlhofer S, Vrtikapa K, Stangl H, Hermann M, Zwiauer K, et al. Effect of obesity on plasma clusterin, [corrected] a proposed modulator of leptin action. Pediatr Res. 2011;69:237–42. doi: 10.1203/PDR.0b013e31820930cb. [DOI] [PubMed] [Google Scholar]
- 43.Seo JA, Lima IS, Chung M, Kim MJ, Kim YB. Targeted Disruption of the ApolipoproteinJ Selectively in Liver Causes Insulin Resistance and Glucose Intolerance. Diabetes. 2014;63(Suppl 1):A851. [Google Scholar]
- 44.Kim BM, Han YM, Shin YJ, Min BH, Park IS. Clusterin expression during regeneration of pancreatic islet cells in streptozotocin-induced diabetic rats. Diabetologia. 2001;44:2192–202. doi: 10.1007/s001250100029. [DOI] [PubMed] [Google Scholar]
- 45.Kim SY, Lee S, Min BH, Park IS. Functional association of the morphogenic factors with the clusterin for the pancreatic beta-cell differentiation. Diabetes Res Clin Pract. 2007;77(Suppl 1):S122–6. doi: 10.1016/j.diabres.2007.01.045. [DOI] [PubMed] [Google Scholar]
- 46.Kang ES, Yun YS, Park SW, Kim HJ, Ahn CW, Song YD, et al. Limitation of the validity of the homeostasis model assessment as an index of insulin resistance in Korea. Metabolism. 2005;54:206–11. doi: 10.1016/j.metabol.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 47.Tripathy D, Almgren P, Tuomi T, Groop L. Contribution of insulin-stimulated glucose uptake and basal hepatic insulin sensitivity to surrogate measures of insulin sensitivity. Diabetes Care. 2004;27:2204–10. doi: 10.2337/diacare.27.9.2204. [DOI] [PubMed] [Google Scholar]
- 48.Nathan DM. Diabetes: Advances in Diagnosis and Treatment. JAMA. 2015;314:1052–62. doi: 10.1001/jama.2015.9536. [DOI] [PubMed] [Google Scholar]
- 49.Phillips SA, Ciaraldi TP, Kong AP, Bandukwala R, Aroda V, Carter L, et al. Modulation of circulating and adipose tissue adiponectin levels by antidiabetic therapy. Diabetes. 2003;52:667–74. doi: 10.2337/diabetes.52.3.667. [DOI] [PubMed] [Google Scholar]