Abstract
Miniaturized mass spectrometry (MMS) is optimal for a wide variety of applications that benefit from field-portable instrumentation. Like MMS, field asymmetric ion mobility spectrometry (FAIMS) has proven capable of providing in situ analysis, allowing researchers to bring the lab to the sample. FAIMS compliments MMS very well, but has the added benefit of operating at atmospheric pressure, unlike MS. This distinct advantage makes FAIMS uniquely suited for portability.
Since its inception, FAIMS has been envisioned as a field-portable device, as it affords less expense and greater simplicity than many similar methods. Ideally, these are simple, robust devices that may be operated by non-professional personnel, yet still provide adequate data when in the field. While reducing the size and complexity tends to bring with it a loss of performance and accuracy, this is made up for by the incredibly high throughput and overall convenience of the instrument. Moreover, the FAIMS device used in the field can be brought back to the lab, and coupled to a conventional mass spectrometer to provide any necessary method development and compound validation.
This work discusses the various considerations, uses, and applications for portable FAIMS instrumentation, and how the future of each applicable field may benefit from the development and acceptance of such a device.
Keywords: FAIMS, DMS, Portable, Biomedical, Law Enforcement, Environmental
Graphical abstract
1. Introduction
Arguably, the principal purpose of miniaturizing a mass spectrometer is to provide in situ analysis, allowing researchers to bring the lab to the sample, rather than the other way around. Ideally, these are simple, robust instruments that may be operated by non-professional personnel, yet still provide adequate data while in the field. The trade-offs that come from reducing the size and complexity of such an instrument tend to be a loss of performance, accuracy, and efficiency. Therefore, while size and cost are reduced, so is data quality.
Despite that, miniaturized mass spectrometry (MMS) is optimal for many specific applications that benefit from field-portable instrumentation. There is a wide range of uses for MMS, from directly analyzing bioaccumulative contaminants in marine environments,1 to mounting an analyzer onto a moving vehicle,2 to probing for life on other planets,3, 4 as well as many applications in between.5–8 Harsh environments are a staple for MMS, but it is not limited to such uses. Still, given the limited performance of MMS, using MMS in tandem with an additional stage of analysis is attractive; e.g., gas chromatography (GC) or tandem MS (MS/MS).9 Furthermore, an alternative to these more traditional approaches, such as ion mobility spectrometry (IMS) or, more specifically, field asymmetric ion mobility spectrometry (FAIMS), could prove more appealing.
While pairing techniques to use in tandem produces the most comprehensive data, utilizing each technique separately would allow for less expensive, more portable, and simpler analyses to be performed. IMS and FAIMS compliment MMS very well, and have proven capable of also providing in situ analysis in a variety of environments. Whether analyzing compounds in the upper atmosphere,10, 11 detecting illegal substances in a person’s breath,12–15 or being utilized for detection of chemical warfare agents,12, 13, 16, 17 IMS and FAIMS have proven useful for analyzing samples similar to those seen with MMS. Portable IMS has been well defined in previous studies,12, 17 hence, this work will focus on portable FAIMS.
To begin with, FAIMS has one distinct advantage over many techniques that makes it well suited for portability, being able to operate at atmospheric pressure. MMS can function at higher pressures than conventional MS, yet still requires pressures lower than 1 atmosphere, whereas FAIMS does not require any vacuum pumps to operate. Additionally, the fundamental hardware used in FAIMS (at its minimum, simply two parallel electrodes) can be fabricated on the microscale; thus, a FAIMS device is not only inherently reduced in size, but the electronics and hardware needed to power such a device can be manufactured relatively small.
While portability is valuable, FAIMS offers something even more imperative to field-accessible devices: validation of field-collected data on a lab-scale mass spectrometer. Coupling a FAIMS device directly to the front of an MS provides in-depth method development, molecular identification, and compound validation while running analysis in the lab. This same device can be subsequently decoupled from the MS and brought out into the field for rapid, inexpensive, simple, and portable analysis.
Since its inception, FAIMS has been envisioned as a field-portable device. FAIMS lends itself well to simultaneously affording less expense and greater simplicity than many similar methods. This work discusses the various considerations, uses, and applications for portable FAIMS instrumentation, and how the future of each applicable field may benefit from the development and acceptance of such a device.
2. Overview and Considerations
2.1 FAIMS and IMS
FAIMS, also called differential mobility spectrometry or DMS, is a form of ion mobility spectrometry. IMS and FAIMS both separate ions by propelling them through a collision gas by way of an electric field. Separation of ions is based on their mobility, which is a function of their mass, size, shape, and charge.18–21 The principal difference between these two techniques being that IMS separates ions by their mobility in a constant, low-field environment, while FAIMS alternates between high and low-electric field strengths, therefore separating ions based on the difference in their high- and low-field (or differential) mobility. Basic operating fundamentals of FAIMS can be found in other works, and in much more detail than will be provided here.18, 19 An asymmetric voltage, or dispersion voltage (DV), is applied to one of two parallel electrodes while the other is grounded (or near ground). As ions drift longitudinally through the cell, they are orthogonally propelled by the electric field toward the electrodes. The asymmetry of the waveform causes the ions to traverse back and forth, from one electrode to the other, with each cycle. Upon contact with an electrode, the ion is annihilated. To compensate for this displacement, a secondary DC voltage (or compensation voltage, CV) is applied which allows ions of interest to reach the detector. By holding the operational and conditional parameters inside the cell constant, the DV and CV can be scanned (or held steady) to allow monitoring (or isolation) of specific compounds and analytes.
The primary topic of this work is to discuss field-portable alternatives to current techniques, and as such, limitations in portability need to be addressed. The limiting factor for resolution with IMS is the drift tube length.22 Therefore, portability will always be a concern since the drift tube can only become so small before even satisfactory separation is compromised. In contrast, the resolving power lost by miniaturizing a FAIMS cell may be overcome by other means. Reducing the analytical gap between the FAIMS electrodes subsequently lowers the voltage required to separate ions; hence, a smaller waveform generator could be used, reducing power requirements. Moreover, increasing the waveform frequency permits a greater number of ion oscillations per unit of length, which affords higher resolution. Therefore, the FAIMS cell is not required to be as large, and the overall device can be manufactured on a much smaller scale.
However, it should be noted that because the “mode” of separation by IMS is well understood (i.e., molecules are separated based on their drift times, which are influenced by their chemical and physical properties), it is possible to predict the mobility of ions based on theoretically calculated values.12 Conversely, ion mobilities based on FAIMS analysis are much more difficult to predict.22, 23 Correlation of molecular features and the difference in mobility at high and low field is challenging at best; additionally, the effects of ion-atmospheric molecule interactions on mobility are not well understood.19, 24 Consequently, the merits of IMS should not be overlooked as it is still a useful technique in its own right.
2.2 Instrumental Considerations for Miniaturization of FAIMS
There are many factors that must be taken into account when aiming to miniaturize any instrument. For instance, shrinking down a mass spectrometer for field use is one of the more daunting tasks that scientists have been pursuing since the 1950s and 60s,25–27 and has made a resurgence in the last decade and a half. The difficulty in this task lies in the fact that most of the crucial components in a mass spectrometer only function properly under high vacuum (< 10−4 Torr or < 10−7 atm). This entails the use of large, cumbersome vacuum pumps, expensive electronics and optics, and significant power requirements. IMS and FAIMS carry their own burdens as well, yet they are easier to overcome than others. Primarily, maintaining a vacuum chamber to house the analyzer is not an issue with IMS and FAIMS as they function under ambient conditions. The concern of a diminishing mass range as the device is shrunk down is another potential issue that, in contrast to mass spectrometry, does not affect FAIMS. There are many publications demonstrating the application of small FAIMS systems both to lowmass compounds, such as metabolites and lipids,28 and high-mass compounds such as proteins.29
Another consideration of note is the ionization source equipped to the compact device. Ambient ionization methods have become highly attractive for portable instrumentation, largely because they enable real-time analysis with little to no sample preparation.30, 31 Electrospray ionization (ESI) and atmospheric pressure chemical ionization (APCI) are some of the most common techniques used today and can be employed for a variety of samples, yet they both require high voltages and ESI demands large quantities of solvents.31 Nano-ESI would alleviate the solvent waste, and is great for analysis when sample quantity is limited, but still operates at higher than ideal voltages and is only useful for liquid samples.32 Desorption electrospray ionization (DESI) has many of the same drawbacks as ESI, but with one distinct advantage of providing mass spectrometric imaging of the sample.33 Direct analysis in real time (DART) would allow direct ionization of any sample type (solid, liquid, or gas), but requires similarly high voltages as well as a superheated gas stream (regularly above 300 °C).34, 35 One more familiar technique, electron ionization (EI), is not only one of the first ionization techniques ever developed, but remains one of the most widely used to this day.31 Like DART, it can be performed on any sample type, and is useful for its predictable and efficient operation.31 On the other hand, it has a limited mass and volatility range, and it is known for its harsh fragmentation which would be detrimental to many biological applications that benefit from softer ionization techniques. As it stands, several portable instruments today rely on radioactive ionization (e.g., Nickel-63 β-decay) since there are no power requirements and sources can be produced at incredibly small scales. However, radioactive sources lack ionization efficiency and dynamic mass range.12 Furthermore, the paperwork and restrictions that come with using radioactive sources can be a nuisance, if not problematic, for many users.
Ideally, several key factors need to be addressed in creation of the most amenable ionization source. These include size of the source, operational requirements (e.g., solvent use and power), potential waste products, and user/operator safety. Specifically, the source would need to be small and lightweight, made of inexpensive materials for mass production, and all essential requirements would need to be as minimal as possible, with a keen eye kept on voltage and power as that directly impacts the final point of safety. For example, utilizing a portable device for breath analysis would mandate well maintained safety conditions to not allow the source to accidentally harm the user breathing into the device, and a high voltage applied to a source placed in close proximity to the user’s face would not be prudent. Additionally, a portable device would preferably be powered by a common battery, meaning power requirements would be less than that of conventional techniques.
Employing an ionization source that lends itself easily to portability is advantageous, however, the component on the opposite end of the FAIMS device is perhaps the most crucial element. The detector is what makes the device portable. A mass spectrometer has the benefit of vacuum systems to deter interference from other ions, conversion dynodes and electron multiplier tubes to enhance sensitivity, and ion optics to focus ions for better resolution, but none of these can be utilized at ambient pressures, meaning portability is limited.31 Yet, the detector strongly influences instrument sensitivity and selectivity. Thus, a simpler, more robust detector must be developed. Most ion detectors are designed based on the Faraday plate (or cup) in which ions collide with a metal electrode, where the charge is collected for a set period of time on a capacitor and the resulting current is measured.36 The electronics, particularly the capacitor and amplifier, are the primary determination of sensitivity. Developing a detector that can differentiate extremely small amounts of charge while reducing inherent electrical noise, especially at atmospheric pressure, has been a challenge.
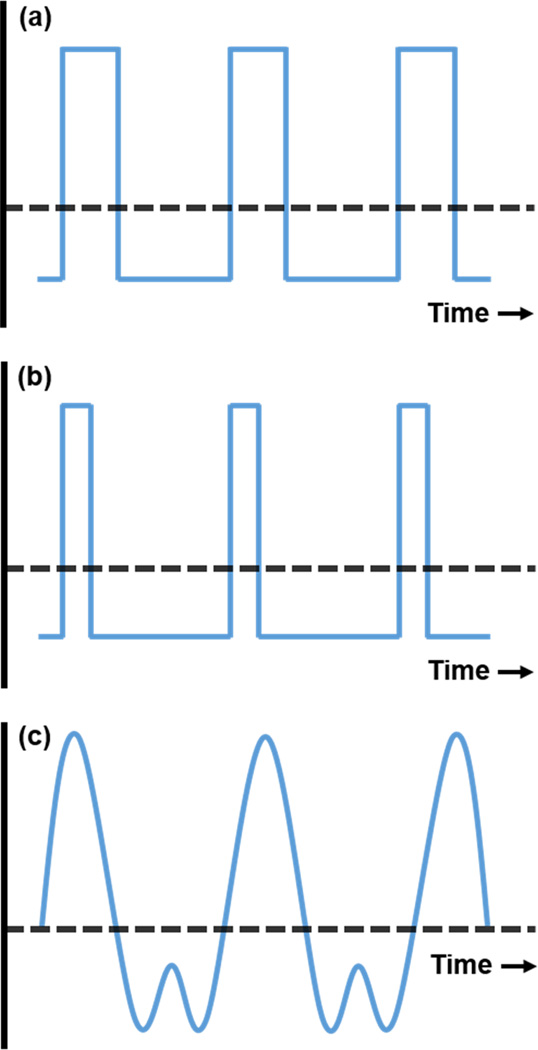
While the previous two considerations would be worth noting no matter what type of field-portable device desired, FAIMS has explicit issues to address. Namely, the electronic waveform supplied to the cell plays a critical role in the size and power required of the device. The basis of FAIMS separation is the difference in ion mobility between high-field and low-field; thus, an asymmetric waveform applied to the FAIMS cell must be capable of achieving both field components, and typically in the RF range. The ideal waveform would provide the quickest transition from high-field component to low-field component, i.e., a square wave (Figure 1a). However, the near-immediate high-voltage transitions of a square-shaped wave are difficult to achieve at RF frequencies.
Figure 1.
Various asymmetric FAIMS waveforms. (a) square, (b) square at a lower duty cycle - 50% of (a), (c) bisinusoidal.
Conventional generators, both commercially available and lab-built, apply electric fields described as “sum-of-sines,” whereby a simple sinusoidal wave is combined with its 2nd harmonic at twice the frequency (Figure 1c). These come close to approximating a square wave, but generally do not perform as well. Previous studies suggest application of a true high-voltage square-waveform (HVSW), or close to, would afford improved sensitivity and selectivity.37 Inherently, a more precipitous drop/increase in voltage with each cycle of the waveform would cause greater differences in the mobility of an ion in the FAIMS cell, producing an increase in separation as well as resolving power. Therefore, a HVSW generator would lead to smaller FAIMS cells, since an increase in differential mobility allows for a decrease in analytical gap dimensions.
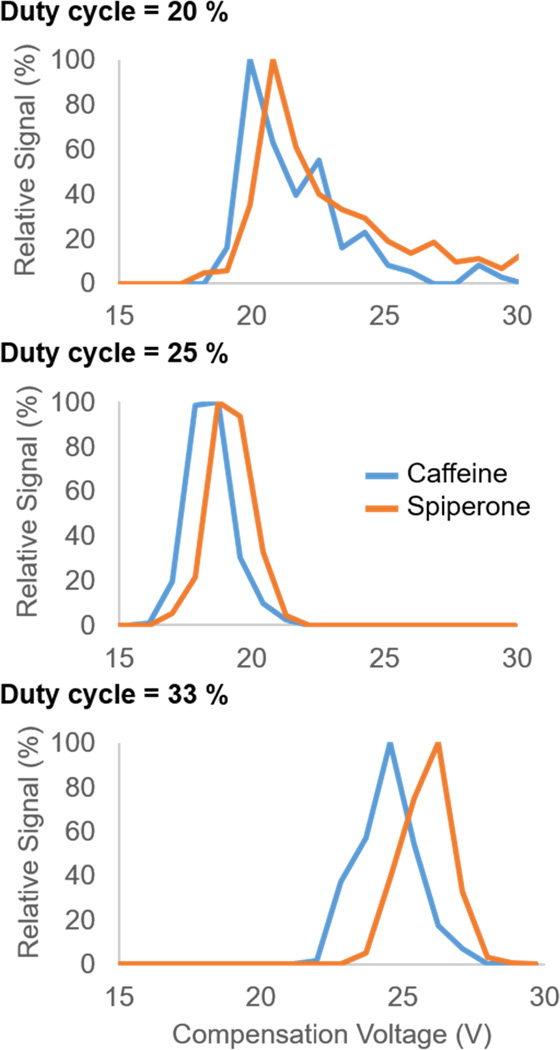
HVSW offers additional opportunities to improve analytical separation by altering the duty cycle of the waveform, i.e. the ratio of the high-field strength portion of each waveform period to the total waveform (Figure 1b). The duty cycle in the bisinusoidal waveform is fixed at 33%. Altering the duty cycle away from 33% in conventional FAIMS is greatly limited by the number of possible harmonics that can be used to generate the waveform; i.e., generating 3rd or 4th harmonics would produce distinct duty cycles, but these become increasingly difficult to produce with each additional harmonic. However, altering the duty cycle is more straightforward with a square waveform. Preliminary data separating a mixture of caffeine and THC in methanol on a miniature-size home-built FAIMS cell (QS-FAIMS, described below in more detail) have shown changes in the resolution, peak width, and analyte signal by altering the duty cycle away from 33% (Figure 2). Likewise, previous studies indicate that the resolving power and signal intensity of TNT improve when operating square-wave FAIMS at a 25% duty as opposed to the 33% duty cycle.37 These changes are thought to result from both the difference in the amount of time analytes spend at each mobility, and the change in the field strength of the low-field portion of the square waveform. Thus, duty cycle has potential as a powerful experimental parameter that can enhance analytical separation without additional instrumentation.
Figure 2.
Compensation voltage (CV) spectrum of caffeine and spiperone. Shows efficiency of separation of [M+H]+ ions of caffeine (m/z 195) and spiperone (m/z 396) using an asymmetric square waveform set to three different duty cycles. Collected at a set dispersion voltage (DV) of 282.52 V.
3. Experimental
3.1 Chemicals and Materials
HPLC-grade acetonitrile, methanol, and water were purchased from Fischer-Scientific (Fair Lawn, NJ). Caffeine and spiperone were purchased from Sigma-Aldrich (St. Louis, MO). THC, (-)-trans-Δ9-tetrahydrocannabinol, was purchased from Cerilliant (Round Rock, TX). Metal salts of chloride (mercury and lead), as well as 2-butanone (>99%), 2-pentanone (>98%), and 2-methylmalonic acid were all purchased from Fischer-Scientific (Fair Lawn, NJ). Indole and succinic acid were purchased from Acros Organics (NJ).
3.2 Instrumentation
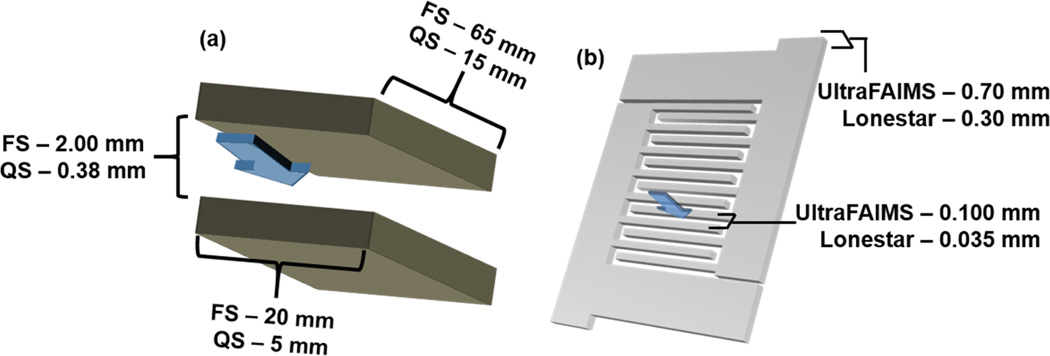
Several mass spectrometers and FAIMS cells/systems were utilized in this work. A total of three mass spectrometers were used in collection of the data: a Thermo Finnigan LCQ Classic (LCQ), a Thermo Finnigan LCQ Deca (DECA), and an Agilent 6460 triple quadrupole (QQQ); all employing ESI. Two home-built planar geometry FAIMS cells were used: a “full-size” cell (FS-FAIMS) with a length of 65 mm, width of 20 mm, and analytical gap of 2mm; and a “quarter-size” cell (QS-FAIMS) with a length of 15 mm, a width of 5 mm, and an analytical gap of 0.38 mm (Figure 3a). Two miniaturized, chip-based FAIMS systems obtained from Owlstone Nanotech Inc. were also utilized in this work: the UltraFAIMS A1 and the Lonestar Gas Analyzer. Both chips in each device are comprised of two interdigitated electrodes that create a serpentine geometry across the face of the chip, where each row is a distinct planar FAIMS channel (Figure 3b). The UltraFAIMS chip consists of 15 total channels, whereas the Lonestar chip contains 47 total channels. As long as ions pass through any one of these channels, they will be acted upon by the electric field and undergo separation. The dimensions of the microchips inside the Owlstone systems are 0.70 mm length×0.10 mm analytical gap and 0.30 mm length×0.035 mm analytical gap for the UltraFAIMS and Lonestar, respectively.
Figure 3.
3D rendering of basic FAIMS cell geometries for the (a) home-built FAIMS cells and (b) Owlstone UltraFAIMS microfabricated chip. The dimensions for the full-size (FS) and quarter-size (QS) cells are displayed in (a), and the dimensions for both of the Owlstone chip systems are displayed in (b). The blue arrow represents the flow of ions through the cell.
All of the data presented on the FS-FAIMS employed the LCQ as the detector and a Thermo Scientific waveform generator (sine, 750 kHz). All data collected on the QS-FAIMS employed the DECA as the detector and a home-built high-voltage square-waveform (HVSW) generator (750 kHz). Data shown for the Owlstone UltraFAIMS system was paired exclusively to the QQQ and utilized its own built-in waveform generator (sine, 28.6 MHz). The Lonestar system is a standalone FAIMS device complete with ionization source (63Ni β-decay), microfabricated FAIMS chip, waveform generator (sine, 28.6 MHz), and Faraday-cup detector in a single system.
3.3 Methods
Standard solutions and mixtures of caffeine, spiperone, and THC were prepared at concentrations ranging from 50 ppm to 300 ppb, all using methanol as the solvent. A solution comprised of 1 ppm mercury chloride and 1 ppm lead chloride was also prepared in methanol. Solutions were infused directly into a home-built custom ESI source at a flow rate of 10 µL/min. The ESI emitter tip was positioned in-line with inlet of the FAIMS cell at a few millimeters away.
MMA and SA were weighed and dissolved in methanol to a concentration of 1 ppm. Standards were directly infused into the ESI source using a syringe pump at a flow rate of 15 µL/min, and analyzed by the Owlstone UltraFAIMS/QQQ setup.
Simulated breath experiments were performed to determine if standalone FAIMS had the capability to detect analytes of interest in a gaseous sample with high solvent concentrations present. 2-butanone, 2-pentanone, and indole were each spiked into 100 mL water in a 1L glass bottle containing a bubbler system. Nitrogen gas was blown into the bubbler below the water level, generating a headspace containing the VOCs. The exhaust of the bubbler was directed toward the inlet of the Lonestar system, which has a vacuum pump on the back with a flow controller set at 1.5 L/min. The temperature of the FAIMS chip was approximately 45 °C, and all measurements were performed under atmospheric conditions, and collected in both positive and negative mode.
4. Application Areas, Results, and Discussion
Traditional, lab-scale mass spectrometers are regularly considered the workhorse instruments for many laboratories, and are used for a variety of applications, over numerous fields of interest. Though they may be cumbersome, complicated to run, and require expensive pumps to function, no tool is better suited to provide the most accurate data possible for many types of biological and environmental samples. Whether running blood or plasma samples, analyzing polluted lake water, or identifying markers of disease, analysis by MS is often the best and most preferred choice, even if it tends cost more time and money.
However, if there was a simple and compact device available that could cut down on the number of samples needing to be shipped to an off-site lab for extensive analysis, it would be of great benefit to many fields of interest, as well as the mass spectrometric community at large. More so if that device could not only work as a standalone field-portable apparatus, but could also couple to a mass spectrometer for method development and result validation. Such a device could become a reality by utilizing FAIMS, and in this work, three notable areas of application will be discussed.
4.1 Counterterrorism and Law Enforcement
At present, most IMS and FAIMS devices are in use by the government and military, and of those, a great number have been developed for explosives detection. There are IMS and FAIMS devices used to sniff out bombs by armed forces abroad and detect chemical warfare agents,12, 13, 15, 17 as well as those used by the U.S. Transportation Security Administration to analyze the hands, shoes, and accessories of passengers wishing to depart from many airports around the country for explosives residue.16 Previous work performed in the Yost Laboratory in recent years, both published38 and unpublished, corroborates these findings. Separation of explosives was observed by FAIMS/MS using both planar and cylindrical cell geometries, on home-built and commercial FAIMS units of comparable size to FS-FAIMS, but is not detailed here. Continued improvement of these instruments is beneficial to the safety and wellbeing of soldiers and citizens alike, yet there are other applications for which FAIMS could prove equally valuable.
An important function that could be widely applied on a daily basis would be detecting the presence of illegal substances on a person’s breath. For instance, giving a police officer the ability to analyze the breath of someone they suspect of DUI while out on the road. Typically, officers rely on visual cues and field sobriety tests to determine impairment,39 but a method to scientifically quantify impairment by various substances is needed. Analysis of breath has become the gold standard for monitoring blood alcohol, but it is not currently used for any other substances; such as marijuana, cocaine, other illegal narcotics, and many over-the-counter or prescription medications that can cause impairment when operating a motor vehicle. This type of instrument could ultimately reduce the number of motor vehicle incidents involving intoxicated and impaired drivers. Additionally, law enforcement officers would have the ability to scientifically determine if a person is impaired, providing an important assessment in a court of law.
Furthermore, a portable breath monitor could just as easily be mounted in a civilian vehicle to be used as an interlock device. Currently, if a person is convicted of driving under the influence of alcohol, they may be instructed to have their vehicle equipped with a device that will only allow it to start if they blow into the device revealing no trace of alcohol on their breath. A FAIMS interlock device could be set to monitor any drug, allowing more inclusive penalties for DUIs outside of alcohol. Similarly, a portable drug-monitoring device could be used in the workplace by employers to test their employees on a regular basis. A simple device could be used by the employers themselves, alleviating much of the cost of expensive third-party blood, urine, and hair testing, and only requiring such extensive, off-site analysis in the event of a positive breath test result.
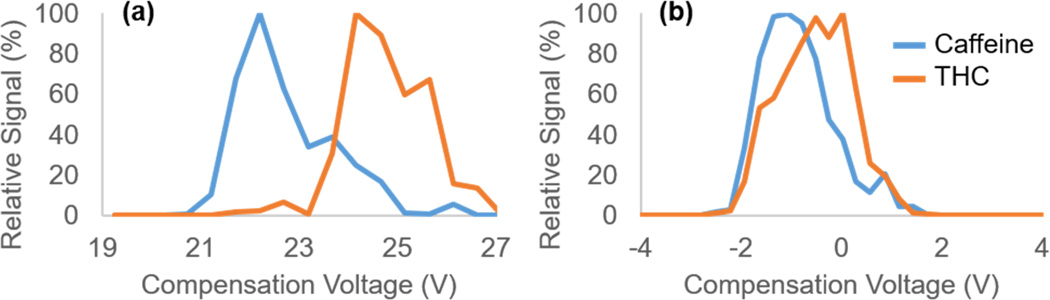
Preliminary data using our current square-wave FAIMS prototype on the QS-FAIMS has produced superior separation compared to those obtained using sine-wave FAIMS for a number of drug molecules. It should be noted that this improved separation occurs even when we operate our square-wave FAIMS at lower field strengths than in our sine-wave FAIMS setup. For example, Figure 4 illustrates the improvement afforded by HVSW in the analysis of a two-component mixture of caffeine (m/z 195) and (−)-trans-Δ9-tetrahydrocannabinol (THC, m/z 315). Despite the field strength of the sine-wave analysis being more than twice that of the square-wave analysis, the resolution between analytes is significantly improved in the square-wave FAIMS case. Further improvements in resolution and resolving power are expected as the field strengths attainable by the square waveform increase and voltage transition times decrease.
Figure 4.
Compensation voltage (CV) spectrum of caffeine and THC. Shows efficiency of separation of [M+H]+ ions of caffeine (m/z 195) and THC (m/z 315) using (a) an asymmetric square waveform at a set dispersion voltage (DV) of 282.52 V and (b) a conventional sinusoidal waveform at a DV of 576.45 V.
4.2 Environmental
The second field of application that could benefit from FAIMS is the analysis of environmental hazards and pollutants. Acute pesticide poisoning (APP) is a global health concern, and is responsible for millions of reported incidents and hundreds of thousands of deaths annually, and that is in developed countries alone.40, 41 The adverse effects related to pharmaceutical waste found at high levels in drinking water is another major factor for public wellness. Therefore, having the ability to provide high-speed trace analysis is a necessity for environmental health and safety. FAIMS offers the ability to rapidly and portably test for ecological contaminants such in the air and water.32, 42, 43
In addition to pharmaceuticals and pesticides, many metals are found as pollutants in water and soil and can be damaging to the environment. These substances, mostly transition metals and otherwise known as “heavy” metals, originate from a various sources. Pollution from trash, byproducts from industrial processes, and mining operations are all potential hazards that could lead to serious health concerns if not regularly monitored. FAIMS has the potential to augment currently established methods for the detection of trace metallic elements in environmental or nuclear applications.
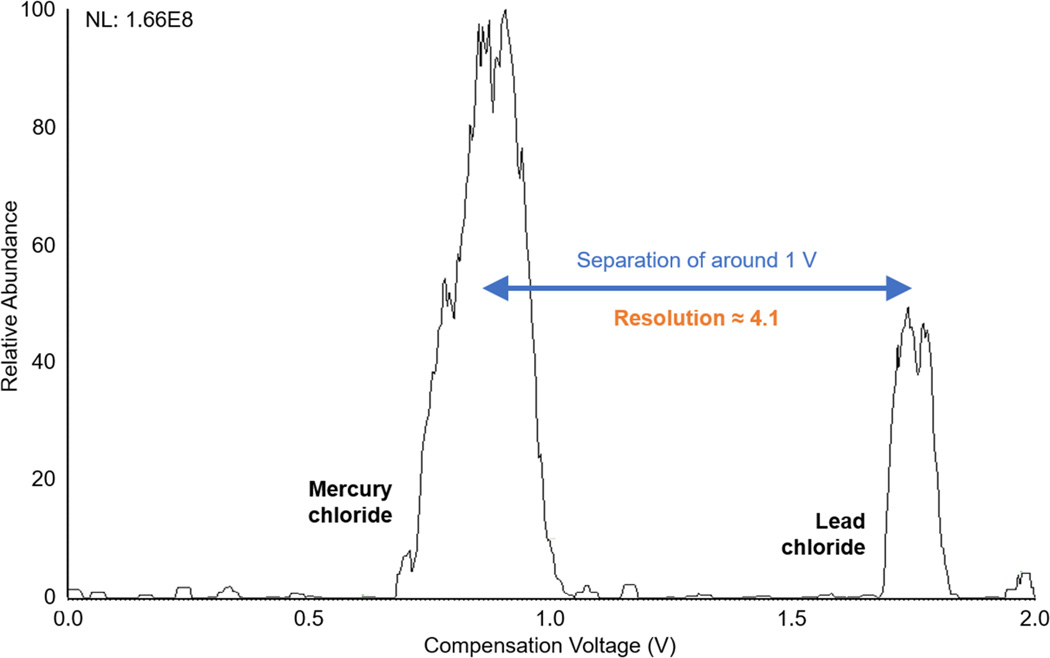
Standards of several chlorinated compounds were analyzed by the home-built FS FAIMS cell over a range of dispersion (DV) and compensation voltages (CV). Figure 5 shows a negative-mode CV spectrum where separated peaks from chlorides of mercury and lead are observed. FAIMS provides baseline resolution of these chloride compounds in a matter of minutes. What’s more, under the same conditions and operational parameters, these compounds can be monitored in real time by holding the CV at a set value. For instance, setting the CV to 0.85 V or 1.85 V allows detection of mercury or lead, respectively, while filtering out the other metal. Thus, with FAIMS, in situ analysis of environmental samples is a promising prospect as the instrumentation continues to improve.
Figure 5.
Compensation voltage (CV) spectrum of mercury chloride and lead chloride. Produced by scanning the CV from 0 to 2 V over a span of 10 minutes, at a set dispersion voltage (DV) of −4500 V. Demonstrates baseline resolution of the chlorides of mercury (left) and lead (right).
4.3 Biomedical
Today, most diagnostic tests require drawing blood, analyzing urine, or in extreme cases collecting a biopsy.44 These methods are often invasive and costly. Moreover, testing and analysis regularly take a minimum of several hours or days, and biological specimens require special safety and storage conditions.45, 46 A swab might be taken to check for bacterial and viral infections, but must be cultured over the course of days, if not weeks. Conversely, breath analysis is noninvasive, requires minimal to no special safety conditions, and can be collected anytime, anywhere. Analyzing breath provides a safer and more rapid alternative to the typical tests conducted for many diseases. Furthermore, the supply of breath that could be collected from the body is not finite, unlike the other sample types mentioned. Analysis of breath has even been shown to provide sufficient separation of blood-borne biomarkers;47–50 thus, it could offer real-time assessment for companion diagnostics as well as early diagnosis of disease, providing invaluable information for every physician, clinician, and biomedical researcher. Such a paradigm shift would save millions of dollars in medical and labor costs, and, more importantly, has the potential to save countless lives. Although breath analysis has the potential to conduct these types of analyses, current techniques do not provide such capabilities in a rapid and cost-effective clinical setting.45, 51–53 FAIMS could prove the ideal solution to this dilemma.54 There have been several successful reports of conducting breath analysis by FAIMS,48, 55–58 and our own research reveals similar results.50, 54, 59, 60
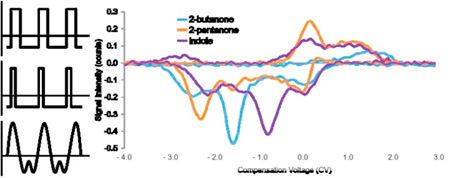
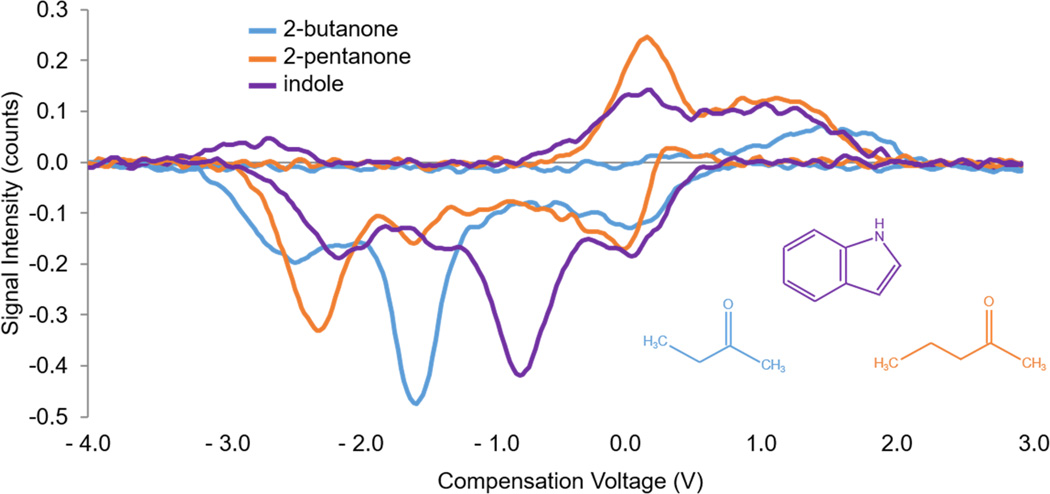
As mentioned, unique patterns of exhaled volatile organic compounds (VOCs), as well as less volatile compounds, could be used to characterize or identify diseases.61 A well-known example is the endogenous VOC acetone, which is present with significant elevated levels in exhaled breath of patients with diabetes mellitus.62–65 What’s more, patients with diabetes mellitus may be at a higher risk of developing liver disease, which left undiagnosed can lead to further damage to the liver.66 Cirrhosis of the liver involves the continuous replacement or encapsulation of damaged tissue by scar tissue, preventing the liver from functioning properly. Rearrangements of damaged blood vessels and the portal vein result in recirculation of toxic compounds in the body.67 For this work, the Lonestar, a standalone FAIMS instrument, was used to analyze several potential biomarkers related to liver cirrhosis in exhaled breath. Many notable compounds48, 68 were studied by analysis of their standards in water vapor, with three revealed to be most significant: 2-butanone, 2-pentanone, and indole. Separation by FAIMS of these key compounds can be observed in Figure 6, without use of MS as the detector.
Figure 6.
Compensation voltage (CV) spectrum. Produced by scanning the CV from −4 to 3 V, at a set dispersion voltage (DV) of 195 V (positive mode) and −132 V (negative mode). Demonstrates good separation, in negative mode, of three potential markers for liver cirrhosis: 2-butanone, 2-pentanone, and indole (100, 150, and 150 ppm in water, respectively) by standalone FAIMS. Standards were analysed separately and their spectra overlaid.
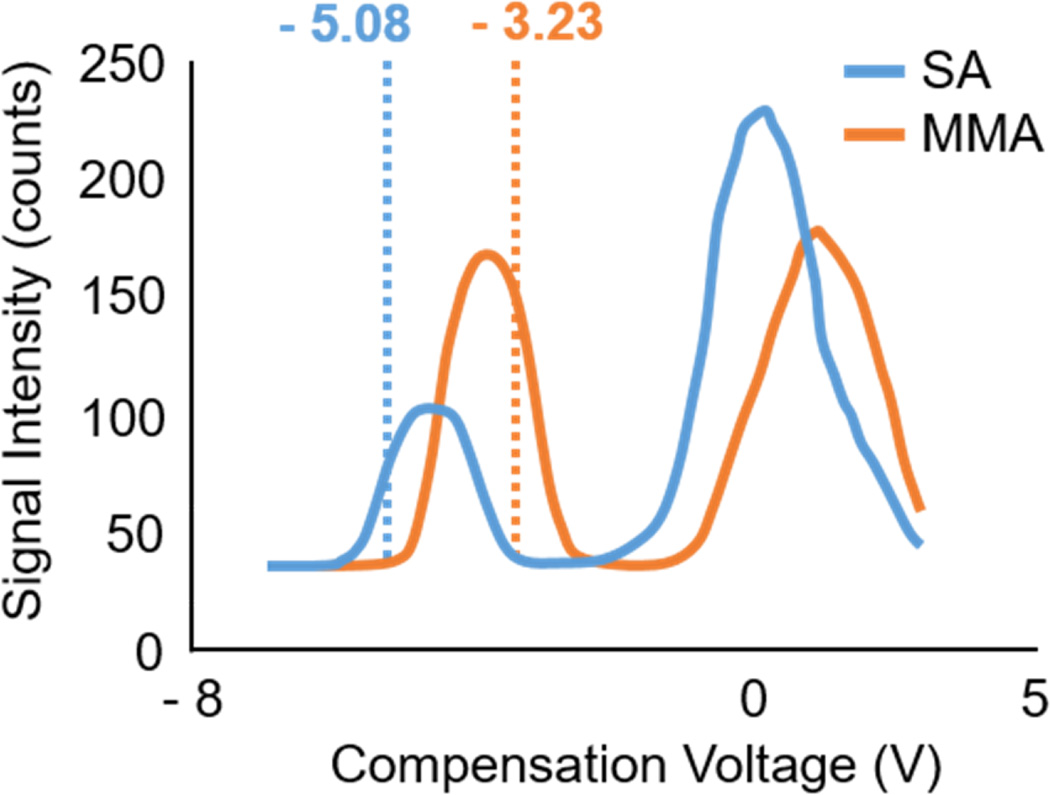
These small FAIMS systems, both standalone and FAIMS/MS, are great at separating and detecting some compounds, like those for cirrhosis above, but may prove problematic with others, especially under the wrong analytical conditions. For instance, methylmalonic acid (MMA) and succinic acid (SA) two biomedical isomers part of the metabolic pathway for cobalamin (vitamin B12),69 do not separate under standard conditions using an Owlstone UltraFAIMS/QQQ system. In the absence of vitamin B12, the MMA concentration will become elevated indicating a vitamin B12 deficiency and methylmalonic acidemia,69–71 making it important to be able to quantify MMA without interference from SA. Conventional methods for MMA detection involve extensive LC/MS/MS or GC/MS/MS assays, both methods involve extensive sample preparation and analysis time, resulting in low throughput.69, 70 Providing a more rapid alternative, such as the FAIMS/MS system above, would drastically improve the output of such assays. Fortunately, by modifying the gas/solvent system inside the FAIMS cell, the desired separation can be achieved. With the addition of solvent vapor modifier or a different auxiliary gas to the cell, the differential mobility is affected,38 often producing increased resolution under the right conditions. Figure 7 demonstrates how the addition of 1,000 ppm ACN and 2,000 ppm water vapour to the FAIMS cell can improve the separation between MMA and SA. Although base line separation has not been achieved, complete isolation of these two compounds can be by selecting targeted CV values, similar to how a quadrupole isolates m/z. For a more comprehensive study into solvent-modifier effects, please refer to Rorrer, et. al.38
Figure 7.
Compensation voltage (CV) spectrum. Produced by scanning the CV from −8 to 4 V, at a set dispersion voltage (DV) of 404.06 V. Reveals MMA and SA can be isolated at distinct CV values; −3.23 V (orange dotted line) and −5.08 V (blue dotted line), respectively.
No matter how practical FAIMS may prove in the field, it would be impossible to compete with the raw analytical power of a mass spectrometer in the laboratory. However, a standalone FAIMS device affords the requisite convenience of a compact device, while also having the ability to remove the standalone detector and place it on the front of a MS, using it as the detector instead. What this allows is rapid fingerprint screening in the field on a device that can then be taken back to the lab, where data can be verified and validated on a mass spectrometer. This marriage of FAIMS and MS could prove most valuable for work in biomarker discovery.
5. Summary and Future Perspectives
5.1 Summary
Both classic drift tube IMS and FAIMS offer their own distinct advantages for the separation and isolation of small molecules. IMS offers predictability and relative ease of use, whereas FAIMS provides the most rapid analysis possible in the most compact device. Each technique offers a wide range of applications both as a standalone instrument and coupled to a mass spectrometer. This potential for coupling/decoupling gives IMS and FAIMS the greatest advantage over most other portable techniques. Utilizing IMS or FAIMS prior to MS analysis affords better method development, compound identification, and biomarker validation on a device that is inexpensive, simple, and field-portable. This is especially advantageous when compared to electronic sensors, often seen as the ideal devices for use outside of the laboratory due to their nanoscale technology and rapid analysis. Performing analyses on the same device that was used in the field, that is then run on the MS in the lab provides the most accurate and efficient validation possible. Using a sensor and verifying with GC/MS can give an idea of what might be seen with that sensor, but will never provide absolute certainty. FAIMS can deliver that confidence, providing a field instrument with compound verification and elimination of common interferences.
5.2 Future Perspectives
Overall, there are a variety of diverse applications to which FAIMS would be uniquely applicable. A standalone FAIMS system could easily fit in a police car to provide on-site DUI testing for a single or multiple illicit or abused substances. Moreover, smaller handheld devices could be used for specific drugs of interest. FAIMS would be also well suited for several environmental applications, such as monitoring toxic pesticides in the air around vital crops, or detecting trace amount of heavy metals in water and soil. Likewise, trace analysis could be performed by the pharmaceutical industry for research and development, as well as for clinical compliance. Additionally, a portable FAIMS device could be revolutionary to the medical field, providing a low-risk, less invasive, more rapid alternative for diagnostic screening, thus affording real-time assessment for companion diagnostics as well as early diagnosis of disease. This would not only benefit medical professionals in hospitals and clinics, but an inexpensive handheld device that is simple to operate would improve current methods for diagnosis and monitoring of pandemic diseases in countries where medical care is not as ubiquitous as in the U.S.
FAIMS instruments and methodologies have come a long way but are still not reached their true potential. The best prospects for this field may involve thinking outside the box in both analytical methodology and instrumental design. Important areas to focus for instrumentation would be additional customizations, but with increased instrument stability to help build reproducible results and validated analytical methods. Combining IMS with FAIMS could provide a uniquely portable yet highly selective device.72 Adding solvent vapor to the FAIMS cell has proven to increase resolving power.38 Investigations involving dimer and trimer analyte species and their potential for expanding ion mobility separation strategies when monomeric analyte species cannot be resolved should be explored. Additionally, the use of cations (e.g., sodium, potassium, cesium, lithium, etc.) for analyte adduct formation would be interesting to investigate as another potential separation strategy, as it would require very little sample preparation in comparison to LC derivatization methodologies.73
Use of HVSW for FAIMS separation is essential as FAIMS instrumentation continues to reduce in size. Although developing a high-frequency and high-voltage square wave supply for a large FAIMS cell would be a daunting task, it is straightforward for a small cell.37 Reducing the size of the FAIMS cell makes developing a HVSW generator practical, since peak voltages applied across a smaller analytical gap would not need to be as high. Consequently, a lower voltage means less power required, and less power implies reduced size of the supply, which is often responsible for the largest portion of the footprint of a portable instrument. Therefore, outside of the intrinsic fundamental benefits of a HVSW afforded to selectivity and sensitivity of FAIMS, there would also be reductions in size and cost of the overall device.
Finally, all of the components surrounding the FAIMS cell need to be addressed. Reproducible, reliable, and efficient techniques for breath sampling, ionization, and ion detection are all key in creation of a portable device. Each method must be compact, but remain accurate and provide adequate sampling and detection to be able to parse out the low concentrations of compounds of interest, in a variety of matrices, often associated with breath and gas sampling.
Acknowledgments
The authors would like thank and acknowledge Joaquin Cassanova and the Tech Toybox of Gainesville, FL for their help with design and fabrication of the electronics used to power the home-built FAIMS cells, the University of Florida Chemistry Department’s machine shop for their work machining the home-built FAIMS cells, and Owlstone Nanotech Inc. for providing the Lonestar Gas Analyzer and UltraFAIMS system.
Funding:
This work was supported by the Southeast Center for Integrated Metabolomics (SECIM) - National Institute of Health (NIH) [grant number U24 DK097209]; and funding from Breathtec Biomedical, Cannabix Technologies, and Agilent Technologies.
Abbreviations
- DMS
Differential mobility spectrometry
- DUI
Driving under the influence (of drugs, alcohol, etc.)
- FAIMS
High-field asymmetric-waveform ion mobility spectrometry
- HVSW
High-voltage square wave
- IMS
Ion mobility spectrometry
- MMS
Miniaturized mass spectrometry
- THC
(−)-trans-Δ9-tetrahydrocannabinol
- VOC
Volatile organic compound
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hoh E, Dodder NG, Lehotay SJ, Pangallo KC, Reddy CM, Maruya KA. Environ. Sci. Technol. 2012;46:8001–8008. doi: 10.1021/es301139q. [DOI] [PubMed] [Google Scholar]
- 2.Mach PM, McBride EM, Sasiene ZJ, Brigance KR, Kennard SK, Wright KC, Verbeck GF. Anal. Chem. 2015;87:11501–11508. doi: 10.1021/acs.analchem.5b03269. [DOI] [PubMed] [Google Scholar]
- 3.Chela-Flores J, Cicuttin A, Crespo ML, Tuniz C. Int. J. Astrobiol. 2015;14:427–434. [Google Scholar]
- 4.Neuland MB, Grimaudo V, Mezger K, Moreno-Garcia P, Riedo A, Tulej M, Wurz P. Meas. Sci. Technol. 2016;27:035904/1–035904/13. [Google Scholar]
- 5.Ouyang Z, Cooks RG. Annu. Rev. Anal. Chem. 2009;2:187–214. doi: 10.1146/annurev-anchem-060908-155229. [DOI] [PubMed] [Google Scholar]
- 6.Snyder DT, Pulliam CJ, Ouyang Z, Cooks RG. Anal. Chem. 2015 doi: 10.1021/acs.analchem.5b03070. Ahead of Print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pulliam CJ, Wei P, Snyder DT, Wang X, Ouyang Z, Pielak RM, Graham Cooks R. Analyst. 2016;141:1633–1636. doi: 10.1039/c5an02575c. [DOI] [PubMed] [Google Scholar]
- 8.Ma Q, Bai H, Li W, Wang C, Cooks RG, Ouyang Z. Talanta. 2015;142:190–196. doi: 10.1016/j.talanta.2015.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lebedev AT. Annu. Rev. Anal. Chem. 2013;6:163–189. doi: 10.1146/annurev-anchem-062012-092604. [DOI] [PubMed] [Google Scholar]
- 10.Eiceman GA, Salazar MR, Rodriguez MR, Limero TF, Beck SW, Cross JH, Young R, James JT. Anal. Chem. 1993;65:1696–1702. doi: 10.1021/ac00061a011. [DOI] [PubMed] [Google Scholar]
- 11.Morris AKR, Ringrose TJ, Sheridan S, Wright I, Parris R, Boyle B, Morgan GH. In-situ monitoring of compounds in planetary atmospheres using a miniaturized field asymmetric ion mobility spectrometer. Milton Keynes, UK: Planetary and Space Sciences Research Institute, The Open University; [Accessed August 2016]. p. 6. [Google Scholar]
- 12.Armenta S, Alcala M, Blanco M. Anal. Chim. Acta. 2011;703:114–123. doi: 10.1016/j.aca.2011.07.021. [DOI] [PubMed] [Google Scholar]
- 13.Kolakowski BM, Mester Z. Analyst. 2007;132:842–864. doi: 10.1039/b706039d. [DOI] [PubMed] [Google Scholar]
- 14.Pollard M, Hilton C, Li H, Kaplan K, Yost R, Hill H. Int. J. Ion. Mobil. Spectrom. 2011;14:15–22. [Google Scholar]
- 15.Li L, Wang Y, Chen C, Wang X, Luo J. J. Mass Spectrom. 2015;50:792–801. doi: 10.1002/jms.3580. [DOI] [PubMed] [Google Scholar]
- 16.Ewing RG, Atkinson DA, Eiceman GA, Ewing GJ. Talanta. 2001;54:515–529. doi: 10.1016/s0039-9140(00)00565-8. [DOI] [PubMed] [Google Scholar]
- 17.Zimmermann S, Barth S, Baether WKM, Ringer J. Anal. Chem. 2008;80:6671–6676. doi: 10.1021/ac800559h. [DOI] [PubMed] [Google Scholar]
- 18.Buryakov IA, Krylov EV, Nazarov EG, Rasulev UK. Int. J. Ion Mobil. Spectrom. 1993;128:143–148. [Google Scholar]
- 19.Guevremont R. J. Chromatogr. A. 2004;1058:3–19. [PubMed] [Google Scholar]
- 20.Purves RW, Guevremont R, Day S, Pipich CW, Matyjaszczyk MS. Rev. Sci. Instrum. 1998;69:4094–4105. [Google Scholar]
- 21.Lapthorn C, Pullen F, Chowdhry BZ. Mass Spectrom. Rev. 2013;32:43–71. doi: 10.1002/mas.21349. [DOI] [PubMed] [Google Scholar]
- 22.Lanucara F, Holman SW, Gray CJ, Eyers CE. Nat. Chem. 2014;6:281–294. doi: 10.1038/nchem.1889. [DOI] [PubMed] [Google Scholar]
- 23.Shvartsburg AA, editor. Differential Ion Mobility Spectrometry: Nonlinear Ion Transport and Fundamentals of FAIMS. CRC Press; 2009. p. 322. [Google Scholar]
- 24.Cumeras R, Figueras E, Davis CE, Baumbach JI, Gracia I. Analyst (Cambridge U.K.) 2015;140:1391–1410. doi: 10.1039/c4an01101e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McNarry LR. Natl. Research Council Can. 1956;(4259):18. [Google Scholar]
- 26.Doil'nitsyn EF, Trubetskoi AI, Shcherbakova MY. Prib. Tekh. Eksp. 1959:81–82. [Google Scholar]
- 27.Bailey AD, Narcisi RS. Miniature mass spectrometers for upper atmosphere composition measurements. Air Force Cambridge Research Labs: L.G. Hanscom Field; 1966. [Google Scholar]
- 28.Griffiths RL, Dexter A, Creese AJ, Cooper HJ. Analyst (Cambridge U.K.) 2015;140:6879–6885. doi: 10.1039/c5an00933b. [DOI] [PubMed] [Google Scholar]
- 29.Shvartsburg AA, Smith RD. Anal. Chem. (Washington, DC, US) 2012;84:7297–7300. doi: 10.1021/ac3018636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Domin M, Cody R, editors. Ambient Ionization Mass Spectrometry. London, UK: Royal Society of Chemistry; 2014. p. 508. [Google Scholar]
- 31.Watson JT, Sparkman OD. Introduction to Mass Spectrometry: Instrumentation, Applications, and Strategies for Data Interpretation. 4th. West Sussex, UK: Wiley Inc.; 2007. [Google Scholar]
- 32.Zhao YY, Liu X, Boyd JM, Qin F, Li J, Li X-F. J. Chromatogr. Sci. 2009;47:92–96. doi: 10.1093/chromsci/47.1.92. [DOI] [PubMed] [Google Scholar]
- 33.Takats Z, Wiseman JM, Cooks RG. J. Mass. Spectrom. 2005;40:1261–1275. doi: 10.1002/jms.922. [DOI] [PubMed] [Google Scholar]
- 34.Cody RB, Laramee JA, Durst HD. Anal. Chem. 2005;77:2297–2302. doi: 10.1021/ac050162j. [DOI] [PubMed] [Google Scholar]
- 35.Monge ME, Harris GA, Dwivedi P, Fernandez FM. Chem. Rev. 2013;113:2269–2308. doi: 10.1021/cr300309q. [DOI] [PubMed] [Google Scholar]
- 36.Busch KL. Spectroscopy. 2011;26(12):14–18. [Google Scholar]
- 37.Prieto M, Tsai C-W, Boumsellek S, Ferran R, Kaminsky I, Harris S, Yost RA. Anal. Chem. 2011;83:9237–9243. doi: 10.1021/ac200948b. [DOI] [PubMed] [Google Scholar]
- 38.Rorrer LC, Yost RA. Int. J Ion Mobil. Spectrom. 2011;300:173–181. [Google Scholar]
- 39.DWI detection and standardized field sobriety testing. National Highway Traffic Safety Administration; 2013. Mar, p. 353. HS 178 R5/13 ed. [Google Scholar]
- 40.Jafari MT. Talanta. 2006;69:1054–1058. doi: 10.1016/j.talanta.2005.12.017. [DOI] [PubMed] [Google Scholar]
- 41.Alarcon WA, Calvert GM, Blondell JM, Mehler LN, Sievert J, Propeck M, Tibbetts DS, Becker A, Lackovic M, Soileau SB, Das R, Beckman J, Male DP, Thomsen CL, Stanbury M. J. Am. Med. Assoc. 2005;294:455–465. doi: 10.1001/jama.294.4.455. [DOI] [PubMed] [Google Scholar]
- 42.Monitoring solvent levels in pharmaceutical waste water using a Lonestar portable analyzer. Cambridge, UK: Owlstone Nanotech Inc.; [Accessed August 2016]. p. 26. [Google Scholar]
- 43.Detection of metaldehyde in water using a Lonestar portable analyzer. Cambridge, UK: Owlstone Nanotech Inc.; [Accessed August 2016]. p. 18. [Google Scholar]
- 44.Costanzo MT, Ulmer CZ, Mohan S, Gravenstein N, Yost RA. presented at Proceedings of the 62nd ASMS Conference on Mass Spectrometry and Allied Topics. Baltimore, MD: 2014. [Google Scholar]
- 45.Cao W, Duan Y. Cr. Rev. Anal. Chem. 2007;37:3–13. [Google Scholar]
- 46.Murugan A, Prys-Picard C, Calhoun WJ. Curr. Opin. Pulm. Med. 2009;15:12–18. doi: 10.1097/MCP.0b013e32831de235. [DOI] [PubMed] [Google Scholar]
- 47.Berchtold C, Meier L, Zenobi R. Int. J. Ion Mobil. Spectrom. 2011;299:145–150. [Google Scholar]
- 48.Corradi M, Gergelova P, Mutti A. Eur. Respir. Monogr. 2010:140–151. [Google Scholar]
- 49.Wehinger A, Schmid A, Mechtcheriakov S, Ledochowski M, Grabmer C, Gastl GA, Amann A. Int. J. Ion Mobil. Spectrom. 2007;265:49–59. [Google Scholar]
- 50.Kemperman RHJ, Costanzo MT, Beekman CR, Chouinard CD, Yost RA. presented at Proceedings of the 63rd ASMS Conference on Mass Spectrometry and Allied Topics. St Louis, MO: 2015. [Google Scholar]
- 51.Modak AS. J. Breath R. 2010;4:017002/1–017002/6. doi: 10.1088/1752-7155/4/1/017002. [DOI] [PubMed] [Google Scholar]
- 52.Popov TA. Ann. Allerg. Asthma. Im. 2011;106:451–456. doi: 10.1016/j.anai.2011.02.016. [DOI] [PubMed] [Google Scholar]
- 53.Phillips M. Sci. Am. 1992;267:74–79. doi: 10.1038/scientificamerican0792-74. [DOI] [PubMed] [Google Scholar]
- 54.Costanzo MT, Tsai C, Yost RA. presented at Proceedings of the 60th ASMS Conference on Mass Spectrometry and Allied Topics. Vancouver, BC, CA: 2012. [Google Scholar]
- 55.Bessa V, Darwiche K, Teschler H, Sommerwerck U, Rabis T, Baumbach J, Freitag L. Int. J. Ion Mobil. Spectrom. 2011;14:7–13. [Google Scholar]
- 56.Maddula S, Rabis T, Sommerwerck U, Anhenn O, Darwiche K, Freitag L, Teschler H, Baumbach J. Int. J. Ion Mobil. Spectrom. 2011;14:197–206. [Google Scholar]
- 57.Fink T, Baumbach JI, Kreuer S. J. Breath Res. 2014;8 doi: 10.1088/1752-7155/8/2/027104. 027104/1-027104/11, 11 pp. [DOI] [PubMed] [Google Scholar]
- 58.Covington JA, van. der Schee MP, Edge ASL, Boyle B, Savage RS, Arasaradnam RP. Analyst. 2015;140:6775–6781. doi: 10.1039/c5an00868a. [DOI] [PubMed] [Google Scholar]
- 59.Costanzo MT, Kazaleh MS, Kemperman RHJ, Yost RA. presented at Proceedings of the 63rd ASMS Conference on Mass Spectrometry and Allied Topics. St. Louis, MO: 2015. [Google Scholar]
- 60.Wei MS, Costanzo MT, Casanova J, Boock JJ, Yost RA. presented at Proceedings of the 64th ASMS Conference on Mass Spectrometry and Allied Topics. San Antonio, TX: 2016. [Google Scholar]
- 61.Westhoff M, Litterst P, Freitag L, Urfer W, Bader S, Baumbach JI. Thorax. 2009;64:744–748. doi: 10.1136/thx.2008.099465. [DOI] [PubMed] [Google Scholar]
- 62.Manolis A. Clin. Chem. 1983;29:5–15. [PubMed] [Google Scholar]
- 63.Miekisch W, Schubert JK, Noeldge-Schomburg GF. E. Clin. Chim. Acta. 2004;347:25–39. doi: 10.1016/j.cccn.2004.04.023. [DOI] [PubMed] [Google Scholar]
- 64.van den Velde S, Quirynen M, van Hee P, van Steenberghe D. J. Chromatogr. B: Anal. Technol. Biomed. Life Sci. 2007;853:54–61. doi: 10.1016/j.jchromb.2007.02.048. [DOI] [PubMed] [Google Scholar]
- 65.van den Velde S, Quirynen M, van Hee P, van Steenberghe D. Anal. Chem. 2007;79:3425–3429. doi: 10.1021/ac062009a. [DOI] [PubMed] [Google Scholar]
- 66.Levinthal GN, Tavill AS. Clinical Diabetes. 1999;17 [Google Scholar]
- 67.Schuppan D, Afdhal NH. Lancet. 2008;371:838–851. doi: 10.1016/S0140-6736(08)60383-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.van den Velde S, Nevens F, van Hee P, van Steenberghe D, Quirynen M. J. Chromatogr. B: Anal. Technol. Biomed. Life Sci. 2008;875:344–348. doi: 10.1016/j.jchromb.2008.08.031. [DOI] [PubMed] [Google Scholar]
- 69.Magera MJ, Helgeson JK, Matern D, Rinaldo P. Clin. Chem. 2000;46:1804–1810. [PubMed] [Google Scholar]
- 70.Marcell PD, Stabler SP, Podell ER, Allen RH. Anal. Biochem. 1985;150:58–66. doi: 10.1016/0003-2697(85)90440-3. [DOI] [PubMed] [Google Scholar]
- 71.Norman EJ, Martelo OJ, Denton MD. Blood. 1982;59:1128–31. [PubMed] [Google Scholar]
- 72.Pollard MJ, Hilton CK, Li H, Kaplan K, Yost RA, Hill HH. Int. J. Ion Mobil. Spectrom. 2011;14:15–22. [Google Scholar]
- 73.Chouinard CD, Wei MS, Beekman CR, Kemperman RHJ, Yost RA. Clin. Chem. 2016;62:124–33. doi: 10.1373/clinchem.2015.238840. [DOI] [PubMed] [Google Scholar]