Abstract
Purpose
Racial disparity in breast cancer outcomes exists between African American and white women in the United States. We have evaluated the impact of genetically determined ancestry on disparity in efficacy and therapy-induced toxicity for patients with breast cancer in the context of a randomized, phase III adjuvant trial.
Methods
This study compared outcomes between 386 patients of African ancestry (AA) and 2,473 patients of European ancestry (EA) in a randomized, phase III breast cancer trial, ECOG-ACRIN-5103. The primary efficacy end point, invasive disease–free survival (DFS), and clinically significant toxicities were compared, including anthracycline-induced congestive heart failure, taxane-induced peripheral neuropathy (TIPN), and bevacizumab-induced hypertension.
Results
Overall, AAs had significantly inferior DFS (P = .002; hazard ratio, 1.5) compared with EAs. This was significant in the estrogen receptor–positive subgroup (P = .03), with a similar, nonsignificant trend for those who had triple-negative breast cancer (P = .12). AAs also had significantly more grades 3 to 4 TIPN (odds ratio [OR], 2.9; P = 2.4 × 10−11) and grades 3 to 4 bevacizumab-induced hypertension (OR, 1.6; P = .02), with a trend for more congestive heart failure (OR, 1.8; P = .08). AAs had significantly more dose reductions in paclitaxel (P = 6.6 × 10−6). In AAs, dose reductions in paclitaxel had a significant negative impact on DFS (P = .03), whereas in EAs, dose reductions did not have an impact on outcome (P = .35).
Conclusion
AAs had inferior DFS, with more clinically important toxicities, in ECOG-ACRIN-5103. The altered risk-to-benefit ratio for adjuvant breast cancer chemotherapy should lead to additional research with the focus on the impact of genetic ancestry on both efficacy and toxicity. Strategies to minimize dose reductions in paclitaxel, especially as the result of TIPN, are warranted for this population.
INTRODUCTION
African American patients with breast cancer have inferior efficacy outcomes compared with other races.1,2 The reason for this imbalance is multifactorial and includes higher stage, higher grade, more triple-negative breast cancer (TNBC), and poorer responsiveness to chemotherapy.3,4 These clinical imbalances have previously been attributed to both socioeconomic factors and a different underlying biology of the tumor.2,5 Patients of African ancestry (AA) also experience more adverse drug reactions, including an increase in clinically important toxicities for chemotherapeutic agents. Although less well characterized, these toxicity disparities are also likely multifactorial and include inherited genetic variation and comorbidities, among other factors.6-10
Much of the prior work has been based on self-reported race. Race, when assessed in this fashion, is typically on the basis of skin color and often neglects the genetic ancestry.11 Recent studies suggest that there is substantial admixture and misclassification of race in the United States when it is done on the basis of self-reported skin color.12 Genetic ancestry can be accurately determined using well-characterized ancestry informative germline markers.13
This correlative work aims to determine genetic ancestry accurately and to elucidate its impact on efficacy and toxicity in the context of a randomized, phase III adjuvant breast cancer trial, ECOG-ACRIN-5103.14
METHODS
ECOG-ACRIN-5103 Overview
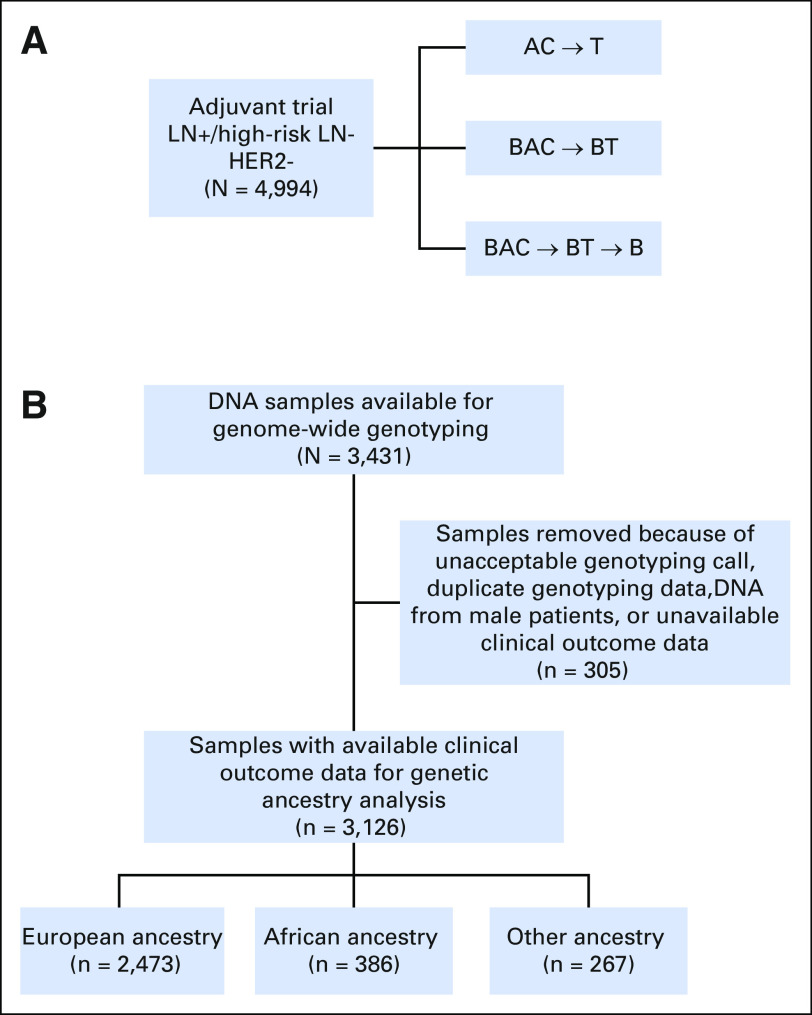
ECOG-ACRIN-5103 was a phase III adjuvant breast cancer trial that randomly assigned 4,994 patients with node-positive or high-risk node-negative breast cancer to intravenous doxorubicin and cyclophosphamide (AC) every 2 or 3 weeks (at discretion of treating physician) for four cycles followed by 12 weeks of paclitaxel (80 mg/m2 once per week) alone (arm A) or to the same chemotherapy with either concurrent bevacizumab (arm B) or concurrent plus sequential bevacizumab (arm C); Figure 1A. Patients were all human epidermal growth factor receptor 2 negative. Patients with TNBC had a tumor ≥ 1 cm or were lymph node (LN) positive. Patients with estrogen receptor (ER)–positive disease were LN positive, had a tumor ≥ 5 cm or a tumor measuring 1 to 5 cm, with a recurrence score ≥ 11.
Fig 1.
(A) Schematic for ECOG-ACRIN-5103 and (B) CONSORT diagram for ECOG-ACRIN-5103. AC, intravenous doxorubicin and cyclophosphamide; B, bevacizumab; BAC, bevacizumab concurrent with intravenous doxorubicin and cyclophosphamide; BT, bevacizumab concurrent with paclitaxel; HER2, human epidermal growth factor receptor 2; LN, lymph node; T, paclitaxel, +, positive; -, negative.
Genome-Wide Association Study
Germline DNA from whole blood and companion clinical outcome data were available in 3,126 patients. Genome-wide single-nucleotide polymorphism arrays (either Illumina HumanOmni1-Quad or Human OmniExpress) were performed in two distinct study subsets as described previously.15 A principal component analysis was performed using Eigenstrat and reference data from 11 HapMap phase III populations to identify clusters using the first two eigenvectors computed using all single-nuclotide polymorphisms.16 Samples clustering with those of AA and those of European Ancestry (EA) were used in these analyses (Data Supplement).
Efficacy Analyses
ECOG-ACRIN-5103 involved random assignment of patients to a control treatment arm and two experimental treatment arms. The primary objective of this phase III trial was to determine whether the addition of bevacizumab improves disease-free survival (DFS). A two-step hierarchical approach was used to assess this objective. In the first step, arm C was compared with arm A. If arm C significantly improved DFS relative to arm A, then in the second step, a comparison of arm B with arm A was performed.14 In this correlative study, DFS and overall survival (OS) were evaluated using Kaplan-Meier methodology. The differences in outcomes between AAs and EAs were compared with the application of Cox proportional-hazards models using either univariate or multivariate analysis, which were corrected with significant covariates. A multivariable Cox proportional-hazards model was used to test associations between independent variables and DFS. To identify the best regression model, a forward and a backward step-wise selection procedure were performed separately to evaluate variable associations, and the Akaike Information Criterion was used to determine the inclusion of potential confounders such as race, menopausal status, age, weight, height, side of cancer involvement, ER status, histologic grade, nuclear grade, LN status, type of surgery, types of AC schedule, and dose reductions in AC or paclitaxel. Both procedures returned the same model with six predictors, which are listed in the Data Supplement.
DFS was the primary end point of the parent trial; it was defined as invasive DFS and calculated from the date of random assignment to the date of first treatment failure (invasive ipsilateral, local/regional invasive, distant recurrence, invasive contralateral breast cancer, invasive nonbreast second primary, or death from any cause [whichever occurred first]). Cases with incomplete follow-up, without a documented invasive DFS event (including those who developed squamous or basal cell skin cancers or in situ carcinomas of any site as their only event) were censored at the date of last disease evaluation. OS, a secondary end point, was calculated from the date of random assignment to the date of death.
Toxicity Analyses
We analyzed the classic and most severe toxicities (Common Toxicity Criteria Adverse Event [CTCAE] version 3.0) associated with doxorubicin (congestive heart failure [CHF]), paclitaxel (taxane-induced peripheral neuropathy [TIPN]), and bevacizumab (hypertension; Data Supplement). We previously published biomarker data on each of these toxicities.15,17,18 In the present study, we sought to compare the frequency of all toxicities between AAs and EAs and to assess the impact of race on dose modifications. Statistical analysis was performed using R (version 3.3.0) and the χ2 test. Odds ratio (OR) was used to evaluate the significance and magnitude of the differences in the toxicity frequency between AAs and EAs.
CHF cases for this study included individuals who had centrally reviewed, cardiologist-adjudicated CHF. To be selected for inclusion in ECOG-ACRIN-5103, patients must have had no history of clinically significant cardiovascular disease. Cardiovascular health was monitored at the start and during the trial by multigated angiograms or echocardiograms. In addition, a cardiac symptoms assessment was performed 2 years after registration. Cardiac events included CHF, decrease in left ventricle ejection fraction, acute coronary syndrome, supraventricular tachycardia, and myocardial dysfunction diagnosed by a cardiologist.
TIPN cases for this study were defined as those experiencing either grades 2 to 4 or grades 3 to 4 TIPN as assessed by CTCAE. To serve as a TIPN case, the patient must have received at least one dose of paclitaxel, and the neuropathy event must have occurred during treatment or within 3 months of the last dose of therapy.
Hypertension cases for this study were defined as those experiencing a systolic blood pressure (SBP) > 160 mmHg (moderate), SBP > 180 mmHg (severe), or grades 3 to 5 hypertension as determined by CTCAE in arms B or C. Of note, a baseline SBP > 160 mmHg was an exclusion criterion for eligibility to enroll in the parent trial. Blood pressure values were collected as part of standard clinical assessment before administration of therapy throughout the conduct of the trial.
RESULTS
Genotyped Group From ECOG-ACRIN-5103 and Genetic Ancestry
A total of 3,394 germline DNA samples from patients were collected as part of the planned correlative protocol within ECOG-ACRIN-5103 (Fig 1B). Genome-wide assessment was conducted and enabled the determination of genetic ancestry in 3,126 patients who had clinical outcome data. Three hundred eighty-six patients (12.3%) were classified as AA and 2,473 patients (79.1%) were classified as EA. Among the 386 patients of AA, 352 (91.1%) were self-reported African American. Among the 2,473 patients of EA, 2,467 (99.8%) were self-reported white. The outcomes of the entire genotyped cohort (all ancestries combined) used in this correlative study were almost identical to the parent trial4 (Data Supplement).
Demographic Data and Disease Comparisons
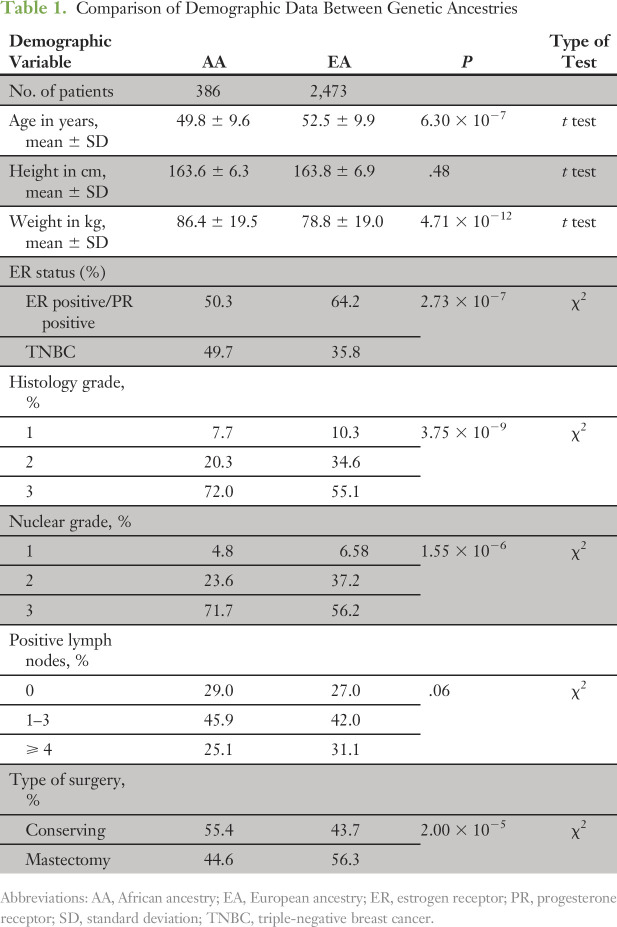
Table 1 summarizes the important demographic comparisons between AAs and EAs. We assessed for the differences in the parent trial stratification factors as well as other known outcome predictors, including age, hormone receptor status (ER positive v TNBC), LN status, height, weight, type of surgery, and tumor grade. Compared with EAs, AAs had a higher proportion of TNBC, and higher nuclear and histologic grades. AAs were younger, heavier, and more likely to undergo lumpectomy compared with EAs. There were no significant differences overall in LN status.
Table 1.
Comparison of Demographic Data Between Genetic Ancestries
Genetic Ancestry as a Predictor of Efficacy in ECOG-ACRIN-5103
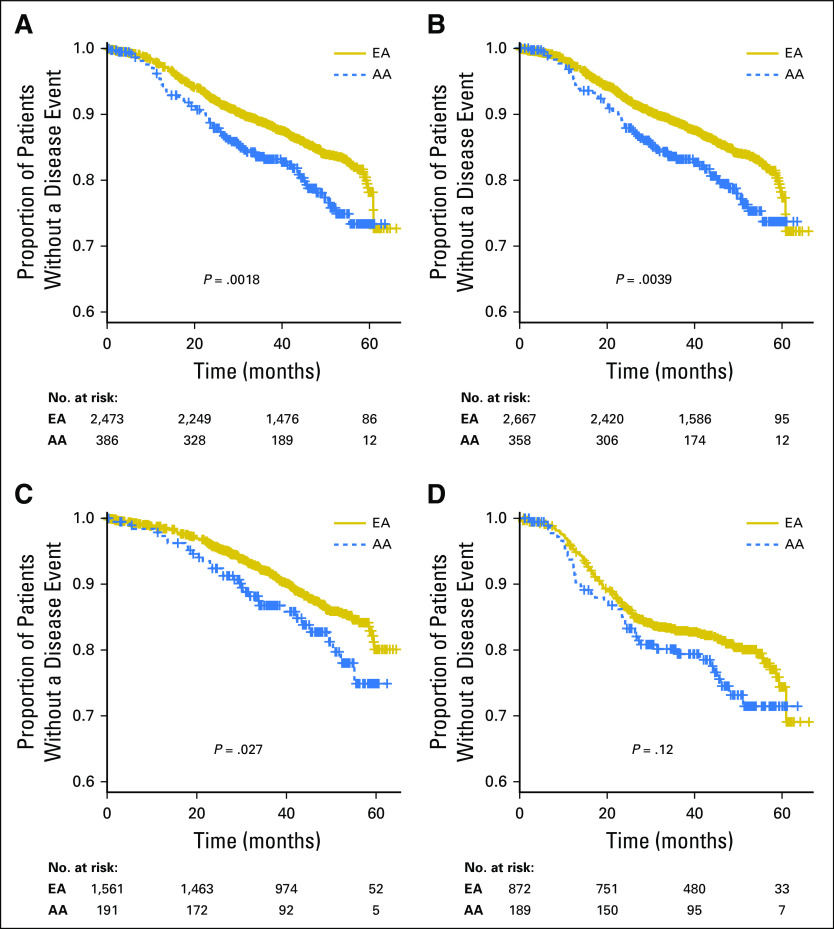
DFS was the primary efficacy end point of the parent trial. When combining all arms of the study and with a median follow-up of 47.5 months, AAs had an inferior DFS compared with EAs on univariable analysis (hazard ratio [HR], 1.5; P = .002; Fig 2A).
Fig 2.
Disease-free survival (DFS) for African ancestry (AA) compared with European ancestry (EA) for all patients (A), DFS for all self-reported patients (B), DFS for genetic ancestry with estrogen receptor– or progesterone receptor–positive disease (C), and DFS for genetic ancestry with triple-negative breast cancer (D).
The finding remained significant (HR, 1.4; P = .013) after correction for ER status, histology grade, LN status, type of surgery, and dose modification of cyclophosphamide; these covariates were significantly associated with DFS in multivariable analysis (Data Supplement). Both genetic ancestry (Fig 2A) and self-reported race (Fig 2B) demonstrated an inferior DFS for AAs compared with EAs, with similar conclusions. The difference in DFS did not result in a significant difference in a secondary end point of OS (P = .22; Data Supplement). When further evaluating the impact of genetic ancestry on the basis of tumor subtype, the ER-positive subgroup demonstrated a significantly inferior DFS for AAs (HR, 1.5; P = .027; Fig 2C and Table 1). The inferior DFS for AAs in the TNBC subgroup did not reach statistical significance, but was in favor of better outcomes for EAs (HR, 1.3; P = .12; Fig 2D). An imbalance in associated comorbidities or environmental factors cannot be excluded as a contributing cause, and unfortunately, those data were not collected in ECOG-ACRIN-5103. Thus, we evaluated the HR across the principal components and found an increasing hazard toward AA (HR, 1.5; P = .004).
Genetic Ancestry as a Predictor of Toxicity and Dose Modifications in ECOG-ACRIN-5103
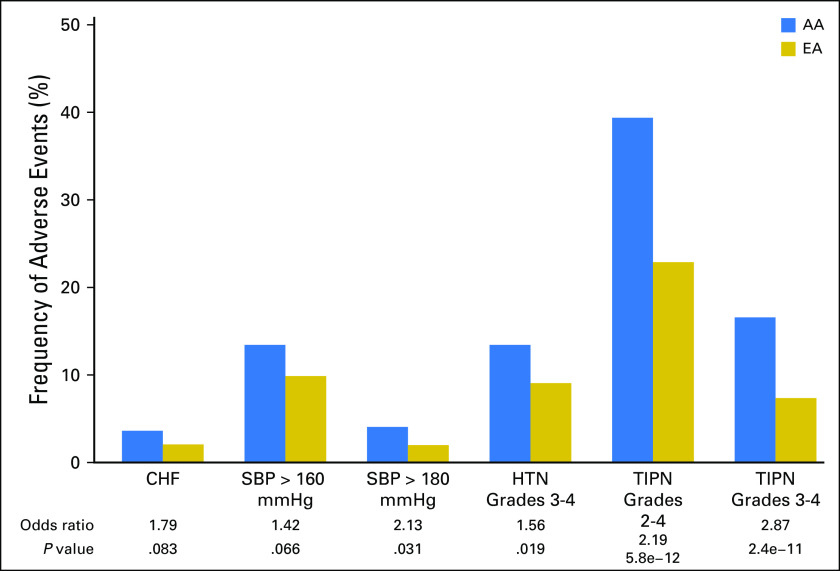
We assessed for the impact of genetic ancestry on likelihood of TIPN and found that AAs had markedly higher rates of grades 2 to 4 (OR, 2.2; P = 5.8 × 10−12) and grades 3 to 4 (OR, 2.9; P = 2.4 × 10−11) compared with EAs (Fig 3). Previously, we reported that compared with all other races, AAs had higher risk of experiencing grades 2 to 4 TIPN (HR, 2.1; P = 9.4 × 10−15) and grades 3 to 4 TIPN (HR, 2.7; P = 7.4 × 10−13).17 When assessed for the impact of genetic ancestry on the risk of various definitions of hypertension in the bevacizumab-containing arms (B and C), AA was associated with significantly more grades 3 to 4 hypertension (OR, 1.6; P = .02), as well as a trend toward more patients with one SBP measurement > 160 mmHg (OR, 1.4; P = .07) or one SBP measurement > 180 mmHg (OR, 2.1; P = .03; Fig 3). Finally, there was a trend for more AA patients with CHF (OR, 1.8; P = .08; Fig 3).
Fig 3.
Frequency of anthracycline-induced congestive heart failure (CHF), systolic blood pressure (SBP) > 160 mmHg, SBP > 180 mmHg, hypertension (HTN) grades 3 to 4, and paclitaxel (taxane)-induced peripheral neuropathy (TIPN) in patients of African ancestry (blue bar) versus those of European ancestry (gold bar) in genotyped patients.
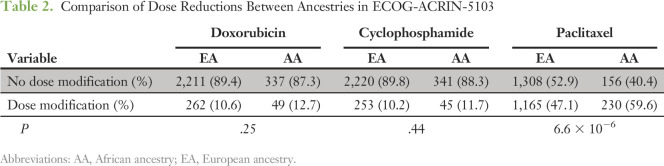
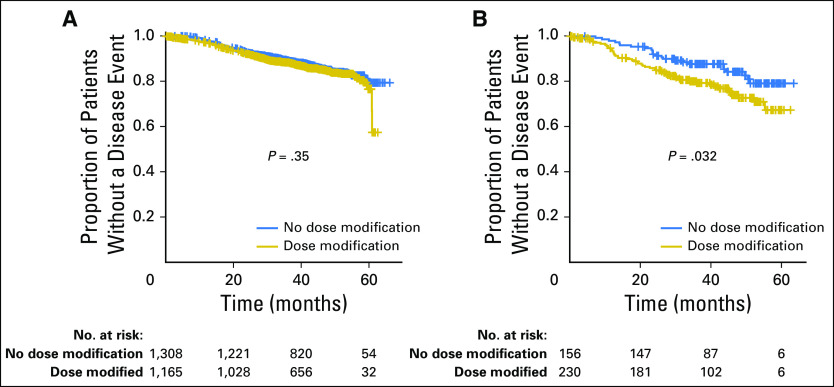
We also assessed whether increased toxicity affected dose reductions, modifications, or premature cessation of therapy. When comparing AAs with EAs, there was no significant difference in the proportion of patients requiring dose reductions in doxorubicin (P = .25) or cyclophosphamide (P = .44); however, there were substantially more dose reductions in paclitaxel in AAs compared with EAs (P = 6.6 × 10−6; Table 2). When considering all genetic ancestries combined, dose reductions in doxorubicin and cyclophosphamide negatively affected DFS (P = 4.9 × 10−4 and P = 3.3 × 10−5, respectively) for all patients. However, because there was no difference in the percentage of AAs who experienced dose reductions in the doxorubicin and cyclophosphamide portion, this did not account for the difference in DFS between ancestries (Data Supplement). Dose reductions in paclitaxel also negatively affected DFS for all ancestries (P = .02; Data Supplement); however, having a dose modification in paclitaxel did not affect DFS for EAs, but it did significantly cause an inferior DFS for AAs (Fig 4). The difference may have been explained by more severe dose reductions in AAs. When comparing the mean normalized cumulative dose exposure of paclitaxel for those who had dose modifications, AAs had a significantly lower cumulative dose (548 mg/m2) than EAs (603 mg/m2; P = .03).
Table 2.
Comparison of Dose Reductions Between Ancestries in ECOG-ACRIN-5103
Fig 4.
Disease-free survival for patients of European ancestry (left) and African ancestry (right) who experienced any dose reduction or early cessation of paclitaxel compared with those who did not have a dose reduction or early cessation.
DISCUSSION
This correlative study from ECOG-ACRIN-5103 supports prior findings that AAs have inferior outcomes compared with EAs.1,2 Although there was a statistically significant decrease in DFS for AAs, this did not translate to a difference in OS. This is probably because of insufficient events, statistical power, and length of follow-up. The inferior DFS was apparent for both the ER-positive and the TNBC subgroups, although the latter was not statistically significant. Recent data have also supported a worse outcome for the ER-positive subgroup of patients of AA.19,20 These findings support a fundamental difference in the biology of the disease in AAs compared with EAs, not just an imbalance in percentage of the more aggressive TNBC subtype.2,21
This study evaluated a subgroup of patients whose race was defined through genetic ancestry determination rather than self-assignment. The 91% and 99.8% concordance rates between the genetic ancestry and the self-reported race for AAs and EAs, respectively, were in agreement with the 1000 Genomes Project.22 The biologic differences in tumors and drug toxicities are probably a reflection of the underlying and nuanced genetic differences rather than differences in skin color.
Ancestry determined with specific genetic markers, rather than self-reported race, should be more helpful for elucidating biologic differences.23 This is particularly true in populations largely composed of patients from the United States, where admixture is common.22,24 Because genetic ancestry information was only available in a subgroup of the parent trial, sample bias was possible. The outcomes for the subgroup genotyped, however, were similar to the parent population, thus minimizing the concern for subgroup bias.
We also compared the results of the genetic ancestry with self-reported race, and there was no significant difference in the conclusion. This supports that for this phenotype, prior conclusions from self-reported race are likely valid. Work in other disease phenotypes, however, has demonstrated that use of self-reported race as a surrogate for genetic ancestry was not perfect.25-27 Prior work has demonstrated that > 9% of patients cannot supply, or choose not to supply, ancestry information.28 Thus, the real impact of accurately self-reported race is probably larger than the 9% that was observed, as the result of misclassification alone. This suggests that for research questions that center on racial disparity, investigators should consider the use of ancestry informative markers,29 and self-reported race should only be used as a surrogate when genetic ancestry data are unavailable. Although genotype may provide more insight toward underlying biologic diversity, self-reported race still likely represents a reasonable surrogate for genotypic variation in the routine clinical setting for consideration of metabolism and drug toxicity.
We previously evaluated genetic markers to predict some of the most clinically important toxicities in ECOG-ACRIN-5103: bevacizumab-induced hypertension (using various definitions), TIPN, and cardiologist-adjudicated anthracycline-induced CHF.15,17,18 In this study we compared the likelihood of each of these clinically relevant toxicities between AAs and EAs. We report a numerically higher likelihood for each of these toxicities for AAs, with ORs ranging from 1.4 to 2.9. This supports a marked genetic difference in therapeutic tolerability. These data support prior work from a single-institution retrospective analysis as well as data in pediatric populations that revealed higher likelihood of anthracycline-induced CHF in AAs. Similarly, a prior single-institution retrospective analysis demonstrated higher risk of TIPN for AAs.8-10
Because AAs experience greater toxicity, it is highly unlikely that the imbalance in efficacy is a result of exposure as the result of pharmacokinetic considerations, as the end organs are clearly being affected. We further assessed whether the adverse effect on toxicity and resultant increase in dose reductions may have accounted for inferior outcomes. As expected, all ancestries that had dose reductions in the AC portion of the chemotherapy had inferior DFS; this difference, however, was present for both EAs and AAs. The inferior difference (as defined by P value) in DFS observed for those who had dose reductions in paclitaxel (P = .02) was less significant for the whole population compared with those who had dose reductions in doxorubicin (P = 4.9 × 10−4) or cyclophosphamide (P = 3.3 × 10−5). The inferior DFS, however, seemed to be uniformly driven by the AA subgroup, implying that dose intensity of paclitaxel is more important in the AA population. The significant difference in DFS as the result of paclitaxel dose modifications for AAs may have been because of the markedly more severe dose reductions; this was evidenced by a lower mean normalized dose in those who had dose reductions (P = .03), in large part because of TIPN. This study did not evaluate socioeconomic factors, which are known to be important variables in outcomes.5 However, in the context of a randomized phase III trial, many of these inequities are minimized.
In conclusion, this study highlights the need to better understand the biologic differences in normal breast biology, tumor biology, and the inherited genetic differences between women of AA and EA. It also highlights the need to better personalize counseling when discussing the risk-to-benefit ratio for AAs, for whom the disease-specific outcomes are inferior and drug-specific toxicities are higher. These data suggest that lack of the full, intended doses of paclitaxel is at least one factor in inferior outcomes in ECOG-ACRIN-5103. Taxanes have been proven to be important in the curative setting for breast cancer, and that point is further illustrated here. ECOG-1199 previously demonstrated that the dosing of docetaxel every 3 weeks was as effective but had fewer dose reductions for AAs in a similar clinical setting.20 Future trials should investigate whether another taxane with less risk of TIPN, such as docetaxel, might be more effective for AAs in the adjuvant breast cancer setting. These data also highlight the need to validate predictive biomarkers for toxicities specific to AAs. Most importantly, these findings underscore the need to include more AAs in clinical trials.
Footnotes
Coordinated by the ECOG-ACRIN Cancer Research Group (Robert L. Comis, MD and Mitchell D. Schnall, MD, PhD, Group Co-Chairs) and supported by the National Cancer Institute of the National Institutes of Health under the following award numbers: CA180820, CA21115, CA180794, CA23318, CA66636, CA180795, CA49883, CA180816, CA189859, and CA14958. The study was also supported by a Susan G. Komen for the Cure Promise Award (B.P.S.) and the Vera Bradley Foundation (B.P.S.).
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, nor does mention of trade names, commercial products, or organizations imply endorsement by the US government.
AUTHOR CONTRIBUTIONS
Conception and design: Bryan P. Schneider, Fei Shen, Guanglong Jiang, Tatiana Foroud, Joseph A. Sparano, Kathy D. Miller
Administrative support: Lang Li, Joseph A. Sparano
Provision of study material or patients: Joseph A. Sparano, Kathy D. Miller
Collection and assembly of data: Bryan P. Schneider, Fei Shen, Anne O’Neill, Laura Gardner, Kathy D. Miller
Data analysis and interpretation: Bryan P. Schneider, Guanglong Jiang, Milan Radovich, Lang Li, Dongbing Lai, Joseph A. Sparano, George W. Sledge
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO’s conflict of interest policy, please refer to www.asco.org/rwc or po.ascopubs.org/site/ifc.
Bryan P. Schneider
No relationship to disclose
Fei Shen
No relationship to disclose
Guanglong Jiang
No relationship to disclose
Anne O’Neill
No relationship to disclose
Milan Radovich
No relationship to disclose
Lang Li
No relationship to disclose
Laura Gardner
No relationship to disclose
Dongbing Lai
No relationship to disclose
Tatiana Foroud
No relationship to disclose
Joseph A. Sparano
Stock and Other Ownership Interests: MetaStat
Consulting or Advisory Role: Genentech, Novartis, AstraZeneca, Celgene, Eli Lilly, Celldex, Pfizer, Prescient Therapeutics, Juno Therapeutics, Merrimack
Research Funding: Prescient Therapeutics (Inst), Deciphera (Inst), Genentech (Inst), Merck (Inst), Novartis (Inst), Merrimack (Inst)
George W. Sledge Jr
Leadership: Syndax
Stock and Other Ownership Interests: Syndax
Honoraria: Symphogen
Consulting or Advisory Role: Symphogen, Coherus Biosciences, Radius Health, Peregrine Pharmaceuticals, Taiho Pharmaceutical
Research Funding: Genentech (Inst)
Travel, Accommodations, Expenses: Nektar, Radius Health, Taiho Pharmaceutical
Kathy D. Miller
Consulting or Advisory Role: Nektar, TESARO, Merck
Research Funding: Genentech (Inst), Merrimack (Inst), EntreMed (Inst), Taiho Pharmaceutical (Inst), MacroGenics (Inst), Medivation (Inst), Novartis (Inst), Seattle Genetics (Inst)
REFERENCES
- 1.Albain KS, Unger JM, Crowley JJ, et al. : Racial disparities in cancer survival among randomized clinical trials patients of the Southwest Oncology Group. J Natl Cancer Inst 101:984-992, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Keenan T, Moy B, Mroz EA, et al. : Comparison of the genomic landscape between primary breast cancer in African American versus white women and the association of racial differences with tumor recurrence. J Clin Oncol 33:3621-3627, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Silber JH, Rosenbaum PR, Clark AS, et al. : Characteristics associated with differences in survival among black and white women with breast cancer. JAMA 310:389-397, 2013 [DOI] [PubMed] [Google Scholar]
- 4.Carey LA, Perou CM, Livasy CA, et al. : Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA 295:2492-2502, 2006 [DOI] [PubMed] [Google Scholar]
- 5.DeSantis C, Jemal A, Ward E: Disparities in breast cancer prognostic factors by race, insurance status, and education. Cancer Causes Control 21:1445-1450, 2010 [DOI] [PubMed] [Google Scholar]
- 6.Dooley MA, Hogan S, Jennette C, et al. : Cyclophosphamide therapy for lupus nephritis: Poor renal survival in black Americans. Kidney Int 51:1188-1195, 1997 [DOI] [PubMed] [Google Scholar]
- 7.Kidd RS, Curry TB, Gallagher S, et al. : Identification of a null allele of CYP2C9 in an African-American exhibiting toxicity to phenytoin. Pharmacogenetics 11:803-808, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Bhatnagar B, Gilmore S, Goloubeva O, et al. : Chemotherapy dose reduction due to chemotherapy induced peripheral neuropathy in breast cancer patients receiving chemotherapy in the neoadjuvant or adjuvant settings: A single-center experience. Springerplus 3:366, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hasan S, Dinh K, Lombardo F, et al. : Doxorubicin cardiotoxicity in African Americans. J Natl Med Assoc 96:196-199, 2004 [PMC free article] [PubMed] [Google Scholar]
- 10.Krischer JP, Epstein S, Cuthbertson DD, et al. : Clinical cardiotoxicity following anthracycline treatment for childhood cancer: The Pediatric Oncology Group experience. J Clin Oncol 15:1544-1552, 1997 [DOI] [PubMed] [Google Scholar]
- 11.Cobb RJ, Thomas CS, Laster Pirtle WN, et al. : Self-identified race, socially assigned skin tone, and adult physiological dysregulation: Assessing multiple dimensions of “race” in health disparities research. SSM Popul Health. 2:595-602, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bryc K, Durand EY, Macpherson JM, et al. : The genetic ancestry of African Americans, Latinos, and European Americans across the United States. Am J Hum Genet 96:37-53, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kosoy R, Nassir R, Tian C, et al. : Ancestry informative marker sets for determining continental origin and admixture proportions in common populations in America. Hum Mutat 30:69-78, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Miller K, O’Neill AM, Dang CT, et al: Bevacizumab (Bv) in the adjuvant treatment of HER2-negative breast cancer: Final results from Eastern Cooperative Oncology Group E5103. J Clin Oncol 32, 2014 (suppl; abstr 500)
- 15.Schneider BP, Li L, Shen F, et al. : Genetic variant predicts bevacizumab-induced hypertension in ECOG-5103 and ECOG-2100. Br J Cancer 111:1241-1248, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Price AL, Patterson NJ, Plenge RM, et al. : Principal components analysis corrects for stratification in genome-wide association studies. Nat Genet 38:904-909, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Schneider BP, Li L, Radovich M, et al. : Genome-wide association studies for taxane-induced peripheral neuropathy in ECOG-5103 and ECOG-1199. Clin Cancer Res 21:5082-5091, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gardner L, Shen F, Radovich M, et al: Genome wide association study for anthracycline-induced congestive heart failure. J Clin Oncol 34, 2016 (suppl; abstr 1017) [DOI] [PMC free article] [PubMed]
- 19.Tichy JR, Deal AM, Anders CK, et al. : Race, response to chemotherapy, and outcome within clinical breast cancer subtypes. Breast Cancer Res Treat 150:667-674, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sparano JA, Wang M, Zhao F, et al. : Race and hormone receptor-positive breast cancer outcomes in a randomized chemotherapy trial. J Natl Cancer Inst 104:406-414, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Killelea BK, Yang VQ, Wang SY, et al. : Racial differences in the use and outcome of neoadjuvant chemotherapy for breast cancer: Results from the National Cancer Data Base. J Clin Oncol 33:4267-4276, 2015 [DOI] [PubMed] [Google Scholar]
- 22.Mersha TB, Abebe T: Self-reported race/ethnicity in the age of genomic research: Its potential impact on understanding health disparities. Hum Genomics 9:1, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Halder I, Shriver M, Thomas M, et al. : A panel of ancestry informative markers for estimating individual biogeographical ancestry and admixture from four continents: Utility and applications. Hum Mutat 29:648-658, 2008 [DOI] [PubMed] [Google Scholar]
- 24.Ding L, Wiener H, Abebe T, et al. : Comparison of measures of marker informativeness for ancestry and admixture mapping. BMC Genomics 12:622, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barnholtz-Sloan JS, Chakraborty R, Sellers TA, et al. : Examining population stratification via individual ancestry estimates versus self-reported race. Cancer Epidemiol Biomarkers Prev 14:1545-1551, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Louwers YV, Lao O, Fauser BC, et al. : The impact of self-reported ethnicity versus genetic ancestry on phenotypic characteristics of polycystic ovary syndrome (PCOS). J Clin Endocrinol Metab 99:E2107-E2116, 2014 [DOI] [PubMed] [Google Scholar]
- 27.Kittles RA, Weiss KM: Race, ancestry, and genes: Implications for defining disease risk. Annu Rev Genomics Hum Genet 4:33-67, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Ritchie MD, Denny JC, Crawford DC, et al. : Robust replication of genotype-phenotype associations across multiple diseases in an electronic medical record. Am J Hum Genet 86:560-572, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jorde LB, Wooding SP: Genetic variation, classification and ‘race’. Nat Genet 36:S28-S33, 2004. (suppl 11) [DOI] [PubMed] [Google Scholar]