Summary
Temporal or giant cell arteritis is an inflammation of medium and small extracranial vessels that may result in ocular ischemia, an aortitis followed by aortic dissection and peripheral limb ischemia. It should be considered a medical emergency due to the seriousness of end organ damage, in particular visual symptoms. While the presentation may be nonspecific, the presence of a tender temporal artery mandates a temporal artery biopsy. High-dose steroids should be begun the moment the diagnosis is considered and only withdrawn once it has been excluded. A gradual tapering of the steroid dose should occur over at least 1 year, with the consideration of the use of steroid-sparing agents if iatrogenic steroid complications occur. Careful monitoring of the response both clinically as well as with serial inflammatory markers is required.
Temporal arteritis, also termed giant cell arteritis (GCA), is an immune-mediated vasculitis that affects medium and large vessels. It affects mainly Caucasians, especially those of Scandinavian descent, and individuals over age 50 years. The incidence from population-based studies is estimated at between 15 and 25 per 100,000 per year.1
While the presentation of temporal arteritis can vary, the most important complication is ocular ischemia leading to blindness. Cerebral ischemia, particularly within the posterior circulation, has also been reported. While noninvasive imaging such as duplex ultrasonography is used, the gold standard for diagnosis is the temporal artery biopsy. The treatment of temporal arteritis revolves around high-dose initial IV or oral steroids followed by a tapering steroid dose depending upon the clinical response. In the event that the temporal arteritis is nonresponsive, more potent immunosuppressives may be used. In this review article, we discuss the clinical presentation, investigations, epidemiology, and treatment.
Case
A 62-year-old woman presented to the ophthalmology unit with sudden left-sided monocular visual loss. In the previous 6 months leading up to her presentation, she had seen a number of specialists with nonspecific symptoms of frontotemporal headache, jaw pain on eating, shoulder tenderness, fatigue, and intermittent elevated body temperature. On examination, her visual acuity was light perception only on the left-hand side with a left afferent papillary defect. The left temporal artery was tender to palpation and had no pulsation, as opposed to the right temporal artery, which was pulsatile. Her erythrocyte sedimentation rate (ESR) (102) and C-reactive protein (CRP) (190) were elevated, and the platelet count was 214,000. All other autoimmune connective tissue screens were normal. A temporal artery biopsy was performed. The fundus examination (figure 1) showed a pale swollen left optic nerve. She was subsequently started on IV methylprednisolone 1 g for 3 days followed by a weaning dose starting at 100 mg of oral prednisolone over a 12-month period. She had permanent loss of vision in the left eye but the right eye maintained normal vision and she had no further vascular events.

Fundus photograph demonstrates a chalky white optic disc in an arteritic anterior ischemic optic neuropathy
Clinical presentation
Temporal arteritis may present in 1 of 3 ways: 1) a cranial arteritis; 2) an extracranial arteritis; and 3) a polymyalgia syndrome. Consequently, different specialists including the internist, the rheumatologist, the neurologist, and the ophthalmologist may be involved in a patient's care. The most common manifestations include headache, an abnormal temporal artery with temporal artery pulsation and pain, jaw claudication, scalp tenderness, and constitutional symptoms such as fever, malaise, anorexia, and weight loss.2,3
The most feared complication is related to an arteritic anterior ischemic optic neuropathy (AAION), which can result in permanent visual loss. Jaw claudication, diplopia, and temporal artery abnormalities may predict ischemic neuro-ophthalmic complications.4 Classically, AAION presents with a sudden, painless, profound unilateral or bilateral vision loss with the presence of a pale chalky white swollen optic nerve head, hemorrhages, and cotton-wool spots (figure 2). It may also present with a central retinal or cilioretinal artery occlusion.5 Other neuro-ophthalmic features include upper cranial nerve palsies and a visual field defect.6,7

Fundus photograph of a patient who presented with acute loss of vision in the left eye and amaurosis fugax in the right eye
Figure 2. (A) The left optic nerve is swollen with light perception vision only. (B) The right optic disc showed sectoral swelling but with pale disc appearance suggestive of acute infarction. Vision in the right eye was 6/18. Following prompt IV steroid treatment, the vision in the right eye returned to 6/7.5 with resolution of optic disc swelling but the left eye vision did not recover.
In 30% of individuals, neurologic manifestations occur such as mononeuropathies or polyneuropathies, an altered mental status or memory dysfunction, depression, and dementia. GCA on rare occasion may involve the cerebrovascular tree, most commonly the vertebro-basilar circulations.8 It may also involve coronary arteries resulting in myocardial ischemia.
Between 20% and 80% have extracranial involvement of large arteries of the upper and lower limbs. Ischemia of these arteries may precede the diagnosis of GCA by 12 months. In the upper limbs, digital ischemia and Raynaud phenomenon may be the presenting feature, while in the lower limbs, the presence of gangrene and leg ulcers are the result of ischemia.9
Involvement of the aorta with an aortitis complicated by aortic dissection is a potentially life-threatening extracranial complication of GCA. Often it may be asymptomatic or present with a vague systemic inflammatory syndrome consisting of fever of unknown origin.10 Due to the lack of specific symptoms, it may remain undetected for a number of years, until such time that a catastrophic complication occurs, such as aortic dissection, or it is picked up incidentally on imaging of the aorta, either with a chest x-ray or CT scan.11,12
Investigations
The gold standard for diagnosis is tissue confirmation from a temporal artery biopsy. At the same time, the presence of elevated inflammatory markers such as an ESR or CRP, in combination with symptoms and clinical signs outlined above, may be suggestive of temporal arteritis. The American College of Rheumatology criteria for the diagnosis of temporal arteritis include the following13:
Age at onset greater than 50 years
Onset of a new headache
Elevated ESR of greater than 50 mm/h
Temporal artery abnormality such as tenderness or reduced pulsation
An abnormal arterial biopsy confirming the presence of temporal arteritis
The British Society of Rheumatology suggests the following 7 features may aid in early diagnosis14:
An abrupt onset, usually unilateral headache within the temporal area
Scalp tenderness
Jaw and tongue claudication
Visual disturbances including diplopia
Constitutional symptoms
Polymyalgia symptoms
Limb claudication
Using the American College of Rheumatology criteria, the presence of 3 or more of the 5 is sufficient to make a diagnosis of temporal arteritis with a sensitivity of 93.5% and specificity of 91.2%. Nevertheless, some have expressed caution in using these criteria as they do not mandate temporal artery biopsy. In a retrospective chart review of a large cohort of temporal arteritis patients presenting to a quaternary neuro-ophthalmology service, a quarter of biopsy-proven patients would not have satisfied the criteria for the diagnosis of temporal arteritis. In addition, 28% of individuals who had a negative biopsy (and therefore did not have temporal arteritis), but would have been diagnosed with temporal arteritis according to the criteria, may have received steroids unnecessarily for a long period of time. In this series, the sensitivity (74.3%) and specificity (74.3%) were lower.15 Therefore the diagnosis of temporal arteritis is still based on clinical suspicion and a biopsy is mandatory unless there are contraindications.
Temporal artery biopsy
Temporal artery biopsy is the gold standard test and should be considered in any individual over age 50 years with new onset headache, systemic symptoms such as fever, malaise, and weight loss, and jaw claudication, where there is a considerable suspicion of temporal arteritis. Due to the fact that temporal arteritis may have skip lesions, a minimum 2-cm biopsy should be performed.4 The sensitivity of a temporal artery biopsy ranges from 70% to 90%, thus a negative biopsy does not exclude the diagnosis. In such cases where there is a high clinical suspicion, bilateral temporal artery biopsies should be performed. This may yield a positive specimen in 4% to 15% of cases if the first biopsy is negative.16,17 Treatment with corticosteroid should not be delayed while waiting for tissue confirmation as histopathologic findings persist at least 6 to 8 weeks following steroid use.6
Histopathology
Typical features of temporal arteritis include an inflammatory infiltrate affecting the adventitia and media and fragmentation of the elastic lamina (figure 3). This may be associated with giant cells, although the absence of these cells does not preclude the diagnosis. The inflammation may be focal and segmental in nature resulting in skip lesions.18 It is these skip lesions that account for the small percentage of biopsy-negative patients and hence the recommendation to have at least a 2-cm sample. Some suggest that following a temporal artery biopsy, a frozen section should be analyzed, and if this is negative, then to proceed to sample the contralateral temporal artery. Bilateral temporal artery sampling in a simultaneous fashion does not yield any further information and is difficult to justify on a routine basis.19
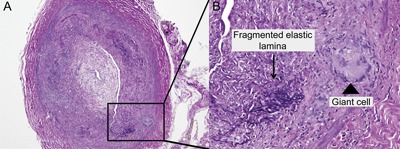
Temporal artery biopsy
Figure 3. (A) Temporal artery biopsy shows cross-section of an artery with inflammatory infiltrates affecting the adventitia and media with narrowing of the lumen (L). (B) Enlargement of a section of the biopsy (square) shows fragmentation of the internal elastic lamina (black arrows) and the presence of giant cells (arrowhead).
Laboratory parameters
Increased levels of acute phase reactants such as the ESR, CRP, and interleukin-6 may occur in the presence of temporal arteritis. Conversely, if these markers are within normal limits, then the diagnosis of temporal arteritis cannot be discounted. In fact, as many as 20% of individuals with biopsy-proven temporal arteritis have a normal ESR prior to therapy.20 In addition, other inflammatory markers such as normochromic, normocytic anemia, leukocytosis, and a thrombocytosis may be present.
The degree to which these biomarkers are elevated has diagnostic significance. For example, in a large population-based study, a positive temporal artery biopsy was most strongly predicted with a CRP that was more than 2.45 mg/dL and a platelet count that was more than 400,000.21 CRP elevation is the more sensitive marker, but the combination of the 2 results in a greater specificity.
Noninvasive vascular imaging
The use of color Doppler and duplex ultrasonography is controversial. Some maintain that it is a valid alternative to performing a temporal artery biopsy. Thickening of the vessel wall results in a dark hypoechoic signal that has been described as a “halo sign” and persists for 2 to 3 weeks following therapy. The presence of a halo sign has a specificity of 89% to 91% with a sensitivity of 69% to 75%. If the halo sign is bilateral, the specificity increases to 100% but the sensitivity is reduced to 43%. The main controversy revolves around reproducibility of these findings given that diagnostic ultrasound is highly operator-dependent. The best results are achieved with an experienced sonographer using industrial standard ultrasound machinery.22
Fluorescein angiography may be of value in selected cases demonstrating prolonged choroidal and central retinal artery filling time. There may also be choroidal nonperfusion or filling defects (figure 4) due to involvement of medium-sized vessels affecting the posterior ciliary artery supplying the choroid. This may be useful in the diagnosis of temporal arteritis given that these findings are not present in nonarteritic ischemic optic neuropathy, which is predominantly due to small-vessel ischemia.6

Fundus fluorescein angiogram of a patient with giant cell arteritis shows patchy choroidal filling defect (black areas)
Therapy
The mainstay of treatment is corticosteroids, which may be supplemented with other immunosuppressives or steroid-sparing agents. There are few randomized controlled trial data guiding a clinician as to the amount, duration, or even method of administration of steroid, be it IV or oral. The dose is also dependent upon whether the clinician is treating a temporal or giant cell arteritis as opposed to the more systemic features of a polymyalgia rheumatica.
Therapy of temporal arteritis and cranial GCA
As visual impairment is the most important symptom, treatment needs to be intense and immediate. It is recommended that high-dose steroids be used. Some regimens recommend IV methylprednisolone of 1 mg/kg per day for 3 days followed by oral steroids. Other regimens suggest the use of 40 to 60 mg per day of oral steroids initially. Treatment should be begun immediately following the clinical suspicion of the diagnosis and should not be delayed while waiting for the results of a temporal artery biopsy.19 This is even more so if visual loss has been documented.
Therapy of extracranial GCA
Due to the potential involvement of the aortic arch resulting in complications such as an aortic dissection, some guidelines have recommended that routine screening of the aortic arch with CT angiography occur.14 Once again, corticosteroids are the mainstay of treatment. Unlike cranial GCA, the response to steroids is not as rapid or as complete. In a population cohort study, just under half of participants showed evidence of improvement of the aortitis at 6 months following treatment. Predictors of an increased aortic thoracic dissection risk include hypertension at the time of diagnosis, symptoms of polymyalgia rheumatic, and severe abnormalities on laboratory markers of inflammation with evidence of aortic insufficiency, hyperlipidemia, and coronary artery disease at any time during follow-up.23
Length of steroid therapy
Treatment response to high-dose steroids tends to occur within 24 to 72 hours of therapy. ESR normalization tends to lag behind the clinical response and at times may take several weeks to normalize. The consensus is that high-dose oral steroids be maintained for a minimum of 4 weeks and thereafter a tapering of the dose is initiated, depending upon symptom and inflammatory markers returning to normal. Once a clinical response is achieved, tapering of the steroid dose may occur at a rate of 10 mg per month until a maintenance dose of 10 to 15 mg per day is reached. At that point, reduction of the steroids by 1 to 2.5 mg per month should occur. In most cases, the total duration of steroids may be a year. Rarely, a maintenance dose of steroid is indicated.6
Adjuvant and steroid-sparing therapies
Long-term steroid treatment is not without risk and may be associated with systemic complications such as osteoporosis, diabetes, hypertension, and an increased risk of infection, as well as local ocular complications such as cataract.24 Azathioprine, methotrexate, cyclophosphamide, cyclosporine, and cytokine blockade with various tumor necrosis factor antagonists have been tried. Methotrexate has been shown to reduce the risk of first and second relapse as well as reduce the total dose of steroid.25 Azathioprine has also shown a steroid-sparing effect, but due to the heterogeneous nature of the subjects enrolled, some of whom were GCA patients, more generalized conclusions cannot be made.26
Unlike ischemic stroke or nonarteritic ischemic optic neuropathy, thromboembolic events are not as prominent in temporal arteritis. Nevertheless, low-dose aspirin has been shown to reduce ischemic complications in some cohorts. Further randomized controlled trials may clarify this issue.19
STUDY FUNDING
No targeted funding reported.
DISCLOSURES
The authors report no disclosures. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp http://cp.neurology.org/lookup/doi/10.1212/CPJ.0b013e3182a9c62a.
Correspondence to: andrew.lee@health.sa.gov.au
Funding information and disclosures are provided at the end of the article. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp http://cp.neurology.org/lookup/doi/10.1212/CPJ.0b013e3182a9c62a.
Footnotes
Correspondence to: andrew.lee@health.sa.gov.au
Funding information and disclosures are provided at the end of the article. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp http://cp.neurology.org/lookup/doi/10.1212/CPJ.0b013e3182a9c62a.
REFERENCES
- 1.Bengtsson B, Malmvall B. The epidemiology of giant cell arteritis, including temporal arteritis and polymyalgia rheumatica: incidences of different clinical presentations and eye complications. Arthritis Rheum. 1981;24:899–904. doi: 10.1002/art.1780240706. [DOI] [PubMed] [Google Scholar]
- 2.Gonzalez-Gay MA, Barros S, Lopez-Diaz MJ, Garcia-Porrua C, Sanchez-Andrade A, Llorca J. Giant cell arteritis: disease patterns of clinical presentation in a series of 240 patients. Medicine. 2005;84:269–276. doi: 10.1097/01.md.0000180042.42156.d1. [DOI] [PubMed] [Google Scholar]
- 3.Chaudhry IA, Shamsi FA, Elzaridi E, Arat YO, Bosley TM, Riley FC. Epidemiology of giant-cell arteritis in an Arab population: a 22-year study. Br J Ophtalmol. 2007;91:715–718. doi: 10.1136/bjo.2006.108845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kale N, Eggenberger E. Diagnosis and management of giant cell arteritis: a review. Curr Opin Ophthalmol. 2010;21:417–422. doi: 10.1097/ICU.0b013e32833eae8b. [DOI] [PubMed] [Google Scholar]
- 5.Carroll SC, Gaskin BJ, Danesh-Meyer HV. Giant cell arteritis. Clin Experiment Ophthalmol. 2006;34:159–173. doi: 10.1111/j.1442-9071.2006.01186.x. [DOI] [PubMed] [Google Scholar]
- 6.Chew SS, Kerr NM, Danesh-Meyer HV. Giant cell arteritis. J Clin Neurosci. 2009;16:1263–1268. doi: 10.1016/j.jocn.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 7.Wang X, Hu Z, Lu W. Giant cell arteritis. Rheumatol Int. 2008;29:1–7. doi: 10.1007/s00296-008-0662-0. [DOI] [PubMed] [Google Scholar]
- 8.García-García J, Ayo-Martín Ó, Argandoña-Palacios L, Segura T. Vertebral artery halo sign in patients with stroke: a key clue for the prompt diagnosis of giant cell arteritis. Stroke. 2011;42:3287–3290. doi: 10.1161/STROKEAHA.111.625152. [DOI] [PubMed] [Google Scholar]
- 9.Assie C, Janvresse A, Plissonnier D, Levesque H, Marie I. Long-term follow-up of upper and lower extremity vasculitis related to giant cell arteritis: a series of 36 patients. Medicine. 2011;90:40–51. doi: 10.1097/MD.0b013e318206af16. [DOI] [PubMed] [Google Scholar]
- 10.Czihal M, Tato F, Forster S, Rademacher A, Schulze-Koops H, Hoffmann U. Fever of unknown origin as initial manifestation of large vessel giant cell arteritis: diagnosis by colour-coded sonography and 18-FDG-PET. Clin Exp Rheumatol. 2010;28:549–552. [PubMed] [Google Scholar]
- 11.Säve-Söderbergh J, Malmvall B, Andersson R, Bengtsson B. Giant cell arteritis as a cause of death: report of nine cases. JAMA. 1986;255:493–496. doi: 10.1001/jama.1986.03370040067025. [DOI] [PubMed] [Google Scholar]
- 12.Nordborg E, Bengtsson BA. Death rates and causes of death in 284 consecutive patients with giant cell arteritis confirmed by biopsy. BMJ. 1989;299:549–550. doi: 10.1136/bmj.299.6698.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hunder G, Bloch D, Michel B. The American College of Rheumatology 1990 criteria for the classification of giant cell arteritis. Arthritis Rheum. 1990;33:1122–1128. doi: 10.1002/art.1780330810. [DOI] [PubMed] [Google Scholar]
- 14.Dasgupta B, Borg FA, Hassan N. BSR and BHPR guidelines for the management of giant cell arteritis. Rheumatology. 2010;49:1594–1597. doi: 10.1093/rheumatology/keq039a. [DOI] [PubMed] [Google Scholar]
- 15.Murchison AP, Gilbert ME, Bilyk JR. Validity of the American College of Rheumatology criteria for the diagnosis of giant cell arteritis. Am J Ophthalmol. 2012;154:722–729. doi: 10.1016/j.ajo.2012.03.045. [DOI] [PubMed] [Google Scholar]
- 16.Breuer GS, Nesher G, Nesher R. Rate of discordant findings in bilateral temporal artery biopsy to diagnose giant cell arteritis. J Rheumatol. 2009;36:794–796. doi: 10.3899/jrheum.080792. [DOI] [PubMed] [Google Scholar]
- 17.Gonzalez-Gay MA, Miranda-Filloy JA, Llorca J. Predictors of positive temporal artery biopsy in patients with giant cell arteritis and polymyalgia rheumatica (comment on the article by Marí et al.) Eur J Intern Med. 2010;21:51. doi: 10.1016/j.ejim.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 18.Chatelain D, Duhaut P, Schmidt J. Pathological features of temporal arteries in patients with giant cell arteritis presenting with permanent visual loss. Ann Rheum Dis. 2009;68:84–88. doi: 10.1136/ard.2007.084947. [DOI] [PubMed] [Google Scholar]
- 19.Mukhtyar C, Guillevin L, Cid MC. EULAR recommendations for the management of large vessel vasculitis. Ann Rheum Dis. 2009;68:318–323. doi: 10.1136/ard.2008.088351. [DOI] [PubMed] [Google Scholar]
- 20.Salvarani C, Hunder GG. Giant cell arteritis with low erythrocyte sedimentation rate: frequency of occurrence in a population-based study. Arthritis Care Res. 2001;45:140–145. doi: 10.1002/1529-0131(200104)45:2<140::AID-ANR166>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 21.Walvick MD, Walvick MP. Giant cell arteritis: laboratory predictors of a positive temporal artery biopsy. Ophthalmology. 2011;118:1201–1204. doi: 10.1016/j.ophtha.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 22.Arida A, Kyprianou M, Kanakis M, Sfikakis PP. The diagnostic value of ultrasonography-derived edema of the temporal artery wall in giant cell arteritis: a second meta-analysis. BMC Musculoskelet Disord. 2010;11:44. doi: 10.1186/1471-2474-11-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gonzalez-Gay MA, Garcia-Porrua C, Pineiro A, Pego-Reigosa R, Llorca J, Hunder GG. Aortic aneurysm and dissection in patients with biopsy-proven giant cell arteritis from northwestern Spain: a population-based study. Medicine. 2004;83:335–341. doi: 10.1097/01.md.0000145366.40805.f8. [DOI] [PubMed] [Google Scholar]
- 24.Proven A, Gabriel SE, Orces C, O'Fallon WM, Hunder GG. Glucocorticoid therapy in giant cell arteritis: duration and adverse outcomes. Arthritis Rheum. 2003;49:703–708. doi: 10.1002/art.11388. [DOI] [PubMed] [Google Scholar]
- 25.Mahr AD, Jover JA, Spiera RF. Adjunctive methotrexate for treatment of giant cell arteritis: an individual patient data meta-analysis. Arthritis Rheum. 2007;56:2789–2797. doi: 10.1002/art.22754. [DOI] [PubMed] [Google Scholar]
- 26.De Silva M, Hazleman BL. Azathioprine in giant cell arteritis/polymyalgia rheumatica: a double-blind study. Ann Rheum Dis. 1986;45:136–138. doi: 10.1136/ard.45.2.136. [DOI] [PMC free article] [PubMed] [Google Scholar]


