Summary
Health care costs continue to rise toward unsustainable levels that will affect our nation's ability to support other key funding priorities for education, military, and infrastructure. Changing the way we deliver health care is critical to mitigating this financial crisis. This review highlights opportunities for redesigning care of acute ischemic stroke and TIA to maintain quality while substantially lowering costs. The recent innovations described are (1) adopting teleneurology networks to improve access to thrombolysis for acute ischemic stroke; (2) improving efficiency of emergency care for acute ischemic stroke; and (3) providing alternatives to inpatient care for TIA. Applying such process innovations will enable us to achieve the goal of patients and the nation—high-quality care at an affordable cost.
As government and private payer budgets tighten, and competing public sector goods continue to vie for limited financial resources from the government and private sectors, all stakeholders are taking a close look at health care value. Cost of care continues to rise and health insurance premiums continue to rise faster than inflation.1 Under the Affordable Care Act of 2010, more Americans will become insured, thus increasing the demand for health care resources.2 This will overtax an already overburdened yet inefficient delivery system.
Clinical and cost burden of ischemic stroke
Stroke is the leading cause of disability in the United States and the fourth leading cause of death.3 It also hits people in their working years, with one-third of patients having their first stroke prior to age 65 years.2,3
Ischemic stroke is a large health care burden, accounting for 87% of all strokes,3 with an estimated 700,000 events3 annually in the United States, costing an estimated $22.8 billion in direct health care expenditures in the United States annually3 and an estimated €18.5 billion in the European Union.4 By 2030, the direct health care costs are projected to grow to $184 billion.3
Health care delivery innovation, or the development of new ways to organize care around patients and their conditions, is thought to reduce substantial waste in health care spending5 and is key to relieving some this burden.6 If designed and implemented well, health care delivery innovations in acute ischemic stroke and TIA could have a considerable effect in this regard.
Teleneurology networks to improve access to thrombolysis for acute ischemic stroke
From the early 2000s, technology for remote, interactive audiovisual interactions with patients has grown in all aspects of medicine, but perhaps nowhere as much as in acute stroke neurology (telestroke care). Ischemic stroke is a time-sensitive condition and IV thrombolysis (tissue plasminogen activator [tPA]) is available only in the first 4.5 hours after onset of symptoms. Thus, it is vital to have early access to neurologists who can evaluate and determine the appropriateness of treatment. Improvement in telemedicine technology and networks allows expert supervision of care for stroke patients from the initial thrombolysis decision and beyond to poststroke care.7
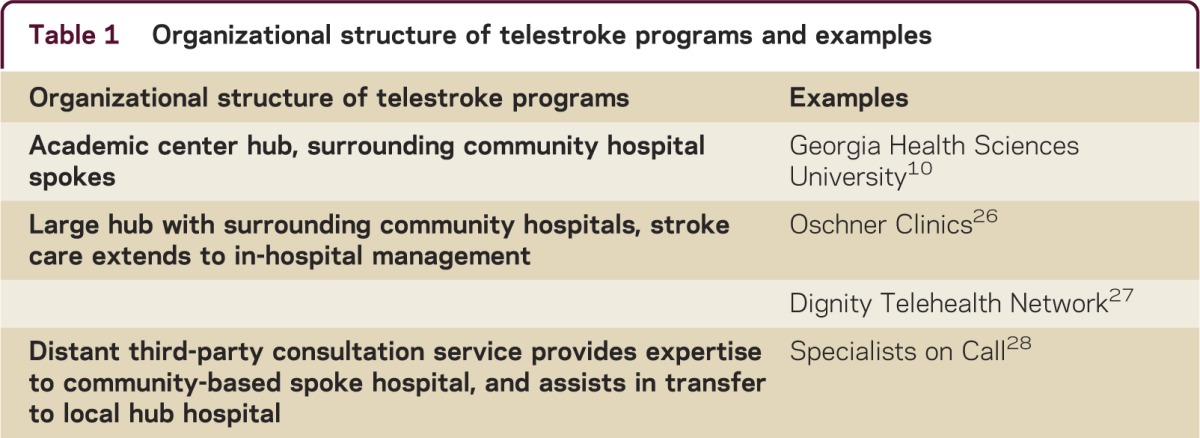
A telemedicine model typically couples an expert consultant in a hub facility (geographically remote from the patient) with a spoke facility where the patient is present and needs evaluation by specialty services. Different models of telestroke have been established, varying in size, geographic reach of the hub hospitals or consultancy service groups, and workflows.8 Examples of different organizational structures of teleneurology networks are shown in table 1.
Table 1 Organizational structure of telestroke programs and examples
Telestroke has been shown to be cost-saving by facilitating treatment of more patients with IV tPA and increasing number of direct to home discharges, thus reducing long-term rehabilitation stays, saving up to $44,000 annually per hospital within the network.9 Still, cost-sharing measures or support from the state and federal level are necessary to make telemedicine networks economically feasible, as current reimbursement for telemedicine is limited by law to certain specialties and low-access communities.10 Fee-for-service reimbursement traditionally requires in-person encounters, which teleneurology is not. The American Academy of Neurology supports the reimbursement of telemedicine encounters in the same fashion as face-to-face, telephonic, and e-mail clinical encounters.11
Currently, many hub and spoke models work using a subscription fee model, where a hospital pays a flat access fee, sometimes with an additional per-patient consultation fee.8 Substantial technology capital investments can be incurred by both hub and spoke sites. As hospital reimbursements for acute stroke admissions are based on discharged patients, one potential benefit to a spoke hospital would be to increase the number of patients they can ship and keep, where the spoke hospital may benefit from the expert advice from a remote consulting neurologist, but still benefit from the insurer reimbursements of acute IV tPA-treated stroke patients (thrombolysis-treated acute stroke patients have a higher remuneration than a nonthrombolyzed patient). The hub hospitals may be incentivized to provide this service by adopting a subscription fee and increasing referrals for patients who benefit from transfer to a higher level of medical care.
State licensure is another barrier to interstate practice of teleneurology. Current legislation and compact initiatives led by the Federation of State Medical Boards is underway to try to reduce the hurdles of interstate practice of medicine. This would allow expedited credentialing pathways for physicians who desire to practice telemedicine in other states, after designating one state as their home state and demonstrating board certification in a specialty.
In the future, teleneurology can expand to beyond just the acute IV tPA decision-making evaluation to care in the acute stroke hospitalization, other neurologic evaluations, and in the postacute care setting such as rehabilitation or clinic.8
Improved efficiency of local emergency care for acute ischemic stroke
As described, acute ischemic stroke is an emergency, requiring expeditious evaluation and treatment to reduce disability. IV tPA treatment is Food and Drug Administration–approved up to 3 hours after symptom onset, but national and international guidelines recommend treatment up to 4.5 hours after symptom onset.12 However, delaying IV tPA administration to 3–4.5 hours after symptom onset drastically reduces odds of clinical benefit.12 Furthermore, only 5%13 of all patients with acute ischemic stroke receive IV tPA. A literature review suggests that fewer than half of potentially eligible patients are receiving IV tPA.14 Several system-level factors (not related to individual patient clinical characteristics) identified as causes of potential delay15 include lack of patient education regarding symptoms of stroke, delays in prehospital emergency care including dispatch and transport, and delays within emergency departments.
Several groups have developed interventions that reduced treatment times considerably. One group in Finland16 adopted a strategy that enabled efficient use of ambulance transportation time by preregistering ischemic stroke patients during transport and having the neurologist obtain essential clinical history by mobile phone during transport. On arrival in the emergency department, patients bypass the triage area and go directly to the CT scanner, where they receive point-of-care laboratory testing. By providing only essential diagnostic and evaluation services that would lead to patient treatment, and avoiding or delaying other treatment steps until after thrombolysis treatment, they were able to reduce their door-to-needle time to 20 minutes.16 In St. Louis, Missouri, emergency department reorganization borrowing from manufacturing principles have also considerably reduced time for acute ischemic stroke treatment.17 Systems of care changes have demonstrated reproducible benefits of increasing the number of patients receiving IV tPA under 60 minutes to 41% (previously only 26%).18 Key elements of evidence-based redesigned rapid thrombolysis are identified in table 2. These changes can potentially increase the number of patients receiving this disability-reducing medication.
Table 2 Optimal care for rapid tissue plasminogen activator delivery

Alternatives to inpatient care for TIA
Closely related to ischemic stroke, TIA is a syndrome of focal neurologic deficits caused by temporary lack of blood flow to a part of the brain. While time-limited in nature, TIA portends a higher risk of stroke, so these patients should be quickly evaluated and treated with preventive medicines or surgeries.19 The traditional model of TIA care in the United States results in the large majority of patients being admitted to the hospital.20 Innovators around the world have looked for safe alternative solutions for rapid evaluation and treatment of TIA.
Protocol-based, expedited care for TIA in observation units results in good quality at lower cost.21 Borrowing from colleagues who safely evaluate and treat transient cardiac symptoms using this extension of the emergency department, it seems natural to extend observation units to transient cerebrovascular events as well. An accelerated diagnostic protocol in the observation unit was found to provide safe TIA care with equally low rates of recurrent events at 90 days compared with inpatient care, and at a median reduced cost of $1,643 per patient.21
TIA clinics provide an even more efficient care method. For example, the Monash clinic in Melbourne, Australia, instituted a triage program22 in their emergency department to select low-risk patients who could safely return home with timely outpatient follow-up. Most patients were agreeable to this disposition and all willingly returned to the clinic for further care and were found to have equally low rates of stroke in both the admitted and discharged groups of patients. Similarly, clinician innovators in Spain demonstrated an 80% reduction in spending and safe outcomes with TIA clinic follow-up rather than inpatient care.23 Electronic clinical support tools24 to assist non-neurologists in providing appropriate outpatient TIA care may also improve access to high-quality neurologic care in communities with limited neurologist access. Creating a TIA clinic is not without challenges.
Ideally, a TIA clinic pathway would be both expedient and efficient, being able to identify those patients who are most appropriate for directly to clinic, have patients attend clinic urgently (ideally in less than 48 hours), and have the clinic be staffed optimally by providing top of license care with a midlevel provider (such as nurse practitioner or physician assistant) with physician supervision and review of complex cases. The patient should have access to urgent diagnostic testing that is commonly used for TIA patients such as MRI, cardiac imaging, and echocardiograms. This will require coordination with other departments and services such as radiology and cardiology.
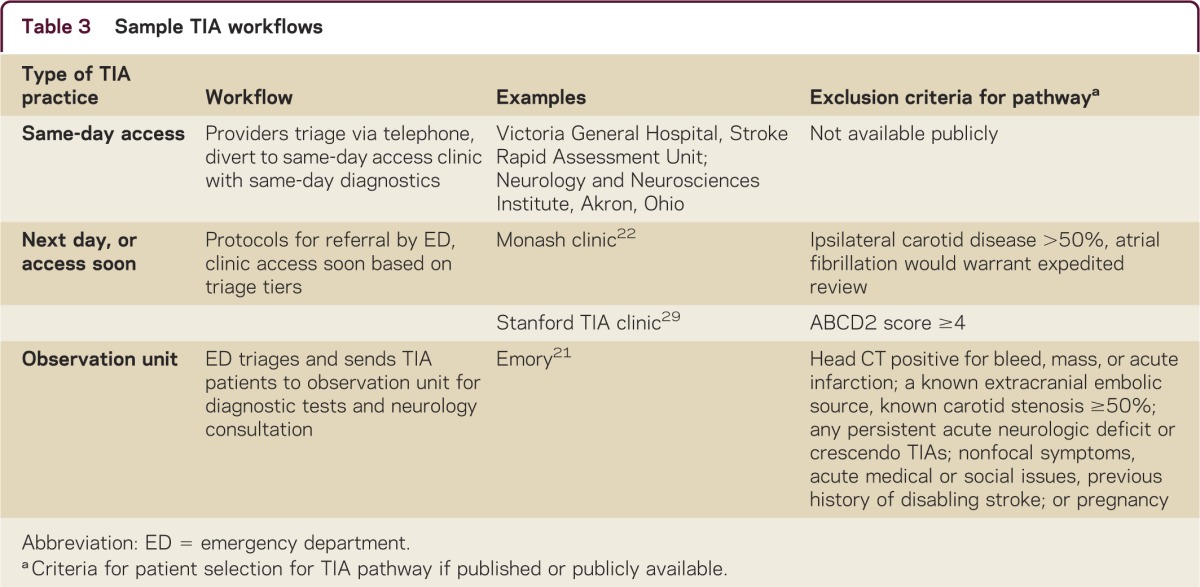
One possibility of restructuring patient flow may include assistance by a physician extender such as a nurse practitioner or other advanced practice provider first, then completion of diagnostic testing, followed by review of test results by neurologist of advance practice provider with neurologist oversight. Examples of different workflows are listed in table 3.
Table 3 Sample TIA workflows
Emerging payment reform to spur health care delivery innovation
While innovations are exciting unto themselves, one of the main reasons these delivery improvements are developing is that the financial context in which physicians work has evolved. The Centers for Medicare and Medicaid Services (in essence, the largest health insurer in the country) is moving towards bundled payments.2 Bundled payments are set on the basis of expected costs for episodes of care. Physicians will be increasingly rewarded by payers for the value they bring to patients as measured by quality outcomes and cost of care. Thus practicing neurologists should consider both cost and additional yield of diagnostic testing, as well as direct treatment decisions, in a safe, but lowest cost manner. Care will be measured not in discrete input units (fee-for-service) but rather in the outputs of health care: mortality and morbidity, quality of life, and patient satisfaction.
Merging a continuum of stroke care from acute neurologic event to postacute stroke care will allow more opportunities to identify upstream interventions to reduce disability (such as faster IV tPA delivery, perhaps with the aid of teleneurology) as well as evaluations of neurologic patients in a lower-cost setting (outpatient clinic or observation units vs costly hospitalizations or a remote consultant via teleneurology). This new payment reimbursement model will allow systems to reorganize to deliver the best value care to patients, even if it means a reduction in in-hospital charges.
From the patient perspective, emerging models of value-based insurance design encourage patients to choose providers who provide high-quality care at relatively low cost. Private insurers are moving to tiering neurologists by value. This means they are grouping neurologists by metrics of quality and value, and making some physicians more preferred than others in terms of copayments for their services.25
In the near future, provision of the best quality care at the lowest cost will thus benefit all stakeholders—patients, physicians, and health care delivery systems. Ultimately, payment reform and the push for transparency in quality and cost will drive adoption of health care delivery innovations, including some of the above, to improve the affordability of high-quality care.
Working together, neurologists and their health care delivery systems are well-equipped to further improve the value of the stroke care they provide, and increase access to such care, while meeting the challenges of the rapidly evolving regulatory and economic climate.
Take-home points
Implement and broaden telestroke systems to increase access to thrombolysis (IV tPA) for acute ischemic stroke.
Allow expert supervision at a distance to improve emergency stroke care delivery in economical fashion.
Redesign workflows to improve speed of thrombolysis (IV tPA) to improve clinical outcomes (and decrease long-term costs) for acute ischemic stroke.
Offer patients with TIA the right intensity and location of care (observation unit or outpatient), avoiding admission for patients who have appropriately low risk for near term stroke or recurrent event.
STUDY FUNDING
No targeted funding reported.
DISCLOSURES
W.A. Tai has served as a consultant for Acupera Inc. L. Kalanithi received a Spectrum Innovation Accelerator Seed Grant through Stanford University. J. Conley reports no disclosures. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp http://cp.neurology.org/lookup/doi/10.1212/CPJ.0000000000000081.
Correspondence to: wtai@stanford.edu
Funding information and disclosures are provided at the end of the article. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp http://cp.neurology.org/lookup/doi/10.1212/CPJ.0000000000000081.
Footnotes
Correspondence to: wtai@stanford.edu
Funding information and disclosures are provided at the end of the article. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp http://cp.neurology.org/lookup/doi/10.1212/CPJ.0000000000000081.
REFERENCES
- 1.Claxton G, Rae M, Panchal N. Health benefits in 2013: moderate premium increases in employer-sponsored plans. Health Aff. 2013;32:1667–1676. doi: 10.1377/hlthaff.2013.0644. [DOI] [PubMed] [Google Scholar]
- 2.Skolarus LE, Jones DK, Lisabeth LD, Burke JF. The Affordable Care Act and stroke. Stroke. 2014;45:2488–2492. doi: 10.1161/STROKEAHA.114.005315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Go AS, Mozaffarian D, Roger VL. Heart disease and stroke statistics: 2014 update: a report from the American Heart Association. Circulation. 2014;129:e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Di Carlo A. Human and economic burden of stroke. Age Ageing. 2009;38:4–5. doi: 10.1093/ageing/afn282. [DOI] [PubMed] [Google Scholar]
- 5.Berwick DM, Hackbarth AD. Eliminating waste in US health care. JAMA. 2012;307:1513–1516. doi: 10.1001/jama.2012.362. [DOI] [PubMed] [Google Scholar]
- 6.Berwick DM, Nolan TW, Whittington J. The triple aim: care, health, and cost. Health Aff. 2008;27:759–769. doi: 10.1377/hlthaff.27.3.759. [DOI] [PubMed] [Google Scholar]
- 7.Rubin MN, Wellik KE, Channer DD, Demaerschalk BM. Systematic review of telestroke for post-stroke care and rehabilitation. Curr Atheroscler Rep. 2013;15:343. doi: 10.1007/s11883-013-0343-7. [DOI] [PubMed] [Google Scholar]
- 8.Hess DC, Audebert HJ. The history and future of telestroke. Nat Rev Neurol. 2013;9:340–350. doi: 10.1038/nrneurol.2013.86. [DOI] [PubMed] [Google Scholar]
- 9.Switzer JA, Demaerschalk BM, Xie J, Fan L, Villa KF, Wu EQ. Cost-effectiveness of hub-and-spoke telestroke networks for the management of acute ischemic stroke from the hospitals' perspectives. Circ Cardiovasc Qual Outcomes. 2013;6:18–26. doi: 10.1161/CIRCOUTCOMES.112.967125. [DOI] [PubMed] [Google Scholar]
- 10.Kulcsar M, Gilchrist S, George MG. Improving stroke outcomes in rural areas through telestroke programs: an examination of barriers, facilitators, and state policies. Telemed J E Health. 2014;20:3–10. doi: 10.1089/tmj.2013.0048. [DOI] [PubMed] [Google Scholar]
- 11.AAN Legislative Position Statement on Telemedicine [online]. Available at: https://www.aan.com/uploadedFiles/Website_Library_Assets/Documents/6.Public_Policy/1.Stay_Informed/2.Position_Statements/3.PDFs_of_all_Position_Statements/FinalTelemedicineLegislativePositionStatement.pdf. Accessed July 7, 2014.
- 12.Saver JL, Fonarow GC, Smith EE. Time to treatment with intravenous tissue plasminogen activator and outcome from acute ischemic stroke. JAMA. 2013;309:2480–2488. doi: 10.1001/jama.2013.6959. [DOI] [PubMed] [Google Scholar]
- 13.Adeoye O, Hornung R, Khatri P, Kleindorfer D. Recombinant tissue-type plasminogen activator use for ischemic stroke in the United States: a doubling of treatment rates over the course of 5 years. Stroke. 2011;42:1952–1955. doi: 10.1161/STROKEAHA.110.612358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eissa A, Krass I, Bajorek BV. Optimizing the management of acute ischaemic stroke: a review of the utilization of intravenous recombinant tissue plasminogen activator (tPA) J Clin Pharm Ther. 2012;37:620–629. doi: 10.1111/j.1365-2710.2012.01366.x. [DOI] [PubMed] [Google Scholar]
- 15.Meretoja A, Kaste M. Pre- and in-hospital intersection of stroke care. Ann NY Acad Sci. 2012;1268:145–151. doi: 10.1111/j.1749-6632.2012.06664.x. [DOI] [PubMed] [Google Scholar]
- 16.Meretoja A, Strbian D, Mustanoja S, Tatlisumak T, Lindsberg PJ, Kaste M. Reducing in-hospital delay to 20 minutes in stroke thrombolysis. Neurology. 2012;79:306–313. doi: 10.1212/WNL.0b013e31825d6011. [DOI] [PubMed] [Google Scholar]
- 17.Ford AL, Williams JA, Spencer M. Reducing door-to-needle times using Toyota's lean manufacturing principles and value stream analysis. Stroke. 2012;43:3395–3398. doi: 10.1161/STROKEAHA.112.670687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fonarow GC, Zhao X, Smith EE. Door-to-needle times for tissue plasminogen activator administration and clinical outcomes in acute ischemic stroke before and after a quality improvement initiative. JAMA. 2014;311:1632–1640. doi: 10.1001/jama.2014.3203. [DOI] [PubMed] [Google Scholar]
- 19.Easton JD, Saver JL, Albers GW. Definition and evaluation of transient ischemic attack: a scientific statement for health care professionals from the American Heart Association/American Stroke Association Stroke Council; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing; and the Interdisciplinary Council on Peripheral Vascular Disease: the American Academy of Neurology affirms the value of this statement as an educational tool for neurologists. Stroke. 2009;40:2276–2293. doi: 10.1161/STROKEAHA.108.192218. [DOI] [PubMed] [Google Scholar]
- 20.Durrani-Tariq S, Eskin B, Allegra JR. Admission rates of ED patients with transient ischemic attack have increased since 2000. Am J Emerg Med. 2013;31:1349–1351. doi: 10.1016/j.ajem.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 21.Nahab F, Leach G, Kingston C. Impact of an emergency department observation unit transient ischemic attack protocol on length of stay and cost. J Stroke Cerebrovasc Dis. 2012;21:673–678. doi: 10.1016/j.jstrokecerebrovasdis.2011.02.017. [DOI] [PubMed] [Google Scholar]
- 22.Sanders LM, Srikanth VK, Jolley DJ. Monash transient ischemic attack triaging treatment: safety of a transient ischemic attack mechanism-based outpatient model of care. Stroke. 2012;43:2936–2941. doi: 10.1161/STROKEAHA.112.664060. [DOI] [PubMed] [Google Scholar]
- 23.Martínez-Martínez MM, Martínez-Sánchez P, Fuentes B. Transient ischaemic attacks clinics provide equivalent and more efficient care than early in-hospital assessment. Eur J Neurol. 2013;20:338–343. doi: 10.1111/j.1468-1331.2012.03858.x. [DOI] [PubMed] [Google Scholar]
- 24.Ranta A, Cariga P. Who should manage transient ischemic attacks? A comparison between stroke experts, generalists, and electronic decision support. NZ Med J. 2013;126:25–31. [PubMed] [Google Scholar]
- 25.UnitedHealth Premium Program [online]. Available at: https://www.uhctools.com/assets/100-7315%20Premium%20FAQs.pdf. Accessed July 7, 2014.
- 26.Oschner Teleneurology [online]. Available at: http://www.ochsner.org/news/story/ochsners_telestroke_program_reaches_1000th_consult_in_two_and_a_half_years/. Accessed July 7, 2014.
- 27.Dignity Telehealth Network [online]. Available at: http://www.dignityhealth.org/sacramento/services/telehealth-network. Accessed June 30, 2014.
- 28.Specialists On Call [online]. Available at: http://www.specialistsoncall.com/en/. Accessed June 30, 2014.
- 29.Olivot JM, Wolford C, Castle J. Two aces: transient ischemic attack work-up as outpatient assessment of clinical evaluation and safety. Stroke. 2011;42:1839–1843. doi: 10.1161/STROKEAHA.110.608380. [DOI] [PubMed] [Google Scholar]


