Abstract
Background
To compare the outcome and complication rates of femoral artery closure and surgical cutdown for endovascular aortic repair procedures (EVAR).
Material/Methods
Patients underwent either percutaneous femoral artery closure (PA group) or surgical cutdown (SC group) for EVAR between July 2011 and June 2016 and EVAR procedures were used for all cases. Data on outcomes and complications were collected and compared.
Results
The SC group contained 55 patients and the PA group contained 60 patients and the technical success rates were 100.0% and 98.0%, respectively. The mean operation time, time to ambulation, and postoperative hospital stay were significantly shorter in the PA group (P<0.01). The estimated intraoperative blood loss and wound pain scores were significantly higher in the SC group (P<0.01). However, the PA procedure was more expensive (P<0.01). The overall incidence rate of complications was higher in the SC group (P=0.026).
Conclusions
The PA technique had a high success rate, shorter operation time and hospital stay, and fewer wound complications compared to SC. Thus, PA might be the preferred choice for selected EVAR procedures.
MeSH Keywords: Aortic Aneurysm, Abdominal; Aortic Diseases; Endovascular Procedures
Background
Endovascular aortic repair (EVAR) improves clinical outcomes, as evidenced by reduced operative morbidity and mortality and shorter hospital stays. Thus, this procedure has become the preferred option for treatment of several aortic pathologies, such as aortic dissection, aneurysms and ruptured aneurysms, and traumatic injury, during the past decade [1–6]. The common femoral artery must be exposed during conventional endovascular repair, and this surgical cutdown (SC) technique increases the incidence of complications associated with the incision [7]. Conversely, the total percutaneous access (PA) technique is minimally invasive and decreases the incidence of complications associated with the incision; moreover, the PA technique has been shown to improve perioperative outcomes [8]. Recently, several randomized and non-randomized clinical trials revealed that the PA technique was associated with shorter operation times, fewer incision complications, and shorter hospital stays compared to the SC technique [2,8–11], and these advantages have been verified in a larger-scale study [12]. Although various femoral closure devices have been proven to be safe and effective [13,14], few studies have examined the PA technique in Asian patients undergoing endovascular aortic repair. Thus, the present case-control study examined consecutive patients with aortic dissection and aneurysm undergoing endovascular repair. The perioperative outcome and complication rates of the PA and SC techniques were compared.
Material and Methods
Patients
This was a non-randomized observational study of 55 consecutive patients (43 male) with a mean age of 61.11±12.78 years (range, 54–80 years) who underwent endovascular abdominal aortic aneurysm repair (n=29) or uncomplicated thoracic aortic dissection type B (n=26) between July 2011 and June 2016. Consecutive patients who underwent surgical cutdown for femoral artery access were allocated to the SC group. The percutaneous access group (PA group) consisted of 60 consecutive patients (51 male) with mean age of 58.27±11.60 years (range, 50–78 years) who underwent abdominal (n=39) or thoracic (n=21) endovascular aortic repair using the totally percutaneous technique with Perclose Proglide devices (Abbott Vascular, Inc., Redwood City, CA). All patients were examined using pre-procedural planning computed tomography angiography (CTA). This study was approved by the local ethics committee and institutional review board, and all patients provided written informed consent. All endovascular procedures were performed in the hybrid catheterization room under fluoroscopy by a team of vascular surgeons and interventionists. All data from patients were rendered anonymous.
Inclusion and exclusion criteria
The inclusion criteria were defined as follows: aged older than 18 but younger than 80 years, provided informed consent, abdominal aortic aneurysm with a maximum diameter >5 cm or rapidly expanding abdominal aortic aneurysm, uncomplicated aortic dissection type B (non-acute stage), a suitable ipsilateral common femoral artery for percutaneous access with a Preclose technique, and life expectancy >1 year as judged by the investigator.
Patients with the following conditions were excluded: emergency procedure for aortic disease; circumferential femoral artery calcification; previous surgery on the groin triangle; severe iliac-femoral artery tortuosity; groin infection; traumatic vascular injury; femoral artery aneurysm, arteriovenous fistula, or pseudoaneurysm; allergy to contrast; hemorrhagic disease; active vasculitis; and cerebrovascular accident or myocardial infarction within 3 months.
Procedural
Most patients received local anesthesia, whereas 7 and 5 patients underwent general anesthesia in the SC and PA groups, respectively. Intravenous heparin (50 U/kg) was routinely administered before the procedure and reversed using protamine at the end of the procedure.
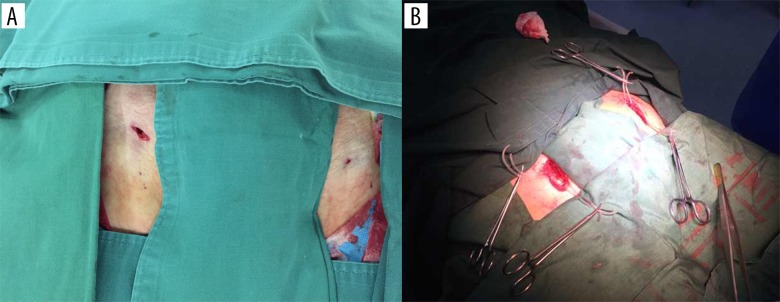
In the PA group (Figure 1A), all endografts were placed with a total percutaneous technique using a percutaneous arterial closure device, the 6 French (Fr) Perclose Proglide (Abbott Vascular, Inc., Redwood City, Calif). The Proglide device and its use have been previously described in detail [15–17]. Briefly, a stab wound (approximately 1 cm) was created to enlarge the puncture site, and a subcutaneous tunnel was carefully fashioned with a hemostatic clamp. The common femoral artery can be directly palpated in this tunnel, and an 18-gauge needle was then used to puncture the anterior aspect of each common femoral artery. An 8-Fr introducer sheath was initially inserted using the Seldinger technique, a straight-tip soft hydrophilic 0.035 guide wire was introduced, and the 8-Fr introducer sheath was temporarily removed. The first device was then laterally rotated at a 30–45° angle and deployed in the standard manner, but the Perclose suture strands were carefully extracted from the device and extracorporeally tagged with hemostatic forceps. Before completely removing the first carrier device, a 0.035-inch hydrophilic guide wire was reinserted into the femoral artery via a marked monorail wire tube in situ, and the second Perclose device was then activated. The guide wire was reinserted, followed by the temporary insertion of a 10-Fr sheath to maintain hemostasis. All endovascular repair procedures continued thereafter according to routine practice.
Figure 1.
The femoral arteries accessed during endovascular aortic repair. (A) Femoral arteries exposed with the percutaneous access technique. (B) Femoral arteries exposure with the conventional surgical cutdown technique.
At the end of the procedure, the preformed knots were tightened using a knot pusher according to the recommendations provided by the manufacturer, and temporary hemostasis was achieved by manual compression. After verification of hemostasis, the guide wire was removed. In case of persistent bleeding, a third or even fourth Perclose device was deployed before removal of the guide wire. Distal perfusion was checked, and manual compression was maintained for an additional 5–10 min following banding with elastic adhesive tape or a bandage, and the patients were confined to bed for 6 h.
In the SC group (control group, Figure 1B), patients underwent endovascular repair procedures via a 4- to 6-cm-long transverse oblique incision located in the groin just below the inguinal ligaments. Both common femoral arteries were exposed, the proximal and distal ends of the vessel were controlled with vessel loops, and the endovascular procedures were then continued according to routine practice. After the sheath was removed, the artery was repaired with 5-0 polypropylene sutures using standard technique. Vacuum drains were placed in the incision, which was closed in layers.
Data collection
A clinical database was prospectively analyzed to obtain patient demographics, operative risks, procedural details, complications, costs, and follow-up information. Local and regional complications were detected clinically and then evaluated by Doppler ultrasound when necessary. The primary endpoint was the technical success rate, which was defined as successful femoral artery closure without secondary surgeries or endovascular therapy within 30 days of the procedure. The secondary endpoint included early access site-related complications (e.g., access site hematoma, pseudoaneurysm, arteriovenous fistula, infection, lymphatic leak, wound dehiscence, and fat liquefaction), anesthesia method, operation time, time to ambulation, postoperative hospital stay (decided by the therapy team), and incision procedure expense (USD). Moreover, postoperative incision pain was assessed using VAS scores (visual analogue scale) 24 h after the operation, in a blinded fashion.
Statistical analysis
Continuous variables are expressed as the mean ± standard deviation (SD), and discrete data are reported as counts and percentages. Continuous variables were compared with the two-sided t test. Differences in categorical variables were analyzed with the chi-squared test or Fisher’s exact test. Each access site served as an independent binomial categorical variable relating to the success or failure of closure. Significance was set at P<0.05 and reflected 2-tailed distributions in all cases. Statistical analyses were performed using SPSS software (version 11.0, SPSS, Chicago, IL, USA).
Results
Demographic and baseline data
A total of 180 patients were evaluated, then 115 patients were enrolled according to the criteria (Table 1); 60 patients who underwent total percutaneous endograft placement using the Preclose Proglide closure device were enrolled in the PA group, whereas 55 patients treated using a traditional surgical cutdown approach were included in the SC group. The demographic data and baseline data did not significantly differ between the 2 groups (Table 1). High-risk factors were similarly distributed in the 2 groups.
Table 1.
The demographic characteristics of the two groups.
| SC (n=55) | PA (n=60) | P value* | |
|---|---|---|---|
| Gender (F/M) | 12/43 | 9/51 | 0.34 |
| Age (years) | 61.11±12.78 | 58.27±11.60 | 0.21 |
| Weight (Kg) | 68.78±7.62 | 70.11±6.49 | 0.31 |
| Smoking | 36 | 41 | 0.74 |
| Hypertension | 40 | 38 | 0.28 |
| 2-diabetes | 6 | 7 | 0.90 |
| CAD | 12 | 17 | 0.42 |
| PAD | 6 | 9 | 0.52 |
| Hyperlipidemia | 25 | 31 | 0.51 |
| COPD | 2 | 3 | 0.91 |
| CRI | 2 | 2 | 0.56 |
SC – surgical cutdown; PA – percutaneous access; CAD – coronary atherosclerotic disease; PAD – peripheral arterial disease; COPD – chronic obstructive pulmonary disease; CRI – chronic renal insufficiency.
P value, SC compared with PA.
Procedure parameters
Thoracic endovascular aortic repair procedures were performed in 26 patients in the SC group and 21 patients in the PA group (Table 2, P>0.05). Moreover, 29 and 39 patients underwent abdominal endovascular aortic repair procedures in the SC and PA groups, respectively (P>0.05), and the use of endografts did not significantly differ between the 2 groups (Table 2, P>0.05). Local anesthesia was used in 48 and 55 patients in the SC and PA groups, respectively, whereas 7 and 5 patients underwent general anesthesia in the SC and PA groups, respectively (Table 2, P>0.05). The mean diameter was (20.7±1.6) Fr in the SC group and (21.0±1.5) Fr in the PA group, and this difference was not significant (Table 2, P=0.30). Partial cases needed more than 2 grafts to complete the procedures, and the number of stent grafts used in the procedures was similar between the 2 groups (1.74±0.91 vs. 1.82±0.87, P=0.43).
Table 2.
The Procedure parameters for the two groups.
| SC (n=55) | PA (n=60) | P value* | |
|---|---|---|---|
| Type of procedure | |||
| Thoracic/abdominal | 26/29 | 21/39 | 0.18 |
|
| |||
| Thoracic graft | |||
| Valiant/capitivia | 8/18 | 3/18 | 0.18 |
|
| |||
| Graft of abdominal | |||
| Aegis | 3 | 5 | 0.95 |
| Hercules B | 7 | 9 | 0.92 |
| Endurant | 19 | 25 | 0.95 |
|
| |||
| Number of graft | 1.74±0.91 | 1.82±0.87 | 0.43 |
|
| |||
| Diameter of sheath | 30 | ||
| 18 Fr | 7 | 4 | |
| 20 Fr | 25 | 29 | |
| 22 Fr | 19 | 22 | |
| 24 Fr | 4 | 5 | |
| Mean diameter | 20.7±1.6 | 21.0±1.5 | |
|
| |||
| Anesthesia | 0.44 | ||
| Local/general | 48/7 | 55/5 | |
SC – surgical cutdown; PA – percutaneous access; Fr – French.
P value, SC compared with PA.
The outcome of procedures
The technical details of the procedures are listed in Table 3. Specifically, 55 patients (84 groin incisions) underwent SC technique with a technical success rate of 100%, and 60 patients (99 puncture sites) underwent PA technique with a success rate of 97.9%. One patient in the PA group experienced femoral artery occlusion after use of the closure device and was converted to conventional surgical repair, and another patient developed a pseudoaneurysm of the puncture site 1 week after the procedure and was treated with an injection of thrombin under ultrasound guidance. In 3 patients, more than 2 Preclose closure devices were used. Severe complications, such as death, systematic infection, myocardial infarction, stroke, and paraplegia, did not occur in either group. One patient in the PA group developed deep-vein thrombosis (1.67%) 10 days after the procedure; the patient developed calf swelling, which was diagnosed as venous thrombosis by an ultrasound examination, and oral anticoagulant therapy was recommended.
Table 3.
The outcome of procedures for the two groups.
| SC (n=55) | PA (n=60) | P value* | |
|---|---|---|---|
| Death (%) | 0 | 0 | Null |
| Sever complication (%) | 0 | 0 | Null |
| DVT (%) | 0 | 1.67 | 0.97 |
| Technique success rate (%) | 100 | 98.0 | 0.55 |
| Operation time (min) | 120.36±26.31 | 94.17±23.24 | <0.01 |
| Estimated blood loss (ml) | 175.45±77.33 | 126.55±61.83 | <0.01 |
| Time to ambulation (hr) | 36.45±9.40 | 21.12±5.61 | <0.01 |
| Stay of postoperative (days) | 6.85±1.55 | 5.12±1.35 | <0.01 |
| Pain score (VAS) | 4.41±1.41 | 2.52±1.31 | <0.01 |
| Cost of femoral access (USD) | 524.94±170.31 | 1353.97±471.01 | <0.01 |
SC – surgical cutdown; PA – percutaneous access; DVT – deep venous thrombosis; VAS – visual analogue scale score.
P value, SC compared with PA.
The mean operation times were significantly shorter in the PA group than in the SC group (P<0.01), and the intraoperative blood loss was lower in the PA group (P<0.01). Nevertheless, blood loss was estimated at the end of the procedure and was not an exact value. Patients in stable condition were discharged, and postoperative stays were longer in the SC group (P<0.01). Incision pain after the procedure was assessed using VAS scores. The patients were administered oral analgesics, and the pain score 24 h after surgery was significantly higher in the SC group (P<0.01). However, the cost of femoral access was higher in the PA group (P<0.01).
Complications associated with femoral artery access
The results of complications are listed in Table 4. All femoral arteries were assessed with CTA before the procedure and ultrasound after the procedure. Distal embolization or arterial wall dissection was not observed during the study, and arterial-venous fistulae and iliac-femoral artery ruptures were not observed in either group. Two patients developed an incision hematoma in the PA group, which did not require specific treatment and healed completely (2.03% vs. 0%, P>0.05). Other wound complications included 2 lymphatic leaks (2.38%), 2 incision infections (2.38%), 1 incision dehiscence (1.19%), and 2 cases of fat liquefaction (2.38%) in the SC group, whereas none of these complications occurred in the PA group. The incidence of these complications did not differ between groups (P>0.05). In patients who experienced lymphatic leaks and fat liquefaction, wound dressings were applied and nursing care was administered, which allowed incisions to heal within 1 month after surgery. The incision dehiscence was small and healed completely after secondary suturing under local anesthesia. The incision infection was a surface infection of the wound that healed completely after the administration of oral antibiotics and the application of a wound dressing. Surgical incisions in the groin area can also develop skin paresthesia, which was observed in 4 patients in the SC group (4.76% vs. 0%, P>0.05). Although the rates of complications did not significantly differ between groups, the overall incidence rate of complications was higher in the SC group than in the PA group (13.10% vs. 4.04%, P=0.026).
Table 4.
The complications associated with femoral artery access in both group.
| SC (n=84) | PA (n=99) | P value* | |
|---|---|---|---|
| Hematoma | 0 | 2 | 0.55 |
| Lymphatic leak | 2 | 0 | 0.41 |
| Incision infection | 2 | 0 | 0.41 |
| Incision dehiscence | 1 | 0 | 0.93 |
| Fat liquefaction | 2 | 0 | 0.41 |
| Paresthesia | 4 | 0 | 0.09 |
| Artery occlusion | 0 | 1 | 0.93 |
| Pseudoaneurysm | 0 | 1 | 0.93 |
| Arterial-venous fistula | 0 | 0 | Null |
| Artery rupture/dissection | 0 | 0 | Null |
| Artery embolization | 0 | 0 | Null |
| Total complication | 11 | 4 | 0.026 |
SC – surgical cutdown; PA – percutaneous access.
P value, SC compared with PA.
Discussion
Minimally invasive surgery has become popular in vascular surgery, and endovascular therapy is becoming increasingly important for the treatment thoracic and abdominal aortic disease. In fact, endovascular therapy is the main treatment for ruptured aortic aneurysms and aortic injury, and several published reports demonstrated that endovascular and surgical repair result in similar mid- and long-term outcomes [18–20]. Therefore, totally percutaneous repair may be a better choice for patients with aortic diseases. These findings were confirmed in a recent large-scale study; a high success rate, shorter procedure time, shorter hospital stay, and fewer access complications were observed in patients treated with percutaneous procedures [8,12]. In our study, the clinical outcomes proved the safety and efficacy of percutaneous procedures with the Preclose technique using the Proglide femoral artery device; in the PA group, the success rate was 98.9% and the clinical success rate was 97.9% 30 days after the procedure. Moreover, the operation times, shorter times until ambulation, shorter hospital stays, and fewer complications were observed in our results.
The surgical cutdown of the common femoral artery is the traditional therapy for endovascular aortic repair and is associated with significant advantages, but wound complications are discouraging, especially in patients with diabetes and obesity. Specifically, the incidence of wound complications varies (2.1% to 22.8%) [7,19–21], and the incidence of access-related complications ranges from 0% to 11% [7,22,23]. In the present study, the incidence of complications associated with surgical cutdown was 13.1%. These complications may require interventional therapy and prolonged hospital stay, which increased the cost of care. Most complications, such as infection, fat liquefaction, and dehiscence, completely resolved after intervention consisting of wound drainage, secondary sutures, and oral antibiotics, whereas the lymphatic leaks required careful wound dressing and other treatment to prevent serious complications. Regarding other wound complications, most studies showed that surgical technique is associated with a higher incidence of complications, and some studies reported that the complications were roughly equivalent in severity [9,20–23]. Moreover, the surgical cutdown technique may also be associated with more surgical blood loss, but blood loss was evaluated based on the use of surgical suction and gauze in our study, and this value may consequently not be exact. Specifically, blood loss due to surgical cutdown (mean value 175 ml) was higher than blood loss due to the percutaneous technique (mean value 126 ml), which is consistent with previous findings [24].
Although the percutaneous technique avoids most complications associated with surgical cutdown, it resulted in other femoral access-related complications, which may cause femoral artery closure or technique failure. Access-related complication included groin hematoma, femoral artery stenosis or occlusion, pseudoaneurysm, and fistula, and the incidence of these complications in our study was 4.04%. Previous studies reported that compared to surgical cutdown, use of the femoral artery closure device was associated with larger femoral pseudoaneurysms and no response to ultrasound compression, which resulted in more blood loss and increased transfusion, which are more with surgical procedures [25]. A study report that femoral artery closure devices are associated few technical failures and few late complications occur, and these complications are usually benign [2]. Although groin hematomas do not require further surgical management except for manual compression and limited activity, pseudoaneurysms and arterial-venous fistulae require further therapy, which increases treatment cost and hospitalization time.
The severe complications associated with the femoral artery result in the complete failure of the percutaneous technique. In our study, 1 patient experienced femoral artery occlusion during the procedure and was converted to surgical therapy, and 1 patient developed a pseudoaneurysm and received secondary treatment. In addition, the femoral artery diameter, artery calcification, artery tortuosity, and operator experience are associated with total percutaneous technique failure [26,27], and the type of closure device might may also affect the success rate of the technique [2,8,13,28]. In our study, all target and access arteries were evaluated using CTA and ultrasound before the procedures, and we selected the optimal access technique for patients undergoing endovascular procedures according to the inclusion criteria, which avoided failure by selecting appropriate doctors. Moreover, all percutaneous procedures were conducted using the Preclose technique and the Proglide device in our study; our team routinely uses closure devices with sheaths greater than 6 Fr at the end of endovascular procedures, which avoids technical failures due to learning curves. Given the differences in closure devices, identifying the optimal device for percutaneous procedures requires additional studies in the future.
In the present study, most patients who underwent endovascular therapy received local anesthesia, whereas 7 patients (12.7%) and 5 patients (8.3%) in the SC and PA groups, respectively, received general anesthesia. General anesthesia was used based on patient need and the evaluation of the surgical team but was not necessary for percutaneous procedures, which can be performed under local anesthesia with sedation in most cases. Local anesthesia is safe and offers several advantages, and additional data suggest that local anesthesia limits postoperative complications and decreases the overall cost of endovascular aortic repair without affecting morbidity and mortality [29–31]. Although Geisbüsch et al. reported that local anesthesia is used in 75% of patients, local anesthesia may affect the imaging quality and precise endograft placement [32]. According to our experience over the years, most patients prefer local anesthesia, which also avoids nausea, vomiting, and other complications caused by general anesthesia.
Postoperative incision pain is used to evaluate surgical comfort. In our study, we assessed pain 24 h after surgery using the VAS scores system, which demonstrated that the percutaneous procedure was associated with less pain than the cutdown procedure because the percutaneous procedure only requires a small stab wound approximately 1 cm in size and no scar tissues formed at the groin triangle. Despite the confirmed advantages of percutaneous procedures, this technique is more expensive than the conventional surgical technique. Specifically, the cost of percutaneous closure was $1354 USD in this study, whereas the cost of the surgical procedure was $525 USD. A previous report revealed that femoral access with the percutaneous procedure is associated with higher cost, but the overall cost of the endovascular therapy was similar in the percutaneous and surgical cutdown groups [9,11].
Conclusions
In conclusion, patients undergoing EVAR with favorable features for percutaneous closure have a low incidence of wound-related complications, but this technique was more expensive. Thus, PA procedures might be the preferred choice for selected EVAR procedures. However, our results were obtained from a non-randomized study of a small sample, and these results should be confirmed in future studies.
Acknowledgements
This manuscript was proofread by Prof. Eric Verhoeven (Klinikum Nuremberg, German), and the language of the paper was edited by the American Journal Experts (AJE) group.
Footnotes
Source of support: This project was supported by the Natural Science Foundation of China (No. 8140 0660) and Shaanxi Province (No. 2015JM8423 and No. 2012K15-02-05)
References
- 1.Raval MV, Eskandari MK. Outcomes of elective abdominal aortic aneurysm repair among the elderly: Endovascular versus opern repair. Surgery. 2012;151:245–60. doi: 10.1016/j.surg.2010.10.022. [DOI] [PubMed] [Google Scholar]
- 2.Skagius E, Bosnjak M, Björck M, et al. Percutaneous closure of large femoral artery access with Prostar XL in thoracic endovascular aortic repair. Eur J Vasc Endovasc Surg. 2013;46:558–63. doi: 10.1016/j.ejvs.2013.08.009. [DOI] [PubMed] [Google Scholar]
- 3.Kim WH, Shin S, Ko Y-G, et al. Efficacy and safety of the preclose technique following percutaneous aortic stentgraft implantation. J Endovasc Ther. 2013;20:350–55. doi: 10.1583/12-4103MR2.1. [DOI] [PubMed] [Google Scholar]
- 4.Najjar SF, Mueller KH, Ujiki MB, et al. Percutaneous endovascular repair of ruptured abdominal aortic aneurysms. Arch Surg. 2007;142:1049–52. doi: 10.1001/archsurg.142.11.1049. [DOI] [PubMed] [Google Scholar]
- 5.Takagi H, Umemoto T ALICE (All-Literature Investigation of Cardiovascular Evidence Group) A meta-analysis of adjusted observational studies and randomized controlled trials of endovascular versus open surgical repair for ruptured abdominal aortic aneurysm. Int Angiol. 2016;35:534–45. [PubMed] [Google Scholar]
- 6.Steuer J, Wanhainen A, Thelin S, et al. Outcome of endovascular treatment of traumatic aortic transection. J Vasc Surg. 2012;56:973–78. doi: 10.1016/j.jvs.2012.03.259. [DOI] [PubMed] [Google Scholar]
- 7.Morasch MD, Kibbe MR, Evans ME, et al. Percutaneous repair of abdominal aortic aneurysm. J Vasc Surg. 2004;40:12–16. doi: 10.1016/j.jvs.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 8.Nelson PR, Kracjer Z, Kansal N, et al. A multicenter, randomized, controlled trial of totally percutaneous access versus open femoral exposure for endovascular aortic aneurysm repair (the PEVAR trial) J Vasc Surg. 2014;59:1181–93. doi: 10.1016/j.jvs.2013.10.101. [DOI] [PubMed] [Google Scholar]
- 9.Torsello GB, Kasprzak B, Klenk E, et al. Endovascular suture versus cutdown for endovascular aneurysm repair: A prospective randomized pilot study. J Vasc Surg. 2003;38:78–82. doi: 10.1016/s0741-5214(02)75454-2. [DOI] [PubMed] [Google Scholar]
- 10.Jean-Baptiste E, Hassen-Khodja R, Haudebourg P, et al. Percutaneous closure devices for endovascular repair of infrarenal abdominal aortic aneurysms: A prospective, non-randomized comparative study. Eur J Vasc Endovasc Surg. 2008;35:422–28. doi: 10.1016/j.ejvs.2007.10.021. [DOI] [PubMed] [Google Scholar]
- 11.Ni ZH, Luo JF, Huang WH, et al. Totally percutaneous thoracic endovascular aortic repair with the preclosing technique: A case-control study. Chin Med J (Engl) 2011;124:851–55. [PubMed] [Google Scholar]
- 12.Buck DB, Karthaus EG, Soden PA, et al. Percutaneous versus femoral cutdown access for endovascular ansurysm repair. J Vasc Surg. 2015;62:16–21. doi: 10.1016/j.jvs.2015.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haulon S, Hassen Khodja R, Proudfoot CW, Samuels E. A systematic literature review of the efficacy and safety of the Prostar XL device for the closure of large femoral arterial access sites in patients undergoing percutaneous endovascular aortic procedures. Eur J Vasc Endovasc Surg. 2011;41:201–13. doi: 10.1016/j.ejvs.2010.10.015. [DOI] [PubMed] [Google Scholar]
- 14.Howell M, Villareal R, Krajcer Z. Percutaneous access and closure of femoral artery access sites associated with endoluminal repair of abdominal aortic aneurysms. J Endovasc Ther. 2001;8:68–74. doi: 10.1177/152660280100800112. [DOI] [PubMed] [Google Scholar]
- 15.Dosluoglu HH, Cherr GS, Harris LM, Dryjski ML. Total percutaneous endovascular repair of abdominal aortic aneurysms using Perclose ProGlide closure devices. J Endovasc Ther. 2007;14:184–88. doi: 10.1177/152660280701400210. [DOI] [PubMed] [Google Scholar]
- 16.Ichihashi T, Ito T, Kinoshita Y, et al. Safety and utility of total percutaneous endovascular aortic repair with a single Perclose ProGlide closure device. J Vasc Surg. 2016;63:585–88. doi: 10.1016/j.jvs.2015.08.111. [DOI] [PubMed] [Google Scholar]
- 17.Hwang JW, Yang JH, Sung K, et al. Percutaneous removal using Perclose ProGlide closure devices versus surgical removal for weaning after percutaneous cannulation for venoarterial extracorporeal membrane oxygenation. J Vasc Surg. 2016;63:998–1003. doi: 10.1016/j.jvs.2015.10.067. [DOI] [PubMed] [Google Scholar]
- 18.Lederle FA, Freischlag JA, Kyriakides TC, et al. Long-term comparison of endovascular and open repair of abdominal aortic aneurysm. N Engl J Med. 2012;367:1988–97. doi: 10.1056/NEJMoa1207481. [DOI] [PubMed] [Google Scholar]
- 19.Thrumurthy SG, Karthikesalingam A, Patterson BO, et al. A systematic review of mid-term outcomes of thoracic endovascular repair (TEVAR) of chronic type B aortic dissection. Eur J Vasc Endovasc Surg. 2011;42:632–47. doi: 10.1016/j.ejvs.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 20.van Beek SC, Vahl A, Wisselink W, et al. Midterm re-interventions and survial after endovascular versus open repair for ruptured abdominal aortic aneurysm. Eur J Vasc Endovasc Surg. 2015;49:661–68. doi: 10.1016/j.ejvs.2015.02.015. [DOI] [PubMed] [Google Scholar]
- 21.Aljabri B, Obrand DI, Montreuil B, et al. Early vascular complications after endovascular repair of aortoiliac aneurysms. Ann Vasc Surg. 2001;15:608–14. doi: 10.1007/s10016-001-0092-x. [DOI] [PubMed] [Google Scholar]
- 22.Jean-Baptiste E, Hassen-Khodja R, Haudebourg P, et al. Percutaneous closure devices for endovascular repair of infrarenal abdominal aortic aneurysms: A prospective, non-randomized comparative study. Eur J Vasc Endovasc Surg. 2008;35:422–28. doi: 10.1016/j.ejvs.2007.10.021. [DOI] [PubMed] [Google Scholar]
- 23.Eisenack M, Umscheid T, Tessarek J, et al. Percutaneous endovascular aortic aneurysm repair: A prospective evaluation of safety, efficiency, and risk factors. J Endovasc Ther. 2009;16:708–13. doi: 10.1583/08-2622.1. [DOI] [PubMed] [Google Scholar]
- 24.Howell M, Villareal R, Krajcer Z. Percutaneous access and closure of femoral artery access sites associated with endoluminal repair of abdominal aortic aneurysms. J Endovasc Ther. 2001;8:68–74. doi: 10.1177/152660280100800112. [DOI] [PubMed] [Google Scholar]
- 25.Sprouse LR, 2, Botta DM, Jr, Hamilton IN., Jr The management of peripheral vascular complications associated with the use of percutaneous suture-mediated closure devices. J Vasc Surg. 2001;33:688–93. doi: 10.1067/mva.2001.112324. [DOI] [PubMed] [Google Scholar]
- 26.Mousa AY, Campbell JE, Broce M, et al. Predictors of percutaneous access failure requiring open femoral surgical conversion during endovascularaortic aneurysm repair. J Vasc Surg. 2013;58:1213–19. doi: 10.1016/j.jvs.2013.04.065. [DOI] [PubMed] [Google Scholar]
- 27.Metcalfe MJ, Brownrigg JR, Black SA, et al. Unselected percutaneous access with large vessel closure for endovascular aortic surgery: Experience andpredictors of technical success. Eur J Vasc Endovasc Surg. 2012;43:378–81. doi: 10.1016/j.ejvs.2011.12.025. [DOI] [PubMed] [Google Scholar]
- 28.Lee CH, Huang JK, Yang TF. Use of a Perclose ProGlide device for percutaneous endovascular aortic aneurysm repair in a general hospital experience. Discov Med. 2016;22:173–79. [PubMed] [Google Scholar]
- 29.Bettex DA, Lachat M, Pfammatter T, et al. To compare general, epidural and local anaesthesia for endovascular aneurysm repair (EVAR) Eur J Vasc Endovasc Surg. 2001;21:179–84. doi: 10.1053/ejvs.2000.1295. [DOI] [PubMed] [Google Scholar]
- 30.Edwards MS, Andrews JS, Edwards AF, et al. Results of endovascular aorticaneurysm repair with general, regional,and local/monitored anesthesia care in the American College of Surgeons National quality improvent program database. J Vasc Surg. 2011;54:1273–82. doi: 10.1016/j.jvs.2011.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De Virgilio C, Romero L, Donayre C, et al. Endovascular abdominal aortic aneurysm repair with general versus local anesthesia: A comparison of cardiopulmonary morbidity and mortality rates. J Vasc Surg. 2002;36:988–91. doi: 10.1067/mva.2002.128314. [DOI] [PubMed] [Google Scholar]
- 32.Geisbüsch P, Katzen BT, Machado R, et al. Local anaesthesia for endovascular repair of infrarenal aortic aneurysms. Eur J Vasc Endovasc Surg. 2011;42:467–73. doi: 10.1016/j.ejvs.2011.05.018. [DOI] [PubMed] [Google Scholar]