Abstract
Backgrounds: As one of the major public health problems, the hepatitis B virus (HBV) infection would activate the immune system. The outcome of HBV infection was affect significantly by the interactions between HBV and host immune response. Interleukins play important role in anti-viral immunity. Here we investigated the role of interleukin-35 (IL-35) in chronic HBV infection patients.
Methods/Results: Serum IL-35 in 72 chronic hepatitis B virus infection patients and 41 healthy control subjects were analyzed by ELISA assay. The mRNA level of IL-35 in PBMCs was determined by RT-qPCR. In this study, we found that both protein and mRNA levels of IL-35 were significantly decreased in chronic HBV patients compared to the healthy controls. Furthermore, the statistical analysis found that serum IL-35 was significantly associated with HBV DNA (P =0.0158), ALT (P =0.0003), AST (P =0.0216), TB (P =0.0270) and AFP (P =0.0369). Importantly, correlation analysis also found that serum IL-35 level was negatively correlated with HBV DNA copies, ALT, AST, TB and AFP. Meanwhile, IL-35 treatment inhibited the level of HBV DNA, HBsAg and HBeAg in HepAD38 cells.
Conclusion: Our study identified that IL-35 may be a novel marker associated with HBV infection and hepatocytes injury. These data suggested the potential use of IL-35 in the HBV treatment.
Keywords: Interleukin-35, Hepatitis B virus
Introduction
Chronic hepatitis B virus infection is a major global health problem. Approximately 2 billion people have been infected with hepatitis B virus (HBV) and 350-400 million people have been identified as carriers of the chronic HBV 1. HBV consists of a 3.2kb partially double-stranded, relaxed-circular DNA (rcDNA) genome. The rcDNA could converted into covalently closed circular DNA (cccDNA) which serve as the template for the synthesis of all viral RNAs, including the 3.5kb, 2.4kb, 2.1kb and 0.7kb mRNAs. Although HBV is a DNA virus, there is reverse transcription process in HBV life cycle. Briefly, the pregenomic RNA (3.5kb mRNA) could reverse transcript to generate DNA. And the DNA is further encapsulated by viral proteins to form mature viral particles which would be the potential reason for the failure of viral clearance and relapse of viral activity 2. It is reported that HBV infection could activate the immune system and induce extensive inflammatory response. Growing evidence supported that cytokine-mediated immune response plays an important role in clinical outcomes of HBV infection 3 4. Many immune factors were involved in the HBV infection including classical immune factors, transforming growth factor-β (TGF-β) 5, tumor necrosis factor-α (TNF-α) 6, and members of the interleukin family such as interleukin-6 7 and interluekin-23 8.
Interleukin-35 (IL-35) has been recognized as a novel member of IL-12 family which composed of IL-12α and IL-27β chains and encoded by two separate genes called IL-12A and EBI3, respectively. IL-35 is a responsive anti-inflammatory cytokine 9 implicated in many pathology processes. It has been reported that serum IL-35 level was significantly decreased in patients with acute motor axonal neuropathy 10 and rheumatoid arthritis 11. Meanwhile, other studies showed that elevated serum levels of IL-35 associated with the immune tolerance in normal pregnancy 12 and autoimmune encephalomyelitis 13. As for virus, interleukin-35 is upregulated in response to influenza virus infection 14. However, the association between IL-35 and HBV infection has not been reported yet. In this study, we analyzed the relationship between IL-35 and HBV infection. Serum IL-35 and IL-35 mRNA were significantly downregulated in chronic HBV patients compared to the healthy controls. Serum IL-35 was significantly associated with HBV DNA, ALT, AST, TB and AFP. This research aims to understand the interactions between the IL-35 and the virus in vivo, providing a new aspect for the prevention and treatment of the virus.
Materials and methods
Patients selection
This study examined 72 cases chronic hepatitis B virus infection patients including 31 cases hepatitis B virus e antigen (HBeAg) positive and 41 cases HBeAg negative ones. All patients were hepatitis B virus s antigen (HBsAg) positive. The patients with other virus infection (hepatitis C virus, hepatitis D virus, human immunodeficiency virus type 1 [HIV-1], and HIV-2) were excluded. These patients were not suffering from any other chronic liver disease or autoimmune disease. Relevant pathological information and outcome data were collected retrospectively. 41 sex- and age-matched healthy subjects were recruited as normal control group. Informed consents were obtained from each patient. This study was approved by the Clinical Research Ethics Committee of the Chongqing Medical University.
Enzyme linked immunosorbent assay (ELISA)
The chronic HBV infection patients' full blood were collected and centrifuged to obtain the serum. Serum levels of IL-35 in patients were determined by IL-35 ELISA kits (Catalog no. JYM2045Hu, ColorfulGene Biological Technology, Wuhan, China) according to the manufacturer's instructions.
The cell culture medium was collected at the indicated time point and the level of HBsAg and HBeAg were detected by ELISA kits (KHB).
Peripheral blood mononuclear cells (PBMCs) preparation
The PBMCs were isolated from blood of chronic HBV patients by using Ficoll kit (Catalog no. LTS1077).
Real-time PCR
The total RNAs in PBMCs were extracted by TRNzol (Catalog no. P5019, TIANGEN, Beijing, China) methods. The expression values of target genes were determined according to the 2-△△Ct method 15. The following primers were used for RT-qPCR: IL-35 specific primers: forward primer, 5'-TGTTCTCCATGGCTCCCTA-3', reverse primer, 5'-TTATGAAAGGCACGAAGCTG-3'. β-actin specific primers: forward primer, 5'-CTCTTCCAGCCTTCCTTCCT-3', reverse primer, 5'-AGCACTGTGTTGGCGTACAG-3'.
The absolute quantification of the HBV DNA was detected using Fast Start Universal SYBR Green Master (Catalog no. 06924204001, Roche, Mannheim, Germany). The sequences of the primers are as follows: forward, 5'-CCTAGTAGTCAGTTATGTCAAC-3', reverse, 5'-TCTATAAGCTGGAGGAGTGCGA-3'.
Cell culture
HepAD38 cells were purchased from the Shanghai Second Military Medical University and cultured in Dulbecco's modified Eagle medium (DMEM) (Catalog no. 10-010-CVR, Corning, New York, USA) with 10% fetal bovine serum (FBS) and 400 ug of G418 (Catalog no. 345810, Merck, Germany) per ml.
Statistical analysis
Results of patients are expressed as medium (interquartile range). Manne-Whitney rank test was used to assess the difference between two groups. Correlations between IL-35 and clinicopathologic parameters were analyzed by Spearman's rank test and nonparametric χ2 test. Results of cell models are expressed as mean±SD and compared by the Student's t-test. P<0.05 was considered statistically different. The SPSS 19.0 software was used to analyze the data.
Results
Characteristics of the subjects enrolled in the study
This study consisted of 72 chronic HBV patients and 41 healthy control subjects. The clinical background of the patients and healthy controls were described in Table 1. As shown in Table 1, all the patients in HBV group were HBsAg and HBV DNA positive. Comparison with control group, the serum level of total protein and albumin in HBV group were obviously decreased. As the total protein and albumin is mainly synthesized in liver, the decreased level of those two proteins suggesting the impaired physiological function of the liver. Furthermore, liver injury index was detected. The serum concentrations of alkanine aminotransferase (ALT), aspartate aminotransferase (AST), total bilirubin (TB), direct bilirubin (DB) and alpha-fetoprotein (AFP) were significantly increased in chronic HBV patient group compared to the healthy control group, indicating the hepatocytes injury caused by HBV infection.
Table 1.
Clinical and virological characteristics of the subjects enrolled in the study.
| Baseline characteristics | Healthy controls (n=41) |
Chronic hepatitis B (n=72) |
P value |
|---|---|---|---|
| Age, years | 39 (24-61) | 35 (18-69) | ns |
| Male/female | 18/23 | 38/34 | ns |
| HBsAg positive | 0/41 | 72/72 | |
| HBeAg positive | 0/41 | 31/72 | |
| HBcAb positive | 0/41 | 18/72 | |
| Total protein g/L [median (IQ range)] | 75.3 (67.3-82.6) | 69.9 (49.2-91.5) | 0.0004 |
| Albumin g/L [median (IQ range)] | 50.5 (45.7-56.4) | 38.2 (25.4-48.5) | <0.0001 |
| ALT, U/L [median (IQ range)] | 17 (4-54) | 389 (25-1792) | <0.0001 |
| AST, U/L [median (IQ range)] | 22 (11-36) | 195 (19-1539) | <0.0001 |
| TB, μmol/L [median (IQ range)] | 11.30 (4.80-39.29) | 56.00 (5.6.00-613.00) | <0.0001 |
| DB, μmol/L [median (IQ range)] | 2.76 (1.20-10.37) | 35.00 (1.50-437.00) | <0.0001 |
| AFP, ug/L | 0.24 (0.01-7.85) | 61.80 (2.78-377.80) | <0.0001 |
| HBV DNA log10 (IU/ml) | 0 | 5.68 (3.00-9.24) | |
| Autoimmune diseases | None | None |
IQ: interquartile, ALT: alkanine aminotransferase, AST: aspartate aminotransferase, TB: total bilirubin, DB: direct bilirubin, AFP: Alpha-fetoprotein. n: number of individuals. P when compared with healthy controls.
IL-35 expression in HBV patients
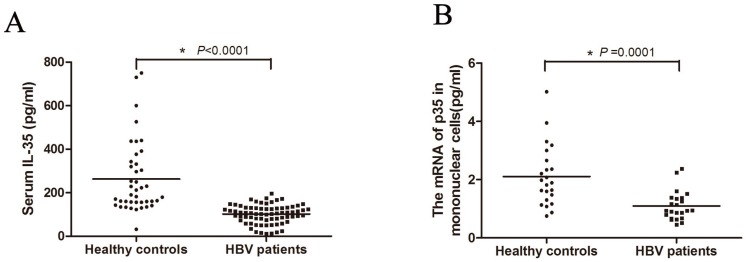
To investigate whether IL-35 plays a role in the process of HBV infection, we first determined the serum concentrations of IL-35 in HBV patients (Figure 1A). The serum IL-35 in HBV patient group (median: 107.01 pg/ml) was significantly decreased compared to control group (median: 190.41 pg/ml), which suggesting the potential relationship between IL-35 and HBV infection. For further study, we isolated the PBMCs from patients. Total RNAs were extracted and the mRNA level of IL-35 was determined by RT-qPCR (Figure 1B). Consistently, the mRNA levels of IL-35 in PBMCs from HBV patients were markedly decreased compared to healthy subjects. These data suggested that IL-35 play an important role in the process of HBV infection.
Figure 1.
IL-35 expression in HBV patients. (A) The serum IL-35 were determined by ELISA assay both in HBV patients and healthy controls. (B) The mRNA level of IL-35 in PBMCs were detected by RT-qPCR. The β-actin was used as the internal control.
Correlation between IL-35 expression and HBV DNA copies
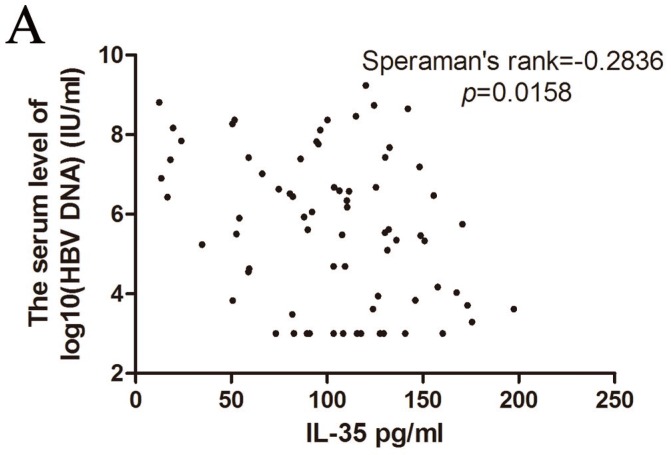
The HBV genome is a relaxed circular partially double-stranded DNA (rcDNA) of approximately 3200bp. Determination of the serum HBV DNA level could predict the efficacy of antiviral therapy 16 17. Therefore, we analyzed the correlation of IL-35 and HBV DNA copies (Figure 2A). The serum concentrations of IL-35 was negatively correlated with HBV DNA copies (Speraman's rank = -0.2836, p = 0.0158). These data indicated that determination of the levels of IL-35 could provide crucial information to point the HBV DNA level.
Figure 2.
The correlation of HBV DNA copies with IL-35 expression was analyzed by Speraman's rank test.
The correlation between IL-35 and liver injuries
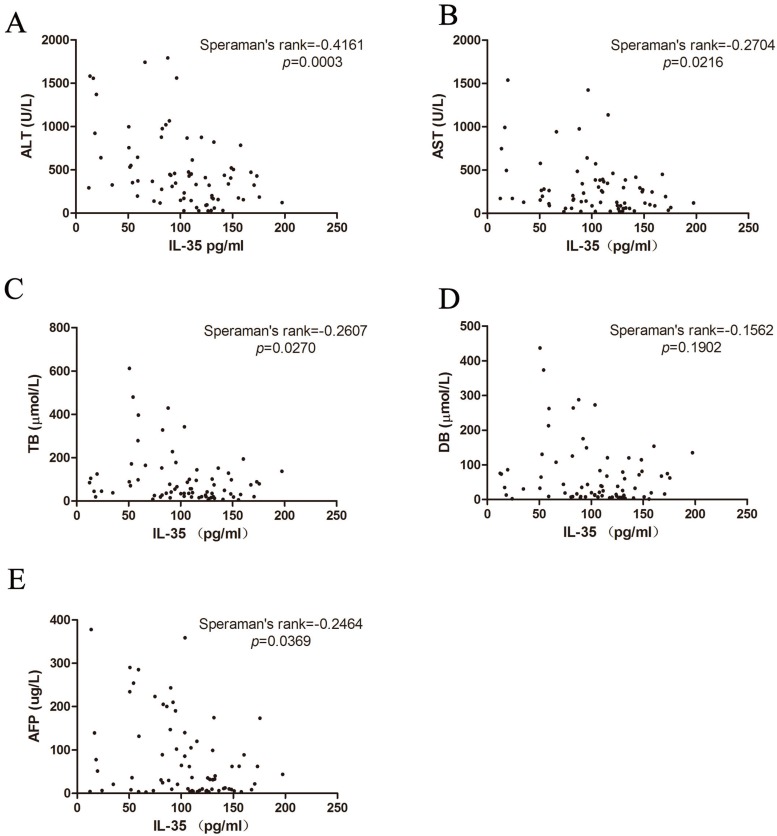
ALT, AST, TB and DB are the common index to reflect the liver damage in clinical practice. Therefore, we studied the association between IL-35 expression and liver injuries (Figure 3A-D). We found that there was a negative correlation between IL-35 and liver injury markers (ALT: Speraman's rank = -0.4161, p = 0.0003; AST: Speraman's rank = -0.2704, p = 0.0216; TB: Speraman's rank = -0.2607, p = 0.0270). All the results demonstrated that IL-35 might be involved in the pathological process of HBV infection and related liver injury.
Figure 3.
Correlation of IL-35 expression with ALT (A), AST (B), TB (C), DB (D) and AFP (E) were analyzed by Speraman's rank test.
Considering that HBV infection is one of the leading causes of hepatocellular carcinoma (HCC) 18, we also analyzed the HCC marker AFP. IL-35 was negatively related to AFP concentration (Figure 3E, Speraman's rank = -0.2464, p = 0.0369). These data indicated that IL-35 may be used for HBV prognosis prediction.
Correlation between IL-35 and clinical parameters in chronic HBV patients
To elucidate the potential correlation between IL-35 and clinical parameters, we divided the HBV patients into two groups (low expression: IL-35<100 pg/ml, high expression: IL-35>100 pg/ml) according to the serum concentrations of IL-35. Table 2 provided an overview of the included analyses. Among the 72 cases, 31 cases showed low expression of IL-35 and 41 cases showed high expression of IL-35. As shown in Table 2, ALT (p = 0.0082), AST (p = 0.0192), TB (p = 0.0445), DB (p = 0.0265), AFP (p = 0.0489) and HBV DNA (p = 0.0489) were significantly associated with serum IL-35. Taken together, those data suggested that IL-35 plays an important role in the interaction between HBV infection and host response.
Table 2.
Correlative analysis of serum IL-35 level with clincopathologic features in chronic HBV patients.
| IL-35 Expression | |||
|---|---|---|---|
| Clincopathologic features | Low (n=31) |
High (n=41) |
P value |
| HBeAg | |||
| Negative | 11 | 15 | 1.0000 |
| Positive | 20 | 26 | |
| ALT, U/L | |||
| < 100 | 0 | 9 | 0.0082 |
| > 100 | 31 | 32 | |
| AST, U/L | |||
| < 100 | 5 | 15 | 0.0192 |
| > 100 | 36 | 26 | |
| TB, μmol/L | |||
| < 100 | 17 | 32 | 0.0445 |
| > 100 | 14 | 9 | |
| DB, μmol/L | |||
| < 100 | 19 | 35 | 0.0265 |
| > 100 | 12 | 6 | |
| AFP, ug/L | |||
| < 20 | 7 | 19 | 0.0489 |
| > 20 | 24 | 22 | |
| HBV DNA log10 (IU/ml) | |||
| < 5 | 7 | 19 | 0.0489 |
| > 5 | 24 | 22 | |
IL-35 inhibited HBV replication in vitro
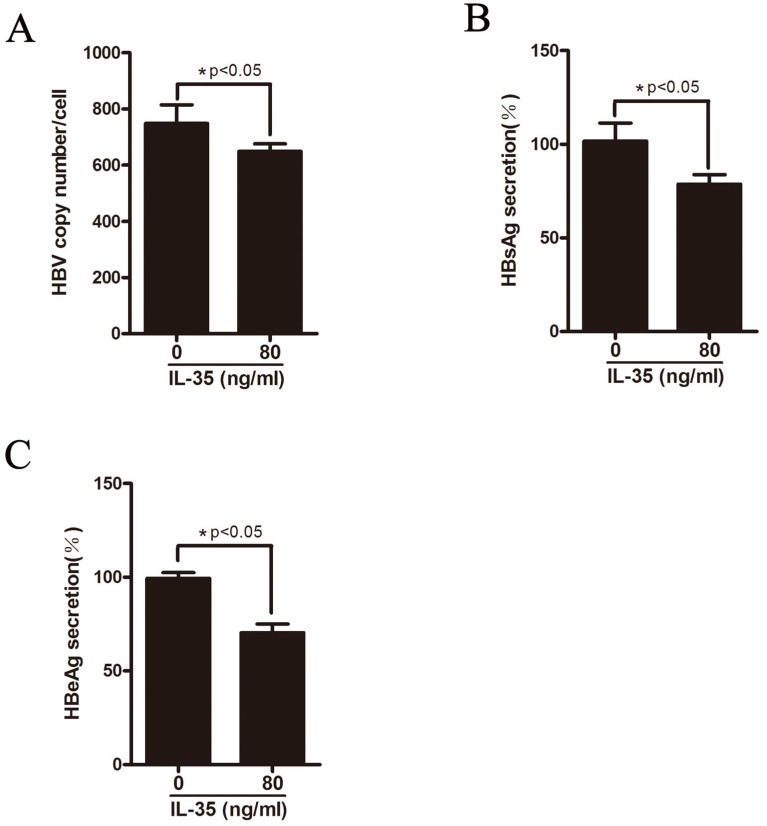
HepAD38 cells can stably expressing HBV where HBV genome integrates into host genome and the expression of HBV can be regulated by tetracycline. To further explore the functional role of IL-35 in HBV replication in vitro, HepAD38 cells were treated with different concentrations of rhIL-35 and the level of HBV DNA replicative intermediates were detected by real-time PCR (Figure 4A). As shown in Figure 4A, IL-35 treatment resulted in decreased of HBV DNA level. Furthermore, the secretion levels of HBsAg and HBeAg in supernatant were moderately decreased which detected by ELISA assay (Figure 4B-C).
Figure 4.
IL-35 inhibited HBV replication in vitro. HepAD38 cells were treated with IL-35. (A) The level of HBV DNA was detected by absolute quantification PCR. (B-C) The secretion level of HBsAg (B) and HBeAg (C) in supernatants were determined by ELISA assay.
Discussion
As HBV is a non-cytopathic virus, immune-mediated host reactions play a pivotal role in both HBV-related liver damage and viral control. IL-12 family includes interleukin-6 (IL-6), interleukin-12 (IL-12), interleukin-23 (IL-23), interleukin-27 (IL-27) and interleukin-35 (IL-35). Many members of this family are reported to be involved in the HBV infection. IL-6 expression is upregulated by hepatitis B virus core antigen and middle S antigen via p38 MAPK/NF-κB pathways 19 20. Meanwhile, serum IL-23 level in HBeAg-positive HBV infection patients before therapy could be used to predict the therapeutic response to interferon (IFN) 21. In addition, serum IL-27 is elevated in HBV patients 22 and IL-27 treatment could inhibit HBV replication 23. It is also reported that IL-12 expression in HBV patients is significantly different from the healthy subjects 24. However, the relationship between IL-35 and HBV replication has not been reported yet. In this study, we found that serum IL-35 and IL-35 mRNA were significantly decreased in chronic HBV infection patients, suggesting the possible role of IL-35 in HBV infection.
In the field of hepatitis virus, Langhans et al. 25 have reported that hepatitis C virus (HCV) core specific Treg cells could suppress the immune response through IL-35, indicating that IL-35 may serve as an immune suppressor in HCV infection. To further elucidate the function of abnormally expression of IL-35 in HBV infection patients, we analyzed the correlations between IL-35 concentration and liver injury markers. Due to the differences in patient screening criteria and the restrictions of patient number, Shi M et al. 26 found that serum IL-35 levels in patients with hepatitis B-related liver cirrhosis were negatively correlated with albumin, but not with ALT. However, our study found that serum IL-35 level negatively correlated with ALT and AST. The correlation between IL-35 and ALT and AST demonstrated the possibility that IL-35 may work as a new marker of liver damage. In addition, some studies report that IL-35 is associated with hepatocellular carcinoma (HCC) progression 27, aggressiveness and recurrence 28 which support our finding that IL-35 was negatively related to tumor marker AFP.
The recent studies demonstrate that IL-35 could function through multiple pathways. It is reported that increased interleukin-35 level in colorectal cancer could activate STAT1/STAT3 to suppress T cell proliferation 29. Meanwhile, Epstein-Barr virus-induced gene 3 (EBI3), the subunit of IL-35, may assist the tumor escape immune surveillance via STAT3 signaling pathway 30. Importantly, the STATx pathways is involved in the pathology process of HBV infection. Matrix metalloproteinase-9 (MMP-9) could repress the STAT signaling pathway to facilitates HBV replication 31. Additionally, IL-6 could reactivate HBV in liver through STAT3 signaling pathway 32. In vitro, ubiquitin-specific protease 18 33, transmembrane protein TMEM2 34 and the novel liver-targeting interferon (IFN-CSP) 35 could inhibit the HBV infection through the JAK-STAT signaling pathway. The above studies strongly suggest that IL-35 may regulate the HBV infection through STATx pathways.
PBMCs play an important role in anti-viral responses. In vivo, it has found that the dysfunction of PBMC may monitor HBV load and the immune states to regulate the HBV chronic infection and tolerance 36. In vitro, it showed that PBMC-derived dendritic cells from HBV patients would suppress HBV replication 37. Consistently, our data showed that the IL-35 mRNA in PBMCs of chronic HBV patients was obviously decreased compared to healthy subjects. This change would alter the immune response to HBV infection.
In summary, this study deeps our understanding of the role of IL-35 in HBV infection, especially on the outcomes and clinical significance of HBV infection. The relationship between IL-35 and clinical parameters may be a potential marker in HBV infection. Further studies are needed to investigate the underlying mechanism of IL-35 in HBV infection.
Acknowledgments
All the authors declare no potential conflicts of interest. This study was supported by the National Natural Science Foundation of China (81672012, JC) and Chongqing Natural Science Foundation (cstc2016jcyjA0183, JC).
Research and ethical clearance
The Clinical Research Ethics Committee of the Chongqing Medical University approved the clinical research.
References
- 1.European Association For The Study Of The L. EASL clinical practice guidelines: Management of chronic hepatitis B virus infection. Journal of hepatology. 2012;57:167–85. doi: 10.1016/j.jhep.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 2.Wang J, Shen T, Huang X, Kumar GR, Chen X, Zeng Z. et al. Serum hepatitis B virus RNA is encapsidated pregenome RNA that may be associated with persistence of viral infection and rebound. Journal of hepatology. 2016;65:700–10. doi: 10.1016/j.jhep.2016.05.029. [DOI] [PubMed] [Google Scholar]
- 3.Bertoletti A, Ferrari C. Innate and adaptive immune responses in chronic hepatitis B virus infections: towards restoration of immune control of viral infection. Gut. 2012;61:1754–64. doi: 10.1136/gutjnl-2011-301073. [DOI] [PubMed] [Google Scholar]
- 4.Seetharam A, Perrillo R, Gish R. Immunosuppression in Patients with Chronic Hepatitis B. Current hepatology reports. 2014;13:235–44. doi: 10.1007/s11901-014-0238-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pang J, Zhang G, Lin Y, Xie Z, Liu H, Tang L. et al. Transforming growth factor beta-activated kinase 1 transcriptionally suppresses hepatitis B virus replication. Scientific reports. 2017;7:39901. doi: 10.1038/srep39901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matsumoto T, Takahashi K, Inuzuka T, Kim SK, Kurosaki T, Kawakami S. et al. Activation of TNF-alpha-AID axis and co-inhibitory signals in coordination with Th1-type immunity in a mouse model recapitulating hepatitis B. Antiviral research. 2017;139:138–45. doi: 10.1016/j.antiviral.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 7.Attar M, Azar SS, Shahbazi M. Interleukin-6-174 Promoter Polymorphism and Susceptibility to Hepatitis B Virus Infection as a Risk Factor for Hepatocellular Carcinoma in Iran. Asian Pacific journal of cancer prevention: APJCP. 2016;17:2395–9. [PubMed] [Google Scholar]
- 8.Bao S, Zheng J, Li N, Huang C, Chen M, Cheng Q, Role of interleukin-23 in monocyte-derived dendritic cells of HBV-related acute-on-chronic liver failure and its correlation with the severity of liver damage. Clinics and research in hepatology and gastroenterology; 2016. [DOI] [PubMed] [Google Scholar]
- 9.Li X, Mai J, Virtue A, Yin Y, Gong R, Sha X. et al. IL-35 is a novel responsive anti-inflammatory cytokine-a new system of categorizing anti-inflammatory cytokines. PloS one. 2012;7:e33628. doi: 10.1371/journal.pone.0033628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang LJ, Guo HY, Zhang DQ, Wang R, Li T, Li LM. et al. Analysis of serum interleukin-27 and interleukin-35 concentrations in patients with Guillain-Barre syndrome. Clinica chimica acta; international journal of clinical chemistry. 2017;468:5–9. doi: 10.1016/j.cca.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 11.Ning X, Jian Z, Wang W. Low Serum Levels of Interleukin 35 in Patients with Rheumatoid Arthritis. The Tohoku journal of experimental medicine. 2015;237:77–82. doi: 10.1620/tjem.237.77. [DOI] [PubMed] [Google Scholar]
- 12.Yue CY, Zhang B, Ying CM. Elevated Serum Level of IL-35 Associated with the Maintenance of Maternal-Fetal Immune Tolerance in Normal Pregnancy. PloS one. 2015;10:e0128219. doi: 10.1371/journal.pone.0128219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu JQ, Liu Z, Zhang X, Shi Y, Talebian F, Carl JW Jr. et al. Increased Th17 and regulatory T cell responses in EBV-induced gene 3-deficient mice lead to marginally enhanced development of autoimmune encephalomyelitis. Journal of immunology. 2012;188:3099–106. doi: 10.4049/jimmunol.1100106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen Y, Wang CJ, Lin SH, Zhang M, Li SY, Xu F. Interleukin-35 is upregulated in response to influenza virus infection and secondary bacterial pneumonia. Cytokine. 2016;81:23–7. doi: 10.1016/j.cyto.2016.01.016. [DOI] [PubMed] [Google Scholar]
- 15.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods (San Diego, Calif) 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 16.Degertekin B, Lok AS. Indications for therapy in hepatitis B. Hepatology. 2009;49:S129–37. doi: 10.1002/hep.22931. [DOI] [PubMed] [Google Scholar]
- 17.Lim SG, Mohammed R, Yuen MF, Kao JH. Prevention of hepatocellular carcinoma in hepatitis B virus infection. Journal of gastroenterology and hepatology. 2009;24:1352–7. doi: 10.1111/j.1440-1746.2009.05985.x. [DOI] [PubMed] [Google Scholar]
- 18.Ott JJ, Stevens GA, Groeger J, Wiersma ST. Global epidemiology of hepatitis B virus infection: new estimates of age-specific HBsAg seroprevalence and endemicity. Vaccine. 2012;30:2212–9. doi: 10.1016/j.vaccine.2011.12.116. [DOI] [PubMed] [Google Scholar]
- 19.Chen Z, Li YX, Fu HJ, Ren YL, Zou L, Shen SZ. et al. Hepatitis B Virus Core Antigen Stimulates IL-6 Expression via p38, ERK and NF-kappaB Pathways in Hepatocytes. Cellular physiology and biochemistry: international journal of experimental cellular physiology, biochemistry, and pharmacology. 2017;41:91–100. doi: 10.1159/000455954. [DOI] [PubMed] [Google Scholar]
- 20.Li YX, Ren YL, Fu HJ, Zou L, Yang Y, Chen Z. Hepatitis B Virus Middle Protein Enhances IL-6 Production via p38 MAPK/NF-kappaB Pathways in an ER Stress-Dependent Manner. PloS one. 2016;11:e0159089. doi: 10.1371/journal.pone.0159089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu C, Gong X, Yang Q, Lian J, Xu K, Ruan B. et al. The serum IL-23 level predicts the response to pegylated interferon therapy in patients with chronic hepatitis B. Liver international: official journal of the International Association for the Study of the Liver. 2015;35:1549–56. doi: 10.1111/liv.12701. [DOI] [PubMed] [Google Scholar]
- 22.Wang HL, Zhang HY, Zhai ZL, Zhou X. The correlation between hepatitis B virus infection and IL-27. Bio-medical materials and engineering. 2012;22:187–93. doi: 10.3233/BME-2012-0706. [DOI] [PubMed] [Google Scholar]
- 23.Tan G, Xiao Q, Song H, Ma F, Xu F, Peng D, Type I IFN augments IL-27-dependent TRIM25 expression to inhibit HBV replication. Cellular & molecular immunology; 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zare A, Rashki A, Ghahari S, Ghayoori B. The analysis of correlation between IL-12 gene expression and hepatitis B virus in the affected patients. Virusdisease. 2015;26:196–9. doi: 10.1007/s13337-015-0261-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Langhans B, Braunschweiger I, Arndt S, Schulte W, Satoguina J, Layland LE. et al. Core-specific adaptive regulatory T-cells in different outcomes of hepatitis C. Clinical science. 2010;119:97–109. doi: 10.1042/CS20090661. [DOI] [PubMed] [Google Scholar]
- 26.Shi M, Wei J, Dong J, Meng W, Ma J, Wang T. et al. Function of interleukin-17 and -35 in the blood of patients with hepatitis B-related liver cirrhosis. Molecular medicine reports. 2015;11:121–6. doi: 10.3892/mmr.2014.2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Long J, Guo H, Cui S, Zhang H, Liu X, Li D. et al. IL-35 expression in hepatocellular carcinoma cells is associated with tumor progression. Oncotarget. 2016;7:45678–86. doi: 10.18632/oncotarget.10141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fu YP, Yi Y, Cai XY, Sun J, Ni XC, He HW. et al. Overexpression of interleukin-35 associates with hepatocellular carcinoma aggressiveness and recurrence after curative resection. British journal of cancer. 2016;114:767–76. doi: 10.1038/bjc.2016.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ma Y, Chen L, Xie G, Zhou Y, Yue C, Yuan X. et al. Elevated level of interleukin-35 in colorectal cancer induces conversion of T cells into iTr35 by activating STAT1/STAT3. Oncotarget. 2016;7:73003–15. doi: 10.18632/oncotarget.12193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liang Y, Chen Q, Du W, Chen C, Li F, Yang J. et al. Epstein-Barr Virus-Induced Gene 3 (EBI3) Blocking Leads to Induce Antitumor Cytotoxic T Lymphocyte Response and Suppress Tumor Growth in Colorectal Cancer by Bidirectional Reciprocal-Regulation STAT3 Signaling Pathway. Mediators of inflammation. 2016;2016:3214105. doi: 10.1155/2016/3214105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen J, Xu W, Chen Y, Xie X, Zhang Y, Ma C, MMP-9 Facilitates Hepatitis B Virus Replication through Binding with IFNAR1 to Repress IFN/JAK/STAT Signaling. Journal of virology; 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chou CH, Chen PJ, Jeng YM, Cheng AL, Huang LR, Cheng JC. Synergistic effect of radiation and interleukin-6 on hepatitis B virus reactivation in liver through STAT3 signaling pathway. International journal of radiation oncology, biology, physics. 2009;75:1545–52. doi: 10.1016/j.ijrobp.2008.12.072. [DOI] [PubMed] [Google Scholar]
- 33.Li L, Lei QS, Zhang SJ, Kong LN, Qin B. Suppression of USP18 Potentiates the Anti-HBV Activity of Interferon Alpha in HepG2.2.15 Cells via JAK/STAT Signaling. PloS one. 2016;11:e0156496. doi: 10.1371/journal.pone.0156496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhu X, Xie C, Li YM, Huang ZL, Zhao QY, Hu ZX. et al. TMEM2 inhibits hepatitis B virus infection in HepG2 and HepG2.2.15 cells by activating the JAK-STAT signaling pathway. Cell death & disease. 2016;7:e2239. doi: 10.1038/cddis.2016.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lu X, Wang J, Jin X, Huang Y, Zeng W, Zhu J. IFN-CSP inhibiting hepatitis B virus in HepG2.2.15 cells involves JAK-STAT signal pathway. BioMed research international. 2015;2015:959684. doi: 10.1155/2015/959684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peng Y, Xiong X, Tong X, Li M, Yang X, Hu G. et al. Correlation of the content of hepatitis B core antigen in peripheral blood mononuclear cells with HBV virus load. Diagnostic microbiology and infectious disease. 2016;85:154–8. doi: 10.1016/j.diagmicrobio.2015.09.017. [DOI] [PubMed] [Google Scholar]
- 37.Liu T, Song HL, Zheng WP, Shen ZY. Inhibition of HBV Replication in HepG2.2.15 Cells by Human Peripheral Blood Mononuclear Cell-Derived Dendritic Cells. Annals of clinical and laboratory science. 2015;45:495–501. [PubMed] [Google Scholar]