Abstract
Mycobacteria and Candida species include significant human pathogens that can cause localized or disseminated infections. Although these organisms may appear to have little in common, several shared pathways of immune recognition and response are important for both control and infection-related pathology. In this article, we compare and contrast the innate and adaptive components of the immune system that pertain to these infections in humans and animal models. We also explore a relatively new concept in the mycobacterial field: biological commensalism. Similar to the well-established model of Candida infection, Mycobacterial species colonize their human hosts in equilibrium with the immune response. Perturbations in the immune response permit the progression to pathologic disease at the expense of the host. Understanding the immune factors required to maintain commensalism may aid with the development of diagnostic and treatment strategies for both categories of pathogens.
Graphical abstract

1. INTRODUCTION
Species in the genera Mycobacteria and Candida include etiological agents of globally significant diseases, as well as non-pathogens that live in either soil and aquatic environments (i.e. Mycobacteria) or on the surface of animals, plants and insects (i.e. Candida). Candida and Mycobacteria species (spp) have very little in common from a strictly biological perspective: Candida spp are eukaryotes with a diploid genome that is sensitive to external stress and extensively heterozygous1, replicate primarily via asexual cell division and hyphal extension, and have polysaccharide-rich cell wall. Mycobacteria are prokaryotes with a haploid genome that is relatively stable, divide asymmetrically2, and have a multilayered hydrophobic cell wall. However, despite their biological differences Candida and Mycobacteria spp share something in common: both are chronic colonizers of large numbers of humans, but elicit disease in a relative minority of colonized humans. Specifically, an estimated >30% of the world population is colonized with Candida and/or Mycobacteria spp, ~90% of whom show no clinical signs of disease.
Several flavors of disease can occur following Candida or Mycobacteria infection. Candida infections are categorized as mucocutaneous or disseminated candidiasis. Mucocutaneous candidiasis is typified by the hallmark infection of oropharyngeal candidiasis, also known as thrush. This disease form can also present as an invasive infection on barrier surfaces of the skin, nails, esophagus, or vulvovaginal mucosa. Disseminated candidiasis includes bloodstream infections (candidemia) and infection of normally sterile organs including liver, spleen, kidney, heart, and brain. The case-fatality rates for disseminated candidiasis are high, with reports of 30–50%, while mucocutaneous candidiasis carries high morbidity for patients3,4. Globally, there are an estimated 400,000 cases of candidemia, 10 million cases of thrush, and 2 million cases of esophageal candidiasis annually3. Mycobacterial infections similarly impact large portions of the globe, and include nontuberculous mycobacterial infection (NTMI), leprosy, swimming pool granuloma, buruli ulcer, and tuberculosis (TB). TB is particularly significant at the global level, and is caused by aerogenic transmission of Mycobacterium tuberculosis, which primarily infects macrophages in the lung alveoli5. In its active form, TB is associated with “consumption” of the lung tissue and dissemination of M. tuberculosis to other organs; in its latent form, TB is asymptomatic and not infectious. Improved public health practices and the use of effective drug treatment have reduced exposure and disease rates in many countries. However, the efforts to control TB in many other countries are not optimal, casting doubt on the World Health Organization’s goal of halving TB incidence by 20506. For these reasons, it is important to have a data-informed framework for understanding the relationship between humans and Candida and Mycobacteria pathogens.
Here we will introduce a novel concept regarding the biological relationship between immune cells and Mycobacterial pathogens that is modeled on established concepts regarding the host relationship with Candida spp. Namely, we advocate that humans’ relationship with Candida and Mycobacteria spp is best described in terms of biological commensalism, and that most individuals maintain the human:commensal equilibrium via innate and T cell-associated cytokines. In a relative minority of individuals, too little or too much of select cytokines offsets this equilibrium and leads to a diseased state. We term this model the “Goldilocks Model.” To support this model, we will review data demonstrating that recognition of Candida and Mycobacteria spp by overlapping pattern recognition receptors (PRRs) leads to similar innate cytokine profiles, which consequently direct T cell differentiation. We also review data concerning how the T cells govern Candida and Mycobacterial disease outcome, as well as the polymorphisms in PRR and cytokine response genes that associate with disease susceptibility.
2. HUMAN COLONIZATION AS A SURVIVAL STRATEGY FOR CANDIDA AND MYCOBACTERIA
Natural selection is the force behind both prokaryotic and eukaryotic evolution, and is the process whereby heritable traits that increase a species likelihood of survival become more common over successive generations. We can therefore assume that during human history the ancestors of what are now Candida and Mycobacteria pathogens found the human niche to give them a selective advantage. Their adaptation to humans is understandable, as the human niche is stable relative to many other environments, with a regulated temperature and wealth of nutrients from the food we ingest. Competition with other bacteria is also limited in the human niche: while a common microbial environment such as soil may contain up to ~3 ×108 CFU per gram of soil7, the human niche is relatively sterile (the gut being an exception, with ~3 × 1011 CFU per gram8). For several Mycobacterial and Candida spp, co-evolution has led to humans now being their only niche. Since most individuals fail to develop disease following initial Mycobacteria or Candida colonization, Mycobacteria and Candida spp are best described in ecological terms as commensals in the majority of chronically colonized individuals, and exploiters in the minority of chronically colonized individuals (FIG 1A).
FIGURE 1.
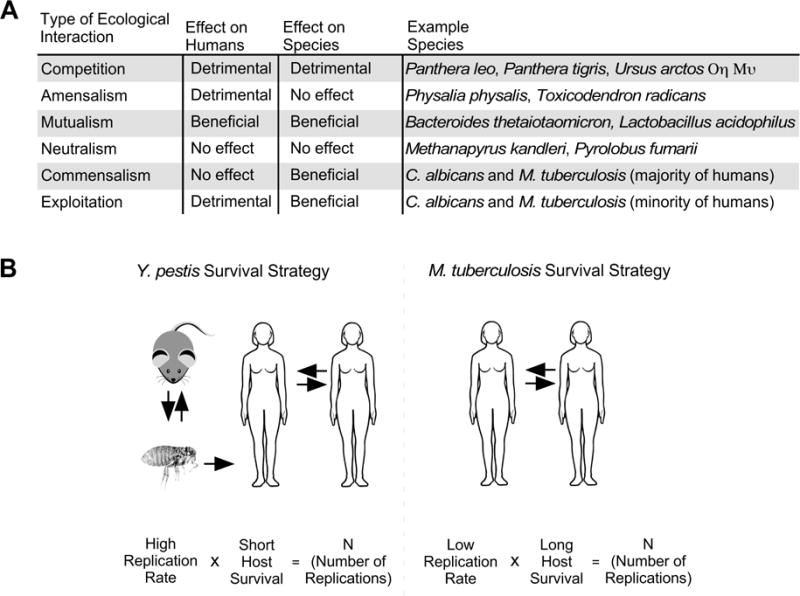
Candida and Mycobacteria species exist as commensals in the majority of infected individuals. (A) A table listing the six types of ecological relationships that can exist between two biological species, as well as examples of such species in relation to human. (B) A comparison of the survival strategies of two human pathogens: Yersinia pestis and Mycobacterium tuberculosis. Y. pestis can transmit via flea bite to rodents or a human, in whom disease disseminates and can then spread to other humans via cough. Y. pestis has a high replication rate at the expense of the human host, whose post-infection survival is short; in this manner, Y. pestis reaches an arbitrary number (N) of replications. In most individuals, M. tuberculosis survive via a different approach. Namely, M. tuberculosis combines a low replication rate with long prolonged host survival. In this manner, M. tuberculosis can reach the same N replications without causing disease in most individuals.
Candida and Mycobacteria spp use of chronic colonization as a survival strategy contrasts with pathogens that solely cause acute disease. FIGURE 1B depicts the life cycle of two human pathogens that employ distinct survival mechanisms: Yersinia pestis and Mycobacterium tuberculosis. Y. pestis caused the Black Death of the 14th century, and passages between rodents and fleas until infecting a human via a flea bite. Human to human transmission of pneumonic plague can also occur. Y. pestis employs a rapid bacterial replication rate at the expense of host survival, and is best described in ecological terms as an exploiter of humans (FIG 1A). By the time its human host dies (a matter of days), Y. pestis will have replicated N times (N being an arbitrary number). Contrasting with the life cycle of Y. pestis is that of M. tuberculosis, which does not have a non-human host and passes between humans via aerosolization of infected sputum. In most infected individuals, M. tuberculosis enters a slowly replicating, dormant state that does not cause clinical disease. By the time it’s latently infected host dies (a matter of years and/or decades), M. tuberculosis will have also replicated N times. A minority of infected individuals (~10%) develop active disease and produce the infected sputum that allows for M. tuberculosis transmission to a new generation of hosts. For these reasons, we propose that M. tuberculosis is best described in ecological terms as a commensal of most infected individuals, and an exploiter in the minority of infected individuals (FIG 1A). In contrast to the relative novelty of the commensal status of Mycobacteria spp, it is well-established that Candida spp live primarily as commensals. The ecological niche for Candida spp is primarily on animal or plant hosts, and the relationship is generally benign to the host. Certain species are commensals in humans that transmit via physical contact (e.g. the touch of caregivers), which correlates with the species that can act as the most common opportunistic pathogens in humans (including C. albicans, C. glabrata, C. parapsilosis, C. tropicalis, C. lusitaniae and C. krusei). Candida spp colonize the majority of humans in the oral cavity, gastrointestinal tract or genital tract. Colonization usually precedes invasive infection and is a key predictor of subsequent disease9. In spite of this and the moderate replication rate of Candida spp, the majority of colonized humans never develop invasive infection and do not require anti-fungal treatment to maintain normal health. Invasive infection occurs with breaches in normal host immune or barrier function. Thus, through three distinct survival strategies Y. pestis, M. tuberculosis and C. albicans will have both replicated the same N times. However, we consider the strategy of M. tuberculosis and C. albicans to be more successful in the long term (a matter of millennia) given that neither species cause the rapid, deleterious effects on their host population that were characteristic of the Black Death.
The concept of Mycobacteria spp being human commensals may initially seem to conflict with the fact that Mycobacteria spp also cause significant global mortality. Historical perspective is needed to reconcile this dissonance. Using M. tuberculosis as an example: the relationship between M. tuberculosis and humans is ancient, having evolved alongside humans for the past 10K–70K years10. The organism’s ability to enter latency is important for this long relationship, allowing it to infect an individual without impacting her/his evolutionary fitness until she/he becomes immunocompromised with age11. While latent TB is asymptomatic and not infectious, active TB is associated with “consumption” of the lung tissue and dissemination of M. tuberculosis into the airways; the extensive coughing that ensues can then spread M. tuberculosis into the air and enable its infection of a new generation of hosts12. The organism’s need to balance between activity versus latency not only ensures the survival of M. tuberculosis: it has also selected against M. tuberculosis variants that are more pathogenic and, thus, more capable of killing its only natural reservoir10. Whatever equilibrium may have existed between M. tuberculosis and its human hosts was altered in the 1970s, the beginning of the HIV/AIDS pandemic13. HIV is a retrovirus that is transmitted through sexual intercourse (via semen or vaginal fluid), blood contamination (via shared needles), or from mother to child perinatally (intrauterine, intrapartum or via breastmilk). The ability of HIV to infect and affect immune lineages that keep M. tuberculosis in latency, including T cells and macrophages14, results in HIV-positive (HIV+) individuals being more likely to develop active TB than HIV-negative (HIV−) individuals15–18. The HIV/AIDS pandemic has also allowed for the outgrowth of more pathogenic clinical isolates since selective barriers are diminished10,19. Thus, whereas the organism’s transition from latency to active disease may take decades in HIV− individuals (a phenotype consistent with the ecological commensals), the recent evolution of HIV and its ability to accelerate the loss of T cells and macrophage lineages results in higher rates of active disease (a phenotype consistent with ecological exploiters).
In many respects, our immune symbiosis with M. tuberculosis mirrors that we have with non-tuberculous mycobacteria (NTM). NTM include species that colonize human epithelia, as well as species that are ubiquitous in soil and aquatic environments20,21. NTM spp that colonize human epithelia are rarely pathogenic, include constituents of the healthy microbiota, and can be found along the urogenital tract (M. smegmatis, M. lentiflavum)22–24, gastrointestinal tract (M. lentiflavum)24, mouth or respiratory tract (M. confluentis, M. branderi, M. bohemicum, M. interjectum, M. intermedium, M. conspicuum)25–31, and skin (M. smegmatis, M. bohemicum, M. intermedium)32–34. NTM spp that are found primarily in soil and aquatic environments include M. vaccae, the M. avium complex (MAC, M. avium and M. intracellulare), and M. abscessus complex (MABSC, M. abscessus subspecies abscessus, massiliense, and bolletii). M. vaccae is unique among soil NTM insomuch that it is also a transient human colonizer35, and benefits the host in a manner that resembles ecological mutualism (FIG 1). Specifically, ingested M. vaccae inhibits pulmonary allergic inflammation in mice36, and decreases anxiety in both mice37 and humans38 via an as-yet-undefined gut-brain-microbiota axis39. MAC was first isolated from wood pigeons40, but is now known to be ubiquitous in the environment and found in freshwater and salt-water, soil, food, dust, domestic and wild animals41–44. In both water and soil, MAC and MABSC species (MAC/MAB) can be free-living, biofilm-associated, or amoeba-associated45,46. Infection with MAC/MAB can follow exposure to aerosols of MAC/MAB-containing water while bathing47,48, or to aerosols of MAC/MAB-containing soil while gardening49 or during a natural disaster50. The first case of human disease due to MAC/MAB was reported in 1943, in a patient with underlying lung disease51. After an incubation period suspected to be weeks to months, infection of humans with MAC/MAB can lead to three basic forms of disease: pulmonary disease, lymphadenitis and disseminated disease. However, as with M. tuberculosis, most individuals infected with MAC/MAB do not develop disease, but instead contain or resolve infection within histiocytic infiltrates or granulomas52–56. Whether MAC/MAB colonizes healthy individuals is debatable, as there is little primary science literature testing for their presence on healthy individuals. Winthrop et al57 demonstrated that among 283 Oregon residents with respiratory NTM isolates, 53% of participants did not have NTM lung disease and could be considered healthy, colonized individuals. A recent survey of the healthy human microbiome at 18 body habitats failed to detect MAC via 16S rRNA sequencing, but did detect MABSC in the vaginal posterior fornix58. Regardless of whether MAC/MAB colonizes healthy individuals, the frequency of individuals who have unknowingly been infected with MAC/MAB is likely high. This is best illustrated by the work of von Reyn et al59–62, who demonstrated that 40% of US-born health care workers have a positive delayed type hypersensitivity (DTH) response to M. avium Sensitin without previously experiencing mycobacterial disease, and that NTM are responsible for most positive Mantoux reactions in the same study population61. Rather than affecting the immunocompetent, individuals who are immunocompromised due to either an inherited or acquired immunodeficiency are most likely to develop MAC disease. Prior to antiretroviral therapy, disseminated MAC infection was the most frequent bacterial complication of AIDS, constituting 45% or more of AIDS-defining infection in the USA, Japan and Europe63. When these MAC/MAB infection data are considered along with TB incidence data in HIV/AIDS communities, they indicate that intact innate and T cell responses are necessary to maintain Mycobacteria spp in a commensal state and limit their transition to becoming ecological exploiters.
In summary, clinical and microbiological data support a model wherein most individuals infected with Candida and Mycobacteria spp do not develop disease, and that instead these species function as commensals. Their commensal status is kept in check by innate and adaptive immune lineages, which when absent (due to either an acquired or inherited immunodeficiency) predispose humans to Candida and Mycobacteria disease. Given their biological divergence, it is remarkable that Candida and Mycobacteria spp both elicit similar innate and adaptive immune responses. In the following sections, we will review the bases of these overlapping innate and adaptive responses, as well as literature demonstrating the deleterious impact of hyporesponsive innate and adaptive cells. Importantly, we will also review literature demonstrating that hyperresponsive innate and adaptive cells are also deleterious to the host. Collectively, these literature support a model wherein Mycobacteria and Candida spp are kept in a commensal state when there is a balance of two opposing forces: immune hyporesponsiveness and immune hyperresponsiveness.
3. INNATE RESPONSES TO CANDIDA AND MYCOBACTERIA SPECIES
Overview of innate responses to Candida and Mycobacteria spp
Cells of the innate immune response are at the interface of Candida and Mycobacterial colonizers and the human host. The best-characterized innate responses are those of phagocytic lineages: monocytes, macrophages/dendritic cells (DCs) and neutrophils, which each respond to prokaryotic and eukaryotic colonizers following the physical interaction of pattern recognition receptors (PRRs) with pathogen associated molecular patterns (PAMPs). Candida and Mycobacteria spp relationship to innate immune cells has been described as both “immune-evasive” and “immunostimulatory” 64, and the immunogenicity of Candida and Mycobacteria PAMPs varies depending on their environmental conditions65–70. C. albicans and most pathogenic Candida spp are pleomorphic fungi that grow as yeast, pseudohyphae or hyphae forms, and have an outer membrane comprising a lipid bilayer and polysaccharide-rich cell wall that promotes adherence to epithelial cells71. The majority of Candida PAMPs reside within the cell wall of the yeast form, and include mannose-rich O- and N-linked glycoproteins, chitin and β-glucans72 (FIG 2A). The relative abundance of these PAMPs declines with Candida spp transition to hyphal form73,74; the greater abundance of PAMPs in Candida spp yeast form may influence the relative pro-inflammatory capacity compared to that of hyphae. However, the transition to the hyphal form has been shown to be required for virulence75. Recent evidence indicates that a Candida hyphal-derived cytolytic peptide damages epithelial membranes and initiates the epithelial immune response76. In addition to differences in PAMPs and virulence factors, the hyphal form of, C. albicans can physically escape phagocytosis by DCs70. Mycobacterial PAMPs are similarly enriched in the cell wall (FIG 2B), and their immunogenicity is also influenced by environmental conditions77–80. For example, when M. tuberculosis grows at an air-liquid interface (resembling the lung environment) it produces and accumulates high levels of trehalose-6,6′dimycolate (TDM), which is pro-inflammatory and a regulator of M. tuberculosis-elicited pathology in animals81.
FIGURE 2.
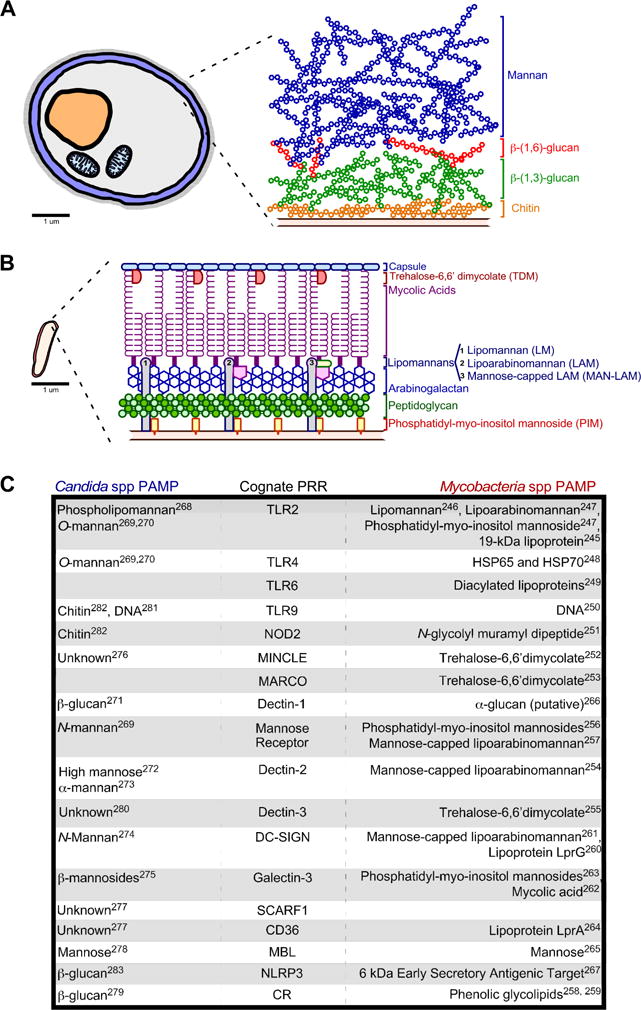
(A–B) Candida and Mycobacteria spp. differ substantially in morphology and size: Candida spp (A) are eukaryotes that have fimbriae, a cell wall, cell membrane, intracellular organelles (e.g. nucleus and mitrochondria), and a large size relative to Mycobacterial spp (B) that are prokaryotes with an extracellular hydrophobic cell wall, cell membrane and intracellular compartment. The depiction and relative sizes of Candida and Mycobacteria spp are based on the electron microscopy studies of Fekete et al241 and Shoonmaker et al242, respectively. (A) Depiction of Candida spp cell wall and the relative localization of common Candida PAMPs. (B) Depiction of Mycobacteria spp cell wall and the relative localization of common Mycobacteria PAMPs, based on recent reviews of the cell wall structure243,244. (C) Listed are the human PRRs activated by Candida and Mycobacteria spp, alongside their cognate PAMPs from each pathogen class. The superscript number(s) next to each mycobacterial PAMP indicates a corresponding reference that documents its association with or activation of TLR2245–247, TLR4248, TLR6249, TLR9250, NOD2251, MINCLE252, MARCO253, Dectin-2254, Dectin-3255, Mannose Receptor256,257, CR258,259, DC-SIGN260,261, Galectin-3262,263, CD36264, MBL265, Dectin-1266, NLRP3267. The superscript number(s) next to each Candida PAMP indicates a corresponding reference that documents its activation of either TLR2268–270, TLR4269,270, Dectin-1271, Mannose Receptor269, Dectin-2272,273, DC-SIGN274, Galectin-3275, MINCLE276, SCARF1277, CD36277, MBL278, CR3279, Dectin-3280, TLR9281,282, NLRP3283, NOD2282.
Remarkably, despite their biological differences and distinct membrane compositions, Candida and Mycobacteria PAMPs activate similar host PRRs (FIG 2C). The PRRs activated by Candida and Mycobacteria spp include membrane-associated Toll-like receptors (TLRs) and C-type lectin receptors (CLRs), as well as cytosolic NOD-like receptors (NLRs) and RIG-I-like receptors (RLRs). The PRRs that recognize Candida and Mycobacteria spp are listed in FIG 2C, alongside their cognate PAMPs. Because of their recognition by similar PRRs, Candida and Mycobacteria spp also trigger overlapping signal pathways in innate lineages. After Dectin-1 recognition of Candida spp, both spleen tyrosine kinase (SYK) and the serine/threonine kinase RAF1 are independently activated; downstream targets of SYK activity include PKCδ, which integrates with RAF1 to activate NFκB82–85. The activities of AKT, PI3K and MTOR are also triggered by β-glucan, albeit independent of Dectin-1 and Complement Receptor (CR)86,87. Candida PAMP stimulation of Dectin-1, NLRP3 and NLRC4 each cause activation of the canonical caspase-1 containing inflammasome67,88,89, whereas Dectin-1 can also activate a non-canonical caspase-8 containing inflammasome90. MAPK activity in macrophages varies depending on whether they have encountered Candida spp in either their yeast or hyphal form91,92. The extent to which neutrophil extracellular traps (NETs) are released by PMNs is also governed by Candida and Mycobacteria spp aggregation93.
Phagocytic innate cells’ secretion of cytokines is an important consequence of Candida and Mycobacteria spp recognition. As anticipated based on their activating similar PRRs, Candida and Mycobacteria spp stimulate secretion of similar cytokines at disease sites in humans94–101. Among the more genetically important are the IL12B-dependent cytokines IL12 and IL23 (IL12/23), which positively-regulate the differentiation of human T helper (TH) cells into TH1 and TH17 lineages102–104. Reflecting the multi-faceted influence of IL12/23, individuals who are insensitive to IL12/23 are susceptible to numerous intracellular pathogens105,106; paradoxically, IL12/23 also contributes to mycobacteria-driven pathology, as repeat BCG-immunization of tuberculous mice causes IL23-dependent lung damage107. IL23 is required to prevent mucosal Candida infection. Mice deficient in the p19 subunit of IL23 are susceptible to oropharyngeal candidiasis with a phenotype similar to humans with chronic mucocutaneous candidiasis (CMC)108. Not surprisingly, a subset of humans with CMC are deficient in IL23 or its receptor. When the deficiency is in the subunits shared with IL12 signaling (p40 subunit of IL23 or IL12Rβ1 receptor subunit), patients are susceptible to both Candida and mycobacterial diseases109,110. These inborn errors of IL12/IL23 comprise a portion of patients with Mendelian susceptibility to mycobacterial diseases (MSMD)111. The IL23 pathway is also involved in control of disseminated candidiasis. Mice deficient in IL17 signaling (downstream of IL23) have increased mortality, higher fungal kidney fungal loads and lower neutrophil recruitment in a model of disseminated candidiasis than wildtype mice112.
Non-phagocytic innate cells at barrier surfaces also respond to Candida and direct the phagocytic response. Specifically, γδ T cells and innate TCRαβ+ cells are essential to the initial control of Candida in the oral cavity and/or skin113,114. γδ-T cells are a minor subset (~1–5%) of T cells whose receptors are distinct from the αβ-TCRs found on the majority of human T cells. In mice γδ-T cells can be classified into two subsets based on their TCR diversity115. The first subset is found in lymphoid tissues and, like αβ-T cells, displays a highly diversified TCR repertoire116. The second γδ-T cell subset is intraepithelial and expresses a more restricted TCR repertoire117,118. This is particularly true in the skin and the female reproductive tract of mice, where the γδ-T cells are essentially homogeneous115,119. The TCR diversity of human peripheral blood γδ-T cells is also limited, as the predominant γδ-T cell subset in the peripheral blood expresses Vγ9 Vδ2115. Human γδ-T cells are nevertheless activated by a diverse range of antigens, including non-peptide antigens120, isoprenoid pathway intermediates121, alkylamines derived from numerous bacteria and plants122, mycobacterial ligands123, heat shock proteins124, nucleotide conjugates125 and prenyl pyrophosphates126. However, unlike αβ-T cells which must be activated by antigen in the context of MHC, the mechanism by which γδ-T cells recognize antigen remains obscure127. The oral cavity also has a resident population of innate TCRαβ+ cells, also termed “natural TH17 cells” or nTH17, that expand in the face of oropharyngeal Candida infection113. Both γδ-T cells and innate TCRαβ+ cells secrete IL17 in response Candida challenge without requiring previous priming. Similar to the adaptive immune response, innate IL17-producing cells direct immune clearance of Candida through neutrophil recruitment and the promotion of antimicrobial peptide production by epithelial cells. In contrast to Candida infection, the precise role of γδ-T cells and their effector mechanisms during mycobacteria infection remains to be completely defined. Following intravenous administration of M. tuberculosis, γδ-T cell deficient mice are more susceptible to infection than wild type controls128; however after respiratory infection with the same organism the absence γδ-T cells had no effect on bacterial burden129. Nevertheless, increased numbers of neutrophils are observed in the granulomas of M. tuberculosis-infected γδ-T cell deficient mice130, suggesting that γδ-T cells may regulate immunopathology during chronic infection.
Innate hyporesponsiveness to Candida and Mycobacteria spp is detrimental to the host
Candida and Mycobacteria disease can occur in any tissue, and can be exacerbated by either ablation of innate responses in experimental disease models, or the inheritance of human alleles that confer innate hyporesponsiveness. Neutrophils are among the first recruits to an infected site131–135. Neutrophils, or polymorphonuclear cells (PMNs), are the most abundant leukocyte in the circulation; following bacterial infection of the lung, alveolar epithelial cells near the infected site express several PMN-attractants (e.g. IL8) and PMN-growth factors (e.g. GCSF) that promote the recruitment of PMNs from proximal capillaries into the infected space136. Once in the infected space, they are capable of killing pathogens via a variety of effector mechanisms137. These mechanisms include NADPH oxidase-dependent production of reactive oxygen species (ROS), myeloperoxidase (MPO)-dependent production of hypochlorus acid (HOCl), elastase (ELA2)-dependent proteolysis and gelatinase B (MMP9)-dependent proteolysis137. These and other effector enzymes are held within one of three PMN granule types (azurophilic, specific and gelatinase granules); upon recruitment and activation of PMNs to an infected site, degranulation results in their release to the extracellular space138. An additional mechanism by which PMNs kill bacteria is by attracting monocytes139, which release even greater amounts of ROS than PMNs when normalized for cellular content140. For both mucocutaneous and disseminated candidiasis, disease susceptibility is induced with neutrophil depletion in animal models135,141,142. Neutropenia is a well-described risk factor for disseminated candidiasis in humans143. Phagocytosis of Candida by macrophages is also key to control of disseminated candidiasis144. People with dysfunctional neutrophils, such as with chronic granulomatous disease, are susceptible to fungal infections including candidiasis145. High frequency of TH17 cells and associated cytokines does not compensate for the abnormal phagocytes146. In contrast to PMNs’ protective role in mucocutaneous and disseminated candidiasis, PMNs’ contribution to mycobacterial control in immunocompetent animals is nil to modest depending on the model system used147. Specifically, while neutrophils can kill M. tuberculosis in vitro147, and are capable of promoting DC presentation of M. tuberculosis antigens148, their depletion from immunocompetent mice results in either no or modest changes to standard disease readouts147.
In humans, the inheritance of genomic polymorphisms that decrease innate responsiveness can increase Candida and/or Mycobacteria disease susceptibility, either by causing a change in the amino acid sequence of immune signals and their receptors, or altering the mRNA expression levels via cis- or trans-factors. A consequence of this sequence polymorphism is variation in disease susceptibility. Non-HLA genes with alleles that associate with Candida and/or Mycobacteria disease susceptibility or severity are listed in FIG 3, and can be broadly categorized as regulators of pattern recognition, cytokine responsiveness, and antimicrobial effectors. For most of the genes in FIG 3, the strength of an association between a given allele and disease susceptibility is strongly influenced by ethnicity and gender, as well as the presence or absence of a comorbidity149. Although the mechanism by which a polymorphism influences disease susceptibility is not always known, those for which the mechanism is known influence innate responsiveness do so by either introducing a nonsense mutation (Dectin-1150)a missense mutation (MDA5151), absent protein expression (CARD9152), altered PAMP affinity (TLR3153), impaired transcription factor activity via a disruption in DNA binding (IRF8154), decreased mRNA expression (DC-SIGN155), lowered protein expression (MBL2156; TLR2157; CD14158; SP-A and SP-D159), disrupted association with accessory host molecules (MRC1160), attenuated TLR2 signal transduction (TIRAP161), and altered signal peptide sequence (TLR8162). Importantly, animal models of Candida and Mycobacteria infection demonstrate that the PRRs that respond to Candida and Mycobacteria PAMPs are genetically redundant, and a deficiency in one PRR allele is often insufficient to confer disease susceptibility. Rather, disease susceptibility is conferred by the combinatorial impact of multiple hyporesponsive PRR alleles.
FIGURE 3.
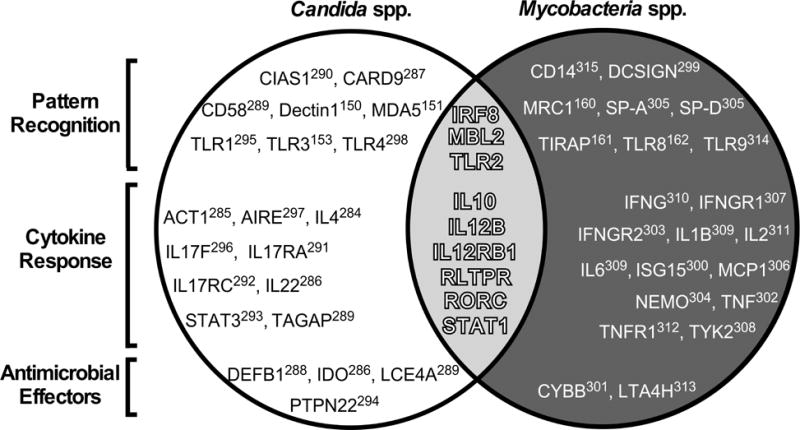
Human susceptibility to Candida and Mycobacteria disease associates with polymorphic alleles of genes involved in pathogen recognition, cytokine responsiveness, and antimicrobial effector mechanisms. Listed in the white portion of the Venn diagram are non-HLA genes with alleles that associate with Candida disease susceptibility150,151,153,284–298. Likewise, listed in the black portion are non-HLA genes with alleles that associate with Mycobacteria disease susceptibility160–162,299–315. Listed in the gray overlap are nine genes with alleles that associate with both Candida and Mycobacteria disease susceptibility: IRF8154, MBL2156,316,317, TLR2157,314,318, IL10319–321, IL12B110,320, IL12RB1322–325, RLTPR326, RORC327 and STAT1206,207,328–332. As indicated by labels on the left, each gene is further classified as being a regulator of either pathogen recognition (top row), cytokine responsiveness (middle row) or antimicrobial effector mechanisms (bottom row).
Innate hyperresponsiveness to Candida and Mycobacteria spp is detrimental to the host
Paradoxically, just as innate hyporesponsiveness promotes Candida and Mycobacterial disease susceptibility, so too can innate hyperresponsiveness. PMNs’ contribution to host defense from Candida spp is tissue-specific and varies with stage of infection. Although the PMN response is required for Candida clearance and control of infection in the oral cavity, PMNs appear to be responsible for the pathology and symptoms associated with vulvovaginal candidiasis163. The influx of PMNs also does not reduce fungal burden during vulvovaginal candidiasis. In the context of disseminated candidiasis, the protective effective of neutrophils early in infection is reversed later in infection164. Regarding Mycobacteria spp, clinical and experimental data demonstrate that PMNs are detrimental in the context of TB with comorbid immunodeficiency, and contribute to the latent-to-active TB transition. In individuals with active TB/AIDS, the number of BAL PMNs is elevated relative to HIV-seropositive TB patients without previous AIDS-defining illnesses165–167. PMNs are also present at high frequencies in the BAL of HIV− individuals with advanced TB168, as well as in the cerebrospinal fluid of individuals with disseminated, tuberculosis meningitis169–171. High frequencies of circulating PMNs at the time of TB diagnosis associates with a poor-prognosis172, and a PMN-driven, IFN-inducible transcript signature in adult whole blood correlates with clinical TB severity and distinguishes between those with active and latent TB173. Lung PMN infiltrations are prominent in genetically susceptible strains of mice infected with M. tuberculosis174–177. Importantly, the survival of M. tuberculosis-infected, genetically susceptible DBA/2 mice is extended following depletion of PMNs using anti-Ly6G, or neutralization of the PMN growth factor GCSF174. Similar to genetically susceptible strains, engineered TB-susceptible strains also have prominent PMN-associated pathology, and can be rescued by PMN-depletion. For example, in the absence of the IFNγR, ifngr−/− mouse neutrophils fail to apoptose and accumulate in large numbers in the lung178; PMN depletion extends the life of ifngr−/− mice, without affecting M. tuberculosis burden178. PMN-depletion also extends the life of M. tuberculosis -infected card9−/− mice 179. Collectively, these clinical and experimental data demonstrate that immunodeficiency, whether acquired or inherited, results in an environment where PMNs become a significant source of tissue pathology during active mycobacterial disease.
4. ACQUIRED IMMUNITY TO CANDIDA AND MYCOBACTERIAL SPECIES
Acquired immunity to Candida and Mycobacterial spp is that which develops following activation of Candida or Mycobacteria antigen-specific T cells and B cells. Having first arisen in jawed vertebrates, cells of the adaptive immune system are activated by the evolutionarily older innate immune system, and express surface receptors that are specific to non-self antigens. The enormous diversity of surface receptors is brought about through V(D)J recombination and somatic hypermutation. Adaptive immunity to Candida or Mycobacteria is an essential compliment to innate immunity, as deficiencies in various aspects of adaptive immunity confer fungal- and mycobacterial-disease susceptibility. The T cell subsets that respond to Candida and/or Mycobacteria spp include CD8+ αβ-T cells, CD4+ αβ-T cells, and CD1-restricted T cells. Below we will review those aspects of Candida and Mycobacteria-specific T cell activation and effector mechanisms that prevent disease. Although antibody responses to Candida and Mycobacteria are measurable in infected individuals, B cells’ influence on fungal and mycobacterial disease outcome is not reviewed here, but has been recently reviewed elsewhere180,181.
Overview of T cell responses to Candida and Mycobacteria spp
CD8+ αβ T-cells
Pathogen clearance by CD8+ αβ-T cells has historically associated with viral infections, since the MHC Class I pathway samples and presents proteins that are present in the cell cytosol (viral components typically localize to cytosol, whereas bacterial components typically localize to the phagolysosome and are thus accessible to the MHC Class II pathway). However, CD8+ T cells contribute the control of chronic M. tuberculosis infection129, and mycobacterial antigens can escape into the cytosol and access the MHC Class I pathway182. CD8+ T cells are protective in vivo, and direct cytolytic activity toward M. tuberculosis infected cells in a manner that is perforin-dependent and promoted by IL12183–185. In children, the frequency of circulating IFNγ-producing M. tuberculosis-specific CD8+ T cells is cellular biomarker of primary TB186. In adults, M. tuberculosis-specific CD8+ T cells are found in high frequency in infected individuals and recognize antigen both in the context of classical human leukocyte antigen (HLA) alleles187 and the major histocompatibility complex class I-related gene protein (MR1)188. MR1-restricted CD8+ cells are enriched in mucosal sites, have a limited TCR diversity, and are “innate-like” insomuch as they rapidly respond to pathogens soon after thymic egress189. MR1-restricted CD8 cells are also referred to as Mucosal Associated Invariant T (MAIT) cells, and are also negatively impacted by HIV infection190.
CD4+ αβ T-cells
CD4+ αβ-T cells, or TH cells, play an important role in maximizing the capabilities of the adaptive immune response. Unlike CD8+ αβ-T cells, CD4+ αβ-T cells have limited bactericidal, fungicidal or cytotoxic abilities; rather, they manage and direct other adaptive and innate cells to perform these tasks. CD4+ αβ-T cells express TCRs that recognize antigen bound to Class II MHC molecules. The activation of a naive CD4+ αβ-T cell causes it to release cytokines, which influences the activity of many cell types, including the APC that activated it. Several types of effector CD4+ αβ-T cell responses can be induced by APCs, with each influencing the host’s response to pathogens through secretion of cytokines; these types of effector CD4+ αβ-T cell responses are designated as TH1, TH2, TH17, TFH and TH9. TH1 cells secrete IFNγ and TNFα, both of which are critical for the eradication of chronic intracellular pathogens such as M. tuberculosis. TH2 cells produce the cytokines IL4, IL5, IL6, and IL13, which are essential for optimal antibody production and for the elimination of extracellular organisms including helminthes and nematodes. TH17 cells produce IL17 (also known as IL17A), IL17F and IL22 and, on initial characterization, were broadly implicated in autoimmune disease; a more natural role for TH17 cells is suggested by studies that have demonstrated preferential induction of IL17 in cases of host infection with various bacterial and fungal species including C. albicans108. TFH cells are a subset of CD4+ T cells that migrate to B cell follicles after activation and promote germinal center formation and B cell Ig isotype switching. Finally, TH9 cells secrete IL9 and IL10 and are a relatively recent addition to the list of known CD4+ αβ-T cell responses; while their transfer into a lymphopenic host results in autoimmune colitis, a role for TH9 cells during the course of a bacterial infection has yet to be demonstrated. Like CD8+ αβ-T cells, most of the CD4+ αβ-T cells apoptose upon resolution of infection, with a few remaining as CD4+ memory cells.
In oropharyngeal candidiasis, antigen-specific IL17 producing CD4+ cells (Th17 cells) are induced in response to repeated exposure to Candida191. Th17 cells confer protection to susceptible mice. In the absence of CD4+ cells, IL17 production is transferred to CD8+ cells or CD3+CD4−CD8− cells with similar protective function. As mentioned above, CD4+ T cells are critical for the control the chronic bacterial pathogen M. tuberculosis. In the absence of CD4-expressing cells at the initiation of M. tuberculosis infection typical mononuclear granulomatous lesions do not form; depletion of CD4+ T cells from mice that have controlled infection results in the recrudescence of M. tuberculosis growth, resulting in increased bacterial numbers in various organs, increased lung pathology and decreased survival. Reflecting the requirement for CD4+ T cells, the cessation of bacterial growth correlates with their arrival into the infected lung. Despite the abundant evidence that CD4 T cells play in protecting against bacterial growth, we’ve yet to fully define the mechanisms by which they mediate immunity. Of the various CD4 T cell subtypes mentioned above, TH1 cells are considered most often to be necessary for control of M. tuberculosis. The TH1 response results in the production of IFNγ, a cytokine that is critical for activation of bactericidal mechanisms in infected macrophages (i.e. production of NO and O− radicals).
NKT cells
CD1-restricted T-cells (referred to as NKT cells) are a heterogeneous group of T cells that recognize the non-polymorphic CD1 molecules, antigen-presenting complexes that binds self- and foreign lipids and glycolipids. They constitute a small fraction of all peripheral blood T cells, but are found in abundance in the liver. NKT cells express an αβ-TCR and sometimes co-express a variety of molecular markers typically associated with NK cells such as NK1.1. They differ from conventional αβ-T cells in that their TCR repertoire is far more restricted and that they recognize lipids and/or glycolipids instead of peptides. Upon activation, NKT cells rapidly down-regulate their TCR and produce large quantities of IFNγ, IL4, GMCSF and multiple other cytokines and chemokines. Although CD1 is dispensable for host defense from oropharyngeal candidiasis and experimental TB113,192, the activation of NKT cells via α-galactosylceramide affords some protection from subsequent M. tuberculosis infection143. Human CD1 can present mycobacterial mycolic acid to human NKT cells in vitro193, and human CD1 transgenic mice present mycobacterial lipids in vivo194. Antigen-specific human NKT cell lines can lyse M. tuberculosis-infected macrophages, as well as kill mycobacteria directly via release of the antimicrobial peptide granulysin195. Circulating NKT cells are dysfunctional in active TB patients relative to non-diseased controls196, further suggesting that generation of NKT cells is part of protective immune response that occurs in most infected individuals.
T cell hyporesponsiveness to Candida and Mycobacteria spp is detrimental to the host
When T cells are either absent or functionally-impaired, Candida and Mycobacteria infections are almost universally detrimental to the host, and best described in ecological terms as exploitive (FIG 1A). The importance of T cells to mycobacterial resistance was established in the 1980s at the Trudeau Institute (Saranac Lake, NY). During that period, Orme and Collins demonstrated that thymectomized mice were susceptible to infection with Mycobacteria spp, and that resistance could be restored by adoptive transfer of spleen T cells197,198. In the absence of thymus-derived cells, mycobacterial growth in multiple organs is unrestricted and mice quickly become moribund. The essential nature of T cells is also true in the guinea pig TB model199. Sadly, in humans the detrimental effect of T cell hyporesponsiveness is demonstrated by the morbidity and mortality associated with TB/HIV co-infection, as HIV depletes the T cells that are needed to limit M. tuberculosis growth and dissemination. Recent studies of HIV-infected humanized mice demonstrate that acquired T cell deficiency causes an increase in proinflammatory cytokine expression, which in turn attract PMNs that cause tissue destruction200. This immunological chain of events is supported by histological studies of granuloma composition in TB/HIV+ individuals, which are enriched in PMNs relative to those of TB/HIV- individuals201. Acquired and inherited forms of T cell hyporesponsiveness also result in susceptibility to CMC in humans and mice. Inherited defects range from autoimmune diseases with antibodies directed against the T-cell derived cytokines (Autoimmune polyendocrinopathy syndrome-I or APS-I) to deletions along the IL23/IL17 signaling pathway. CMC is nearly universal in APS-I patients, but they are not prone to other infections202,203. Defects in the signaling pathway that result in CMC susceptibility include mutations or deficiency in the IL17 receptor (IL17RA), IL17, IL17F, signal transducer and activator of transcription 3 (STAT3), dedicator of cytokinesis 8 (DOCK8), Tyrosine kinase 2 (TYK2), IL-12Rβ1, IL12p40, Caspase recruitment domain-containing protein 9 (CARD9), and IκBα (FIG 3). Susceptibility to oropharyngeal candidiasis is universal in untreated HIV/AIDS204, solid organ transplant recipients and those undergoing cancer chemotherapy205, again highlighting the importance of normal TH cell responses.
T cell hyperresponsiveness to Candida and Mycobacteria spp is detrimental to the host
At the interface of Candida and Mycobacteria susceptibility are cytokine signaling pathways that influence disease by either directing or inhibiting TH cell differentiation. For example, signaling through STAT1 results in a TH1 phenotype characterized by IFNα/β, IFNγ and IL27 secretion under normal conditions. With a gain-of-function STAT1 mutation, this phenotype is further skewed resulting in deficient Th17 signaling and susceptibility to CMC206,207. The impact of TH cell cytokine signaling on Candida and Mycobacteria disease susceptibility is also illustrated by the fact that most susceptibility alleles encode regulators of TH cell differentiation (FIG3). When inhibition of TH1 and TH17 responses is absent – either due to repeated mycobacterial stimulation (e.g. Koch’s phenomenon)107 or a genetic deficiency in inhibitory ligands208 – T cell hyperresponsiveness develops and leads to pathology at sites of infection. In humans, the detrimental impact of T cell hyperresponsiveness is observed in TB patients with immune reconstitution inflammatory syndrome (TB-IRIS). IRIS is an inflammatory response in Mycobacteria/HIV co-infected individuals that develops after commencing anti-retroviral therapy (ART): concomitant with declines in HIV viral loads, there is a rapid expansion of TH cells specific to M. tuberculosis or nontuberculous mycobacteria (NTM)209. Accompanying this expansion are high circulating levels TH1- and TH17-associated cytokines210–212, which contribute to progressive organ dysfunction in animal models of TB-IRIS209.
Among animal models of TB, the negative impact of T cell hyperresponsiveness is most notable in M. tuberculosis infected IL27 knockout mice. IL27 is a heterodimeric cytokine of the IL12 family that consists of the Epstein-Barr virus-induced gene 3 (EBI3) and IL27p28 proteins that are expressed from distinct genes213. IL27 is primarily produced by macrophages and DCs following activation, but the repertoire of cells that secrete the cytokine is expanding. IL27 signals through a receptor that is a heterodimer of WSX-1 and gp130 expressed on monocytes, macrophages, DCs, NK cells and lymphocytes214,215. IL27 is unique in that it can both activate and suppress immune responses. IL27 was originally described as a cytokine that promotes differentiation of TH1 cells and production of IFNγ216. However, WSX-1-deficient animals mount TH1 responses suggesting that IL27 is compensated for in this regard217–220. The anti-inflammatory effects of IL27 have been documented in animal models of infectious and chronic disease, including tuberculosis217–223. WSX-1 deficient mice exhibit improved control of mycobacterial growth218,219. However, these mice are also susceptible to severe immunopathology during chronic infection that compromises survival. This includes enhanced T cell proliferation, T cell activation, and heightened IFN-γ production217–220. IL-27 is involved with both the generation of anti-inflammatory T cells (Tr1) that produce large amounts of IL-10224,225, and suppression of TH17 cells that mediate inflammation218,219. IL-27 also has anti-inflammatory activity toward human macrophages with consequences to control of bacterial growth, including M. tuberculosis and M. bovis BCG226–229.
6. THE GOLDILOCKS MODEL OF IMMUNE SYMBIOSIS WITH CANDIDA AND MYCOBACTERIA COLONIZERS
In one telling of the fairy tale “Goldilocks and the Three Bears”, Goldilocks is an innocent young girl who wanders into a forest, and happens upon the house and home of three bears (a Father Bear, Mother Bear and Child Bear). The three bears were not home, and Goldilocks enters the home to discover – among other items – three beds which vary in softness. Having grown tired from her wanderings, Goldilocks settles into the bed of Child Bear, which is neither too hard (as was Father Bear’s bed and demeanor) nor too soft (as was Mother Bear’s bed and demeanor). The three bears return home to discover the sleeping Goldilocks, who upon waking reacts to sight of Father Bear and Mother Bear by jumping out a window, falling to her death. The tale of Goldilocks has been used by multiple academic fields to model how homeostasis or maintenance of an ideal state (represented by the sleeping Goldilocks) is maintained by a balance of two opposing forces (represented by the hardness of Father Bear and softness of Mother Bear), and that a detrimental outcome happens upon an imbalance of these opposing forces (represented by the death of Goldilocks). Academic fields that have applied a “Goldilocks Model” include materials science230, evolutionary biology231, auditory development232, visual development233, vaccinology234 and workplace safety235.
Based on our above review of literature regarding innate and adaptive responses to Mycobacteria and Candida spp, we propose a “Goldilocks model of immune symbiosis” (FIG 4). In most infected individuals, Candida and Mycobacteria spp are best described as commensals, since they benefit from a second species (i.e. the human host) without damaging the host. In a relative minority of individuals, Candida and Mycobacteria spp transition from being biological commensals to being biological exploiters. The percentage of individuals in whom this commensal-to-exploiter transition occurs is ~12% of M. tuberculosis infected individuals236, and virtually no C. albicans infected individuals in the absence of an immune or barrier defect. The commensal state of Mycobacteria and Candida spp is represented by the sleeping Goldilocks at the curve maximum in FIG 4, while the commensal-to-exploiter transition is represented by red curve minima. Mycobacteria and Candida spp are kept in a commensal state when there is a balance of two opposing forces: immune hypo-responsiveness (represented by Mother Bear) and immune hyper-responsiveness (represented by Father Bear). The commensal-to-exploiter transition occurs when there is an imbalance between these two forces, such as that caused by acquired immunodeficiency (e.g. HIV) or reoccurring immune stimulation (e.g. Koch’s phenomenon). As depicted by the steep slope on either side of sleeping Goldilocks (FIG 4), the commensal-to-exploiter transition is irreversible in the absence of medical intervention.
FIGURE 4.
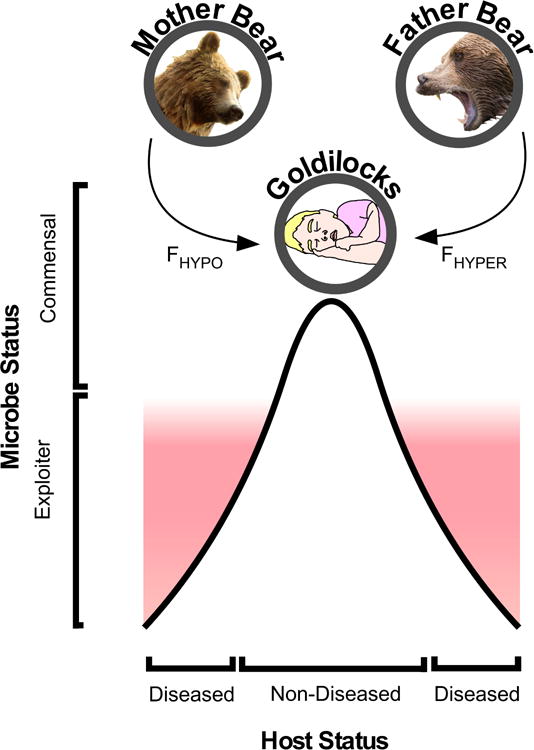
Goldilocks model of immune symbiosis with Candida and Mycobacteria Colonizers. Depicted by Mother Bear and Father Bear are two equally important but opposing forces: immune-hyporesponsiveness (FHYPO) and immune-hyperresponsiveness (FHYPER). In the majority of humans these two forces are balanced, a consequence of which is maintenance of Candida and Mycobacteria spp in a commensal state (represented by sleeping Goldilocks). Depicted below sleeping Goldilocks is a bell curve, the maxima of which represents those individuals in whom Candida and Mycobacteria spp remain in perfect equilibrium with the forces of immune-hyporesponsiveness and immune-hyperresponsiveness. Proximity to this equilibrium is a characteristic of most individuals infected with Candida or Mycobacteria spp, and is not associated with disease. In a relative minority of individuals, an imbalance exists between the forces of immune-hyporesponsiveness and immune-hyperresponsiveness (examples of which are provided in our review for both innate and adaptive immune cells), leading Candida and Mycobacteria spp to transition from a commensal to exploitive state. This transition from commensal to exploiter leads to disease, and is irreversible in absence of medical intervention.
Based on our model we can make several predictions that impact upon Candida and Mycobacteria disease prevention and treatment. First, we would predict that boosting immune responses in hypo-responsive individuals will maintain Mycobacteria and Candida spp in a commensal state and prevent disease. This prediction is supported by clinical data in children, whose immune system is hypo-responsive to mycobacterial stimuli relative to adults. Specifically, BCG-vaccination increases resistance to mycobacterial disease in children237. Similarly, infants less than 6 months of age are unable to maintain oral Candida in a commensal state and are therefore prone to thrush. A second and more surprising prediction is that immunosuppression would be effective in some individuals to prevent Candida- and Mycobacteria-related pathology. Vitamin D3 suppresses human TH1/TH17 differentiation, yet promotes resolution of TB pathology in humans and experimental models238,239. There are also situations in which steroidal and non-steroidal anti-inflammatory drugs are effective adjuncts to TB chemotherapy238,239, as well as host directed therapy for treating TB-IRIS209. In terms of Candida infection, there is a surprising disconnect between the severity of immune dysfunction in HIV/AIDS infection and the susceptibility and/or severity of vulvovaginal candidiasis. The discrepancy along with the association of exuberant neutrophil responses with this form of candidiasis again suggests a role for dampening the immune response to treat symptomatic infection240. While using anti-inflammatory drugs in any infected patient should of course be done cautiously, their documented value in some individuals supports the model depicted in FIG 4.
In conclusion, despite the obvious differences between Candida and Mycobacteria spp, there is remarkable overlap in the pattern recognition pathways that respond to these species, which in turn lead to similar innate and adaptive responses. Candida and Mycobacteria spp include pathogens that can cause localized or disseminated infections in a relative minority of colonized individuals. However, since the majority of colonized individuals are asymptomatic, Candida and Mycobacteria spp are best described in ecological terms as commensals which can transition to an exploitive state following immune perturbations. These perturbations can be either hyporesponsive or hyperresponsive in nature, and can originate from innate and adaptive lineages. Although the commensal status of Mycobacteria spp is a newer concept in the field of mycobacterial immunology, the commensal status of Candida spp is a well-accepted concept in the field of fungal immunology. Based on this model, understanding the immune factors that maintain Candida and Mycobacteria spp in a commensal state may aid the development of diagnostic and treatment strategies for both categories of pathogens.
Acknowledgments
This work was supported by funds from the Medical College of Wisconsin (MCW) Department of Microbiology and Immunology, MCW Center for Infectious Disease Research, and the National Institutes of Health (R01AI121212 to RTR), as well as the MCW Department of Pediatrics, MCW Research Affairs Committee and Children’s Hospital of Wisconsin Research Institute (to ARH).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
BIBLIOGRAPHY
- 1.Whelan WL, Magee PT. Natural heterozygosity in Candida albicans. J Bacteriol. 1981;145:896–903. doi: 10.1128/jb.145.2.896-903.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aldridge BB, Fernandez-Suarez M, Heller D, Ambravaneswaran V, Irimia D, Toner M, Fortune SM. Asymmetry and aging of mycobacterial cells lead to variable growth and antibiotic susceptibility. Science. 2012;335:100–104. doi: 10.1126/science.1216166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown GD, Denning DW, Gow NA, Levitz SM, Netea MG, White TC. Hidden killers: human fungal infections. Sci Transl Med. 2012;4:165rv113. doi: 10.1126/scitranslmed.3004404. [DOI] [PubMed] [Google Scholar]
- 4.Pfaller MA, Diekema DJ. Epidemiology of invasive candidiasis: a persistent public health problem. Clin Microbiol Rev. 2007;20:133–163. doi: 10.1128/CMR.00029-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Russell DG. Mycobacterium tuberculosis and the intimate discourse of a chronic infection. Immunol Rev. 2011;240:252–268. doi: 10.1111/j.1600-065X.2010.00984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bloom BR, Atun R. Back to the future: Rethinking global control of tuberculosis. Sci Transl Med. 2016;8:329ps327. doi: 10.1126/scitranslmed.aaf2944. [DOI] [PubMed] [Google Scholar]
- 7.Vieira FC, Nahas E. Comparison of microbial numbers in soils by using various culture media and temperatures. Microbiol Res. 2005;160:197–202. doi: 10.1016/j.micres.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 8.Whitman WB, Coleman DC, Wiebe WJ. Prokaryotes: the unseen majority. Proc Natl Acad Sci U S A. 1998;95:6578–6583. doi: 10.1073/pnas.95.12.6578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perlroth J, Choi B, Spellberg B. Nosocomial fungal infections: epidemiology, diagnosis, and treatment. Med Mycol. 2007;45:321–346. doi: 10.1080/13693780701218689. [DOI] [PubMed] [Google Scholar]
- 10.Gagneux S. Host-pathogen coevolution in human tuberculosis. Philosophical transactions of the Royal Society of London. Series B, Biological sciences. 2012;367:850–859. doi: 10.1098/rstb.2011.0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ernst JD. The immunological life cycle of tuberculosis. Nat Rev Immunol. 2012;12:581–591. doi: 10.1038/nri3259. [DOI] [PubMed] [Google Scholar]
- 12.Hardy MA, Schmidek HH. Epidemiology of tuberculosis aboard a ship. JAMA : the journal of the American Medical Association. 1968;203:175–179. [PubMed] [Google Scholar]
- 13.Worobey M, Watts TD, McKay RA, Suchard MA, Granade T, Teuwen DE, Koblin BA, Heneine W, Lemey P, Jaffe HW. 1970s and ‘Patient 0’ HIV-1 genomes illuminate early HIV/AIDS history in North America. Nature. 2016;539:98–101. doi: 10.1038/nature19827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diedrich CR, Flynn JL. HIV-1/mycobacterium tuberculosis coinfection immunology: how does HIV-1 exacerbate tuberculosis? Infection and immunity. 2011;79:1407–1417. doi: 10.1128/IAI.01126-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glynn JR, Murray J, Bester A, Nelson G, Shearer S, Sonnenberg P. Effects of duration of HIV infection and secondary tuberculosis transmission on tuberculosis incidence in the South African gold mines. AIDS. 2008;22:1859–1867. doi: 10.1097/QAD.0b013e3283097cfa. [DOI] [PubMed] [Google Scholar]
- 16.Sonnenberg P, Glynn JR, Fielding K, Murray J, Godfrey-Faussett P, Shearer S. How soon after infection with HIV does the risk of tuberculosis start to increase? A retrospective cohort study in South African gold miners. The Journal of infectious diseases. 2005;191:150–158. doi: 10.1086/426827. [DOI] [PubMed] [Google Scholar]
- 17.Whalen CC, Nsubuga P, Okwera A, Johnson JL, Hom DL, Michael NL, Mugerwa RD, Ellner JJ. Impact of pulmonary tuberculosis on survival of HIV-infected adults: a prospective epidemiologic study in Uganda. AIDS. 2000;14:1219–1228. doi: 10.1097/00002030-200006160-00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Selwyn PA, Sckell BM, Alcabes P, Friedland GH, Klein RS, Schoenbaum EE. High risk of active tuberculosis in HIV-infected drug users with cutaneous anergy. JAMA : the journal of the American Medical Association. 1992;268:504–509. [PubMed] [Google Scholar]
- 19.Fenner L, Egger M, Bodmer T, Furrer H, Ballif M, Battegay M, Helbling P, Fehr J, Gsponer T, Rieder HL, Zwahlen M, Hoffmann M, Bernasconi E, Cavassini M, Calmy A, Dolina M, Frei R, Janssens JP, Borrell S, Stucki D, Schrenzel J, Bottger EC, Gagneux S. HIV infection disrupts the sympatric host-pathogen relationship in human tuberculosis. PLoS genetics. 2013;9:e1003318. doi: 10.1371/journal.pgen.1003318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang W, Zhai Y, Cao L, Tan H, Zhang R. Illumina-based analysis of core actinobacteriome in roots, stems, and grains of rice. Microbiol Res. 2016;190:12–18. doi: 10.1016/j.micres.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 21.Pontiroli A, Khera TT, Oakley BB, Mason S, Dowd SE, Travis ER, Erenso G, Aseffa A, Courtenay O, Wellington EM. Prospecting environmental mycobacteria: combined molecular approaches reveal unprecedented diversity. PLoS One. 2013;8:e68648. doi: 10.1371/journal.pone.0068648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alvarado-Esquivel C, Garcia-Corral N, Carrero-Dominguez D, Enciso-Moreno JA, Gurrola-Morales T, Portillo-Gomez L, Rossau R, Mijs W. Molecular analysis of Mycobacterium isolates from extrapulmonary specimens obtained from patients in Mexico. BMC Clin Pathol. 2009;9:1. doi: 10.1186/1472-6890-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gordon RE, Smith MM. Rapidly growing, acid fast bacteria. I. Species’ descriptions of Mycobacterium phlei Lehmann and Neumann and Mycobacterium smegmatis (Trevisan) Lehmann and Neumann. J Bacteriol. 1953;66:41–48. doi: 10.1128/jb.66.1.41-48.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Springer B, Wu WK, Bodmer T, Haase G, Pfyffer GE, Kroppenstedt RM, Schroder KH, Emler S, Kilburn JO, Kirschner P, Telenti A, Coyle MB, Bottger EC. Isolation and characterization of a unique group of slowly growing mycobacteria: description of Mycobacterium lentiflavum sp. nov. J Clin Microbiol. 1996;34:1100–1107. doi: 10.1128/jcm.34.5.1100-1107.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kirschner P, Teske A, Schroder KH, Kroppenstedt RM, Wolters J, Bottger EC. Mycobacterium confluentis sp. nov. Int J Syst Bacteriol. 1992;42:257–262. doi: 10.1099/00207713-42-2-257. [DOI] [PubMed] [Google Scholar]
- 26.Koukila-Kahkola P, Springer B, Bottger EC, Paulin L, Jantzen E, Katila ML. Mycobacterium branderi sp. nov., a new potential human pathogen. Int J Syst Bacteriol. 1995;45:549–553. doi: 10.1099/00207713-45-3-549. [DOI] [PubMed] [Google Scholar]
- 27.Lumb R, Goodwin A, Ratcliff R, Stapledon R, Holland A, Bastian I. Phenotypic and molecular characterization of three clinical isolates of Mycobacterium interjectum. J Clin Microbiol. 1997;35:2782–2785. doi: 10.1128/jcm.35.11.2782-2785.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meier A, Kirschner P, Schroder KH, Wolters J, Kroppenstedt RM, Bottger EC. Mycobacterium intermedium sp. nov. Int J Syst Bacteriol. 1993;43:204–209. doi: 10.1099/00207713-43-2-204. [DOI] [PubMed] [Google Scholar]
- 29.Reischl U, Emler S, Horak Z, Kaustova J, Kroppenstedt RM, Lehn N, Naumann L. Mycobacterium bohemicum sp. nov., a new slow-growing scotochromogenic mycobacterium. Int J Syst Bacteriol. 1998;48(Pt 4):1349–1355. doi: 10.1099/00207713-48-4-1349. [DOI] [PubMed] [Google Scholar]
- 30.van Ingen J, Boeree MJ, Stals FS, Pitz CC, Rooijmans-Rietjens JJ, van der Zanden AG, Dekhuijzen PN, van Soolingen D. Clinical Mycobacterium conspicuum isolation from two immunocompetent patients in The Netherlands. J Clin Microbiol. 2007;45:4075–4076. doi: 10.1128/JCM.00867-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wolfe J, Turenne C, Alfa M, Harding G, Thibert L, Kabani A. Mycobacterium branderi from both a hand infection and a case of pulmonary disease. J Clin Microbiol. 2000;38:3896–3899. doi: 10.1128/jcm.38.10.3896-3899.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Singh AK, Marak RS, Maurya AK, Das M, Nag VL, Dhole TN. Mixed Cutaneous Infection Caused by Mycobacterium szulgai and Mycobacterium intermedium in a Healthy Adult Female: A Rare Case Report. Case Rep Dermatol Med. 2015;2015:607519. doi: 10.1155/2015/607519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Torkko P, Suomalainen S, Iivanainen E, Suutari M, Paulin L, Rudback E, Tortoli E, Vincent V, Mattila R, Katila ML. Characterization of Mycobacterium bohemicum isolated from human, veterinary, and environmental sources. J Clin Microbiol. 2001;39:207–211. doi: 10.1128/JCM.39.1.207-211.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wallace RJ, Jr, Nash DR, Tsukamura M, Blacklock ZM, Silcox VA. Human disease due to Mycobacterium smegmatis. J Infect Dis. 1988;158:52–59. doi: 10.1093/infdis/158.1.52. [DOI] [PubMed] [Google Scholar]
- 35.Rook GA, Brunet LR. Microbes, immunoregulation, and the gut. Gut. 2005;54:317–320. doi: 10.1136/gut.2004.053785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hunt JR, Martinelli R, Adams VC, Rook GA, Brunet LR. Intragastric administration of Mycobacterium vaccae inhibits severe pulmonary allergic inflammation in a mouse model. Clin Exp Allergy. 2005;35:685–690. doi: 10.1111/j.1365-2222.2005.02239.x. [DOI] [PubMed] [Google Scholar]
- 37.Matthews DM, Jenks SM. Ingestion of Mycobacterium vaccae decreases anxiety-related behavior and improves learning in mice. Behav Processes. 2013;96:27–35. doi: 10.1016/j.beproc.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 38.O’Brien ME, Anderson H, Kaukel E, O’Byrne K, Pawlicki M, Von Pawel J, Reck M, Group SOS SRL172 (killed Mycobacterium vaccae) in addition to standard chemotherapy improves quality of life without affecting survival, in patients with advanced non-small-cell lung cancer: phase III results. Ann Oncol. 2004;15:906–914. doi: 10.1093/annonc/mdh220. [DOI] [PubMed] [Google Scholar]
- 39.Mayer EA, Knight R, Mazmanian SK, Cryan JF, Tillisch K. Gut microbes and the brain: paradigm shift in neuroscience. J Neurosci. 2014;34:15490–15496. doi: 10.1523/JNEUROSCI.3299-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wayne LG, Sramek HA. Agents of newly recognized or infrequently encountered mycobacterial diseases. Clin Microbiol Rev. 1992;5:1–25. doi: 10.1128/cmr.5.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Inderlied CB, Kemper CA, Bermudez LE. The Mycobacterium avium complex. Clin Microbiol Rev. 1993;6:266–310. doi: 10.1128/cmr.6.3.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wolinsky E. Nontuberculous mycobacteria and associated diseases. Am Rev Respir Dis. 1979;119:107–159. doi: 10.1164/arrd.1979.119.1.107. [DOI] [PubMed] [Google Scholar]
- 43.Wolinsky E, Rynearson TK. Mycobacteria in soil and their relation to disease-associated strains. Am Rev Respir Dis. 1968;97:1032–1037. doi: 10.1164/arrd.1968.97.6P1.1032. [DOI] [PubMed] [Google Scholar]
- 44.Yajko DM, Chin DP, Gonzalez PC, Nassos PS, Hopewell PC, Reingold AL, Horsburgh CR, Jr, Yakrus MA, Ostroff SM, Hadley WK. Mycobacterium avium complex in water, food, and soil samples collected from the environment of HIV-infected individuals. J Acquir Immune Defic Syndr Hum Retrovirol. 1995;9:176–182. [PubMed] [Google Scholar]
- 45.Drancourt M. Looking in amoebae as a source of mycobacteria. Microb Pathog. 2014;77:119–124. doi: 10.1016/j.micpath.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 46.Delafont V, Mougari F, Cambau E, Joyeux M, Bouchon D, Hechard Y, Moulin L. First evidence of amoebae-mycobacteria association in drinking water network. Environ Sci Technol. 2014;48:11872–11882. doi: 10.1021/es5036255. [DOI] [PubMed] [Google Scholar]
- 47.Thomson R, Tolson C, Carter R, Coulter C, Huygens F, Hargreaves M. Isolation of nontuberculous mycobacteria (NTM) from household water and shower aerosols in patients with pulmonary disease caused by NTM. J Clin Microbiol. 2013;51:3006–3011. doi: 10.1128/JCM.00899-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.von Reyn CF, Maslow JN, Barber TW, Falkinham JO, 3rd, Arbeit RD. Persistent colonisation of potable water as a source of Mycobacterium avium infection in AIDS. Lancet. 1994;343:1137–1141. doi: 10.1016/s0140-6736(94)90239-9. [DOI] [PubMed] [Google Scholar]
- 49.De Groote MA, Pace NR, Fulton K, Falkinham JO., 3rd Relationships between Mycobacterium isolates from patients with pulmonary mycobacterial infection and potting soils. Appl Environ Microbiol. 2006;72:7602–7606. doi: 10.1128/AEM.00930-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Honda JR, Bernhard JN, Chan ED. Natural disasters and nontuberculous mycobacteria: a recipe for increased disease? Chest. 2015;147:304–308. doi: 10.1378/chest.14-0974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Feldman WH, Davies R, Moses HE, Andberg W. An unusual mycobacterium isolate from a sputum of a man suffering from pulmonary disease of long duration. American Review of Tuberculosis. 1943;48:272–290. [Google Scholar]
- 52.Dubbioso R, Cerillo I, D’Arco F, D’Amico A, Pettinato G, Boldorini R, Santoro L, Manganelli F. Isolated intracranial Mycobacterium avium complex granulomas in an immune-competent man. J Neurol Sci. 2015;349:264–265. doi: 10.1016/j.jns.2015.01.020. [DOI] [PubMed] [Google Scholar]
- 53.Farhi DC, Mason UG, 3rd, Horsburgh CR., Jr Pathologic findings in disseminated Mycobacterium avium-intracellulare infection. A report of 11 cases. Am J Clin Pathol. 1986;85:67–72. doi: 10.1093/ajcp/85.1.67. [DOI] [PubMed] [Google Scholar]
- 54.Florido M, Cooper AM, Appelberg R. Immunological basis of the development of necrotic lesions following Mycobacterium avium infection. Immunology. 2002;106:590–601. doi: 10.1046/j.1365-2567.2002.01459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Perisson C, Nathan N, Thierry B, Corvol H. Endobronchial avium mycobacteria infection in an immunocompetent child. BMJ Case Rep. 2013;2013 doi: 10.1136/bcr-2013-200776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rives P, Joubert M, Launay E, Guillouzouic A, Espitalier F, Malard O. Cervicofacial non-tuberculous mycobacteria: A report of 30 cases. Eur Ann Otorhinolaryngol Head Neck Dis. 2016;133:107–111. doi: 10.1016/j.anorl.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 57.Winthrop KL, McNelley E, Kendall B, Marshall-Olson A, Morris C, Cassidy M, Saulson A, Hedberg K. Pulmonary nontuberculous mycobacterial disease prevalence and clinical features: an emerging public health disease. Am J Respir Crit Care Med. 2010;182:977–982. doi: 10.1164/rccm.201003-0503OC. [DOI] [PubMed] [Google Scholar]
- 58.Human Microbiome Project C. Structure, function and diversity of the healthy human microbiome. Nature. 2012;486:207–214. doi: 10.1038/nature11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Marsh BJ, San Vicente J, von Reyn CF. Utility of dual skin tests to evaluate tuberculin skin test reactions of 10 to 14 mm in healthcare workers. Infect Control Hosp Epidemiol. 2003;24:821–824. doi: 10.1086/502143. [DOI] [PubMed] [Google Scholar]
- 60.von Reyn CF, Barber TW, Arbeit RD, Sox CH, O’Connor GT, Brindle RJ, Gilks CF, Hakkarainen K, Ranki A, Bartholomew C, et al. Evidence of previous infection with Mycobacterium avium-Mycobacterium intracellulare complex among healthy subjects: an international study of dominant mycobacterial skin test reactions. J Infect Dis. 1993;168:1553–1558. doi: 10.1093/infdis/168.6.1553. [DOI] [PubMed] [Google Scholar]
- 61.von Reyn CF, Horsburgh CR, Olivier KN, Barnes PF, Waddell R, Warren C, Tvaroha S, Jaeger AS, Lein AD, Alexander LN, Weber DJ, Tosteson AN. Skin test reactions to Mycobacterium tuberculosis purified protein derivative and Mycobacterium avium sensitin among health care workers and medical students in the United States. Int J Tuberc Lung Dis. 2001;5:1122–1128. [PubMed] [Google Scholar]
- 62.von Reyn CF, Marsh BJ, Waddell R, Lein AD, Tvaroha S, Morin P, Modlin JF. Cellular immune responses to mycobacteria in healthy and human immunodeficiency virus-positive subjects in the United States after a five-dose schedule of Mycobacterium vaccae vaccine. Clin Infect Dis. 1998;27:1517–1520. doi: 10.1086/515024. [DOI] [PubMed] [Google Scholar]
- 63.Horsburgh CR., Jr Mycobacterium avium complex infection in the acquired immunodeficiency syndrome. N Engl J Med. 1991;324:1332–1338. doi: 10.1056/NEJM199105093241906. [DOI] [PubMed] [Google Scholar]
- 64.Cheng SC, Joosten LA, Kullberg BJ, Netea MG. Interplay between Candida albicans and the mammalian innate host defense. Infect Immun. 2012;80:1304–1313. doi: 10.1128/IAI.06146-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Arcos J, Diangelo LE, Scordo JM, Sasindran SJ, Moliva JI, Turner J, Torrelles JB. Lung Mucosa Lining Fluid Modification of Mycobacterium tuberculosis to Reprogram Human Neutrophil Killing Mechanisms. J Infect Dis. 2015;212:948–958. doi: 10.1093/infdis/jiv146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Arcos J, Sasindran SJ, Fujiwara N, Turner J, Schlesinger LS, Torrelles JB. Human lung hydrolases delineate Mycobacterium tuberculosis-macrophage interactions and the capacity to control infection. J Immunol. 2011;187:372–381. doi: 10.4049/jimmunol.1100823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cheng SC, van de Veerdonk FL, Lenardon M, Stoffels M, Plantinga T, Smeekens S, Rizzetto L, Mukaremera L, Preechasuth K, Cavalieri D, Kanneganti TD, van der Meer JW, Kullberg BJ, Joosten LA, Gow NA, Netea MG. The dectin-1/inflammasome pathway is responsible for the induction of protective T-helper 17 responses that discriminate between yeasts and hyphae of Candida albicans. J Leukoc Biol. 2011;90:357–366. doi: 10.1189/jlb.1210702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kallenius G, Correia-Neves M, Buteme H, Hamasur B, Svenson SB. Lipoarabinomannan, and its related glycolipids, induce divergent and opposing immune responses to Mycobacterium tuberculosis depending on structural diversity and experimental variations. Tuberculosis (Edinb) 2016;96:120–130. doi: 10.1016/j.tube.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 69.Lowman DW, Greene RR, Bearden DW, Kruppa MD, Pottier M, Monteiro MA, Soldatov DV, Ensley HE, Cheng SC, Netea MG, Williams DL. Novel structural features in Candida albicans hyphal glucan provide a basis for differential innate immune recognition of hyphae versus yeast. J Biol Chem. 2014;289:3432–3443. doi: 10.1074/jbc.M113.529131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.d’Ostiani CF, Del Sero G, Bacci A, Montagnoli C, Spreca A, Mencacci A, Ricciardi-Castagnoli P, Romani L. Dendritic cells discriminate between yeasts and hyphae of the fungus Candida albicans. Implications for initiation of T helper cell immunity in vitro and in vivo. J Exp Med. 2000;191:1661–1674. doi: 10.1084/jem.191.10.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yu L, Lee KK, Sheth HB, Lane-Bell P, Srivastava G, Hindsgaul O, Paranchych W, Hodges RS, Irvin RT. Fimbria-mediated adherence of Candida albicans to glycosphingolipid receptors on human buccal epithelial cells. Infect Immun. 1994;62:2843–2848. doi: 10.1128/iai.62.7.2843-2848.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Netea MG, Joosten LA, van der Meer JW, Kullberg BJ, van de Veerdonk FL. Immune defence against Candida fungal infections. Nat Rev Immunol. 2015;15:630–642. doi: 10.1038/nri3897. [DOI] [PubMed] [Google Scholar]
- 73.Munro CA, Schofield DA, Gooday GW, Gow NA. Regulation of chitin synthesis during dimorphic growth of Candida albicans. Microbiology. 1998;144(Pt 2):391–401. doi: 10.1099/00221287-144-2-391. [DOI] [PubMed] [Google Scholar]
- 74.Shibata N, Suzuki A, Kobayashi H, Okawa Y. Chemical structure of the cell-wall mannan of Candida albicans serotype A and its difference in yeast and hyphal forms. Biochem J. 2007;404:365–372. doi: 10.1042/BJ20070081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lo HJ, Kohler JR, DiDomenico B, Loebenberg D, Cacciapuoti A, Fink GR. Nonfilamentous C. albicans mutants are avirulent. Cell. 1997;90:939–949. doi: 10.1016/s0092-8674(00)80358-x. [DOI] [PubMed] [Google Scholar]
- 76.Moyes DL, Wilson D, Richardson JP, Mogavero S, Tang SX, Wernecke J, Hofs S, Gratacap RL, Robbins J, Runglall M, Murciano C, Blagojevic M, Thavaraj S, Forster TM, Hebecker B, Kasper L, Vizcay G, Iancu SI, Kichik N, Hader A, Kurzai O, Luo T, Kruger T, Kniemeyer O, Cota E, Bader O, Wheeler RT, Gutsmann T, Hube B, Naglik JR. Candidalysin is a fungal peptide toxin critical for mucosal infection. Nature. 2016;532:64–68. doi: 10.1038/nature17625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bacon J, Alderwick LJ, Allnutt JA, Gabasova E, Watson R, Hatch KA, Clark SO, Jeeves RE, Marriott A, Rayner E, Tolley H, Pearson G, Hall G, Besra GS, Wernisch L, Williams A, Marsh PD. Non-replicating Mycobacterium tuberculosis elicits a reduced infectivity profile with corresponding modifications to the cell wall and extracellular matrix. PLoS One. 2014;9:e87329. doi: 10.1371/journal.pone.0087329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Brennan PJ. Structure, function, and biogenesis of the cell wall of Mycobacterium tuberculosis. Tuberculosis (Edinb) 2003;83:91–97. doi: 10.1016/s1472-9792(02)00089-6. [DOI] [PubMed] [Google Scholar]
- 79.Dietrich J, Roy S, Rosenkrands I, Lindenstrom T, Filskov J, Rasmussen EM, Cassidy J, Andersen P. Differential influence of nutrient-starved Mycobacterium tuberculosis on adaptive immunity results in progressive tuberculosis disease and pathology. Infect Immun. 2015;83:4731–4739. doi: 10.1128/IAI.01055-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Venkataswamy MM, Goldberg MF, Baena A, Chan J, Jacobs WR, Jr, Porcelli SA. In vitro culture medium influences the vaccine efficacy of Mycobacterium bovis BCG. Vaccine. 2012;30:1038–1049. doi: 10.1016/j.vaccine.2011.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hunter RL, Olsen MR, Jagannath C, Actor JK. Multiple roles of cord factor in the pathogenesis of primary, secondary, and cavitary tuberculosis, including a revised description of the pathology of secondary disease. Ann Clin Lab Sci. 2006;36:371–386. [PubMed] [Google Scholar]
- 82.Gringhuis SI, den Dunnen J, Litjens M, van der Vlist M, Wevers B, Bruijns SC, Geijtenbeek TB. Dectin-1 directs T helper cell differentiation by controlling noncanonical NF-kappaB activation through Raf-1 and Syk. Nat Immunol. 2009;10:203–213. doi: 10.1038/ni.1692. [DOI] [PubMed] [Google Scholar]
- 83.Gross O, Gewies A, Finger K, Schafer M, Sparwasser T, Peschel C, Forster I, Ruland J. Card9 controls a non-TLR signalling pathway for innate anti-fungal immunity. Nature. 2006;442:651–656. doi: 10.1038/nature04926. [DOI] [PubMed] [Google Scholar]
- 84.Strasser D, Neumann K, Bergmann H, Marakalala MJ, Guler R, Rojowska A, Hopfner KP, Brombacher F, Urlaub H, Baier G, Brown GD, Leitges M, Ruland J. Syk kinase-coupled C-type lectin receptors engage protein kinase C-sigma to elicit Card9 adaptor-mediated innate immunity. Immunity. 2012;36:32–42. doi: 10.1016/j.immuni.2011.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Whitney PG, Bar E, Osorio F, Rogers NC, Schraml BU, Deddouche S, LeibundGut-Landmann S, Reis e Sousa C. Syk signaling in dendritic cells orchestrates innate resistance to systemic fungal infection. PLoS Pathog. 2014;10:e1004276. doi: 10.1371/journal.ppat.1004276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Moyes DL, Shen C, Murciano C, Runglall M, Richardson JP, Arno M, Aldecoa-Otalora E, Naglik JR. Protection against epithelial damage during Candida albicans infection is mediated by PI3K/Akt and mammalian target of rapamycin signaling. J Infect Dis. 2014;209:1816–1826. doi: 10.1093/infdis/jit824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Smeekens SP, Gresnigt MS, Becker KL, Cheng SC, Netea SA, Jacobs L, Jansen T, van de Veerdonk FL, Williams DL, Joosten LA, Dinarello CA, Netea MG. An anti-inflammatory property of Candida albicans beta-glucan: Induction of high levels of interleukin-1 receptor antagonist via a Dectin-1/CR3 independent mechanism. Cytokine. 2015;71:215–222. doi: 10.1016/j.cyto.2014.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gross O, Poeck H, Bscheider M, Dostert C, Hannesschlager N, Endres S, Hartmann G, Tardivel A, Schweighoffer E, Tybulewicz V, Mocsai A, Tschopp J, Ruland J. Syk kinase signalling couples to the Nlrp3 inflammasome for anti-fungal host defence. Nature. 2009;459:433–436. doi: 10.1038/nature07965. [DOI] [PubMed] [Google Scholar]
- 89.Tomalka J, Ganesan S, Azodi E, Patel K, Majmudar P, Hall BA, Fitzgerald KA, Hise AG. A novel role for the NLRC4 inflammasome in mucosal defenses against the fungal pathogen Candida albicans. PLoS Pathog. 2011;7:e1002379. doi: 10.1371/journal.ppat.1002379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ganesan S, Rathinam VA, Bossaller L, Army K, Kaiser WJ, Mocarski ES, Dillon CP, Green DR, Mayadas TN, Levitz SM, Hise AG, Silverman N, Fitzgerald KA. Caspase-8 modulates dectin-1 and complement receptor 3-driven IL-1beta production in response to beta-glucans and the fungal pathogen, Candida albicans. J Immunol. 2014;193:2519–2530. doi: 10.4049/jimmunol.1400276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Moyes DL, Murciano C, Runglall M, Islam A, Thavaraj S, Naglik JR. Candida albicans yeast and hyphae are discriminated by MAPK signaling in vaginal epithelial cells. PLoS One. 2011;6:e26580. doi: 10.1371/journal.pone.0026580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Moyes DL, Runglall M, Murciano C, Shen C, Nayar D, Thavaraj S, Kohli A, Islam A, Mora-Montes H, Challacombe SJ, Naglik JR. A biphasic innate immune MAPK response discriminates between the yeast and hyphal forms of Candida albicans in epithelial cells. Cell Host Microbe. 2010;8:225–235. doi: 10.1016/j.chom.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Branzk N, Lubojemska A, Hardison SE, Wang Q, Gutierrez MG, Brown GD, Papayannopoulos V. Neutrophils sense microbe size and selectively release neutrophil extracellular traps in response to large pathogens. Nat Immunol. 2014;15:1017–1025. doi: 10.1038/ni.2987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Aubert-Pivert EM, Chedevergne FM, Lopez-Ramirez GM, Colle JH, Scheinmann PL, Gicquel BM, de Blic JM. Cytokine transcripts in pediatric tuberculosis: a study with bronchoalveolar cells. Tuber Lung Dis. 2000;80:249–258. doi: 10.1054/tuld.2000.0259. [DOI] [PubMed] [Google Scholar]
- 95.Herrera MT, Torres M, Nevels D, Perez-Redondo CN, Ellner JJ, Sada E, Schwander SK. Compartmentalized bronchoalveolar IFN-gamma and IL-12 response in human pulmonary tuberculosis. Tuberculosis (Edinb) 2009;89:38–47. doi: 10.1016/j.tube.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lev-Sagie A, Nyirjesy P, Tarangelo N, Bongiovanni AM, Bayer C, Linhares IM, Giraldo PC, Ledger WJ, Witkin SS. Hyaluronan in vaginal secretions: association with recurrent vulvovaginal candidiasis. Am J Obstet Gynecol. 2009;201(206):e201–205. doi: 10.1016/j.ajog.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 97.Mwandumba HC, Bertel Squire S, White SA, Nyirenda MH, Kampondeni SD, Rhoades ER, Zijlstra EE, Molyneux ME, Russell DG. Association between sputum smear status and local immune responses at the site of disease in HIV-infected patients with pulmonary tuberculosis. Tuberculosis (Edinb) 2008;88:58–63. doi: 10.1016/j.tube.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 98.Nolan A, Fajardo E, Huie ML, Condos R, Pooran A, Dawson R, Dheda K, Bateman E, Rom WN, Weiden MD. Increased production of IL-4 and IL-12p40 from bronchoalveolar lavage cells are biomarkers of Mycobacterium tuberculosis in the sputum. PLoS One. 2013;8:e59461. doi: 10.1371/journal.pone.0059461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Spear GT, Kendrick SR, Chen HY, Thomas TT, Bahk M, Balderas R, Ghosh S, Weinberg A, Landay AL. Multiplex immunoassay of lower genital tract mucosal fluid from women attending an urban STD clinic shows broadly increased IL1ss and lactoferrin. PLoS One. 2011;6:e19560. doi: 10.1371/journal.pone.0019560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Spear GT, Zariffard MR, Cohen MH, Sha BE. Vaginal IL-8 levels are positively associated with Candida albicans and inversely with lactobacilli in HIV-infected women. J Reprod Immunol. 2008;78:76–79. doi: 10.1016/j.jri.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tsao TC, Hong J, Li LF, Hsieh MJ, Liao SK, Chang KS. Imbalances between tumor necrosis factor-alpha and its soluble receptor forms, and interleukin-1beta and interleukin-1 receptor antagonist in BAL fluid of cavitary pulmonary tuberculosis. Chest. 2000;117:103–109. doi: 10.1378/chest.117.1.103. [DOI] [PubMed] [Google Scholar]
- 102.Schmitt N, Bustamante J, Bourdery L, Bentebibel SE, Boisson-Dupuis S, Hamlin F, Tran MV, Blankenship D, Pascual V, Savino DA, Banchereau J, Casanova JL, Ueno H. IL-12 receptor beta1 deficiency alters in vivo T follicular helper cell response in humans. Blood. 2013;121:3375–3385. doi: 10.1182/blood-2012-08-448902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ramos HJ, Davis AM, Cole AG, Schatzle JD, Forman J, Farrar JD. Reciprocal responsiveness to interleukin-12 and interferon-alpha specifies human CD8+ effector versus central memory T-cell fates. Blood. 2009;113:5516–5525. doi: 10.1182/blood-2008-11-188458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.de Beaucoudrey L, Puel A, Filipe-Santos O, Cobat A, Ghandil P, Chrabieh M, Feinberg J, von Bernuth H, Samarina A, Janniere L, Fieschi C, Stephan JL, Boileau C, Lyonnet S, Jondeau G, Cormier-Daire V, Le Merrer M, Hoarau C, Lebranchu Y, Lortholary O, Chandesris MO, Tron F, Gambineri E, Bianchi L, Rodriguez-Gallego C, Zitnik SE, Vasconcelos J, Guedes M, Vitor AB, Marodi L, Chapel H, Reid B, Roifman C, Nadal D, Reichenbach J, Caragol I, Garty BZ, Dogu F, Camcioglu Y, Gulle S, Sanal O, Fischer A, Abel L, Stockinger B, Picard C, Casanova JL. Mutations in STAT3 and IL12RB1 impair the development of human IL-17-producing T cells. The Journal of experimental medicine. 2008;205:1543–1550. doi: 10.1084/jem.20080321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ouederni M, Sanal O, Ikinciogullari A, Tezcan I, Dogu F, Sologuren I, Pedraza-Sanchez S, Keser M, Tanir G, Nieuwhof C, Colino E, Kumararatne D, Levy J, Kutukculer N, Aytekin C, Herrera-Ramos E, Bhatti M, Karaca N, Barbouche R, Broides A, Goudouris E, Franco JL, Parvaneh N, Reisli I, Strickler A, Shcherbina A, Somer A, Segal A, Angel-Moreno A, Lezana-Fernandez JL, Bejaoui M, Bobadilla-Del Valle M, Kachboura S, Sentongo T, Ben-Mustapha I, Bustamante J, Picard C, Puel A, Boisson-Dupuis S, Abel L, Casanova JL, Rodriguez-Gallego C. Clinical features of Candidiasis in patients with inherited interleukin 12 receptor beta1 deficiency. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2014;58:204–213. doi: 10.1093/cid/cit722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.de Beaucoudrey L, Samarina A, Bustamante J, Cobat A, Boisson-Dupuis S, Feinberg J, Al-Muhsen S, Janniere L, Rose Y, de Suremain M, Kong XF, Filipe-Santos O, Chapgier A, Picard C, Fischer A, Dogu F, Ikinciogullari A, Tanir G, Al-Hajjar S, Al-Jumaah S, Frayha HH, AlSum Z, Al-Ajaji S, Alangari A, Al-Ghonaium A, Adimi P, Mansouri D, Ben-Mustapha I, Yancoski J, Garty BZ, Rodriguez-Gallego C, Caragol I, Kutukculer N, Kumararatne DS, Patel S, Doffinger R, Exley A, Jeppsson O, Reichenbach J, Nadal D, Boyko Y, Pietrucha B, Anderson S, Levin M, Schandene L, Schepers K, Efira A, Mascart F, Matsuoka M, Sakai T, Siegrist CA, Frecerova K, Bluetters-Sawatzki R, Bernhoft J, Freihorst J, Baumann U, Richter D, Haerynck F, De Baets F, Novelli V, Lammas D, Vermylen C, Tuerlinckx D, Nieuwhof C, Pac M, Haas WH, Muller-Fleckenstein I, Fleckenstein B, Levy J, Raj R, Cohen AC, Lewis DB, Holland SM, Yang KD, Wang X, Jiang L, Yang X, Zhu C, Xie Y, Lee PP, Chan KW, Chen TX, Castro G, Natera I, Codoceo A, King A, Bezrodnik L, Di Giovani D, Gaillard MI, de Moraes-Vasconcelos D, Grumach AS, da Silva Duarte AJ, Aldana R, Espinosa-Rosales FJ, Bejaoui M, Bousfiha AA, Baghdadi JE, Ozbek N, Aksu G, Keser M, Somer A, Hatipoglu N, Aydogmus C, Asilsoy S, Camcioglu Y, Gulle S, Ozgur TT, Ozen M, Oleastro M, Bernasconi A, Mamishi S, Parvaneh N, Rosenzweig S, Barbouche R, Pedraza S, Lau YL, Ehlayel MS, Fieschi C, Abel L, Sanal O, Casanova JL. Revisiting human IL-12Rbeta1 deficiency: a survey of 141 patients from 30 countries. Medicine. 2010;89:381–402. doi: 10.1097/MD.0b013e3181fdd832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cruz A, Fraga AG, Fountain JJ, Rangel-Moreno J, Torrado E, Saraiva M, Pereira DR, Randall TD, Pedrosa J, Cooper AM, Castro AG. Pathological role of interleukin 17 in mice subjected to repeated BCG vaccination after infection with Mycobacterium tuberculosis. J Exp Med. 2010;207:1609–1616. doi: 10.1084/jem.20100265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Conti HR, Shen F, Nayyar N, Stocum E, Sun JN, Lindemann MJ, Ho AW, Hai JH, Yu JJ, Jung JW, Filler SG, Masso-Welch P, Edgerton M, Gaffen SL. Th17 cells and IL-17 receptor signaling are essential for mucosal host defense against oral candidiasis. J Exp Med. 2009;206:299–311. doi: 10.1084/jem.20081463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.de Beaucoudrey L, Samarina A, Bustamante J, Cobat A, Boisson-Dupuis S, Feinberg J, Al-Muhsen S, Janniere L, Rose Y, de Suremain M, Kong XF, Filipe-Santos O, Chapgier A, Picard C, Fischer A, Dogu F, Ikinciogullari A, Tanir G, Al-Hajjar S, Al-Jumaah S, Frayha HH, AlSum Z, Al-Ajaji S, Alangari A, Al-Ghonaium A, Adimi P, Mansouri D, Ben-Mustapha I, Yancoski J, Garty BZ, Rodriguez-Gallego C, Caragol I, Kutukculer N, Kumararatne DS, Patel S, Doffinger R, Exley A, Jeppsson O, Reichenbach J, Nadal D, Boyko Y, Pietrucha B, Anderson S, Levin M, Schandene L, Schepers K, Efira A, Mascart F, Matsuoka M, Sakai T, Siegrist CA, Frecerova K, Bluetters-Sawatzki R, Bernhoft J, Freihorst J, Baumann U, Richter D, Haerynck F, De Baets F, Novelli V, Lammas D, Vermylen C, Tuerlinckx D, Nieuwhof C, Pac M, Haas WH, Muller-Fleckenstein I, Fleckenstein B, Levy J, Raj R, Cohen AC, Lewis DB, Holland SM, Yang KD, Wang X, Wang X, Jiang L, Yang X, Zhu C, Xie Y, Lee PP, Chan KW, Chen TX, Castro G, Natera I, Codoceo A, King A, Bezrodnik L, Di Giovani D, Gaillard MI, de Moraes-Vasconcelos D, Grumach AS, da Silva Duarte AJ, Aldana R, Espinosa-Rosales FJ, Bejaoui M, Bousfiha AA, Baghdadi JE, Ozbek N, Aksu G, Keser M, Somer A, Hatipoglu N, Aydogmus C, Asilsoy S, Camcioglu Y, Gulle S, Ozgur TT, Ozen M, Oleastro M, Bernasconi A, Mamishi S, Parvaneh N, Rosenzweig S, Barbouche R, Pedraza S, Lau YL, Ehlayel MS, Fieschi C, Abel L, Sanal O, Casanova JL. Revisiting human IL-12Rbeta1 deficiency: a survey of 141 patients from 30 countries. Medicine (Baltimore) 2010;89:381–402. doi: 10.1097/MD.0b013e3181fdd832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Prando C, Samarina A, Bustamante J, Boisson-Dupuis S, Cobat A, Picard C, AlSum Z, Al-Jumaah S, Al-Hajjar S, Frayha H, Alangari A, Al-Mousa H, Mobaireek KF, Ben-Mustapha I, Adimi P, Feinberg J, de Suremain M, Janniere L, Filipe-Santos O, Mansouri N, Stephan JL, Nallusamy R, Kumararatne DS, Bloorsaz MR, Ben-Ali M, Elloumi-Zghal H, Chemli J, Bouguila J, Bejaoui M, Alaki E, AlFawaz TS, Al Idrissi E, ElGhazali G, Pollard AJ, Murugasu B, Wah Lee B, Halwani R, Al-Zahrani M, Al Shehri MA, Al-Zahrani M, Bin-Hussain I, Mahdaviani SA, Parvaneh N, Abel L, Mansouri D, Barbouche R, Al-Muhsen S, Casanova JL. Inherited IL-12p40 deficiency: genetic, immunologic, and clinical features of 49 patients from 30 kindreds. Medicine (Baltimore) 2013;92:109–122. doi: 10.1097/MD.0b013e31828a01f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Filipe-Santos O, Bustamante J, Chapgier A, Vogt G, de Beaucoudrey L, Feinberg J, Jouanguy E, Boisson-Dupuis S, Fieschi C, Picard C, Casanova JL. Inborn errors of IL-12/23- and IFN-gamma-mediated immunity: molecular, cellular, and clinical features. Semin Immunol. 2006;18:347–361. doi: 10.1016/j.smim.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 112.van de Veerdonk FL, Kullberg BJ, Verschueren IC, Hendriks T, van der Meer JW, Joosten LA, Netea MG. Differential effects of IL-17 pathway in disseminated candidiasis and zymosan-induced multiple organ failure. Shock. 2010;34:407–411. doi: 10.1097/SHK.0b013e3181d67041. [DOI] [PubMed] [Google Scholar]
- 113.Conti HR, Peterson AC, Brane L, Huppler AR, Hernandez-Santos N, Whibley N, Garg AV, Simpson-Abelson MR, Gibson GA, Mamo AJ, Osborne LC, Bishu S, Ghilardi N, Siebenlist U, Watkins SC, Artis D, McGeachy MJ, Gaffen SL. Oral-resident natural Th17 cells and gammadelta T cells control opportunistic Candida albicans infections. J Exp Med. 2014;211:2075–2084. doi: 10.1084/jem.20130877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hirota K, Duarte JH, Veldhoen M, Hornsby E, Li Y, Cua DJ, Ahlfors H, Wilhelm C, Tolaini M, Menzel U, Garefalaki A, Potocnik AJ, Stockinger B. Fate mapping of IL-17-producing T cells in inflammatory responses. Nat Immunol. 2011;12:255–263. doi: 10.1038/ni.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bonneville M, O’Brien RL, Born WK. Gammadelta T cell effector functions: a blend of innate programming and acquired plasticity. Nat Rev Immunol. 2010;10:467–478. doi: 10.1038/nri2781. [DOI] [PubMed] [Google Scholar]
- 116.Olive C. Expression of the T cell receptor delta-chain repertoire in mouse lymph node. Immunol Cell Biol. 1996;74:313–317. doi: 10.1038/icb.1996.56. [DOI] [PubMed] [Google Scholar]
- 117.Asarnow DM, Kuziel WA, Bonyhadi M, Tigelaar RE, Tucker PW, Allison JP. Limited diversity of gamma delta antigen receptor genes of Thy-1+ dendritic epidermal cells. Cell. 1988;55:837–847. doi: 10.1016/0092-8674(88)90139-0. [DOI] [PubMed] [Google Scholar]
- 118.O’Brien RL, Born WK. Dermal gammadelta T cells–What have we learned? Cell Immunol. 2015;296:62–69. doi: 10.1016/j.cellimm.2015.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Itohara S, Farr AG, Lafaille JJ, Bonneville M, Takagaki Y, Haas W, Tonegawa S. Homing of a gamma delta thymocyte subset with homogeneous T-cell receptors to mucosal epithelia. Nature. 1990;343:754–757. doi: 10.1038/343754a0. [DOI] [PubMed] [Google Scholar]
- 120.Tanaka Y, Morita CT, Tanaka Y, Nieves E, Brenner MB, Bloom BR. Natural and synthetic non-peptide antigens recognized by human gamma delta T cells. Nature. 1995;375:155–158. doi: 10.1038/375155a0. [DOI] [PubMed] [Google Scholar]
- 121.Hintz M, Reichenberg A, Altincicek B, Bahr U, Gschwind RM, Kollas AK, Beck E, Wiesner J, Eberl M, Jomaa H. Identification of (E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate as a major activator for human gammadelta T cells in Escherichia coli. FEBS Lett. 2001;509:317–322. doi: 10.1016/s0014-5793(01)03191-x. [DOI] [PubMed] [Google Scholar]
- 122.Bukowski JF, Morita CT, Brenner MB. Human gamma delta T cells recognize alkylamines derived from microbes, edible plants, and tea: implications for innate immunity. Immunity. 1999;11:57–65. doi: 10.1016/s1074-7613(00)80081-3. [DOI] [PubMed] [Google Scholar]
- 123.Constant P, Davodeau F, Peyrat MA, Poquet Y, Puzo G, Bonneville M, Fournie JJ. Stimulation of human gamma delta T cells by nonpeptidic mycobacterial ligands. Science. 1994;264:267–270. doi: 10.1126/science.8146660. [DOI] [PubMed] [Google Scholar]
- 124.O’Brien RL, Fu YX, Cranfill R, Dallas A, Ellis C, Reardon C, Lang J, Carding SR, Kubo R, Born W. Heat shock protein Hsp60-reactive gamma delta cells: a large, diversified T-lymphocyte subset with highly focused specificity. Proc Natl Acad Sci U S A. 1992;89:4348–4352. doi: 10.1073/pnas.89.10.4348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Poquet Y, Constant P, Halary F, Peyrat MA, Gilleron M, Davodeau F, Bonneville M, Fournie JJ. A novel nucleotide-containing antigen for human blood gamma delta T lymphocytes. Eur J Immunol. 1996;26:2344–2349. doi: 10.1002/eji.1830261011. [DOI] [PubMed] [Google Scholar]
- 126.Morita CT, Lee HK, Wang H, Li H, Mariuzza RA, Tanaka Y. Structural features of nonpeptide prenyl pyrophosphates that determine their antigenicity for human gamma delta T cells. J Immunol. 2001;167:36–41. doi: 10.4049/jimmunol.167.1.36. [DOI] [PubMed] [Google Scholar]
- 127.Born WK, Kemal Aydintug M, O’Brien RL. Diversity of gammadelta T-cell antigens. Cell Mol Immunol. 2013;10:13–20. doi: 10.1038/cmi.2012.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ladel CH, Blum C, Dreher A, Reifenberg K, Kaufmann SH. Protective role of gamma/delta T cells and alpha/beta T cells in tuberculosis. Eur J Immunol. 1995;25:2877–2881. doi: 10.1002/eji.1830251025. [DOI] [PubMed] [Google Scholar]
- 129.Mogues T, Goodrich ME, Ryan L, LaCourse R, North RJ. The relative importance of T cell subsets in immunity and immunopathology of airborne Mycobacterium tuberculosis infection in mice. J Exp Med. 2001;193:271–280. doi: 10.1084/jem.193.3.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.D’Souza CD, Cooper AM, Frank AA, Mazzaccaro RJ, Bloom BR, Orme IM. An anti-inflammatory role for gamma delta T lymphocytes in acquired immunity to Mycobacterium tuberculosis. J Immunol. 1997;158:1217–1221. [PubMed] [Google Scholar]
- 131.Rhoades ER, Frank AA, Orme IM. Progression of chronic pulmonary tuberculosis in mice aerogenically infected with virulent Mycobacterium tuberculosis. Tubercle and lung disease : the official journal of the International Union against Tuberculosis and Lung Disease. 1997;78:57–66. doi: 10.1016/s0962-8479(97)90016-2. [DOI] [PubMed] [Google Scholar]
- 132.Tateda K, Moore TA, Newstead MW, Tsai WC, Zeng X, Deng JC, Chen G, Reddy R, Yamaguchi K, Standiford TJ. Chemokine-dependent neutrophil recruitment in a murine model of Legionella pneumonia: potential role of neutrophils as immunoregulatory cells. Infection and immunity. 2001;69:2017–2024. doi: 10.1128/IAI.69.4.2017-2024.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Moore TA, Newstead MW, Strieter RM, Mehrad B, Beaman BL, Standiford TJ. Bacterial clearance and survival are dependent on CXC chemokine receptor-2 ligands in a murine model of pulmonary Nocardia asteroides infection. Journal of immunology. 2000;164:908–915. doi: 10.4049/jimmunol.164.2.908. [DOI] [PubMed] [Google Scholar]
- 134.Tsai WC, Strieter RM, Wilkowski JM, Bucknell KA, Burdick MD, Lira SA, Standiford TJ. Lung-specific transgenic expression of KC enhances resistance to Klebsiella pneumoniae in mice. Journal of immunology. 1998;161:2435–2440. [PubMed] [Google Scholar]
- 135.Trautwein-Weidner K, Gladiator A, Nur S, Diethelm P, LeibundGut-Landmann S. IL-17-mediated antifungal defense in the oral mucosa is independent of neutrophils. Mucosal Immunol. 2015;8:221–231. doi: 10.1038/mi.2014.57. [DOI] [PubMed] [Google Scholar]
- 136.Gomez MI, Prince A. Airway epithelial cell signaling in response to bacterial pathogens. Pediatr Pulmonol. 2008;43:11–19. doi: 10.1002/ppul.20735. [DOI] [PubMed] [Google Scholar]
- 137.Amulic B, Cazalet C, Hayes GL, Metzler KD, Zychlinsky A. Neutrophil function: from mechanisms to disease. Annual review of immunology. 2012;30:459–489. doi: 10.1146/annurev-immunol-020711-074942. [DOI] [PubMed] [Google Scholar]
- 138.Borregaard N. Neutrophils, from marrow to microbes. Immunity. 2010;33:657–670. doi: 10.1016/j.immuni.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 139.Soehnlein O, Lindbom L, Weber C. Mechanisms underlying neutrophil-mediated monocyte recruitment. Blood. 2009;114:4613–4623. doi: 10.1182/blood-2009-06-221630. [DOI] [PubMed] [Google Scholar]
- 140.Campbell EJ, Silverman EK, Campbell MA. Elastase and cathepsin G of human monocytes. Quantification of cellular content, release in response to stimuli, and heterogeneity in elastase-mediated proteolytic activity. Journal of immunology. 1989;143:2961–2968. [PubMed] [Google Scholar]
- 141.Huppler AR, Conti HR, Hernandez-Santos N, Darville T, Biswas PS, Gaffen SL. Role of neutrophils in IL-17-dependent immunity to mucosal candidiasis. J Immunol. 2014;192:1745–1752. doi: 10.4049/jimmunol.1302265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Basu S, Quilici C, Zhang HH, Grail D, Dunn AR. Mice lacking both G-CSF and IL-6 are more susceptible to Candida albicans infection: critical role of neutrophils in defense against Candida albicans. Growth Factors. 2008;26:23–34. doi: 10.1080/08977190801987513. [DOI] [PubMed] [Google Scholar]
- 143.Maertens J, Vrebos M, Boogaerts M. Assessing risk factors for systemic fungal infections. Eur J Cancer Care (Engl) 2001;10:56–62. doi: 10.1046/j.1365-2354.2001.00241.x. [DOI] [PubMed] [Google Scholar]
- 144.Qian Q, Jutila MA, Van Rooijen N, Cutler JE. Elimination of mouse splenic macrophages correlates with increased susceptibility to experimental disseminated candidiasis. J Immunol. 1994;152:5000–5008. [PubMed] [Google Scholar]
- 145.Cohen MS, Isturiz RE, Malech HL, Root RK, Wilfert CM, Gutman L, Buckley RH. Fungal infection in chronic granulomatous disease. The importance of the phagocyte in defense against fungi. Am J Med. 1981;71:59–66. doi: 10.1016/0002-9343(81)90259-x. [DOI] [PubMed] [Google Scholar]
- 146.Horvath R, Rozkova D, Lastovicka J, Polouckova A, Sedlacek P, Sediva A, Spisek R. Expansion of T helper type 17 lymphocytes in patients with chronic granulomatous disease. Clin Exp Immunol. 2011;166:26–33. doi: 10.1111/j.1365-2249.2011.04449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Lowe DM, Redford PS, Wilkinson RJ, O’Garra A, Martineau AR. Neutrophils in tuberculosis: friend or foe? Trends in immunology. 2012;33:14–25. doi: 10.1016/j.it.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 148.Blomgran R, Desvignes L, Briken V, Ernst JD. Mycobacterium tuberculosis inhibits neutrophil apoptosis, leading to delayed activation of naive CD4 T cells. Cell host & microbe. 2012;11:81–90. doi: 10.1016/j.chom.2011.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Plantinga TS, van der Velden WJ, Ferwerda B, van Spriel AB, Adema G, Feuth T, Donnelly JP, Brown GD, Kullberg BJ, Blijlevens NM, Netea MG. Early stop polymorphism in human DECTIN-1 is associated with increased candida colonization in hematopoietic stem cell transplant recipients. Clin Infect Dis. 2009;49:724–732. doi: 10.1086/604714. [DOI] [PubMed] [Google Scholar]
- 150.Ferwerda B, Ferwerda G, Plantinga TS, Willment JA, van Spriel AB, Venselaar H, Elbers CC, Johnson MD, Cambi A, Huysamen C, Jacobs L, Jansen T, Verheijen K, Masthoff L, Morre SA, Vriend G, Williams DL, Perfect JR, Joosten LA, Wijmenga C, van der Meer JW, Adema GJ, Kullberg BJ, Brown GD, Netea MG. Human dectin-1 deficiency and mucocutaneous fungal infections. N Engl J Med. 2009;361:1760–1767. doi: 10.1056/NEJMoa0901053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Jaeger M, van der Lee R, Cheng SC, Johnson MD, Kumar V, Ng A, Plantinga TS, Smeekens SP, Oosting M, Wang X, Barchet W, Fitzgerald K, Joosten LA, Perfect JR, Wijmenga C, van de Veerdonk FL, Huynen MA, Xavier RJ, Kullberg BJ, Netea MG. The RIG-I-like helicase receptor MDA5 (IFIH1) is involved in the host defense against Candida infections. Eur J Clin Microbiol Infect Dis. 2015;34:963–974. doi: 10.1007/s10096-014-2309-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Drewniak A, Gazendam RP, Tool AT, van Houdt M, Jansen MH, van Hamme JL, van Leeuwen EM, Roos D, Scalais E, de Beaufort C, Janssen H, van den Berg TK, Kuijpers TW. Invasive fungal infection and impaired neutrophil killing in human CARD9 deficiency. Blood. 2013;121:2385–2392. doi: 10.1182/blood-2012-08-450551. [DOI] [PubMed] [Google Scholar]
- 153.Nahum A, Dadi H, Bates A, Roifman CM. The L412F variant of Toll-like receptor 3 (TLR3) is associated with cutaneous candidiasis, increased susceptibility to cytomegalovirus, and autoimmunity. J Allergy Clin Immunol. 2011;127:528–531. doi: 10.1016/j.jaci.2010.09.031. [DOI] [PubMed] [Google Scholar]
- 154.Hambleton S, Salem S, Bustamante J, Bigley V, Boisson-Dupuis S, Azevedo J, Fortin A, Haniffa M, Ceron-Gutierrez L, Bacon CM, Menon G, Trouillet C, McDonald D, Carey P, Ginhoux F, Alsina L, Zumwalt TJ, Kong XF, Kumararatne D, Butler K, Hubeau M, Feinberg J, Al-Muhsen S, Cant A, Abel L, Chaussabel D, Doffinger R, Talesnik E, Grumach A, Duarte A, Abarca K, Moraes-Vasconcelos D, Burk D, Berghuis A, Geissmann F, Collin M, Casanova JL, Gros P. IRF8 mutations and human dendritic-cell immunodeficiency. N Engl J Med. 2011;365:127–138. doi: 10.1056/NEJMoa1100066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Vannberg FO, Chapman SJ, Khor CC, Tosh K, Floyd S, Jackson-Sillah D, Crampin A, Sichali L, Bah B, Gustafson P, Aaby P, McAdam KP, Bah-Sow O, Lienhardt C, Sirugo G, Fine P, Hill AV. CD209 genetic polymorphism and tuberculosis disease. PLoS One. 2008;3:e1388. doi: 10.1371/journal.pone.0001388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Soborg C, Madsen HO, Andersen AB, Lillebaek T, Kok-Jensen A, Garred P. Mannose-binding lectin polymorphisms in clinical tuberculosis. J Infect Dis. 2003;188:777–782. doi: 10.1086/377183. [DOI] [PubMed] [Google Scholar]
- 157.Yim JJ, Lee HW, Lee HS, Kim YW, Han SK, Shim YS, Holland SM. The association between microsatellite polymorphisms in intron II of the human Toll-like receptor 2 gene and tuberculosis among Koreans. Genes Immun. 2006;7:150–155. doi: 10.1038/sj.gene.6364274. [DOI] [PubMed] [Google Scholar]
- 158.Kang YA, Lee HW, Kim YW, Han SK, Shim YS, Yim JJ. Association between the −159C/T CD14 gene polymorphism and tuberculosis in a Korean population. FEMS Immunol Med Microbiol. 2009;57:229–235. doi: 10.1111/j.1574-695X.2009.00602.x. [DOI] [PubMed] [Google Scholar]
- 159.Sorensen GL, Husby S, Holmskov U. Surfactant protein A and surfactant protein D variation in pulmonary disease. Immunobiology. 2007;212:381–416. doi: 10.1016/j.imbio.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 160.Alter A, de Leseleuc L, Van Thuc N, Thai VH, Huong NT, Ba NN, Cardoso CC, Grant AV, Abel L, Moraes MO, Alcais A, Schurr E. Genetic and functional analysis of common MRC1 exon 7 polymorphisms in leprosy susceptibility. Hum Genet. 2010;127:337–348. doi: 10.1007/s00439-009-0775-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Khor CC, Chapman SJ, Vannberg FO, Dunne A, Murphy C, Ling EY, Frodsham AJ, Walley AJ, Kyrieleis O, Khan A, Aucan C, Segal S, Moore CE, Knox K, Campbell SJ, Lienhardt C, Scott A, Aaby P, Sow OY, Grignani RT, Sillah J, Sirugo G, Peshu N, Williams TN, Maitland K, Davies RJ, Kwiatkowski DP, Day NP, Yala D, Crook DW, Marsh K, Berkley JA, O’Neill LA, Hill AV. A Mal functional variant is associated with protection against invasive pneumococcal disease, bacteremia, malaria and tuberculosis. Nat Genet. 2007;39:523–528. doi: 10.1038/ng1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Salie M, Daya M, Lucas LA, Warren RM, van der Spuy GD, van Helden PD, Hoal EG, Moller M. Association of toll-like receptors with susceptibility to tuberculosis suggests sex-specific effects of TLR8 polymorphisms. Infect Genet Evol. 2015;34:221–229. doi: 10.1016/j.meegid.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 163.Fidel PL, Jr, Barousse M, Espinosa T, Ficarra M, Sturtevant J, Martin DH, Quayle AJ, Dunlap K. An intravaginal live Candida challenge in humans leads to new hypotheses for the immunopathogenesis of vulvovaginal candidiasis. Infect Immun. 2004;72:2939–2946. doi: 10.1128/IAI.72.5.2939-2946.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Romani L, Mencacci A, Cenci E, Del Sero G, Bistoni F, Puccetti P. An immunoregulatory role for neutrophils in CD4+ T helper subset selection in mice with candidiasis. J Immunol. 1997;158:2356–2362. [PubMed] [Google Scholar]
- 165.Werneck-Barroso E, Bonecini-de-Almeida MD, Vieira MA, Carvalho CE, Teixeira AK, Kritski AL, Ho JL. Preferential recruitment of phagocytes into the lung of patients with advanced acquired immunodeficiency syndrome and tuberculosis. Respiratory medicine. 2000;94:64–70. doi: 10.1053/rmed.1999.0669. [DOI] [PubMed] [Google Scholar]
- 166.Fischl MA, Daikos GL, Uttamchandani RB, Poblete RB, Moreno JN, Reyes RR, Boota AM, Thompson LM, Cleary TJ, Oldham SA, et al. Clinical presentation and outcome of patients with HIV infection and tuberculosis caused by multiple-drug-resistant bacilli. Annals of internal medicine. 1992;117:184–190. doi: 10.7326/0003-4819-117-3-184. [DOI] [PubMed] [Google Scholar]
- 167.Plaza V, Jimenez P, Xaubet A, Picado C, Torres A, Agusti C, Agusti-Vidal A. Bronchoalveolar lavage cell analysis in patients with human immunodeficiency virus related diseases. Thorax. 1989;44:289–291. doi: 10.1136/thx.44.4.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Condos R, Rom WN, Liu YM, Schluger NW. Local immune responses correlate with presentation and outcome in tuberculosis. American journal of respiratory and critical care medicine. 1998;157:729–735. doi: 10.1164/ajrccm.157.3.9705044. [DOI] [PubMed] [Google Scholar]
- 169.Jongeling AC, Pisapia D. Pearls and Oy-sters: tuberculous meningitis: not a diagnosis of exclusion. Neurology. 2013;80:e36–39. doi: 10.1212/WNL.0b013e31827f0832. [DOI] [PubMed] [Google Scholar]
- 170.Green JA, Tran CT, Farrar JJ, Nguyen MT, Nguyen PH, Dinh SX, Ho ND, Ly CV, Tran HT, Friedland JS, Thwaites GE. Dexamethasone, cerebrospinal fluid matrix metalloproteinase concentrations and clinical outcomes in tuberculous meningitis. PLoS One. 2009;4:e7277. doi: 10.1371/journal.pone.0007277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Torok ME, Chau TT, Mai PP, Phong ND, Dung NT, Chuong LV, Lee SJ, Caws M, de Jong MD, Hien TT, Farrar JJ. Clinical and microbiological features of HIV-associated tuberculous meningitis in Vietnamese adults. PLoS One. 2008;3:e1772. doi: 10.1371/journal.pone.0001772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Barnes PF, Leedom JM, Chan LS, Wong SF, Shah J, Vachon LA, Overturf GD, Modlin RL. Predictors of short-term prognosis in patients with pulmonary tuberculosis. The Journal of infectious diseases. 1988;158:366–371. doi: 10.1093/infdis/158.2.366. [DOI] [PubMed] [Google Scholar]
- 173.Berry MP, Graham CM, McNab FW, Xu Z, Bloch SA, Oni T, Wilkinson KA, Banchereau R, Skinner J, Wilkinson RJ, Quinn C, Blankenship D, Dhawan R, Cush JJ, Mejias A, Ramilo O, Kon OM, Pascual V, Banchereau J, Chaussabel D, O’Garra A. An interferon-inducible neutrophil-driven blood transcriptional signature in human tuberculosis. Nature. 2010;466:973–977. doi: 10.1038/nature09247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Keller C, Hoffmann R, Lang R, Brandau S, Hermann C, Ehlers S. Genetically determined susceptibility to tuberculosis in mice causally involves accelerated and enhanced recruitment of granulocytes. Infection and immunity. 2006;74:4295–4309. doi: 10.1128/IAI.00057-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Majorov KB, Eruslanov EB, Rubakova EI, Kondratieva TK, Apt AS. Analysis of cellular phenotypes that mediate genetic resistance to tuberculosis using a radiation bone marrow chimera approach. Infection and immunity. 2005;73:6174–6178. doi: 10.1128/IAI.73.9.6174-6178.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Eruslanov EB, Lyadova IV, Kondratieva TK, Majorov KB, Scheglov IV, Orlova MO, Apt AS. Neutrophil responses to Mycobacterium tuberculosis infection in genetically susceptible and resistant mice. Infection and immunity. 2005;73:1744–1753. doi: 10.1128/IAI.73.3.1744-1753.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Mitsos LM, Cardon LR, Fortin A, Ryan L, LaCourse R, North RJ, Gros P. Genetic control of susceptibility to infection with Mycobacterium tuberculosis in mice. Genes Immun. 2000;1:467–477. doi: 10.1038/sj.gene.6363712. [DOI] [PubMed] [Google Scholar]
- 178.Nandi B, Behar SM. Regulation of neutrophils by interferon-gamma limits lung inflammation during tuberculosis infection. The Journal of experimental medicine. 2011;208:2251–2262. doi: 10.1084/jem.20110919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Dorhoi A, Desel C, Yeremeev V, Pradl L, Brinkmann V, Mollenkopf HJ, Hanke K, Gross O, Ruland J, Kaufmann SH. The adaptor molecule CARD9 is essential for tuberculosis control. The Journal of experimental medicine. 2010;207:777–792. doi: 10.1084/jem.20090067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Achkar JM, Chan J, Casadevall A. B cells and antibodies in the defense against Mycobacterium tuberculosis infection. Immunol Rev. 2015;264:167–181. doi: 10.1111/imr.12276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Casadevall A, Pirofski LA. Immunoglobulins in defense, pathogenesis, and therapy of fungal diseases. Cell Host Microbe. 2012;11:447–456. doi: 10.1016/j.chom.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Grotzke JE, Siler AC, Lewinsohn DA, Lewinsohn DM. Secreted immunodominant Mycobacterium tuberculosis antigens are processed by the cytosolic pathway. J Immunol. 2010;185:4336–4343. doi: 10.4049/jimmunol.1000801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Booty MG, Nunes-Alves C, Carpenter SM, Jayaraman P, Behar SM. Multiple Inflammatory Cytokines Converge To Regulate CD8+ T Cell Expansion and Function during Tuberculosis. J Immunol. 2016;196:1822–1831. doi: 10.4049/jimmunol.1502206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Kamath AB, Woodworth J, Xiong X, Taylor C, Weng Y, Behar SM. Cytolytic CD8+ T cells recognizing CFP10 are recruited to the lung after Mycobacterium tuberculosis infection. J Exp Med. 2004;200:1479–1489. doi: 10.1084/jem.20041690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Woodworth JS, Wu Y, Behar SM. Mycobacterium tuberculosis-specific CD8+ T cells require perforin to kill target cells and provide protection in vivo. J Immunol. 2008;181:8595–8603. doi: 10.4049/jimmunol.181.12.8595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Lancioni C, Nyendak M, Kiguli S, Zalwango S, Mori T, Mayanja-Kizza H, Balyejusa S, Null M, Baseke J, Mulindwa D, Byrd L, Swarbrick G, Scott C, Johnson DF, Malone L, Mudido-Musoke P, Boom WH, Lewinsohn DM, Lewinsohn DA, Tuberculosis Research U CD8+ T cells provide an immunologic signature of tuberculosis in young children. Am J Respir Crit Care Med. 2012;185:206–212. doi: 10.1164/rccm.201107-1355OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Lewinsohn DA, Winata E, Swarbrick GM, Tanner KE, Cook MS, Null MD, Cansler ME, Sette A, Sidney J, Lewinsohn DM. Immunodominant tuberculosis CD8 antigens preferentially restricted by HLA-B. PLoS Pathog. 2007;3:1240–1249. doi: 10.1371/journal.ppat.0030127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Gold MC, Cerri S, Smyk-Pearson S, Cansler ME, Vogt TM, Delepine J, Winata E, Swarbrick GM, Chua WJ, Yu YY, Lantz O, Cook MS, Null MD, Jacoby DB, Harriff MJ, Lewinsohn DA, Hansen TH, Lewinsohn DM. Human mucosal associated invariant T cells detect bacterially infected cells. PLoS Biol. 2010;8:e1000407. doi: 10.1371/journal.pbio.1000407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Gold MC, Eid T, Smyk-Pearson S, Eberling Y, Swarbrick GM, Langley SM, Streeter PR, Lewinsohn DA, Lewinsohn DM. Human thymic MR1-restricted MAIT cells are innate pathogen-reactive effectors that adapt following thymic egress. Mucosal Immunol. 2013;6:35–44. doi: 10.1038/mi.2012.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Wong EB, Akilimali NA, Govender P, Sullivan ZA, Cosgrove C, Pillay M, Lewinsohn DM, Bishai WR, Walker BD, Ndung’u T, Klenerman P, Kasprowicz VO. Low levels of peripheral CD161++CD8+ mucosal associated invariant T (MAIT) cells are found in HIV and HIV/TB co-infection. PLoS One. 2013;8:e83474. doi: 10.1371/journal.pone.0083474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Hernandez-Santos N, Huppler AR, Peterson AC, Khader SA, McKenna KC, Gaffen SL. Th17 cells confer long-term adaptive immunity to oral mucosal Candida albicans infections. Mucosal Immunol. 2013;6:900–910. doi: 10.1038/mi.2012.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Chackerian A, Alt J, Perera V, Behar SM. Activation of NKT cells protects mice from tuberculosis. Infect Immun. 2002;70:6302–6309. doi: 10.1128/IAI.70.11.6302-6309.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Beckman EM, Porcelli SA, Morita CT, Behar SM, Furlong ST, Brenner MB. Recognition of a lipid antigen by CD1-restricted alpha beta+ T cells. Nature. 1994;372:691–694. doi: 10.1038/372691a0. [DOI] [PubMed] [Google Scholar]
- 194.Felio K, Nguyen H, Dascher CC, Choi HJ, Li S, Zimmer MI, Colmone A, Moody DB, Brenner MB, Wang CR. CD1-restricted adaptive immune responses to Mycobacteria in human group 1 CD1 transgenic mice. J Exp Med. 2009;206:2497–2509. doi: 10.1084/jem.20090898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Gansert JL, Kiessler V, Engele M, Wittke F, Rollinghoff M, Krensky AM, Porcelli SA, Modlin RL, Stenger S. Human NKT cells express granulysin and exhibit antimycobacterial activity. J Immunol. 2003;170:3154–3161. doi: 10.4049/jimmunol.170.6.3154. [DOI] [PubMed] [Google Scholar]
- 196.Kee SJ, Kwon YS, Park YW, Cho YN, Lee SJ, Kim TJ, Lee SS, Jang HC, Shin MG, Shin JH, Suh SP, Ryang DW. Dysfunction of natural killer T cells in patients with active Mycobacterium tuberculosis infection. Infect Immun. 2012;80:2100–2108. doi: 10.1128/IAI.06018-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Orme IM, Collins FM. Protection against Mycobacterium tuberculosis infection by adoptive immunotherapy. Requirement for T cell-deficient recipients. J Exp Med. 1983;158:74–83. doi: 10.1084/jem.158.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Orme IM, Collins FM. Crossprotection against nontuberculous mycobacterial infections by Mycobacterium tuberculosis memory immune T lymphocytes. J Exp Med. 1986;163:203–208. doi: 10.1084/jem.163.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Orme IM, Ordway DJ. Mouse and Guinea Pig Models of Tuberculosis. Microbiol Spectr. 2016;4 doi: 10.1128/microbiolspec.TBTB2-0002-2015. [DOI] [PubMed] [Google Scholar]
- 200.Nusbaum RJ, Calderon VE, Huante MB, Sutjita P, Vijayakumar S, Lancaster KL, Hunter RL, Actor JK, Cirillo JD, Aronson J, Gelman BB, Lisinicchia JG, Valbuena G, Endsley JJ. Pulmonary Tuberculosis in Humanized Mice Infected with HIV-1. Sci Rep. 2016;6:21522. doi: 10.1038/srep21522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.de Noronha AL, Bafica A, Nogueira L, Barral A, Barral-Netto M. Lung granulomas from Mycobacterium tuberculosis/HIV-1 co-infected patients display decreased in situ TNF production. Pathol Res Pract. 2008;204:155–161. doi: 10.1016/j.prp.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 202.Kisand K, Lilic D, Casanova JL, Peterson P, Meager A, Willcox N. Mucocutaneous candidiasis and autoimmunity against cytokines in APECED and thymoma patients: clinical and pathogenetic implications. Eur J Immunol. 2011;41:1517–1527. doi: 10.1002/eji.201041253. [DOI] [PubMed] [Google Scholar]
- 203.Ahonen P, Myllarniemi S, Sipila I, Perheentupa J. Clinical variation of autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy (APECED) in a series of 68 patients. N Engl J Med. 1990;322:1829–1836. doi: 10.1056/NEJM199006283222601. [DOI] [PubMed] [Google Scholar]
- 204.Klein RS, Harris CA, Small CB, Moll B, Lesser M, Friedland GH. Oral candidiasis in high-risk patients as the initial manifestation of the acquired immunodeficiency syndrome. N Engl J Med. 1984;311:354–358. doi: 10.1056/NEJM198408093110602. [DOI] [PubMed] [Google Scholar]
- 205.Kullberg BJ, Oude Lashof AM. Epidemiology of opportunistic invasive mycoses. Eur J Med Res. 2002;7:183–191. [PubMed] [Google Scholar]
- 206.Liu L, Okada S, Kong XF, Kreins AY, Cypowyj S, Abhyankar A, Toubiana J, Itan Y, Audry M, Nitschke P, Masson C, Toth B, Flatot J, Migaud M, Chrabieh M, Kochetkov T, Bolze A, Borghesi A, Toulon A, Hiller J, Eyerich S, Eyerich K, Gulacsy V, Chernyshova L, Chernyshov V, Bondarenko A, Grimaldo RM, Blancas-Galicia L, Beas IM, Roesler J, Magdorf K, Engelhard D, Thumerelle C, Burgel PR, Hoernes M, Drexel B, Seger R, Kusuma T, Jansson AF, Sawalle-Belohradsky J, Belohradsky B, Jouanguy E, Bustamante J, Bue M, Karin N, Wildbaum G, Bodemer C, Lortholary O, Fischer A, Blanche S, Al-Muhsen S, Reichenbach J, Kobayashi M, Rosales FE, Lozano CT, Kilic SS, Oleastro M, Etzioni A, Traidl-Hoffmann C, Renner ED, Abel L, Picard C, Marodi L, Boisson-Dupuis S, Puel A, Casanova JL. Gain-of-function human STAT1 mutations impair IL-17 immunity and underlie chronic mucocutaneous candidiasis. J Exp Med. 2011;208:1635–1648. doi: 10.1084/jem.20110958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.van de Veerdonk FL, Plantinga TS, Hoischen A, Smeekens SP, Joosten LA, Gilissen C, Arts P, Rosentul DC, Carmichael AJ, Smitsvan der Graaf CA, Kullberg BJ, van der Meer JW, Lilic D, Veltman JA, Netea MG. STAT1 mutations in autosomal dominant chronic mucocutaneous candidiasis. N Engl J Med. 2011;365:54–61. doi: 10.1056/NEJMoa1100102. [DOI] [PubMed] [Google Scholar]
- 208.Lazar-Molnar E, Chen B, Sweeney KA, Wang EJ, Liu W, Lin J, Porcelli SA, Almo SC, Nathenson SG, Jacobs WR., Jr Programmed death-1 (PD-1)-deficient mice are extraordinarily sensitive to tuberculosis. Proc Natl Acad Sci U S A. 2010;107:13402–13407. doi: 10.1073/pnas.1007394107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Lai RP, Meintjes G, Wilkinson RJ. HIV-1 tuberculosis-associated immune reconstitution inflammatory syndrome. Semin Immunopathol. 2016;38:185–198. doi: 10.1007/s00281-015-0532-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Chakrabarti LA, Boucherie C, Bugault F, Cumont MC, Roussillon C, Breton G, Patey O, Chene G, Richert L, Lortholary O, Anrs 129 Bkvir-Cytok Study G Biomarkers of CD4+ T-cell activation as risk factors for tuberculosis-associated immune reconstitution inflammatory syndrome. AIDS. 2014;28:1593–1602. doi: 10.1097/QAD.0000000000000311. [DOI] [PubMed] [Google Scholar]
- 211.Grant PM, Komarow L, Lederman MM, Pahwa S, Zolopa AR, Andersen J, Asmuth DM, Devaraj S, Pollard RB, Richterman A, Kanthikeel S, Sereti I. Elevated interleukin 8 and T-helper 1 and T-helper 17 cytokine levels prior to antiretroviral therapy in participants who developed immune reconstitution inflammatory syndrome during ACTG A5164. J Infect Dis. 2012;206:1715–1723. doi: 10.1093/infdis/jis604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Tadokera R, Wilkinson KA, Meintjes GA, Skolimowska KH, Matthews K, Seldon R, Rangaka MX, Maartens G, Wilkinson RJ. Role of the interleukin 10 family of cytokines in patients with immune reconstitution inflammatory syndrome associated with HIV infection and tuberculosis. J Infect Dis. 2013;207:1148–1156. doi: 10.1093/infdis/jit002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Devergne O, Hummel M, Koeppen H, Le Beau MM, Nathanson EC, Kieff E, Birkenbach M. A novel interleukin-12 p40-related protein induced by latent Epstein-Barr virus infection in B lymphocytes. J Virol. 1996;70:1143–1153. doi: 10.1128/jvi.70.2.1143-1153.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Pflanz S, Hibbert L, Mattson J, Rosales R, Vaisberg E, Bazan JF, Phillips JH, McClanahan TK, de Waal Malefyt R, Kastelein RA. WSX-1 and glycoprotein 130 constitute a signal-transducing receptor for IL-27. J Immunol. 2004;172:2225–2231. doi: 10.4049/jimmunol.172.4.2225. [DOI] [PubMed] [Google Scholar]
- 215.Sprecher CA, Grant FJ, Baumgartner JW, Presnell SR, Schrader SK, Yamagiwa T, Whitmore TE, O’Hara PJ, Foster DF. Cloning and characterization of a novel class I cytokine receptor. Biochem Biophys Res Commun. 1998;246:82–90. doi: 10.1006/bbrc.1998.8576. [DOI] [PubMed] [Google Scholar]
- 216.Pflanz S, Timans JC, Cheung J, Rosales R, Kanzler H, Gilbert J, Hibbert L, Churakova T, Travis M, Vaisberg E, Blumenschein WM, Mattson JD, Wagner JL, To W, Zurawski S, McClanahan TK, Gorman DM, Bazan JF, de Waal Malefyt R, Rennick D, Kastelein RA. IL-27, a heterodimeric cytokine composed of EBI3 and p28 protein, induces proliferation of naive CD4+ T cells. Immunity. 2002;16:779–790. doi: 10.1016/s1074-7613(02)00324-2. [DOI] [PubMed] [Google Scholar]
- 217.Hamano S, Himeno K, Miyazaki Y, Ishii K, Yamanaka A, Takeda A, Zhang M, Hisaeda H, Mak TW, Yoshimura A, Yoshida H. WSX-1 is required for resistance to Trypanosoma cruzi infection by regulation of proinflammatory cytokine production. Immunity. 2003;19:657–667. doi: 10.1016/s1074-7613(03)00298-x. [DOI] [PubMed] [Google Scholar]
- 218.Holscher C, Holscher A, Ruckerl D, Yoshimoto T, Yoshida H, Mak T, Saris C, Ehlers S. The IL-27 receptor chain WSX-1 differentially regulates antibacterial immunity and survival during experimental tuberculosis. J Immunol. 2005;174:3534–3544. doi: 10.4049/jimmunol.174.6.3534. [DOI] [PubMed] [Google Scholar]
- 219.Pearl JE, Khader SA, Solache A, Gilmartin L, Ghilardi N, deSauvage F, Cooper AM. IL-27 signaling compromises control of bacterial growth in mycobacteria-infected mice. J Immunol. 2004;173:7490–7496. doi: 10.4049/jimmunol.173.12.7490. [DOI] [PubMed] [Google Scholar]
- 220.Villarino A, Hibbert L, Lieberman L, Wilson E, Mak T, Yoshida H, Kastelein RA, Saris C, Hunter CA. The IL-27R (WSX-1) is required to suppress T cell hyperactivity during infection. Immunity. 2003;19:645–655. doi: 10.1016/s1074-7613(03)00300-5. [DOI] [PubMed] [Google Scholar]
- 221.Batten M, Li J, Yi S, Kljavin NM, Danilenko DM, Lucas S, Lee J, de Sauvage FJ, Ghilardi N. Interleukin 27 limits autoimmune encephalomyelitis by suppressing the development of interleukin 17-producing T cells. Nat Immunol. 2006;7:929–936. doi: 10.1038/ni1375. [DOI] [PubMed] [Google Scholar]
- 222.Stumhofer JS, Laurence A, Wilson EH, Huang E, Tato CM, Johnson LM, Villarino AV, Huang Q, Yoshimura A, Sehy D, Saris CJ, O’Shea JJ, Hennighausen L, Ernst M, Hunter CA. Interleukin 27 negatively regulates the development of interleukin 17-producing T helper cells during chronic inflammation of the central nervous system. Nat Immunol. 2006;7:937–945. doi: 10.1038/ni1376. [DOI] [PubMed] [Google Scholar]
- 223.Wirtz S, Tubbe I, Galle PR, Schild HJ, Birkenbach M, Blumberg RS, Neurath MF. Protection from lethal septic peritonitis by neutralizing the biological function of interleukin 27. J Exp Med. 2006;203:1875–1881. doi: 10.1084/jem.20060471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Awasthi A, Carrier Y, Peron JP, Bettelli E, Kamanaka M, Flavell RA, Kuchroo VK, Oukka M, Weiner HL. A dominant function for interleukin 27 in generating interleukin 10-producing anti-inflammatory T cells. Nat Immunol. 2007;8:1380–1389. doi: 10.1038/ni1541. [DOI] [PubMed] [Google Scholar]
- 225.Pot C, Apetoh L, Awasthi A, Kuchroo VK. Molecular pathways in the induction of interleukin-27-driven regulatory type 1 cells. J Interferon Cytokine Res. 2010;30:381–388. doi: 10.1089/jir.2010.0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Jung JY, Robinson CM. Interleukin-27 inhibits phagosomal acidification by blocking vacuolar ATPases. Cytokine. 2013;62:202–205. doi: 10.1016/j.cyto.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Kraft JD, Horzempa J, Davis C, Jung JY, Pena MM, Robinson CM. Neonatal macrophages express elevated levels of interleukin-27 that oppose immune responses. Immunology. 2013;139:484–493. doi: 10.1111/imm.12095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Robinson CM, Jung JY, Nau GJ. Interferon-gamma, tumor necrosis factor, and interleukin-18 cooperate to control growth of Mycobacterium tuberculosis in human macrophages. Cytokine. 2012;60:233–241. doi: 10.1016/j.cyto.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Robinson CM, Nau GJ. Interleukin-12 and interleukin-27 regulate macrophage control of Mycobacterium tuberculosis. J Infect Dis. 2008;198:359–366. doi: 10.1086/589774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Omogo B, Gao F, Bajwa P, Kaneko M, Heyes CD. Reducing Blinking in Small Core-Multishell Quantum Dots by Carefully Balancing Confinement Potential and Induced Lattice Strain: The “Goldilocks” Effect. ACS Nano. 2016;10:4072–4082. doi: 10.1021/acsnano.5b06994. [DOI] [PubMed] [Google Scholar]
- 231.Rosenblum EB, Sarver BA, Brown JW, Des Roches S, Hardwick KM, Hether TD, Eastman JM, Pennell MW, Harmon LJ. Goldilocks Meets Santa Rosalia: An Ephemeral Speciation Model Explains Patterns of Diversification Across Time Scales. Evol Biol. 2012;39:255–261. doi: 10.1007/s11692-012-9171-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Kidd C, Piantadosi ST, Aslin RN. The Goldilocks effect in infant auditory attention. Child Dev. 2014;85:1795–1804. doi: 10.1111/cdev.12263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Kidd C, Piantadosi ST, Aslin RN. The Goldilocks effect: human infants allocate attention to visual sequences that are neither too simple nor too complex. PLoS One. 2012;7:e36399. doi: 10.1371/journal.pone.0036399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Slansky JE, Jordan KR. The Goldilocks model for TCR-too much attraction might not be best for vaccine design. PLoS Biol. 2010;8 doi: 10.1371/journal.pbio.1000482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Goldenhar LM, Hecker S, Moir S, Rosecrance J. The “Goldilocks model” of overtime in construction: not too much, not too little, but just right. J Safety Res. 2003;34:215–226. doi: 10.1016/s0022-4375(03)00010-0. [DOI] [PubMed] [Google Scholar]
- 236.Dheda K, Barry CE, 3rd, Maartens G. Tuberculosis. Lancet. 2016;387:1211–1226. doi: 10.1016/S0140-6736(15)00151-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Trunz BB, Fine P, Dye C. Effect of BCG vaccination on childhood tuberculous meningitis and miliary tuberculosis worldwide: a meta-analysis and assessment of cost-effectiveness. Lancet. 2006;367:1173–1180. doi: 10.1016/S0140-6736(06)68507-3. [DOI] [PubMed] [Google Scholar]
- 238.Prasad K, Singh MB, Ryan H. Corticosteroids for managing tuberculous meningitis. Cochrane Database Syst Rev. 2016;4:CD002244. doi: 10.1002/14651858.CD002244.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Zumla A, Maeurer M, Host-Directed Therapies N. Chakaya J, Hoelscher M, Ntoumi F, Rustomjee R, Vilaplana C, Yeboah-Manu D, Rasolof V, Munderi P, Singh N, Aklillu E, Padayatchi N, Macete E, Kapata N, Mulenga M, Kibiki G, Mfinanga S, Nyirenda T, Maboko L, Garcia-Basteiro A, Rakotosamimanana N, Bates M, Mwaba P, Reither K, Gagneux S, Edwards S, Mfinanga E, Abdulla S, Cardona PJ, Russell JB, Gant V, Noursadeghi M, Elkington P, Bonnet M, Menendez C, Dieye TN, Diarra B, Maiga A, Aseffa A, Parida S, Wejse C, Petersen E, Kaleebu P, Oliver M, Craig G, Corrah T, Tientcheu L, Antonio M, Rao M, McHugh TD, Sheikh A, Ippolito G, Ramjee G, Kaufmann SH, Churchyard G, Steyn A, Grobusch M, Sanne I, Martinson N, Madansein R, Wilkinson RJ, Mayosi B, Schito M, Wallis RS. Towards host-directed therapies for tuberculosis. Nat Rev Drug Discov. 2015;14:511–512. doi: 10.1038/nrd4696. [DOI] [PubMed] [Google Scholar]
- 240.Ohmit SE, Sobel JD, Schuman P, Duerr A, Mayer K, Rompalo A, Klein RS, Group, H. I. V. E. R. S. Longitudinal study of mucosal Candida species colonization and candidiasis among human immunodeficiency virus (HIV)-seropositive and at-risk HIV-seronegative women. J Infect Dis. 2003;188:118–127. doi: 10.1086/375746. [DOI] [PubMed] [Google Scholar]
- 241.Fekete A, Emri T, Gyetvai A, Gazdag Z, Pesti M, Varga Z, Balla J, Cserhati C, Emody L, Gergely L, Pocsi I. Development of oxidative stress tolerance resulted in reduced ability to undergo morphologic transitions and decreased pathogenicity in a t-butylhydroperoxide-tolerant mutant of Candida albicans. FEMS Yeast Res. 2007;7:834–847. doi: 10.1111/j.1567-1364.2007.00244.x. [DOI] [PubMed] [Google Scholar]
- 242.Schoonmaker MK, Bishai WR, Lamichhane G. Nonclassical transpeptidases of Mycobacterium tuberculosis alter cell size, morphology, the cytosolic matrix, protein localization, virulence, and resistance to beta-lactams. J Bacteriol. 2014;196:1394–1402. doi: 10.1128/JB.01396-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Ouellet H, Johnston JB, de Montellano PR. Cholesterol catabolism as a therapeutic target in Mycobacterium tuberculosis. Trends Microbiol. 2011;19:530–539. doi: 10.1016/j.tim.2011.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Hett EC, Rubin EJ. Bacterial growth and cell division: a mycobacterial perspective. Microbiol Mol Biol Rev. 2008;72:126–156. doi: 10.1128/MMBR.00028-07. table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Brightbill HD, Libraty DH, Krutzik SR, Yang RB, Belisle JT, Bleharski JR, Maitland M, Norgard MV, Plevy SE, Smale ST, Brennan PJ, Bloom BR, Godowski PJ, Modlin RL. Host defense mechanisms triggered by microbial lipoproteins through toll-like receptors. Science. 1999;285:732–736. doi: 10.1126/science.285.5428.732. [DOI] [PubMed] [Google Scholar]
- 246.Gilleron M, Nigou J, Nicolle D, Quesniaux V, Puzo G. The acylation state of mycobacterial lipomannans modulates innate immunity response through toll-like receptor 2. Chem Biol. 2006;13:39–47. doi: 10.1016/j.chembiol.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 247.Jones BW, Means TK, Heldwein KA, Keen MA, Hill PJ, Belisle JT, Fenton MJ. Different Toll-like receptor agonists induce distinct macrophage responses. J Leukoc Biol. 2001;69:1036–1044. [PubMed] [Google Scholar]
- 248.Bulut Y, Michelsen KS, Hayrapetian L, Naiki Y, Spallek R, Singh M, Arditi M. Mycobacterium tuberculosis heat shock proteins use diverse Toll-like receptor pathways to activate pro-inflammatory signals. J Biol Chem. 2005;280:20961–20967. doi: 10.1074/jbc.M411379200. [DOI] [PubMed] [Google Scholar]
- 249.Marinho FA, de Paula RR, Mendes AC, de Almeida LA, Gomes MT, Carvalho NB, Oliveira FS, Caliari MV, Oliveira SC. Toll-like receptor 6 senses Mycobacterium avium and is required for efficient control of mycobacterial infection. Eur J Immunol. 2013;43:2373–2385. doi: 10.1002/eji.201243208. [DOI] [PubMed] [Google Scholar]
- 250.Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, Matsumoto M, Hoshino K, Wagner H, Takeda K, Akira S. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740–745. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 251.Coulombe F, Divangahi M, Veyrier F, de Leseleuc L, Gleason JL, Yang Y, Kelliher MA, Pandey AK, Sassetti CM, Reed MB, Behr MA. Increased NOD2-mediated recognition of N-glycolyl muramyl dipeptide. J Exp Med. 2009;206:1709–1716. doi: 10.1084/jem.20081779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Ishikawa E, Ishikawa T, Morita YS, Toyonaga K, Yamada H, Takeuchi O, Kinoshita T, Akira S, Yoshikai Y, Yamasaki S. Direct recognition of the mycobacterial glycolipid, trehalose dimycolate, by C-type lectin Mincle. J Exp Med. 2009;206:2879–2888. doi: 10.1084/jem.20091750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Bowdish DM, Sakamoto K, Kim MJ, Kroos M, Mukhopadhyay S, Leifer CA, Tryggvason K, Gordon S, Russell DG. MARCO, TLR2, and CD14 are required for macrophage cytokine responses to mycobacterial trehalose dimycolate and Mycobacterium tuberculosis. PLoS Pathog. 2009;5:e1000474. doi: 10.1371/journal.ppat.1000474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Yonekawa A, Saijo S, Hoshino Y, Miyake Y, Ishikawa E, Suzukawa M, Inoue H, Tanaka M, Yoneyama M, Oh-Hora M, Akashi K, Yamasaki S. Dectin-2 is a direct receptor for mannose-capped lipoarabinomannan of mycobacteria. Immunity. 2014;41:402–413. doi: 10.1016/j.immuni.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 255.Zhao XQ, Zhu LL, Chang Q, Jiang C, You Y, Luo T, Jia XM, Lin X. C-type lectin receptor dectin-3 mediates trehalose 6,6′-dimycolate (TDM)-induced Mincle expression through CARD9/Bcl10/MALT1-dependent nuclear factor (NF)-kappaB activation. J Biol Chem. 2014;289:30052–30062. doi: 10.1074/jbc.M114.588574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Torrelles JB, Azad AK, Schlesinger LS. Fine discrimination in the recognition of individual species of phosphatidyl-myo-inositol mannosides from Mycobacterium tuberculosis by C-type lectin pattern recognition receptors. J Immunol. 2006;177:1805–1816. doi: 10.4049/jimmunol.177.3.1805. [DOI] [PubMed] [Google Scholar]
- 257.Schlesinger LS, Kaufman TM, Iyer S, Hull SR, Marchiando LK. Differences in mannose receptor-mediated uptake of lipoarabinomannan from virulent and attenuated strains of Mycobacterium tuberculosis by human macrophages. J Immunol. 1996;157:4568–4575. [PubMed] [Google Scholar]
- 258.Arbues A, Malaga W, Constant P, Guilhot C, Prandi J, Astarie-Dequeker C. Trisaccharides of Phenolic Glycolipids Confer Advantages to Pathogenic Mycobacteria through Manipulation of Host-Cell Pattern-Recognition Receptors. ACS Chem Biol. 2016;11:2865–2875. doi: 10.1021/acschembio.6b00568. [DOI] [PubMed] [Google Scholar]
- 259.Ferguson JS, Weis JJ, Martin JL, Schlesinger LS. Complement protein C3 binding to Mycobacterium tuberculosis is initiated by the classical pathway in human bronchoalveolar lavage fluid. Infect Immun. 2004;72:2564–2573. doi: 10.1128/IAI.72.5.2564-2573.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Carroll MV, Sim RB, Bigi F, Jakel A, Antrobus R, Mitchell DA. Identification of four novel DC-SIGN ligands on Mycobacterium bovis BCG. Protein Cell. 2010;1:859–870. doi: 10.1007/s13238-010-0101-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Wu T, Guo S, Wang J, Li L, Xu L, Liu P, Ma S, Zhang J, Xu L, Luo Y. Interaction between mannosylated lipoarabinomannan and dendritic cell-specific intercellular adhesion molecule-3 grabbing nonintegrin influences dendritic cells maturation and T cell immunity. Cell Immunol. 2011;272:94–101. doi: 10.1016/j.cellimm.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 262.Barboni E, Coade S, Fiori A. The binding of mycolic acids to galectin-3: a novel interaction between a host soluble lectin and trafficking mycobacterial lipids? FEBS Lett. 2005;579:6749–6755. doi: 10.1016/j.febslet.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 263.Beatty WL, Rhoades ER, Hsu DK, Liu FT, Russell DG. Association of a macrophage galactoside-binding protein with Mycobacterium-containing phagosomes. Cell Microbiol. 2002;4:167–176. doi: 10.1046/j.1462-5822.2002.00183.x. [DOI] [PubMed] [Google Scholar]
- 264.Drage MG, Pecora ND, Hise AG, Febbraio M, Silverstein RL, Golenbock DT, Boom WH, Harding CV. TLR2 and its co-receptors determine responses of macrophages and dendritic cells to lipoproteins of Mycobacterium tuberculosis. Cell Immunol. 2009;258:29–37. doi: 10.1016/j.cellimm.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Bartlomiejczyk MA, Swierzko AS, Brzostek A, Dziadek J, Cedzynski M. Interaction of lectin pathway of complement-activating pattern recognition molecules with mycobacteria. Clin Exp Immunol. 2014;178:310–319. doi: 10.1111/cei.12416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Yadav M, Schorey JS. The beta-glucan receptor dectin-1 functions together with TLR2 to mediate macrophage activation by mycobacteria. Blood. 2006;108:3168–3175. doi: 10.1182/blood-2006-05-024406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Wong KW, Jacobs WR., Jr Critical role for NLRP3 in necrotic death triggered by Mycobacterium tuberculosis. Cell Microbiol. 2011;13:1371–1384. doi: 10.1111/j.1462-5822.2011.01625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Jouault T, Ibata-Ombetta S, Takeuchi O, Trinel PA, Sacchetti P, Lefebvre P, Akira S, Poulain D. Candida albicans phospholipomannan is sensed through toll-like receptors. J Infect Dis. 2003;188:165–172. doi: 10.1086/375784. [DOI] [PubMed] [Google Scholar]
- 269.Netea MG, Gow NA, Munro CA, Bates S, Collins C, Ferwerda G, Hobson RP, Bertram G, Hughes HB, Jansen T, Jacobs L, Buurman ET, Gijzen K, Williams DL, Torensma R, McKinnon A, MacCallum DM, Odds FC, Van der Meer JW, Brown AJ, Kullberg BJ. Immune sensing of Candida albicans requires cooperative recognition of mannans and glucans by lectin and Toll-like receptors. J Clin Invest. 2006;116:1642–1650. doi: 10.1172/JCI27114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Tada H, Nemoto E, Shimauchi H, Watanabe T, Mikami T, Matsumoto T, Ohno N, Tamura H, Shibata K, Akashi S, Miyake K, Sugawara S, Takada H. Saccharomyces cerevisiae- and Candida albicans-derived mannan induced production of tumor necrosis factor alpha by human monocytes in a CD14- and Toll-like receptor 4-dependent manner. Microbiol Immunol. 2002;46:503–512. doi: 10.1111/j.1348-0421.2002.tb02727.x. [DOI] [PubMed] [Google Scholar]
- 271.Brown GD, Taylor PR, Reid DM, Willment JA, Williams DL, Martinez-Pomares L, Wong SY, Gordon S. Dectin-1 is a major beta-glucan receptor on macrophages. J Exp Med. 2002;196:407–412. doi: 10.1084/jem.20020470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.McGreal EP, Rosas M, Brown GD, Zamze S, Wong SY, Gordon S, Martinez-Pomares L, Taylor PR. The carbohydrate-recognition domain of Dectin-2 is a C-type lectin with specificity for high mannose. Glycobiology. 2006;16:422–430. doi: 10.1093/glycob/cwj077. [DOI] [PubMed] [Google Scholar]
- 273.Saijo S, Ikeda S, Yamabe K, Kakuta S, Ishigame H, Akitsu A, Fujikado N, Kusaka T, Kubo S, Chung SH, Komatsu R, Miura N, Adachi Y, Ohno N, Shibuya K, Yamamoto N, Kawakami K, Yamasaki S, Saito T, Akira S, Iwakura Y. Dectin-2 recognition of alpha-mannans and induction of Th17 cell differentiation is essential for host defense against Candida albicans. Immunity. 2010;32:681–691. doi: 10.1016/j.immuni.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 274.Cambi A, Netea MG, Mora-Montes HM, Gow NA, Hato SV, Lowman DW, Kullberg BJ, Torensma R, Williams DL, Figdor CG. Dendritic cell interaction with Candida albicans critically depends on N-linked mannan. J Biol Chem. 2008;283:20590–20599. doi: 10.1074/jbc.M709334200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Jouault T, El Abed-El Behi M, Martinez-Esparza M, Breuilh L, Trinel PA, Chamaillard M, Trottein F, Poulain D. Specific recognition of Candida albicans by macrophages requires galectin-3 to discriminate Saccharomyces cerevisiae and needs association with TLR2 for signaling. J Immunol. 2006;177:4679–4687. doi: 10.4049/jimmunol.177.7.4679. [DOI] [PubMed] [Google Scholar]
- 276.Bugarcic A, Hitchens K, Beckhouse AG, Wells CA, Ashman RB, Blanchard H. Human and mouse macrophage-inducible C-type lectin (Mincle) bind Candida albicans. Glycobiology. 2008;18:679–685. doi: 10.1093/glycob/cwn046. [DOI] [PubMed] [Google Scholar]
- 277.Means TK, Mylonakis E, Tampakakis E, Colvin RA, Seung E, Puckett L, Tai MF, Stewart CR, Pukkila-Worley R, Hickman SE, Moore KJ, Calderwood SB, Hacohen N, Luster AD, El Khoury J. Evolutionarily conserved recognition and innate immunity to fungal pathogens by the scavenger receptors SCARF1 and CD36. J Exp Med. 2009;206:637–653. doi: 10.1084/jem.20082109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Brouwer N, Dolman KM, van Houdt M, Sta M, Roos D, Kuijpers TW. Mannose-binding lectin (MBL) facilitates opsonophagocytosis of yeasts but not of bacteria despite MBL binding. J Immunol. 2008;180:4124–4132. doi: 10.4049/jimmunol.180.6.4124. [DOI] [PubMed] [Google Scholar]
- 279.van Bruggen R, Drewniak A, Jansen M, van Houdt M, Roos D, Chapel H, Verhoeven AJ, Kuijpers TW. Complement receptor 3, not Dectin-1, is the major receptor on human neutrophils for beta-glucan-bearing particles. Mol Immunol. 2009;47:575–581. doi: 10.1016/j.molimm.2009.09.018. [DOI] [PubMed] [Google Scholar]
- 280.Wang T, Pan D, Zhou Z, You Y, Jiang C, Zhao X, Lin X. Dectin-3 Deficiency Promotes Colitis Development due to Impaired Antifungal Innate Immune Responses in the Gut. PLoS Pathog. 2016;12:e1005662. doi: 10.1371/journal.ppat.1005662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Miyazato A, Nakamura K, Yamamoto N, Mora-Montes HM, Tanaka M, Abe Y, Tanno D, Inden K, Gang X, Ishii K, Takeda K, Akira S, Saijo S, Iwakura Y, Adachi Y, Ohno N, Mitsutake K, Gow NA, Kaku M, Kawakami K. Toll-like receptor 9-dependent activation of myeloid dendritic cells by Deoxynucleic acids from Candida albicans. Infect Immun. 2009;77:3056–3064. doi: 10.1128/IAI.00840-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Wagener J, Malireddi RK, Lenardon MD, Koberle M, Vautier S, MacCallum DM, Biedermann T, Schaller M, Netea MG, Kanneganti TD, Brown GD, Brown AJ, Gow NA. Fungal chitin dampens inflammation through IL-10 induction mediated by NOD2 and TLR9 activation. PLoS Pathog. 2014;10:e1004050. doi: 10.1371/journal.ppat.1004050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Kumar H, Kumagai Y, Tsuchida T, Koenig PA, Satoh T, Guo Z, Jang MH, Saitoh T, Akira S, Kawai T. Involvement of the NLRP3 inflammasome in innate and humoral adaptive immune responses to fungal beta-glucan. J Immunol. 2009;183:8061–8067. doi: 10.4049/jimmunol.0902477. [DOI] [PubMed] [Google Scholar]
- 284.Babula O, Lazdane G, Kroica J, Linhares IM, Ledger WJ, Witkin SS. Frequency of interleukin-4 (IL-4) -589 gene polymorphism and vaginal concentrations of IL-4, nitric oxide, and mannose-binding lectin in women with recurrent vulvovaginal candidiasis. Clin Infect Dis. 2005;40:1258–1262. doi: 10.1086/429246. [DOI] [PubMed] [Google Scholar]
- 285.Boisson B, Wang C, Pedergnana V, Wu L, Cypowyj S, Rybojad M, Belkadi A, Picard C, Abel L, Fieschi C, Puel A, Li X, Casanova JL. An ACT1 mutation selectively abolishes interleukin-17 responses in humans with chronic mucocutaneous candidiasis. Immunity. 2013;39:676–686. doi: 10.1016/j.immuni.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.De Luca A, Carvalho A, Cunha C, Iannitti RG, Pitzurra L, Giovannini G, Mencacci A, Bartolommei L, Moretti S, Massi-Benedetti C, Fuchs D, De Bernardis F, Puccetti P, Romani L. IL-22 and IDO1 affect immunity and tolerance to murine and human vaginal candidiasis. PLoS Pathog. 2013;9:e1003486. doi: 10.1371/journal.ppat.1003486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Glocker EO, Hennigs A, Nabavi M, Schaffer AA, Woellner C, Salzer U, Pfeifer D, Veelken H, Warnatz K, Tahami F, Jamal S, Manguiat A, Rezaei N, Amirzargar AA, Plebani A, Hannesschlager N, Gross O, Ruland J, Grimbacher B. A homozygous CARD9 mutation in a family with susceptibility to fungal infections. N Engl J Med. 2009;361:1727–1735. doi: 10.1056/NEJMoa0810719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Jurevic RJ, Bai M, Chadwick RB, White TC, Dale BA. Single-nucleotide polymorphisms (SNPs) in human beta-defensin 1: high-throughput SNP assays and association with Candida carriage in type I diabetics and nondiabetic controls. J Clin Microbiol. 2003;41:90–96. doi: 10.1128/JCM.41.1.90-96.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Kumar V, Cheng SC, Johnson MD, Smeekens SP, Wojtowicz A, Giamarellos-Bourboulis E, Karjalainen J, Franke L, Withoff S, Plantinga TS, van de Veerdonk FL, van der Meer JW, Joosten LA, Sokol H, Bauer H, Herrmann BG, Bochud PY, Marchetti O, Perfect JR, Xavier RJ, Kullberg BJ, Wijmenga C, Netea MG. Immunochip SNP array identifies novel genetic variants conferring susceptibility to candidaemia. Nat Commun. 2014;5:4675. doi: 10.1038/ncomms5675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Lev-Sagie A, Prus D, Linhares IM, Lavy Y, Ledger WJ, Witkin SS. Polymorphism in a gene coding for the inflammasome component NALP3 and recurrent vulvovaginal candidiasis in women with vulvar vestibulitis syndrome. Am J Obstet Gynecol. 2009;200(303):e301–306. doi: 10.1016/j.ajog.2008.10.039. [DOI] [PubMed] [Google Scholar]
- 291.Levy R, Okada S, Beziat V, Moriya K, Liu C, Chai LY, Migaud M, Hauck F, Al Ali A, Cyrus C, Vatte C, Patiroglu T, Unal E, Ferneiny M, Hyakuna N, Nepesov S, Oleastro M, Ikinciogullari A, Dogu F, Asano T, Ohara O, Yun L, Della Mina E, Bronnimann D, Itan Y, Gothe F, Bustamante J, Boisson-Dupuis S, Tahuil N, Aytekin C, Salhi A, Al Muhsen S, Kobayashi M, Toubiana J, Abel L, Li X, Camcioglu Y, Celmeli F, Klein C, AlKhater SA, Casanova JL, Puel A. Genetic, immunological, and clinical features of patients with bacterial and fungal infections due to inherited IL-17RA deficiency. Proc Natl Acad Sci U S A. 2016;113:E8277–E8285. doi: 10.1073/pnas.1618300114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Ling Y, Cypowyj S, Aytekin C, Galicchio M, Camcioglu Y, Nepesov S, Ikinciogullari A, Dogu F, Belkadi A, Levy R, Migaud M, Boisson B, Bolze A, Itan Y, Goudin N, Cottineau J, Picard C, Abel L, Bustamante J, Casanova JL, Puel A. Inherited IL-17RC deficiency in patients with chronic mucocutaneous candidiasis. J Exp Med. 2015;212:619–631. doi: 10.1084/jem.20141065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Milner JD, Brenchley JM, Laurence A, Freeman AF, Hill BJ, Elias KM, Kanno Y, Spalding C, Elloumi HZ, Paulson ML, Davis J, Hsu A, Asher AI, O’Shea J, Holland SM, Paul WE, Douek DC. Impaired T(H)17 cell differentiation in subjects with autosomal dominant hyper-IgE syndrome. Nature. 2008;452:773–776. doi: 10.1038/nature06764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Nahum A, Bates A, Sharfe N, Roifman CM. Association of the lymphoid protein tyrosine phosphatase, R620W variant, with chronic mucocutaneous candidiasis. J Allergy Clin Immunol. 2008;122:1220–1222. doi: 10.1016/j.jaci.2008.10.027. [DOI] [PubMed] [Google Scholar]
- 295.Plantinga TS, Johnson MD, Scott WK, van de Vosse E, Velez Edwards DR, Smith PB, Alexander BD, Yang JC, Kremer D, Laird GM, Oosting M, Joosten LA, van der Meer JW, van Dissel JT, Walsh TJ, Perfect JR, Kullberg BJ, Netea MG. Toll-like receptor 1 polymorphisms increase susceptibility to candidemia. J Infect Dis. 2012;205:934–943. doi: 10.1093/infdis/jir867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Puel A, Cypowyj S, Bustamante J, Wright JF, Liu L, Lim HK, Migaud M, Israel L, Chrabieh M, Audry M, Gumbleton M, Toulon A, Bodemer C, El-Baghdadi J, Whitters M, Paradis T, Brooks J, Collins M, Wolfman NM, Al-Muhsen S, Galicchio M, Abel L, Picard C, Casanova JL. Chronic mucocutaneous candidiasis in humans with inborn errors of interleukin-17 immunity. Science. 2011;332:65–68. doi: 10.1126/science.1200439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Puel A, Doffinger R, Natividad A, Chrabieh M, Barcenas-Morales G, Picard C, Cobat A, Ouachee-Chardin M, Toulon A, Bustamante J, Al-Muhsen S, Al-Owain M, Arkwright PD, Costigan C, McConnell V, Cant AJ, Abinun M, Polak M, Bougneres PF, Kumararatne D, Marodi L, Nahum A, Roifman C, Blanche S, Fischer A, Bodemer C, Abel L, Lilic D, Casanova JL. Autoantibodies against IL-17A, IL-17F, and IL-22 in patients with chronic mucocutaneous candidiasis and autoimmune polyendocrine syndrome type I. J Exp Med. 2010;207:291–297. doi: 10.1084/jem.20091983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Van der Graaf CA, Netea MG, Morre SA, Den Heijer M, Verweij PE, Van der Meer JW, Kullberg BJ. Toll-like receptor 4 Asp299Gly/Thr399Ile polymorphisms are a risk factor for Candida bloodstream infection. Eur Cytokine Netw. 2006;17:29–34. [PubMed] [Google Scholar]
- 299.Barreiro LB, Neyrolles O, Babb CL, Tailleux L, Quach H, McElreavey K, Helden PD, Hoal EG, Gicquel B, Quintana-Murci L. Promoter variation in the DC-SIGN-encoding gene CD209 is associated with tuberculosis. PLoS Med. 2006;3:e20. doi: 10.1371/journal.pmed.0030020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Bogunovic D, Byun M, Durfee LA, Abhyankar A, Sanal O, Mansouri D, Salem S, Radovanovic I, Grant AV, Adimi P, Mansouri N, Okada S, Bryant VL, Kong XF, Kreins A, Velez MM, Boisson B, Khalilzadeh S, Ozcelik U, Darazam IA, Schoggins JW, Rice CM, Al-Muhsen S, Behr M, Vogt G, Puel A, Bustamante J, Gros P, Huibregtse JM, Abel L, Boisson-Dupuis S, Casanova JL. Mycobacterial disease and impaired IFN-gamma immunity in humans with inherited ISG15 deficiency. Science. 2012;337:1684–1688. doi: 10.1126/science.1224026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Bustamante J, Arias AA, Vogt G, Picard C, Galicia LB, Prando C, Grant AV, Marchal CC, Hubeau M, Chapgier A, de Beaucoudrey L, Puel A, Feinberg J, Valinetz E, Janniere L, Besse C, Boland A, Brisseau JM, Blanche S, Lortholary O, Fieschi C, Emile JF, Boisson-Dupuis S, Al-Muhsen S, Woda B, Newburger PE, Condino-Neto A, Dinauer MC, Abel L, Casanova JL. Germline CYBB mutations that selectively affect macrophages in kindreds with X-linked predisposition to tuberculous mycobacterial disease. Nat Immunol. 2011;12:213–221. doi: 10.1038/ni.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Correa PA, Gomez LM, Cadena J, Anaya JM. Autoimmunity and tuberculosis. Opposite association with TNF polymorphism. J Rheumatol. 2005;32:219–224. [PubMed] [Google Scholar]
- 303.Dorman SE, Holland SM. Mutation in the signal-transducing chain of the interferon-gamma receptor and susceptibility to mycobacterial infection. J Clin Invest. 1998;101:2364–2369. doi: 10.1172/JCI2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Filipe-Santos O, Bustamante J, Haverkamp MH, Vinolo E, Ku CL, Puel A, Frucht DM, Christel K, von Bernuth H, Jouanguy E, Feinberg J, Durandy A, Senechal B, Chapgier A, Vogt G, de Beaucoudrey L, Fieschi C, Picard C, Garfa M, Chemli J, Bejaoui M, Tsolia MN, Kutukculer N, Plebani A, Notarangelo L, Bodemer C, Geissmann F, Israel A, Veron M, Knackstedt M, Barbouche R, Abel L, Magdorf K, Gendrel D, Agou F, Holland SM, Casanova JL. X-linked susceptibility to mycobacteria is caused by mutations in NEMO impairing CD40-dependent IL-12 production. J Exp Med. 2006;203:1745–1759. doi: 10.1084/jem.20060085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Floros J, Lin HM, Garcia A, Salazar MA, Guo X, DiAngelo S, Montano M, Luo J, Pardo A, Selman M. Surfactant protein genetic marker alleles identify a subgroup of tuberculosis in a Mexican population. J Infect Dis. 2000;182:1473–1478. doi: 10.1086/315866. [DOI] [PubMed] [Google Scholar]
- 306.Ganachari M, Ruiz-Morales JA, Gomez de la Torre Pretell JC, Dinh J, Granados J, Flores-Villanueva PO. Joint effect of MCP-1 genotype GG and MMP-1 genotype 2G/2G increases the likelihood of developing pulmonary tuberculosis in BCG-vaccinated individuals. PLoS One. 2010;5:e8881. doi: 10.1371/journal.pone.0008881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Jouanguy E, Altare F, Lamhamedi S, Revy P, Emile JF, Newport M, Levin M, Blanche S, Seboun E, Fischer A, Casanova JL. Interferon-gamma-receptor deficiency in an infant with fatal bacille Calmette-Guerin infection. N Engl J Med. 1996;335:1956–1961. doi: 10.1056/NEJM199612263352604. [DOI] [PubMed] [Google Scholar]
- 308.Kreins AY, Ciancanelli MJ, Okada S, Kong XF, Ramirez-Alejo N, Kilic SS, El Baghdadi J, Nonoyama S, Mahdaviani SA, Ailal F, Bousfiha A, Mansouri D, Nievas E, Ma CS, Rao G, Bernasconi A, Sun Kuehn H, Niemela J, Stoddard J, Deveau P, Cobat A, El Azbaoui S, Sabri A, Lim CK, Sundin M, Avery DT, Halwani R, Grant AV, Boisson B, Bogunovic D, Itan Y, Moncada-Velez M, Martinez-Barricarte R, Migaud M, Deswarte C, Alsina L, Kotlarz D, Klein C, Muller-Fleckenstein I, Fleckenstein B, Cormier-Daire V, Rose-John S, Picard C, Hammarstrom L, Puel A, Al-Muhsen S, Abel L, Chaussabel D, Rosenzweig SD, Minegishi Y, Tangye SG, Bustamante J, Casanova JL, Boisson-Dupuis S. Human TYK2 deficiency: Mycobacterial and viral infections without hyper-IgE syndrome. J Exp Med. 2015;212:1641–1662. doi: 10.1084/jem.20140280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Mao X, Ke Z, Liu S, Tang B, Wang J, Huang H, Chen S. IL-1beta+3953C/T, −511T/C and IL-6 −174C/G polymorphisms in association with tuberculosis susceptibility: A meta-analysis. Gene. 2015;573:75–83. doi: 10.1016/j.gene.2015.07.025. [DOI] [PubMed] [Google Scholar]
- 310.Rossouw M, Nel HJ, Cooke GS, van Helden PD, Hoal EG. Association between tuberculosis and a polymorphic NFkappaB binding site in the interferon gamma gene. Lancet. 2003;361:1871–1872. doi: 10.1016/S0140-6736(03)13491-5. [DOI] [PubMed] [Google Scholar]
- 311.Selvaraj P, Alagarasu K, Harishankar M, Vidyarani M, Nisha Rajeswari D, Narayanan PR. Cytokine gene polymorphisms and cytokine levels in pulmonary tuberculosis. Cytokine. 2008;43:26–33. doi: 10.1016/j.cyto.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 312.Stein CM, Zalwango S, Chiunda AB, Millard C, Leontiev DV, Horvath AL, Cartier KC, Chervenak K, Boom WH, Elston RC, Mugerwa RD, Whalen CC, Iyengar SK. Linkage and association analysis of candidate genes for TB and TNFalpha cytokine expression: evidence for association with IFNGR1, IL-10, and TNF receptor 1 genes. Hum Genet. 2007;121:663–673. doi: 10.1007/s00439-007-0357-8. [DOI] [PubMed] [Google Scholar]
- 313.Tobin DM, Vary JC, Jr, Ray JP, Walsh GS, Dunstan SJ, Bang ND, Hagge DA, Khadge S, King MC, Hawn TR, Moens CB, Ramakrishnan L. The lta4h locus modulates susceptibility to mycobacterial infection in zebrafish humans. Cell. 2010;140:717–730. doi: 10.1016/j.cell.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Velez DR, Wejse C, Stryjewski ME, Abbate E, Hulme WF, Myers JL, Estevan R, Patillo SG, Olesen R, Tacconelli A, Sirugo G, Gilbert JR, Hamilton CD, Scott WK. Variants in toll-like receptors 2 and 9 influence susceptibility to pulmonary tuberculosis in Caucasians, African-Americans, and West Africans. Hum Genet. 2010;127:65–73. doi: 10.1007/s00439-009-0741-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Yuan Q, Chen H, Zheng X, Chen X, Li Q, Zhang Y, Zhang X, Shi T, Zhou J, Chen Q, Yu S. The association between C-159T polymorphism in CD14 gene and susceptibility to tuberculosis: a meta-analysis. Mol Biol Rep. 2014;41:7623–7629. doi: 10.1007/s11033-014-3652-1. [DOI] [PubMed] [Google Scholar]
- 316.Babula O, Lazdane G, Kroica J, Ledger WJ, Witkin SS. Relation between recurrent vulvovaginal candidiasis, vaginal concentrations of mannose-binding lectin, and a mannose-binding lectin gene polymorphism in Latvian women. Clin Infect Dis. 2003;37:733–737. doi: 10.1086/377234. [DOI] [PubMed] [Google Scholar]
- 317.Giraldo PC, Babula O, Goncalves AK, Linhares IM, Amaral RL, Ledger WJ, Witkin SS. Mannose-binding lectin gene polymorphism, vulvovaginal candidiasis, and bacterial vaginosis. Obstet Gynecol. 2007;109:1123–1128. doi: 10.1097/01.AOG.0000260386.17555.a5. [DOI] [PubMed] [Google Scholar]
- 318.Woehrle T, Du W, Goetz A, Hsu HY, Joos TO, Weiss M, Bauer U, Brueckner UB, Marion Schneider E. Pathogen specific cytokine release reveals an effect of TLR2 Arg753Gln during Candida sepsis in humans. Cytokine. 2008;41:322–329. doi: 10.1016/j.cyto.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 319.Delgado JC, Baena A, Thim S, Goldfeld AE. Ethnic-specific genetic associations with pulmonary tuberculosis. J Infect Dis. 2002;186:1463–1468. doi: 10.1086/344891. [DOI] [PubMed] [Google Scholar]
- 320.Johnson MD, Plantinga TS, van de Vosse E, Velez Edwards DR, Smith PB, Alexander BD, Yang JC, Kremer D, Laird GM, Oosting M, Joosten LA, van der Meer JW, van Dissel JT, Walsh TJ, Perfect JR, Kullberg BJ, Scott WK, Netea MG. Cytokine gene polymorphisms and the outcome of invasive candidiasis: a prospective cohort study. Clin Infect Dis. 2012;54:502–510. doi: 10.1093/cid/cir827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Oral HB, Budak F, Uzaslan EK, Basturk B, Bekar A, Akalin H, Ege E, Ener B, Goral G. Interleukin-10 (IL-10) gene polymorphism as a potential host susceptibility factor in tuberculosis. Cytokine. 2006;35:143–147. doi: 10.1016/j.cyto.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 322.Altare F, Durandy A, Lammas D, Emile JF, Lamhamedi S, Le Deist F, Drysdale P, Jouanguy E, Doffinger R, Bernaudin F, Jeppsson O, Gollob JA, Meinl E, Segal AW, Fischer A, Kumararatne D, Casanova JL. Impairment of mycobacterial immunity in human interleukin-12 receptor deficiency. Science. 1998;280:1432–1435. doi: 10.1126/science.280.5368.1432. [DOI] [PubMed] [Google Scholar]
- 323.Aytekin C, Dogu F, Tuygun N, Tanir G, Guloglu D, Boisson-Dupuis S, Bustamante J, Feinberg J, Casanova JL, Ikinciogullari A. Bacille Calmette-Guerin lymphadenitis and recurrent oral candidiasis in an infant with a new mutation leading to interleukin-12 receptor beta-1 deficiency. J Investig Allergol Clin Immunol. 2011;21:401–404. [PMC free article] [PubMed] [Google Scholar]
- 324.de Jong R, Altare F, Haagen IA, Elferink DG, Boer T, van Breda Vriesman PJ, Kabel PJ, Draaisma JM, van Dissel JT, Kroon FP, Casanova JL, Ottenhoff TH. Severe mycobacterial and Salmonella infections in interleukin-12 receptor-deficient patients. Science. 1998;280:1435–1438. doi: 10.1126/science.280.5368.1435. [DOI] [PubMed] [Google Scholar]
- 325.Ouederni M, Sanal O, Ikinciogullari A, Tezcan I, Dogu F, Sologuren I, Pedraza-Sanchez S, Keser M, Tanir G, Nieuwhof C, Colino E, Kumararatne D, Levy J, Kutukculer N, Aytekin C, Herrera-Ramos E, Bhatti M, Karaca N, Barbouche R, Broides A, Goudouris E, Franco JL, Parvaneh N, Reisli I, Strickler A, Shcherbina A, Somer A, Segal A, Angel-Moreno A, Lezana-Fernandez JL, Bejaoui M, Bobadilla-Del Valle M, Kachboura S, Sentongo T, Ben-Mustapha I, Bustamante J, Picard C, Puel A, Boisson-Dupuis S, Abel L, Casanova JL, Rodriguez-Gallego C. Clinical features of Candidiasis in patients with inherited interleukin 12 receptor beta1 deficiency. Clin Infect Dis. 2014;58:204–213. doi: 10.1093/cid/cit722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Wang Y, Ma CS, Ling Y, Bousfiha A, Camcioglu Y, Jacquot S, Payne K, Crestani E, Roncagalli R, Belkadi A, Kerner G, Lorenzo L, Deswarte C, Chrabieh M, Patin E, Vincent QB, Muller-Fleckenstein I, Fleckenstein B, Ailal F, Quintana-Murci L, Fraitag S, Alyanakian MA, Leruez-Ville M, Picard C, Puel A, Bustamante J, Boisson-Dupuis S, Malissen M, Malissen B, Abel L, Hovnanian A, Notarangelo LD, Jouanguy E, Tangye SG, Beziat V, Casanova JL. Dual T cell- and B cell-intrinsic deficiency in humans with biallelic RLTPR mutations. J Exp Med. 2016;213:2413–2435. doi: 10.1084/jem.20160576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Okada S, Markle JG, Deenick EK, Mele F, Averbuch D, Lagos M, Alzahrani M, Al-Muhsen S, Halwani R, Ma CS, Wong N, Soudais C, Henderson LA, Marzouqa H, Shamma J, Gonzalez M, Martinez-Barricarte R, Okada C, Avery DT, Latorre D, Deswarte C, Jabot-Hanin F, Torrado E, Fountain J, Belkadi A, Itan Y, Boisson B, Migaud M, Arlehamn CS, Sette A, Breton S, McCluskey J, Rossjohn J, de Villartay JP, Moshous D, Hambleton S, Latour S, Arkwright PD, Picard C, Lantz O, Engelhard D, Kobayashi M, Abel L, Cooper AM, Notarangelo LD, Boisson-Dupuis S, Puel A, Sallusto F, Bustamante J, Tangye SG, Casanova JL. IMMUNODEFICIENCIES. Impairment of immunity to Candida and Mycobacterium in humans with bi-allelic RORC mutations. Science. 2015;349:606–613. doi: 10.1126/science.aaa4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Dupuis S, Dargemont C, Fieschi C, Thomassin N, Rosenzweig S, Harris J, Holland SM, Schreiber RD, Casanova JL. Impairment of mycobacterial but not viral immunity by a germline human STAT1 mutation. Science. 2001;293:300–303. doi: 10.1126/science.1061154. [DOI] [PubMed] [Google Scholar]
- 329.Dupuis S, Jouanguy E, Al-Hajjar S, Fieschi C, Al-Mohsen IZ, Al-Jumaah S, Yang K, Chapgier A, Eidenschenk C, Eid P, Al Ghonaium A, Tufenkeji H, Frayha H, Al-Gazlan S, Al-Rayes H, Schreiber RD, Gresser I, Casanova JL. Impaired response to interferon-alpha/beta and lethal viral disease in human STAT1 deficiency. Nat Genet. 2003;33:388–391. doi: 10.1038/ng1097. [DOI] [PubMed] [Google Scholar]
- 330.Kristensen IA, Veirum JE, Moller BK, Christiansen M. Novel STAT1 alleles in a patient with impaired resistance to mycobacteria. J Clin Immunol. 2011;31:265–271. doi: 10.1007/s10875-010-9480-8. [DOI] [PubMed] [Google Scholar]
- 331.Smeekens SP, Plantinga TS, van de Veerdonk FL, Heinhuis B, Hoischen A, Joosten LA, Arkwright PD, Gennery A, Kullberg BJ, Veltman JA, Lilic D, van der Meer JW, Netea MG. STAT1 hyperphosphorylation and defective IL12R/IL23R signaling underlie defective immunity in autosomal dominant chronic mucocutaneous candidiasis. PLoS One. 2011;6:e29248. doi: 10.1371/journal.pone.0029248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Toubiana J, Okada S, Hiller J, Oleastro M, Lagos Gomez M, Aldave Becerra JC, Ouachee-Chardin M, Fouyssac F, Girisha KM, Etzioni A, Van Montfrans J, Camcioglu Y, Kerns LA, Belohradsky B, Blanche S, Bousfiha A, Rodriguez-Gallego C, Meyts I, Kisand K, Reichenbach J, Renner ED, Rosenzweig S, Grimbacher B, van de Veerdonk FL, Traidl-Hoffmann C, Picard C, Marodi L, Morio T, Kobayashi M, Lilic D, Milner JD, Holland S, Casanova JL, Puel A, International, S. G.-o.-F. S. G Heterozygous STAT1 gain-of-function mutations underlie an unexpectedly broad clinical phenotype. Blood. 2016;127:3154–3164. doi: 10.1182/blood-2015-11-679902. [DOI] [PMC free article] [PubMed] [Google Scholar]


