Abstract
Aim
To investigate adiponectin levels and cardiovascular (CV) outcomes in patients with diabetes and recent acute coronary syndrome (ACS).
Materials and methods
We analysed baseline adiponectin concentration and CV outcomes in 5213 patients with type 2 diabetes enrolled in the EXAMINE trial of alogliptin vs placebo 15 to 90 days (median 45 days) after ACS. Event rates at 18 months are reported.
Results
The median (interquartile range) baseline adiponectin concentration was 5.2 (3.5–7.9) µg/mL. Patients with the highest baseline adiponectin concentration (quartile [Q]4) were at significantly higher risk of death from a CV event (8.4% vs 1.7%; P < .0001), hospitalization for heart failure (HF; 7.5% vs 1.7%; P < .0001), and all-cause mortality (10.8% vs 2.4%; P < .0001) compared with those in Q1. After adjusting for age, sex, index event, HF, estimated glomerular filtration rate and hypertension, adiponectin concentration in Q4 remained associated with an increased risk of death from CV causes (hazard ratio [HR] 2.43, 95% confidence interval [CI] 1.52, 3.88), all-cause mortality (HR 2.45, 95% CI 1.65, 3.64), and HF (HR 2.44, 95% CI 1.47, 4.05), without change after stratification by body mass index. There was no significant difference in the rate of myocardial infarction or stroke.
Conclusions
In this contemporary population of patients with diabetes and ACS, adiponectin concentration was independently associated with increased risk of death from CV causes, all-cause mortality, and hospitalization for HF. The relationship between adiponectin and CV outcomes is complex and deserves further study.
Keywords: cardiovascular disease, clinical trial, DPP-4 inhibitor, type 2 diabetes
1 | INTRODUCTION
Adiponectin is the most abundant adipocytokine and is involved in numerous pathways impacting the metabolic syndrome and atherosclerosis.1,2 Low adiponectin concentrations are associated with an unfavourable cardiovascular (CV) and metabolic risk profile, including insulin resistance, type 2 diabetes mellitus and obesity,2–9 and strong inverse correlations have been observed between adiponectin, prevalent coronary artery disease,10,11 and incident CV events.2,12–14 Adiponectin has also been found in animal and in vitro studies to have directly beneficial anti-inflammatory and vasculo-protective actions.2,15–17 As such, adiponectin has been considered a prognostic biomarker as well as an important potential mediator of protective vascular and metabolic effects.
Because adiponectin has been studied in increasingly diverse populations and clinical settings, however, the association with CV outcomes has been mixed.1,10,14,18–29 Among patients with acute coronary syndrome (ACS) enrolled in the PROVE IT-TIMI 22 (Pravastatin or Atorvastatin Evaluation and Infection Trial – Thrombolysis in Myocardial Infarction 22) trial,30 patients with the highest adiponectin levels had a higher rate of death and CV events.31 Similarly, adiponectin level drawn at the time of intervention correlates positively with CV events in patients undergoing percutaneous coronary intervention for ST-elevation myocardial infarction (STEMI).23 Eighteen months after ACS, however, adiponectin may again have a protective association.1
While adiponectin has been studied immediately after and late after ACS, it has not been investigated in the sub-acute post-ACS phase or among patients with diabetes after ACS. Moreover, given the heterogeneity in the results across previous studies, further evaluation in a robustly sized data set is warranted. The EXAMINE (Examination of Cardiovascular Outcomes with Alogliptin versus Standard of Care in Patients with Type 2 Diabetes Mellitus and Acute Coronary Syndrome) trial, which randomized patients with type 2 diabetes 15 to 90 days after ACS to the dipeptidyl peptidase-4 (DPP-4) inhibitor alogliptin or to placebo, provides an opportunity to further the understanding of adiponectin in the post-ACS period.
2 | MATERIALS AND METHODS
2.1 | Study design and participants
The design, baseline patient characteristics and primary results of the EXAMINE trial (NCT00968708) have been published previously.32,33 EXAMINE was a multicentre, randomized, controlled, double-blind trial of the DPP-4 inhibitor alogliptin vs placebo among patients with type 2 diabetes and ACS within the preceding 15 to 90 days (median 45 days). Patients were followed for a median of 18 months. The diabetes inclusion criterion was glycated haemoglobin HbA1c 6.5% to 11.0% (48–97 mmol/mol), and 7.0% to 11.0% (53–97 mmol/mol) for patients on insulin, and ACS was defined as myocardial infarction (MI) or unstable angina requiring hospitalization.32 Patients were excluded for New York Heart Association (NYHA) class IV heart failure (HF), uncontrolled hypertension, uncontrolled angina, and severe valvular heart disease, among other exclusion criteria.32,33 All participants provided written informed consent.
2.2 | Endpoints
The primary trial endpoint was the composite of death from CV causes, MI or stroke. On the basis of our previous work,31 the endpoints of death and HF were of principal interest in this analysis. An independent clinical events committee adjudicated each component of the primary endpoint according to prespecified criteria.32,33 Hospitalization for HF was additionally recorded and adjudicated.
2.3 | Measurement of adiponectin
Venous blood samples were obtained in EDTA-treated tubes at study entry as part of the study protocol. Plasma samples were transported refrigerated to the TIMI Clinical Trials Laboratory (Boston, Massachusetts) and stored at −80°C or colder until analysed after one preceding freeze–thaw. Adiponectin was measured at baseline in all available samples (n = 5213) using a validated enzyme-linked immunosorbent assay (R&D Systems, Minneapolis, Minnesota). Performance characteristics include a mean minimal detectable concentration of 0.246 ng/mL and inter-assay imprecision (coefficient of variation) values of 6.8%, 5.8% and 6.9% at adiponectin concentrations of 20.5, 74.4 and 157 ng/mL, respectively.
Prior to measurement of adiponectin, all specimens had been assayed for high-sensitivity C-reactive protein (hsCRP; Denka Seiken, Tokyo, Japan), high-sensitivity cardiac troponin I (hsTnI), B-type natriuretic peptide (BNP; Abbott Laboratories), LDL cholesterol, HDL cholesterol and triglycerides using validated methods. All assays were performed by laboratory personnel blinded to treatment allocation and clinical outcome.
2.4 | Statistical analysis
Adiponectin concentrations at baseline were categorized by quartiles (Q1–4). All patients with available adiponectin results were included. Baseline characteristics by adiponectin quartile were recorded with mean ± standard deviation (s.d.) or median and interquartile range for continuous variables and number and percent for categorical variables. Comparisons of baseline characteristics were made with the Student t-test or Wilcoxon’s rank sum test for continuous variables and the chi-squared or Fisher’s exact test for categorical variables.
Event rates at 18 months were calculated using the Kaplan–Meier method. Cox proportional hazards and 95% confidence intervals (CIs) were calculated for each of the clinical outcomes. The covariates included in the adjusted model were age, sex, index event type, history of HF, estimated glomerular filtration rate (eGFR), prior hypertension, and randomized treatment. This analysis was repeated stratifying by baseline body mass index (BMI) category (<25, 25 to <30 or ≥30 kg/m2), and as a sensitivity analysis, with the addition of BNP into the model. Spearman correlation coefficients were calculated between adiponectin concentration and other biomarker levels at baseline.
Assessment of the treatment effect of alogliptin was performed on an intention-to-treat basis. A 2-sided P value of .05 was taken to indicate statistical significance for all tests. All analyses were performed with SAS version 9.4 (SAS institute, Cary, North Carolina) and were performed by the Harvard Clinical Research Institute and the TIMI Study Group. The academic authors take full responsibility for the integrity of the database and the analyses.
3 | RESULTS
3.1 | Baseline characteristics
Adiponectin levels were available at baseline for 5213 patients with 523 primary endpoint events, including 184 deaths from CV causes, 315 MI events, and 58 strokes. There were 164 hospitalizations for HF in this cohort. The median (25th–75th percentile) adiponectin concentration was 5.2 (3.5–7.9) µg/mL. The mean age was 61 years, the mean HbA1c was 8.0% (64 mmol/mol), and the mean duration of diabetes was 9.2 years. The qualifying ACS was MI in 77% of patients (Table 1). The baseline distributions of BMI and HbA1c are provided in Figures S1 and S2, respectively, along with the mean adiponectin concentration for each BMI/HbA1c bin.
TABLE 1.
Baseline patient characteristics by adiponectin quartile
| Characteristics | Adiponectin, µg/mL | P | |||
|---|---|---|---|---|---|
| Q1 (N = 1301) | Q2 (N = 1306) | Q3 (N = 1302) | Q4 (N = 1304) | ||
| Age, years | 56 (50, 63) | 60 (53, 66) | 62 (56, 69) | 65 (59, 73) | <.001 |
| Non-white, % (n/N) | 33.5 (436/1301) | 25.8 (337/1306) | 23.1 (301/1302) | 26.2 (341/1304) | <.001 |
| Female, % (n/N) | 14.1 (183/1301) | 25.7 (336/1306) | 38.3 (499/1302) | 50.4 (657/1304) | <.001 |
| BMI, kg/m2 | 29.0 (26.0, 32.6) | 29.4 (26.1, 32.9) | 29.3 (26.1, 33.0) | 27.4 (24.4, 31.6) | <.001 |
| Range (min, max) | (16.9, 55.9) | (16.9, 68.3) | (15.6, 65.9) | (15.7, 67.2) | |
| <25 kg/m2 | 25.0 (272/1086) | 25.0 (272/1086) | 24.9 (270/1086) | 25.0 (272/1086) | |
| 25 to <30 kg/m2 | 24.9 (493/1983) | 25.1 (498/1983) | 25.0 (496/1983) | 25.0 (496/1983) | |
| ≥30 kg/m2 | 24.8 (532/2143) | 25.2% (541/2143) | 25.0 (534/2143) | 25.0 (536/2143) | |
| Duration of diabetes, years | 5.3 (1.8, 10.3) | 6.4 (2.3, 12.0) | 7.8 (3.3, 14.7) | 10.1 (4.2, 16.8) | <.001 |
| Hypertension, % (n/N) | 77.2 (1004/1301) | 82.5 (1077/1306) | 86.4 (1125/1302) | 86.7 (1131/1304) | <.001 |
| Dyslipidemia, % (n/N) | 28.0 (364/1301) | 25.4 (332/1306) | 29.0 (378/1302) | 26.8 (349/1304) | .188 |
| Current smoker, % (n/N) | 19.2 (250/1301) | 14.9 (194/1306) | 12.5 (163/1302) | 8.2 (107/1304) | <.001 |
| Prior MI, % (n/N) | 88.2 (1148/1301) | 87.8 (1147/1306) | 87.8 (1143/1302) | 88.2 (1150/1304) | .976 |
| Prior percutaneous coronary intervention or CABG, % (n/N) | 72.2 (939/1301) | 71.0 (927/1306) | 67.6 (880/1302) | 61.6 (803/1304) | <.001 |
| Prior HF, % (n/N) | 18.1 (235/1301) | 24.0 (314/1306) | 30.3 (394/1302) | 39.5 (515/1304) | <.001 |
| NYHA classification at screening, % (n/N) | .260 | ||||
| I | 27.2 (64/235) | 22.0 (69/313) | 20.6 (81/394) | 20.0 (103/515) | |
| II | 55.7 (131/235) | 61.0 (191/313) | 57.4 (226/394) | 57.3 (295/515) | |
| III | 15.3 (36/235) | 15.7 (49/313) | 20.6 (81/394) | 21.6 (111/515) | |
| IV | 1.7 (4/235) | 1.3 (4/313) | 1.5 (6/394) | 1.2 (6/515) | |
| Systolic blood pressure, mm Hg | 125 (116, 136) | 130 (120, 140) | 130 (120, 140) | 130 (120, 140) | <.001 |
| Heart rate, bpm | 70 (64, 78) | 70 (63, 77) | 70 (64, 78) | 72 (64, 80) | <.001 |
| Estimated CrCl, mL/min | 78 (65, 89) | 74 (61, 86) | 69 (56, 83) | 62 (48, 77) | <.001 |
| eGFR, mL/min/1.73 m2 | 77.0 (64.6, 89.0) | 74.8 (61.2, 87.0) | 68.8 (55.6, 83.4) | 62.3 (47.2, 76.2) | <.001 |
| Index inclusion ACS | .062 | ||||
| Myocardial infarction, % (n/N) | 79.6 (1034/1299) | 77.5 (1011/1304) | 75.2 (977/1299) | 76.8 (998/1299) | |
| Time post-index ACS to randomization, days | 43 (29, 61) | 43 (30, 62) | 44 (29, 66) | 46 (30, 66) | .009 |
| Percutaneous coronary intervention for index event, % (n/N) | 8.7% (90/1035) | 9.4% (95/1013) | 7.1% (70/980) | 5.4% (54/999) | .004 |
| BNP, pmol/L | 13.4 (5.4, 31.0) | 16.8 (7.3, 36.9) | 24.4 (10.7, 52.3) | 40.7 (16.5, 97.3) | <.001 |
Abbreviations: bpm, beats per minute; CABG, coronary artery bypass graft; CrCl, creatinine clearance.
Values are median (interquartile range), unless otherwise indicated.
At baseline, patients with adiponectin levels in Q4 vs Q1 were older (mean age 65.3 ± 9.7 vs 56.3 ± 9.2 years; P < .001), had a lower BMI (median [25th–75th percentile] BMI 27.4 [24.4, 31.6] vs 29.0 [26.0, 32.6] kg/m2), and had a higher BNP (median BNP 40.7 [16.5, 97.3] vs 13.4 [5.4, 31.0] pmol/L). They were also less likely to be current smokers or have a history of coronary revascularization and were more likely to have a history of hypertension or HF, with P < .001 for each (Table 1). There was no difference based on adiponectin quartile in the rate of dyslipidaemia.
3.2 | Adiponectin and other biomarkers
Spearman correlation coefficients were calculated with baseline cardiac and inflammatory biomarkers (Table 2). Continuous adiponectin concentration showed weak positive correlations with BNP (r = 0.34, P < .0001), hsTnI (r = 0.17, P < .0001), and cystatin C (r = 0.29, P < .0001) and weak negative correlations with HbA1c (r = −0.03, P = 0.019) and BMI (r = −0.10, P < .0001). No significant correlation was observed with hsCRP (r = −0.01, P = 0.41; Table 2).
TABLE 2.
Spearman correlation coefficients between baseline adiponectin and other biomarkers
| Biomarker | |r| | P |
|---|---|---|
| hsTnI, ng/L | 0.17 | <.0001 |
| BNP, pg/mL | 0.31 | <.0001 |
| hsCRP, mg/L | −0.01 | .40 |
| MPO, ng/mL | 0.06 | <.0001 |
| ST-2, ng/mL | 0.11 | <.0001 |
| GDF-15, pg/mL | 0.25 | <.0001 |
| Galectin-3, ng/mL | 0.26 | <.0001 |
| Cystatin C, mg/L | 0.29 | <.0001 |
| HbA1c, % | −0.03 | .02 |
| BMI, kg/m2 | −0.10 | <.0001 |
Abbreviations: GDF-15, growth differentiation factor-15; MPO, myeloperoxidase.
3.3 | Adiponectin and cardiovascular outcomes
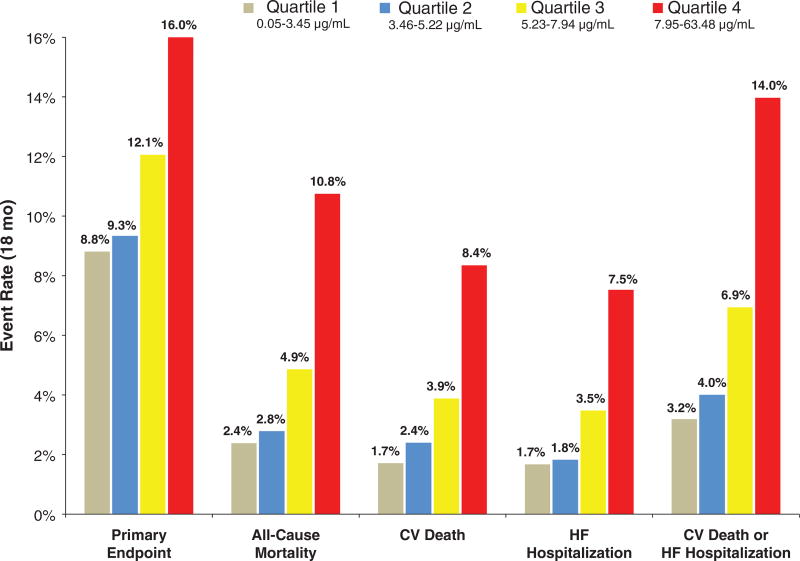
In the unadjusted analysis, patients with adiponectin concentrations in Q4 had significantly higher rates of the primary endpoint (16.0% vs 8.8%; P < .0001), death from CV causes, all-cause mortality, hospitalization for HF, and death from CV causes or hospitalization for HF than did patients in Q1 (Figure 1). There was no significant difference in the rate of MI (7.7% vs 6.6%; P = 0.31) or stroke (1.7% vs 1.4%; P = 0.65).
Figure 1.
Kaplan–Meier event rates at 18 months are shown by quartile of adiponectin concentration for the primary endpoint (death from CV causes, MI, or ischemic stroke), all-cause mortality, death from CV causes, HF hospitalization, and death from CV causes or HF hospitalization
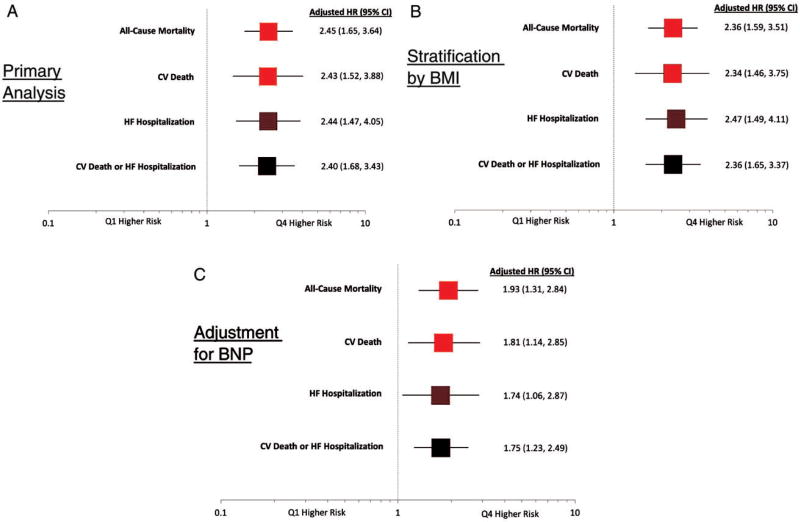
After adjustment for potential confounders (age, sex, index event type, history of HF, eGFR, prior hypertension and randomized treatment), patients in Q4 had significantly higher risk of death from CV causes (HR 2.43 [95% CI 1.52, 3.88]), all-cause mortality (HR 2.45 [95% CI 1.65, 3.64]), hospitalization for HF (HR 2.44 [95% CI 1.47, 4.05]), and death from CV causes or hospitalization for HF (HR 2.40 [95% CI 1.68, 3.43]; Figure 2A).
Figure 2.
A–C, Adjusted hazard ratios are shown for Q4 vs Q1 adiponectin concentration for all-cause mortality, death from CV causes, HF hospitalization, and death from CV causes or HF hospitalization for the (A) primary analysis, (B) with stratification by BMI, and (C) with inclusion of BNP in the adjustment model. The covariates used for adjustment were age, sex, index event, prior heart failure, eGFR, HTN, treatment arm, and BNP (panel C only)
Adiponectin remained significantly associated with risk within high and low BMI strata. After stratification by baseline BMI category (<25, 25 to <30, or ≥30 kg/m2), patients with adiponectin concentrations in Q4 were still found to have a higher risk of death from CV causes (HR 2.34 [95% CI 1.46, 3.75]), all-cause mortality, hospitalization for HF, and death from CV causes or hospitalization for HF as compared with patients in Q1 (Figure 2B and Tables S1A–C).
After adjustment for BNP, patients with adiponectin concentrations in Q4 remained at higher risk of death from CV causes, all-cause mortality, hospitalization for HF, and death from CV causes or hospitalization for HF as compared with patients in Q1 (Figure 2C). The potential interaction between adiponectin and BNP was further explored in analyses stratified by BNP level < or ≥ the median. There was a homogeneous risk of death from CV causes or hospitalization for HF associated with elevated adiponectin concentration (Q4) across strata defined by BNP <median level (HR 2.20 [95% CI 0.50–10.08]) and BNP ≥ median (HR 2.27 [95% CI 1.39–3.71]) with P-interaction = 0.29.
Adjusted risk was calculated by adiponectin quartile after stratification by sex. Adiponectin concentration in the highest quartile continued to be associated with increased risk of death from CV causes or hospitalization for HF in the female (HR 2.25 [95% CI 1.36–3.72]) and male (HR 2.25 [95% CI 1.45–3.50]) strata (P-interaction = 0.31).
Among patients with highest CV risk identified by adiponectin levels in Q4, there was no difference in trial endpoints based on assignment to alogliptin or placebo. Relative to patients randomized to placebo, patients assigned to alogliptin had HRs of 0.94 (95% CI 0.71, 1.23) for the primary endpoint and 1.03 (95% CI 0.77, 1.37) for the combined outcome of CV death or hospitalization for HF. There was similarly no difference in the risk of hospitalization for HF based on treatment assignment (Table S2).
4 | DISCUSSION
In this contemporary trial population of adult patients with type 2 diabetes and recent ACS, we found baseline adiponectin concentration to be strongly directly, rather than inversely, associated with adverse cardiovascular events, including CV death, all-cause mortality, and hospitalization for HF. This association was independent of the patient’s presentation and relevant clinical characteristics, including BMI. Adiponectin concentration showed only weak correlations with baseline biomarkers of inflammation, glycometabolic status, and myocardial stress.
4.1 | Adiponectin and cardiovascular disease
Previous studies have shown mixed associations between adiponectin and CV outcomes. Whereas a protective association has been observed in numerous cohorts of patients without manifest CV disease,1,10,14,18,29 other samples of patients with HF,21,25 stable coronary artery disease,19,28 and advanced age20,22,24,26 have found adiponectin to be directly correlated with adverse events. In the setting of ACS, adiponectin level has been positively correlated with adverse CV events at the time of percutaneous coronary intervention for STEMI (n = 735),23 within 10 days of ACS (n = 3931),30 and at 4 months after ACS (n = 3366).30 By 18 months after ACS, however, this association was seen to reverse (n = 102).1 Further complicating understanding of this relationship is the recent observation of a higher risk of CV events associated with high adiponectin concentrations in a cohort of relatively young, otherwise healthy patients.27 Our study adds further evidence of increased risk with elevated adiponectin in the sub-acute post-ACS period in patients with diabetes irrespective of BMI.
Interpretation of the clinical and translational investigation of adiponectin to date has been difficult. Given the direct beneficial effects of adiponectin in animal and in vitro studies, one explanation offered for the association between elevated adiponectin and adverse outcomes in the acute setting is that adiponectin increases in response to cardiovascular derangement or systemic stress in a counter-regulatory manner, as most clearly evident in the setting of acute MI or HF.19,23,30,34 In fact, increased adiponectin has been found to be a marker of poor prognosis in severe non-cardiovascular illness as well.35 The mechanism for such a response is not well understood, although increased catecholamine production may be at least partly responsible.36 Under this hypothesis, elevated adiponectin level would be expected to be associated with markers of disease severity such as NYHA class, reduced left ventricular ejection fraction, cardiac-specific troponin, and BNP, as some of the series described above have shown.21,25,37–39
In our analysis, patients with high adiponectin levels were more likely to have prior HF than patients with low adiponectin levels, perhaps supporting the counter-regulatory hypothesis. There was no association with NYHA class, however, and the correlations between continuous adiponectin and BNP and hsTnI were weak. Other biomarkers of inflammation, such as hsCRP, might be expected to provide a measure of systemic illness. Similar to findings in PROVE IT-TIMI 22,31 adiponectin was not correlated with hsCRP in our sample and showed only weak correlations with the remainder of the biomarker panel.
Another possible mechanism is failure of a compensatory response by adiponectin, either by decreased responsiveness to or insufficient production of adiponectin following a systemic perturbation. It is conceivable that some patients with CV disease develop resistance to circulating adiponectin, analogous to insulin insensitivity. Given the assumed protective effects of adiponectin, these patients would be expected to face increased CV risk and would be identified by elevated adiponectin levels. While our data do not allow the direct examination of this hypothesis, the weak correlation between adiponectin and the measured inflammatory markers is of interest. Adiponectin has demonstrable anti-inflammatory effects, including reducing TNF-α,2 and it is notable that the patients in the highest adiponectin quartile in the EXAMINE cohort were observed to have 1.5 times the adiponectin concentration of those in the lowest quartile yet a similar inflammatory state, as indicated by the biomarker panel.
While inappropriately low adiponectin production is an intriguing theoretical mechanism for adiponectin failure and may help explain the inverse association between adiponectin and adverse outcomes in stable patients, our finding of an overall positive association does not support this as being the dominant mechanism post-ACS in patients with diabetes.
4.2 | Relationship to BNP
Recent work has identified important relationships between adiponectin and BNP.19,37,38 Observationally, BNP is known to be a strongly predictive biomarker in patients with HF, as has also been seen recently with adiponectin.21,25,37 Furthermore, Tsukamoto et al.38 have shown increased adiponectin messenger ribonucleic acid production in cultured human adipocytes in response to exposure to BNP or atrial natriuretic peptide. This finding may, in part, explain the association seen between natriuretic peptide levels and favourable adipose distribution.37,40
While we observed an increase in BNP from Q1 to Q4 of adiponectin concentration, the degree of correlation between adiponectin and BNP was only weak. Moreover, the association between adiponectin and adverse cardiovascular outcomes was consistent after adjusting for BNP and after stratifying by BNP level < or ≥ the median. Nonetheless, the possibility that an interrelation of natriuretic peptide release and adiponectin production may be tied to underlying mechanisms for poorer cardiovascular outcomes is intriguing and warrants continued investigation. Overall, the findings presented here are consistent with the trend in other reports of adiponectin as a stronger predictor of death and HF than of atherothrombotic events such as MI or stroke.
4.3 | Limitations
The present study has several limitations. While the EXAMINE trial provides a large, well-characterized study sample with adjudicated outcomes, the data reported result from an exploratory, observational analysis. Additionally, because one of the principal hypotheses connecting elevated adiponectin to adverse outcomes in the setting of CV disease is disease severity itself, and particularly severity of HF, it would be desirable to have left ventricular ejection fraction data. Similarly, it would have been ideal to have TNF-α measurements for the assessment of the inflammatory state, as TNF-α appears to be directly suppressed by adiponectin,2 as well as catecholamine levels. Finally, we only measured total adiponectin rather than specific subclasses, such as high-molecular-weight adiponectin. Data on the relevance of this distinction have been mixed.22,25 There may be sex-based differences in the high-molecular-weight fraction41 that are a consideration given the increased proportion of women among those with higher adiponectin concentration. Importantly, the sex-based difference in adiponectin concentration in our cohort was similar to that seen in other cohorts and sex was a covariate in all adjusted analyses in our report.19,21,23,27,31,35 Additionally, results were consistent in female and male patients after stratification by sex.
4.4 | Conclusion
In conclusion, in this contemporary cohort of adult patients with diabetes and recent ACS, baseline adiponectin concentration was strongly associated with adverse cardiovascular events, adding to prior investigations of adiponectin in acute illness. This association is directionally opposite to that seen in patients at risk of CV disease. The role of adiponectin in manifest cardiovascular disease is complex and deserves further study.
Supplementary Material
Acknowledgments
The EXAMINE trial was sponsored by Takeda Development Center Americas. David A. Morrow and Brian A. Bergmark take full responsibility for the accuracy and contents of this article.
C. P. C. reports research grants from (all >10K) Amgen, Arisaph, Boehringer-Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, Janssen, Merck, and Takeda, and consulting fees from Alnylam, Amgen, Arisaph, Astra Zeneca, Boehringer-Ingelheim, Bristol-Myers Squibb, GlaxoSmithKline, Kowa, Lipimedix*, Merck, Pfizer, Regeneron*, Sanofi* and Takeda (* denotes > 10K). W. B. W. reports personal fees from Takeda Development Center. P. J. has received research support through his institution from Abbott Laboratories, AstraZeneca, LP, Daiichi-Sankyo, Inc., GlaxoSmithKline, Janssen Scientific Affairs, LLC, Merck & Co., Inc., Roche Diagnostics Corporation, Takeda Global Research and Development Center, and Waters Technologies Corporation. M. P. B. was supported by a Research Career Development Award (K12 HL083786) from the National Heart, Lung, and Blood Institute and reports consulting for Merck and Co, AstraZeneca, Bayer, and Roche diagnostics. F. Z. reports personal fees from Takeda Development Center. D. A. M. reports grants to the TIMI Study Group from Abbott Laboratories, Amgen, AstraZeneca, Daiichi Sankyo, Eisai, GlaxoSmithKline, Merck, Novartis, Roche Diagnostics and consultant fees from Abbott Laboratories, AstraZeneca, diaDexus, GlaxoSmithKline, Merck, Peloton, Roche Diagnostics and Verseon.
Footnotes
Conflict of interest
B. B. and Y. L have no conflict of interest to declare.
Author contributions
All authors provided feedback on the manuscript, including final approval. Additional contributions are as follows: B. B. assisted in interpretation of the data and writing of the manuscript; C. P. C. assisted in the design and analysis of the EXAMINE trial and interpretation of data for this study; W. B. W. assisted in the design and analysis of the EXAMINE trial and interpretation of data for this study; Y. L. performed the data analysis; P. J. oversaw the biomarker measurements; M. P. B. assisted in the design of the biomarker analysis; F. Z. assisted in the design and analysis of the EXAMINE trial; and D. A. M. secured funding for the biomarker sub-study and assisted in the design and analysis of the EXAMINE trial, interpretation of data for this study, and writing of the manuscript.
Additional Supporting Information may be found online in the supporting information tab for this article.
References
- 1.Huang S-S, Huang P-H, Chen Y-H, Chiang K-H, Chen J-W, Lin S-J. Association of adiponectin with future cardiovascular events in patients after acute myocardial infarction. J Atheroscler Thromb. 2010;17:295–303. doi: 10.5551/jat.3533. [DOI] [PubMed] [Google Scholar]
- 2.Okamoto Y, Kihara S, Funahashi T, Matsuzawa Y, Libby P. Adiponectin: a key adipocytokine in metabolic syndrome. Clin Sci. 2006;110:267–278. doi: 10.1042/CS20050182. [DOI] [PubMed] [Google Scholar]
- 3.Hotta K, Funahashi T, Arita Y, et al. Plasma concentrations of a novel, adipose-specific protein, adiponectin, in type 2 diabetic patients. Arterioscler Thromb Vasc Biol. 2000;20:1595–1599. doi: 10.1161/01.atv.20.6.1595. [DOI] [PubMed] [Google Scholar]
- 4.Kunita E, Yamamoto H, Kitagawa T, et al. Association between plasma high-molecular-weight adiponectin and coronary plaque characteristics assessed by computed tomography angiography in conditions of visceral adipose accumulation. Circ J. 2012;76:1687–1696. doi: 10.1253/circj.cj-11-1442. [DOI] [PubMed] [Google Scholar]
- 5.Lindsay RS, Funahashi T, Hanson RL, et al. Adiponectin and development of type 2 diabetes in the Pima Indian population. Lancet. 2002;360:57–58. doi: 10.1016/S0140-6736(02)09335-2. [DOI] [PubMed] [Google Scholar]
- 6.Matsubara M, Maruoka S, Katayose S. Decreased plasma adiponectin concentrations in women with dyslipidemia. J Clin Endocrinol Metab. 2002;87:2764–2769. doi: 10.1210/jcem.87.6.8550. [DOI] [PubMed] [Google Scholar]
- 7.Peri–Okonny PA, Ayers C, Maalouf N, et al. Adiponectin predicts incident hypertension independent of body fat distribution: observations from the Dallas Heart Study. Diabetes Metab Res Rev. 2017;33:e2840–e2846. doi: 10.1002/dmrr.2840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sawada T, Shite J, Shinke T, et al. Low plasma adiponectin levels are associated with presence of thin-cap fibroatheroma in men with stable coronary artery disease. Int J Cardiol. 2010;142:250–256. doi: 10.1016/j.ijcard.2008.12.216. [DOI] [PubMed] [Google Scholar]
- 9.Weyer C, Funahashi T, Tanaka S, et al. Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia. J Clin Endocrinol Metab. 2001;86:1930–1935. doi: 10.1210/jcem.86.5.7463. [DOI] [PubMed] [Google Scholar]
- 10.Schulze MB, Shai I, Rimm EB, Li T, Rifai N, Hu FB. Adiponectin and future coronary heart disease events among men with type 2 diabetes. Diabetes. 2005;54:534–539. doi: 10.2337/diabetes.54.2.534. [DOI] [PubMed] [Google Scholar]
- 11.Kajikawa Y, Ikeda M, Takemoto S, Tomoda J, Ohmaru N, Kusachi S. Association of circulating levels of leptin and adiponectin with metabolic syndrome and coronary heart disease in patients with various coronary risk factors. Int Heart J. 2011;52:17–22. doi: 10.1536/ihj.52.17. [DOI] [PubMed] [Google Scholar]
- 12.Nakamura Y, Shimada K, Fukuda D, et al. Implications of plasma concentrations of adiponectin in patients with coronary artery disease. Heart. 2004;90:528–533. doi: 10.1136/hrt.2003.011114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kojima S, Funahashi T, Sakamoto T, et al. The variation of plasma concentrations of a novel, adipocyte derived protein, adiponectin, in patients with acute myocardial infarction. Heart. 2003;89:667–668. doi: 10.1136/heart.89.6.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pischon T, Girman CJ, Hotamisligil GS, Rifai N, Hu FB, Rimm EB. Plasma adiponectin levels and risk of myocardial infarction in men. JAMA. 2004;291:1730–1737. doi: 10.1001/jama.291.14.1730. [DOI] [PubMed] [Google Scholar]
- 15.Aprahamian TR, Sam F. Adiponectin in cardiovascular inflammation and obesity. Int J Inflam. 2011;2011:1–8. doi: 10.4061/2011/376909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Okamoto Y, Kihara S, Ouchi N, et al. Adiponectin reduces atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2002;106:2767–2770. doi: 10.1161/01.cir.0000042707.50032.19. [DOI] [PubMed] [Google Scholar]
- 17.Shibata R, Izumiya Y, Sato K, et al. Adiponectin protects against the development of systolic dysfunction following myocardial infarction. J Mol Cell Cardiol. 2007;42:1065–1074. doi: 10.1016/j.yjmcc.2007.03.808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ai M, Otokozawa S, Asztalos BF, et al. Adiponectin: an independent risk factor for coronary heart disease in men in the Framingham offspring Study. Atherosclerosis. 2011;217:543–548. doi: 10.1016/j.atherosclerosis.2011.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beatty AL, Zhang MH, Ku IA, Na B, Schiller NB, Whooley MA. Adiponectin is associated with increased mortality and heart failure in patients with stable ischemic heart disease: data from the Heart and Soul Study. Atherosclerosis. 2012;220:587–592. doi: 10.1016/j.atherosclerosis.2011.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karas MG, Benkeser D, Arnold AM, et al. Relations of plasma total and high-molecular-weight adiponectin to new-onset heart failure in adults >/=65 years of age (from the Cardiovascular Health study) Am J Cardiol. 2014;113:328–334. doi: 10.1016/j.amjcard.2013.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kistorp C, Faber J, Galatius S, et al. Plasma adiponectin, body mass index, and mortality in patients with chronic heart failure. Circulation. 2005;112:1756–1762. doi: 10.1161/CIRCULATIONAHA.104.530972. [DOI] [PubMed] [Google Scholar]
- 22.Kizer JR, Benkeser D, Arnold AM, et al. Associations of total and high-molecular-weight adiponectin with all-cause and cardiovascular mortality in older persons: the Cardiovascular Health Study. Circulation. 2012;126:2951–2961. doi: 10.1161/CIRCULATIONAHA.112.135202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lindberg S, Pedersen SH, Møgelvang R, et al. Usefulness of adiponectin as a predictor of all cause mortality in patients with ST-segment elevation myocardial infarction treated with primary percutaneous coronary intervention. Am J Cardiol. 2012;109:492–496. doi: 10.1016/j.amjcard.2011.09.041. [DOI] [PubMed] [Google Scholar]
- 24.Poehls J, Wassel CL, Harris TB, et al. Association of adiponectin with mortality in older adults: the Health, Aging, and Body Composition Study. Diabetologia. 2009;52:591–595. doi: 10.1007/s00125-009-1261-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsutamoto T, Tanaka T, Sakai H, et al. Total and high molecular weight adiponectin, haemodynamics, and mortality in patients with chronic heart failure. Eur Heart J. 2007;28:1723–1730. doi: 10.1093/eurheartj/ehm154. [DOI] [PubMed] [Google Scholar]
- 26.Wannamethee SG, Whincup PH, Lennon L, Sattar N. Circulating adiponectin levels and mortality in elderly men with and without cardiovascular disease and heart failure. Arch Intern Med. 2007;167:1510–1517. doi: 10.1001/archinte.167.14.1510. [DOI] [PubMed] [Google Scholar]
- 27.Witberg G, Ayers CR, Turer AT, et al. Relation of adiponectin to all-cause mortality, cardiovascular mortality, and major adverse cardiovascular events (from the Dallas Heart Study) Am J Cardiol. 2016;117:574–579. doi: 10.1016/j.amjcard.2015.11.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu ZJ, Cheng YJ, Gu WJ, Aung LH. Adiponectin is associated with increased mortality in patients with already established cardiovascular disease: a systematic review and meta-analysis. Metabolism. 2014;63:1157–1166. doi: 10.1016/j.metabol.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 29.Zoccali C, Mallamaci F, Tripepi G, et al. Adiponectin, metabolic risk factors, and cardiovascular events among patients with end-stage renal disease. J Am Soc Nephrol. 2002;13:134–141. doi: 10.1681/ASN.V131134. [DOI] [PubMed] [Google Scholar]
- 30.Cannon CP, Braunwald E, McCabe CH, et al. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2004;350:149–1504. doi: 10.1056/NEJMoa040583. [DOI] [PubMed] [Google Scholar]
- 31.Wilson SR, Sabatine MS, Wiviott SD, et al. Assessment of adiponectin and the risk of recurrent cardiovascular events in patients presenting with an acute coronary syndrome: observations from the Pravastatin Or atorVastatin Evaluation and Infection Trial-Thrombolysis in Myocardial Infarction 22 (PROVE IT-TIMI 22) Am Heart J. 2011;161:1147–1155. doi: 10.1016/j.ahj.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 32.White WB, Bakris GL, Bergenstal RM, et al. EXamination of cArdiovascular outcoMes with alogliptIN versus standard of carE in patients with type 2 diabetes mellitus and acute coronary syndrome (EXAMINE): a cardiovascular safety study of the dipeptidyl peptidase 4 inhibitor alogliptin in patients with type 2 diabetes with acute coronary syndrome. Am Heart J. 2011;162:620–626 e1. doi: 10.1016/j.ahj.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 33.White WB, Cannon CP, Heller SR, et al. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med. 2013;369:1327–1335. doi: 10.1056/NEJMoa1305889. [DOI] [PubMed] [Google Scholar]
- 34.Witberg G, Ayers C, Turer A, et al. The association of adiponectin with increased all-cause and cardiovascular mortality and major adverse cardiovascular events: insights from the Dallas Heart Study. J Am Coll Cardiol. 2016;67:1907. doi: 10.1016/j.amjcard.2015.11.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Walkey AJ, Rice TW, Konter J, Ouchi N, Shibata R, Walsh K. Plasma adiponectin and mortality in critically ill subjects with acute respiratory failure. Crit Care Med. 2010;38:2329–2334. doi: 10.1097/CCM.0b013e3181fa0561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Simpson KA, Singh MAF. Effects of exercise on adiponectin: a systematic review. Obesity. 2008;16:241–256. doi: 10.1038/oby.2007.53. [DOI] [PubMed] [Google Scholar]
- 37.Lindberg S, Jensen JS, Bjerre M, et al. Cardio-adipose tissue crosstalk: relationship between adiponectin, plasma pro brain natriuretic peptide and incident heart failure. Eur J Heart Fail. 2014;16:633–638. doi: 10.1002/ejhf.82. [DOI] [PubMed] [Google Scholar]
- 38.Tsukamoto O, Fujita M, Kato M, et al. Natriuretic peptides enhance the production of adiponectin in human adipocytes and in patients with chronic heart failure. J Am Coll Cardiol. 2009;53:2070–2077. doi: 10.1016/j.jacc.2009.02.038. [DOI] [PubMed] [Google Scholar]
- 39.Cavusoglu E, Chopra V, Battala V, et al. Baseline plasma adiponectin levels as a predictor of left ventricular systolic dysfunction in patients referred for coronary angiography. Am J Cardiol. 2008;101:1073–1078. doi: 10.1016/j.amjcard.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 40.Neeland IJ, Winders BR, Ayers CR, et al. Higher natriuretic peptide levels associate with a favorable adipose tissue distribution profile. J Am Coll Cardiol. 2013;62:752–760. doi: 10.1016/j.jacc.2013.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu A, Chan KW, Hoo RL, et al. Testosterone selectively reduces the high molecular weight form of adiponectin by inhibiting its secretion from adipocytes. J Biol Chem. 2005;280:18073–18080. doi: 10.1074/jbc.M414231200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.