Abstract
The aim of the study was to investigate the efficacy of 6 week Mediterranean diet or 30% calorie restriction on the fatty acid profile and eicosanoids (hydroxyoctadecadienoi acids and hydroxyeicosatetraenoic acids) concentration. Furthermore, basic biochemical variables such as insulin, glucose, HOMA-IR, and a lipid profile were estimated. The study enrolled 94 Caucasian former athletes aged 20-42, with body height of 179 ± 16.00 cm and body mass of 89.26 ± 13.25 kg who had not been active for at least 5 years. The subjects were randomly assigned to one of the three intervention groups: CR group – the 30% calorie restriction (n = 32), MD group - the Mediterranean diet (n = 34), and C group - a control group (n = 28). The pattern of nutrition was analysed before and after the experiment using the 72 h food diaries. In order to evaluate the effect of diet intervention, the following variables were measured: anthropometrics, basic biochemical variables (insulin, fasting glucose, HOMA-IR, lipid profile), fatty acids and their blood derivatives profiles. The CR group showed significantly lower levels of several biochemical variables, i.e., BMI, total cholesterol LDL, TG, total lipids, insulin and HOMA – IR (p < 0.05). Subjects consuming the MD diet significantly decreased their BMI and reduced the level of total lipids (p < 0.05). We did not find any significant changes in the C group. The analysis of the fatty acid profile revealed that the CR group had a significantly decreased EPA level (p < 0.05). The MD group showed a significantly increased level of the DHA (p < 0.05) and improvement in the omega - 3 index (p < 0.05). Subjects following the MD also showed significantly lower concentrations of 15 - hydroxyicosatetraenoic acid (15-HETE). We did not observe any significant differences between the CR and C groups. Within short time, calorie restriction helps to improve lipid variables and insulin resistance. The MD diet seems to be more advantageous in the decrease of inflammation, but does not improve basic biochemical variables. We can conclude that calorie restriction can be a good choice for former athletes, although EPA and DHA supplementation is needed.
Key words: calorie restriction, Mediterranean diet, nutrition, former athletes
Introduction
Numerous studies have shown that intense physical activity is essential for health and helps prevent hypertension, atherosclerosis (Kohl, 2010) and diabetes (Kelley and Goodpaster, 2001). The level of physical activity which allows to improve basic biochemical variables such as insulin, lipids or glucose depends on many factors and should be personalised (Leon and Sanchez, 2001; Roczniok et al., 2013; Czuba et al., 2014). Some of the athletes who end their professional career, especially those of international level, remain physically active and lead a healthy lifestyle. The risk of acquiring the metabolic syndrome or cardiovascular disease is much lower for them than for the general population; however, most studies on former athletes focus on elite players (Backmand et al., 2010; Kujala et al., 1994; Sarna et al., 1997; Ostrowski et al., 2012).
There are only few studies which show the condition of former athletes who achieved success at a local or national level. Many former athletes reduce their physical activity and do not change their dietary habits. It causes difficulties in maintaining normal body mass and may lead to obesity, diabetes and many other diseases. A study conducted on a group of 100 former soccer players from Brazil showed that 78% of them were overweight and 4% suffered from obesity (Arliani et al., 2014).
The generally recommended diet associated with a decreased risk of cardiovascular disease, cancer and obesity is the Mediterranean diet (MD) (Assmann et al., 1997). It has been shown that this type of diet can be recommended to all populations worldwide (Kouris-Blazos et al., 1999). The beneficial effect of the MD is based on various mechanisms associated with increased supply of dietary components such as vitamins, antioxidants, monounsaturated fatty acids (MUFA), and polyunsaturated fatty acids (PUFA). The MD also includes increased amounts of fish, whole grain products, vegetables, fruits and nuts. The main source of fat in MD is olive oil, which provides most of the energy coming from fat (Dontas et al., 2007).
Another nutritional approach that allows to achieve many health benefits and extends a lifespan is the calorie restriction diet (CR). The diet is based on a 20-30% calorie cut from the basic metabolic rate. Despite the restriction, the CR diet should provide all of the nutrients needed for proper body functioning (Leanne and Ravussin, 2011). Scientific research has shown that this type of diet helps improve insulin resistance, hypertension, obesity, and basic biochemical variables. The CR diet has a significant impact on gene expression, particularly genes related to the skeletal muscle metabolism, brain and heart muscle (Heilbronn and Ravussin, 2003). However, there is little data about the type and effectiveness of the diet among former athletes. Therefore, it seems important to search for optimal nutrition for this population.
The Mediterranean diet has a significant impact on the improvement of lipid variables and insulin resistance, therefore it has cardiovascular protective effects. Some of these effects are caused by an increased intake of unsaturated fatty acids. Research has shown that the Mediterranean diet can improve the fatty acid profile in the serum and help reduce inflammation (Hagfors et al., 2005). A one year dietary intervention based on the Mediterranean diet in patients with rheumatoid arthritis improved EPA and DHA in the serum phospholipid. This research confirmed that these changes contributed to prevention of inflammation (Ambring et al., 2006). A study conducted on the Swedish population showed that even short term Mediterranean diet (4 weeks) could improve serum fatty acid concentration related to omega 3 and omega 6 families (Sala- Vila et al., 2011). Research based on the Mediterranean population reveals that cardiovascular events in this group are less frequent than in other populations. Furthermore, due to the high consumption of sea-food and fish, the omega – 3 index (the sum of eicosapentaenoic acid and docosahexaenoic acid) is higher in this group in comparison to the American population (Djuric, 2011). The high intake of EPA and DHA could, in part, explain the paradox of low rates of both cardiovascular events and cardiac death, despite a high background prevalence of cardiovascular risk factors (Calder, 2010).
We already know that some fatty acids such as linoleic acid (LA) and arachidonic acid (AA) can be transformed into immunomodulators, i.e., prostaglandins, leukotrienes, thromboxanes, and hydroxyeicosatetraenoic and hydroxyoctadecadienoic acids (HETE and HODE, respectively). Both EPA and DHA are the source of anti-inflammatory mediators while AA derivatives are strongly pro-inflammatory. EPA competes with AA for the same enzymes, therefore a higher level of EPA can reduce the level of AA products and inflammation. One of the most powerful inflammatory mediators are HETE and HODE. They are involved in many inflammation processes such as neutrophils and macrophages regulation, endothelial cells adhesion, reorganization of the cytoskeleton and production of cytokines (Rossaint et al., 2012).
The fatty acid profile as well as the eicosanoids profile can provide essential knowledge about diet, however, there is little data about fatty acids or their derivatives’ status during the calorie restriction diet.
The aim of the study was to investigate the effects of 6 week Mediterranean diet or 30% calorie restriction on the fatty acid profile and eicosanoids (hydroxyoctadecadienoi acids and hydroxyeicosatetraenoic acids) concentration. Furthermore, basic biochemical variables such as insulin, glucose, HOMA-IR and lipid profile were estimated.
Methods
Subjects
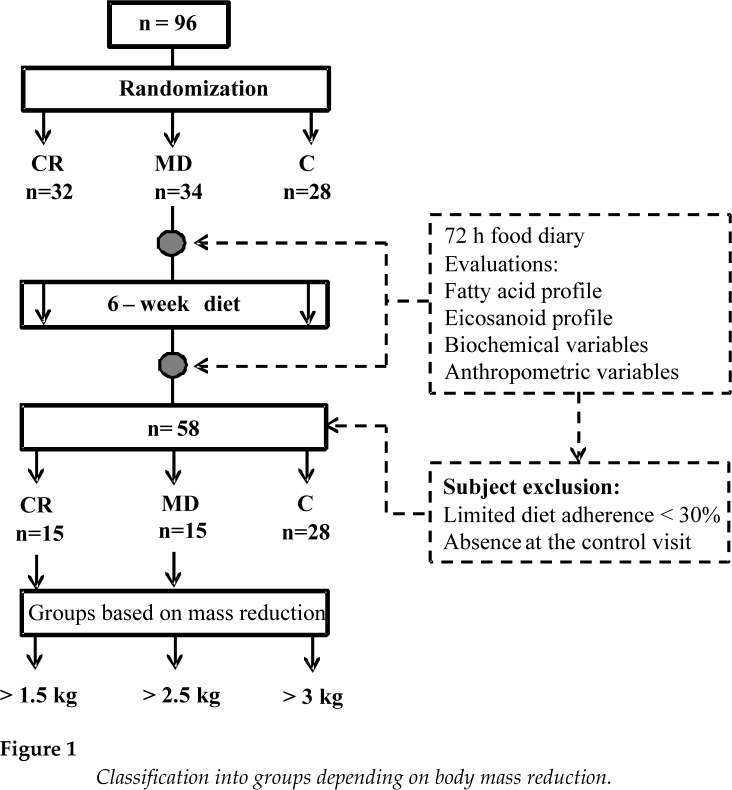
The study enrolled 94 Caucasian former athletes aged 20-42, with body height of 179 ± 16.00 cm and body mass of 89.26 ± 13.25 kg, who had not been active for at least 5 years. The study participants represented the following sport disciplines: canoeing, rowing, swimming, athletics, soccer, and different strength sports. The subjects were randomly assigned to one of the three intervention groups: CR group - calorie restriction (n = 32), MD group - Mediterranean diet (n = 34), and C group – a control group (n = 28). In the CR and MD groups, the subjects consumed daily less than 30% of their Total Daily Energy Expenditure (TDEE). Former athletes who suffered from celiac disease, hypertension, hypercholesterolemia or glucose intolerance were excluded from the study. The study protocol was approved by the ethics committee of the Pomeranian Medical University and conformed to the ethical guidelines of the 1975 Declaration of Helsinki. After the 6 week dietary intervention, 36 subjects were excluded from the study because they followed the diet in less than 30% (Figure 1).
Figure 1.
Classification into groups depending on body mass reduction.
Dietary intervention
The subjects were randomly divided into the CR, MD or C group during the first visit to the laboratory (Figure 1). The diet was programmed individually for each subject according to his/her caloric needsby a certificated nutritionist. Total daily energy expenditure (TDEE) was calculated using the direct measurement of the resting metabolic rate (RMR) that was measured twice, i.e., during the first and control visit, with a Fitmate apparatus (Pro, COSMED Italy). The activity factor (AF) was calculated using the standard formula (TDEE = AF x RMR). Subjects in the CR and MD groups with a BMI indicating overweight or obesity (BMI over 25 kg/m2) received a reduced calorie diet of 30% of their TDEE. Caloric restriction was established by reducing the diet by 30% considering the resting metabolic rate (RMR) (Table 1). The control group was not submitted to any type of diet. All individuals received a weekly dietary plan and guidelines on the composition and size of portions as well the times of meals throughout the day. The menu was prepared in the form of five daily meals, for seven days of the week. The recommended source of fat in all groups was olive oil. Animal fats such as lard were excluded, while usage of butter and margarine was permissible. The recommended source of carbohydrates included whole wheat bread, whole-wheat pasta, cereal and brown rice. Sweets were excluded. The recommended protein source comprised poultry, fish, fermented dairy products, eggs, lean cottage cheese, cheese with reduced fat content. Fat meat and other high fat products were excluded. Three daily portions of vegetables and two portions of fruit were recommended in the diets. The amount of fluid intake was calculated as 35 ml/kg of actual body mass per day. The details of particular diets are presented in Table 1.
Table 1.
Diet specification
| Diet type | Mediterranean diet | CR diet |
|---|---|---|
| Protein | 1g/kg body mass | 1g/kg body mass |
| Fats: | 25-35% of TDEE | <20% of TDEE |
| SFA | <7% | <10% |
| MUFA | >60% | >50% |
| PUFA | >20-35% | >20-35% |
| Cholesterol | 300 mg/day | 300 mg/day |
| Carbohydrates | 50-60% from TDEE | 50-60% from TDEE |
| Simple sugars | <10% from TDEE | <10% from TDEE |
| Fibre | 30-35 g/day | 30-35 g/day |
| Fish consumption | 3 times/week | 2 times/week |
| Snacks | Fruit and nuts | Fruit and natural yogurt |
Dietary control
After the 6 week intervention, all participants were evaluated for fatty acid content and their derivatives’ (eicosanoids) profiles, as well as basic anthropometric and biochemical variables. The pattern of nutrition was analysed using the 72 h food diary (including two working days and one weekend day) during the visits to the laboratory. The amount of food consumed was recorded in household units, by volume or by measuring with a ruler. The dietary records were validated by a nutritionist on the basis of the corresponding food table and the nutrient database.
Biochemical blood analysis
After an overnight fast, venous blood was collected and placed in tubes with anticoagulant for lipid analyses. Whole blood was collected and placed in ethylenediaminetetraacetic acid (EDTA) tubes. Blood was immediately placed on ice or in a refrigerator, and the samples were centrifuged at 3500 rpm for 10 min at 4°C within 2 h of collection. Plasma was then immediately stored under conditions to minimize artificial oxidation (i.e., with an antioxidant cocktail in an inert atmosphere). Standard blood biochemical analyses were carried out at the University Hospital Laboratory.
Isolation of fatty acids and analysis of fatty acid esters
Plasma was obtained from blood (taken at blood clot) by centrifugation for 10 min at 1200 G. Fatty acids were extracted with chloroform/methanol solution. About 0.5 ml of plasma was saponified with 1 ml of 2M KOH methanolic solution at 70°C for 20 min and then methylated with 2 ml of 14% solution of boron trifluoride in methanol under the same conditions. Then 2 ml of n-hexane and 10 ml saturated NaCl solution were added. 1 ml of the n-hexane phase was collected for analysis.
Gas chromatography was performed using an Agilent Technologies 7890A GC System (SUPELCOWAX™ 10 Capillary GC Column (15 m × 0.10 mm, 0.10 μm), Supelco, Bellefonte, PA, USA). Chromatographic conditions were as follows: the initial temperature was 60°C for 0 min, increased at the rate of 40°C/min to 160°C (0 min), then increased at the rate of 30°C/min to 190°C (0.5 min) and finally increased at the rate of 30°C/min to 230°C for 2.6 min, where it was maintained for 4.9 min. The total analysis lasted approximately 8 min and the gas flow rate was 0.8 ml/min with hydrogen as the carrier gas. Fatty acids were identified by comparing their retention times with those of commercially available standards.
Isolation and analyses of fatty acid derivatives
5(S),6(R)-Lipoxin A4, 5(S),6(R), 15(R)- Lipoxin A4, 5(S)-HETE, 5(S)-oxoETE, 12(S)-HETE, 15(S)-HETE, 16(R)/16(S)-HETE, 9(S)-HODE and 13(S)-HODE were extracted from the 0.5 ml of plasma by using a solid-phase extraction RP-18 SPE columns (Agilent Technologies, UK).
The HPLC separations were performed using Agilent Technologies 1260 liquid chromatography. Agilent ChemStation software (Agilent Technologies, Cheadle, UK) was employed for instrument control, data acquisition and analysis. The separation was completed on a Thermo Scientific Hypersil BDS C18 column 100x4.6mm 3μm (cat no. 28103-104630). The temperature of the column oven was set at 25°C. A gradient method was used where the mobile phase was composed of a mixture of solvent A (methanol/water/acetic acid, 50/50/0.1, v/v/v) and B (methanol/water/acetic acid, 100/0/0.1, v/v/v). The content of buffer B in the mobile phase was 30% at 0.0 min of separation, then it increased linearly to 80% at 20 min, was 98% between 20.1 and 23.9 min, and 30% between 24 and 28 min. The flow rate was 1.0 mL/min. The sample injection volume was 60 uL. The absorbance spectra of peaks were analysed to confirm the identification of analytes. The quantification was based on peak areas with internal standard calibration.
Statistical analysis
Statistica 7.1 software was used for the statistical analysis and the results are expressed as mean ± standard deviation. As distribution in most cases was normal (Shapiro-Wilk test), parametric tests were used: a t test for comparisons between groups, and p < 0.05 was considered as statistically significant.
Results
Thirty-six subjects were excluded from the study because they followed the diet in less than 30% or did not attend the control visit. Subjects who completed the experiment (n = 58) were analysed according to groups: MD, CR or C, and there were no significant differences between biochemical variables. Therefore we rearranged the groups and classified them depending on body mass reduction (>1.5 kg, >2.5 kg, >3 kg). Division into particular groups corresponded to the percentage of the completion of the dietary plan. The classification is shown in Table 2 and Figure 1.
Table 2.
Subject classification
| 6 weeks = 42 days of diet | ||
|---|---|---|
| Body mass reduction | CR group | MD group |
| Mean (SD) of calorie amount | 2173 (231) kcal | 2293 (269) kcal |
| > 1.5 kg | >31.25%# n = 15 | >50%# n = 15 |
| > 2.5 kg | >52%# n = 10 | >83%# n = 11 |
| > 3 kg | >62.5%# n = 7 | 100%# n = 8 |
The percentages of completion of a dietary plan
Biochemical variables, fatty acid and eicosanoid profiles were evaluated in the blood before and after the 6 week dietary intervention. The results showed that the following biochemical variables were significantly reduced in the CR group: LDL-cholesterol, triacylglycerols and total lipids. These results were observed at the lowest level of body mass reduction (>1.5 kg). In the case of > 3 kg reduction, we also observed an improvement in insulin and HOMA-IR levels. The MD group showed significantly lower concentration of total lipids, but only at the level of the reduction > 3 kg. We did not notice significant changes in the control group. The results of biochemical variables are shown in Table 3.
Table 3.
Biochemical variables of subjects from the CR and MD groups
| Variables | CR group | MD group | ||
|---|---|---|---|---|
| Reduction > 1.5 kg | Before intervention | After intervention | Before intervention | After intervention |
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | |
| Body mass index (BMI) [kg/m2] | 28.5 (1.25)* | 27 (1.05)* | 28.8 (8.5)* | 28.15 (14.15)* |
| Total cholesterol [mg/dl] | 203 (36) | 175 (20) | 186 (39.5) | 172 (36.7) |
| HDL-cholesterol [mg/dl] | 51 (11.5) | 46 (12.3) | 51 (16) | 44.5 (25.5) |
| LDL - cholesterol [mg/dl] | 120 (30.5)* | 107 (24)* | 120 (42.75) | 105 (32.25) |
| Triacylglycerols [mg/dl] | 176 (35.6)* | 165 (26)* | 104 (62.5) | 74.5 (65.5) |
| Fasting glucose [mg/ml] | 95 (33) | 97 (29) | 99 (66) | 95 (35.5) |
| Fasting insulin [U/ml] | 7.5 (4.2) | 6 (3.8) | 8 (3.2) | 7.7 (4.9) |
| HOMA-IR | 1.88 (0.56) | 1.85 (0.51) | 2.35 (2.16) | 1.77 (1.37) |
| Reduction > 2.5 kg | CR group | MD group | ||
| Body mass index (BMI) [kg/m2] | 30 (4.2)* | 28.3 (3.9)* | 30.6 (4.3)* | 29.7 (4)* |
| Total cholesterol [mg/dl] | 183 (16.2)* | 165 (19.5)* | 200 (51.7) | 192 (60) |
| HDL-cholesterol [mg/dl] | 50 (14.7) | 48.8 (13.4) | 46.4 (14.4) | 47.7 (15.8) |
| LDL - cholesterol [mg/dl] | 109 (95.9)* | 99.9 (15.4)* | 139 (90) | 143 (107.2) |
| Triacyloglycerols [mg/dl] | 122 (96.6)* | 82.6 (48.4)* | 247 (72) | 244 (46) |
| Fasting glucose [mg/ml] | 97 (5.8) | 92.1 (7) | 99 (8.1) | 96.1 (10.6) |
| Fasting insulin [U/ml] | 11 (7.1) | 10.2 (6.4) | 13.5 (7.35) | 13.8 (9.7) |
| HOMA-IR | 3 (1.8)* | 2.4 (1.6)* | 3.36 (1.9) | 2.17 (1.41) |
| Reduction > 3 kg | CR group | MD group | ||
| Body mass index (BMI) [kg/m2] | 32 (5.4)* | 31 (5.1)* | 30 (4.2)* | 28.3 (3.9)* |
| Total cholesterol [mg/dl] | 207 (29)* | 178 (8.4)* | 183 (16.2) | 165 (19.5) |
| HDL-cholesterol [mg/dl] | 50 (9.3) | 51 (13.6) | 50 (14.7) | 48.8 (13.4) |
| LDL - cholesterol [mg/dl] | 135 (28.9)* | 112 (14.5)* | 109 (25.9) | 25.9 (48.4) |
| Triacylglycerols [mg/dl] | 109 (56.1)* | 74 (26.3)* | 122 (48) | 82.6 (126) |
| Fasting glucose [mg/ml] | 94 (9.3) | 99 (5.3) | 97 (5.8) | 92.1 (7) |
| Fasting insulin [U/ml] | 15 (11.8)* | 10 (5.7)* | 11 (7.1) | 10.2 (6.4) |
| HOMA-IR | 4 (3.1)* | 2 (1.5)* | 2.35 (2.1) | 1.77 (1.37) |
p < 0.05
Analysis of the fatty acid profile showed significant changes in the concentration of polyunsaturated fatty acids. In both the CR and MD groups, an over 1.5 kg reduction did not reveal any changes. The CR group showed a significantly lower level of gamma-linoleic acid (GLA) and eicosapentaenoic acid (EPA). Subjects that followed the MD had significantly higher concentrations of docosahexaenoic acid (DHA). We did not observe any changes in the control group. The fatty acids profile is shown in Table 4. On the basis of changes in the fatty acid profile, the basic biomarker of coronary heart disease was calculated (Harris, 2009; Inoue et al., 2013; Oto et al., 2014). Regardless of the body mass reduction, both the control and CR group did not show any significant changes. Regarding the omega – 3 index, we observed an improvement in the MD group. This trend was found in subjects with the mass reduction of >2.5 kg and >3 kg (Table 4).
Table 4.
Fatty acid profile
| CR group | MD group | |||||||
|---|---|---|---|---|---|---|---|---|
| Before intervention | After intervention | Before intervention | After intervention | |||||
| Mean | (SD) | Mean | (SD) | Mean | (SD) | Mean | (SD) | |
| Reduction >1.5 kg | No significant changes | |||||||
| Reduction >2.5 kg | CR group | MD group | ||||||
| C14:0 myristic acid | 5.58 | 2.12 | 4.61 | 1.94 | 6.29 | 5.29 | 7.23 | 9.74 |
| C14:1 miristoleic acid | 2.11 | 0.79 | 1.85 | 0.42 | 1.76 | 0.60 | 2.13 | 0.72 |
| C15:0 pentadecanoic acid | 0.73 | 0.47 | 0.59 | 0.58 | 0.73 | 0.79 | 0.99 | 1.35 |
| C16:0 palmitic acid | 192.45 | 98.86 | 147.82 | 62.64 | 155.94 | 96.75 | 211.39 | 211.72 |
| C16:1 palmitoleic acid | 4.10 | 1.76 | 2.61 | 0.91 | 5.11 | 5.98 | 7.09 | 12.60 |
| C17:0 heptadecanoic acid | 1.13 | 5.70 | 1.13 | 0.27 | 0.99 | 0.61 | 1.27 | 1.25 |
| C18:0 stearic acid | 135.96 | 77.87 | 108.49 | 57.28 | 94.07 | 43.30 | 120.32 | 64.75 |
| C18:1w9 oleic acid | 26.92 | 17.50 | 18.88 | 7.02 | 28.78 | 28.88 | 44.23 | 77.38 |
| C18:1trans11 trans-vaccenic acid | 4.23 | 2.21 | 2.98 | 1.38 | 3.82 | 3.11 | 5.72 | 7.11 |
| C18:2n-6 linoleic acid | 68.39 | 43.91 | 51.14 | 21.43 | 49.10 | 31.22 | 74.15 | 97.69 |
| C18:3n-6 gamma-linolenic acid | 0.80* | 0.36 | 0.55* | 0.32 | 0.82 | 0.39 | 1.03 | 1.42 |
| C18:3n-3 linolenic acid | 2.32 | 2.11 | 1.73 | 0.54 | 2.41 | 1.65 | 3.84 | 6.83 |
| C20:4 n-6 arachidonic acid | 19.51 | 10.25 | 14.94 | 6.29 | 13.62 | 6.39 | 19.58 | 15.59 |
| C20:5 n-3 eicosapentaenoic acid (EPA) | 1.46* | 1.05 | 0.85* | 0.33 | 0.81 | 0.56 | 1.12 | 0.78 |
| C22:6 n-3 docosahexaenoic acid (DHA) | 9.23 | 5.60 | 7.15 | 2.33 | 5.71* | 3.73 | 8.95* | 6.65 |
| Omega 3 index (EPA+DHA) | 9.89 | 3.58 | 8.10 | 2.83 | 7.18* | 4.43 | 9.24* | 7.26 |
| Reduction >3 kg | CR group | MD group | ||||||
| C10:0 Lauric acid | 4.29 | 1.45 | 4.37 | 1.55 | 3.69 | 1.26 | 3.66 | 0.53 |
| C14:0 myristic acid | 4.76 | 0.51 | 4.79 | 2.31 | 4.89 | 1.96 | 4.17 | 1.43 |
| C14:1 miristoleic acid | 1.73 | 0.18 | 2.00 | 0.51 | 1.55 | 0.41 | 1.84 | 0.21 |
| C15:0 pentadecanoic acid | 0.47 | 0.36 | 0.59 | 0.62 | 0.59 | 0.50 | 0.56 | 0.33 |
| C16:0 palmitic acid | 142.61 | 25.87 | 153.59 | 70.76 | 141.59 | 51.09 | 148.71 | 30.53 |
| C16:1 palmitoleic acid | 3.39 | 0.94 | 3.29 | 1.42 | 3.63 | 2.01 | 3.00 | 1.33 |
| C17:0 heptadecanoic acid | 1.00 | 0.31 | 1.17 | 0.32 | 0.93 | 0.26 | 0.88 | 0.35 |
| C18:0 stearic acid | 99.50 | 27.72 | 111.85 | 62.07 | 97.14 | 37.39 | 108.62 | 28.73 |
| C18:1w9 oleic acid | 20.66 | 3.71 | 20.62 | 7.96 | 23.16 | 12.69 | 19.53 | 4.92 |
| C18:1trans11 trans-vaccenic acid | 3.34 | 0.41 | 3.06 | 1.67 | 3.28 | 1.37 | 3.27 | 0.81 |
| C18:2n-6 linoleic acid | 47.69 | 7.63 | 50.13 | 23.93 | 47.43 | 19.46 | 41.71 | 9.93 |
| C18:3n-6 gamma-linolenic acid | 0.80* | 0.09 | 0.59* | 0.29 | 0.77 | 0.22 | 0.64 | 0.30 |
| C18:3n-3 linolenic acid | 1.82 | 0.29 | 1.81 | 0.50 | 2.18 | 1.06 | 1.65 | 0.24 |
| C20:4 n-6 arachidonic acid | 16.08 | 2.44 | 16.57 | 6.50 | 13.40 | 2.75 | 14.23 | 3.27 |
| C20:5 n-3 eicosapentaenoic acid (EPA) | 1.16* | 0.50 | 0.78* | 0.31 | 0.85 | 0.48 | 0.98 | 0.30 |
| C22:6 n-3 docosahexaenoic acid (DHA) | 7.13 | 1.84 | 6.40 | 1.90 | 5.58* | 2.74 | 6.84* | 2.26 |
| Omega 3 index (EPA+DHA) | 8.30 | 2.31 | 7.19 | 2.15 | 7.09* | 3.93 | 7.95* | 2.64 |
p < 0.05
The main eicosanoids (fatty acid derivatives) were measured in the serum of each subject. The reductions of body mass of >1.5 kg and >2.5 kg did not induce any changes in any of the groups. The subjects from the MD group who lost over 3 kg reduced the level of 15- hydroxyeicosatetraenoic acid (15 – HETE). We did not observe any differences in the control and CR groups. Table 5 shows the eicosanoids profile.
Table 5.
Eicosanoids profile
| CR group | MD group | |||||||
|---|---|---|---|---|---|---|---|---|
| Eicosanoids | Before intervention | After intervention | Before intervention | After intervention | ||||
| Mean | (SD) | Mean | (SD) | Mean | (SD) | Mean | (SD) | |
| Reduction of >1.5 kg | No significant changes | |||||||
| Reduction of > 2.5 kg | No significant changes | |||||||
| Reduction of >3 kg | Before intervention | After intervention | Before intervention | After intervention | ||||
| Mean | (SD) | Mean | (SD) | Mean | (SD) | Mean | (SD) | |
| LTX5S6R15R | 0.087 | 0.091 | 0.07 | 0.083 | 0.16 | 0.129 | 0.12 | 0.148 |
| 16RS-HETE | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 |
| 13S-HODE | 0.012 | 0.017 | 0.007 | 0.01 | 0.015 | 0.012 | 0.015 | 0.017 |
| 9S-HODE | 0.045 | 0.054 | 0.023 | 0.031 | 0.048 | 0.037 | 0.042 | 0.049 |
| 15S-HETE | 0.068 | 0.064 | 0.04 | 0.049 | 0.102* | 0.067 | 0.051* | 0.054 |
| 12S-HETE | 0.265 | 0.267 | 0.157 | 0.172 | 0.378 | 0.301 | 0.191 | 0.23 |
| 5-oxo-ETE | 0.015 | 0.018 | 0.01 | 0.011 | 0.016 | 0.014 | 0.015 | 0.016 |
p<0.05
Discussion
Former athletes constitute a specific population as previous intense physical activity has significantly changed their metabolism, yet little is known about the consequences of these changes after the end of their sports carriers. There are no dietary recommendations for this population. Our study examined the effects of two types of diets on former athletes. One of the diets was based on a 30% calorie restriction, while the other was the Mediterranean diet containing increased amounts of monounsaturated fatty acids (MUFA, from olive oil). The difference between the MD and CR groups was also in their total fat consumption. The diet intervention was relatively short and lasted 6 weeks (42 days). The evaluation of the effectiveness of a diet usually starts with basic anthropometric measurements, including calculation of the BMI. The next step consists of the analysis of basic biochemical variables such as the lipid profile, fasting glucose and insulin level. These simple variables allow to assess the general condition of subjects, the risk of common diseases, and the effectiveness of dietary intervention (Huang, 2009).
Based on the results of biochemical tests and body mass reduction, it can be concluded that the calorie restriction is more appropriate for subjects with dyslipidemia or insulin resistance. It should be highlighted that even a modest body mass reduction of 1.5 kg significantly improves the bloods’ lipid profile, while such changes are not observed in case of the MD (Fontana et al., 2004).
Body mass reduction in the CR group of more than 3 kg helped improve the lipid profile and normalized the level of insulin and insulin resistance in subjects who completed more than 60% of the established dietary plan. Recent studies confirm that caloric restriction helps reduce insulin resistance and insulin levels. This relationship was observed even in former athletes who did not exercise (Larson-Meyer et al., 2006).
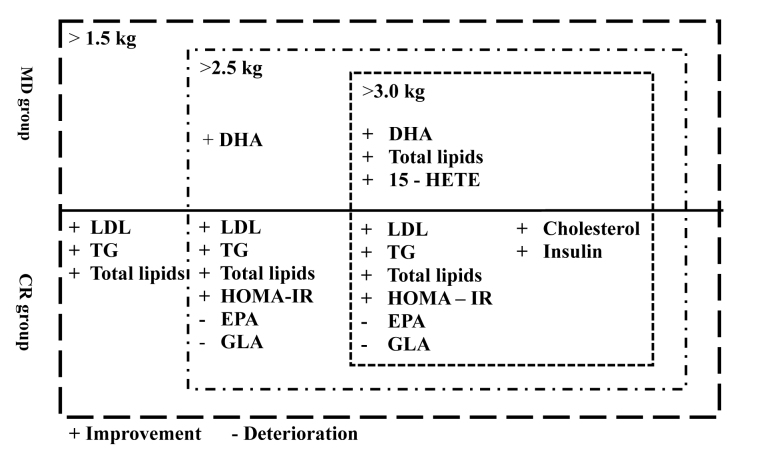
In the MD group, most of the biochemical variables did not change significantly (Table 3, Figure 2). Only in the subgroup with a reduction of more than 3 kg we registered a lower concentration of total lipids, yet the other lipid variables and insulin resistance did not improve significantly. The lack of changes in biochemical variables can be caused by a relatively short time of the dietary intervention.Greco et al. (2014) showed that even short-term application of the Mediterranean diet (4 weeks) improved insulin resistance and reduced insulin secretion. The authors of that study also established significant calorie restrictions, with the total amount of calorie intake ranging from 1400 to 1600 kcal/day. In our experiment, the average energy intake was 2293 kcal (SD = 269) (Greco et al., 2014). Considering the results of other studies and our observations, we can conclude that a high calorie restriction is the major factor in improving the lipid profile and insulin sensitivity. Yet, the mechanism of insulin secretion during calorie restriction is not fully understood. It is known that the improvement is associated with a decreased amount of free fatty acids circulating in the blood and cytokines released by adipose tissue (Boden, 2001; Jacob et al., 1999). Calorie restriction also increases the glucose infusion rate which helps maintain a proper level of glucose during hyperinsulinemia and results in increased peripheral insulin sensitivity (Johnson et al., 2016).
Figure 2.
Effects of the MD and CR diets on the lipid profile
Analysis of the fatty acid profile revealed that subjects consuming the MD had a significantly higher level of DHA after the dietary intervention, whereas the CR group showed significantly lower concentrations of EPA and GLA. Both EPA and DHA are very important in the human diet as they regulate many essential metabolic processes, gene expression and build cell membranes in body structures (Conquer et al., 2000; Lazzarin et al., 2009; Smith et al., 2011). One of the most important roles of EPA and DHA includes the regulation of inflammatory processes. Enzymatic products of these acids help suppress the inflammatory process (Calder, 2010) and play a crucial role in cardiovascular disease prevention. EPA and DHA supplementation decreases endothelial activation, improves plaque stability and vascular permeability (Dawczynski et al., 2010; Swanson et al., 2012; Superko et al., 2013). The former athletes that were subjected to the Mediterranean diet increased the level of DHA, and improved the basic index related to the fatty acid profile and cardiovascular diseases (Figure 2). We did not observe such changes in the CR group.
Subjects who followed the Mediterranean diet and reduced their body mass by more than 3 kg also revealed lower levels of 15-HETE, which is an enzymatic product of arachidonic acid oxidation. The increased production of 15-HETE has been confirmed in the process of atherosclerosis, both in the animal model, as well as in human trials. Henrikson et al. (1985) conducted a study where rabbits were fed a high cholesterol diet in order to develop atherosclerosis. The study showed that the main metabolite of arachidonic acid during the process of atherosclerosis was 15-HETE (Henriksson et al., 1985). 15 HETE is synthesised primarily by macrophages during the process initiated by 15 lipoxygenase (LOX 15) and 12 lipoxygenase (LOX 12). 12 and 15 LOX have the ability to oxidize phospholipids and cholesterol in membranes. The oxidized form of cholesterol esters induces a number of pathological processes which lead to pro-inflammatory cytokine synthesis. 15 HETE increases adhesion of chemokines and adhesion of molecules to the endothelial cells. This process has a significant impact on monocyte circulation, as well as the migration and proliferation of smooth muscle cells. Additionally, 15 HETE activates NADPH oxidase, which is regarded as a key enzyme in the pathogenesis of atherosclerosis (Funk, 2006).
Both the MD and diet based on calorie restriction helped reduce body mass in former athletes. However, it seems that the mechanism of action in both diets was quite different. A CR diet was very effective in the improvement of the lipid profile and reduction of insulin resistance. We observed a significant decrease in crucial biochemical variables after only 6 weeks of the dietary intervention even in subjects with a 2 kg body mass reduction. However, it should be also noted that the 6-week calorie restriction caused unfavourable changes in the fatty acid profile (through lower concentrations of EPA) and did not result in the reduction of inflammation. On the other hand, our study indicates that even a short term MD can improve these variables (Figure 2). According to previous research, the Mediterranean diet has a special cardio-protective effect, which was confirmed by our study (Perez-Martínez et al., 2017).
Programming optimal nutrition plans for former athletes is a very difficult issue because of great diversity in the exercise metabolism of particular sport disciplines and different anthropometric characteristics. We can conclude that calorie restriction is a healthy solution for former athletes. Even a short period of a calorie restriction diet can significantly improve most basic biochemical variables. The novel aspect of our research includes the finding that calorie restriction diets contain an insufficient amount of omega 3 and 6 fatty acids. For practical implications, we can suggest that former athletes and other subjects following the calorie restriction diet should consider omega – 3 fatty acid supplementation, especially EPA and DHA, to reduce inflammatory processes and prevent cardiovascular diseases.
Acknowledgements
The study was supported by the grant from the NCN No. KB-0012/53/11.
References
- Ambring A, Johansson M, Axelsen M, Gan L, Strandvik B, Friberg P.. Mediterranean-inspired diet lowers the ratio of serum phospholipid n 6 to n 3 fatty acids, the number of leukocytes and platelets, and vascular endothelial growth factor in healthy subjects. Am J Clin Nutr. 2006;83(3):575–581. doi: 10.1093/ajcn.83.3.575. [DOI] [PubMed] [Google Scholar]
- Arliani GG, Lara PS, Astur DC, Cohen M, Gonçalves JPP, Ferretti M.. Impact of sports on health of former professional soccer players in Brazil. Acta Ortop Bras. 2014;22:188–90. doi: 10.1590/1413-78522014220400954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assmann G, de Basker G, Bagnara S.. International consensus statement on olive oil and the Mediterranean diet- implications for health in Europe. Eur J Cancer Prev. 1997;6:418–21. doi: 10.1097/00008469-199710000-00002. [DOI] [PubMed] [Google Scholar]
- Backmand H, Kujala U, Sarna S, Kaprio J.. Former athletes’ health-related lifestyle behaviours and self-rated health in late adulthood. Int J Sports Med. 2010;31:751–8. doi: 10.1055/s-0030-1255109. [DOI] [PubMed] [Google Scholar]
- Boden G.. Free fatty acids- the link between obesity and insulin resistance. Endocr Pract. 2001;7:44–51. doi: 10.4158/EP.7.1.44. [DOI] [PubMed] [Google Scholar]
- Calder P.. Omega-3 Fatty Acids and Inflammatory Processes. Nutrients. 2010;2:355–374. doi: 10.3390/nu2030355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conquer JA, Tierney MC, Zecevic J, Bettger WJ, Fisher RH.. Fatty acid analysis of blood plasma of patients with Alzheimer’s disease, other types of dementia, and cognitive impairment. Lipids. 2000;35:1305–12. doi: 10.1007/s11745-000-0646-3. [DOI] [PubMed] [Google Scholar]
- Czuba M, Maszczyk A, Gerasimuk D, Roczniok R, Fidos-Czuba O, Zajac A, Gołas A, Mostowik A, Langfort J.. The Effects of Hypobaric Hypoxia on Erythropoiesis, Maximal Oxygen Uptake and Energy Cost of Exercise Under Normoxia in Elite Biathletes. J Sport Scie Med. 2014;13(4):912–920. [PMC free article] [PubMed] [Google Scholar]
- Dawczynski C, Martin L, Wagner A, Jahreis G.. n-3 LC-PUFA-enriched dairy products are able to reduce cardiovascular risk factors: a doubleblind, cross-over study. Clin Nutr. 2010;29:592–9. doi: 10.1016/j.clnu.2010.02.008. [DOI] [PubMed] [Google Scholar]
- Djuric Z.. The Mediterranean diet: Effects on proteins that mediate fatty acid metabolism in the colon. Nutr Rev. 2011;69(12):730–744. doi: 10.1111/j.1753-4887.2011.00439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dontas A, Zerefos N, Panagiotakos D, Valis D.. Mediterranean diet and prevention of coronary heart disease in the elderly. Clin Interv Aging. 2007;2:109–115. doi: 10.2147/ciia.2007.2.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana L, Meyer TE, Klein S, Holloszy JO.. Long-term calorie restriction is highly effective in reducing the risk for atherosclerosis in humans. Proc Natl Acad Sci USA. 2004;101:6659–6663. doi: 10.1073/pnas.0308291101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk CD.. Lipoxygenase pathways as mediators of early inflammatory events in atherosclerosis. Arterioscler Thromb Vasc Biol. 2006;26:1204–6. doi: 10.1161/01.ATV.0000222960.43792.ff. [DOI] [PubMed] [Google Scholar]
- Greco M, Chiefari E, Montalcini T, Accattato F, Costanzo FS, Pujia A, Foti D, Brunetti A, Early GE.. Effects of a Hypocaloric, Mediterranean Diet on Laboratory Parameters in Obese Individuals. Mediators of Inflamm. 2014;750-860:8. doi: 10.1155/2014/750860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagfors L, Nilsson I, Sköldstam L, Johansson G.. Fat intake and composition of fatty acids in serum phospholipids in a randomized, controlled, Mediterranean dietary intervention study on patients with rheumatoid arthritis. Nutr Metab (Lond) 2005;2:26. doi: 10.1186/1743-7075-2-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris WS.. The omega-3 index: from biomarker to risk marker to risk factor. Curr Atheroscler Rep. 2009;116:411–7. doi: 10.1007/s11883-009-0062-2. [DOI] [PubMed] [Google Scholar]
- Heilbronn LK, Ravussin E.. Calorie restriction and aging: review of the literature and implications for studies in humans. Am J Clin Nutr. 2003;78:361–369. doi: 10.1093/ajcn/78.3.361. [DOI] [PubMed] [Google Scholar]
- Henriksson P, Hamberg M, Diczfalusy U.. Formation of 15-HETE as a major hydroxyeicosatetraenoic acid in the atherosclerotic vessel wall. Biochim Biophys Acta. 1985;834:272–4. doi: 10.1016/0005-2760(85)90166-3. [DOI] [PubMed] [Google Scholar]
- Huang PL.. A comprehensive definition for metabolic syndrome. Dis Model Mech. 2009;2:231–237. doi: 10.1242/dmm.001180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue K, Kishida K, Hirata A, Funahashi A, Shimomura I.. Low serum eicosapentaenoic acid/ arachidonic acid ratio in male subjects with visceral obesity. Nutr Metab. 2013;10:25. doi: 10.1186/1743-7075-10-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito R, Satoh-Asahara N, Yamakage H, Sasaki Y, Odori S, Kono S, Wada H, Suganami T, Ogawa Y, Hasegawa K, Shimatsu A.. An increase in the EPA/AA ratio is associated with improved arterial stiffness in obese patients with dyslipidemia. J Atheroscler Thromb. 2014;21:248–60. doi: 10.5551/jat.19976. [DOI] [PubMed] [Google Scholar]
- Jacob S, Machann J, Rett K, Brechtel K, Volk A, Renn W, Maerker E, Matthaei S, Schick F, Claussen CD, Häring HU.. Association of increased intramyocellular lipid content with insulin resistance in lean nondiabetic offspring of type 2 diabetic subjects. Diabetes. 1999;48:1113–9. doi: 10.2337/diabetes.48.5.1113. [DOI] [PubMed] [Google Scholar]
- Johnson ML, Distelmaier K, Lanza IR, Irving BA, Robinson MM, Konopka AR, Shulman GI, Nair KS.. Mechanism by Which Caloric Restriction Improves Insulin Sensitivity in Sedentary Obese Adults. Diabetes. 2016;65:74–84. doi: 10.2337/db15-0675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley DE, Goodpaster BH.. Effects of exercise on glucose homeostasis in type 2 diabetes mellitus. Med Sci Sports Exerc. 2001;33:495–501. doi: 10.1097/00005768-200106001-00020. [DOI] [PubMed] [Google Scholar]
- Kohl HW.. Physical activity and cardiovascular disease: evidence for a dose response. Med Sci Sports Exerc. 2010;33:472–83. doi: 10.1097/00005768-200106001-00017. [DOI] [PubMed] [Google Scholar]
- Kouris-Blazos A, Gnardellis C, Wahlqvist ML.. Are the advantages of the Mediterranean diet transferable to other populations? A cohort study in Melbourne, Australia. Br J Nutr. 1999;82:57–61. doi: 10.1017/s0007114599001129. [DOI] [PubMed] [Google Scholar]
- Kujala UM, Kaprio J, Taimela S, Sarna S.. Prevalence of diabetes, hypertension, and ischemic heart disease in former elite athletes. Metabolism. 1994;43:1255–60. doi: 10.1016/0026-0495(94)90219-4. [DOI] [PubMed] [Google Scholar]
- Larson-Meyer DE, Heilbronn LK, Redman LM, Newcomer BR, Frisard MI, Anton S, Smith SR, Alfonso A, Ravussin E.. Effect of Calorie Restriction With or Without Exercise on Insulin Sensitivity, β-Cell Function, Fat Cell Size, and Ectopic Lipid in Overweight Subjects. Diabetes Care. 2006;29:1337–1344. doi: 10.2337/dc05-2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazzarin N, Vaquero E, Exacoustos C, Bertonotti E, Romanini ME, Arduini D.. Low-dose aspirin and omega- 3 fatty acids improve uterine artery blood flow velocity in women with recurrent miscarriage due to impaired uterine perfusion. Fertil Steril. 2009;92:296–300. doi: 10.1016/j.fertnstert.2008.05.045. [DOI] [PubMed] [Google Scholar]
- Leanne M, Eric Ravussin. Caloric Restriction in Humans: Impact on Physiological, Psychological, and Behavioral Outcomes. Antioxid Redox Signal. 2011;14:275–28. doi: 10.1089/ars.2010.3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon AS, Sanchez OA.. Response of blood lipids to exercise training alone and combined with dietary intervention. Med Sci Sports Exerc. 2001;33:502–15. doi: 10.1097/00005768-200106001-00021. [DOI] [PubMed] [Google Scholar]
- Ostrowski A, Strzala M, Stanula A, Juszkiewicz M, Pilch W, Maszczyk A.. The Role of Training in the Development of Adaptive Mechanisms in Freedivers. J Hum Kinet. 2012;32:197–210. doi: 10.2478/v10078-012-0036-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Martínez P, Mikhailidis DP, Athyros VG, Bullo M, Couture P, Covas MI, de Koning L, Delgado-Lista J, Díaz-López A, Drevon CA, Estruch R, Esposito K, Fitó M, Garaulet M, Giugliano D, García-Ríos A, Katsiki N, Kolovou G, Lamarche B, Maiorino MI, Mena-Sánchez G, Muñoz-Garach A, Nikolic D, Ordovás JM, Pérez-Jiménez F, Rizzo M, Salas-Salvadó J, Schröder H, Tinahones FJ, de la Torre R, van Ommen B, Wopereis S, Ros E, López-Miranda J.. Lifestyle recommendations for the prevention and management of metabolic syndrome: an international panel recommendation. Nutr Rev. 2017;75:307–326. doi: 10.1093/nutrit/nux014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roczniok R, Stanula A, Maszczyk A, Mostowik A, Kowalczyk A, Fidos-Czuba O, Zając A.. Physiological and physical profiles and on-ice performance approach to predict talent in male youth ice hockey players during draft to hockey team. IES. 2013;21(2):121–127. [Google Scholar]
- Rossaint J, Nadler JL, Ley K, Zarbock A. Eliminating or blocking 12/15-lipoxygenase reduces neutrophil recruitment in mouse models of acute lung injury. Crit Care. 2012;13-16(5):166. doi: 10.1186/cc11518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sala- Vila A, Harris WS, Cofan M, Perez-Heras AN, Pinto X, Lamuela- Ravento RM, Covas MR, Estruch R, Ros E.. Determinants of the omega-3 index in a Mediterranean population at increased risk for CHD. Br J Nutr. 2011;106:425–431. doi: 10.1017/S0007114511000171. [DOI] [PubMed] [Google Scholar]
- Sarna S, Kaprio J, Kujala UM, Koskenvuo M.. Health status of former elite athletes. The Finnish experience. Aging. 1997;9:35–41. doi: 10.1007/BF03340126. [DOI] [PubMed] [Google Scholar]
- Smith GI, Atherton P, Reeds DN, Mohammed BS, Rankin D, Rennie MJ, Mittendorfer B.. Dietary omega-3 fatty acid supplementation increases the rate of muscle protein synthesis in older adults: a randomized controlled trial. Am J Clin Nutr. 2011;93:402–12. doi: 10.3945/ajcn.110.005611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Superko RH, Faha MD, Superko SM, Nasir K, Agatston A, Garrett BC.. Contemporary Reviews in Cardiovascular Medicine. Omega-3 Fatty Acid Blood Levels. Clinical Significance and Controversy. Circulation. 2013;128:2154–2161. doi: 10.1161/CIRCULATIONAHA.113.002731. [DOI] [PubMed] [Google Scholar]
- Swanson D, Block R, Mousa SA.. Omega-3 fatty acids EPA and DHA: health benefits throughout life. Adv Nutr. 2012;3:1–7. doi: 10.3945/an.111.000893. [DOI] [PMC free article] [PubMed] [Google Scholar]