Abstract
Owing to their great diversity and abundance, ammonites and belemnites represented key elements in Mesozoic food webs. Because of their extreme ontogenetic size increase by up to three orders of magnitude, their position in the food webs likely changed during ontogeny. Here, we reconstruct the number of eggs laid by large adult females of these cephalopods and discuss developmental shifts in their ecologic roles. Based on similarities in conch morphology, size, habitat and abundance, we suggest that similar niches occupied in the Cretaceous by juvenile ammonites and belemnites were vacated during the extinction and later partially filled by holoplanktonic gastropods. As primary consumers, these extinct cephalopod groups were important constituents of the plankton and a principal food source for planktivorous organisms. As victims or, respectively, profiteers of this case of ecological replacement, filter feeding chondrichthyans and cetaceans likely filled the niches formerly occupied by large pachycormid fishes during the Jurassic and Cretaceous.
Keywords: Belemnitida, Ammonoidea, Cretaceous, Fecundity, Palaeogene, Filter feeders, Holoplanktonic gastropoda, Pachycormiformes, Mass extinctions
Introduction
The fate of individual groups of marine organisms at mass extinctions is of considerable interest (e.g., Jablonski & Raup, 1994; Jablonski, 2008). By contrast, the disappearance of entire communities or ecological associations or food webs or important parts of any of these structures from the geologic past still requires a lot of palaeontological research (Hautmann, 2014; Hofmann et al., 2014; Roopnarine & Angielczyk, 2015). Extinctions of entire communities or ecosystems are most conspicuous during the great mass extinctions (e.g., Foster & Twitchett, 2014), when usually vast new ecospace was freed and thereby, new ecological niches could form during recovery periods.
Although it is not the most severe of the Big Five, the end-Cretaceous mass extinction is likely the most famous among those with the greatest severity (McGhee et al., 2013). This fame roots in the facts that popular groups of organisms such as dinosaurs (Sloan et al., 1986; Archibald & Fastovsky, 2004) and ammonites (Goolaerts, 2010; Kennedy, 1993; Landman et al., 2015) were erased by the consequences of an impact in Mexico and/or flood basalt-eruptions in India (Keller et al., 2009; Miller et al., 2010; Schulte et al., 2010; Tobin et al., 2012).
Marine communities were heavily affected as reflected in a significant reduction of diversity or a partial or even total disappearance of major groups such as ammonoids and belemnites (Doyle, 1992; Marshall & Ward, 1996; Iba et al., 2011; Olivero, 2012; Landman et al., 2014) as well as planktonic foraminifers (Alvarez et al., 1980; Smit, 1982) and bivalves (Jablonski & Raup, 1994). Before this extinction, ammonoids were both highly diverse and had evolved a great disparity in the course of the Cretaceous (Ward & Signor, 1983; Ward, 1996); some of the most bizarre forms (heteromorphs) such as Nipponites, Diplomoceras and Didymoceras appeared, some of which reached impressive sizes (e.g., Diplomoceras, Emericiceras). Furthermore, members of the family Puzosiidae, which comprises the largest ammonoids of all times, also lived during Cretaceous times (Landois, 1895; Olivero & Zinsmeister, 1989; Kennedy & Kaplan, 1995). Puzosiids are not only gigantic but they also abundantly occurred worldwide. In addition to this family, members of other ammonite families reached sizes of around one meter in the Late Cretaceous as well.
The sometimes extreme variation in conch size and morphological disparity makes it likely that their modes of life and habitats differed as well. This is reflected in hatchling conch morphology, which may produce shapes unknown from adult ammonoid conchs (Klug, De Baets & Korn, 2016). A further line of reasoning supporting ontogenetic changes in ecology is the change in hydrodynamic properties due to size changes (Jacobs, 1992; Jacobs & Chamberlain, 1996; Naglik et al., 2015).
The great abundance, wide geographical distribution, extreme diversity, middle to giant size in combination with the likely high fecundity of ammonites raises a number of questions: (i) what respective roles did juvenile and adult ammonites and belemnites play in Cretaceous marine food webs? (ii) The adults were probably planktotrophic consumers, but what was the role of their minute offspring? (iii) What groups filled the ecospace occupied by ammonites, belemnites and their predators after the Cretaceous? (iv) How did this ecological replacement occur?
Methods
We estimated the fecundity of large Cretaceous ammonites such as Parapuzosia seppenradensis (Fig. 1) using the following facts, assumptions and measurements, which probably applied to all members of the Ammonoidea. (i) we know that the major part of egg-development happened in the body chamber (De Baets, Landman & Tanabe, 2015; Mironenko & Rogov, 2016); (ii) there is good evidence that the ammonitella represents the embryonic part of the conch (De Baets, Landman & Tanabe, 2015); (iii) we suggest that egg-size only slightly exceeded ammonitella-size because of their dense packing in fossils with embryos preserved in the body chamber (De Baets, Landman & Tanabe, 2015; Mironenko & Rogov, 2016); and (iv) we followed the proportion of 8% of the soft body volume being occupied by the gonads according to the proportions known from Recent Nautilus (Tanabe & Tsukahara, 1987; Korn & Klug, 2007; De Baets, Landman & Tanabe, 2015). As far as (iv) is concerned, there is some uncertainty because the proportions of the ovaries are poorly known from ammonoids due to the extremely rare and fragmentary preservation of soft parts (Mironenko & Rogov, 2016; Lehmann, 1981; Lehmann, 1985; Klug & Lehmann, 2015; Klug, Riegraf & Lehmann, 2012). When regarding the specimens figured by Mironenko & Rogov (2016), one tends to assume that the gonads filled a much larger portion of the body chamber. This hypothesis finds further support in symmetric bulges in the posterior body chamber in mature Pachydesmoceras (Fig. 1) and scaphitid conchs (Kennedy, 1989). These bulges may have offered space for the growing ovaries. Owing to these materials and morphological adult modifications of ammonoid conchs, we calculated alternative maximum egg-numbers using a body chamber volume proportion occupied by gonads of 30%.
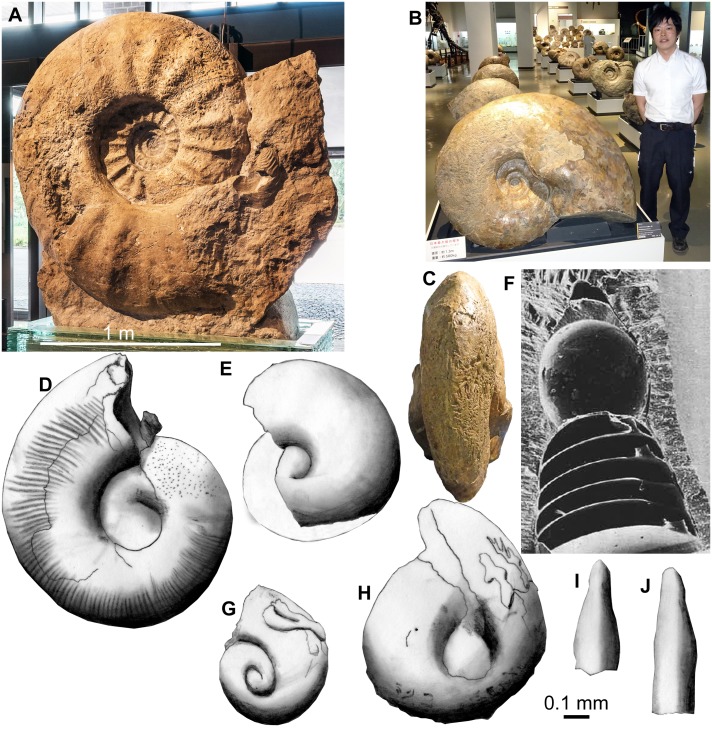
Figure 1. Adult ammonites (A–C), juvenile ammonites (D, E), and an embryonic belemnite (F) compared to fossil conchs of Thecosomata from the Eocene of India (G–J). 0.1 mm-scale bar applies to figures D to J.
Photo in (A) courtesy C Steinweg, L Schöllmann and J-O Kriegs (all Mnster); (D and E) redrawn after Tanabe, Kulicki & Landman (2008); (F) from Bandel et al. (1984); (G to J) redrawn after Lokho & Kumar (2008). (A) Parapuzosia seppenradensis, Campanian, Seppenrade. (B) C. Pachydesmoceras sp., Campanian, Hokkaido, diameter 1.3 m, D. Aiba (Mikasa) for scale. Note the symmetrical bulges in the posterior body chamber in C. (D) juvenile conch of Scaphites whitfieldi, AMNH 44833, Turonian, USA. (E) embryonic conch of Aconeceras cf. trautscholdi, UMUT MM 29439–4, Aptian, Russia. (F) embryonic conch of Hibolithes sp., GPIT Ce 1599, Callovian, Lithuania. (G) to (J), Upper Disang Formation, Phek District, Nagaland. (G, H) Limacinidae spp. (I, J) Creseidae spp.
The largest specimen of the largest ammonite species Parapuzosia seppenradensis is incomplete (Landois, 1895; Kennedy & Kaplan, 1995). This specimen measures 1,740 mm (Fig. 1). We estimated the adult body chamber volume and the surface area of the terminal aperture assuming a body chamber length of about 180 degrees because of shell traces of the missing conch part along the umbilical seam. Accordingly, the maximum diameter dm can be reconstructed to have reached 2,200 mm with a whorl height wh of about 800 mm and a whorl width ww of about 500 mm. The radiuses would then measure 1,250 mm (r1) at the terminal aperture and 950 mm (r2) on the opposite side. Using the wh and ww values, we reconstructed a whorl cross section in CorelDraw and measured the area; accordingly, the cross section area K amounts to almost 320,000 mm2.
As shown by De Baets et al. (2012), derived ammonoids (i.e., with fully coiled embryonic conchs) likely had a high fecundity. This is corroborated by the great differences between embryo size and adult conch size. For example, in the largest specimen of Parapuzosia seppenradense from the Late Cretaceous of Germany, the embryonic conch measured about one millimeter in diameter at hatching, while the adult conch exceeded two meters in diameter (Kennedy & Kaplan, 1995; De Baets et al., 2012; De Baets, Landman & Tanabe, 2015; Korn & Klug, 2007; Landman, Tanabe & Shigeta, 1996; Tanabe, Kulicki & Landman, 2008). This implies a factor of at least 2,000 in diameter increase between embryos and adult macroconchs. Embryonic conch size (ammonitella size) is well documented for most ammonoid clades (De Baets, Landman & Tanabe, 2015). In Cretaceous ammonoids, ammonitella size ranges between 0.5 and 1.5 mm with the average being smaller than 1 mm (De Baets, Landman & Tanabe, 2015).
In order to estimate the absolute gonad volume, we determined the body chamber volume VBC, which can be achieved by applying an equation introduced by Raup & Chamberlain (1967) and also used by De Baets et al. (2012):
| (1) |
with K—area of the last aperture, Ra—distance coiling axis to center of mass (estimated 200 mm based on comparisons with species with similar conch shape: Tajika et al., 2015; Naglik, Rikhtegar & Klug, 2016), θ—angular length of the body chamber in radians (equals π here, because the body chamber is about 180° long), the whorl expansion rate for this particular body chamber length
| (2) |
with r1—radius at maximum conch diameter and r2—radius at conch diameter 180° behind the aperture.
In order to compare the embryonic conch size of Cretaceous ammonites to early Thecosomata, we collected data from various publications (for values and references, see Tables 1, 2 and 3. We plotted these values in box plots using Excel.
Table 1. Measurements of embryonic conchs of Cretaceous ammonites.
| Species | Stage | AD mean |
|---|---|---|
| Acanthoplites sp. | Albian | 0.75 |
| Aconeceras (Sanmartinoceras) sp. | Aptian | 0.84 |
| Aconeceras trautscholdi | Aptian | 0.63 |
| Aconeceras trautscholdi | Aptian | 0.77 |
| Anagaudryceras limatum | Coniacian | 1.28 |
| Anagaudryceras matsumotoi | Maastrichtian | 1.32 |
| Anagaudryceras nanum | Campanian | 1.26 |
| Anagaudryceras tetragonum | Maastrichtian | 1.26 |
| Anagaudryceras yokoyamai | Santonian | 1.41 |
| Baculites sp. | Santonian | 0.78 |
| Beudanticeras beudanti | Albian | 1.06 |
| Beudanticeras laevigatum | Albian | 0.88 |
| Boreophylloceras densicostatum | Berriasian | 2.37 |
| Boreophylloceras praeinfundibulum | Berriasian | 2.9 |
| Calliphylloceras subalpinum | Albian | 0.8 |
| Calliphylloceras velledae | Aptian | 0.84 |
| Calycoceras orientale | Cenomanian | 0.93 |
| Canadoceras kossmati | Campanian | 0.89 |
| Canadoceras mystricum | Campanian | 1 |
| Clioscaphites vermiformis | Santonian | 0.71 |
| Collignoniceras woollgari | Turonian | 0.82 |
| Colombiceras sp. | Aptian | 0.62 |
| Damesites ainuanus | Turonian | 0.7 |
| Damesites damesi | Santonian | 0.89 |
| Damesites latidorsatus | Santonian | 0.85 |
| Damesites semicostatus | Santonian | 0.84 |
| Damesites sugata | Santonian | 0.83 |
| Deshayesites deshayesi | Albian | 1 |
| Desmoceras dawsoni | Albian | 1.52 |
| Desmoceras ezoanum | Cenomanian | 1.22 |
| Desmoceras japonicum | Turonian | 0.97 |
| Desmoceras kossmati | Cenomanian | 0.85 |
| Desmoceras kossmati | Cenomanian | 0.9 |
| Desmoceras poronaicum | Albian | 0.84 |
| Desmophyllites diphylloides | Santonian | 0.86 |
| Desmophyllites diphylloides | Santonian | 0.81 |
| Desmophyllites sp. | Santonian | 0.84 |
| Desmophyllites sp. | Santonian | 0.89 |
| Diadochoceras nodosocostatiforme | Aptian | 0.77 |
| Diadochoceras sinuosocostatus | Aptian | 0.74 |
| Diadochoceras sp. | Aptian | 0.75 |
| Discoscaphites conradi | Maastrichtian | 0.76 |
| Discoscaphites gulosus | Maastrichtian | 0.72 |
| Discoscaphites rossi | Maastrichtian | 0.69 |
| Eogaudryceras (Eotetragonites) aurarium | Albian | 0.93 |
| Eogaudryceras (Eotetragonites) balmensis | Albian | 0.98 |
| Eogunnarites unicus | Cenomanian | 0.76 |
| Eupachydiscus haradai | Campanian | 0.9 |
| Gabbioceras angulatum | Aptian | 0.88 |
| Gabbioceras latericarinatum | Albian | 0.93 |
| Gabbioceras michelianum | Albian | 0.9 |
| Gaudryceras cf. denseplicatum | Turonian | 1.34 |
| Gaudryceras cf. tenuiliratum | Campanian | 1.4 |
| Gaudryceras denseplicatum | Turonian | 1.52 |
| Gaudryceras stefaninii | Cenomanian | 0.93 |
| Gaudryceras striatum | Campanian | 1.26 |
| Gaudryceras tombetsense | Maastrichtian | 1.42 |
| Hauericeras angustum | Campanian | 0.7 |
| Hauericeras gardeni | Campanian | 0.72 |
| Holcophylloceras guettardi | Aptian | 0.81 |
| Holcophylloceras sp. | Aptian | 0.81 |
| Hoploscaphites comprimus | Maastrichtian | 0.66 |
| Hoploscaphites nebrascensis | Maastrichtian | 0.68 |
| Hoploscaphites nicolletii | Maastrichtian | 0.77 |
| Hoploscaphites spedeni | Maastrichtian | 0.76 |
| Hypacanthohoplites sp. | Aptian | 0.84 |
| Hypacanthohoplites subcornuenianus | Aptian | 0.93 |
| Hypophylloceras hetonaiensis | Maastrichtian | 0.9 |
| Hypophylloceras ramosum | Maastrichtian | 1.02 |
| Hypophylloceras subramosum | Santonian | 1.03 |
| Karsteniceras obatai | Barremian | 0.75 |
| Kossmatella agassiziana | Albian | 1.11 |
| Luppovia sp. | Aptian | 0.7 |
| Mantelliceras japonicum | Cenomanian | 0.89 |
| Marshallites compressus | Cenomanian | 0.97 |
| Melchiorites sp. | Albian | 0.69 |
| Menuites pusilus | Maastrichtian | 0.87 |
| Menuites yezoensis | Maastrichtian | 0.76 |
| Metaplacenticas subtilistriatum | Campanian | 1.14 |
| Microdesmoceras tetragonum | Cenomanian | 0.94 |
| Nolaniceras sp. | Aptian | 0.79 |
| Parahoplites melchioris | Aptian | 1.21 |
| Parajaubertella kawakitana | Cenomanian | 1.12 |
| Phylloceras japonicum | Cenomanian | 0.92 |
| Phyllopachyceras ezoense | Santonian | 1.08 |
| Phyllopachyceras ezoense | Campanian | 0.91 |
| Phyllopachyceras sp. | Aptian | 0.76 |
| Protexanites minimus | Santonian | 0.74 |
| Pseudohaploceras nipponicus | Aptian | 0.79 |
| Pseudophyllites indra | Campanian | 1.48 |
| Ptychoceras renngarteni | Aptian | 0.85 |
| Ptychophylloceras ptychoicum | Berriasian | 0.69 |
| Pusozia takahashii | Turonian | 0.89 |
| Puzosia orientale | Turonian | 0.83 |
| Puzosia pacifica | Turonian | 0.84 |
| Puzosia yubarensis | Turonian | 0.61 |
| Saghalinites teshioensis | Campanian | 1.19 |
| Scaphites carlilensis | Turonian | 0.6 |
| Scaphites corvensis | Turonian | 0.67 |
| Scaphites depressus | Coniacian | 0.76 |
| Scaphites larvaeformis | Turonian | 0.59 |
| Scaphites nigricollensis | Turonian | 0.68 |
| Scaphites planus | Turonian | 0.84 |
| Scaphites preventitricosus | Turonian | 0.65 |
| Scaphites pseudoaequalis | Coniacian | 0.72 |
| Scaphites warreni | Turonian | 0.66 |
| Scaphites whitfieldi | Turonian | 0.65 |
| Scaphites yonekurai | Coniacian | 0.87 |
| Simbirskites coronatiformis | Hauterivian | 1.09 |
| Simbirskites discofalcatus | Hauterivian | 1.17 |
| Simbirskites elatus | Hauterivian | 1.06 |
| Simbirskites sp. | Hauterivian | 1.26 |
| Simbirskites sp. | Hauterivian | 0.98 |
| Simbirskites versicolor | Hauterivian | 1.09 |
| Subprionocyclus bakeri | Turonian | 0.75 |
| Subprionocyclus minimum | Turonian | 0.74 |
| Subprionocyclus neptuni | Turonian | 0.78 |
| Teshioites sp. | Campanian | 0.92 |
| Tetragonites duvalianus | Albian | 1.06 |
| Tetragonites glabrus | Turonian | 1.08 |
| Tetragonites hulensis | Albian | 1.04 |
| Tetragonites minimus | Turonian | 0.98 |
| Tetragonites popetensis | Campanian | 0.97 |
| Tetragonites popetensis | Campanian | 1.08 |
| Tetragonites terminus | Maastrichtian | 1.8 |
| Texanites kawasakii | Santonian | 0.93 |
| Tragodesmoceroides subcostatus | Turonian | 0.88 |
| Valdedorsella akuschaensis | Aptian | 0.68 |
| Yezoites klamathensis | Coniacian | 0.75 |
| Yezoites matsumotoi | Coniacian | 0.8 |
| Yezoites puerculus | Coniacian | 0.82 |
| Yokoyamaoceras ishikawai | Turonian | 0.89 |
| Zelandites aff. inflatus | Cenomanian | 1.22 |
| Zelandites kawanoii | Santonian | 1.19 |
| Zelandites mihoensis | Coniacian | 1 |
| Zelandites varuna | Maastrichtian | 1.22 |
| Zuercherella falcistriata | Aptian | 0.75 |
Table 2. Size of conchs of Thecosomata with coiled conchs.
Data sources are indicated in the table.
| Taxon | Age | Conch size (mm) | Source |
|---|---|---|---|
| Limacinidae | Eocene | 1.125 | Lokho & Kumar (2008) |
| Limacinidae | Eocene | 1.05 | Lokho & Kumar (2008) |
| Limacinidae | Eocene | 0.733333 | Lokho & Kumar (2008) |
| Limacinidae | Eocene | 0.75 | Lokho & Kumar (2008) |
| Limacinidae | Eocene | 0.32 | Lokho & Kumar (2008) |
| Limacinidae | Eocene | 0.568182 | Lokho & Kumar (2008) |
| Limacinidae | Eocene | 0.857143 | Lokho & Kumar (2008) |
| Altaspiratella elongatoidea | Eocene | 1.416667 | Janssen & Goedert (2016) |
| Altaspiratella elongatoidea | Eocene | 1.833333 | Janssen & Goedert (2016) |
| Altaspiratella elongatoidea | Eocene | 1.233333 | Janssen & Goedert (2016) |
| Heliconoides mercinensis | Eocene | 1.764706 | Janssen & Goedert (2016) |
| Limacina aegis | Eocene | 1.4375 | Janssen & Goedert (2016) |
| Limacina aegis | Eocene | 1.71875 | Janssen & Goedert (2016) |
| Limacina aegis | Eocene | 1.03125 | Janssen & Goedert (2016) |
| Limacina aegis | Eocene | 1.53125 | Janssen & Goedert (2016) |
| Limacina aegis | Eocene | 1.84375 | Janssen & Goedert (2016) |
| Limacina aegis | Eocene | 1.28125 | Janssen & Goedert (2016) |
| Limacina novacaesarea | Eocene | 1.382353 | Janssen & Goedert (2016) |
| Limacina novacaesarea | Eocene | 1.588235 | Janssen & Goedert (2016) |
| Limacina novacaesarea | Eocene | 1.470588 | Janssen & Goedert (2016) |
| Heliconoides lillebaeltensis | Eocene | 2.4 | Janssen, Schnetler & Heilmann-Clausen (2007) |
| Heliconoides lillebaeltensis | Eocene | 2.5 | Janssen, Schnetler & Heilmann-Clausen (2007) |
| Heliconoides lillebaeltensis | Eocene | 2.5 | Janssen, Schnetler & Heilmann-Clausen (2007) |
| Heliconoides lillebaeltensis | Eocene | 2 | Janssen, Schnetler & Heilmann-Clausen (2007) |
| Heliconoides lillebaeltensis | Eocene | 1.7 | Janssen, Schnetler & Heilmann-Clausen (2007) |
| Heliconoides lillebaeltensis | Eocene | 1.8 | Janssen, Schnetler & Heilmann-Clausen (2007) |
| Heliconoides mercinensis | Eocene | 2 | Janssen, Schnetler & Heilmann-Clausen (2007) |
| Heliconoides mercinensis | Eocene | 2.6 | Janssen, Schnetler & Heilmann-Clausen (2007) |
| Limacina pygmaea | Eocene | 1.4 | Janssen, Schnetler & Heilmann-Clausen (2007) |
| Heliconoides sp. | Campanian | 1.56 | Janssen & Goedert (2016) |
| Heliconoides mercinensis | Pleistocene | 2 | Guess |
Table 3. Size of conchs of Thecosomata with straight conchs.
Data sources are indicated in the table.
| Family | Age | Conch size (mm) | Source |
|---|---|---|---|
| Creseidae | Eocene | 1.875 | Lokho & Kumar (2008) |
| Creseidae | Eocene | 1.25 | Lokho & Kumar (2008) |
| Creseidae | Eocene | 1.25 | Lokho & Kumar (2008) |
| Creseidae | Eocene | 0.75 | Lokho & Kumar (2008) |
| Creseidae | Eocene | 2.142857 | Lokho & Kumar (2008) |
| Creseidae | Eocene | 0.416667 | Lokho & Kumar (2008) |
| Creseidae | Eocene | 0.597826 | Lokho & Kumar (2008) |
| Creseidae | Eocene | 0.597826 | Lokho & Kumar (2008) |
| Creseidae | Eocene | 0.480769 | Lokho & Kumar (2008) |
| Creseidae | Eocene | 0.477273 | Lokho & Kumar (2008) |
| Creseidae | Eocene | 0.575 | Lokho & Kumar (2008) |
| Creseidae | Eocene | 0.5 | Lokho & Kumar (2008) |
| Cliidae | Eocene | 1.857143 | Lokho & Kumar (2008) |
| Cliidae | Eocene | 1.145833 | Lokho & Kumar (2008) |
| Cliidae | Eocene | 0.9375 | Lokho & Kumar (2008) |
| Cliidae | Eocene | 1.5625 | Lokho & Kumar (2008) |
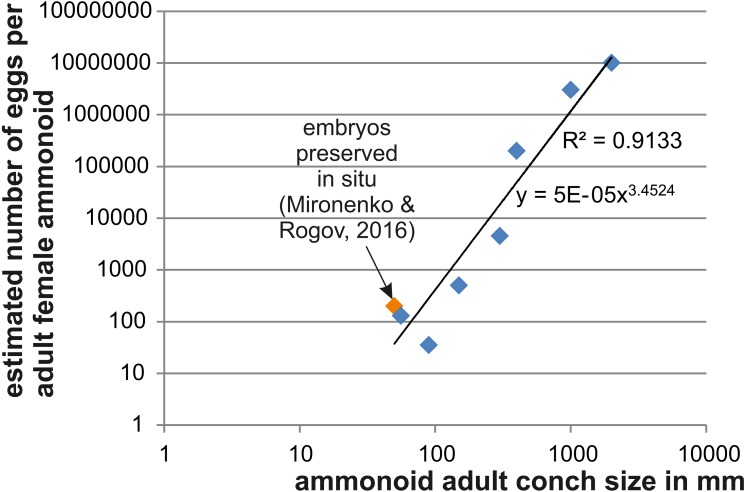
As a further approach to estimate ammmonoid fecundity, we gathered published data on the numbers of eggs per adult female and list those in Table 4 (references of the data sources are given there). Using Excel, we produced a loglog-biplot depicting the relationship between the estimated number of eggs and the mature conch size in various ammonoids of Devonian to Cretaceous age. Unsurprisingly, there is an exponential relationship between the two parameters.
Table 4. Estimates of numbers of eggs produced by various ammonoid taxa.
| Genus | Age | Adult size (mm) | Fecundity | Source |
|---|---|---|---|---|
| Parapuzosia | Cretaceous | 2,000 | 10,000,000 | This paper |
| Pachydesmoceras | Cretaceous | 1,000 | 3,000,000 | This paper |
| Sinzovia | Jurassic | 50 | 200 | Mironenko & Rogov (2016) |
| Manticoceras | Devonian | 400 | 200,000 | De Baets et al. (2012) |
| Erbenoceras | Devonian | 150 | 500 | De Baets et al. (2012) |
| Mimosphinctes | Devonian | 90 | 35 | De Baets et al. (2012) |
| Gyroceratites | Devonian | 56 | 130 | De Baets et al. (2012) |
| Agoniatites | Devonian | 300 | 4,500 | De Baets et al. (2012) |
Results
Estimating ammonoid fecundity
Applying the data and calculations listed in the method section to the lectotype of Parapuzosia seppenradense, we obtain a whorl expansion rate W of 1.73 and then an according body chamber volume VBC of 137,075,470 mm3. Depending on the proportion of the gonads (between 8 and 30%; see discussion in ‘Methods’), we obtain gonad volumes varying between about 10,000,000 mm3 and 40,000,000 mm3. Assuming an egg-volume of 1 mm3, we obtain numbers of 10,000,000 to 40,000,000 eggs per adult female Parapuzosia seppenradensis (see also Fig. 2) if they were semelparous. If we assume iteroparity, these numbers increase by the factor of the number of reproductive cycles. Also, if we assume that the eggs and embryos continued to grow after they were laid (see discussion in Walton, Korn & Klug, 2010), ammonoid fecundity would further increase, but evidence for this is missing in ammonoids (Mironenko & Rogov, 2016). For an adult female of half the diameter, we would still obtain egg-numbers of between 3,000,000 (8% gonad volume) and 10,000,000 eggs (30% gonad volume) at semelparity. Puzosiids and other large Cretaceous ammonoids in the size range between 500 and 1,000 mm are quite common worldwide (e.g., Pachydesmoceras).
Figure 2. Relationship between adult conch size and the estimated number of eggs.
Data are displayed in Table 4. The variation seen in smaller species likely roots in differences of the embryo size.
In Fig. 2, we depict the relationship between the number of eggs per female adult ammonoid using mostly published data and the results presented here. The loglog-biplot shows an exponential relationship between these two parameters, which is not surprising taking into account how the estimates were achieved (De Baets et al., 2012). It also shows some variation scattering around the exponential trend line, which likely roots in the variation of the ratio of embryonic versus adult conch size (e.g., Gyroceratites and Sinzovia have similar adult sizes, but the embryonic conch is 50% larger in Gyroceratites; De Baets et al., 2012; De Baets, Landman & Tanabe, 2015; Mironenko & Rogov, 2016).
The role of r-strategy in ammonite and belemnite ecology
Depending on the proportional gonad size and whether or not ammonites were semelparous or iteroparous, it appears likely that adult females of the largest puzosiid ammonites such as Parapuzosia seppenradensis laid between 10,000,000 and 100,000,000 eggs and ammonoids about half the size still over 1,000,000 eggs. The simple calculation above highlights the likelihood that derived ammonites were extreme r-strategists (respectively fast life strategy), which produced vast amounts of offspring, likely contributing an important part of the plankton in size at the limit from micro- to macroplankton. R-strategy corresponded with high mortality and it is likely that hatchlings and juveniles of ammonites formed a major source of food in the marine realm.
As far as belemnites are concerned, their global abundance had already decreased during the Late Cretaceous (prior to the main extinction event), freeing ecospace for, e.g., some other coleoids (Iba et al., 2011). Nevertheless, coleoids with conical phragmocones such as belemnites, diplobelids, Groenlandibelus or Naefia share a small initial chamber and likely small embryonic conchs (Bandel et al., 1984). Above all, there are a lot of similarities between belemnite and ammonite hatchlings such as their small initial chambers, their overall hatchling size, their supposed habitats including the planktonic mode of life and the r-strategy reproductive mode (Ward & Bandel, 1987; Arkhipkin & Laptikhovsky, 2012; Doguzhaeva et al., 2014). Accordingly, we assume that belemnite fecundity was also high, although much lower than those of the puzosiid ammonites because of the much lower size difference between adults and embryos (about 100–1,000 eggs per female).
Which animals ate ammonites?
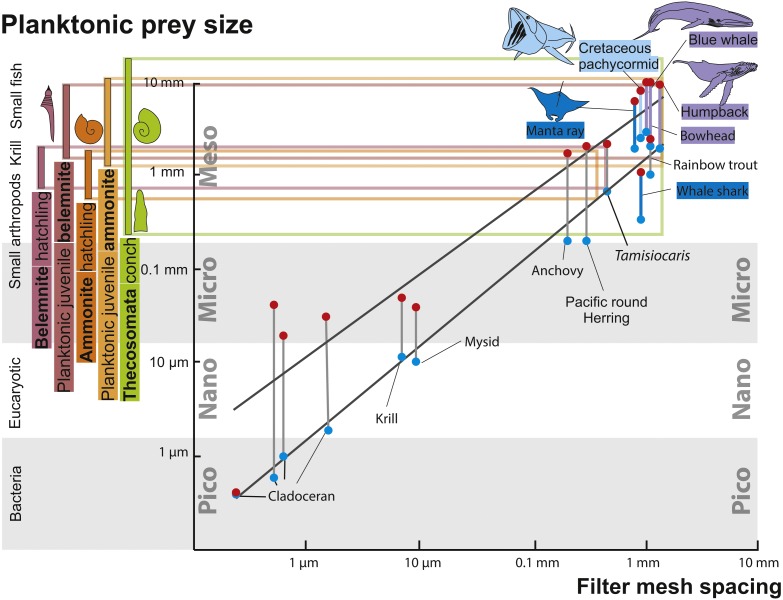
Evidence for successful and unsuccessful predation on medium to large-sized ammonites is not rare but identifying the actual predator is possible only in very few cases (Keupp, 2012; Hoffmann & Keupp, 2015). Additionally, most hard parts of ammonites (conch and lower jaw) were likely crushed by the predators and quickly dissolved in the digestive tract, making ammonites as fossilized stomach contents improbable, although a few cases have been reported where juvenile ammonoid remains are preserved in stomachs of Jurassic ammonites (Keupp & Schweigert, 2015; Klug & Lehmann, 2015). It is even more difficult to find evidence for predators that fed on hatchlings and neanic juveniles of ammonites (dm < 10 mm), which must have occurred in vast numbers in the world’s oceans of the Mesozoic. These early post-hatching developmental stages probably lived in the water column because their conchs already had functional phragmocones and they are often found in black shales, which were deposited under hypoxic to anoxic bottom water conditions and therefore, a strictly benthic mode of life was impossible (Shigeta, 1993; Nützel & Mapes, 2001; Mapes & Nützel, 2009). Thus, pelagic nektonic animals (including older growth stages of ammonites) are the likeliest candidates as predators feeding on these young ammonites: Fig. 3 depicts the fitting of juvenile planktonic ammonites and holoplanktonic gastropods with the mesh size of filter feeders of the corresponding time intervals. See Fig. 4 for the stratigraphic distribution of the groups discussed here.
Figure 3. Zooplankton size ranks and filter mesh spacing of planktivorous filter feeders.
Modified after Vinther et al. (2014), using data from Lokho & Kumar (2008), Friedman et al. (2010) and De Baets, Landman & Tanabe (2015). The horizontal coloured lines connect the size ranges of zooplanktonic prey organisms with the mesh size of large filter-feeding marine vertebrates. The black lines mark the range in prey size organisms filtered by the respective filter feeders. Note how the size range of hatchlings of ammonites and belemnites match that of Thecosomata and, on the filter feeder-side, that of the mesh-size of the filtering organ.
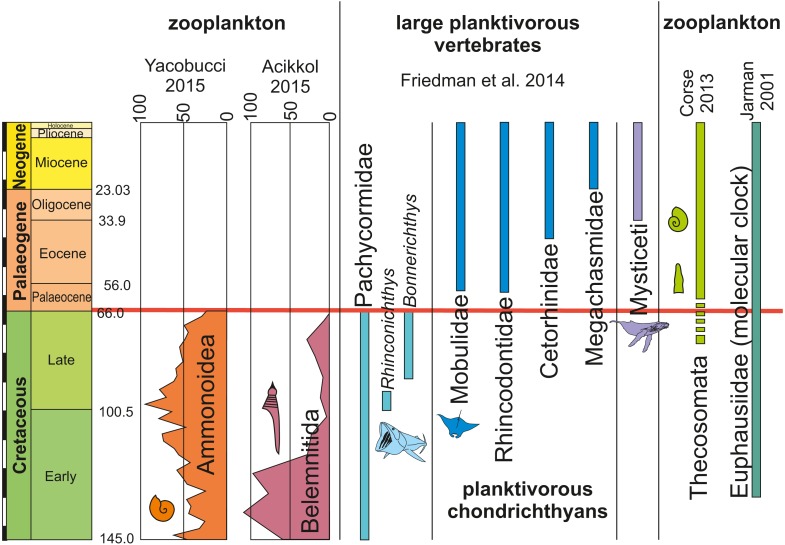
Figure 4. Occurrences, extinctions, originations and diversity changes in plankton and large planktotrophic suspension feeders from the Cretaceous to the Palaeogene (mass extinction marked by red bar).
Ammonite and belemnite diversity show the number of species. Data from Friedman et al. (2010), Bristow, Ellison & Wood (1980), Corse et al. (2013), Yacobucci (2015) and Jarman (2001). Note the relative timing of extinctions of cephalopods with small planktonic juveniles and filter-feeding bony fish and rediations of holoplanktonic gastropods, krill as well as filter-feeding chondrichthyans and whales.
For abundant and easy prey like juvenile ammonites, a broad range of predators can be hypothesized. Like plankton today, these masses of juvenile ammonites represent perfect food sources for medium-sized to large suspension feeders (invertebrates and vertebrates). From the Cretaceous, giant planktivorous bony fishes (pachycormids: Friedman et al., 2010) have been suggested to be nektonic suspension feeders, which might have fed on plankton comprising a wealth of juvenile ammonites. In the SOM of their paper, Friedman et al. (2010) show a fragment of the gill rakers; their filaments have a spacing of about 1 mm, which is suitable to filter out hatchlings and juvenile ammonites with conchs of one millimeter in diameter or slightly more (Fig. 3). This trophic relationship is further suggested by the extinction of this group synchronous with the demise of the Ammonoidea and Belemnitida but direct evidence is missing. Taking the direct fossil evidence from the Jurassic into account, it appears likely that ammonites also played a role as micropredators feeding on early juvenile ammonite offspring (Jäger & Fraaye, 1997; Klug & Lehmann, 2015; Keupp, 2012; Kruta et al., 2011; Keupp & Schweigert, 2015; Keupp et al., 2016).
The extreme differences in size (up to three orders in magnitude) between adults and juveniles in large ammonites indicate that the range of potential predators changed significantly throughout the life history of these cephalopods. As hatchlings and small juvenile planktonic forms, moderate-sized to large suspension feeders and small predators likely used them as a food source but for adult puzosiids and other large ammonites, only large predators such as mosasaurs, pliosaurs and large fishes can be considered, although the seeming direct evidence for such a trophic relationship is still under debate (Kauffman & Kesling, 1960; Tsujita & Westermann, 2001; Kauffman, 2004; Gale, Kennedy & Martill, 2017). Latest Cretaceous juvenile ammonites were possibly not the primary food source of ichthyosaurs since the latter became extinct already in the Cenomanian and are unknown from stomach contents to our knowledge. After the demise of ichthyosaurs, ammonites persisted to be diverse and abundant. In spite of a better link of their extinction with that of the belemnites in the North Pacific near the end of the Early Cretaceous (Iba et al., 2011), belemnite decline in the Tethys at the Cenomanian–Turonian boundary (Doyle, 1992; Christensen, 2002) and direct evidence for a trophic relationship between phragmocone-bearing coleoids and ichthyosaurs (Kear, Briggs & Donovan, 1995 and references therein), Acikkol (2015) suggested that a link between the severe reduction of belemnite diversity and ichthyosaur extinction is unlikely.
An additional point we want to raise is the role of adult ammonites as micropredators feeding on juvenile ammonites. It has been claimed by various authors that ammonites, particularly heteromorphs, lived in the water column (Cecca, 1997; Guex, 2006), i.e., with direct access to meso- and microplankton. As pointed out above, a microphagous diet has been shown for several ammonite species including heteromorphs (Jäger & Fraaye, 1997; Klug & Lehmann, 2015; Keupp, 2012; Kruta et al., 2011; Keupp & Schweigert, 2015; Keupp et al., 2016), thus making ammonites as predators feeding on juvenile ammonites in the Cretaceous likely.
Which groups filled the ecospace freed by the extinction of ammonite hatchlings and planktivorous actinopterygians?
Association of the extinctions of large marine reptiles, large planktivorous fish and those of ammonites and belemnites suggest trophic relationships between these groups; their extinction freed ecospace for both small zooplankton and various suspension feeders as well as predators. This association is followed by major changes in the planktonic realm such as the rise of holoplanktonic gastropods. Although a few Early Jurassic to Cretaceous heteropods are known (Bandel & Hemleben, 1995; Nützel, 2014; Teichert & Nützel, 2015; Janssen & Goedert, 2016; Nützel et al., 2016; Burridge et al., 2017; Janssen & Peijnenburg, 2017), the major expansion of heteropods and ‘pteropods’ falls into the Cenozoic (Lalli & Gilmer, 1989; Tracey, Todd & Erwin, 1993; Janssen & Goedert, 2016; Janssen & Peijnenburg, 2013).
In size and their coiled form, many fossil Limacinidae (Thecosomata, planktonic opisthobranch gastropods) resemble ammonites. Similarly, the conchs of fossil Creseidae morphologically and in size (at least roughly) correspond to hatchlings of belemnites, diplobelids and other phragmocone-bearing coleoids of the Cretaceous (Bandel et al., 1984; Lokho & Kumar, 2008). In addition to these morphologic similarities, these groups shared the planktonic habitat. According to Janssen & King (1988), Janssen & Peijnenburg (1988), ‘pteropods’ were already present at least as early as the latest Palaeocene (see also Janssen & Goedert, 2016). Janssen & Goedert (2016) even claimed a Cretaceous origin of the Thecosomata. A number of Eocene pteropod occurrences are known worldwide (Bristow, Ellison & Wood, 1980; King, 1981; Curry, 1982; Zorn, 1991; Hodgkinson, Garvie & Bé, 1992; Janssen, Schnetler & Heilmann-Clausen, 2007; Lokho & Kumar, 2008; Ando, Ujihara & Ichihara, 2009; Cahuzac & Janssen, 2010). An early Palaeogene origin is also supported by a combination of palaeontological and molecular clock data published by Corse et al. (2013). Remarkably, the latter authors compare the uncoiling of the conch of Thecosomata with the coiling of ammonites, but they did not discuss macroecological implications such as the ecological replacements suggested here. As far as abundance of these fossils is concerned, pteropods are much less frequent than subadult to adult ammonites and belemnites, while their hatchlings are similarly rare. This is probably due to the combination of their small body size as well as their thin and fragile aragonitic shells (Janssen & King, 1988), which did not provide a high fossilization potential. The great majority of these thin aragonitic shells was undoubtedly rapidly dissolved during early diagenesis and as a consequence not fossilized (Berner, 1977; Janssen & Peijnenburg, 2017). Nevertheless, the fact that numerous pteropods have been reported from the Eocene implies that they were abundant and widely distributed at least since that period of time.
Similarities in size, overall morphology, habitat, abundance as well as the timing of their respective extinction and origination suggest that hatchlings and small individuals of ammonites as well as belemnites were ecologically replaced, at least partially, by planktonic opisthobranchs (Thecosomata) and other holoplanktonic gastropods. In turn, the ecological installation of the Thecosomata together with other planktonic organisms contributed to the dietary basis for the evolution of new groups of large planktivorous suspension feeders (Lalli & Gilmer, 1989; Armstrong et al., 2005; Hunt et al., 2008). As suggested by Friedman et al. (2010), the Cretaceous ‘giant planktivorous bony fishes’ found an ecological replacement in both large suspension-feeding chondrichthyans and baleen whales. Several of these groups are known to take in important amounts of planktonic gastropods, although arthropods such as krill and other plankton plays important roles as well. Today, thecosomes may regionally contribute up to 50% of the zooplanktonic biomass and thus are ecologically important (Mackas & Galbraith, 2012). However, today’s Manta rays (Mobulidae) are known to feed predominantly on small Crustaceans, small fish and other plankton and the same holds true for several baleen whales (e.g. Sims & Merrett, 1997; Motta et al., 2010; De la Parra Venegas et al., 2011; Slater et al., 2017). Nevertheless, it is unknown what exactly these Palaeogene suspension feeders ate, but at least the filter mesh spacing of both planktivorous chondrichthyans and several baleen whales fits well with the size range of thecosomes (Fig. 3). Also, it is conceivable that early filter feeding species of these groups did not discriminate in their planktonic diet as much as more derived modern relatives. In any case, the niche of filterers catching prey of a size of a few millimetres was occupied by the chondrichthyan and marine mammal filter feeders mentioned above. The Thecosomata can thus be understood as an example for the new plankton that occurred after the end-Cretaceous mass extinction.
What caused this ecological replacement?
The fossil record comprises quite a few cases of ecological replacements (which are often called ‘biotic replacements’; e.g., Benton, 1987; Benton, 1991; Briggs, 1998). Some famous examples include replacements of brachiopods by bivalves (Gould & Calloway, 1980; Payne et al., 2014), dinosaurs by mammals (e.g., Sloan et al., 1986), hybodonts by modern sharks (Schaeffer, 1965), and ‘straight-necked’ turtles by ‘flexible-neck’ turtles (Rosenzweig & McCord, 1991; for a comprehensive list of ecological replacement, see Benton, 1987; Benton, 1991). These examples are also used to discuss possible mechanisms, which explain what drove the replacement and in turn, facilitated speciation or macroevolution of clades. Benton (1991) presented several models of ecological replacements, in which the role of competition, key adaptation and mass extinction was discussed as main cause of such replacements. He concluded that ‘competition’ and ‘key adaptation’ can rarely be the main cause of such replacements, although the role of mass extinction also needs to be further examined. By contrast, some other researchers have different views on mechanisms of ecological replacements. For instance, Rosenzweig & McCord (1991) wrote the following: “species from the new clade produce new species to replace already extinct species from the old clade. The key adaptation gives them a higher competitive speciation rate than old-clade sources of replacement”. They presented the example of the straight-necked turtles (Amphichelydia), which were replaced by cryptodiran turtles with the capability of neck retraction and termed this model of replacement ‘incumbent replacement’. In this mechanism, key adaptations play a major role. Also, Briggs (1998) argued that several cases of ecological replacement, which are cited as evidence for mass-extinction-induced replacements, took place over a long period of time, and thus, biological interaction played a primary role.
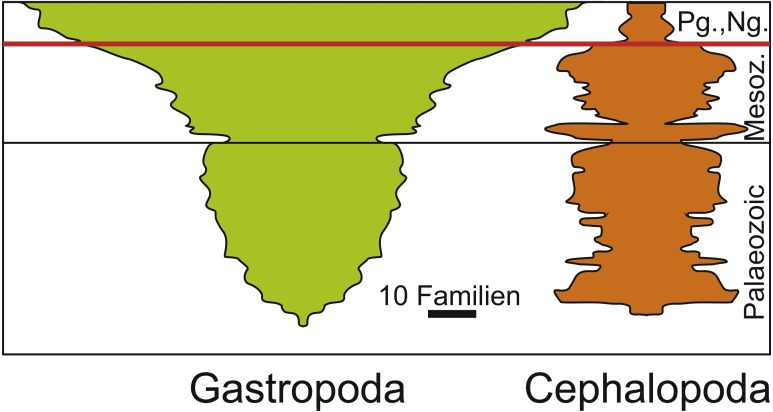
In the case of ammonoids and belemnites being ecologically replaced by holoplanktonic gastropods, it partially depends on the phylogenetic and temporal scale that is examined and on the fossil record. When focusing only on ammonoids and belemnites, no species remained after the Cretaceous to compete with Palaeogene holoplanktonic gastropods. By contrast, when including the whole clade, this looks different since cephalopods continued to exist and gastropods were also present long before this faunal change (Fig. 5; Sepkoski, 1981). What makes this more relevant is the end-Cretaceous diversity reduction among the Cephalopoda in a phase where the whole clade Gastropoda diversified (a longterm process that started already in the Triassic, which was largely a result of the expansion of Neogastropda). Naturally, both Cephalopoda and Gastropoda contain species with a broad range of different modes of life and thus, their differing diversity patterns cannot be explained by the ecological replacement of juvenile ammonites by holoplanktonic gastropods alone—especially although holoplanktonic gastropods are abundant and widely distributed, their diversity is not high. On the other hand, the removal of ammonites and belemnites from the planktonic realm offered certain gastropods the opportunity to flourish in this realm and to long for new opportunities including availability of food and space resources.
Figure 5. Diversity of Cephalopoda and Gastropoda through the Phanerozoic. Redrawn after Sepkoski (1981).
Note the diversity reduction in cephalopods at the end of the Cretaceous and the subsequent radiation of gastropods. The Cretaceous Palaeogene mass extinction is marked by the red line.
As far as key adaptations (e.g., Simpson, 1943; Benton, 1983; Benton, 1987; Rosenzweig & McCord, 1991; Briggs, 1998; Payne et al., 2014) are concerned, they were already present in the group of species assuming the ecological role of the previously incumbent species after their extinction (Rosenzweig & McCord, 1991). Although some researchers report the presence of holoplanktonic gastropods in the Cretaceous (Janssen & Goedert, 2016; Burridge et al., 2017), to date, there is no fossil evidence, which suggests that they acquired all key adaptations that ensured the success of radiation in the Cenozoic except the small, thin-shelled conch. Also, due to a lack of data, it is not possible yet to conclude whether or not a direct biotic interaction between the old (ammonite and belemnite hatchlings) and the new (holoplanktonic gastropods) clades occurred in the Cretaceous, although the Thecosomata originated already in the late Campanian (Janssen & Goedert, 2016; Burridge et al., 2017). At least, the fossil record suggests that this case of replacement was triggered by a mass extinction event. In Fig. 6, we show the distribution of sizes of embryonic conchs of ammonites compared to the earliest occurrences of Thecosomata in the fossil record demonstrating the size-overlap between the two groups. Moreover, the evolution of a holoplanktonic mode of life is particularly easy in gastropods with a planktotrophic veliger larva. Bandel et al. (1984) have convincingly shown that pteropods evolved through neotenic extension of larval life of a benthic ancestor. A similar model has been proposed by Teichert & Nützel (2015) for Jurassic heteropods, which are superabundant in the Early Jurassic Posidonia Shale. Here, repeated Early Jurassic Anoxia resulted in neotenic prolongation of larval life to a holoplanktonic adult life. Thus, the free-swimming veliger larvae may be seen as an preadaptation for a holoplanktonic mode of life in these gastropods and this way the timely overlap that is required for in the incumbency model applies.
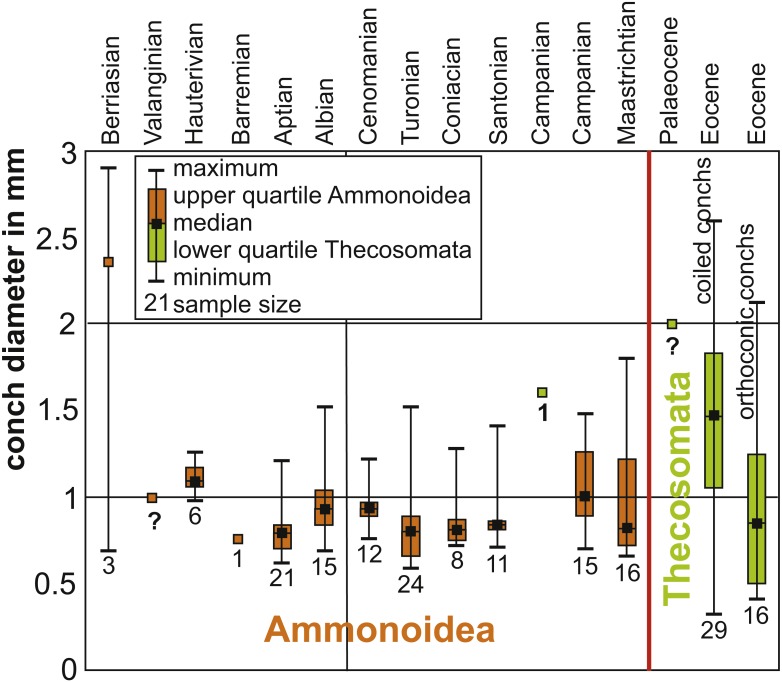
Figure 6. Box plots of Cretaceous ammonitella diameters and Cretaceous to Palaeogene Thecosomata conch size.
Data (Table 1) from De Baets, Landman & Tanabe (2015), Laptikhovsky, Nikolaeva & Rogov (2017), Lokho & Kumar (2008), Janssen, Schnetler & Heilmann-Clausen (2007), Janssen, Sessa & Thomas (2016), Janssen & Goedert (2016), and Janssen & Peijnenburg (2017). The red line marks the Cretaceous-Palaeogene boundary. The numbers below the box plots give the sample size. Orange marks ammonitella data, while green marks gastropod data. Question marks indicate that fossils are present but the values indicate guesses: in the case of Valanginian ammonites, protoconchs are known and from that size, an ammonitella size close to 1 mm appears likely. In the case of Campanian and late Palaeocene Thecosomata, the only genus ranging back into the Campanian is Heliconoides (Janssen & Goedert, 2016; Janssen & Peijnenburg, 2017); Eocene specimens measure approximately 2 mm, hence this size estimate.
Nevertheless, the main radiation of the Thecosomata appears delayed until the Palaeocene-Eocene boundary judging from their fossil record (Janssen, Schnetler & Heilmann-Clausen, 2007; Janssen & Peijnenburg, 2017). This raises the question whether this delay in radiation is an artefact of the poor fossil record of the Thecosomata or whether the main radiation really occurred several million years after the Cretaceous. Considering that a species loss caused by a mass extinction event can require as long as 5–10 My for community recovery (Copper, 1989), it appears likely that the radiation of holoplanktonic gastropods began in the Palaeocene. It must be taken into account that adaptation to the new life style including optimization of resource exploitation needed time.
Although we cannot rule out key adaptations and biotic interaction as main causes of the ecological exchange of ammonites and belemnites by Thecosomata and other holoplantonic gastropods after the Cretaceous, it is probably safe to claim that the holoplanktonic gastropods opportunistically benefited from the ecospace vacated by the extinction of these two groups of cephalopods. Possibly, new finds of holoplanktonic gastropods from the early Palaeocene will push their radiation back in time closer to the extinction of ammonoids.
Conclusions
Large Late Cretaceous ammonites such as puzosiids reached sizes exceeding two meters in diameter. Their offspring has a conch size that is in stark contrast to the adult size; the embryonic conchs of many Cretaceous ammonites measure only about 1 mm in diameter at the time of hatching. This size relationship, conch geometry and anatomical proportions allow estimates of the number of offspring per female. Accordingly, the largest females might have laid between 10,000,000 (semelparity, small gonads) and 100,000,000 eggs (iteroparity, large gonads). Apart from this extreme example, the great abundance of ammonites, many of them of considerable size as adults, throughout the Mesozoic and the generally small size of their offspring implies that juvenile ammonites and belemnites played a fundamental role near the base of Mesozoic food webs, both as primary consumers and as food source for secondary consumers. We assume that Mesozoic oceans were full of small hatchlings and juveniles of ammonites and belemnites in the mm to cm size range. This part of the planktonic food chain vanished with the extinction of ammonites and belemnites but may have enabled the evolutionary and ecological rise of holoplanktonic gastropods, which occupy a similar size range, conch morphologies (coiled and straight) and trophic role. This underlines the importance of ecological differentiation between different ontogenetic stages. Gill raker filament spacing in huge pachycormids correspond in size to these juvenile ammonites and belemnites, potentially suggesting a trophic link in the light of the synchronous extinction at the end of the Cretaceous.
Here, we suggest that the ecospace formerly occupied by ammonite and belemnite juveniles was taken over during the post-Mesozoic rise of the Thecosomata (holoplanktonic heterobranchs) and other holoplanktonic gastropods. The ecological replacement of the pachycormids is a bit more difficult to explain. During the early Palaeogene, three important large planktivorous lineages of chondrichthyans occur; however, modern mobulids (Manta rays), for instance, are known to feed on planktonic crustaceans. Perhaps, stomach contents of exceptionally preserved specimens of Palaeogene planktivorous chondrichthyans will shed more light on the suspension feeders that, at least in their function as primary consumers, benefited from the thecosomes that ecologically replaced juvenile ammonites. In any case, it is likely that ammonite hatchlings had occupied a significant part of their particular plankton size class and were important prey for some filter feeders. This size class niche of small plankton was vacant after the Cretaceous and holoplanktonic gastropods are one important group filling this ecospace, probably in concert with other plankton.
Independent of the filter feeder-side, we conclude that in r-strategists, the young offspring can play a more important ecological role than their large adults. This case of ecological replacement underlines the significance of differences at which developmental stage the acme in ecological importance of an organism occurs.
Acknowledgments
Jakob Vinther (Bristol) generously provided an illustration, which was a valuable basis for one of our illustrations. We thank Christoph Steinweg, Lothar Schöllmann and Jan-Ole Kriegs (all Münster), Kishor Kumar (Uttarakhand, India), Kazushige Tanabe (Tokyo), and Klaus Bandel (Hamburg) for providing illustrations and allowing us to use them. We greatly appreciate the input of the reviewers (Margaret Yacobucci, Bowling Green State University and two anonymous reviewers) and the editor Kenneth De Baets (Erlangen).
Funding Statement
This study is supported by the Swiss National Science Foundation SNF (project number 200021_149119). This study was also supported by the Deutsche Forschungsgemeinschaft NU 96/13-1. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript the Deutsche Forschungsgemeinschaft NU 96/13-1.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Amane Tajika and Christian Klug conceived and designed the experiments, performed the experiments, analyzed the data, wrote the paper, prepared figures and/or tables, reviewed drafts of the paper.
Alexander Nützel analyzed the data, wrote the paper, reviewed drafts of the paper.
Animal Ethics
The following information was supplied relating to ethical approvals (i.e., approving body and any reference numbers):
Only fossil specimens were included, so animal ethics approval was not required.
Data Availability
The following information was supplied regarding data availability:
Specimens are on display in the following museums: LWL-Museum für Naturkunde Münster, Germany, and Mikasa City Museum, Mikasa, Japan.
References
- Acikkol (2015).Acikkol N. Degree project at the department of earth sciences. Uppsala University; Uppsala: 2015. Testing the Cretaceous diversity of ichthyosaurs and their extinction Hypotheses using a quantitative approach; p. 40. [Google Scholar]
- Alvarez et al. (1980).Alvarez LW, Alvarez W, Asaro F, Michel HV. Extraterrestrial cause for the Cretaceous-Tertiary extinction. Science. 1980;208:1095–1108. doi: 10.1126/science.208.4448.1095. [DOI] [PubMed] [Google Scholar]
- Ando, Ujihara & Ichihara (2009).Ando Y, Ujihara A, Ichihara T. First occurrence of Paleogene pteropods (Gastropoda; Thecosomata) from Japan. Journal of the Geological Society of Japan. 2009;115:187–190. doi: 10.5575/geosoc.115.187. [DOI] [Google Scholar]
- Archibald & Fastovsky (2004).Archibald D, Fastovsky D. Dinosaur extinction. In: Weishampel DB, Dodson P, Osmólska H, editors. The Dinosauria. Second Edition University of California Press; Berkeley: 2004. pp. 672–684. [Google Scholar]
- Arkhipkin & Laptikhovsky (2012).Arkhipkin AI, Laptikhovsky VV. Impact of ocean acidification on plankton larvae as a cause of mass extinctions in ammonites and belemnites. Neues Jahrbuch für Geologie und Paläontologie, Abhandlungen. 2012;266:39–50. doi: 10.1127/0077-7749/2012/0268. [DOI] [Google Scholar]
- Armstrong et al. (2005).Armstrong JL, Boldt JL, Cross AD, Moss JH, Davis ND, Myers KW, Walker RV, Beauchamp DA, Haldorson LJ. Distribution, size, and interannual, seasonal and diel food habits of northern Gulf of Alaska juvenile pink salmon, Oncorhynchus gorbuscha. Deep Sea Research Part II: Topical Studies in Oceanography. 2005;52:247–265. doi: 10.1016/j.dsr2.2004.09.019. [DOI] [Google Scholar]
- Bandel et al. (1984).Bandel K, Almogi-Labin A, Hemleben C, Deuser WG. The conch of Limacina and Peraclis (Pteropoda) and a model for the evolution of planktonic gastropods. Neues Jahrbuch für Geologie und Paläontologie, Abhandlungen. 1984;168:87–107. [Google Scholar]
- Bandel & Hemleben (1995).Bandel K, Hemleben C. Observations on the ontogeny of thecosomatous pteropods (holoplanktic Gastropoda) in the southern Red Sea and from Bermuda. Marine Biology. 1995;124:225–243. doi: 10.1007/BF00347127. [DOI] [Google Scholar]
- Benton (1983).Benton MJ. Large-scale replacements in the history of life. Nature. 1983;302:16–17. doi: 10.1038/302016a0. [DOI] [Google Scholar]
- Benton (1987).Benton MJ. Progress and competition in macroevolution. Biological Reviews of the Cambridge Philosophical Society. 1987;62:305–338. doi: 10.1111/j.1469-185X.1987.tb00666.x. [DOI] [Google Scholar]
- Benton (1991).Benton MJ. The unity of evolutionary biology. Dioscorides Press; Portland: 1991. Extinction, biotic replacements, and clade interactions; pp. 89–102. [Google Scholar]
- Berner (1977).Berner RA. Sedimentation and dissolution of pteropods in the ocean. In: Andersen RT, Malahoff A, editors. The fate of fossil fuel CO2 in the oceans. Plenum; New York: 1977. pp. 243–260. [Google Scholar]
- Briggs (1998).Briggs JC. Biotic replacements: extinction or clade interaction? Bioscience. 1998;48:389–395. doi: 10.2307/1313378. [DOI] [Google Scholar]
- Bristow, Ellison & Wood (1980).Bristow CR, Ellison RA, Wood CJ. The Claygate beds of essex. Proceedings of the Geological Association. 1980;91:261–277. doi: 10.1016/S0016-7878(80)80022-8. [DOI] [Google Scholar]
- Burridge et al. (2017).Burridge AK, Hörnlein C, Janssen AW, Hughes M, Bush SL, Marlétaz F, Gasca R, Pierrot-Bults AC, Michel E, Todd JA, Young JR, Osborn KJ, Menken SBJ, Peijnenburg KTCA. Time-calibrated molecular phylogeny of pteropods. PLOS ONE. 2017;12:e0177325. doi: 10.1371/journal.pone.0177325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahuzac & Janssen (2010).Cahuzac B, Janssen AW. Eocene to Miocene holoplanktonic Mollusca (Gastropoda) of the Aquitaine Basin, southwest France. Scripta Geologica. 2010;141:1–193. [Google Scholar]
- Cecca (1997).Cecca F. Late Jurassic and Early Cretaceous uncoiled ammonites: trophism-related evolutionary processes. Comptes Rendus de l’Académie des Sciences, Ser. IIA, Earth and Planetary Science. 1997;325:629–634. doi: 10.1016/S1251-8050(97)89465-7. [DOI] [Google Scholar]
- Christensen (2002).Christensen WK. Palaeobiology, phylogeny and palaeobiogeography of belemnoids and related coleoids. Berliner paläobiologische Abhandlungen. 2002;1:18–21. [Google Scholar]
- Copper (1989).Copper P. Enigmas in Phanerozoic reef development. Fossil Cnidaria. 1989;5:371–385. [Google Scholar]
- Corse et al. (2013).Corse E, Rampal J, Cuoc C, Pech N, Perez Y, Gilles A. Phylogenetic analysis of Thecosomata Blainville, 1824 (holoplanktonic Opisthobranchia) using morphological and molecular data. PLOS ONE. 2013;8:e59439. doi: 10.1371/journal.pone.0059439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curry (1982).Curry D. Ptéropodes éocènes de la tuilerie de Gan (Pyrénées–Atlantiques) et de quelques autres localités de SW de la France. Cahiers de Micropaléontologie. 1982;4:35–44. [Google Scholar]
- De Baets et al. (2012).De Baets K, Klug C, Korn D, Landman NH. Early evolutionary trends in ammonoid embryonic development. Evolution. 2012;66:1788–1806. doi: 10.1111/j.1558-5646.2011.01567.x. [DOI] [PubMed] [Google Scholar]
- De Baets, Landman & Tanabe (2015).De Baets K, Landman NH, Tanabe K. Ammonoid embryonic development. In: Klug C, Korn D, De Baets K, Kruta I, Mapes RH, editors. Ammonoid paleobiology: from anatomy to paleoecology. Springer; Dordrecht: 2015. pp. 113–205. [Google Scholar]
- De la Parra Venegas et al. (2011).De la Parra Venegas R, Hueter R, González Cano J, Tyminski J, Gregorio Remolina J, Maslanka M, Ormos A, Weigt L, Carlson B, Dove A. An unprecedented aggregation of Whale Sharks, Rhincodon typus, in Mexican Coastal Waters of the Caribbean Sea. PLOS ONE. 2011;6(4):e18994. doi: 10.1371/journal.pone.0018994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doguzhaeva et al. (2014).Doguzhaeva LA, Weis R, Delsate D, Mariotti N. Embryonic shell structure of Early–Middle Jurassic belemnites, and its significance for belemnite expansion and diversification in the Jurassic. Lethaia. 2014;47:49–65. doi: 10.1111/let.12037. [DOI] [Google Scholar]
- Doyle (1992).Doyle PA. Review of the biogeography of Cretaceous belemnites. Palaeogeography, Palaeoclimatology, Palaeoecology. 1992;92:207–216. doi: 10.1016/0031-0182(92)90082-G. [DOI] [Google Scholar]
- Foster & Twitchett (2014).Foster WJ, Twitchett RJ. Functional diversity of marine ecosystems after the Late Permian mass extinction event. Nature Geoscience. 2014;7:233–238. doi: 10.1038/ngeo2079. [DOI] [Google Scholar]
- Friedman et al. (2010).Friedman M, Shimada K, Martin LD, Everhart MJ, Liston J, Maltese A, Triebold M. 100-million-year dynasty of giant planktivorous bony fishes in the Mesozoic seas. Science. 2010;327:990–993. doi: 10.1126/science.1184743. [DOI] [PubMed] [Google Scholar]
- Gale, Kennedy & Martill (2017).Gale AS, Kennedy WJ, Martill D. Mosasauroid predation on an ammonite–Pseudaspidoceras–from the Early Turonian of south-eastern Morocco. Acta Geologica Polonica. 2017;67:31–46. doi: 10.1515/agp-2017-0003. [DOI] [Google Scholar]
- Goolaerts (2010).Goolaerts S. Late Cretaceous ammonites from Tunisia: chronology and causes of their extinction and extrapolation to other areas. Aardkundige Mededelingen. 2010;21:1–220. [Google Scholar]
- Gould & Calloway (1980).Gould SJ, Calloway CB. Clams and brachiopods–ships that pass in the night. Paleobiology. 1980;6:383–396. doi: 10.1017/S0094837300003572. [DOI] [Google Scholar]
- Guex (2006).Guex J. Reinitialization of evolutionary clocks during sublethal environmental stress in some invertebrates. Earth and Planetary Science Letters. 2006;242:240–253. doi: 10.1016/j.epsl.2005.12.007. [DOI] [Google Scholar]
- Hautmann (2014).Hautmann M. Diversification and diversity partitioning. Paleobiology. 2014;40:162–176. doi: 10.1666/13041. [DOI] [Google Scholar]
- Hodgkinson, Garvie & Bé (1992).Hodgkinson KA, Garvie CL, Bé AWH. Eocene euthecosomatous Pteropoda (Gastropoda) of the Gulf and eastern coasts of North America. Bulletin of American Paleontology. 1992;103:5–62. [Google Scholar]
- Hoffmann & Keupp (2015).Hoffmann R, Keupp H. Ammonoid paleopathology. In: Klug C, Korn D, De Baets K, Kruta I, Mapes RH, editors. Ammonoid paleobiology, volume I: from anatomy to ecology. vol. 43. Springer; Dordrecht: 2015. pp. 877–926. (Topics in geobiology). [Google Scholar]
- Hoffmann et al. (2014).Hofmann R, Hautmann M, Brayard A, Nützel A. Bylund, KG, Jenks JF, Vennin E, Olivier N, Bucher H. Recovery of benthic marine communities from the end-Permian mass extinction at the low-latitudes of Eastern Panthalassa. Palaeontology. 2014;57:547–558. doi: 10.1111/pala.12076. [DOI] [Google Scholar]
- Hunt et al. (2008).Hunt BPV, Pakhomov EA, Hosie GW, Siegel V, Ward P, Bernard K. Pteropods in Southern Ocean ecosystems. Progress in Oceanography. 2008;78:193–221. doi: 10.1016/j.pocean.2008.06.001. [DOI] [Google Scholar]
- Iba et al. (2011).Iba Y, Mutterlose J, Tanabe K, Sano S, Misaki A, Terabe K. Belemnite extinction and the origin of modern cephalopods 35 m.y. prior to the Cretaceous-Paleogene event. Geology. 2011;39:483–486. doi: 10.1130/G31724.1. [DOI] [Google Scholar]
- Jablonski (2008).Jablonski D. Extinction and the spatial dynamics of biodiversity. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:11528–11535. doi: 10.1073/pnas.0801919105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jablonski & Raup (1994).Jablonski D, Raup DM. Selectivity of end-Cretaceous marine bivalve extinctions. Science. 1994;268:389–391. doi: 10.1126/science.11536722. [DOI] [PubMed] [Google Scholar]
- Jacobs (1992).Jacobs DK. Shape, drag, and power in ammonoid swimming. Paleobiology. 1992;18:203–220. doi: 10.1017/S009483730001397X. [DOI] [Google Scholar]
- Jacobs & Chamberlain (1996).Jacobs DK, Chamberlain JA. Buoyancy and hydrodynamics in ammonoids. In: Landman NH, Tanabe K, Davis RA, editors. Ammonoid paleobiology. Topics in geobiology. Vol. 13. Plenum; New York: 1996. [Google Scholar]
- Jäger & Fraaye (1997).Jäger M, Fraaye R. The diet of the Early Toarcian ammonite Harpoceras falciferum. Palaeontology. 1997;40:557–574. [Google Scholar]
- Janssen & Goedert (2016).Janssen AW, Goedert JL. Notes on the systematics, morphology and biostratigraphy of fossil holoplanktonic Mollusca, 24. First observation of a genuinely Late Mesozoic thecosomatous pteropod. Basteria. 2016;80:59–63. [Google Scholar]
- Janssen & King (1988).Janssen AW, King C. Planktonic mollusks (pteropods) In: Vinken R, editor. The Northwest European Tertiary Basin, results of the International Geological Correlation Programme Project no. 124. Schweizerbart; Stuttgart: 1988. pp. 356–368. (Geologisches Jahrbuch 100). [Google Scholar]
- Janssen & Peijnenburg (2013).Janssen AW, Peijnenburg KTCA. Holoplanktonic Mollusca: development in the Mediterranean Basin during the last 30 Ma and their future. In: Goffredo S, Dubinsky Z, editors. The Mediterranean Sea. Its history and present challenges. Springer; Dordrecht: 2013. pp. 341–362. [Google Scholar]
- Janssen & Peijnenburg (2017).Janssen AW, Peijnenburg KTCA. An overview of the fossil record of Pteropoda (Mollusca, Gastropoda, Heterobranchia) Cainozoic Research. 2017;17:3–10. [Google Scholar]
- Janssen, Schnetler & Heilmann-Clausen (2007).Janssen AW, Schnetler KI, Heilmann-Clausen C. Notes on the systematics morphology and biostratigraphy of fossil holoplanktonic Mollusca, 19. Pteropods (Gastropoda, Euthecosomata) from the Eocene Lillebaelt Clay Formation (Denmark, Jylland) Basteria. 2007;71:157–168. [Google Scholar]
- Janssen, Sessa & Thomas (2016).Janssen AW, Sessa JA, Thomas E. Pteropoda (Mollusca, Gastropoda, Thecosomata) from the Paleocene-Eocene Thermal Maximum (United States Atlantic Coastal Plain) Palaeontologia Electronica. 2016;19 doi: 10.26879/689. Article 19.3.47A. [DOI] [Google Scholar]
- Jarman (2001).Jarman SN. The evolutionary history of krill inferred from nuclear large subunit rDNA sequence analysis. Biological Journal of the Linnean Society. 2001;73:199–212. doi: 10.1111/j.1095-8312.2001.tb01357.x. [DOI] [Google Scholar]
- Kauffman (2004).Kauffman EG. Mosasaur predation on Upper Cretaceous nautiloids and ammonites from the United States Pacific Coast. Palaios. 2004;19:96–100. doi: 10.1669/0883-1351(2004)019<0096:MPOUCN>2.0.CO;2. [DOI] [Google Scholar]
- Kauffman & Kesling (1960).Kauffman EG, Kesling RV. An Upper Cretaceous ammonite bitten by a mosasaur. Contributions of the Michigan University, Museum of Paleontology. 1960;15:193–248. [Google Scholar]
- Kear, Briggs & Donovan (1995).Kear AJ, Briggs DEG, Donovan DT. Decay and fossilization of non-mineralized tissue in coleoid cephalopods. Palaeontology. 1995;38:105–131. [Google Scholar]
- Keller et al. (2009).Keller G, Abramovich S, Berner Z, Adatte T. Biotic effects of the Chicxulub impact, K–T catastrophe and sea level change in Texas. Palaeogeography, Palaeoclimatology, Palaeoecology. 2009;271:52–68. doi: 10.1016/j.palaeo.2008.09.007. [DOI] [Google Scholar]
- Kennedy (1989).Kennedy WJ. Thoughts on the evolution and extinction of Cretaceous ammonites. Proceedings of the Geological Association. 1989;100:251–279. doi: 10.1016/S0016-7878(89)80047-1. [DOI] [Google Scholar]
- Kennedy (1993).Kennedy WJ. Ammonite faunas of the European Maastrichtian; diversity and extinction. In: House MR, editor. The Ammonoidea: environment, ecology, and evolutionary change. Oxford University Press; Oxford: 1993. pp. 285–326. (Systematics association special volume 47). [Google Scholar]
- Kennedy & Kaplan (1995).Kennedy WJ, Kaplan U. Parapuzosia (Parapuzosia) seppenradensis (Landois) und die Ammonitenfauna der Dülmener Schichten, unteres Unter-Campan, Westfalen. Geologie und Paläontologie in Westfalen. 1995;33:1–127. [Google Scholar]
- Keupp (2012).Keupp H. Atlas zur Paläopathologie der Cephalopoden. Berliner Paläobiologische Abhandlungen. 2012;12:1–390. [Google Scholar]
- Keupp et al. (2016).Keupp H, Hoffmann R, Stevens K, Albersdörfer R. Key innovations in Mesozoic ammonoids: the multicuspidate radula and the calcified aptychus. Palaeontology. 2016;59:775–791. doi: 10.1111/pala.12254. [DOI] [Google Scholar]
- Keupp & Schweigert (2015).Keupp H, Schweigert G. Kopffüßer (Cephalopoda) In: Arratia G, Schultze H-P, Tischlinger H, Viohl G, editors. Solnhofen. Ein Fenster ind die Jurazeit. Pfeil; München: 2015. pp. 218–238. [Google Scholar]
- King (1981).King C. Tertiary Research Special Papers 6. Backhuys Publishers; Kerkwerve: 1981. The stratigraphy of the London clay and associated deposits; pp. 1–158. [Google Scholar]
- Klug, De Baets & Korn (2016).Klug C, De Baets K, Korn D. Exploring the limits of morphospace: ontogeny and ecology of Late Viséan ammonoids from the Tafilalt (Morocco) Acta Palaeontologica Polonica. 2016;61:1–14. doi: 10.4202/app.00220.2015. [DOI] [Google Scholar]
- Klug & Lehmann (2015).Klug C, Lehmann J. Soft part anatomy of ammonoids: reconstructing the animal based on exceptionally preserved specimens and actualistic comparisons. In: Klug C, Korn D, De Baets K, Kruta I, Mapes RH, editors. Ammonoid paleobiology, volume I: from anatomy to ecology. vol. 43. Springer; Dordrecht: 2015. pp. 539–552. (Topics in geobiology). [Google Scholar]
- Klug, Riegraf & Lehmann (2012).Klug C, Riegraf W, Lehmann J. Soft-part preservation in heteromorph ammonites from the Cenomanian-Turonian Boundary Event (OAE 2) in the Teutoburger Wald (Germany) Palaeontology. 2012;55:1307–1331. doi: 10.1111/j.1475-4983.2012.01196.x. [DOI] [Google Scholar]
- Korn & Klug (2007).Korn D, Klug C. Conch form analysis, variability, and morphological disparity of a Frasnian (Late Devonian) ammonoid assemblage from Coumiac (Montagne Noire, France) In: Landman NH, Davis RA, Manger W, Mapes RH, editors. Cephalopods—present and past. Springer; Dordrecht: 2007. pp. 57–86. [Google Scholar]
- Kruta et al. (2011).Kruta I, Landman NH, Rouget I, Cecca F, Tafforeau P. The role of ammonites in the marine Mesozoic food web revealed by jaw preservation. Science. 2011;311:70–72. doi: 10.1126/science.1198793. [DOI] [PubMed] [Google Scholar]
- Lalli & Gilmer (1989).Lalli CM, Gilmer RW. Pelagic snails: the biology of holoplanktonic gastropod mollusks. Stanford University Press; Stanford: 1989. p. 269. [Google Scholar]
- Landman et al. (2015).Landman NH, Goolaerts S, Jagt JWM, Jagt-Yazykova EA, Machalski M. Ammonites on the brink of extinction: diversity, abundance, and ecology of the Order Ammonoidea at the Cretaceous/Paleogene (K/Pg) boundary. In: Klug C, Korn D, De Baets K, Kruta I, Mapes RH, editors. Ammonoid paleobiology: from macroevcolution to paleogeography. vol. 44. Springer; Dordrecht: 2015. pp. 497–553. (Topics in geobiology). [Google Scholar]
- Landman et al. (2014).Landman NH, Goolaerts G, Jagt JWM, Jagt-Yazykova EA, Machalski M, Yacobucci MM. Ammonite extinction and nautilid survival at the end of the Cretaceous. Geology. 2014;42:707–710. doi: 10.1130/G35776.1. [DOI] [Google Scholar]
- Landman, Tanabe & Shigeta (1996).Landman NH, Tanabe K, Shigeta Y. Ammonoid embryonic development. In: Landman NH, Tanabe K, Davis RA, editors. Ammonoid paleobiology. Plenum; New York: 1996. [Google Scholar]
- Landois (1895).Landois H. Die Riesenammoniten von Seppenrade, Pachydiscus Zittel seppenradensis II. Landois. Jahresbericht Der Zoologischen Section des Westfälischen Provinzial-Vereins für Wissenschaft und Kunst. 1895;23:99–108. [Google Scholar]
- Laptikhovsky, Nikolaeva & Rogov (2017).Laptikhovsky V, Nikolaeva S, Rogov M. Cephalopod embryonic shells as a tool to reconstruct reproductive strategies in extinct taxa. Biological Reviews of the Cambridge Philosophical Society. 2017 doi: 10.1111/brv.12341. Epub ahead of print May 30 2017. [DOI] [PubMed] [Google Scholar]
- Lehmann (1981).Lehmann U. Ammonite jaw apparatus and soft parts. In: House MR, Senior JR, editors. The Ammonoidea. Systematics Association Special Papers 18. Oxford University Press; Oxford: 1981. pp. 275–287. [Google Scholar]
- Lehmann (1985).Lehmann U. Zur Anatomie der Ammoniten: Tintenbeutel, Kiemen, Augen. Paläontologische Zeitschrift. 1985;59:99–108. doi: 10.1007/BF02986003. [DOI] [Google Scholar]
- Lokho & Kumar (2008).Lokho K, Kumar K. Fossil pteropods (Thecosomata, holoplanktonic Mollusca) from the Eocene of Assam-Arakan Basin, northeastern India. Current Science. 2008;94:647–652. [Google Scholar]
- Mackas & Galbraith (2012).Mackas DL, Galbraith MD. Pteropod time-series from the NE Pacific. ICES Journal of Marine Science. 2012;69:448–459. doi: 10.1093/icesjms/fsr163. [DOI] [Google Scholar]
- Mapes & Nützel (2009).Mapes RH, Nützel A. Late Palaeozoic mollusc reproduction: cephalopod egg-laying behavior and gastropod larval palaeobiology. Lethaia. 2009;42:341–356. doi: 10.1111/j.1502-3931.2008.00141.x. [DOI] [Google Scholar]
- Marshall & Ward (1996).Marshall CR, Ward PD. Sudden and gradual molluscan extinctions in the latest Cretaceous in western European Tethys. Science. 1996;274:1360–1363. doi: 10.1126/science.274.5291.1360. [DOI] [PubMed] [Google Scholar]
- McGhee et al. (2013).McGhee Jr GR, Clapham ME, Sheehan PM, Bottjer DJ, Droser ML. A new ecological-severity ranking of major Phanerozoic biodiversity crises. Palaeogeography, Palaeoclimatology, Palaeoecology. 2013;370:260–270. doi: 10.1016/j.palaeo.2012.12.019. [DOI] [Google Scholar]
- Miller et al. (2010).Miller KG, Sherrell RM, Browning JV, Field MP, Gallagher W, Olsson RK, Sugarman PJ, Tuorto S, Wahyudi H. Relationship between mass extinction and iridium across the Cretaceous-Paleogene boundary in New Jersey. Geology. 2010;38:867–870. doi: 10.1130/G31135.1. [DOI] [Google Scholar]
- Mironenko & Rogov (2016).Mironenko AA, Rogov MA. First direct evidence of ammonoid ovoviviparity. Lethaia. 2016;49:245–260. doi: 10.1111/let.12143. [DOI] [Google Scholar]
- Motta et al. (2010).Motta PJ, Maslanka M, Hueter RE, Davis RL, De la Parra R, Mulvany SL, Habegger ML, Strother JA, Mara KR, Gardiner JM, Tyminski JP, Zeigler LD. Feeding anatomy, filter-feeding rate, and diet of whale sharks Rhincodon typus during surface ram filter feeding off the Yucatan Peninsula, Mexico. Zoology. 2010;113:199–212. doi: 10.1016/j.zool.2009.12.001. [DOI] [PubMed] [Google Scholar]
- Naglik, Rikhtegar & Klug (2016).Naglik C, Rikhtegar FN, Klug C. Buoyancy in Palaeozoic ammonoids from empirical 3D-models and their place in a theoretical morphospace. Lethaia. 2016;49:3–12. doi: 10.1111/let.12125. [DOI] [Google Scholar]
- Naglik et al. (2015).Naglik C, Tajika A, Chamberlain J, Klug C. Ammonoid locomotion. In: Klug C, Korn D, De Baets K, Kruta I, Mapes RH, editors. Ammonoid paleobiology, volume I: from anatomy to ecology. vol. 43. Springer; Dordrecht: 2015. pp. 657–696. (Topics in geobiology). [Google Scholar]
- Nützel (2014).Nützel A. Larval ecology and morphology in fossil gastropods. Palaeontology. 2014;1:1–25. doi: 10.1111/pala.12104. [DOI] [Google Scholar]
- Nützel & Mapes (2001).Nützel A, Mapes RH. Larval and juvenile gastropods from a Carboniferous black shale: palaeoecology and implications for the evolution of the Gastropoda. Lethaia. 2001;34:143–162. doi: 10.1080/00241160152418447. [DOI] [Google Scholar]
- Nützel et al. (2016).Nützel A, Schneider S, Hülse P, Kelly SRA, Tilley L, Veit R. A new Early Jurassic gastropod from Ellesmere Island, Canadian Arctic–an ancient example of holoplanktonic gastropods. Bulletin of Geosciences. 2016;91:229–242. [Google Scholar]
- Olivero (2012).Olivero EB. Sedimentary cycles, ammonite diversity and palaeoenvironmental changes in the Upper Cretaceous Marambio Group, Antarctica. Cretaceous Research. 2012;34:348–366. doi: 10.1016/j.cretres.2011.11.015. [DOI] [Google Scholar]
- Olivero & Zinsmeister (1989).Olivero EB, Zinsmeister WJ. Large heteromorph ammonites from the Upper Cretaceous of Seymour Island, Antarctica. Journal of Paleontology. 1989;63:626–636. doi: 10.1017/S0022336000041251. [DOI] [Google Scholar]
- Payne et al. (2014).Payne JL, Heim NA, Knope ML, McClain CR. Metabolic dominance of bivalves predates brachiopod diversity decline by more than 150 million years. Proceedings of the Royal Society B: Biological Sciences. 2014;281 doi: 10.1098/rspb.2013.3122. Article 20133122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raup & Chamberlain (1967).Raup DM, Chamberlain Jr JA. Equations for volume and center of gravity in ammonoids shells. Journal of Paleontology. 1967;41:566–574. [Google Scholar]
- Roopnarine & Angielczyk (2015).Roopnarine PD, Angielczyk KD. Community stability and selective extinction during the Permian-Triassic mass extinction. Science. 2015;350:90–93. doi: 10.1126/science.aab1371. [DOI] [PubMed] [Google Scholar]
- Rosenzweig & McCord (1991).Rosenzweig ML, McCord RD. Incumbent replacement: evidence for long-term evolutionary progress. Paleobiology. 1991;17:202–213. doi: 10.1017/S0094837300010563. [DOI] [Google Scholar]
- Schaeffer (1965).Schaeffer B. The role of experimentation in the origin of higher levels of organization. Systematic Biology. 1965;14:18–336. doi: 10.2307/sysbio/14.4.318. [DOI] [PubMed] [Google Scholar]
- Schulte et al. (2010).Schulte P, Alegret L, Arenillas I, Arz JA, Barton PJ, Bown PR, Bralower TJ, Christeson GL, Claeys P, Cockell CS, Collins GS, Deutsch A, Goldin TJ, Goto K, Grajales-Nishimura JM, Grieve RAF, Gulick SPS, Johnson KR, Kiessling W, Koeberl C, Kring DA, MacLeod KG, Matsui T, Melosh J, Montanari A, Morgan JV, Neal CR, Nichols DJ, Norris RD, Pierazzo E, Ravizza G, Rebolledo-Vieyra M, Reimold WU, Robin E, Salge T, Speijer RP, Sweet AR, Urrutia-Fucugauchi J, Vajda V, Whalen MT, Willumsen PS. The Chicxulub asteroid impact and mass extinction at the Cretaceous-Paleogene Boundary. Science. 2010;327:1214–1218. doi: 10.1126/science.1177265. [DOI] [PubMed] [Google Scholar]
- Sepkoski (1981).Sepkoski Jr JJ. A factor analytic description of the Phanerozoic marine fossil record. Paleobiology. 1981;7:36–53. doi: 10.1017/S0094837300003778. [DOI] [Google Scholar]
- Shigeta (1993).Shigeta Y. Post-hatching early life history of Cretaceous Ammonoidea. Lethaia. 1993;26:133–145. doi: 10.1111/j.1502-3931.1993.tb01804.x. [DOI] [Google Scholar]
- Simpson (1943).Simpson GG. Turtles and the origin of the fauna of Latin America. American Journal of Science. 1943;241:413–429. doi: 10.2475/ajs.241.7.413. [DOI] [Google Scholar]
- Sims & Merrett (1997).Sims DW, Merrett DA. Determination of zooplankton characteristics in the presence of surface feeding basking sharks Cetorhinus maximus. Marine Ecology Progress Series. 1997;158:297–302. doi: 10.3354/meps158297. [DOI] [Google Scholar]
- Slater et al. (2017).Slater GJ, Price SA, Santini F, Alfaro ME. Diversity versus disparity and the radiation of modern cetaceans. Proceedings of the Royal Society B. 2017;277:3097–3104. doi: 10.1098/rspb.2010.0408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan et al. (1986).Sloan RE, Rigby K, Van Valen LM, Diane G. Gradual dinosaur extinction and simultaneous ungulate radiation in the Hell Creek formation. Science. 1986;232:629–633. doi: 10.1126/science.232.4750.629. [DOI] [PubMed] [Google Scholar]
- Smit (1982).Smit J. Extinction and evolution of planktonic formaninifera after a major impact at the Cretaceous/Tertiary boundary. GSA Special Paper. 1982;190:329–352. [Google Scholar]
- Tajika et al. (2015).Tajika A, Naglik C, Morimoto N, Pascual-Cebrian E, Hennhöfer DK, Klug C. Empirical 3D-model of the conch of the Middle Jurassic ammonite microconch Normannites, its buoyancy, the physical effects of its mature modifications and speculations on their function. Historical Biology. 2015;27:181–191. doi: 10.1080/08912963.2013.872097. [DOI] [Google Scholar]
- Tanabe, Kulicki & Landman (2008).Tanabe K, Kulicki C, Landman NH. Development of the embryonic shell structure of Mesozoic ammonoids. American Museum Novitates. 2008;3621:1–19. doi: 10.1206/588.1. [DOI] [Google Scholar]
- Tanabe & Tsukahara (1987).Tanabe K, Tsukahara J. Biometric analysis of Nautilus pompilius from the Philippines and the Fiji Islands. In: Saunders WB, Landman NH, editors. Nautilus. Plenum Press; New York: 1987. pp. 105–113. [Google Scholar]
- Teichert & Nützel (2015).Teichert S, Nützel A. Early Jurassic anoxia triggered the evolution of the oldest holoplanktonic gastropod Coelodiscus minutus by means of heterochrony. Acta Palaeontologica Polonica. 2015;60:269–276. doi: 10.4202/app.00145.2014. [DOI] [Google Scholar]
- Tobin et al. (2012).Tobin TS, Ward PD, Steig EJ, Olivero EB, Hilburn IA, Mitchell RN, Diamond MR, Raub TD, Kirschvink JL. Extinction patterns, δ18O trends, and magnetostratigraphy from a southern high-latitude Cretaceous-Paleogene section: links with Deccan volcanism. Palaeogeography, Palaeoclimatology, Palaeoecology. 2012;350–352:180–188. doi: 10.1016/j.palaeo.2012.06.029. [DOI] [Google Scholar]
- Tracey, Todd & Erwin (1993).Tracey S, Todd JA, Erwin DH. Mollusca: Gastropoda. In: Benton MJ, editor. The fossil record. Chapman and Hall; London: 1993. pp. 131–167. [Google Scholar]
- Tsujita & Westermann (2001).Tsujita CJ, Westermann GEG. Were limpets or mosasaurs responsible fort he perforations in the ammonite Placenticeras? Palaeogeography, Palaeoclimatology, Palaeoecology. 2001;169:245–270. doi: 10.1016/S0031-0182(01)00220-6. [DOI] [Google Scholar]
- Vinther et al. (2014).Vinther J, Stein M, Longrich NR, Harper DAT. A suspension-feeding anomalocarid from the Early Cambrian. Nature. 2014;507:496–497. doi: 10.1038/nature13010. [DOI] [PubMed] [Google Scholar]
- Walton, Korn & Klug (2010).Walton SA, Korn D, Klug C. Size distribution of the Late Devonian ammonoid Prolobites: indication for possible mass spawning events. Swiss Journal of Geoscience. 2010;103:475–494. doi: 10.1007/s00015-010-0036-y. [DOI] [Google Scholar]
- Ward (1996).Ward PD. Ammonoid extinction. In: Landman NH, Tanabe K, Davis RA, editors. Ammonoid Paleobiology. Plenum; New York: 1996. [Google Scholar]
- Ward & Bandel (1987).Ward PD, Bandel K. Life history strategies in fossil cephalopods. In: Boyle PR, editor. Cephalopod life cycles II. Academic Press; London: 1987. pp. 329–352. [Google Scholar]
- Ward & Signor (1983).Ward PD, Signor III PW. Evolutionary tempo in Jurassic and Cretaceous ammonites. Paleobiology. 1983;9:183–198. doi: 10.1017/S0094837300007569. [DOI] [Google Scholar]
- Yacobucci (2015).Yacobucci MM. Macroevolution and paleobiogeography of Jurassic-Cretaceous ammonoids. In: Klug C, Korn D, De Baets K, Kruta I, Mapes RH, editors. Ammonoid paleobiology, volume I: from anatomy to ecology. vol. 43. Springer; Dordrecht: 2015. pp. 539–552. (Topics in geobiology). [Google Scholar]
- Zorn (1991).Zorn I. A systematic account of Tertiary Pteropoda (Gastropoda, Euthecosomata) from Austria. Contributions to Tertiary and Quaternary Geology. 1991;28:95–139. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The following information was supplied regarding data availability:
Specimens are on display in the following museums: LWL-Museum für Naturkunde Münster, Germany, and Mikasa City Museum, Mikasa, Japan.