Summary
Extended video-EEG or 18F-fluorodeoxyglucose PET (FDG-PET) was obtained in 3 adult patients with hemianopia secondary to nonketotic hyperglycemia. Two male patients presented with left hemianopia and episodic left gaze deviation and one male patient presented with right hemianopia and visual hallucinations. None of the 3 patients had a history of seizures or known epilepsy risk factors. All 3 patients were found to have elevated serum glucose (267 mg/dL, 320 mg/dL, and 487 mg/dL) without acidosis or urine ketones. In all 3 patients, video-EEG recorded recurrent ictal discharges originating from the posterior quadrant contralateral to their hemianopia. In 2 patients, FDG-PET demonstrated corresponding focal areas of hypermetabolism. Resolution of visual symptoms was achieved with antiepileptic drugs, hydration, and tight glycemic control.
Homonymous hemianopia (HH) occurs in the setting of nonketotic hyperglycemia (NKH).1 The nature of this particular phenomenon is unclear, as partial seizures are common in NKH. We report evidence of ictal activity from EEG and functional imaging in 3 patients with NKH-associated HH, supporting the hypothesis that this condition is the result of recurrent partial seizures.
Case reports
Case 1
A 45-year-old African American man presented to the emergency department with severe headache, blurred vision, and complaints of bumping into objects with the left side of his body. His fiancée also described episodes of behavioral arrest and intermittent deviation of his head and eyes to the left side. His past medical history was notable for diabetes mellitus, hypertension, and obstructive sleep apnea.
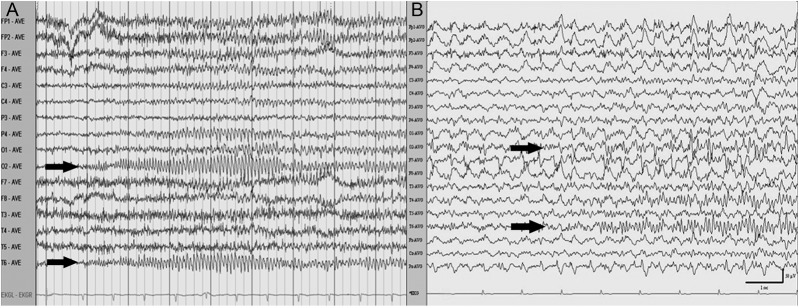
His vital signs were within the normal range. His general and neurologic examinations were unremarkable except for a dense left HH. During the examination, the patient exhibited a 30-second episode of speech and behavioral arrest, with head and eye deviation to the left. He was responsive to vocal and tactile stimulation, but exhibited delayed verbal response, poor comprehension, and inattention. A bedside EEG demonstrated recurrent ictal discharges initiated with beta activity originating from the right posterior quadrant (figure 1A). Laboratory investigation was notable for glucose of 267 mg/dL and calculated osmolality of 293 mOsm/kg. CSF cell count was 0, glucose 92 mg/dL, and protein 40 mg/dL. Bacterial, viral, and fungal cultures and herpes simplex virus PCR were negative.
EEG recording of ictal discharges
Figure 1. EEG demonstrates definite ictal discharges from the right posterior quadrant (arrows) for patient 1 (A) and patient 2 (B).
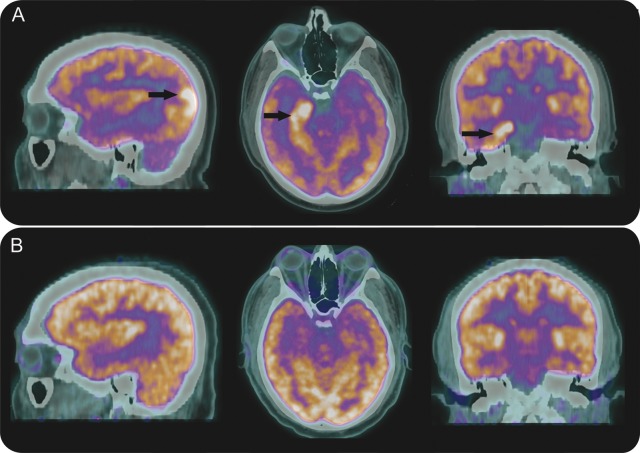
A noncontrasted MRI of the brain showed right temporo-occipital cortical thickening with T2 hyperintensity and subtle gadolinium enhancement of the right hippocampus. 18F-fluorodeoxyglucose PET (FDG-PET) of the brain showed right occipital as well as right mesial temporal hypermetabolism (figure 2A).
Brain FDG-PET images
Figure 2. FDG-PET images of brain in patient 1 demonstrate hypermetabolic foci in the right occipital lobe and right hippocampus (arrows) 2 days after presentation (A), and complete resolution of hypermetabolism 3 months later (B).
Resolution of clinical seizures was achieved with a combination of valproic acid, levetiracetam, and oxcarbazepine, hydration, and IV insulin. The patient was discharged on oxcarbazepine monotherapy. One month after presentation, automated perimetry demonstrated marked visual improvement with only a small residual noncongruous left hemianopia. Three months following his initial presentation, there was no visual field cut to confrontation. Repeat MRI with gadolinium was normal and brain FDG-PET demonstrated symmetric FDG uptake (figure 2B). Oxcarbazepine was eventually tapered and discontinued.
Case 2
A 60-year-old man presented with complaints of colored spots and loss of vision in his left visual field. Two weeks before presentation, he experienced a minor head trauma when his vehicle rear ended another car. He also complained of increased thirst, polyuria, and weight loss of 30 lb for the past 4 months. His medical history indicated hypertension, obesity, and asthma. On examination, vital signs were unremarkable except for a blood pressure of 176/110 mm Hg. Neurologic examination showed left HH and left end-gaze nystagmus. His general examination was unremarkable. Laboratory investigations revealed glucose of 320 mg/dL and calculated serum osmolarity of 290 mOsm/kg. Lumbar puncture revealed 8 leukocytes, 100 erythrocytes, glucose of 110 mg/dL, and protein of 70 mg/dL with negative infectious testing.
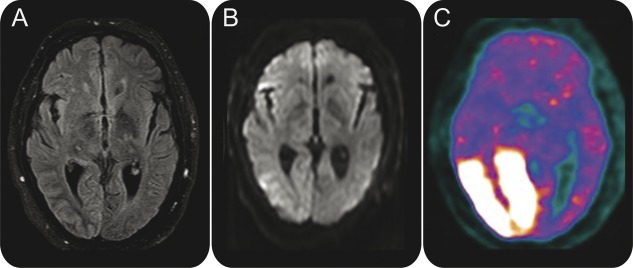
Brain MRI demonstrated T2 hyperintensity in the right temporo-occipital cortex and subtle restricted diffusion in the lateral right occipital lobe (figure 3, A and B). EEG recorded recurrent focal ictal discharges originating from the right occipital and posterior temporal regions coinciding with ictal nystagmus (figure 1B). Brain FDG-PET revealed an area of hypermetabolism involving the right occipital cortex, right posterior and mesial temporal cortex, and right thalamus (figure 3C).
Brain MRI
Figure 3. Brain images of patient 2 demonstrate right occipital cortical hyperintensity and subcortical hypointensity on fluid-attenuated inversion recovery (A), subtle restricted diffusion in lateral right occipital lobe on diffusion-weighted imaging (B), and marked hypermetabolism of right occipital lobe on FDG-PET (C).
The patient's seizures persisted on continuous EEG despite oxcarbazepine and levetiracetam but resolved following aggressive hydration and escalating doses of insulin. He was discharged on levetiracetam monotherapy. Visual field examination on day of discharge demonstrated an incomplete left congruous HH with a temporal crescent. Two weeks after discharge, kinetic perimetry using a Goldmann perimeter revealed no focal defects. EEG performed 2 months later demonstrated only attenuation of the right posterior dominant rhythm. He was ultimately weaned off levetiracetam.
Case 3
A 69-year-old man presented with moderate headache and 5 days history of visual disturbances described as “green circles” in the right lower binocular field. He had experienced unintentional weight loss in recent months and had bulbar myasthenia gravis that was well-controlled on a stable regimen of pyridostigmine and prednisone. He had hypertension, atrial fibrillation, and congestive heart failure. On examination, he had an irregularly irregular heart rhythm and obesity. His neurologic examination was remarkable for a right HH. Laboratory investigation revealed glucose of 487 mg/dL and measured serum osmolality of 315 mOsm/kg.
Brain MRI demonstrated no restricted diffusion or focal parenchymal abnormalities. EEG recorded 13 distinct seizures originating in the left occipital lobe. The patient was given an IV phenytoin load, vigorous fluid hydration, and insulin. When normoglycemia was achieved, there was resolution of clinical and electrographic seizures. Phenytoin was discontinued on his second hospital day. Visual fields were reported full to confrontation 4 days after admission.
DISCUSSION
We report 3 patients, each with HH in the setting of NKH. All 3 patients had focal posterior quadrant seizures recorded on EEG and 2 patients had occipital hypermetabolism on brain FDG-PET scan. These findings strongly support that ongoing partial seizure activity was the underlying pathophysiology of HH associated with NKH.
The association of HH with NKH was well-documented in a report of 4 patients who presented with positive or negative visual phenomena involving a single hemifield. EEGs obtained after treatment demonstrated posterior quadrant nonspecific abnormalities in 2 patients and interictal epileptiform discharges in one patient; only one patient had an EEG before treatment was initiated, documenting recurrent occipital ictal discharges. There is other evidence of occipital seizures in the setting of hyperglycemia in NKH.2,3 Two separate reports in patients with right HH described left occipital hyperperfusion on SPECT in conjunction with left occipital rhythmic beta activity on EEG,4 and fMRI blood oxygenation level–dependent activation in Brodmann area 18 in conjunction with left occipital ictal activity.5 To our knowledge, there are no reports of brain FDG-PET with EEG to confirm the ictal nature of NKH-associated HH. Brain MRI abnormalities mainly consisted of cortical T2 hyperintensity and subtle restricted diffusion, which were reversible. As previously described in NKH-associated seizures,4 these transient changes favor an epileptic rather than an ischemic cause.
Our findings have clinical implications. The development of HH in patients with uncontrolled diabetes, whether known or newly diagnosed, should suggest ictal activity, rather than an ischemic etiology, particularly when visual symptoms are intermittent, whether positive or negative. Alternatively, HH may actually represent a postictal phenomenon akin to Todd paralysis. Prompt recognition of the underlying metabolic state is key to avoid inappropriate therapy and to prevent progression. Although acute antiepileptic drug therapy is needed, correction of the underlying metabolic disorder is also an important component of effective therapy.
The biochemical mechanism underlying the occurrence of seizures in NKH is not well-understood. One hypothesis relates to depletion of gamma aminobutyric acid (GABA).6 In hyperglycemic conditions, Krebs cycle and glucose utilization are depressed, enhancing alternative pathways of energy metabolism, including conversion of GABA to succinic acid via the succinic-semialdehyde pathway (the GABA shunt), which supplies up to 40% of the brain energy requirements.6 The resultant decrease in neuronal GABA leads to proconvulsive activity, even in previously normal brain tissue. This is in contrast to diabetic ketoacidosis, where ketone bodies provide most of the brain energy supply and also convey antiepileptic effect.7
It is not known whether drugs that enhance GABA will be more effective for seizures in the setting of NKH. Aggressive insulin therapy and adequate hydration are crucial. While phenytoin was used in our third patient, it should be avoided as it can exacerbate hyperglycemia by reducing insulin secretion.8 NKH-associated seizures may be resistant to antiepileptic drug therapy, which should be limited to the acute phase of the illness. Reversal of neurologic deficits primarily depends on correction of underlying hyperglycemia. None of our 3 patients had subsequent emergence of unprovoked seizures after antiepileptic drug withdrawal.
STUDY FUNDING
No targeted funding reported.
DISCLOSURES
A. Stayman reports no disclosures. B. Abou-Khalil serves on the editorial board of Epilepsy Research and as a Contributing Editor for Epilepsy Currents; receives publishing royalties for Atlas of EEG and Seizure Semiology (Elsevier, 2005); and receives research support from Valeant Pharmaceuticals International, UCB, Schwarz, Ortho McNeill, GlaxoSmithKline, Pfizer, Upsher Smith, Supernus, and Sunovion. P. Lavin and N. Azar report no disclosures. Full disclosure form information provided by the authors is available with the full text of this article at http://cp.neurology.org/lookup/doi/10.1212/CPJ.0b013e3182a7bb76.
Correspondence to: nabil.azar@vanderbilt.edu
Funding information and disclosures are provided at the end of the article. Full disclosure form information provided by the authors is available with the full text of this article at http://cp.neurology.org/lookup/doi/10.1212/CPJ.0b013e3182a7bb76.
Footnotes
Correspondence to: nabil.azar@vanderbilt.edu
Funding information and disclosures are provided at the end of the article. Full disclosure form information provided by the authors is available with the full text of this article at http://cp.neurology.org/lookup/doi/10.1212/CPJ.0b013e3182a7bb76.
REFERENCES
- 1.Lavin PJ. Hyperglycemic hemianopia: a reversible complication of non-ketotic hyperglycemia. Neurology. 2005;65:616–619. doi: 10.1212/01.wnl.0000173064.80826.b8. [DOI] [PubMed] [Google Scholar]
- 2.Duncan MB, Jabbari B, Rosenberg ML. Gaze-evoked visual seizures in nonketotic hyperglycemia. Epilepsia. 1991;32:221–224. doi: 10.1111/j.1528-1157.1991.tb05248.x. [DOI] [PubMed] [Google Scholar]
- 3.Harden CL, Rosenbaum DH, Daras M. Hyperglycemia presenting with occipital seizures. Epilepsia. 1991;32:215–220. doi: 10.1111/j.1528-1157.1991.tb05247.x. [DOI] [PubMed] [Google Scholar]
- 4.Wang CP, Hsieh PF, Chen CC. Hyperglycemia with occipital seizures: images and visual evoked potentials. Epilepsia. 2005;46:1140–1144. doi: 10.1111/j.1528-1167.2005.56404.x. [DOI] [PubMed] [Google Scholar]
- 5.Del Felice A, Zanoni T, Avesani M. EEG-fMRI coregistration in non-ketotic hyperglycemic occipital seizures. Epilepsy Res. 2009;85:321–324. doi: 10.1016/j.eplepsyres.2009.03.025. [DOI] [PubMed] [Google Scholar]
- 6.Guisado R, Arieff AI. Neurologic manifestations of diabetic comas: correlation with biochemical alterations in the brain. Metabolism. 1975;24:665–679. doi: 10.1016/0026-0495(75)90146-8. [DOI] [PubMed] [Google Scholar]
- 7.Kastrup O, Gerwig M, Frings M, Diener HC. Posterior reversible encephalopathy syndrome (PRES): electroencephalographic findings and seizure patterns. J Neurol. 2012;259:1383–1389. doi: 10.1007/s00415-011-6362-9. [DOI] [PubMed] [Google Scholar]
- 8.Malherbe C, Burrill KC, Levin SR, Karam JH, Forsham PH. Effect of diphenylhydantoin on insulin secretion in man. N Engl J Med. 1972;286:339–342. doi: 10.1056/NEJM197202172860702. [DOI] [PubMed] [Google Scholar]