Summary
Intravenous immunoglobulin (IVIg) has been widely used in the treatment of autoimmune neuromuscular diseases. Compared to other treatment modalities, such as corticosteroids and chemotherapy for autoimmune disorders, IVIg has relatively few side effects and favorable therapeutic outcomes in certain neuromuscular diseases. There is Class I evidence for IVIg as an initial treatment for patients with Guillain-Barré syndrome (GBS), chronic inflammatory demyelinating polyneuropathy (CIDP), and multifocal motor neuropathy. It is as effective as plasma exchange in GBS and CIDP. In myasthenia gravis, IVIg is used for myasthenic crisis and exacerbations, though it is also helpful as maintenance therapy, particularly in patients with a suboptimal response or contraindications to prednisone or other immunosuppressive agents. IVIg has been demonstrated to be beneficial in placebo-controlled, double-blind, randomized studies in dermatomyositis and Lambert-Eaton syndrome. IVIg has also been beneficial in select patients with polymyositis and other autoimmune peripheral neuropathies. Clinical trials in amyotrophic lateral sclerosis, inclusion body myositis, and anti–myelin-associated glycoprotein neuropathy have been negative.
Intravenous immunoglobulin (IVIg) has been widely used in the treatment of neuromuscular disease due to relatively few side effects and favorable therapeutic outcomes. It has multiple effects on the immune system that are responsible for its benefit in autoimmune neuromuscular disorders. Serious side effects, including thromboembolic events, renal failure, aseptic meningitis, and anaphylactic reactions, are rare.
IVIg is prepared from human plasma that is pooled from 3,000 to 10,000 donors. It is purified by enzymatic treatment, followed by fractionation and chromatography and usually stabilized with sugars or amino acids to avoid aggregation. Most products are treated with viral inactivation methods to inactivate hepatitis, retroviruses, and other viruses. These include solvent detergent methods or caprylate incubation. A case of hepatitis C transmission in 1994 was associated with one particular brand of IVIg, which has led to modification with solvent/detergent or 10% caprylate to eliminate viruses from all subsequent IVIg formulations. Since the addition of these viral inactivation methods, there have been no case reports of hepatitis transmission. There have been no confirmed cases of HIV transmission via IVIg. As with any biologic product, the risk of transmission of new and unrecognized blood-borne pathogens remains.1 IVIg contains >95% immunoglobulin G (IgG) and <2.5% immunoglobulin A (IgA). Other plasma components such as CD4, CD8, and human leukocyte antigen (HLA) molecules, as well as coagulation factors, are present in the formulations. The half-life of IVIg is 18–32 days.2
Mechanism of action
The IgG molecule in the IVIg is thought to be responsible for the therapeutic mechanism of IVIg. The IgG molecule can be divided into the antigen binding region, involved in adaptive immunity, and Fc, involved in innate immunity. Small amounts of IgA and immunoglobulin M (IgM) antibodies within the IVIg formulation may also contribute to the therapeutic efficacy. There are several targets that have been identified on which IVIg is thought to act. T cells, which interact with antigen presenting cells, play a crucial role in adaptive immunity. Components of IVIg, such as soluble CD4/CD8, HLA, and anti-TCR, interrupt T cells' interaction with antigen presenting cells. Additionally, they may inactivate, silence, or bring about apoptosis in T cells.
Cytokines are released when T cells are activated. Th1 cytokines are thought to be proinflammatory, while Th2 cytokines are thought to be anti-inflammatory. In many autoimmune disorders, this balance is disrupted and Th1 cells predominate, thus causing a systemic proinflammatory state. IVIg is thought to restore the balance between Th1 and Th2 cells because it contains antibodies to Th1 cytokines, a number of Th2 cytokines, as well as Th1 antagonists, thus shifting toward the anti-inflammatory state. Another mode of IVIg action is the interruption of T cells' migration from the blood to the peripheral nerve, through the blood–nerve barrier. IVIg, therefore may interfere with a build-up of inflammation in target organs.
In addition to T cells, many autoimmune diseases are caused by B-cell production of autoantibodies that IVIg may counteract as well. Several modes are postulated, including downregulation of the production of antibodies by B cells, the neutralization of autoantibodies by anti-idiotypes contained in the IVIg, blocking the activity of specific B-cell populations by anti-CD5 antibodies found in IVIg preparations, as well as a blockade of receptors on the surface of B cells that are responsible for their proliferation. The Fcγ receptor, FcγRIIB, expressed on myeloid and B cells, is thought to have a role in the balance between tolerance and autoimmunity. This receptor is reduced in patients with CIDP and is upregulated with IVIg treatment, thus promoting an anti-inflammatory state vs a proinflammatory state.3 Additionally, IVIg interrupts several steps in the complement activation cascade and interrupts the assembly of the terminal complement complex. Finally, IVIg has been shown to have an effect on the Fc receptor–mediated activity of immunologically relevant cells, thus downregulating the damaging effects of macrophages.4 Different mechanisms are more important than others in individual autoimmune disorders. However, the fact that IVIg can act on multiple paths of immune dysregulation is likely beneficial.
Efficacy
IVIg is widely used in neurologic autoimmune conditions (table). In the management of Guillain-Barré syndrome (GBS), IVIg and plasma exchange are the mainstay of therapy with about equal efficacy. Plasma exchange, however, is not readily available at all institutions, is more difficult to enact, and may have worse outcomes in patients with septicemia. In chronic inflammatory demyelinating polyneuropathy (CIDP), which has a good response to immunomodulatory treatment, maintenance treatment is usually required due to the short-lived effect of the medication5. The frequency and dosage are variable because relapses can occur at irregular time intervals. Some patients respond after the first 2 g/kg bolus; however, the majority of patients require more prolonged treatment before a benefit can be demonstrated. Treatment for 3 months is recommended before it is determined that patients are unresponsive. IVIg is the only treatment that has been shown to be effective in randomized, placebo-controlled studies for multifocal motor neuropathy and is the treatment of choice. Much like CIDP, a therapeutic schedule needs to be designed by the provider to tailor to the needs of the patient. IVIg has been shown to be efficacious in select patients with other chronic autoimmune neuropathies, including the sensory ataxic neuropathy associated with Sjögren disease, as well as other Sjögren neuropathies, sarcoid neuropathy, the neuropathy associated with inflammatory bowel disease, and Churg-Strauss neuropathy. IVIg is beneficial in some patients with IgM anti-nerve antibody–associated neuropathy, such as anti-sulfatide neuropathy and CANOMAD (chronic ataxic neuropathy, ophthalmoplegia, IgM paraprotein, cold agglutinins, and disialosyl antibodies). Some patients with progressive idiopathic neuropathy have improved with IVIg as well.6,7
Table Neuromuscular diseases and evidence supporting IVIg use7,9,11,12
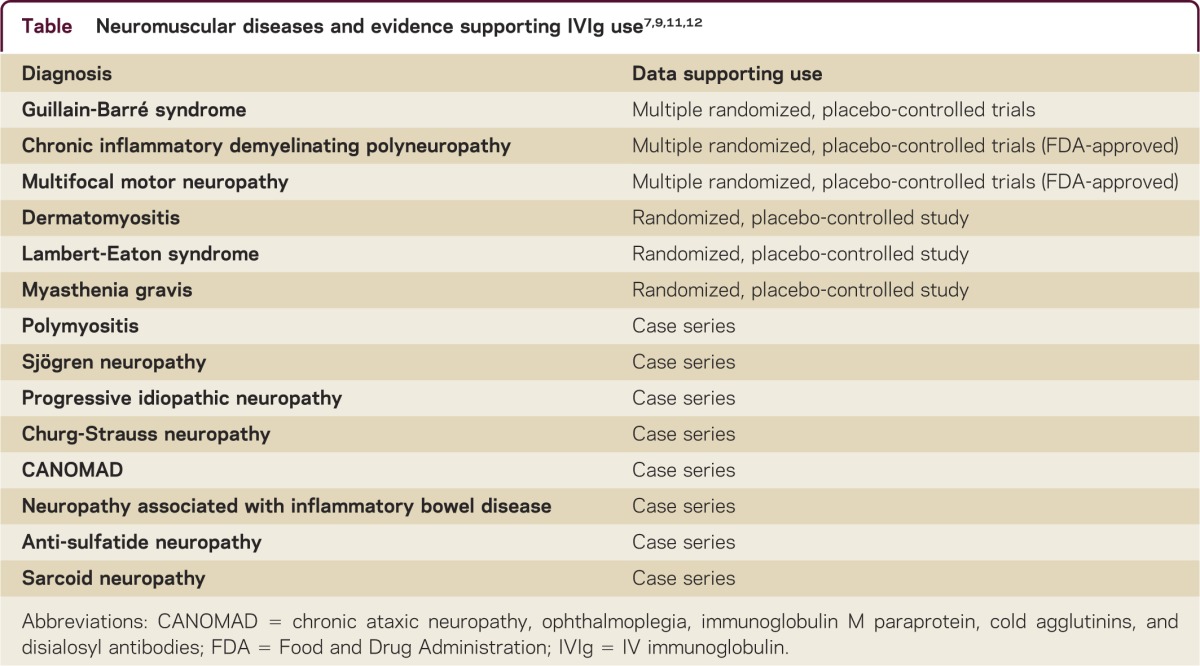
In the management of myasthenia gravis (MG), IVIg is often used for myasthenic crisis or exacerbations and has a similar therapeutic effect as plasma exchange during an acute myasthenic crisis. There is now Class I evidence supporting both treatments. In such patients, repeated infusions are often necessary as the favorable effect is often short-lived. IVIg has also been shown to be beneficial for at least 60 days in treatment of persistently symptomatic MG, in randomized, double-blind, placebo-controlled studies.8 It is also often used for patients who have contraindications or a suboptimal response to prednisone or immunosuppressive medications or as a useful adjunct in persistently symptomatic patients awaiting the long latent response to immunosuppressive therapies. IVIg has been shown to be effective in placebo-controlled, double-blind, randomized trials in dermatomyositis and in other studies in polymyositis. In dermatomyositis and polymyositis, IVIg is usually used when steroid therapy has failed or is unable to be tapered after an initial response, or when side effects occur or contraindications exist.6 The benefit of IVIg in the treatment of Lambert-Eaton myasthenic syndrome (LEMS) has been demonstrated in a small randomized, double-blind, placebo-controlled study. Amelioration of weakness and reduction of the anti-Ca channel antibodies occur.9
A placebo-controlled randomized crossover study was negative for IVIg in inclusion body myositis (IBM), although a minority of patients experienced some benefit. Similarly, 2 placebo-controlled randomized double-blind studies of IVIg in anti–myelin-associated glycoprotein (MAG) neuropathy were negative; however, some patients improved in some of the secondary outcomes. Several small open-label studies have shown no benefit for IVIg in amyotrophic lateral sclerosis (ALS).
For most disorders, the usual initial dose of IVIg is 2 g/kg, divided over 2 to 5 days. For chronic disorders, IVIg is typically given with doses ranging from 0.4 g/kg to 2 g/kg every 1 to 4 weeks, depending on the patient's response. If patients are stable and tapering the dosage is attempted, this can be done by reducing the dosage of the infusion or increasing the interval between infusions. IVIg can affect common laboratory tests; for example, the erythrocyte sedimentation rate can rise to over 100 after IVIg is given.
Adverse events
Although highly effective in treating many autoimmune neuromuscular disorders, there are several known adverse events associated with the administration of IVIg. Chills, nausea, myalgia, headache, and vasomotor symptoms are typically infusion-related and can often be controlled by reducing the infusion rates or with premedication with acetaminophen or diphenhydramine.1 With more severe reactions, premedication with IV hydrocortisone may be given. Rare and more serious side effects are known to occur in some patients as well.
Anaphylaxis has been reported in patients with immunodeficiency and selective IgA deficiency, which is thought to be due to the presence of anti-IgA antibodies. Patients with IgA deficiency have safely received IVIg, including those with IgG antibodies against IgA. The presence of immunoglobulin E antibodies to IgA, however, has been reported in patients who had anaphylaxis with IVIg. The incidence of IgA deficiency is 1:1,000 and screening IgA levels prior to administration is not essential.7
Renal failure may result in those receiving IVIg and is related to a sucrose-containing formulation and preexisting renal insufficiency. Renal status should be closely monitored in those patients who have diabetes, preexisting renal disease, hypovolemia, or sepsis, or are on concomitant therapy with nephrotoxic agents. Slower infusion rates, adequate hydration, as well as the use of products without sucrose are advised to prevent renal failure.1
Thromboembolic events, including stroke, myocardial infarction, central retinal vein occlusion, deep vein thrombosis, and pulmonary embolism, are the most serious of complications associated with IVIg administration. Case-control studies have shown that no single cardiovascular risk factor, such as coronary artery disease, cigarette use, hypertension, cerebrovascular disease, diabetes, and hyperlipidemia, increases the risk of a thromboembolic event, but the risk is elevated when 2 risk factors are present and significant when 4 or more risk factors are present. The increase in viscosity is dose-dependent; patients with monoclonal gammopathies and other causes of increased viscosity may be more susceptible to this effect of IVIg and screening serum viscosity prior to treatment is advised. Thus, increased serum viscosity, procoagulant activity of factor XIa, and the action of IVIg on endothelial cells are postulated to be the factors contributing to thromboembolic events.2,10 Aseptic meningitis has been reported, with an incidence as high as 11%. Patients develop headache, nuchal rigidity, drowsiness, fever, photophobia, painful eye movements, nausea, and vomiting that can last for several days. CSF may reveal a pleocytosis with elevated leukocyte counts with a polymorphonuclear predominance. Patients with a history of migraine are more susceptible. This occurs with different IVIg brands and can recur in the same patient, even if different brands of IVIg are used.9 Pretreatment with steroids or rechallenging patients with other IVIg brands has often not been useful in preventing aseptic meningitis. Patients without history of migraines may be able to tolerate another trial of IVIg.
There is Class I evidence for IVIg as an initial treatment for patients with GBS, CIDP, and multifocal motor neuropathy.9 It is as effective as plasma exchange in GBS and CIDP. In MG, IVIg is recommended for myasthenic exacerbations or crisis and, in some patients, for maintenance therapy, particularly when prednisone or immunosuppressive agents are insufficient or contraindicated. IVIg is also beneficial in LEMS, dermatomyositis, polymyositis, and some chronic autoimmune peripheral neuropathies. Clinical trials have not shown benefit in IBM, anti-MAG neuropathy, and ALS. Serious side effects, such as thromboembolic events, are rare but may occur.
STUDY FUNDING
No targeted funding reported.
DISCLOSURES
K. Ruzhansky reports no disclosures. T.H. Brannagan serves as a consultant for Grifols, Pfizer, CSL, and Baxter and on the speakers' bureau for Grifols. Full disclosure form information provided by the authors is available with the full text of this article at http://cp.neurology.org/lookup/doi/10.1212/CPJ.0b013e3182a78ecf.
ACKNOWLEDGMENT
The authors thank Toni Brannagan for comments on the manuscript.
Correspondence to: Tb2325@columbia.edu
Funding information and disclosures are provided at the end of the article. Full disclosure form information provided by the authors is available with the full text of this article at http://cp.neurology.org/lookup/doi/10.1212/CPJ.0b013e3182a78ecf.
Footnotes
Correspondence to: Tb2325@columbia.edu
Funding information and disclosures are provided at the end of the article. Full disclosure form information provided by the authors is available with the full text of this article at http://cp.neurology.org/lookup/doi/10.1212/CPJ.0b013e3182a78ecf.
REFERENCES
- 1.Brannagan TH. Intravenous gammaglobulin (IVIg) for treatment of CIDP and related immune-mediated neuropathies. Neurology. 2002;59:S33–S40. doi: 10.1212/wnl.59.12_suppl_6.s33. [DOI] [PubMed] [Google Scholar]
- 2.Brannagan TH. Current treatments of chronic immune-mediated demyelinating polyneuropathies. Muscle Nerve. 2009;39:563–578. doi: 10.1002/mus.21277. [DOI] [PubMed] [Google Scholar]
- 3.Tackenberg B, Jelcic I, Baerenwaldt A. Impaired inhibitory Fcgamma receptor IIB expression on B cells in chronic inflammatory demyelinating polyneuropathy. Proc Natl Acad Sci USA. 2009;106:4788–4792. doi: 10.1073/pnas.0807319106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hartung HP. Advances in the understanding of the mechanism of action of IVIg. J Neurol. 2008;255:3–6. doi: 10.1007/s00415-008-3002-0. [DOI] [PubMed] [Google Scholar]
- 5.Hughes RA, Donofrio P, Bril V. Intravenous immune globulin (10% caprylate-chromatography purified) for the treatment of chronic inflammatory demyelinating polyradiculoneuropathy (ICE study): a randomised placebo-controlled trial. Lancet Neurol. 2008;7:136–144. doi: 10.1016/S1474-4422(07)70329-0. [DOI] [PubMed] [Google Scholar]
- 6.Stangel M, Hartung HP, Marx P, Gold R. Intravenous immunoglobulin treatment of neurological autoimmune diseases. J Neurol Sci. 1998;153:203–214. doi: 10.1016/s0022-510x(97)00292-x. [DOI] [PubMed] [Google Scholar]
- 7.Donofrio PD, Berger A, Brannagan TH. Consensus statement: the use of intravenous immunoglobulin in the treatment of neuromuscular conditions report of the AANEM ad hoc committee. Muscle Nerve. 2009;40:890–900. doi: 10.1002/mus.21433. [DOI] [PubMed] [Google Scholar]
- 8.Barth D, Nabavi Nouri M, Ng E, Nwe P, Bril V. Comparison of IVIg and PLEX in patients with myasthenia gravis. Neurology. 2011;76:2017–2023. doi: 10.1212/WNL.0b013e31821e5505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dalakas MC. The use of intravenous immunoglobulin in the treatment of autoimmune neuromuscular diseases: evidence-based indications and safety profile. Pharmacol Ther. 2004;102:177–193. doi: 10.1016/j.pharmthera.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 10.Caress JB, Hobson-Webb L, Passmore LV, Finkbiner AP, Cartwright MS. Case-control study of thromboembolic events associated with IV immunoglobulin. J Neurol. 2009;256:339–342. doi: 10.1007/s00415-009-0969-0. [DOI] [PubMed] [Google Scholar]
- 11.Latov N, Gorson KC, Brannagan TH. Diagnosis and treatment of chronic immune-mediated neuropathies. J Clin Neuromuscul Dis. 2006;7:141–157. doi: 10.1097/01.cnd.0000205575.26451.e4. [DOI] [PubMed] [Google Scholar]
- 12.Hughes RA, Swan AV, van Doorn PA. Intravenous immunoglobulin for Guillain-Barre syndrome. Cochrane Database Syst Rev. 2012;7:CD002063. doi: 10.1002/14651858.CD002063.pub5. [DOI] [PubMed] [Google Scholar]


