Abstract
Our brains represent spatial information in egocentric (self-based) or allocentric (landmark-based) coordinates. Rodent studies have demonstrated a critical role for the caudate in egocentric navigation and the hippocampus in allocentric navigation. We administered tests of egocentric and allocentric working memory to individuals with premotor Huntington’s disease (pmHD), which is associated with early caudate nucleus atrophy, and controls. Each test had 80 trials during which subjects were asked to remember 2 locations over 1-sec delays. The only difference between these otherwise identical tests was that locations could only be coded in self-based or landmark-based coordinates. We applied a multiatlas-based segmentation algorithm and computed point-wise Jacobian determinants to measure regional variations in caudate and hippocampal volumes from 3 T MRI. As predicted, the pmHD patients were significantly more impaired on egocentric working memory. Only egocentric accuracy correlated with caudate volumes, specifically the dorsolateral caudate head, right more than left, a region that receives dense efferents from dorsolateral prefrontal cortex. In contrast, only allocentric accuracy correlated with hippocampal volumes, specifically intermediate and posterior regions that connect strongly with parahippocampal and posterior parietal cortices. These results indicate that the distinction between egocentric and allocentric navigation applies to working memory. The dorsolateral caudate is important for egocentric working memory, which can explain the disproportionate impairment in pmHD. Allocentric working memory, in contrast, relies on the hippocampus and is relatively spared in pmHD.
Keywords: Visuospatial, Working memory, Caudate, Navigation
1. Introduction
Our brains code spatial information separately in allocentric (landmark-based) or egocentric (self-based) coordinates. The allocentric reference frame represents the spatial relationships between landmarks, independent of the position of the self, and is important for developing cognitive maps (Maguire et al., 1998; O’Keefe and Nadel, 1978). The egocentric reference frame represents object locations in reference to the position of the self, is updated during movement, and is important for navigating toward a visible landmark and learning stimulus-response associations and fixed routes (Aguirre and D’Esposito, 1999; Chersi and Burgess, 2015; Redish, 1999). These representational systems operate largely in parallel, and individuals favor one system or the other when solving a navigation task (Bohbot et al., 2007; Iaria et al., 2003).
Egocentric and allocentric representational systems have been studied extensively in the context of navigation learning. Well-established rodent paradigms, including the Morris Water Maze, the plus maze, and the radial arm maze, have been manipulated to test the integrity of either system. For example, maze targets can be positioned relative to other landmarks to measure allocentric navigation, or to the rodent’s starting position to measure egocentric navigation. Lesion and neuronal activation studies show a double dissociation in the neural bases of these strategies such that the hippocampus is critical for allocentric navigation and the caudate for egocentric navigation (Kesner and Gilbert, 2006; McDonald and White, 1994; Packard and Knowlton, 2002; Packard and McGaugh, 1996; Pearce et al., 1998; Vann et al., 2000).
Human studies that have adapted these paradigms in real-space (delpolyi, et al., 2007; Hort et al., 2007) and virtual environments (Doeller et al., 2008; Etchamendy and Bohbot, 2007; Hartley et al., 2003; Iaria et al., 2007; Possin et al., 2016) demonstrate that the role of the hippocampus in allocentric navigation may translate, to some degree, to humans. For example, transgenic mice expressing human amyloid precursor protein and patients with mild cognitive impairment with the predicted pathology of Alzheimer’s disease show similar impairments on analogous versions of an allocentric Morris Maze (Possin et al., 2016). Performance on an allocentric Morris Maze correlates with right hippocampal volumes in patients with amnestic mild cognitive impairment or Alzheimer’s disease (Nedelska et al., 2012) and with the size of CA1 hippocampal lesions in acute transient global amnesia (Bartsch et al., 2010). In functional neuroimaging studies, the medial temporal lobe activates in relationship to the spatial layout of virtual environments (Aguirre et al., 1996; Maguire et al., 1998; Mellet et al., 2000). The hippocampus contribution to human navigation is not only allocentric, however. Direct projections from parietal cortex provide a large amount of egocentric information to the hippocampus (Boccia et al., 2016; Kravitz et al., 2011), and hippocampal hypoactivation in amnestic MCI has been associated with decreased performances in both egocentric and allocentric navigation (Boccia et al., 2016). Beyond navigation, a leading view is that the hippocampus encodes events by mapping the relationships between objects and actions within spatial contexts Eichenbaum and Cohen, 2014).
The caudate appears to play an important role in egocentric forms of human navigation. Navigating based on memory for prior responses is associated with caudate nucleus activation (Iaria et al., 2003) and individuals who spontaneously adopt an egocentric response (versus allocentric) strategy on a radial maze task have larger caudate and smaller hippocampal volumes (Bohbot et al., 2007). Also, patients with early stage Huntington’s disease (HD), which is associated with caudate atrophy, have shown less caudate activation and greater hippocampal activation than controls on a route-learning task, suggesting that the hippocampus may compensate during navigation for functional degradation of the caudate (Voermans et al., 2004). However, in this same study, the HD patients did not differ from controls in their navigation accuracy, and further, Majerová et al. (2012) demonstrated normal navigation in HD patients with mild motor symptoms and parallel deterioration in allocentric and egocentric navigation in patients with moderate motor symptoms.
There are limitations to investigating the allocentric - egocentric distinction in navigation. Navigation learning is complex, involving basic perceptual and memory related processes as well as the integration and manipulation of multisensory information over time and space (Wolbers and Hegarty, 2010). Performance can be compromised by impairment in any of the component processes. Also, although widely-used egocentric and allocentric navigation paradigms usually favor one strategy, they can often be solved by either strategy (Ekstrom et al., 2014; Neggers et al., 2006; Wolbers and Wiener, 2014). Further, egocentric navigation has been inconsistently defined. Judgment of the order of landmarks, the novelty of landmarks, and landmark appearance, and way-finding along habitual routes and memory for responses, have all been considered forms of egocentric navigation (Boccia et al., 2014).
In a model presented by Wolbers and Hegarty (2010), navigation relies on various sensory cues, computational mechanisms, and both online and offline spatial representations. Online spatial representations, in their model, include both egocentric self-to-object directions and distances, and allocentric object-to-object directions and distances. Consistent with their model, there is some evidence that spatial information may be distinctly represented by the egocentric and allocentric reference frames, and there may be caudate-hippocampal underpinnings that parallel those of more complex navigation learning. Postle and D’Esposito (2003) presented healthy young adults with blocks of allocentric working memory trials (the subjects briefly remembered the distance between a square and an adjacent line) and egocentric working memory trials (the subjects remembered where a square was relative to their own gaze with allocentric reference points disrupted). Caudate activity was greater during the delay period of the egocentric trials only. Individuals with HD have shown impaired immediate memory for arm movements or hand movements (Davis et al., 2007, 2003), whereas patients with hippocampal damage have shown impaired perception and brief memory for topographical information (Hartley et al., 2007). Both individuals with hippocampal damage and individuals with HD have shown impaired memory for locations on a grid (Davis et al., 2003; Olson et al., 2006), which can be represented in either allocentric or egocentric coordinates.
The possibility of measuring the allocentric - egocentric distinction in working memory by using an experimental design that facilitates one strategy while preventing the other is compelling. Working memory, when tested in its most basic form with simple stimuli and brief delays, could allow for a more pure measure of the reference frames. Furthermore, working memory is an essential component of all navigation tasks; one cannot navigate using either reference frame without online spatial representations. Thus, the distinction measured in working memory would have relevance for understanding the distinction in navigation learning.
The purpose of this study was to evaluate whether the allocentric - egocentric distinction existed in the context of simple visuospatial working memory with a hippocampal - caudate dissociation that paralleled the literature on navigation learning. We adapted the Postle and D’Esposito working memory tests that require subjects to briefly represent locations relative to a landmark or relative to their own position (2003). The working memory tests were otherwise identical, which allowed us to independently evaluate and compare the integrity of working memory in each reference frame. We compared performance on the tests in individuals with premotor Huntington’s disease (pmHD) and neurologically healthy controls. HD is an auto-somal dominant inherited disorder caused by an expansion of the trinucleotide repeat cytosine-adenine-guanine (CAG). Individuals with pmHD do not yet exhibit the motor symptoms including chorea, but can exhibit cognitive or psychiatric changes (Epping et al., 2015; Paulsen et al., 2013), including working memory impairments (You et al., 2014). Caudate volume loss is present more than a decade before HD diagnosis and contributes robustly to cognitive impairment, whereas hippocampal volumes are relatively preserved and do not predict cognitive impairment (Aylward et al., 2013, 2004). We correlated accuracy on each test with regional caudate and hippocampal volumes. We hypothesized that individuals with pmHD would show greater impairment on egocentric working memory. Also, we hypothesized that atrophy in the dorsolateral head of the caudate, a region that receives projections from dorsolateral prefrontal regions important for goal directed actions (Bonelli and Cummings, 2007), would be associated with egocentric working memory accuracy. We hypothesized that the posterior hippocampus, a region with place cells, strong connections to parahippocampal cortex and posterior parietal cortex, and an important role in spatial processing and navigation (Fanselow and Dong, 2010; Poppenk et al., 2013), would be associated with allocentric working memory accuracy.
2. Method
2.1. Subjects
This study was approved by the UCSF Committee on Human Research. Written informed consent was obtained from each subject. Demographic characteristics, motor scores, and egocentric and allocentric working memory accuracy are presented in Table 1 for the full sample and for the neuroimaging subsample.
Table 1.
Demographic features and egocentric working memory (EWM) and allocentric working memory (AWM) performance of the full sample who performed the tests and the subsample who completed the 3 T MRI.
| N | Age | %female | education | MoCA | UHDRS – motor | EWM | AWM | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||||
| Accuracy | RT | Accuracy | RT | ||||||||
| Full Sample | NC | 17 | 44.2 (15.9) | 51 | 16.8 (1.6) | 27.9 (2.3) | n/a | .88 (.07) | 1204 (211) | .86 (.04) | 1342 (250) |
| pmHD | 16 | 44.9 (12.0) | 56 | 15.3 (2.4) | 26.9 (1.9) | 8.8 (6.5) | .72 (.10) | 1576 (381) | .82 (.07) | 1654 (323) | |
| MRI Subsample | NC | 10 | 41.9 (15.1) | 50 | 17.0 (1.5) | 27.8 (2.5) | n/a | .86 (.08) | 1171 (246) | .86 (.05) | 1278 (234) |
| pmHD | 14 | 45.8 (11.9) | 57 | 15.6 (2.3) | 26.9 (2.0) | 8.5 (5.9) | .72 (.10) | 1544 (399) | .82 (.07) | 1617 (301) | |
Abbreviations: MoCA: Montreal Cognitive Assessment; UHDRS: Unified Huntington’s Disease Rating Scale; RT: reaction time, reported in milliseconds.
Tests of egocentric and allocentric spatial working memory were administered to 16 pmHD subjects recruited through clinic or participation in other research studies, and to 17 neurologically healthy controls (NC) who were selected to match the pmHD individuals on age and sex. The pmHD individuals tested positive for the HD mutation with at least 40 CAG repeats and did not meet criteria for manifest motor HD according to previous methods (Paulsen et al., 2008). Motor symptoms were evaluated by a neurologist using the Unified Huntington’s Disease Rating Scale Motor subscale. NC status was determined on the basis of neurological history and examination. pmHD and NC subjects were excluded if they had current major psychiatric illness or substance abuse disorder, ongoing cancer treatment, known HIV, or history of metabolic abnormalities, major systemic medical illness, traumatic brain injury with > 30 min loss of consciousness, seizure disorder, or diagnosis of developmental learning disability. The groups were closely matched in age and sex, but the NCs obtained higher education (p=.04). Global cognitive scores, as measured by the Montreal Cognitive Assessment (MoCA), did not differ between the groups, p=.17, and the MoCA scores in the pmHD subjects ranged from 23 to 30. All individuals had good visual acuity (Snellen scores between 20/20 to 20/30).
All of the subjects who completed the cognitive testing were invited to complete a 3 T MRI, and this was completed by 14 of the pmHD and 10 of the NC subjects.
2.2. Egocentric and allocentric working memory tests
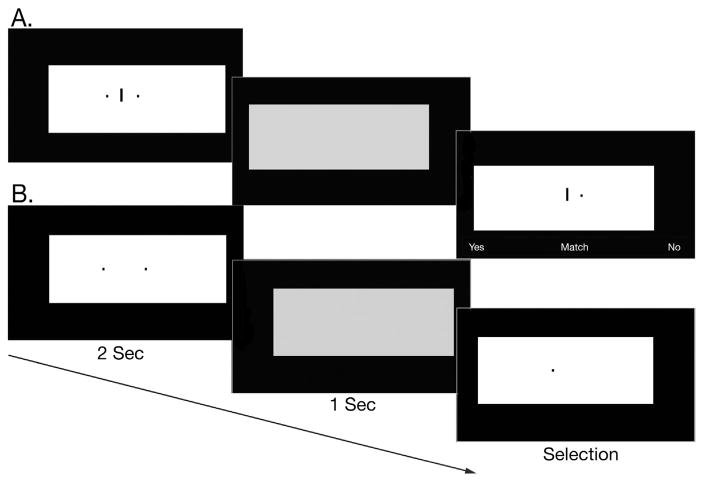
Subjects were seated and positioned with their gaze fixated 18 in. from and perpendicular to the center of a 30″ monitor in a dark room. In both tests, the targets to be remembered were two black rectangles that subtended .75° X .88° in visual angle and were positioned on a large white background rectangle that subtended 55.7°×25°. On the allocentric working memory test, a line appeared between the targets, and the subjects were told to remember the distance of each of these black rectangles from the line. On the egocentric working memory test, there was no line, and subjects remembered the location of each target relative to their own position or gaze. After 2 s, the targets disappeared and the large background rectangle shifted positions and turned gray to minimize afterimages. After a 1 s delay, one of the targets reappeared (on the allocentric version it reappeared with the line) and the large background rectangle shifted positions again and turned white. This probe remained on the screen until the subject responded. Subjects pressed the left arrow key to indicate that yes, the probe was a match with one of the targets, or the right arrow key to indicate that no, the probe was not a match. These keys were labeled with “Y” and “N” stickers accordingly. On allocentric working memory match trials, the relative distance and position (right v left) of the probe rectangle to the line was the same as the relative distance and position of one of the target rectangles to the line (eg, if the probe appeared on the right of the line, it was the same distance to the line as the target that appeared on the right of the line). On egocentric working memory match trials, the probe was in the same location as one of the target rectangles, that is, relative to the subject’s position and relative to the computer monitor. However, because we disrupted the availability of the monitor edge as a landmark (see below), the only available reference was the subject’s own position. Each test had 80 trials that included 32 match trials and 48 non-match trials presented randomly. Test order was counterbalanced to control for order effects. Performance was measured in accuracy and no time limit was placed on responding. See Fig. 1 for sample trials.
Fig. 1.
Sample allocentric working memory (A) and egocentric working memory (B) match trials.
On the Allocentric Working Memory Test, the targets always appeared on either side and on the same horizontal plane as the line. To prevent an egocentric strategy, these stimuli appeared in different positions during target presentation and probe.
On the Egocentric Working Memory Test, subjects were asked to remember the position of the 2 target rectangles relative to their own position or gaze. To prevent an allocentric strategy, we used the shifting background white rectangle, large monitor, and dark room but applied these to both tests for consistency. Although it is possible that subjects may have used the computer monitor edge as a landmark, this would have been difficult because the monitor edge was in 39 degrees from central vision, which is in the range of peripheral vision where visual acuity is poor. Also, they would have had to inhibit the moving white rectangle, and both the monitor edge and the background screen were black and so the contrast was low in the dark room.
2.3. MRI acquisition and image preprocessing
Subjects were scanned on a 3 T MR imaging scanner (GE Healthcare, Milwaukee, Wisconsin) with 8 channel head coils. Volumetric T1-weighted imaging was performed using the following parameters: TR/TE=7/2 ms, flip angle=15°, FOV =23 cm, matrix=256×192, yielding the image resolution of .9×.9×1mm3. Each image underwent automated correction for intensity non-uniformity (Sled et al., 1998) and was then spatially normalized using linear registration to the MNI-ICBM 152 nonlinear template created using an unbiased framework for the construction of nonlinear average templates (Fonov et al., 2011).
Bilateral caudate and hippocampal volumes were segmented automatically based on a multi-atlas and label fusion algorithm (Wang and Yushkevich, 2013a). To increase the automated segmentation accuracy, which can be compromised by modeling of anatomic variability, each individual image to segment was registered to images in previously described template libraries, in which 50 caudates (Kim et al., 2016) and 368 hippocampi (Kim et al., 2012) were manually labeled on these template images. These images were taken from a database comprising healthy subjects, and patients with various brain disorders and presumably have good coverage of shape variability. The manual segmentations in these libraries followed the protocols defined for caudates (Kim, et al.,; 2015; Looi et al., 2008) and hippocampi (Joo et al., 2014). For each structure, we calculated regionally the image similarity between an individual MRI to segment and each template image using a patch-based approach. Image patches were ranked based on the image similarity and their corresponding manual label patches were subsequently fused to segment for a given patch voxel of the respective individual image (0 – background; 1 – the target structural label), using a weighted averaging method that emphasized the most similar patches (Wang and Schmid, 2013). This procedure was iterated across all the voxels within a volume of interest that was a 5×5×5 voxel cube (Wang and Yushkevich, 2013b) that included the target structure (i.e., caudate or hippocampus) and its surrounding areas. After automated segmentation, every image was reviewed and manually corrected when necessary. The resulting labels were converted to a surface, triangulated with 1002 points equally for each structure distributed across subjects using SPHARM-PDM (Styner et al., 2005). Point-wise displacement vectors were computed between each individual structure and a template surface representing the whole cohort. Computing point-wise Jacobian determinants from these vectors quantified local volumes (Kim et al., 2013).
2.4. Statistical analysis
A mixed model ANOVA was performed to evaluate the accuracy of the pmHD individuals, compared to controls, on the egocentric and allocentric working memory tests. The normality and homogeneity of variance assumptions were met. To identify the relationship between regional variations in volume and accuracy on the egocentric and allocentric working memory tests in the mixed sample of pmHD patients and controls, we correlated Jacobian determinants point-wise while correcting for age and gender and with FDR correction for multiple comparisons, α =.05. T-values greater than 2.8 were considered significant.
3. Results
The group (pmHD v NC) by test (egocentric v allocentric) ANOVA revealed a significant interaction effect, F (1, 31)=6.56, p=.02. The pmHD patients were more impaired on the egocentric working memory test, t (31)=4.85, p < .001, d=1.68, pmHD M (SD)=.72 (.10), NC M (SD)=.88 (.07), mean difference (MD)=.15, 95% CI [.09, .22] than the allocentric working memory test, t (31)=2.17, p=.04, d =.75, pmHD M (SD)=.82 (.07), NC M (SD)=.86 (.04), MD=.05, 95% CI [.003, .09]; see Fig. 2. When a Bonferroni correction was applied to the group effects, α =.05/2, only the group effect on egocentric working memory was significant. The main effect of group was also significant, F (1, 31) =30.42, p < .001, d=1.91, MD =.10, 95% CI [.06, .14]. Although there was a tendency for the egocentric working memory test to be more difficult for all subjects combined, F (1, 31)=3.71, p=.06, d=.41, MD =.04, 95% CI [−.01, .08], this was driven entirely by the pmHD group, t (15) =2.53, p=.02, d=.98, MD =.09, 95% CI [.01, .17], as the NCs did not differ in their accuracy on the two tests, t (16) =−.64, p=.53, d=−.21, MD =.01, 95% CI [−.03, .06]. When the ANOVA was repeated with education as a covariate, the interaction remained significant, F (1, 30)=4.49, p=.04.
Fig. 2.
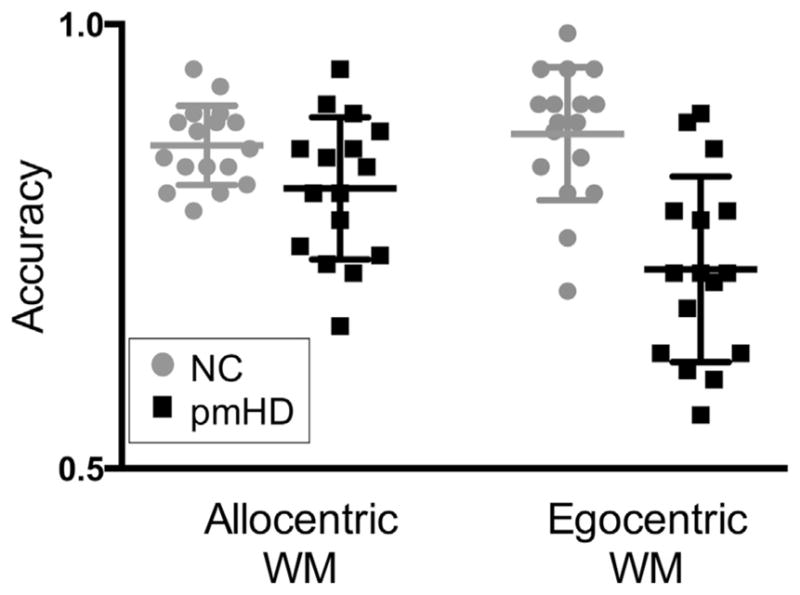
Performance on the allocentric and egocentric working memory (WM) tests in neurologically healthy controls (NC) and individuals with premotor Huntington’s disease (pmHD). Bars represent means and 25th and 75th percentiles.
The group by test ANOVA was repeated for mean reaction time. The interaction effect was not significant, F (1, 31)=.46, p=.50. The pmHD individuals were slower than the NCs across tests, F (1, 31)=13.26, p=.001, d=1.26, MD =342 ms, 95% CI [150,533]. Performance on the allocentric working memory task was slower across groups, F (1, 31)=6.20, p=.02, d=.32, MD=109 ms, 95% CI [21,197].
The mean caudate volume was smaller in the pmHD patients (M=2075+/−1130 mm^3) than in the controls (M=3369+/−584), p=.003, d =1.44. The mean hippocampal volume did not differ between pmHD patients (M=3295+/−424) and controls (M=3346+/−371 mm^3), p=.8, d=.13. Shape analysis of these structures in the same sample was previously reported and revealed volume reductions from controls in the dorsal and medial portions of the caudate head (Kim et al., 2016).
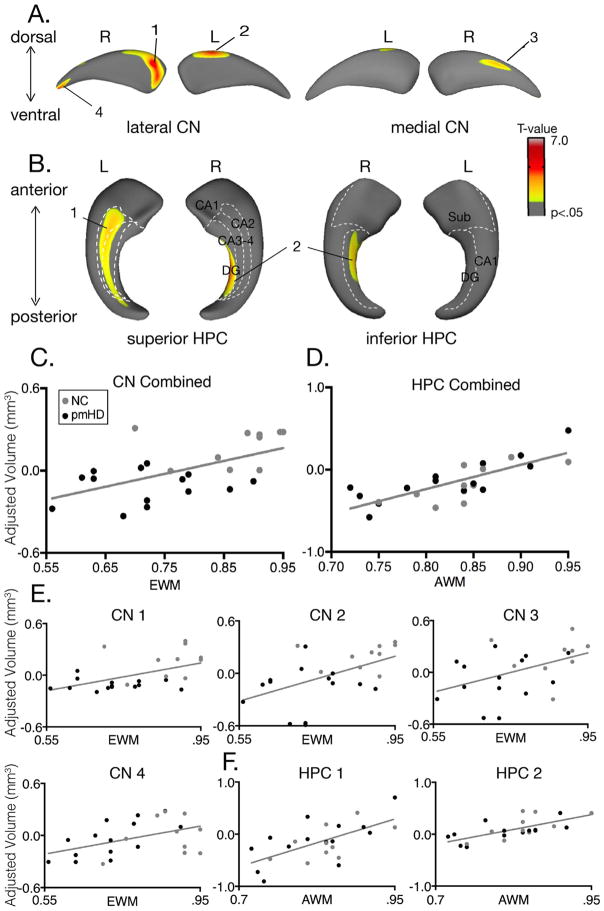
Egocentric working memory accuracy correlated with global caudate (right caudate: t(20)=2.84, r=.54, 95% CI [.18, .77], p=.01; left caudate: t(20)=2.72, r =.52, 95% CI [.15, .76], p=.03). Furthermore, egocentric working memory accuracy correlated with regional variations in caudate volumes. Specifically, significant correlations were seen in the dorsolateral head of the caudate bilaterally. The largest and most significant cluster was in the right dorsolateral head of the caudate, t=4.53, r=.71, 95% CI [.43, .87], p=.00007, followed by the left dorsolateral head, t=3.57, r=.62, 95% CI [.29, .82], p=.002. Smaller and less significant clusters were observed in the right medial body and the right tail (t=3.03, r=.56, 95% CI [.20, .79], p=.008; t=3.28, r =.59, 95% CI [.25, .80], p=.006). Egocentric working memory accuracy did not correlate significantly with hippocampal volumes globally (right: t=.61, r=.14, 95% CI [−.28, .51], p=.28; left: t=.69, r=.15, 95% CI [−.27, .52], p=.31), nor did it correlate with regional variations in hippocampal volumes, even when the relationship was explored without a multiple comparison correction; all t values were less than t=1.70, p > .10 (Fig. 3).
Fig. 3.
Egocentric working memory correlates with regional variations in caudate volumes and allocentric working memory with regional variations in hippocampal volumes. A. Caudate regions that correlate with egocentric working memory accuracy. B. Hippocampal regions that correlate with allocentric working memory accuracy. C. Scatterplot of the correlation between egocentric working memory accuracy and the combined caudate regional volumes that significantly correlated with performance (shown in A). D. Scatterplot of the correlation between allocentric working memory accuracy and the combined hippocampal regional volumes that significantly correlated with performance (shown in B). E. Correlation of each caudate region with egocentric working memory accuracy. F. Correlation of each hippocampus region with allocentric working memory. Abbreviations: CN=caudate nucleus, HPC=hippocampus, EWM=egocentric working memory, AWM = allocentric working memory.
Allocentric working memory accuracy did not correlate with global hippocampal volume (right hippocampus: t(20)=.48, r=.11, 95% CI [−.31, .49], p=.39; left hippocampus: t(20)=1.12, r=.24, 95% CI [−.18, .59], p=.22). Accuracy correlated significantly with regional variations in hippocampal volumes, however, with clusters that extended through intermediate and posterior regions but not including anterior hippocampus. Specifically, significant correlations were observed in right dentate gyrus, t=5.40, r =.77; 95% CI [.53, .90], p < .00001, and left CA2-4, t=5.91, r=.80, 95% CI [.59, .91], p < .00001. Allocentric working memory accuracy did not correlate significantly with global right (t=1.13, r =.24, 95% CI [−.18, .59], p=.22) or left (t=.69, r =.15, 95% CI [−.27, .52], p=.31) caudate volumes, nor did it correlate with regional variations in caudate volumes, even when the relationship was explored without a multiple comparison correction; all t values were less than 1.30, p > .20.
4. Discussion
Research in rodents has demonstrated that allocentric and egocentric navigation learning are dissociable cognitive processes that rely on distinct neural systems, with a critical role for the hippocampus in allocentric navigation and the caudate in egocentric navigation (McDonald and White, 1994; O’Keefe and Nadel, 1978; Packard and McGaugh, 1996). More recently, this dissociation has been shown to extend to some degree to humans, but interpretations from the human data have been limited by the multifactorial nature of the cognitive processes required for successful performance. This is particularly true for egocentric navigation because the paradigms have been altered substantially from the rodent paradigms to increase difficulty for humans, and because the egocentric navigation strategy is inconsistently defined across studies (Boccia et al., 2014). For the present study, we developed analogous tests of allocentric and egocentric working memory with simple stimuli, brief delays, and experimental controls to separately measure the mental representation of spatial locations relative to landmarks (allocentric), and spatial locations relative to the viewer (egocentric). Individuals with pmHD and caudate atrophy were significantly more impaired on the egocentric version. We found a double dissociation with caudate and hippocampal regional volumes such that egocentric working memory correlated with dorsal caudate volume, and allocentric working memory correlated with posterior hippocampal volume. Taken together, the dissociation between allocentric and egocentric spatial processing that has been extensively investigated in the context of navigation learning also extends to working memory, and working memory may allow for a more pure measure of these spatial reference frames in humans.
The egocentric working memory test required subjects to briefly remember locations defined relative to their own position or gaze. Non-egocentric reference points were disrupted using a large monitor, a moving white rectangle superimposed on a black background screen, and a dark room. If subjects had used a landmark such as the monitor edge to solve this task, it would have been more difficult than our allocentric working memory test for which the targets were presented with a landmark in high contrast and central vision. Controls performed similarly on the egocentric and allocentric working memory tests, however, suggesting they did not use an allocentric strategy on the egocentric test. Egocentric working memory performance correlated with volume in the dorsolateral head of the caudate bilaterally, with the largest cluster in the right caudate. The dorsolateral head of the caudate receives dense efferents from dorsolateral frontal regions important for executive and motor control, as well as from parietal sensory regions (Alexander et al., 1986). The dorsolateral head of the caudate may integrate perception and action by coding spatial information represented in parietal cortex in preparation for movement (Chersi and Burgess, 2015; Neggers et al., 2006; Voorn et al., 2004). Consistent with our findings, rats have shown impairment in coding egocentrically defined locations contralateral to dorsal striatum lesions (Brasted et al., 1997) and primates have shown metabolic activation in the caudate head during a right-left alternation spatial working memory test but not an object alternation working memory test, which was associated with caudate body activation (Levy et al., 1997).
The allocentric working memory test required subjects to briefly remember locations defined relative to a landmark. The stimuli moved together relative to the viewer between presentation and probe, preventing egocentric strategies. Performance correlated with volume in the intermediate and posterior regions of the hippocampus but not with any regions in the anterior hippocampus. The posterior and the anterior hippocampus, which correspond to the dorsal and ventral hippocampus in rodents, are anatomically and functionally segregated (Fanselow and Dong, 2010; Moser and Moser, 1998). The posterior hippocampus has long range connections to parahippocampal cortex, anterior and posterior cingulate cortex, cuneus, precuneus, inferior parietal lobe, and dorsolateral prefrontal cortex (Poppenk et al., 2013) and has place cells that underlie cognitive maps of the environment important for spatial memory and navigation (Moser et al., 1995; O’Keefe and Dostrovsky, 1971). Increased c-fos activation has been shown in the dorsal hippocampus of rats across all subfields when the spatial demands of a radial maze working memory test are increased (Vann et al., 2000). Within the intermediate and posterior hippocampus, we found significant correlations with the right dentate gyrus and the left CA 2–4 subfields.
A widely-held idea in cognitive neuroscience is that the medial temporal lobes, including the hippocampi, are not required for working memory unless the retention interval or the memory load exceed working memory capacity such that long-term memory systems are required (Jeneson et al., 2011). Individuals with medial temporal lobe damage, including the famous patient H.M., have been reported to retain information via continuous rehearsal, but to fail when the memoranda exceeds working memory capacity or attention is distracted (Milner et al., 1968; Squire and Wixted, 2011;). An alternative view supported by our results is the caudate and the hippocampus represent distinct types of information (Gaffan, 2002; Ranganath and Blumenfeld, 2005). Our working memory tests had the same short retention intervals (1 s) and spatial memory load (2 spatial relationships), and the only difference between the tests was the reference point of the spatial relationships: the self (egocentric) or a landmark (allocentric). We find that the caudate is not universally involved in spatial working memory, and the hippocampus is important even for subspan allocentric spatial working memory. The role of the caudate in briefly representing locations in self-based coordinates is consistent with its role in procedural learning and stimulus-response learning (Foerde and Shohamy, 2011; Helie et al., 2013), which both require rapid pairing of environmental stimuli with action. Our results are also consistent with the view that the hippocampus is a “convergence zone” that binds relational information between event- or landmark-unique features (Davachi and DuBrow, 2015; Backus et al., 2016; Horner and Burgess, 2013; Eichenbaum, 2004). According to this view, the hippocampus plays a general role in declarative memory, which is fundamentally a relational processing system (Eichenbaum and Cohen, 2014).
Two prior studies have investigated egocentric and allocentric navigation in HD. Using a real-space human analogue of the Morris Water Maze with egocentric and allocentric versions called the Blue Velvet Arena, Majerová et al. (2012) found normal performance on both versions in patients with mild motor impairment, and similar impairments on both versions in patients with at least moderate motor impairment. The authors concluded that striatal degeneration does not differentially impair egocentric navigation, and that the simultaneous impairment of both strategies reflects a more generalized neurodegenerative process extending beyond caudate atrophy. Voermans et al. (2004) applied fMRI to study hippocampal and caudate activation in early stage HD during a route learning test that could be solved by allocentric or egocentric strategies. The HD patients navigated as accurately as neurologically healthy controls, but during the test exhibited reduced caudate activation and greater hippocampal activation, and increased interaction between the caudate and hippocampus, which the authors interpreted to represent hippocampal compensation for caudate dysfunction. The pmHD individuals in the present study were at a milder stage of disease than most of the patients in these prior studies, but exhibited large impairments in egocentric working memory with a dissociation from relatively spared allocentric working memory. The multifactorial nature of navigation learning may allow for variable strategies and require diffuse neural substrates, which weakens its sensitivity to caudate dysfunction and impairments in early stage HD. In contrast, we find that egocentric working memory, which is a skill fundamental to egocentric navigation, relies critically on the dorsolateral head of the caudate and reveals early deficits in these individuals.
The small sample size of this study is a limitation. Future work in a larger sample should examine whole brain contributions to egocentric and allocentric working memory, ideally in a sample of participants who also complete navigation testing to examine the overlapping and distinct neural correlates. This study was conducted in figural space, whereas most navigation paradigms are conducted in vista or environmental space. This scale can influence which processes are recruited (Wolbers and Wiener, 2014), and it is not clear the extent to which the current findings would generalize to larger scales of space. To validate egocentric working memory as a possible disease marker of pmHD, longitudinal study in a larger sample is needed.
Acknowledgments
This work was supported by the National Institute on Aging (Possin: K23AG037566; Kramer: R01AG032289; Miller: P50AG023501), the Larry L. Hillblom Foundation (Miller, 2007/2I), a University of California Discovery ITL-BIO04–10148, and the Hellman Family Foundation. The funding sources did not play a role in the study design, implementation, analysis, or writing of this report. We are grateful to our research participants for their generous time and efforts.
References
- Aguirre GK, D’Esposito M. Topographical disorientation: a synthesis and taxonomy. Brain: A J Neurol. 1999;122(9):1613–1628. doi: 10.1093/brain/122.9.1613. [DOI] [PubMed] [Google Scholar]
- Aguirre GK, Detre JA, Alsop DC, D’Esposito M. The parahippocampus subserves topographical learning in man. Cereb Cortex. 1996;6(6):823–829. doi: 10.1093/cercor/6.6.823. [DOI] [PubMed] [Google Scholar]
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9(1):357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Aylward EH, Sparks BF, Field KM, Yallapragada V, Shpritz BD, Rosenblatt A, Margolis RL. Onset and rate of striatal atrophy in preclinical Huntington disease. Neurology. 2004;63(1):66–72. doi: 10.1212/01.wnl.0000132965.14653.d1. [DOI] [PubMed] [Google Scholar]
- Aylward EH, Harrington DL, Mills JA, Nopoulos PC, Ross CA, Long JD, Paulsen JS. Regional atrophy associated with cognitive and motor function in prodromal Huntington disease. J Huntingt Dis. 2013;2(4):477–489. doi: 10.3233/JHD-130076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backus AR, Bosch SE, Ekman M, Grabovetsky AV, Doeller CF. Mnemonic convergence in the human hippocampus. Nat Commun. 2016;7:11991. doi: 10.1038/ncomms11991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartsch T, Schönfeld R, Müller FJ, Alfke K, Leplow B, Aldenhoff J, Koch JM. Focal lesions of human hippocampal CA1 neurons in transient global amnesia impair place memory. Science. 2010;328(5984):1412–1415. doi: 10.1126/science.1188160. [DOI] [PubMed] [Google Scholar]
- Boccia M, Nemmi F, Guariglia C. Neuropsychology of environmental navigation in humans: review and meta-analysis of fMRI studies in healthy participants. Neuropsychol Rev. 2014;24(2):236–251. doi: 10.1007/s11065-014-9247-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boccia M, Silveri MC, Sabatini U, Guariglia C, Nemmi F. Neural underpinnings of the decline of topographical memory in mild cognitive impairment. Am J Alzheimer’S Dis Other Dement. 2016;31(8):618–630. doi: 10.1177/1533317516654757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohbot VD, Lerch J, Thorndycraft B, Iaria G, Zijdenbos AP. Gray matter differences correlate with spontaneous strategies in a human virtual navigation task. J Neurosci: Off J Soc Neurosci. 2007;27(38):10078–10083. doi: 10.1523/JNEUROSCI.1763-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonelli RM, Cummings JL. Frontal-subcortical circuitry and behavior. Dialogues Clin Neurosci. 2007;9(2):141. doi: 10.31887/DCNS.2007.9.2/rbonelli. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasted PJ, Humby T, Dunnett SB, Robbins TW. Unilateral lesions of the dorsal striatum in rats disrupt responding in egocentric space. J Neurosci: Off J Soc Neurosci. 1997;17(22):8919–8926. doi: 10.1523/JNEUROSCI.17-22-08919.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chersi F, Burgess N. The cognitive architecture of spatial navigation: hippocampal and striatal contributions. Neuron. 2015;88(1):64–77. doi: 10.1016/j.neuron.2015.09.021. [DOI] [PubMed] [Google Scholar]
- Davachi L, DuBrow S. How the hippocampus preserves order: the role of prediction and context. Trends Cogn Sci. 2015;19(2):92–99. doi: 10.1016/j.tics.2014.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JD, Filoteo JV, Kesner RP, Roberts JW. Recognition memory for hand positions and spatial locations in patients with Huntington’s disease: differential visuospatial memory impairment? Cortex. 2003;39(2):239–253. doi: 10.1016/s0010-9452(08)70107-2. [DOI] [PubMed] [Google Scholar]
- Davis JD, Filoteo JV, Kesner RP. Is short-term memory for discrete arm movements impaired in Huntington’s disease? Cortex. 2007;43(2):255–263. doi: 10.1016/s0010-9452(08)70480-5. [DOI] [PubMed] [Google Scholar]
- deIpolyi AR, Rankin KP, Mucke L, Miller BL, Gorno-Tempini ML. Spatial cognition and the human navigation network in AD and MCI. Neurology. 2007;69(10):986–997. doi: 10.1212/01.wnl.0000271376.19515.c6. [DOI] [PubMed] [Google Scholar]
- Doeller CF, King JA, Burgess N. Parallel striatal and hippocampal systems for landmarks and boundaries in spatial memory. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(15):5915–5920. doi: 10.1073/pnas.0801489105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H. Hippocampus: cognitive processes and neural representations that underlie declarative memory. Neuron. 2004;44(1):109–120. doi: 10.1016/j.neuron.2004.08.028. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Cohen NJ. Can we reconcile the declarative memory and spatial navigation views on hippocampal function? Neuron. 2014;83(4):764–770. doi: 10.1016/j.neuron.2014.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekstrom AD, Arnold A, Iaria G. A critical review of the allocentric spatial representation and its neural underpinnings: toward a network-based perspective. Front Human Neurosci. 2014;8(803) doi: 10.3389/fnhum.2014.00803. doi:10.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epping EA, Kim JI, Craufurd D, Brashers-Krug TM, Anderson KE, McCusker E Coordinators of the Huntington Study Group. Longitudinal psychiatric symptoms in prodromal Huntington’s Disease: a decade of data. Am J Psychiatry. 2015 doi: 10.1176/appi.ajp.2015.14121551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etchamendy N, Bohbot VD. Spontaneous navigational strategies and performance in the virtual town. Hippocampus. 2007;17(8):595. doi: 10.1002/hipo.20303. [DOI] [PubMed] [Google Scholar]
- Fanselow MS, Dong H. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron. 2010;65(1):7–19. doi: 10.1016/j.neuron.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foerde K, Shohamy D. The role of the basal ganglia in learning and memory: insight from parkinson’s disease. Neurobiol Learn Mem. 2011;96(4):624–636. doi: 10.1016/j.nlm.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonov V, Evans AC, Botteron K, Almli CR, McKinstry RC, Collins DL Brain Development Cooperative Group. Unbiased average age-appropriate atlases for pediatric studies. NeuroImage. 2011;54(1):313–327. doi: 10.1016/j.neuroimage.2010.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffan D. Against memory systems. Philos Trans R Soc B: Biol Sci. 2002;357(1424):1111–1121. doi: 10.1098/rstb.2002.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley T, Maguire EA, Spiers HJ, Burgess N. The well-worn route and the path less traveled: distinct neural bases of route following and way finding in humans. Neuron. 2003;37(5):877–888. doi: 10.1016/s0896-6273(03)00095-3. [DOI] [PubMed] [Google Scholar]
- Hartley T, Bird CM, Chan D, Cipolotti L, Husain M, Vargha-Khadem F, Burgess N. The hippocampus is required for short-term topographical memory in humans. Hippocampus. 2007;17(1):34–48. doi: 10.1002/hipo.20240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helie S, Chakravarthy S, Moustafa AA. Exploring the cognitive and motor functions of the basal ganglia: an integrative review of computational cognitive neuroscience models. Front Comput Neurosci. 2013;7:174. doi: 10.3389/fncom.2013.00174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horner AJ, Burgess N. The Associative Structure of Memory for Multi-Element Events. J Exp Psychol General. 2013;142(4):1370–1383. doi: 10.1037/a0033626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hort J, Laczo J, Vyhnalek M, Bojar M, Bures J, Vlcek K. Spatial navigation deficit in amnestic mild cognitive impairment. Proc Natl Acad Sci USA. 2007;104(10):4042–4047. doi: 10.1073/pnas.0611314104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iaria G, Petrides M, Dagher A, Pike B, Bohbot VD. Cognitive strategies dependent on the hippocampus and caudate nucleus in human navigation: variability and change with practice. J Neurosci: Off J Soc Neurosci. 2003;23(13):5945–5952. doi: 10.1523/JNEUROSCI.23-13-05945.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iaria G, Chen J, Guariglia C, Ptito A, Petrides M. Retrosplenial and hippocampal brain regions in human navigation: complementary functional contributions to the formation and use of cognitive maps. Eur J Neurosci. 2007;25(3):890–899. doi: 10.1111/j.1460-9568.2007.05371.x. [DOI] [PubMed] [Google Scholar]
- Jeneson A, Mauldin KN, Hopkins RO, Squire LR. The role of the hippocampus in retaining relational information across short delays: the importance of memory load. Learn Mem. 2011;18(5):301–305. doi: 10.1101/lm.2010711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joo EY, Kim H, Suh S, Hong SB. Hippocampal substructural vulnerability to sleep disturbance and cognitive impairment in patients with chronic primary insomnia: magnetic resonance imaging morphometry. Sleep. 2014;37(7):1189–1198. doi: 10.5665/sleep.3836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesner RP, Gilbert PE. The role of the medial caudate nucleus, but not the hippocampus, in a matching-to sample task for a motor response. Eur J Neurosci. 2006;23(7):1888–1894. doi: 10.1111/j.1460-9568.2006.04709.x. [DOI] [PubMed] [Google Scholar]
- Kim H, Mansi T, Bernasconi N, Bernasconi A. Surace-based multi template automated hippocampal segmentation: application to temporal lobe epilepsy. Med Image Anal. 2012;16(7):1445–1455. doi: 10.1016/j.media.2012.04.008. [DOI] [PubMed] [Google Scholar]
- Kim H, Mansi T, Bernasconi N. Disentangling hippocampal shape anomalies in epilepsy. Front Neurol. 2013;4:131. doi: 10.3389/fneur.2013.00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Suh S, Joo EY, Hong SB. Morphological alterations in amygdalo-hippocampal substructures in narcolepsy patients with cataplexy. Brain Imaging Behav. 2015:1–11. doi: 10.1007/s11682-015-9450-0. [DOI] [PubMed] [Google Scholar]
- Kim H, Kim J, Possin KL, Winer J, Geschwind MD, Xu D, Hess CP. Surface-based morphometry reveals caudate subnuclear structural damage in patients with premotor Huntington disease. Brain Imaging Behav. 2016 doi: 10.1007/s11682-016-9616-4. epub Print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravitz DJ, Saleem KS, Baker CI, Mishkin M. A new neural framework for visuospatial processing. Nat Rev Neurosci. 2011;12(4):217–230. doi: 10.1038/nrn3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy R, Friedman HR, Davachi L, Goldman-Rakic PS. Differential activation of the caudate nucleus in primates performing spatial and nonspatial working memory tasks. J Neurosci: Off J Soc Neurosci. 1997;17(10):3870–3882. doi: 10.1523/JNEUROSCI.17-10-03870.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Looi JCL, Lindberg O, Zandbelt BB, Östberg P, Andersen C, Botes L, Wahlund LO. Caudate nucleus volumes in frontotemporal lobar degeneration: differential atrophy in subtypes. Am J Neuroradiol. 2008;29(8):1537–1543. doi: 10.3174/ajnr.A1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire EA, Burgess N, Donnett JG, Frackowiak RS, Frith CD, O’Keefe J. Knowing where and getting there: a human navigation network. Science. 1998;280(5365):921–924. doi: 10.1126/science.280.5365.921. [DOI] [PubMed] [Google Scholar]
- Majerová V, Kalinčík T, Laczó J, Vyhnálek M, Hort J, Bojar M, Roth J. Disturbance of real space navigation in moderately advanced but not in early Huntington’s disease. J Neurol Sci. 2012;312(1):86–91. doi: 10.1016/j.jns.2011.08.016. [DOI] [PubMed] [Google Scholar]
- McDonald RJ, White NM. Parallel information processing in the water maze: evidence for independent memory systems involving dorsal striatum and hippocampus. Behav Neural Biol. 1994;61(3):260–270. doi: 10.1016/s0163-1047(05)80009-3. [DOI] [PubMed] [Google Scholar]
- Mellet E, Bricogne S, Tzourio-Mazoyer N, Ghaem O, Petit L, Zago L, Denis M. Neural correlates of topographic mental exploration: the impact of route versus survey perspective learning. NeuroImage. 2000;12(5):588–600. doi: 10.1006/nimg.2000.0648. [DOI] [PubMed] [Google Scholar]
- Milner B, Corkin S, Teuber HL. Further analysis of the hippocampal amnesic syndrome: 14-year follow-up study of H. M. Neuropsychologia. 1968;6(3):215–234. [Google Scholar]
- Moser MB, Moser EI. Functional differentiation in the hippocampus. Hippocampus. 1998;8(6):608–619. doi: 10.1002/(SICI)1098-1063(1998)8:6<608::AID-HIPO3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Moser MB, Moser EI, Forrest E, Andersen P, Morris RG. Spatial learning with a minislab in the dorsal hippocampus. Proc Natl Acad Sci USA. 1995;92(21):9697–9701. doi: 10.1073/pnas.92.21.9697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedelska Z, Andel R, Laczó J, Vlcek K, Horinek D, Lisy J, Hort J. Spatial navigation impairment is proportional to right hippocampal volume. Proc Natl Acad Sci. 2012;109(7):2590–2594. doi: 10.1073/pnas.1121588109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neggers S, Van der Lubbe R, Ramsey N, Postma A. Interactions between ego-and allocentric neuronal representations of space. NeuroImage. 2006;31(1):320–331. doi: 10.1016/j.neuroimage.2005.12.028. [DOI] [PubMed] [Google Scholar]
- O’Keefe J, Dostrovsky J. The hippocampus as a spatial map preliminary evidence from unit activity in the freely-moving rat. Brain Res. 1971;34(1):171–175. doi: 10.1016/0006-8993(71)90358-1. [DOI] [PubMed] [Google Scholar]
- O’Keefe J, Nadel L. The Hippocampus as a Cognitive Map 3. Clarendon Press; Oxford: 1978. pp. 483–484. [Google Scholar]
- Olson IR, Page K, Moore KS, Chatterjee A, Verfaellie M. Working memory for conjunctions relies on the medial temporal lobe. J Neurosci: Off J Soc Neurosci. 2006;26(17):4596–4601. doi: 10.1523/JNEUROSCI.1923-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packard MG, McGaugh JL. Inactivation of hippocampus or caudate nucleus with lidocaine differentially affects expression of place and response learning. Neurobiol Learn Mem. 1996;65(1):65–72. doi: 10.1006/nlme.1996.0007. [DOI] [PubMed] [Google Scholar]
- Packard MG, Knowlton BJ. Learning and memory functions of the basal ganglia. Annu Rev Neurosci. 2002;25(1):563–593. doi: 10.1146/annurev.neuro.25.112701.142937. [DOI] [PubMed] [Google Scholar]
- Paulsen JS, Langbehn DR, Stout JC, Aylward E, Ross CA, Nance M, Duff K. Detection of Huntington’s disease decades before diagnosis: the Predict-HD study. Journal of Neurology, Neurosurgery & Psychiatry. 2008;79(8):874–880. doi: 10.1136/jnnp.2007.128728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsen JS, Smith MM, Long JD PREDICT HD investigators and coordinators of the Huntington Study Group. Cognitive decline in prodromal Huntington disease: Implications for clinical trials. Journal of Neurology, Neurosurgery, and Psychiatry. 2013;84(11):1233–1239. doi: 10.1136/jnnp-2013-305114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce JM, Roberts AD, Good M. Hippocampal lesions disrupt navigation based on cognitive maps but not heading vectors. Nature. 1998;396(6706):75–77. doi: 10.1038/23941. [DOI] [PubMed] [Google Scholar]
- Poppenk J, Evensmoen HR, Moscovitch M, Nadel L. Long-axis specialization of the human hippocampus. Trends Cogn Sci. 2013;17(5):230–240. doi: 10.1016/j.tics.2013.03.005. [DOI] [PubMed] [Google Scholar]
- Possin KL, Sanchez PE, Anderson-Bergman C, Fernandez R, Kerchner GA, Johnson ET, Fenesy MC. Cross-species translation of the Morris maze for Alzheimer’s disease. J Clin Investig. 2016;126:2. doi: 10.1172/JCI78464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postle BR, D’Esposito M. Spatial working memory activity of the caudate nucleus is sensitive to frame of reference. Cogn, Affect, Behav Neurosci. 2003;3(2):133–144. doi: 10.3758/cabn.3.2.133. [DOI] [PubMed] [Google Scholar]
- Ranganath C, Blumenfeld RS. Doubts about double dissociations between short-and long-term memory. Trends Cogn Sci. 2005;9(8):374–380. doi: 10.1016/j.tics.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Redish AD. Beyond the Cognitive Map: from Place Cells to Episodic Memory. MIT Press; Cambridge, MA: 1999. [Google Scholar]
- Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRIdata. Med Imaging, IEEE Trans on. 1998;17(1):87–97. doi: 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- Squire LR, Wixted JT. The cognitive neuroscience of human memory since H.M. Annu Rev Neurosci. 2011;34:259–288. doi: 10.1146/annurev-neuro-061010-113720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Styner MA, Oguz I, Smith RG, Cascio C, Jomier M. Corpus callosum subdivision based on a probabilistic model of inter-hemispheric connectivity. Med Image Comput Comput-Assist Interv. 2005;2005:765–772. doi: 10.1007/11566489_94. [DOI] [PubMed] [Google Scholar]
- Vann SD, Brown MW, Erichsen JT, Aggleton JP. Fos imaging reveals differential patterns of hippocampal and parahippocampal subfield activation in rats in response to different spatial memory tests. J Neurosci: Off J Soc Neurosci. 2000;20(7):2711–2718. doi: 10.1523/JNEUROSCI.20-07-02711.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voermans NC, Petersson KM, Daudey L, Weber B, Van Spaendonck KP, Kremer HP, Fernández G. Interaction between the human hippocampus and the caudate nucleus during route recognition. Neuron. 2004;43(3):427–435. doi: 10.1016/j.neuron.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Voorn P, Vanderschuren LJ, Groenewegen HJ, Robbins TW, Pennartz CM. Putting a spin on the dorsal–ventral divide of the striatum. Trends Neurosci. 2004;27(8):468–474. doi: 10.1016/j.tins.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Wang H, Schmid C. Action recognition with improved trajectories. Proceedings of the IEEE International Conference on Computer Vision; 2013. pp. 3551–3558. [Google Scholar]
- Wang H, Yushkevich PA. Multi-atlas segmentation with joint label fusion and corrective learning—an open source implementation. Front Neuroinformatics. 2013a;7:27. doi: 10.3389/fninf.2013.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Yushkevich PA. Multi-Atlas Segmentation without Registration: A Supervoxel-based Approach. Medical Image Computing and Computer-Assisted Intervention: in: Proceedings of the MICCAI … International Conference on Medical Image Computing and Computer-Assisted Intervention; 2013b. pp. 535–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolbers T, Hegarty M. What determines our navigational abilities? Trends Cogn Sci. 2010;14(3):138–146. doi: 10.1016/j.tics.2010.01.001. [DOI] [PubMed] [Google Scholar]
- Wolbers T, Wiener JM. Challenges for identifying the neural mechanisms that support spatial navigation: the impact of spatial scale. Front Human Neurosci. 2014;8:571. doi: 10.3389/fnhum.2014.00571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You SC, Geschwind MD, Sha SJ, Apple A, Satris G, Wood KA, Kang GA. Executive functions in premanifest Huntington’s disease. Mov Disord. 2014;29(3):405–409. doi: 10.1002/mds.25762. [DOI] [PMC free article] [PubMed] [Google Scholar]