Abstract
Alcohol-induced brain damage likely contributes to the dysfunctional poor decisions associated with alcohol dependence. Human alcoholics have a global loss of brain volume that is most severe in the frontal cortex. Neuroimmune gene induction by binge drinking increases neurodegeneration through increased oxidative stress, particularly NADPH oxidase-induced oxidative stress. In addition, HMGB1-TLR4 and innate immune NF-κB target genes are increased leading to persistent and sensitized neuroimmune responses to ethanol and other agents that release HMGB1 or directly stimulate TLR receptors and/or NMDA receptors. Neuroimmune signaling and glutamate excitotoxicity are linked to alcoholic neurodegeneration. Models of adolescent alcohol abuse lead to significant frontal cortical degeneration and show the most severe loss of hippocampal neurogenesis. Adolescence is a period of high risk for ethanol-induced neurodegeneration and alterations in brain structure, gene expression, and maturation of adult phenotypes. Together, these findings support the hypothesis that adolescence is a period of risk for persistent and long-lasting increases in brain neuroimmune gene expression that promote persistent and long-term increases in alcohol consumption, neuroimmune gene induction, and neurodegeneration that we find associated with alcohol use disorders.
1. INTRODUCTION
A characteristic of alcohol use disorders is the consumption of large quantities of alcohol despite the knowledge that problems occur during drinking. Approximately 10% of the population drinks 75% of the alcohol consumed in the United States (Li, 2008). In addition to the increased risk of developing a variety of health problems, heavy drinking patterns also increase the risk of developing an addiction to alcohol. Chronic repetitive use of alcohol results in persistent changes in brain and behavior that are characterized by diminished behavioral control, difficulty avoiding negative consequences, increased preoccupation with drinking alcohol, and mounting craving and limbic negative affect. Alcohol dependence and addiction involve difficulty in moderating or stopping drinking even though it has become a problem. The prefrontal cortex (PFC) and limbic system structures are particularly vulnerable to the neurotoxic effects of alcohol, and are critically involved in decision-making, motivation, planning, goal setting, and impulse inhibition. A variety of preclinical and clinical studies suggest that chronic repetitive alcohol consumption persistently changes neurobiology thereby increasing risky decision-making, impulsivity, and anxiety that further drive repeated cycles of binge drinking, disrupting cognitive-limbic circuits through progressive neuroimmune activation and loss of control over alcohol use. Impulsivity, which is regulated by the PFC, increases during intoxication and alcoholism (Potenza & De Wit, 2010). Further, loss of attention, poor decision-making, and increased impulsivity are found in individuals with lesions of the PFC consistent with PFC degeneration contributing to alcoholism and other drug dependence (Bechara, Damasio, Damasio, & Anderson, 1994; Bechara et al., 2005). These studies suggest that alcoholism is at least in part related to heavy alcohol consumption-induced neurodegeneration and other alterations in brain and behavior contributing to the chronic relapsing disorder characterized by repeated use of alcohol and/or other drugs despite problems related to use of the substance (O’Brien, 2008).
Alcoholism is a chronic relapsing disorder that can include extended periods of abstinence followed by relapse to heavy drinking. Relapse rapidly returns to excessive drinking suggesting long-term permanent alterations in neurobiology that can be controlled, but remain persistently altered. A key component of the risks of addiction and relapse are subtle changes in cognitive flexibility, risky decision-making, and mounting alcohol reminders (e.g., cue-induced limbic anxiety and impulsivity). The chronic relapsing nature of alcoholism suggests persistent alterations in neurobiology that continuously increase risk of relapse to heavy drinking. Successful abstinence is most often achieved through complete abstinence and active avoidance of places and situations involving alcohol and associated reminders of alcohol that drive craving and relapse.
Brain neuroimmune signaling is activated in models of binge drinking and neurodegeneration. Neurons and glia (both astrocytes and microglia) contribute to the release and responses to signaling molecules first discovered within the immune system. Neuroimmune factors in this review are defined as proteins such as cytokines, Toll-like receptors (TLRs), and HMGB1 that are known to be peripheral immune signaling molecules that were recently discovered to function as brain signaling proteins and protein receptors. Immune signaling pathways activated by infection are known to remain sensitized for life through adaptive immunity. Similarly, neuroimmune signaling in brain, which consists of innate immune signals, persists for long periods that could contribute to long-lasting changes in neurobiology. Neuroimmune signaling increases alcohol drinking, risky decision-making, and blunts ability to change (e.g., loss of behavior flexibility) in alcohol-treated animals. Postmortem human alcoholic brain studies find neuroimmune gene expression correlates with lifetime alcohol consumption (Crews, Qin, Sheedy, Vetreno, & Zou, 2013) consistent with persistent neuroimmune signaling repeatedly increased by alcohol consumption and signaling contributing to the chronic relapsing nature of alcoholism (Crews et al., 2013; see Table 10.1).
Table 10.1.
Studies linking neuroimmune activation to alcoholism
| Marker | Brain region |
|---|---|
| Rodent | |
| LPS increases ethanol drinking | Blednov et al. (2011) |
| CD14 KO reduces ethanol drinking | Blednov et al. (2012) |
| AIE-induced cognitive inflexibility correlates with neuroimmune expression | Vetreno and Crews (2012) |
| Amygdalar TLR4 siRNA administration reduces ethanol responding in dependent rats | Liu et al. (2011) |
| Age of drinking onset in humans correlates with neuroimmune expression | Vetreno et al. (2013) |
| TLR4 KO blocks ethanol-induced dopamine release | Alfonso-Loeches et al. (2010) |
| Human | |
| Neuroimmune expression correlates with lifetime alcohol consumption | Crews (2012) |
| Neuroimmune expression correlates with age of drinking onset | Vetreno et al. (2013) |
2. ALCOHOL-INDUCED NEURODEGENERATION AND ALCOHOLISM
Human studies find that active, recent, and frequent heavy drinking behaviors are the best indicators of alcoholic brain damage (Shelton & Parsons, 1987; Sullivan & Pfefferbaum, 2005). Over a 5-year interval, the degree of excessive drinking in alcoholics corresponded with gray matter loss, particularly in the frontal lobes (Pfefferbaum et al., 1995). Frontal cortical choline-containing compounds measured by MRI were decreased in alcoholics with significant correlations between alcohol consumption in the last 90 days and decreases in anterior cingulated cortex and frontal white matter (Ende et al., 2006). Although studies suggest a recovery of some brain structure volumes in abstinence (Drake et al., 1995; O’Neill, Cardenas, & Meyerhoff, 2001), there is also evidence of greater vulnerability to damage during relapse (Pfefferbaum et al., 1995). Neuropathology studies have found a 22% reduction in the number of neurons in the superior frontal cortex of alcoholics (Harper, Kril, & Daly, 1987), and reduced brain weight of alcoholics relative to controls that was correlated with the rate and amount of lifetime alcohol consumption (Harding, Halliday, Ng, Harper, & Kril, 1996). Brain imaging studies by Fein et al. (2002) and Fein, Di Sclafani, and Finn (2010) found cortical gray matter volumes in alcohol dependent individuals were negatively associated with age and lifetime duration of alcohol use. Individuals with less severe alcohol abuse histories showed reduced whole brain, prefrontal, and parietal cortical gray matter volumes compared with nonalcoholic control participants. Other studies focused on cortical thickness find abstinent alcoholics have reduced whole brain cortical thickness. Decreases in cortical thickness were largest in frontal lobes and were related to the severity of alcohol abuse (Fortier et al., 2011). Investigations of recently detoxified individuals with chronic alcoholism have consistently shown significant impairments in the cognitive domains of executive function, nonverbal memory, visuospatial function, and gait and balance (Gansler et al., 2000; Oscar-Berman & Marinkovic, 2007, e.g., Parsons, 1993). Deficits in these functions suggest dysfunction in cerebellum, posterior parietal lobe, and most significantly, frontal lobes (specifically, orbitofrontal and PFC) (Deshmukh, Rosenbloom, Pfefferbaum, & Sullivan, 2002; Sullivan, Rosenbloom, & Pfefferbaum, 2000). Multiple studies find the frontal lobes are the most insulted region in the alcoholic brain (Kubota et al., 2001; Sullivan & Pfefferbaum, 2005). The frontal lobes regulate complex cognitive skills such as working memory, temporal ordering, discrimination and reversal learning that underlie judgment, attention, risk taking, motivation, mood, and wanting. Thus, the impaired judgment, blunted affect, poor insight, social withdrawal, reduced motivation, distractibility, and attention- and impulse-control deficits associated with alcohol use disorders are consistent with neurodegeneration, particularly within the cortex and more specifically the frontal cortex (Oscar-Berman & Hutner, 1993; Shelton & Parsons, 1987; Sullivan & Pfefferbaum, 2005; Sullivan et al., 2000).
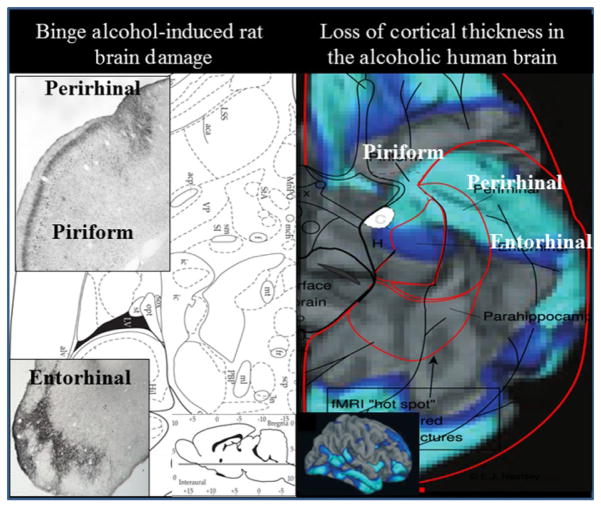
The Crews laboratory and others using preclinical binge drinking models in rats find neurodegeneration in multiple cortical areas that extend from frontal to perirhinal, piriform, and entorhinal cortex (Collins, Zou, & Neafsey, 1998; Corso, Mostafa, Collins, & Neafsey, 1998; Crews et al., 2004; Hamelink, Hampson, Wink, Eiden, & Eskay, 2005; Obernier, Bouldin, & Crews, 2002). The cortical areas damaged in binge drinking rat models of neurodegeneration overlap with those found to be thinner in alcoholics (see Fig. 10.1). Binge alcohol-induced brain damage in adult rats is found in multiple cortical regions, namely agranular insular cortex, anterior piriform cortex, perirhinal cortex, and entorhinal cortex as well as the hippocampal dentate gyrus, particularly ventral dentate gyrus which is close to the amygdala, analogous to human temporal lobe where hippocampus is adjacent to the amygdala. In these regions, dark cell degeneration, a necrotic form of cell death with shrunken soma, is the predominant form of neuronal death (Obernier, Bouldin, et al., 2002). Studies in humans find cortical thinning in alcoholics in multiple cortical areas that overlap with the rat binge neurodegeneration studies (see Fig. 10.1), with several regions showing greater thinning with severity of alcoholism (Fortier et al., 2011). Genetics is implicated in the increased risk for the development of alcohol dependence, and genetically bred high alcohol preferring P rats are more sensitive to binge drinking-induced cortical neurodegeneration than non-preferring low alcohol drinking rats. Interestingly, humans who begin drinking in the early teenage years are more likely to have an alcohol use disorder in their lifetime (Grant & Dawson, 1997) and binge drinking models of neurodegeneration comparing adolescent and adult rats find significantly more frontal cortical neurodegeneration in adolescent animals (Coleman, Liu, Oguz, Styner, & Crews, 2014). Adolescence has been suggested to be a critical period of risk for developing alcoholism since the frontal cortex and executive functions develop during adolescence, and the developing brain is uniquely sensitive to alcohol (Crews, He, & Hodge, 2007). Binge drinking-induced brain damage is associated with a loss of behavioral flexibility (Obernier, White, Swartzwelder, & Crews, 2002). Binge drinking models in rats find no persistent deficits in spatial learning on the Morris water maze (Obernier, White, et al., 2002) or Barnes Maze (Vetreno & Crews, 2012) but show deficits in reversal learning, a task requiring animals to change what was learned and find a new site. Reversal learning deficits are consistent with frontal cortical dysfunction, particularly orbital frontal cortex (Schoenbaum, Roesch, Stalnaker, & Takahashi, 2009). Consistent with adolescence being a critical period of risk, studies find that adolescent binge drinking models in both rats (Vetreno & Crews, 2012) and mice (Coleman, He, Lee, Styner, & Crews, 2011) lead to reversal learning deficits that persists into adulthood. Further, they find persistent changes in brain structure, neurotransmitter, and other gene expression (Coleman et al., 2011, 2014; Vetreno & Crews, 2012; Vetreno, Qin, & Crews, 2013). The preclinical finding that the adolescent brain is particularly sensitive to frontal cortical damage and that adolescent age as well as genetic factors increases sensitivity to alcohol-induced brain damage that is related to a persistent loss of behavioral flexibility is consistent with human studies suggesting neurodegeneration contributes to the neurobiology of alcoholism.
Figure 10.1.
Comparison of human alcoholic cortical thinning with rat binge alcohol treatment-induced neurodegeneration. Shown are horizontal sections through the ventral rat brain and the ventral surface of the human brain. Left: Rat brain anatomical section in the lower right indicates location of ventral horizontal section. The left micrographs shows brain sections with silver cell death stain (black areas) showing neurodegeneration from a binge drinking model that includes the association cortical areas piriform and perirhinal cortex as well as entorhinal cortex (Crews et al., 2004; Obernier, Bouldin, et al., 2002). Right: Human alcoholic ventral cortical thinning is shown as a significance map of group differences in cortical thickness in abstinent alcoholics as compared to nonalcoholic controls with areas showing brain regions with significant cortical thinning in alcoholics highlighted in blue (light blue (light gray in the print version) p <0.01, dark blue (dark gray in the print version) p <0.05). Note: perirhinal, entorhinal, and piriform cortex are areas with cortical thinning in humans and areas of binge drinking neurodegeneration in rats, suggesting binge drinking human alcoholics are damaging association cortical areas. Right: Adapted from Fortier et al. (2011).
3. LOSS OF NEUROGENESIS COULD CONTRIBUTE TO ALCOHOLIC NEURODEGENERATION
New neurons from neural stem cells are continuously produced in at least two regions of the normal adult brain, the subventricular zone of the lateral ventricles and the hippocampal dentate gyrus (Altman & Das, 1965; Alvarez-Buylla & Garcia-Verdugo, 2002). The Crews laboratory and many others have found that both acute and chronic alcohol exposure reduce hippocampal neurogenesis (He, Nixon, Shetty, & Crews, 2005; Herrera et al., 2003; Jang, Shin, Jung, et al., 2002; Jang, Shin, Kim, & Kim, 2002; Nixon & Crews, 2002). Multiple markers covering all stages of neurogenesis, specifically proliferation, neuronal maturation, and survival, have been examined in the 4-day binge model and find all are decreased (Crews, Bechara, et al., 2006; He et al., 2005; Nixon & Crews, 2002, 2004). Further, ethanol treatment during adult neurogenesis blunts the growth of the progenitor’s dendritic arbor (He et al., 2005). Adolescents have high levels of neurogenesis in hippocampus and are more sensitive to acute alcohol inhibition of neurogenesis (Crews, Mdzinarishvili, Kim, He, & Nixon, 2006). Interestingly, adult hippocampal neurogenesis is resilient, recovering over a 30-day period from the 4-day binge alcohol model (Nixon & Crews, 2004) and a 7-week chronic prolonged relapsing model of alcohol dependence, whereas the ventricular neurogenesis is persistently reduced (Hansson et al., 2010). In contrast, in models of underage drinking (e.g., adolescent intermittent ethanol (AIE) treatment), hippocampal neurogenesis is persistently inhibited into adulthood (Broadwater, Liu, Crews, & Spear, 2013; Ehlers, Liu, Wills, & Crews, 2013). AIE exposure reductions in neurogenesis were found to be associated with more “disinhibitory” behavior in the open field conflict test at 2 and 8 weeks following termination of vapor exposure (Ehlers, Liu, et al., 2013). Similarly, AIE exposure of rats found reduced hippocampal volumes assessed using MRI consistent with those found in alcoholism (Ehlers, Oguz, Budin, Wills, & Crews, 2013). Loss of neurogenesis and/or gliogenesis likely contributes to alcoholic neurobiology and the reductions in brain volume found in human alcoholics (Crews & Nixon, 2009).
4. MONOCYTES AND INNATE IMMUNE GENES
Studies on the mechanisms of alcoholic neurodegeneration and loss of neurogenesis led the Crews laboratory to the discovery that innate immune gene induction is involved in alcoholic neurodegeneration. Innate immune genes are associated with rapid monocyte responses to infections (e.g., the acute phase response) that include increases in multiple cytokines as well as increases in their cellular receptors that together amplify expression of a large number of genes through kinase signaling pathways that converge on nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) and activator protein-1 (AP-1) transcription factors. NF-κB and AP-1 transcription factors promote expression of innate immune cytokines, including tumor necrosis factor-alpha (TNFα), interleukin-1β (IL-1β), monocyte chemotactic protein-1 (MCP-1), and IL-6 as well as TLRs and cytokine receptors (see Fig. 10.2). In addition, innate immune proteases and oxidases are induced, particularly cyclooxygenase and nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, as well as major histo-compatibility (MHC) signaling molecules, such as beta-2 microglobulin. NF-κB transcription of proinflammatory genes is amplified within and across cells by induction of TLR and cytokine receptors, many belonging to the IL-1β receptor family, which induce innate immune gene expression. Amplification of innate immune gene induction across cells and tissues can cause pathology, with one example being sepsis. Sepsis and systemic inflammatory response syndrome involve a “cytokine storm,” a potentially fatal innate immune reaction consisting of positive feedback loops between cytokines, and immune and tissue cells, resulting in highly elevated levels of blood cytokines, multiorgan failure, and death (Osterholm, 2005). Sepsis is modeled with high doses of bacterial endotoxin, lipopolysaccharide (LPS), or other polypathogen infections that activate an acute phase-like response. Multiple cytokines increase in blood, with both TNFα and IL-1β increasing in blood during the first several hours after infection and then subsiding. Interestingly, HMGB1, a cytokine-like protein that can activate TLR4 receptors and potentiate cytokine responses, rises in blood about 16 h after infection and remains elevated for several days (Wang, Yang, Czura, Sama, & Tracey, 2001). In mouse models, sepsis-induced death occurs days after infection, which is associated with HMGB1, and is blocked by HMGB1 antibody treatment. Survivors of sepsis show prolonged increases in serum HMGB1 and cognitive deficits that can be prevented with HMGB1 antibody treatment (Chavan et al., 2012). In humans, about half of those released from the hospital after surviving a cytokine storm-sepsis insult die within 5 years (Quartin, Schein, Kett, & Peduzzi, 1997). Thus, innate immune responses can be long lasting and can induce pathology long after initial activation. Although most studies support innate immune signaling converging upon NF-κB transcription of proinflammatory cytokines, proteases, and oxidases, the precise mechanisms that regulate individual cell or cytokine activation, and how different tissues and cells contribute in vivo to amplification of specific innate immune genes is poorly understood.
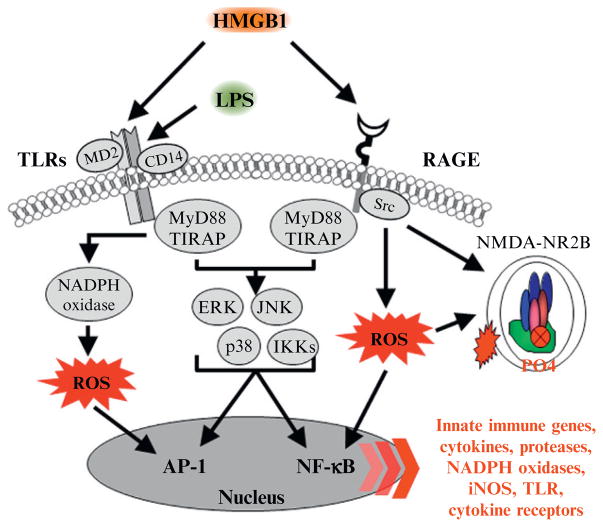
Figure 10.2.
HMGB1-TLR/RAGE signaling leads to activation of NF-κB transcription of innate immune genes and alterations in NMDA receptors. A simplified schematic of the TLR and RAGE signaling cascades. Stimulation of TLRs with HMGB1 and other inflammagens leads to the generation of ROS and downstream activation of NF-κB. Similarly, HMGB1 activation of the RAGE receptor leads to downstream activation of NF-κB and induction of ROS. Nuclear translocation of NF-κB leads to the secretion of proinflammatory gene expression, neuroimmune induction, and cell death. Innate immune receptor stimulation also leads to activation of glutamatergic N-methyl-D-aspartate (NMDA) receptors (Iori et al., 2013; Maroso et al., 2010), which increases Ca2+ flux triggering further induction of neuroimmune genes. AP-1, activator protein-1; CD14, cluster of differentiation 14; ERK, extracellular signal-regulated kinase; HMGB1, high-mobility group box-1; IKK, inhibitor of nuclear factor kappa-B; JNK, c-Jun N-terminal kinases; LPS, lipopolysaccharide; MyD88, myeloid differentiation primary response gene 88; NADPH oxidase, nicotinamide adenine dinucleotide phosphate-oxidase; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; RAGE, receptor for advanced glycation end products; ROS: reactive oxygen species; Src, Proto-oncogene tyrosine-protein kinase; TIRAP, Toll/Interleukin-1 receptor domain-containing adaptor protein; TLRs, Toll-like receptors.
Monocytes are the primary innate immune cell studied in acute proinflammatory responses as well as signaling adaptive immune cells through MHC molecules creating a persistent sensitization to the pathogen (e.g., antibodies that mediate immunization). In addition to responding to pathogens, monocytes and the innate immune system respond to tissue damage, cell death, and degeneration. The innate immune systems response to tissue damage includes the activation of monocyte proinflammatory signaling, termed M1 monocytes or M1 microglia, the brain-specific monocyte. Microglia and other monocyte-like cells consistently express multiple cytokine and TLRs that activate microglia–monocyte-like cells inducing innate immune genes, particularly proinflammatory cytokines, proteases, and oxidases that contribute to the breakdown, processing, and removal of damaged cells and tissue. In addition, monocyte innate immune responses to tissue damage have a delayed response that initiates wound healing trophic signaling, associated with M2 trophic monocyte phenotypes that appear critical for healing. Although poorly understood, both monocytes and brain microglia have M1 and M2, proinflammatory M1, and trophic M2 phenotypes (Colton, 2009; Michelucci, Heurtaux, Grandbarbe, Morga, & Heuschling, 2009). Although monocyte M1 to M2 phenotypes are poorly understood, monocyte proinflammatory activation is clearly linked to NF-κB transcription of multiple innate immune genes. Activation of monocyte NF-κB transcription by pathogens and tissue damage shares common pathways (see Fig. 10.2). Toll-like receptor 4 (TLR4) is a receptor that responds to bacterial endotoxin (e.g., LPS), and HMGB1, a ubiquitously expressed nuclear protein released during necrotic cell death, leading to proinflammatory responses to tissue damage. Proinflammatory gene induction is also amplified by cytokine receptor activated release of HMGB1 that further contributes to innate immune gene induction. The role of these innate immune signaling molecules is well characterized within the immune system, but only recently they have been discovered to contribute to brain signaling. Recent studies find MHC molecules contribute to brain development (Huh et al., 2000), to most neurodegenerative diseases (Gage, 2002; Glass, Saijo, Winner, Marchetto, & Gage, 2010), and alcohol and drug dependence (Crews, 2012). Neuroimmune signaling has not been extensively studied and most knowledge is based on the assumption that monocyte responses represent microglial and brain innate immune responses.
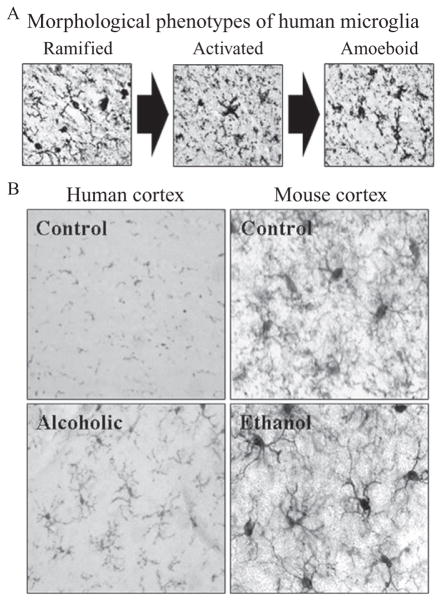
The immune system is not normally active in the healthy brain. The healthy normal brain does not contain antibodies and has only one immune-like cell, the microglia. Neurons, astrocytes, and all other brain cells are formed from ectoderm; whereas, microglia migrates from mesoderm to brain at a specific time during fetal development (Ginhoux et al., 2010). In the healthy brain, ramified or “resting” microglia equal neurons in number and contribute to the integration of sensory systems and overall survey of the brain milieu (Raivich, 2005). Healthy brain microglia are poorly understood, but clearly participate in overall brain health. Microglia, along with astrocytes, modulate important metabolic, trophic, and synaptic functions in addition to responding to brain damage induced neuroimmune responses (Farina, Aloisi, & Meinl, 2007; Streit, Mrak, & Griffin, 2004). Although poorly understood, microglia respond to endogenous or exogenous insults with distinct morphological changes in shape (see Fig. 10.3) as well as marked alterations in gene expression, including proinflammatory innate immune response genes (Graeber, 2010). However, it is sometimes unclear whether microglia are responding to a brain insult or causing the brain insult. Microglial signaling involves both neuroimmune signals and neurotransmitter signals. For example, acetylcholine, an important neuro-transmitter involved in multiple brain functions including cognition, inhibits peripheral monocyte and brain microglial proinflammatory activation and is anti-inflammatory. Our laboratory found an increase in expression of the microglial marker, Iba-1 in the brains of alcoholic individuals (Fig. 10.3 He & Crews, 2007), suggesting that microglia contribute to the neurobiology of alcoholism. Microglia in postmortem human alcoholic brain and chronic alcohol-treated mouse and rat brain show increased MHC gene expression, but not bushy or phagocytic activation profiles (see Fig. 10.3). Thus, microglia increase MHC and likely TLR4 receptor expression during chronic ethanol treatment but are not activated to bushy or phagocytic phenotypes associated with marked brain damage. Thus, microglia are the only immune cells in healthy brain and are integrated into brain responding to both neurotransmitters and neuroimmune signals as well as contributing to chronic alcohol-induced responses.
Figure 10.3.
Increased expression of microglial markers in postmortem alcoholic and mouse brain. (A) Representative photomicrographs depicting the characteristic stages of microglial activation. Ramified or “resting” microglia are characterized by long, highly ramified processes with comparatively small cell bodies. Activated microglia are characterized by swollen, truncated processes, and enlarged cell bodies. Amoeboid or “phagocytic” microglia are characterized by large, amoeba-like cell body with no or few small processes (Kreutzberg, 1996; Raivich et al., 1999). (B) Left, Photomicrographs depicting microglial activation in postmortem human alcoholic brain tissue (He & Crews, 2008). Right: Representative figures depicting microglial activation in the mouse cortex following ethanol treatment. Male C57BL/6 mice were treated with saline or ethanol (5 g/kg, i.g.) for 10 days and were sacrificed 1 h later (Qin et al., 2008).
5. ALCOHOL, NEUROIMMUNE SIGNALING, AND NEURODEGENERATION
Chronic binge drinking models have repeatedly found that ethanol treatment increases the expression of a variety of neuroimmune genes in brain (see Table 10.2 and Fig. 10.4). Cyclooxygenase 2 (COX2) induction by chronic ethanol treatment in brain was found to persist in multiple cortical and limbic brain regions long after physical signs of withdrawal had subsided (Knapp & Crews, 1999). Guerri’s laboratory has found ethanol induction of COX2 is blocked in transgenic mice-lacking TLR4 receptors (Alfonso-Loeches, Pascual-Lucas, Blanco, Sanchez-Vera, & Guerri, 2010). Zou & Crews (2006), using HEC brain slice cultures found that ethanol treatment increased NF-κB binding to DNA probes modeling gene promoter regions and decreased cyclic AMP-responsive element binding protein (CREB) binding to DNA probes modeling CREB gene promoter DNA. The CREB family of transcription factors are activated by phosphorylation and promote neuronal survival, protecting neurons from excitotoxicity and apoptosis through regulating the transcription of prosurvival factors (Lonze & Ginty, 2002; Mantamadiotis et al., 2002). Conversely, NF-κB is a transcriptional factor that is known widely for its ubiquitous roles in inflammatory and immune responses (O’Neill & Kaltschmidt, 1997). As described earlier, NF-κB and CREB have different target genes. For example, neuropeptide Y (NPY) and brain-derived neurotrophic factor (BDNF) are CREB target genes involved in promoting neuronal growth and resilience to insults including protection against excitotoxicity and neuronal death (Lonze & Ginty, 2002). Excitation of neurons increases synaptic plasticity-related to CREB and induction of synaptic proteins and BDNF, whereas excessive excitation triggers excitation of extrasynaptic N-methyl-D-aspartate (NMDA) receptors and excitotoxicity, either rapid or delayed neuronal death, which is associated with reduced CREB (Hardingham & Bading, 2010). Levels of CREB-DNA binding and phosphorylated CREB, as well as the target gene BDNF, are decreased in the rat frontal cortex following a 24-h withdrawal from chronic ethanol exposure (Pandey, Roy, & Mittal, 2001; Pandey, Zhang, Mittal, & Nayyar, 1999). In addition, NPY levels are reduced in the cortex following ethanol treatment, an effect that was complemented by reduced phosphorylated CREB (Bison & Crews, 2003). A reciprocal relationship between NF-κB and CREB transcription sensitizes neurons to excitotoxicity (Zou & Crews, 2006). This reciprocal relationship appears to be due to kinases, such as protein kinase A, which activates CREB transcription and inhibits NF-κB activation. However, the reciprocal relationship may represent neuronal-glial signaling as CREB is principally neuronal and NF-κB activation of proinflammatory genes primarily glial consistent with signals that crossing cells reducing CREB in neurons while activating NF-κB in glia. Thus, ethanol can directly increase NF-κB transcription of proinflammatory genes in brain as well as decreasing trophic protective factor transcription decreasing resilience to insults.
Table 10.2.
Neuroimmune markers in the human alcoholic postmortem brain
| Marker | Brain region | Effect | Method | Citations |
|---|---|---|---|---|
| RAGE | Orbitofrontal cortex | ↑ | IHC, RTPCR, Western blot | Vetreno et al. (2013) |
| TLR2 | Orbitofrontal cortex | ↑ | IHC, Western blot | Crews et al. (2013) |
| TLR3 | Orbitofrontal cortex | ↑ | IHC, Western blot | Crews et al. (2013) |
| TLR4 | Orbitofrontal cortex | ↑ | IHC, Western blot | Crews et al. (2013) |
| HMGB1 | Orbitofrontal cortex | ↑ | IHC, Western blot | Crews et al. (2013) |
| IL-1β | Hippocampus | ↑ | IHC | Zou and Crews (2012) |
| NALP1 | Hippocampus | ↑ | IHC | Zou and Crews (2012) |
| gp91phox (NOX2) | Orbitofrontal cortex | ↑ | IHC | Qin and Crews (2012a, 2012b) |
| MCP-1 | Ventral tegmental area Substantia nigra Hippocampus Amygdala |
↑ ↑ ↑ ↑ |
ELISA | He and Crews (2008) |
| Iba-1 | Cingulate cortex Ventral tegmental area Midbrain Amygdala |
↑ ns ns ns |
IHC | He and Crews (2008) |
| GluT5 | Cingulate cortex Ventral tegmental area Midbrain Amygdala |
↑ ↑ ↑ ns |
IHC | He and Crews (2008) |
| Active NF-κB | Prefrontal cortex | ↓ | EMSA binding activity | Okvist et al. (2007) |
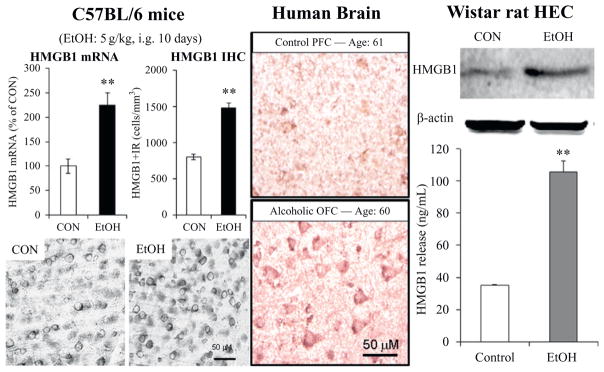
Figure 10.4.
Alcohol increases high-mobility group box 1 (HMGB1) expression in mouse brain, human brain, and releases HMGB1 from rat brain slices. Left: Chronic ethanol treatment of mice for 10 days increases expression of HMGB1 and TLR receptor mRNA and protein. Middle: Postmortem human alcoholic orbitofrontal cortex has significantly more HMGB1-immunoreactive cells than age-matched controls. Right: Ethanol causes the release of HMGB1 into the media from hippocampal-entorhinal cortex (HEC) slice culture. Top: Representative Western blot of HMGB1 protein in medium following control (CON) and ethanol (EtOH) treatment. Bottom: ELISA assessment of HMGB1 protein in medium in control and EtOH-treated HEC slice cultures. **p <0.01, relative to corresponding control group. Adapted from (Crews et al., 2013).
There are multiple mechanisms of neuroimmune gene induction by ethanol. We recently discovered that one mechanism involves alcohol release of HMGB1 (a TLR4 agonist) that increases NF-κB transcription of proinflammatory cytokines (Crews et al., 2013; Zou & Crews, 2014). A number of transmitters and neuroimmune signaling receptors as well as neuronal excitability increase release of HMGB1 (Maroso et al., 2010). HMGB1, also known as amphoterin (Huttunen & Rauvala, 2004), has multiple signaling mechanisms in brain that impact astrocytes, microglia, neurogenesis-neurite growth, and excitability in adjacent neurons. HMGB1 released by neuronal activity stimulates TLR4 receptors inducing IL-1β and increasing phosphorylation of the NR2B subunit of the NMDA receptor increasing excitation to seizures (Maroso et al., 2010; Vezzani, Maroso, Balosso, Sanchez, & Bartfai, 2011). Actively released HMGB1 is acetylated. We found that ethanol increases HMGB1 acetylation and histone deacetylases in brain slice cultures. Acetyl-HMGB1 is increased in cytosolic fractions, likely to vesicles, and is increased progressively in the media consistent with neuronal release (Zou & Crews, 2014). The importance of ethanol release of HMGB1 and activation of TLR4 receptors became apparent due to the elegant experiments of Consuelo Guerri’s laboratory establishing TLR4 receptors as critical to ethanol-induced neurodegeneration and behavioral pathology. Using TLR4 knock-out transgenic cells and mice, her group discovered that chronic ethanol-induced neurodegeneration and induction of proinflammatory gene expression is markedly blunted by knockout of TLR4 receptors (Alfonso-Loeches et al., 2010; Blanco, Valles, Pascual, & Guerri, 2005; Fernandez-Lizarbe, Pascual, & Guerri, 2009; Pascual, Balino, Alfonso-Loeches, Aragon, & Guerri, 2011; Valles, Blanco, Pascual, & Guerri, 2004). Guerri’s laboratory has also shown that ethanol treatment induces neuroimmune genes in microglia and astrocyte primary cultures as well as in vivo in mice that is dependent upon the expression of TLR4 receptors. TLR4 receptors are constitutively expressed on microglia, making microglia a key component of drug-induced neuroimmune activation (Alfonso-Loeches & Guerri, 2011; Schwarz & Bilbo, 2013). More recent studies by Guerri’s laboratory have found that TLR4 receptors are integral to ethanol-induced dopamine release (Alfonso-Loeches & Guerri, 2011), damage to white matter (Alfonso-Loeches et al., 2012), and other pathologies associated with chronic ethanol-induced changes in brain (Pascual et al., 2011). In culture, ethanol treatment increases innate immune gene expression in a time-dependent fashion mimicking responses to LPS or IL-1β administration, although ethanol induces a much smaller response (Crews et al., 2013). In vivo, ethanol induces neuroimmune genes in the brains of wild type mice, but not TLR4 transgenic mice (Alfonso-Loeches et al., 2010). These studies support the hypothesis that TLR4 signaling is critical to many of the effects of alcohol on the brain. The critical role of TLR4 is somewhat surprising since multiple proinflammatory receptors converge upon common signaling through NF-κB. Also, Vetreno et al. (2013) found that chronic intermittent treatment of adolescent rats led to persistent increases in expression of RAGE in brain, a receptor also stimulated by HMGB1 (see Fig. 10.2). It is not understood why TLR4 signaling appears to contribute significantly to ethanol responses since TLR and cytokine receptors generally are within the IL1-receptor family and share kinase cascades in monocytes and microglia that all converge upon NF-κB. These findings suggest that the TLR4 receptors on neurons or other cells in brain may have some unique properties that differ from NF-κB activation by TNFα, IL-1β, and other cytokine receptors known to induce NF-κB transcription of proinflammatory cytokines. One explanation may be related to amplification of overlapping signaling, particularly IL-1β and HMGB1. IL-1β can be induced by HMGB1-TLR signaling, including formation of the inflammasome, a unique intracellular multiprotein organelle containing specific proteases and NLRP proteins. We found increased expression of IL-1β and NLRP-inflammasome proteins within alcoholic human hippocampus (see Fig. 10.5) consistent with neuroimmune gene activation contributing to ethanol-induced loss of neurogenesis (Zou & Crews, 2014). Although the mechanisms remain complicated, together these studies suggest that HMGB1-TLR4, and perhaps RAGE signaling, involves multiple brain cells type, with neuronal-glial neuroimmune signaling and microglial–astrocyte activation contributing to alcohol-induced brain damage.
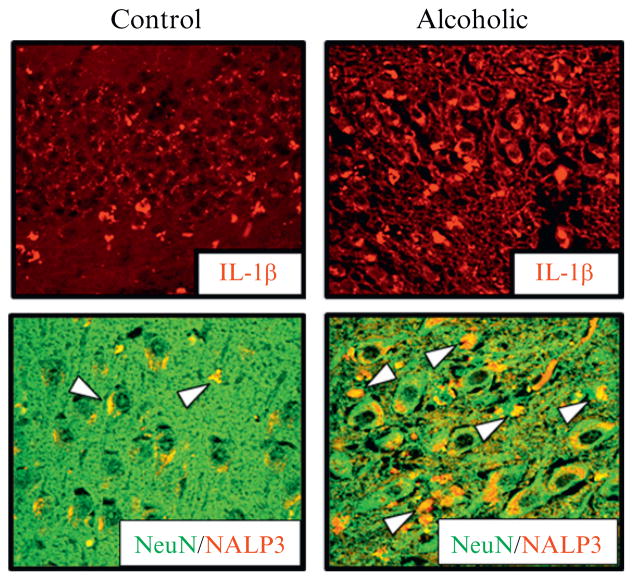
Figure 10.5.
Neuronal expression of IL-1β and inflammasome proteins in the human alcoholic hippocampus. IL-1β can be induced by HMGB1-TLR signaling, including formation of the inflammasome, a unique intracellular multiprotein organelle containing specific proteases and NLRP proteins. We found increased expression of IL-1β and NLRP-inflammasome proteins within alcoholic human hippocampus. Shown is immunohistochemistry for IL-1β in control (left) and alcoholic hippocampus (right) above double immunohistochemistry sections stained for NeuN, a neuronal marker, and NLRP3, an inflammasome protein, that form yellow (white in the print version) when coexpressed consistent with ethanol induction of neuronal inflammasome formation and IL-1β release contributing to ethanol inhibition of neurogenesis. Adapted from (Zou & Crews, 2012).
Although chronic alcohol treatment increases proinflammatory gene expression in brain through activation of TLR4 receptors, this is confounded by alcohol inhibition of TLR4 signaling in monocytes and possibly other cells. Time-dependent acute and chronic opposing effects of ethanol confound many studies (Crews, Bechara, et al., 2006; Crews, Zou, & Qin, 2011; Szabo & Mandrekar, 2009). Ethanol suppresses the innate immune response to LPS, a TLR4 agonist, in both in vivo and in vitro models. For example, LPS-induced TNFα and IL-1β production is blunted in blood monocytes obtained from healthy human volunteers after acute alcohol exposure (2 mL vodka/kg body weight; Crews, Bechara, et al., 2006; Szabo, Mandrekar, & Catalano, 1995; Szabo, Mandrekar, Dolganiuc, Catalano, & Kodys, 2001; Szabo, Verma, & Catalano, 1993). In animal models, acute ethanol exposure attenuated the TNFα, IL-1β, and IL-6 immune response to LPS (Pruett, Zheng, Fan, Matthews, & Schwab, 2004). Similarly, Szabo’s group reported that ethanol (25 mM) in vitro added just before LPS blunts induction of TNFα (Szabo et al., 1993, 1995, 2001). In contrast, chronic in vitro ethanol exposure of astrocytes, microglia, and brain slices induces NF-κB transduction of proinflammatory genes through activation of TLR4 signaling (Blanco, Pascual, Valles, & Guerri, 2004; Blanco et al., 2005; Crews et al., 2013; Fernandez-Lizarbe et al., 2009; Zou & Crews, 2014). While it is not clear if the presence of ethanol antagonizes TLR4 receptors on all cell types, other TLR receptors are not acutely blocked by ethanol (Crews, Bechara, et al., 2006). Upregulation of TLR receptors by alcohol can lead to sensitization. In mice, binge treatment with ethanol for 10 days (5 g/kg/day) followed by LPS 24 h later when alcohol had cleared resulted in a marked increase in proinflammatory gene induction (Qin & Crews, 2012b). Ethanol treatment increased the response to LPS-induced proinflammatory cytokines in liver, blood, and brain. The responses in blood and liver were transient, but were long lasting in brain. Similarly, chronic 10-day alcohol treatment sensitized mice to the proinflammatory response to Poly:IC, a viral mimetic TLR3 agonist (Qin & Crews, 2012a). Thus, the effects of ethanol on brain neuroimmune signaling are in part related to increases in TLR receptors (see Fig. 10.4) that increase neuroimmune signaling and cytokines, such as IL-1β (see Fig. 10.5), during chronic ethanol treatment, although the presence of alcohol can blunt TLR4 responses during intoxication.
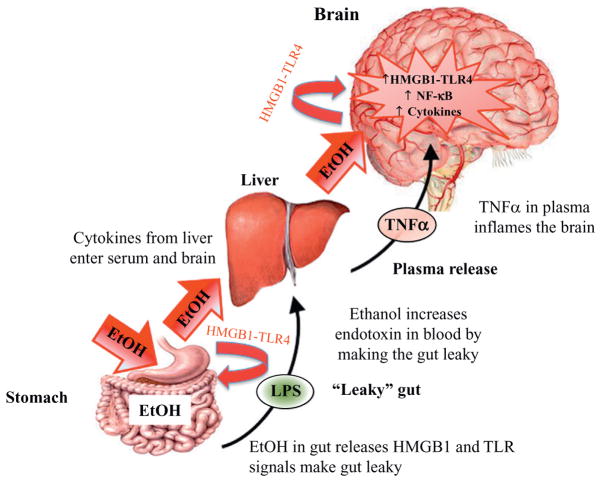
Although ethanol-induced release of HMGB1 from neurons activates TLR4 receptors and NF-κB transcription increasing synthesis of proinflammatory cytokines in brain (see Figs. 10.2, 10.4, and 10.5), a second mechanism involves ethanol-induced increases in blood proinflammatory cytokines that spread and amplify gene induction in brain (see Fig. 10.6). Recent studies indicate that ethanol in the gut releases HMGB1, which activates TLR4 receptors causing the gut to leak endotoxin LPS-like bacterial products stimulating proinflammatory cytokine induction in the liver. Induction of liver cytokines results in increased blood TNFα and other cytokines. Qin and Crews (2012b) discovered that LPS-induced increases in serum TNFα as well as proinflammatory gene induction in brain. Proinflammatory cytokines in the blood can be transported by their receptors across the blood–brain barrier (BBB) (e.g., TNFR; Banks & Erickson, 2010; Qin et al., 2007) as well as activating endothelial cells to release cytokines (Watkins, Maier, & Goehler, 1995). Using LPS–TLR4 stimulation of proinflammatory responses in liver and other tissues induction of proinflammatory cytokines, we discovered that blood and brain levels show parallel increases in TNFα following intraperitoneal injections of LPS (Qin et al., 2007). Using transgenic mice-lacking TNF receptors, we found that LPS increased TNFα in blood, but did not increase TNFα in the brain, suggesting that LPS–TLR4 induction of TNFα in blood leads to TNF transport through its receptors across the BBB-activating pro-inflammatory responses in brain. Transgenic mice without the TNF receptor do not transport TNF to brain so the LPS–TLR4 proinflammatory response amplifies across peripheral tissues, but does not spread to brain. Ethanol can increase blood proinflammatory cytokines through activation of proinflammatory responses in the liver and other tissues. One mechanism appears to involve ethanol causing the gut to become permeable or “leaky” (Ferrier et al., 2006). Only high doses of ethanol, at least 2–3 g/kg ETOH intragastric doses (Ferrier et al., 2006), potentiate gut innate immune signaling, disrupting gut tight junctions, and opening sites that allow the gut biome bacteria and their endotoxins to enter portal circulation leading to the liver where they can initiate a proinflammatory response (Sims, Rowe, Rietdijk, Herbst, & Coyle, 2010). Thus, high doses of ethanol increase systemic proinflammatory responses that can spread the proinflammatory response to brain through TNFα and likely other cytokines (see Fig. 10.6). Although some in vitro studies have suggested ethanol can insult the BBB, most in vivo studies do not show BBB damage following chronic ethanol treatment. Marshall et al. (2013) assessed BBB integrity using immunohistochemistry for albumin, a 140 kDa protein that does not cross an intact BBB, and found no evidence of brain albumin indicating an intact BBB following treatment with the severe intragastric 4-day binge rat alcohol dependence model of alcoholic brain damage. Using this model, Crews and Braun (2003) found that inhibition of NF-κB protected against 4-day binge alcohol-induced brain damage and inhibition of neurogenesis, consistent with proinflammatory responses in brain mediating brain damage without BBB damage, but being induced through direct activation of proinflammatory responses in brain and/or systemic proinflammatory signals being transported across the BBB and contributing to brain proinflammatory responses.
Figure 10.6.
Ethanol in the gut activates systemic cytokine signaling inducing neuroimmune gene expression in the brain. Consumed EtOH enters the stomach and makes it “leaky,” through the release of HMGB1 which activates gut TLR4 receptors that allows bacterial products such as lipopolysaccharide (LPS) to enter the blood. EtOH and LPS lead to induction of liver tumor necrosis factor-alpha (TNFα) and other proinflammatory cytokines. These proinflammatory cytokines in blood enter the brain and increase neuroimmune expression. Chronic ethanol increases expression of HMGB1-TLR4 receptors in brain leading to persistent and progressive increases in neuroimmune gene expression in brain.
Although proinflammatory gene expression in blood and brain parallel each other at early time points, we found the brain response to LPS is much smaller than the liver and blood responses during the first few hours. Surprisingly, the blood and liver responses to LPS return to baseline over about 8–12 h, whereas the increase in proinflammatory gene expression in brain persists for months leading to degeneration of dopamine neurons in substantia nigra (Qin et al., 2007). Binge alcohol-induced liver and blood responses have not been extensively investigated and appear to be small and transient. However, brain expression of the proinflammatory cytokine, MCP-1, persists for at least 1 week (Qin et al., 2008). As mentioned earlier, exposure of C57Bl/6 mice to 10 daily doses of ethanol followed by LPS results in increased LPS induction of proinflammatory cytokines in liver, blood, and brain, compared to control LPS-treated animals (Qin et al., 2008). In these studies, ethanol-sensitized mice to the LPS response that resulted in sustained increases in multiple proinflammatory cytokines, including TNFα, IL-1β, and MCP-1 in brain, but not in the liver. The mechanism of the sustained brain response and transient liver response is not clear, although we found IL-10, an anti-inflammatory factor that inhibits NF-κB, increased in liver 1 week after alcohol treatment, but decreased in brain (Qin et al., 2008) consistent with anti-inflammatory mechanisms contributing to the loss of the liver response. Mice pretreated with ethanol are sensitized to both the TLR4 receptor agonist LPS as well as the TLR3 agonist Poly:IC (Qin & Crews, 2012a). Similar to LPS, Poly:IC induces proinflammatory genes in brain and 24 h after 10 days of daily alcohol administration (5 g/kg/day). These findings suggest that chronic ethanol sensitizes proinflammatory TLR responses that are easily observed after the clearance of alcohol. Thus, chronic ethanol sensitizes systemic and brain responses to neuroimmune gene activation through induction of HMGB1 and TLR proteins. Ethanol-induced leaky gut occurs with high binge drinking doses, with gut ethanol exposure often being equivalent to the beverage content (i.e., 80 proof is 40% ethanol). Bacterial products enter portal circulation activating Kupffer cells, liver monocytes, which produce cytokines including TNFα. TNFα can be transported into brain activating brain neuroimmune signaling that persists for long periods (Qin et al., 2007). Thus, there are at least two mechanisms of ethanol activation of neuroimmune signaling, a direct activation within brain as well as the spread of a systemic innate immune activation to brain.
6. ETHANOL INDUCTION OF HMGB1-TLR SIGNALING IN BRAIN
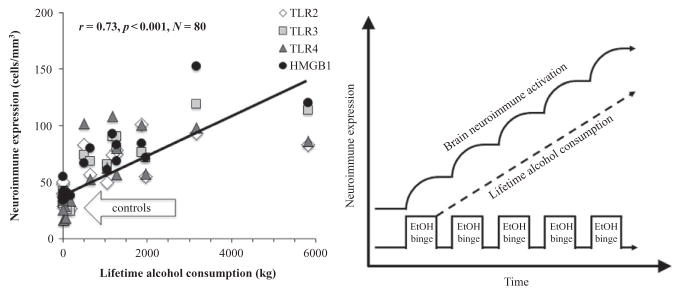
Studies investigating the mechanisms of ethanol induction of proinflammatory genes in brain have led to the discovery that chronic ethanol increases expression of TLR receptors as well as the TLR4 receptor agonist HMGB1. Studies of chronic 10-day ethanol treatment of mice (Crews et al., 2013), chronic in vitro treatment of rat brain slice cultures (Zou & Crews, 2014), and postmortem human alcoholic brain (Crews et al., 2013) find increased expression of HMGB1, TLR4, TLR3, and TLR2 (see Fig. 10.4). There are 13 TLRs that have been identified (i.e., TLRs 1–13) in mammals (Medzhitov, 2001; Takeda, Kaisho, & Akira, 2003) with all signaling through NF-κB transcription of additional proinflammatory cytokines through paracrine and autocrine amplification. Increases in receptors and agonists are common in innate immune signaling and suggest induction of HMGB1 and its receptor, the TLR4 receptor, as well as a less well-characterized receptor, RAGE, contribute to increases in neuroimmune gene expression. Brain slice culture experiments find ethanol releases HMGB1 that increases proinflammatory gene expression that is blocked by pharmacological antagonists or knock down of TLR4 receptors (Crews et al., 2013; Zou & Crews, 2014). Studies in adolescent rats (Vetreno & Crews, 2012), adolescent mice (Coleman et al., 2014), and adult mice (Qin et al., 2008; Qin, Liu, Hong, & Crews, 2013; Qin et al., 2007) find long-lasting increases in neuroimmune gene induction. Interestingly, levels of HMGB1 and TLR receptor expression in OFC correlate with lifetime alcohol consumption (see Fig. 10.7). Moderate drinking humans consume much less alcohol than alcoholics and set the y-axis intercept, whereas alcoholics who are known to vary greatly in the duration and amounts of active drinking bouts show a large variation in lifetime alcohol consumption that correlates with expression of HMGB1-TLR receptor expression in their brains. This interesting correlation could only occur if ethanol induction of HMGB1-TLR receptors was persistent and cumulative with binge drinking episodes (see Fig. 10.7). Together, these studies suggest that HMGB1-TLR4 signaling is increased by chronic binge drinking, contributing to the persistent and sustained induction of HMGB1-TLR4 proinflammatory signaling in brain.
Figure 10.7.
Cycles of chronic alcohol consumption lead to persistently increased neuroimmune gene expression. Left: Depicted are correlations of individual human TLR2 (r =0.66, p <0.01), TLR3 (r =0.83, p <0.001), TLR4 (r =0.62, p <0.01), and HMGB1 (r =0.83, p <0.001) immunoreactivity versus lifetime alcohol consumption (kg) in alcoholics and moderate drinking controls. Across human subjects, lifetime alcohol consumption positively correlated with neuroimmune signal immunoreactivity. Note that moderate drinking control subjects are clustered along the y-axis due to low lifetime alcohol consumption values and similar neuroimmune expression. Alcoholic subjects show considerable variation in neuroimmune expression, and over a 10-fold variation in lifetime alcohol consumption. Right: Repeated ethanol (EtOH) binges result in increased microglial and astrocytic activation as well as upregulated neuroimmune gene expression. In previous studies, we have shown that neuroimmune activation persists for long periods following upregulation in brain (Qin et al., 2008; Vetreno & Crews, 2012; Vetreno et al., 2013).
7. NADPH OXIDASE AND NEURODEGENERATION
One innate immune gene induced by ethanol and endotoxin is NADPH oxidase, a multisubunit enzyme that catalytically makes superoxide. NADPH oxidase was first characterized as a phagocytic oxidase in monocytes where it was hypothesized to contribute to oxidizing infectious agents. NADPH oxidase produces superoxide that can increase NF-κB transcription creating another amplifying loop of proinflammatory signaling (see Fig. 10.2). More recent studies have found that there are multiple genes and forms of NADPH oxidase all innate immune genes. Qin and Crews (2012b) discovered that LPS and ethanol can increase brain expression of NADPH oxidase subunits, particularly gp91phox, the superoxide forming subunit in brain, and that ethanol treatment of mice increased superoxide formation in brain in association with neuronal death. Inhibition of oxidases reduced superoxide formation and protected against alcohol-induced neuronal death. These findings are consistent with oxidative stress through innate immune gene induction making a significant contribution to alcoholic brain damage. In mice, LPS treatment induces neuroimmune gene expression, NADPH oxidase, and oxidative stress that persists for at least 20 months and leads to neurodegeneration (Qin et al., 2013). The prolonged and persistent induction of NADPH oxidase and oxidative stress in brain could contribute to the persistent increase in NF-κB transcription, since oxidative free radicals can activate NF-κB. These studies suggest that oxidative stress contributes to alcoholic neurodegeneration.
8. NEUROIMMUNE SIGNALING, HYPEREXCITABILITY, AND NEURONAL DEATH
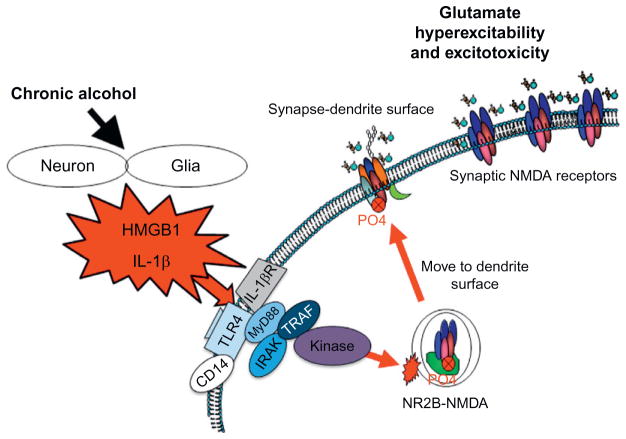
Excitotoxicity is associated with alcoholic neurodegeneration and HMGB1-TLR4 signaling. Chronic ethanol treatment of neurons leads to increased sensitivity to excitotoxicity (Chandler, Guzman, Sumners, & Crews, 1994). Ethanol potentiates glutamate excitotoxicity in brain slice cultures due to blockade of glial transporters (Zou & Crews, 2010). However, in neuronal primary cultures, ethanol blocks NMDA excitotoxicity consistent with many studies finding ethanol inhibition of NMDA receptors (Chandler, Harris, & Crews, 1998). Similar to ethanol blocking TLR4 receptor signaling when present, ethanol blocks NMDA receptors when present (Chandler et al., 1998). Although ethanol can block NMDA responses, glutamate excitotoxicity is increased by ethanol and TNFα in brain slice cultures due in part to glial loss of glutamate uptake (Zou & Crews, 2006) and perhaps release by ethanol. Further, HMGB1-TLR4 signaling has been shown to activate kinase cascades that lead to phosphorylation of the NR2B subunit of NMDA receptors causing the migration of more NMDA receptors to the synapse that increase synaptic NMDA receptors, neuronal excitability, and excitotoxicity (see Fig. 10.8; Balosso, Liu, Bianchi, & Vezzani, 2014; Maroso et al., 2010). Both HMGB1-TLR4 signaling (Balosso et al., 2014) and IL1β-IL1R signaling (Viviani et al., 2003) have been shown to increase NMDA receptor mediated calcium flux, neuronal excitability, and excitotoxicity through activation of kinase cascades. IL1β-IL1R activation of Src kinase has been found to increase NMDA calcium flux, excitability, and excitotoxicity. Many studies have found tyrosine-kinase activation can increase excitability through increases in NR2B-NMDA receptor phosphorylation (see Fig. 10.8). Ron’s group has found that ethanol increases NMDA excitability in hippocampus through kinase activation that alters receptor trafficking, leading to increased NR2B-NMDA receptors and increased excitability (Suvarna et al., 2005). Another mechanism of chronic ethanol-induced hyperexcitability is neuroimmune inhibition of glial glutamate transporters (Zou & Crews, 2005). Ethanol releases HMGB1-creating hyperexcitability that disrupts synaptic plasticity and sensitizes to excitotoxicity. HMGB1 is massively released during brain damage activating persistent neuroimmune gene induction (Kim et al., 2006). Maroso et al. (2010) found increased release of HMGB1 with hippocampal excitability that caused seizures leading to persistent increases in HMGB1 and excitability. Ethanol has modest cumulative effects with repeated chronic exposure increasing excitability and excitotoxicity due to increased neuroimmune signaling (see Fig. 10.8). Thus, the global neurodegeneration with the most severe losses in frontal cortex found in alcoholism is secondary to the persistent and progressive neuroimmune activation that occurs during alcoholism, a chronic relapsing disorder.
Figure 10.8.
HMGB1-TLR signaling-induced hyperexcitability contributes to the neurobiology of addiction. A simplified schematic depicting how neuroimmune signaling leads to hyperexcitability and the neurobiology of addiction. Alcohol and stress activation neurons and glia in the CNS, resulting in the release of various neuroimmune signals (e.g., high-mobility group box 1 (HMGB1) and interleukin-1β (IL-1β)) that activate neuroimmune receptors (i.e., Toll-like receptors (TLRs) and receptor for advanced glycation end products (RAGE)). Neuroimmune receptor stimulation leads to activation of glutamatergic N-methyl-D-aspartate (NMDA) receptors (Iori et al., 2013; Maroso et al., 2010), which increases Ca2+ flux triggering induction of neuroimmune genes. In addition, TLR/RAGE activation leads to downstream transcription of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) signaling that might be accompanied by diminished cyclic AMP-responsive element binding protein (CREB) expression, which contributes to neuroimmune gene induction. These two pathways converge, leading to cycles of neuroimmune gene induction that lead to hyperexcitability, neuronal cell death, and network reorganization that culminates in addiction.
9. ADOLESCENCE: A MAJOR PERIOD OF RISK FOR ALCOHOL DEPENDENCE
Adolescence is a developmental period involving increased play behavior, thrill seeking, risk taking, puberty, and transitions to independence. During adolescence, the brain continues to develop, with the frontal cortex continuing to show structural changes coincident with maturation of adult behaviors and executive functions (Ernst, Romeo, & Andersen, 2009). Adolescence is also a period of experimentation as exemplified by the finding that the highest level of binge drinking among humans occurs during late adolescence. The prevalence of lifetime alcohol use disorders, as well as violence, fights, and injuries associated with alcohol use are all associated with age of drinking onset (Brown et al., 2008; Dawson, Stinson, Chou, & Grant, 2008; Sher & Gotham, 1999). The younger the age of drinking onset, the more likely alcohol use disorder will develop. Although binge drinking peaks in adolescence, the association between age of drinking onset and alcohol use disorder could represent maturation of an “alcoholic destiny” due to the emergence of conduct disorder and/or antisocial personalities, or genetic factors that identify themselves with an early onset of alcohol drinking. Alternatively, the association could be related to binge drinking-induced changes in brain that alter brain maturation thereby increasing the risk of alcohol use disorder, or both (Crews & Boettiger, 2009; Crews et al., 2007). The high prevalence of binge drinking among adolescence increases the importance of understanding how binge drinking might impact the adolescent brain. The correlation of younger age of drinking onset with increased risks of lifetime alcohol use disorder also correlates with a smaller brain and greater expression of HMGB1 and TLR4 receptors as well as other neuroimmune signaling receptors (Vetreno et al., 2013; see Fig. 10.9). The cause and effect of these associations is likely to be due to both preexisting conditions maturing into dysfunctional behavior as well as alcohol-induced factors that change the life course, and increase dysfunctional behavior, perhaps by changing brain maturation. The contributions of these two factors can only be determined by controlled experiments that do not have different genetic or other factors other than adolescent alcohol exposure. These studies cannot be done in humans, but are being done in rats where genetics and environment can be controlled. The essential need to understand the neurobiology of adolescent drinking on adulthood (NADIA) resulted in the formation of the NADIA consortium funded by NIAAA to address the contribution of adolescent alcohol abuse to adult psychopathology.
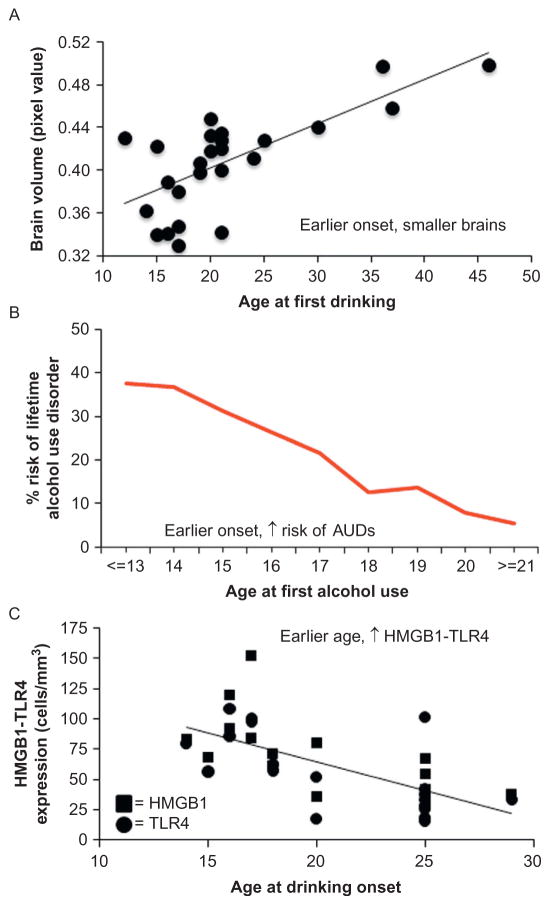
Figure 10.9.
Brain regional volume, risk of alcoholism, and induction of innate immune genes correlates with age of drinking onset in humans. (A) Age of drinking onset correlates positively with gray matter reductions in the middle frontal gyrus, brainstem, and cerebellum. Depicted is correlation of pixel value of cerebellum with age at first drink. (B) An earlier age of drinking onset is predictive of an increased likelihood of developing an alcohol use disorder during an individual’s lifetime. (C) TLR4 and HMGB1 expression in the postmortem human brain are negatively correlated with age of drinking onset. Panel A: Adapted from Chanraud et al. (2007). Panel B: Adapted from Grant (1998). Panel C: Adapted from Vetreno et al. (2013).
Adolescents have an immature response to alcohol, with evidence supporting unique factors that differ from the adult response to alcohol. Adolescent rats show greater ethanol-induced memory impairment on the Morris water maze and in discrimination tasks than do adults (Land & Spear, 2004; Markwiese, Acheson, Levin, Wilson, & Swartzwelder, 1998). Similarly, in humans that initiate drinking in their early 1920s are more sensitive to the effects of ethanol on multiple memory tasks compared to those that start in their late 1920s (Acheson, Stein, & Swartzwelder, 1998). The adolescent hippocampal response to ethanol compared to adults shows more potent inhibition of NMDA receptor-mediated synaptic activity (Swartzwelder, Wilson, & Tayyeb, 1995) and the induction of long-term potentiation (Martin, Tayyeb, & Swartzwelder, 1995). Adolescents, already social, are also uniquely sensitive to the social facilitative effects of ethanol (Varlinskaya & Spear, 2002). Adolescent rats are also more sensitive to binge drinking models of brain damage, particularly in the frontal cortex (Crews, Bechara, et al., 2006; Crews, Mdzinarishvili, et al., 2006). Interestingly, adolescent rats are less sensitive than adults to the sedative (Little, Kuhn, Wilson, & Swartzwelder, 1996; Silveri & Spear, 1998), motor impairing (Little et al., 1996; White, Bae, et al., 2002; White, Truesdale, et al., 2002), social inhibitory (Varlinskaya & Spear, 2002), and aversive (Anderson, Varlinskaya, & Spear, 2010) effects of ethanol. Adolescent rats also show electrophysiological differences from adults in the hippocampus, with reduced sensitivity to γ-aminobutyric acid (GABA) type A (GABAA) receptor-mediated inhibition (Carr, Spence, Peter Eriksson, Lumeng, & Li, 2003; Sullivan et al., 2006; Yan et al., 2009; Yan, Li, Madison, Wilson, & Swartzwelder, 2010). Reduced adolescent sedative sensitivity to alcohol and increased cognitive disruption is consistent with the human findings of high binge drinking coupled with high risk of horrific traffic accidents that have prompted zero tolerance for underage drinking and driving laws across the United States. The continuous increase in high binge drinking levels in human adolescents over the past decade justifies the need to know the long-term consequences of adolescent alcohol abuse.
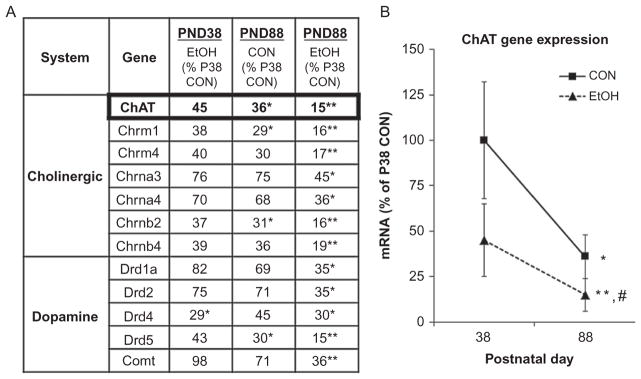
The adolescent brain is structurally different from adult brain with both brain regional volume (Sowell et al., 2004) and prefrontal cortical synaptic changes continuing into the early 1930s in humans. Rat studies have found that the adolescent frontal cortex is more sensitive to ethanol toxicity (Crews, Bechara, et al., 2006). Multiple studies on adults following adolescent behavior done by NADIA investigators Linda Spear and Scott Swartzwelder find an altered adult that has persistent adolescent-like responses in adulthood. Studies of spatial learning in the radial arm maze find adolescence, but not adult, ethanol treatment models result in high adolescent-like sensitivity to ethanol memory impairment persisting into adulthood (Swartzwelder et al., 2014; White, Ghia, Levin, & Swartzwelder, 2000). Similarly, adolescent ethanol binge models produce a long-lasting decrease in sensitivity to the sedative/motor-impairing effects of acute ethanol, i.e., an adolescent-like low response persisting into adulthood (White, Truesdale, et al., 2002). At the cellular level, adolescent alcohol exposure, but not adult, produces an enduring decrease in the magnitude of GABA receptor-mediated tonic current in dentate granule cells (Fleming, Acheson, Moore, Wilson, & Swartzwelder, 2012; Fleming et al., 2013), which is critical for maintaining the balance of excitation and inhibition within hippocampal circuits. In addition, acute ethanol induced decreases in A-type potassium current (IA) in GABAergic hippocampal interneurons, persists after adolescent ethanol treatment resulting in adolescent-like responses in adulthood (Li et al., 2013). Levels of neurotransmitters in adolescent brain are maturing to adult levels and are altered in binge models of adolescent drinking. Expression of multiple cholinergic and dopaminergic genes peaks during adolescence (Spear, 2000). We found adolescence binge drinking models decrease the expression of multiple genes involved in cholinergic and dopaminergic neurotransmission (see Fig. 10.10; Coleman et al., 2011). Interestingly, many of these neuro-transmitter genes undergo maturational decreases in expression that in animals exposed to alcohol continued the maturational decrease resulting in persistent impairment in adulthood in both mice (Coleman et al., 2011) and rats (Ehlers, Oguz, et al., 2013; Vetreno & Crews, 2012; see Fig. 10.11). Adolescent alcohol exposure, like adult alcohol exposure, induces neuroimmune genes in brain, and in humans, correlates with age of drinking onset (see Fig. 10.9). Indeed, Vetreno and Crews (2012) found that adolescent intermittent binge ethanol treatment of rats increases expression of multiple innate immune genes in adult frontal cortex. Interestingly, the critical neuroimmune signaling receptor TLR4 showed decreased maturational expression in controls, and adolescent binge ethanol increased expression and remained elevated into adulthood (Vetreno & Crews, 2012). In contrast, HMGB1 showed a developmental increase in expression in frontal cortex of control subjects, and adolescent binge ethanol led to greater increases in HMGB1 resulting in a persistent increase in adult HMGB1 and TLR4. High TLR4 levels in adults may represent adolescent-like HMGB1-TLR4 signaling in adults.
Figure 10.10.
Adolescent ethanol treatment reduces choline acetyltransferase (ChAT) gene expression across development. Mice received either water or ethanol (5 g/kg) once a day for 10 days during adolescence (P28–P37). (A) The expression of cholinergic and dopaminergic neurotransmitter genes was assessed in whole brain either 24 h after treatment (P39; CON=6, EtOH =6) or in adulthood (P88; CON=5; EtOH =5) using an RT2 Profiler Neurotransmitter Receptor and Regulator SuperassayTM. Gene expression levels are given as the percent of P38 CON. Genes that changed in at least on treatment group are presented. Data analysis was performed using the ΔCt method using the Data Analysis Template provided by SABioscience Corporation as published previously (Lee et al., 2008). A two-way ANOVA with Bonferroni posttests to account for multiple comparisons was used to determine statistical significance. (B) ChAT mRNA levels changed significantly across development. ChAT mRNA in adolescent CON mice (P38 =100%) decreased during maturation to P80 (64% reduction). ChAT mRNA expression 24 h following ethanol treatment revealed a 55% reduction on P38, and a 85% reduction in adulthood on P88. *p <0.05, **p <0.01, relative to P38 CON (Coleman et al., 2011).
Figure 10.11.
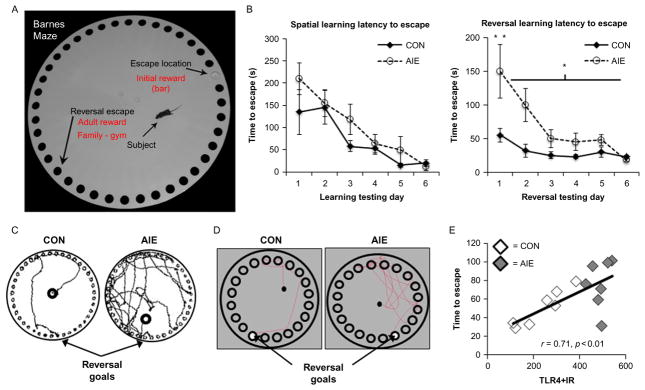
Adolescent intermittent ethanol exposure causes reversal, but not spatial, learning deficits in adult rodents. (A) Photomicrograph of a mouse on the Barnes maze. The subjects and its assigned escape locations are identified. (B). Mice received either water or ethanol (5 g/kg, i.g.) once a day for 10 days during adolescence (P28–P37), and spatial learning was assessed for 6 days from P68 to P73. Adolescent ethanol exposure did not impair spatial learning, as measured by latency to escape, relative to CONs. However, adolescent ethanol exposure did impair reversal learning in adulthood as evidenced by increased time to locate and enter the reversal goal, relative to CONs. (C) Digital reconstruction of the search paths during the first trial of reversal learning in adulthood. (D) Rats received either water or ethanol (5 g/kg, i.g.) on a 2 days on/2 days off intermittent schedule from P25 to P55. Spatial and reversal learning was assessed from P64 to P75. As evidenced in the digital reconstruction, AIE treatment did not affect spatial learning, but did impair reversal learning as evidenced by the increased time to locate and enter the reversal goal. (E) TLR4 receptor expression in the adult prefrontal cortex is positively correlated with time to escape during the first trial of reversal learning on the Barnes maze (Vetreno & Crews, 2012). *p <0.05, **p <0.01, relative to CONs.
As mentioned earlier, HMGB1/TLR4-induced NMDA sensitivity would counteract the direct ethanol inhibitory effects on NMDA receptors resulting in adolescent-like tolerance to sedation and perhaps increased adolescent-like cognitive disruption. These mechanisms are oversimplified, and likely more complex and in need of further study. Regardless of the molecular mechanism, NADIA studies strongly suggest that adolescent alcohol exposure increases risk for alcohol problems in adulthood. One repeated finding in the Crews laboratory is that models of binge drinking lead to long-term disruption of behavioral flexibility or the ability to adjust to changes in environment, which differs from simple learning (see Fig. 10.11). We have used reversal learning tasks and related them to changes in orbital frontal cortex, a brain region involved in predicting the outcome of initiated behaviors. Although we do not find deficits in adult learning after adolescent binge intermittent binge exposure, we have found deficits in reversal learning using in both the rats (Vetreno & Crews, 2012) and mice (Coleman et al., 2011), using both the Barnes maze and Morris water maze, respectively. These studies are consistent with the adolescent brain being vulnerable to long-lasting changes that persist through maturation into adulthood. The persistence of neuroimmune gene induction likely contributes to continuous slow degeneration as well as more specific insults on key neurotransmitters maturing in adolescence (Crews et al., 2007; Vetreno & Crews, 2012). The persistent loss of behavioral flexibility may be related to neuroimmune gene induction (see Fig. 10.11). Together, these findings of persistent loss of ability to adapt to changes and low sedative response to alcohol with increased sensitivity to cognitive disruption are all likely to promote and sustain high alcohol drinking levels that will further promote additional alcohol consumption and the chances that alcohol use disorder will develop in addition to alcoholic neurodegeneration.
10. NEUROIMMUNE GENE EXPRESSION IN POSTMORTEM HUMAN ALCOHOLIC BRAIN
Although this review highlights HMGB1-TLR4 signaling, there are multiple other proinflammatory genes increased and we have found many in postmortem human brain (see Table 10.2). Our first human brain studies looked a microglia and the proinflammatory cytokine MCP-1 (CCL2), which was the cytokine most robustly induced by ethanol in brain slice cultures among those measured (Zou & Crews, 2012). We found that postmortem alcoholic human brain has increased levels of MCP-1 protein in VTA, amygdala, nucleus accumbens, and hippocampus (He & Crews, 2006; see Table 10.2). We also found increased expression of the micro-glial marker Iba-1. These studies indicate that the human alcoholic brain has increased neuroimmune gene expression. In later studies, we focused on the PFC, specifically the orbital frontal cortex, and found that postmortem alcoholic brain has increased levels of HMGB1, and well as TLR receptors, specifically TLR2, TLR3, and TLR4 receptors (Crews et al., 2013). In other studies, we have found increased IL-1β inflammasome markers in hippocampus of postmortem alcoholic brain that could contribute to loss of neurogenesis. In addition, NADPH oxidase is increased in alcoholic OFC consistent with increased oxidative stress as found in mice. Further, RAGE, another HMGB1 receptor, is increased in postmortem human alcoholic brain (Vetreno et al., 2013). These studies indicate that multiple neuroimmune genes are increased in alcoholic brain and likely contribute to neurodegeneration and the neurobiology of alcoholism. We investigated the relationship between alcohol drinking and neuroimmune gene expression across controls and alcoholics. Interestingly, two forms of correlations were found linking neuroimmune gene expression to alcohol consumption and alcoholism. Adolescent drinking is known to increase risk of developing alcohol dependence with risks of alcoholism decreasing with every year of delaying alcohol use across adolescence. In OFC, we found HMGB1-TLR4 expression was lower in individuals who initiated alcohol use having a negative correlation with age of drinking onset. In addition, total lifetime alcohol consumption across groups has a positive correlation with OFC expression of HMGB1, TLR4, TLR3, TLR2, and RAGE. These findings further support the role of neuroimmune signaling in alcoholic brain and alcoholic neurodegeneration.
11. SUMMARY
Alcoholic neurodegeneration is global and defuse with the most severe loss in frontal cortex. Neuroimmune gene induction by binge drinking increases neurodegeneration through increased oxidative stress, particularly NADPH oxidase-induced oxidative stress. In addition, HMGB1-TLR4 and innate immune NF-κB target genes are increased leading to persistent and sensitized neuroimmune responses to ethanol and other agents that release HMGB1 or directly stimulate TLR receptors and/or NMDA receptors. Neuroimmune signaling and glutamate excitotoxicity are linked to alcoholic neurodegeneration. Models of adolescent alcohol abuse lead to significant frontal cortical degeneration and show the most severe loss of hippocampal neurogenesis. Adolescence is a period of high risk for ethanol-induced neurodegeneration and alterations in brain structure, gene expression, and maturation of adult phenotypes. Together, these findings support the hypothesis that adolescence is a period of risk for persistent and long-lasting increases in brain neuroimmune gene expression that promote persistent and long-term increases in alcohol consumption, neuroimmune gene induction, and neurodegeneration that we find associated with alcohol use disorders.
References
- Acheson SK, Stein RM, Swartzwelder HS. Impairment of semantic and figural memory by acute ethanol: Age-dependent effects. Alcoholism, Clinical and Experimental Research. 1998;22:1437–1442. doi: 10.1111/j.1530-0277.1998.tb03932.x. [DOI] [PubMed] [Google Scholar]
- Alfonso-Loeches S, Guerri C. Molecular and behavioral aspects of the actions of alcohol on the adult and developing brain. Critical Reviews in Clinical Laboratory Sciences. 2011;48:19–47. doi: 10.3109/10408363.2011.580567. [DOI] [PubMed] [Google Scholar]
- Alfonso-Loeches S, Pascual M, Gomez-Pinedo U, Pascual-Lucas M, Renau-Piqueras -J, Guerri C. Toll-like receptor 4 participates in the myelin disruptions associated with chronic alcohol abuse. Glia. 2012;60:948–964. doi: 10.1002/glia.22327. [DOI] [PubMed] [Google Scholar]
- Alfonso-Loeches S, Pascual-Lucas M, Blanco AM, Sanchez-Vera I, Guerri C. Pivotal role of TLR4 receptors in alcohol-induced neuroinflammation and brain damage. Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2010;30:8285–8295. doi: 10.1523/JNEUROSCI.0976-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman J, Das GD. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. Journal of Comparative Neurology. 1965;124:319–335. doi: 10.1002/cne.901240303. [DOI] [PubMed] [Google Scholar]
- Alvarez-Buylla A, Garcia-Verdugo JM. Neurogenesis in adult subventricular zone. Journal of Neuroscience. 2002;22:629–634. doi: 10.1523/JNEUROSCI.22-03-00629.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RI, Varlinskaya EI, Spear LP. Ethanol-induced conditioned taste aversion in male Sprague-Dawley rats: Impact of age and stress. Alcoholism, Clinical and Experimental Research. 2010;34:2106–2115. doi: 10.1111/j.1530-0277.2010.01307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balosso S, Liu J, Bianchi ME, Vezzani A. Disulfide-containing high mobility group box-1 promotes N-methyl-d-aspartate receptor function and excitotoxicity by activating Toll-like receptor 4-dependent signaling in hippocampal neurons. Antioxidants & Redox Signaling. 2014 doi: 10.1089/ars.2013.5349. [epub ahead of print]. http://dx.doi.org/10.1089/ars.2013.5349. [DOI] [PubMed]
- Banks WA, Erickson MA. The blood-brain barrier and immune function and dysfunction. Neurobiology of Disease. 2010;37:26–32. doi: 10.1016/j.nbd.2009.07.031. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio AR, Damasio H, Anderson SW. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition. 1994;50:7–15. doi: 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Bechara RI, Pelaez A, Palacio A, Joshi PC, Hart CM, Brown LA, et al. Angiotensin II mediates glutathione depletion, transforming growth factor-beta1 expression, and epithelial barrier dysfunction in the alcoholic rat lung. American Journal of Physiology. Lung Cellular and Molecular Physiology. 2005;289:L363–L370. doi: 10.1152/ajplung.00141.2005. [DOI] [PubMed] [Google Scholar]
- Bison S, Crews F. Alcohol withdrawal increases neuropeptide Y immunoreactivity in rat brain. Alcoholism, Clinical and Experimental Research. 2003;27:1173–1183. doi: 10.1097/01.ALC.0000075827.74538.FE. [DOI] [PubMed] [Google Scholar]
- Blanco AM, Pascual M, Valles SL, Guerri C. Ethanol-induced iNOS and COX-2 expression in cultured astrocytes via NF-kappa B. Neuroreport. 2004;15:681–685. doi: 10.1097/00001756-200403220-00021. [DOI] [PubMed] [Google Scholar]
- Blanco AM, Valles SL, Pascual M, Guerri C. Involvement of TLR4/type I IL-1 receptor signaling in the induction of inflammatory mediators and cell death induced by ethanol in cultured astrocytes. Journal of Immunology. 2005;175:6893–6899. doi: 10.4049/jimmunol.175.10.6893. [DOI] [PubMed] [Google Scholar]
- Blednov YA, Benavidez JM, Geil C, Perra S, Morikawa H, Harris RA. Activation of inflammatory signaling by lipopolysaccharide produces a prolonged increase of voluntary alcohol intake in mice. Brain Behavior, and Immunity. 2011;(Suppl 1):S92–S105. doi: 10.1016/j.bbi.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blednov YA, Ponomarev I, Geil C, Bergeson S, Koob GF, Harris RA. Neuroimmune regulation of alcohol consumption: behavioral validation of genes obtained from genomic studies. Addiction Biology. 2012;17(1):108–120. doi: 10.1111/j.1369-1600.2010.00284.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadwater M, Liu W, Crews F, Spear L. Persistent loss of hippocampal neurogenesis and increased cell death following adolescent, but not adult, chronic ethanol exposure. Developmental Neuroscience. 2013;36(3–4):1–9. doi: 10.1159/000362874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SA, Mcgue M, Maggs J, Schulenberg J, Hingson R, Swartzwelder S, et al. A developmental perspective on alcohol and youths 16 to 20 years of age. Pediatrics. 2008;121(Suppl 4):S290–S310. doi: 10.1542/peds.2007-2243D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr LG, Spence JP, Peter Eriksson CJ, Lumeng L, Li TK. AA and ANA rats exhibit the R100Q mutation in the GABAA receptor alpha 6 subunit. Alcohol. 2003;31:93–97. doi: 10.1016/j.alcohol.2003.07.003. [DOI] [PubMed] [Google Scholar]
- Chandler LJ, Guzman N, Sumners C, Crews FT. Induction of nitric oxide synthase in brain glial cells: Possible interaction with ethanol in reactive neuronal injury. In: Lancaster FE, editor. Alcohol and glial cells. Rockville: NIAAA; 1994. [Google Scholar]
- Chandler LJ, Harris RA, Crews FT. Ethanol tolerance and synaptic plasticity. Trends in Pharmacological Sciences. 1998;19:491–495. doi: 10.1016/s0165-6147(98)01268-1. [DOI] [PubMed] [Google Scholar]
- Chanraud S, Martelli C, Delain F, Kostogianni N, Douaud G, Aubin HJ, et al. Brain morphometry and cognitive performance in detoxified alcohol-dependents with preserved psychosocial functioning. Neuropsychopharmacology. 2007;32(2):429–438. doi: 10.1038/sj.npp.1301219. [DOI] [PubMed] [Google Scholar]
- Chavan SS, Huerta PT, Robbiati S, Valdes-Ferrer SI, Ochani M, Dancho M, et al. HMGB1 mediates cognitive impairment in sepsis survivors. Molecular Medicine. 2012;18:930–937. doi: 10.2119/molmed.2012.00195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman LG, Jr, He J, Lee J, Styner M, Crews FT. Adolescent binge drinking alters adult brain neurotransmitter gene expression, behavior, brain regional volumes, and neurochemistry in mice. Alcoholism, Clinical and Experimental Research. 2011;35:671–688. doi: 10.1111/j.1530-0277.2010.01385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman LG, Jr, Liu W, Oguz I, Styner M, Crews FT. Adolescent binge ethanol treatment alters adult brain regional volumes, cortical extracellular matrix protein and behavioral flexibility. Pharmacology, Biochemistry, and Behavior. 2014;116:142–151. doi: 10.1016/j.pbb.2013.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins MA, Zou JY, Neafsey EJ. Brain damage due to episodic alcohol exposure in vivo and in vitro: Furosemide neuroprotection implicates edema-based mechanism. FASEB Journal: Official Publication of the Federation of American Societies for Experimental Biology. 1998;12:221–230. doi: 10.1096/fasebj.12.2.221. [DOI] [PubMed] [Google Scholar]
- Colton CA. Heterogeneity of microglial activation in the innate immune response in the brain. Journal of Neuroimmune Pharmacology: The Official Journal of the Society on NeuroImmune Pharmacology. 2009;4:399–418. doi: 10.1007/s11481-009-9164-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corso TD, Mostafa HM, Collins MA, Neafsey EJ. Brain neuronal degeneration caused by episodic alcohol intoxication in rats: Effects of nimodipine, 6,7-dinitro-quinoxaline-2,3-dione, and MK-801. Alcoholism, Clinical and Experimental Research. 1998;22:217–224. [PubMed] [Google Scholar]
- Crews FT. Immune function genes, genetics, and the neurobiology of addiction. Alcohol Research: Current Reviews. 2012;34:355–361. doi: 10.35946/arcr.v34.3.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Bechara R, Brown LA, Guidot DM, Mandrekar P, Oak S, et al. Cytokines and alcohol. Alcoholism, Clinical and Experimental Research. 2006;30:720–730. doi: 10.1111/j.1530-0277.2006.00084.x. [DOI] [PubMed] [Google Scholar]
- Crews FT, Boettiger CA. Impulsivity, frontal lobes and risk for addiction. Pharmacology, Biochemistry and Behavior. 2009;93:237–247. doi: 10.1016/j.pbb.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Braun CJ. Binge ethanol treatment causes greater brain damage in alcohol-preferring P rats than in alcohol-nonpreferring NP rats. Alcoholism, Clinical and Experimental Research. 2003;27:1075–1082. doi: 10.1097/01.ALC.0000075826.35688.0D. [DOI] [PubMed] [Google Scholar]
- Crews FT, Collins MA, Dlugos C, Littleton J, Wilkins L, Neafsey EJ, et al. Alcohol-induced neurodegeneration: When, where and why? Alcoholism: Clinical and Experimental Research. 2004;28:350–364. doi: 10.1097/01.alc.0000113416.65546.01. [DOI] [PubMed] [Google Scholar]
- Crews F, He J, Hodge C. Adolescent cortical development: A critical period of vulnerability for addiction. Pharmacology, Biochemistry and Behavior. 2007;86:189–199. doi: 10.1016/j.pbb.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Mdzinarishvili A, Kim D, He J, Nixon K. Neurogenesis in adolescent brain is potently inhibited by ethanol. Neuroscience. 2006;137:437–445. doi: 10.1016/j.neuroscience.2005.08.090. [DOI] [PubMed] [Google Scholar]
- Crews FT, Nixon K. Mechanisms of neurodegeneration and regeneration in alcoholism. Alcohol and Alcoholism. 2009;44:115–127. doi: 10.1093/alcalc/agn079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Qin L, Sheedy D, Vetreno RP, Zou J. High mobility group box 1/Toll-like receptor danger signaling increases brain neuroimmune activation in alcohol dependence. Biological Psychiatry. 2013;73:602–612. doi: 10.1016/j.biopsych.2012.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Zou J, Qin L. Induction of innate immune genes in brain create the neurobiology of addiction. Brain, Behavior, and Immunity. 2011;25(Suppl 1):S4–S12. doi: 10.1016/j.bbi.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson DA, Stinson FS, Chou SP, Grant BF. Three-year changes in adult risk drinking behavior in relation to the course of alcohol-use disorders. Journal of Studies on Alcohol and Drugs. 2008;69:866–877. doi: 10.15288/jsad.2008.69.866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshmukh A, Rosenbloom MJ, Pfefferbaum A, Sullivan EV. Clinical signs of cerebellar dysfunction in schizophrenia, alcoholism, and their comorbidity. Schizophrenia Research. 2002;57:281–291. doi: 10.1016/s0920-9964(01)00300-0. [DOI] [PubMed] [Google Scholar]
- Drake AI, Butters N, Shear PK, Smith TL, Bondi M, Irwin M, et al. Cognitive recovery with abstinence and its relationship to family history for alcoholism. Journal of Studies on Alcohol. 1995;56:104–109. doi: 10.15288/jsa.1995.56.104. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Liu W, Wills DN, Crews FT. Periadolescent ethanol vapor exposure persistently reduces measures of hippocampal neurogenesis that are associated with behavioral outcomes in adulthood. Neuroscience. 2013;244:1–15. doi: 10.1016/j.neuroscience.2013.03.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers CL, Oguz I, Budin F, Wills DN, Crews FT. Peri-adolescent ethanol vapor exposure produces reductions in hippocampal volume that are correlated with deficits in prepulse inhibition of the startle. Alcoholism, Clinical and Experimental Research. 2013;37:1466–1475. doi: 10.1111/acer.12125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ende G, Walter S, Welzel H, Demirakca T, Wokrina T, Ruf M, et al. Alcohol consumption significantly influences the MR signal of frontal choline-containing compounds. NeuroImage. 2006;32:740–746. doi: 10.1016/j.neuroimage.2006.03.049. [DOI] [PubMed] [Google Scholar]
- Ernst M, Romeo RD, Andersen SL. Neurobiology of the development of motivated behaviors in adolescence: A window into a neural systems model. Pharmacology, Biochemistry, and Behavior. 2009;93:199–211. doi: 10.1016/j.pbb.2008.12.013. [DOI] [PubMed] [Google Scholar]
- Farina C, Aloisi F, Meinl E. Astrocytes are active players in cerebral innate immunity. Trends in Immunology. 2007;28:138–145. doi: 10.1016/j.it.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Fein G, Di Sclafani V, Cardenas VA, Goldmann H, Tolou-Shams M, Meyerhoff DJ. Cortical gray matter loss in treatment-naive alcohol dependent individuals. Alcoholism: Clinical and Experimental Research. 2002;26:558–564. [PMC free article] [PubMed] [Google Scholar]
- Fein G, Di Sclafani V, Finn P. Sensation seeking in long-term abstinent alcoholics, treatment-naive active alcoholics, and nonalcoholic controls. Alcoholism: Clinical and Experimental Research. 2010;34:1045–1051. doi: 10.1111/j.1530-0277.2010.01179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Lizarbe S, Pascual M, Guerri C. Critical role of TLR4 response in the activation of microglia induced by ethanol. Journal of Immunology. 2009;183:4733–4744. doi: 10.4049/jimmunol.0803590. [DOI] [PubMed] [Google Scholar]
- Ferrier L, Berard F, Debrauwer L, Chabo C, Langella P, Bueno L, et al. Impairment of the intestinal barrier by ethanol involves enteric microflora and mast cell activation in rodents. American Journal of Pathology. 2006;168:1148–1154. doi: 10.2353/ajpath.2006.050617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming RL, Acheson SK, Moore SD, Wilson WA, Swartzwelder HS. In the rat, chronic intermittent ethanol exposure during adolescence alters the ethanol sensitivity of tonic inhibition in adulthood. Alcoholism: Clinical and Experimental Research. 2012;36:279–285. doi: 10.1111/j.1530-0277.2011.01615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming RL, Li Q, Risher ML, Sexton HG, Moore SD, Wilson WA, et al. Binge-pattern ethanol exposure during adolescence, but not adulthood, causes persistent changes in GABAA receptor-mediated tonic inhibition in dentate granule cells. Alcoholism, Clinical and Experimental Research. 2013;37:1154–1160. doi: 10.1111/acer.12087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortier CB, Leritz EC, Salat DH, Venne JR, Maksimovskiy AL, Williams V, et al. Reduced cortical thickness in abstinent alcoholics and association with alcoholic behavior. Alcoholism, Clinical and Experimental Research. 2011;35:2193–2201. doi: 10.1111/j.1530-0277.2011.01576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage FH. Neurogenesis in the adult brain. Journal of Neuroscience. 2002;22:612–613. doi: 10.1523/JNEUROSCI.22-03-00612.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gansler DA, Harris GJ, Oscar-Berman M, Streeter C, Lewis RF, Ahmed I, et al. Hypoperfusion of inferior frontal brain regions in abstinent alcoholics: A pilot SPECT study. Journal of Studies on Alcohol. 2000;61:32–37. doi: 10.15288/jsa.2000.61.32. [DOI] [PubMed] [Google Scholar]
- Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330:841–845. doi: 10.1126/science.1194637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass CK, Saijo K, Winner B, Marchetto MC, Gage FH. Mechanisms underlying inflammation in neurodegeneration. Cell. 2010;140:918–934. doi: 10.1016/j.cell.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graeber MB. Changing face of microglia. Science. 2010;330:783–788. doi: 10.1126/science.1190929. [DOI] [PubMed] [Google Scholar]
- Grant BF. The impact of a family history of alcoholism on the relationship between age at onset of alcohol use and DSM-IV alcohol dependence: Results from the National Longitudinal Alcohol Epidemiologic Survey. Alcohol Health and Research World. 1998;22:144–147. [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Dawson DA. Age of onset of alcohol use and its association with DSM-IV alcohol abuse and dependence: Results from the national longitudinal alcohol epidemiologic survey. Journal of Substance Abuse. 1997;9:103–110. doi: 10.1016/s0899-3289(97)90009-2. [DOI] [PubMed] [Google Scholar]
- Hamelink C, Hampson A, Wink DA, Eiden LE, Eskay RL. Comparison of cannabidiol, antioxidants, and diuretics in reversing binge ethanol-induced neurotoxicity. Journal of Pharmacology and Experimental Therapeutics. 2005;314:780–788. doi: 10.1124/jpet.105.085779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson AC, Nixon K, Rimondini R, Damadzic R, Sommer WH, Eskay R, et al. Long-term suppression of forebrain neurogenesis and loss of neuronal progenitor cells following prolonged alcohol dependence in rats. International Journal of Neuropsychopharmacology/Official Scientific Journal of the Collegium Internationale Neuropsychopharmacologicum (CINP) 2010;13:583–593. doi: 10.1017/S1461145710000246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding AJ, Halliday GM, Ng JL, Harper CG, Kril JJ. Loss of vasopressin-immunoreactive neurons in alcoholics is dose-related and time-dependent. Neuroscience. 1996;72:699–708. doi: 10.1016/0306-4522(95)00577-3. [DOI] [PubMed] [Google Scholar]
- Hardingham GE, Bading H. Synaptic versus extrasynaptic NMDA receptor signalling: Implications for neurodegenerative disorders. Nature reviews. Neuroscience. 2010;11:682–696. doi: 10.1038/nrn2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper C, Kril J, Daly J. Are we drinking our neurones away? British Medical Journal (Clinical Research Ed) 1987;294:534–536. doi: 10.1136/bmj.294.6571.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J, Crews F. Neurogenesis decreases during brain maturation from adolescence to adulthood. Pharmacology, Biochemistry, and Behavior. 2007a;86(2):327–333. doi: 10.1016/j.pbb.2006.11.003. [DOI] [PubMed] [Google Scholar]
- He J, Crews FT. Neurogenesis decreases during brain maturation from adolescence to adulthood. Pharmacology, Biochemistry and Behavior. 2007b;86:327–333. doi: 10.1016/j.pbb.2006.11.003. [DOI] [PubMed] [Google Scholar]
- He J, Crews FT. Increased MCP-1 and microglia in various regions of the human alcoholic brain. Experimental Neurology. 2008;210:349–358. doi: 10.1016/j.expneurol.2007.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J, Nixon K, Shetty AK, Crews FT. Chronic alcohol exposure reduces hippocampal neurogenesis and dendritic growth of newborn neurons. European Journal of Neuroscience. 2005;21:2711–2720. doi: 10.1111/j.1460-9568.2005.04120.x. [DOI] [PubMed] [Google Scholar]
- Herrera DG, Yague AG, Johnsen-Soriano S, Bosch-Morell F, Collado-Morente L, Muriach M, et al. Selective impairment of hippocampal neurogenesis by chronic alcoholism: Protective effects of an antioxidant. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:7919–7924. doi: 10.1073/pnas.1230907100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh GS, Boulanger LM, Du H, Riquelme PA, Brotz TM, Shatz CJ. Functional requirement for class I MHC in CNS development and plasticity. Science. 2000;290:2155–2159. doi: 10.1126/science.290.5499.2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttunen HJ, Rauvala H. Amphoterin as an extracellular regulator of cell motility: From discovery to disease. Journal of Internal Medicine. 2004;255:351–366. doi: 10.1111/j.1365-2796.2003.01301.x. [DOI] [PubMed] [Google Scholar]
- Iori V, Maroso M, Rizzi M, Iyer AM, Vertemara R, Carli M, et al. Receptor for advanced glycation end products is upregulated in temporal lobe epilepsy and contributes to experimental seizures. Neurobiology of Disease. 2013;58:102–114. doi: 10.1016/j.nbd.2013.03.006. http://dx.doi.org/10.1016/j.nbd.2013.03.006. [DOI] [PubMed] [Google Scholar]
- Jang MH, Shin MC, Jung SB, Lee TH, Bahn GH, Kwon YK, et al. Alcohol and nicotine reduce cell proliferation and enhance apoptosis in dentate gyrus. Neuroreport. 2002;13:1509–1513. doi: 10.1097/00001756-200208270-00004. [DOI] [PubMed] [Google Scholar]
- Jang MH, Shin MC, Kim EH, Kim CJ. Acute alcohol intoxication decreases cell proliferation and nitric oxide synthase expression in dentate gyrus of rats. Toxicology Letters. 2002;133:255–262. doi: 10.1016/s0378-4274(02)00129-7. [DOI] [PubMed] [Google Scholar]
- Kim JB, Sig Choi J, Yu YM, Nam K, Piao CS, Kim SW, et al. HMGB1, a novel cytokine-like mediator linking acute neuronal death and delayed neuroinflammation in the postischemic brain. Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2006;26:6413–6421. doi: 10.1523/JNEUROSCI.3815-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp DJ, Crews FT. Induction of cyclooxygenase-2 in brain during acute and chronic ethanol treatment and ethanol withdrawal. Alcoholism: Clinical and Experimental Research. 1999;23:633–643. [PubMed] [Google Scholar]
- Kreutzberg GW. Microglia: A sensor for pathological events in the CNS. Trends in Neurosciences. 1996;19:312–318. doi: 10.1016/0166-2236(96)10049-7. [DOI] [PubMed] [Google Scholar]
- Kubota M, Nakazaki S, Hirai S, Saeki N, Yamaura A, Kusaka T. Alcohol consumption and frontal lobe shrinkage: Study of 1432 non-alcoholic subjects. Journal of Neurology, Neurosurgery and Psychiatry. 2001;71:104–106. doi: 10.1136/jnnp.71.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land C, Spear NE. Fear conditioning is impaired in adult rats by ethanol doses that do not affect periadolescents. International Journal of Developmental Neuroscience: The Official Journal of the International Society for Developmental Neuroscience. 2004;22:355–362. doi: 10.1016/j.ijdevneu.2004.04.008. [DOI] [PubMed] [Google Scholar]
- Lee JK, McCoy MK, Harms AS, Ruhn KA, Gold SJ, Tansey MG. Regulator of G-protein signaling 10 promotes dopaminergic neuron survival via regulation of the microglial inflammatory response. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2008;28(34):8517–8528. doi: 10.1523/JNEUROSCI.1806-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li TK. Quantifying the risk for alcohol-use and alcohol-attributable health disorders: Present findings and future research needs. Journal of Gastroenterology and Hepatology. 2008;23(Suppl 1):S2–S8. doi: 10.1111/j.1440-1746.2007.05298.x. [DOI] [PubMed] [Google Scholar]
- Li Q, Fleming RL, Acheson SK, Madison RD, Moore SD, Risher ML, et al. Long-term modulation of A-type K(+) conductances in hippocampal CA1 inter-neurons in rats after chronic intermittent ethanol exposure during adolescence or adulthood. Alcoholism: Clinical and Experimental Research. 2013;37:2074–2085. doi: 10.1111/acer.12204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little PJ, Kuhn CM, Wilson WA, Swartzwelder HS. Differential effects of ethanol in adolescent and adult rats. Alcoholism: Clinical and Experimental Research. 1996;20:1346–1351. doi: 10.1111/j.1530-0277.1996.tb01133.x. [DOI] [PubMed] [Google Scholar]
- Liu J, Yang AR, Kelly T, Puche A, Esoqa C, June HL, Jr, et al. Binge alcohol drinking is associated with GABAA alpha2-regulated Toll-like receptor 4 (TLR4) expression in the central amygdala. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(11):4465–4470. doi: 10.1073/pnas.1019020108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonze BE, Ginty DD. Function and regulation of CREB family transcription factors in the nervous system. Neuron. 2002;35:605–623. doi: 10.1016/s0896-6273(02)00828-0. [DOI] [PubMed] [Google Scholar]
- Mantamadiotis T, Lemberger T, Bleckmann SC, Kern H, Kretz O, Martin Villalba A, et al. Disruption of CREB function in brain leads to neurodegeneration. Nature Genetics. 2002;31:47–54. doi: 10.1038/ng882. [DOI] [PubMed] [Google Scholar]
- Markwiese BJ, Acheson SK, Levin ED, Wilson WA, Swartzwelder HS. Differential effects of ethanol on memory in adolescent and adult rats. Alcoholism: Clinical and Experimental Research. 1998;22:416–421. [PubMed] [Google Scholar]
- Maroso M, Balosso S, Ravizza T, Liu J, Aronica E, Iyer AM, et al. Toll-like receptor 4 and high-mobility group box-1 are involved in ictogenesis and can be targeted to reduce seizures. Nature Medicine. 2010;16:413–419. doi: 10.1038/nm.2127. [DOI] [PubMed] [Google Scholar]
- Marshall SA, McClain JA, Kelso ML, Hopkins DM, Pauly JR, Nixon K. Microglial activation is not equivalent to neuroinflammation in alcohol-induced neurodegeneration: The importance of microglia phenotype. Neurobiology of Disease. 2013;54:239–251. doi: 10.1016/j.nbd.2012.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin D, Tayyeb MI, Swartzwelder HS. Ethanol inhibition of AMPA and kainate receptor-mediated depolarizations of hippocampal area CA1. Alcoholism: Clinical and Experimental Research. 1995;19:1312–1316. doi: 10.1111/j.1530-0277.1995.tb01617.x. [DOI] [PubMed] [Google Scholar]
- Medzhitov R. Toll-like receptors and innate immunity. Nature Reviews. Immunology. 2001;1:135–145. doi: 10.1038/35100529. [DOI] [PubMed] [Google Scholar]
- Michelucci A, Heurtaux T, Grandbarbe L, Morga E, Heuschling P. Characterization of the microglial phenotype under specific pro-inflammatory and anti-inflammatory conditions: Effects of oligomeric and fibrillar amyloid-beta. Journal of Neuroimmunology. 2009;210:3–12. doi: 10.1016/j.jneuroim.2009.02.003. [DOI] [PubMed] [Google Scholar]
- Nixon K, Crews FT. Binge ethanol exposure decreases neurogenesis in adult rat hippocampus. Journal of Neurochemistry. 2002;83:1087–1093. doi: 10.1046/j.1471-4159.2002.01214.x. [DOI] [PubMed] [Google Scholar]
- Nixon K, Crews FT. Temporally specific burst in cell proliferation increases hippocampal neurogenesis in protracted abstinence from alcohol. Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2004;24:9714–9722. doi: 10.1523/JNEUROSCI.3063-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obernier JA, Bouldin TW, Crews FT. Binge ethanol exposure in adult rats causes necrotic cell death. Alcoholism: Clinical and Experimental Research. 2002;26:547–557. [PubMed] [Google Scholar]
- Obernier JA, White AM, Swartzwelder HS, Crews FT. Cognitive deficits and CNS damage after a 4-day binge ethanol exposure in rats. Pharmacology, Biochemistry and Behavior. 2002;72:521–532. doi: 10.1016/s0091-3057(02)00715-3. [DOI] [PubMed] [Google Scholar]
- O’Brien CP. Evidence-based treatments of addiction. Philosophical Transactions of the Royal Society of London, Series B: Biological Sciences. 2008;363:3277–3286. doi: 10.1098/rstb.2008.0105. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okvist A, Johansson S, Kuzmin A, Bazov I, Merino-Martinez R, Ponomarev I, et al. Neuroadaptations in human chronic alcoholics: dysregulation of the NF-kappB system. PLoS One. 2007;2(9):e930. doi: 10.1371/journal.pone.0000930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill J, Cardenas VA, Meyerhoff DJ. Effects of abstinence on the brain: Quantitative magnetic resonance imaging and magnetic resonance spectroscopic imaging in chronic alcohol abuse. Alcoholism: Clinical and Experimental Research. 2001;25:1673–1682. [PubMed] [Google Scholar]
- O’Neill LA, Kaltschmidt C. NF-kappa B: A crucial transcription factor for glial and neuronal cell function. Trends in Neurosciences. 1997;20:252–258. doi: 10.1016/s0166-2236(96)01035-1. [DOI] [PubMed] [Google Scholar]
- Oscar-Berman M, Hutner N. Frontal lobe changes after chronic alcohol ingestion. In: Hunt WA, Nixon SJ, editors. Alcohol-induced brain damage. Rockville: NIAAA/NIH; 1993. [Google Scholar]
- Oscar-Berman M, Marinkovic K. Alcohol: Effects on neurobehavioral functions and the brain. Neuropsychology Review. 2007;17:239–257. doi: 10.1007/s11065-007-9038-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterholm MT. Preparing for the next pandemic. New England Journal of Medicine. 2005;352:1839–1842. doi: 10.1056/NEJMp058068. [DOI] [PubMed] [Google Scholar]
- Pandey SC, Roy A, Mittal N. Effects of chronic ethanol intake and its withdrawal on the expression and phosphorylation of the creb gene transcription factor in rat cortex. Journal of Pharmacology and Experimental Therapeutics. 2001;296:857–868. [PubMed] [Google Scholar]
- Pandey SC, Zhang D, Mittal N, Nayyar D. Potential role of the gene transcription factor cyclic AMP-responsive element binding protein in ethanol withdrawal-related anxiety. Journal of Pharmacology and Experimental Therapeutics. 1999;288:866–878. [PubMed] [Google Scholar]
- Parsons OA. Impaired neuropsychological cognitive functioning in sober alcoholics. In: Hunt WA, Nixon SJ, editors. Alcohol-induced brain damage. Rockville: NIAAA/NIH; 1993. [Google Scholar]
- Pascual M, Balino P, Alfonso-Loeches S, Aragon CM, Guerri C. Impact of TLR4 on behavioral and cognitive dysfunctions associated with alcohol-induced neuroinflammatory damage. Brain, Behavior, and Immunity. 2011;25(Suppl 1):S80–S91. doi: 10.1016/j.bbi.2011.02.012. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Mathalon DH, Shear PK, Rosenbloom MJ, Lim KO. Longitudinal changes in magnetic resonance imaging brain volumes in abstinent and relapsed alcoholics. Alcohol, Clinical and Experimental Research. 1995;19:1177–1191. doi: 10.1111/j.1530-0277.1995.tb01598.x. [DOI] [PubMed] [Google Scholar]
- Potenza MN, De Wit H. Control yourself: Alcohol and impulsivity. Alcoholism, Clinical and Experimental Research. 2010;34:1303–1305. doi: 10.1111/j.1530-0277.2010.01214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruett SB, Zheng Q, Fan R, Matthews K, Schwab C. Ethanol suppresses cytokine responses induced through Toll-like receptors as well as innate resistance to Escherichia coli in a mouse model for binge drinking. Alcohol. 2004;33:147–155. doi: 10.1016/j.alcohol.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Qin L, Crews FT. Chronic ethanol increases systemic TLR3 agonist-induced neuroinflammation and neurodegeneration. Journal of Neuroinflammation. 2012a;9:130. doi: 10.1186/1742-2094-9-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin L, Crews FT. NADPH oxidase and reactive oxygen species contribute to alcohol-induced microglial activation and neurodegeneration. Journal of Neuroinflammation. 2012b;9:5. doi: 10.1186/1742-2094-9-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin L, He J, Hanes RN, Pluzarev O, Hong JS, Crews FT. Increased systemic and brain cytokine production and neuroinflammation by endotoxin following ethanol treatment. Journal of Neuroinflammation. 2008;5:10. doi: 10.1186/1742-2094-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin L, Liu Y, Hong JS, Crews FT. NADPH oxidase and aging drive micro-glial activation, oxidative stress, and dopaminergic neurodegeneration following systemic LPS administration. Glia. 2013;61:855–868. doi: 10.1002/glia.22479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin L, Wu X, Block ML, Liu Y, Breese GR, Hong JS, et al. Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia. 2007;55:453–462. doi: 10.1002/glia.20467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quartin AA, Schein RM, Kett DH, Peduzzi PN. Magnitude and duration of the effect of sepsis on survival Department of Veterans Affairs Systemic Sepsis Cooperative Studies Group. JAMA: The Journal of the American Medical Association. 1997;277:1058–1063. [PubMed] [Google Scholar]
- Raivich G. Like cops on the beat: The active role of resting microglia. Trends in Neurosciences. 2005;28:571–573. doi: 10.1016/j.tins.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Raivich G, Bohatschek M, Kloss CU, Werner A, Jones LL, Kreutzberg GW. Neuroglial activation repertoire in the injured brain: Graded response, molecular mechanisms and cues to physiological function. Brain Research. Brain Research Reviews. 1999;30:77–105. doi: 10.1016/s0165-0173(99)00007-7. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Roesch MR, Stalnaker TA, Takahashi YK. A new perspective on the role of the orbitofrontal cortex in adaptive behaviour. Nature Reviews. Neuroscience. 2009;10:885–892. doi: 10.1038/nrn2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz JM, Bilbo SD. Adolescent morphine exposure affects long-term microglial function and later-life relapse liability in a model of addiction. Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2013;33:961–971. doi: 10.1523/JNEUROSCI.2516-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton MD, Parsons OA. Alcoholics’ self-assessment of their neuropsychological functioning in everyday life. Journal of Clinical Psychology. 1987;43:395–403. doi: 10.1002/1097-4679(198705)43:3<395::aid-jclp2270430314>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Sher KJ, Gotham HJ. Pathological alcohol involvement: A developmental disorder of young adulthood. Development and Psychopathology. 1999;11:933–956. doi: 10.1017/s0954579499002394. [DOI] [PubMed] [Google Scholar]
- Silveri MM, Spear LP. Decreased sensitivity to the hypnotic effects of ethanol early in ontogeny. Alcoholism: Clinical and Experimental Research. 1998;22:670–676. doi: 10.1111/j.1530-0277.1998.tb04310.x. [DOI] [PubMed] [Google Scholar]
- Sims GP, Rowe DC, Rietdijk ST, Herbst R, Coyle AJ. HMGB1 and RAGE in inflammation and cancer. Annual Review of Immunology. 2010;28:367–388. doi: 10.1146/annurev.immunol.021908.132603. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Leonard CM, Welcome SE, Kan E, Toga AW. Longitudinal mapping of cortical thickness and brain growth in normal children. Journal of Neuroscience. 2004;24:8223–8231. doi: 10.1523/JNEUROSCI.1798-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neuroscience and Biobehavioral Reviews. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Streit WJ, Mrak RE, Griffin WS. Microglia and neuroinflammation: A pathological perspective. Journal of Neuroinflammation. 2004;1:14. doi: 10.1186/1742-2094-1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EV, Adalsteinsson E, Sood R, Mayer D, Bell R, Mcbride W, et al. Longitudinal brain magnetic resonance imaging study of the alcohol-preferring rat. Part I: Adult brain growth. Alcoholism: Clinical and Experimental Research. 2006;30:1234–1247. doi: 10.1111/j.1530-0277.2006.00145.x. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Pfefferbaum A. Neurocircuitry in alcoholism: A substrate of disruption and repair. Psychopharmacology. 2005;180:583–594. doi: 10.1007/s00213-005-2267-6. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Rosenbloom MJ, Pfefferbaum A. Pattern of motor and cognitive deficits in detoxified alcoholic men. Alcoholism: Clinical and Experimental Research. 2000;24:611–621. [PubMed] [Google Scholar]
- Suvarna N, Borgland SL, Wang J, Phamluong K, Auberson YP, Bonci A, et al. Ethanol alters trafficking and functional N-methyl-D-aspartate receptor NR2 subunit ratio via H-Ras. Journal of Biological Chemistry. 2005;280:31450–31459. doi: 10.1074/jbc.M504120200. [DOI] [PubMed] [Google Scholar]
- Swartzwelder HS, Hogan A, Risher ML, Swartzwelder RA, Wilson WA, Acheson SK. Effect of sub-chronic intermittent ethanol exposure on spatial learning and ethanol sensitivity in adolescent and adult rats. Alcohol. 2014;48(4):353–360. doi: 10.1016/j.alcohol.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartzwelder HS, Wilson WA, Tayyeb MI. Differential sensitivity of NMDA receptor-mediated synaptic potentials to ethanol in immature versus mature hippocampus. Alcoholism: Clinical and Experimental Research. 1995;19:320–323. doi: 10.1111/j.1530-0277.1995.tb01509.x. [DOI] [PubMed] [Google Scholar]
- Szabo G, Mandrekar P. A recent perspective on alcohol, immunity, and host defense. Alcoholism: Clinical and Experimental Research. 2009;33:220–232. doi: 10.1111/j.1530-0277.2008.00842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo G, Mandrekar P, Catalano D. Inhibition of superantigen-induced T cell proliferation and monocyte IL-1 beta, TNF-alpha, and IL-6 production by acute ethanol treatment. Journal of Leukocyte Biology. 1995;58:342–350. doi: 10.1002/jlb.58.3.342. [DOI] [PubMed] [Google Scholar]
- Szabo G, Mandrekar P, Dolganiuc A, Catalano D, Kodys K. Reduced alloreactive T-cell activation after alcohol intake is due to impaired monocyte accessory cell function and correlates with elevated IL-10, IL-13, and decreased IFNgamma levels. Alcoholism: Clinical and Experimental Research. 2001;25:1766–1772. [PubMed] [Google Scholar]
- Szabo G, Verma B, Catalano D. Selective inhibition of antigen-specific T lymphocyte proliferation by acute ethanol exposure: The role of impaired monocyte antigen presentation capacity and mediator production. Journal of Leukocyte Biology. 1993;54:534–544. doi: 10.1002/jlb.54.6.534. [DOI] [PubMed] [Google Scholar]
- Takeda K, Kaisho T, Akira S. Toll-like receptors. Annual Review of Immunology. 2003;21:335–376. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- Valles SL, Blanco AM, Pascual M, Guerri C. Chronic ethanol treatment enhances inflammatory mediators and cell death in the brain and in astrocytes. Brain Pathology. 2004;14:365–371. doi: 10.1111/j.1750-3639.2004.tb00079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Acute effects of ethanol on social behavior of adolescent and adult rats: Role of familiarity of the test situation. Alcoholism: Clinical and Experimental Research. 2002;26:1502–1511. doi: 10.1097/01.ALC.0000034033.95701.E3. [DOI] [PubMed] [Google Scholar]
- Vetreno RP, Crews FT. Adolescent binge drinking increases expression of the danger signal receptor agonist HMGB1 and Toll-like receptors in the adult prefrontal cortex. Neuroscience. 2012;226:475–488. doi: 10.1016/j.neuroscience.2012.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetreno RP, Qin L, Crews FT. Increased receptor for advanced glycation end product expression in the human alcoholic prefrontal cortex is linked to adolescent drinking. Neurobiology of Disease. 2013;59:52–62. doi: 10.1016/j.nbd.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezzani A, Maroso M, Balosso S, Sanchez MA, Bartfai T. IL-1 receptor/Toll-like receptor signaling in infection, inflammation, stress and neurodegeneration couples hyperexcitability and seizures. Brain, Behavior, and Immunity. 2011;25:1281–1289. doi: 10.1016/j.bbi.2011.03.018. [DOI] [PubMed] [Google Scholar]
- Viviani B, Bartesaghi S, Gardoni F, Vezzani A, Behrens MM, Bartfai T, et al. Interleukin-1beta enhances NMDA receptor-mediated intracellular calcium increase through activation of the Src family of kinases. Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2003;23:8692–8700. doi: 10.1523/JNEUROSCI.23-25-08692.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Yang H, Czura CJ, Sama AE, Tracey KJ. HMGB1 as a late mediator of lethal systemic inflammation. American Journal of Respiratory and Critical Care Medicine. 2001;164:1768–1773. doi: 10.1164/ajrccm.164.10.2106117. [DOI] [PubMed] [Google Scholar]
- Watkins LR, Maier SF, Goehler LE. Cytokine-to-brain communication: A review & analysis of alternative mechanisms. Life Sciences. 1995;57:1011–1026. doi: 10.1016/0024-3205(95)02047-m. [DOI] [PubMed] [Google Scholar]
- White AM, Bae JG, Truesdale MC, Ahmad S, Wilson WA, Swartzwelder HS. Chronic-intermittent ethanol exposure during adolescence prevents normal developmental changes in sensitivity to ethanol-induced motor impairments. Alcoholism: Clinical and Experimental Research. 2002;26:960–968. doi: 10.1097/01.ALC.0000021334.47130.F9. [DOI] [PubMed] [Google Scholar]
- White AM, Ghia AJ, Levin ED, Swartzwelder HS. Binge pattern ethanol exposure in adolescent and adult rats: Differential impact on subsequent responsiveness to ethanol. Alcoholism: Clinical and Experimental Research. 2000;24:1251–1256. [PubMed] [Google Scholar]
- White AM, Truesdale MC, Bae JG, Ahmad S, Wilson WA, Best PJ, et al. Differential effects of ethanol on motor coordination in adolescent and adult rats. Pharmacology, Biochemistry, and Behavior. 2002;73:673–677. doi: 10.1016/s0091-3057(02)00860-2. [DOI] [PubMed] [Google Scholar]
- Yan H, Li Q, Fleming R, Madison RD, Wilson WA, Swartzwelder HS. Developmental sensitivity of hippocampal interneurons to ethanol: Involvement of the hyperpolarization-activated current, Ih. Journal of Neurophysiology. 2009;101:67–83. doi: 10.1152/jn.90557.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan H, Li Q, Madison R, Wilson WA, Swartzwelder HS. Differential sensitivity of hippocampal interneurons to ethanol in adolescent and adult rats. Journal of Pharmacology and Experimental Therapeutics. 2010;335:51–60. doi: 10.1124/jpet.110.168450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou JY, Crews FT. TNF alpha potentiates glutamate neurotoxicity by inhibiting glutamate uptake in organotypic brain slice cultures: Neuroprotection by NF kappa B inhibition. Brain Research. 2005;1034:11–24. doi: 10.1016/j.brainres.2004.11.014. [DOI] [PubMed] [Google Scholar]
- Zou J, Crews F. CREB and NF-kappaB transcription factors regulate sensitivity to excitotoxic and oxidative stress induced neuronal cell death. Cellular and Molecular Neurobiology. 2006;26:385–405. doi: 10.1007/s10571-006-9045-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou J, Crews F. Induction of innate immune gene expression cascades in brain slice cultures by ethanol: Key role of NF-kappaB and proinflammatory cytokines. Alcoholism: Clinical and Experimental Research. 2010;34:777–789. doi: 10.1111/j.1530-0277.2010.01150.x. [DOI] [PubMed] [Google Scholar]
- Zou J, Crews FT. Inflammasome-IL-1beta signaling mediates ethanol inhibition of hippocampal neurogenesis. Frontiers in Neuroscience. 2012;6:77. doi: 10.3389/fnins.2012.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou JY, Crews FT. Release of neuronal HMGB1 by ethanol through decreased HDAC activity activates brain neuroimmune signaling. PLoS One. 2014;9:e87915. doi: 10.1371/journal.pone.0087915. [DOI] [PMC free article] [PubMed] [Google Scholar]