Abstract
The collection of evolutionary transformations known as the ‘major transitions’ or ‘transitions in individuality’ resulted in changes in the units of evolution and in the hierarchical structure of cellular life. Volvox and related algae have become an important model system for the major transition from unicellular to multicellular life, which touches on several fundamental questions in evolutionary biology. The Third International Volvox Conference was held at the University of Cambridge in August 2015 to discuss recent advances in the biology and evolution of this group of algae. Here, I highlight the benefits of integrating phylogenetic comparative methods and experimental evolution with detailed studies of developmental genetics in a model system with substantial genetic and genomic resources. I summarize recent research on Volvox and its relatives and comment on its implications for the genomic changes underlying major evolutionary transitions, evolution and development of complex traits, evolution of sex and sexes, evolution of cellular differentiation and the biophysics of motility. Finally, I outline challenges and suggest future directions for research into the biology and evolution of the volvocine algae.
Keywords: algae, biophysics, evolution of sex, major transitions, multicellularity, Volvox
Introduction
As a historical science, evolutionary biology relies heavily on comparative methods to infer when, how, and why changes in organismal form and function have occurred. An integration of phylogenetic, developmental, biomechanical, experimental and genomic approaches to the biology of a diverse lineage can be invaluable within such a framework. Wake (2009) argued for the value of such ‘taxon-centred’ research, and one clade in which it has proven its value is the volvocine green algae, that is, Volvox and its relatives. The Third International Volvox Conference in Cambridge, UK, recently highlighted advances in volvocine biology, the implications of which are relevant to some of the most intractable problems in evolutionary biology.
The volvocine algae include two model organisms that represent the extremes of size and complexity within the clade, unicellular Chlamydomonas reinhardtii and multicellular Volvox carteri forma nagariensis (Fig. 1). Each has a sequenced and annotated genome (Merchant et al. 2007; Prochnik et al. 2010), established protocols for classical genetics (Kirk 1998; Harris 2009) and techniques for stable nuclear transformation (Kindle 1990; Schiedlmeier et al. 1994; Shimogawara et al. 1998; Yamano et al. 2013). The relative simplicity of multicellular organization in V. carteri, with only two cell types, has long made it an attractive system for understanding the evolution of multicellularity and cellular differentiation (e.g. Weismann 1889, 1904; Huxley 1912).
Fig. 1.
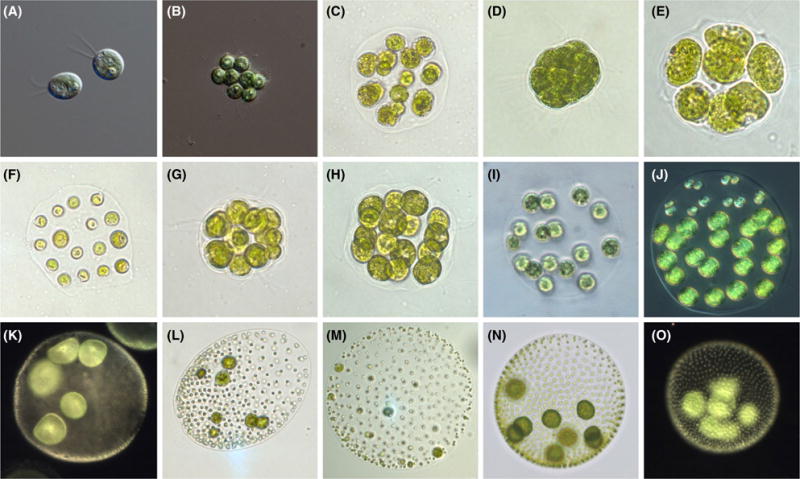
Examples of volvocine species. (A) Chlamydomonas reinhardtii, (B) Gonium pectorale, (C) Astrephomene gubernaculiferum, (D) Pandorina morum, (E) Volvulina compacta, (F) Platydorina caudata, (G) Yamagishiella unicocca, (H) Colemanosphaera charkowiensis, (I) Eudorina elegans, (J) Pleodorina starrii, (K) Volvox barberi, (L) Volvox ovalis, (M) Volvox gigas, (N) Volvox aureus, (O) Volvox carteri. Figure Credit for A and B: Deborah Shelton.
However, the real strength of the volvocine algae as a model system lies not in comparisons between the extremes of unicellular and fully differentiated multicellular species, but in the diversity of species of intermediate size and complexity (Box 1). Cell numbers span five orders of magnitude, with nearly every power of two from 20 to 215 represented. Sizes span a similar range, with organismal volumes from hundreds to billions of μm3. Colonial organization is also highly variable, with body plans including simple clumps of cells, flat or curved sheets, and spheroids with and without cellular differentiation (Fig. 1). Furthermore, some of these traits had multiple independent origins within the volvocine clade (Herron & Michod 2008; Herron et al. 2010), making meaningful comparisons of convergent evolutionary trends possible.
Box 1. Diversity and natural history of the volvocine algae.
The ‘great round particles’ that would eventually be known as Volvox were first described by van Leeuwenhoek (1700, p. 511), who found them ‘a very pleasant sight’, in part because he ‘did fancy at first that they were small animals’. This fancy was shared by Linnaeus, who gave them the name Volvox (‘fierce roller’) and classified them in the order Zoophyta within the class Vermes, along with Cnidarians, Bryozoa and tapeworms (Linnaeus 1758, pp. 821–822). Eleven additional genera of volvocine algae are distinguished on the basis of cell number and organization, cellular differentiation and sexual characters (Table 1).
Typical volvocine habitats are still, fresh water, but they have also been found in rivers, soil, ice and snow. Known ranges are from a single pond to nearly cosmopolitan, but most are probably underestimates. Systematic sampling has been rare, and most species are present in appreciable numbers only in brief, early summer blooms. The known diversity of around 50 extant species is also likely to be an underestimate. New species are being discovered at an alarming rate, including one at this meeting, when Hisayoshi Nozaki described a new species of Volvox from strains formerly considered synonymous with Volvox africanus (Nozaki et al. 2015).
Table 1.
Taxonomy of colonial volvocine algae. Numbers of cells are restricted to powers of 2 (with the possible exception of Volvox), so, for example, ‘8–32’ should be understood as 8, 16 or 32 cells
| Family | Genus | Gametes | No. of cells | Differentiated cells | % somatic | Morphology |
|---|---|---|---|---|---|---|
| Tetrabaenaceae | Basichlamys | Isogamy | 4 | No | 0 | Cluster |
| Tetrabaena | Isogamy | 4 | No | 0 | Cluster | |
| Goniaceae | Astrephomene | Isogamy | 32–128 | Somatic | 6–12 | Spheroid |
| Gonium | Isogamy | 8–32 | No | 0 | Flat/bowl | |
| Volvocaceae | Colemanosphaera | Anisogamy* | 16–32 | No | 0 | Spheroid |
| Eudorina | Anisogamy | 16–32 | No | 0 | Spheroid | |
| Pandorina | Anisogamy | 16–32 | No | 0 | Spheroid | |
| Platydorina | Isogamy | 16–32 | No | 0 | Flattened† | |
| Pleodorina | Anisogamy | 32–128 | Somatic | 12–50 | Spheroid | |
| Volvox | Oogamy | 500–50000 | Somatic and germ | 98+ | Spheroid | |
| Volvulina | Isogamy | 8–16 | No | 0 | Spheroid | |
| Yamagishiella | Isogamy | 16–32 | No | 0 | Spheroid |
Colemanosphaera is anisogamous with external fertilization; all other anisogamous species undergo internal fertilization (Nozaki et al. 2014).
Platydorina develops as a spheroid, undergoing complete inversion, but is secondarily flattened.
The volvocine algae have several advantages as a model system. Multicellularity has evolved dozens of times across the tree of life, but in most cases evidence of the earliest steps in the transition to multicellular life have been lost due to extinction and to the limitations of the fossil record. In the volvocine algae, multicellularity is a relatively recent innovation (c. 200 MY; Herron et al. 2009), and a wealth of information about the changes involved has been retained in extant species with varied combinations of derived and ancestral characters. Experimental work is facilitated by their small size, ease of culture, short generations and prolific asexual reproduction. Haploid genomes and facultative sexuality that can in most cases be controlled by the investigator simplify genetic investigations. In addition to the two available genomes described above, several other genome projects are in progress (Umen & Olson 2012), and techniques for nuclear transformation have been successfully applied to several species beyond C. reinhardtii and V. carteri (Lerche & Hallmann 2009, 2013, 2014). A wide variety of species, geographic isolates and mutant strains are available in culture collections at the University of Texas (UTEX; www.utex.org), University of Minnesota (Chlamydomonas Resource Center; chlamycollection.org) and the National Institute of Environmental Studies in Japan (NIES; mcc.nies.go.jp).
Research using this system has provided novel insights into several fundamental evolutionary processes, including innovation and evolution of complex traits, transitions to multicellular life, the evolution of sex and sexes and the interplay of adaptive processes with biophysical constraints. Here, I report recent developments in evolutionary biology resulting from research on Volvox and related algae. In addition, I highlight challenges faced by the Volvox community and suggest promising future research directions.
Genomics of major transitions
Twenty years ago, John Maynard Smith and Eörs Szathmáry struggled to understand the general principles underlying increases in biological complexity (Maynard Smith & Szathmáry 1995; Szathmáry & Maynard Smith 1995). Although they rejected the idea of increased complexity as an inevitable outcome of natural selection, they nevertheless recognized that ‘…there is surely some sense in which elephants and oak trees are more complex than bacteria, and bacteria than the first replicating molecules’ (Maynard Smith & Szathmáry 1995, p. 3). They identified eight ‘major evolutionary transitions’, increases in biological complexity characterized by the common features of a shift in the unit of replication, division of labour and a change in the mode of information storage and/or transmission. These include the origins of cells, of genomes, of the genetic code, of eukaryotes, of sex, of differentiated multicellular organisms, of colonial superorganisms and of language.
The major transitions framework has become a paradigm for understanding the origins of biological complexity and the hierarchical structure of life (individuals within societies, cells within individuals, organelles within cells and so on). Biologists and philosophers of biology have sought to identify mechanisms underlying particular transitions and general principles that might apply to all. As a model system for the evolution of multicellularity, the volvocine algae have played a large role in these discussions.
One important component of the evolution of complexity is the origin of morphological novelty. The relative importance of mutations in protein-coding gene sequences vs. changes in gene regulation in explaining the evolution of morphological novelty is a subject of debate (e.g. Hoekstra & Coyne 2007; Carroll 2008). The evolution of multicellularity is surely an important source of morphological novelty; what, then, are the genetic mechanisms underlying this transition?
The answer seems to be ‘some of each’. The gene content of the C. reinhardtii and V. carteri genomes is nearly identical despite 200 million years of independent evolution (Merchant et al. 2007; Prochnik et al. 2010). In one sense, important differences between unicellular and differentiated multicellular organisms are bound to involve changes in gene expression: different cell types within a multicellular organism share the same genome, and differences among cell types must therefore involve differences in gene expression. And, in fact, some of the genes essential to Volvox developmental processes that do not occur in unicells, such as inversion and asymmetric division, are so similar that the Chlamydomonas gene can rescue Volvox mutants (Cheng et al. 2003; Nishii et al. 2003).
However, ‘nearly identical’ obscures some potentially important differences. Among the very few differences in gene family content between the two genomes are dramatic expansions of two families known to be involved in building the extracellular matrix in Volvox (Prochnik et al. 2010). Extracellular matrix is a complex organ in Volvox, making up >99% of colony volume and playing important roles in functions as diverse as cell orientation and sex induction (Gilles et al. 1983, 1984; Hallmann & Kirk 2000).
One possible explanation of the small number of differences between unicellular and multicellular genomes is that genes present in unicellular ancestors are recruited for functions related to multicellularity after the transition. A gene known to be involved in cell fate determination in V. carteri (regA) has a homologue in C. reinhardtii, where it temporarily suspends reproduction in response to adverse environmental conditions (Nedelcu & Michod 2004, 2006; Nedelcu 2009). Aurora Nedelcu (University of New Brunswick) reported on recent work showing that the expression of this homologue is affected by cell size, as is regA expression in V. carteri. The importance of this sort of co-option is consistent with findings of genes involved in multicellular functions in the unicellular relatives of animals (King et al. 2008; Manning et al. 2008; Pincus et al. 2008; Rokas 2008; Suga et al. 2013) and of plants (Nedelcu et al. 2006) and may be general to origins of multicellularity.
Erik Hanschen (University of Arizona) and Tara Marriage presented an update of the Volvocales Genome Project headed by Bradley Olson (Kansas State University), which aims to sequence the genomes of key species from all three families of colonial volvocine algae. The release of these additional genomes will substantially reduce uncertainty about which features are ancestral to the multicellular species and which are derived in the V. carteri lineage. Because they include species with and without several key developmental features, these additional genomes will greatly facilitate identification of the causative genetic changes. In addition, they will help to polarize differences between the C. reinhardtii and V. carteri genomes, for example whether a given difference in gene content represents a loss in one lineage or a gain in the other.
Development and evolution of complex traits
Nothing at first can appear more difficult to believe than that the more complex organs and instincts have been perfected, not by means superior to, though analogous with, human reason, but by the accumulation of innumerable slight variations, each good for the individual possessor.(Darwin 1872, p. 404)
Understanding the evolution of complex traits, if not the primary goal of evolutionary biology, is certainly one of the primary goals. And the development of multicellular body plans is among the most complex traits, or suites of traits, we might consider. One of the main attractions of Volvox as a model system is its relative simplicity: with thousands of cells rather than trillions, and two cell types rather than hundreds, a complete account of its origins from a unicellular ancestor seems much more tractable than of, say, animals or plants.
The history of the transition to multicellular life in the volvocine algae has been reconstructed in greater detail than for any other multicellular clade. Kirk (2005) has shown that the transition to differentiated multicellularity in this group can be broken down into a series of developmental changes, each plausibly adaptive, as Darwin did for the vertebrate eye (Darwin 1872; Nilsson & Pelger 1994; Dawkins 1997). In fact, we can say something a bit stronger than just ‘plausibly adaptive’, because most of the combinations of these traits that Kirk proposes actually exist in extant species of volvocine algae. In describing a major transition in terms of its component developmental changes, Kirk showed that the transition to multicellular life is neither irreducibly complex nor requires any saltational explanation. The evolutionary history of these and other developmental changes has been reconstructed in a time-calibrated phylogenetic framework, showing that some traits had multiple, independent origins, as well as reversions from derived back to ancestral states (Herron & Michod 2008; Herron 2009; Herron et al. 2009, 2010). Collectively, these studies make up the most complete history available for any of the major transitions in evolution (Fig. 2).
Fig. 2.
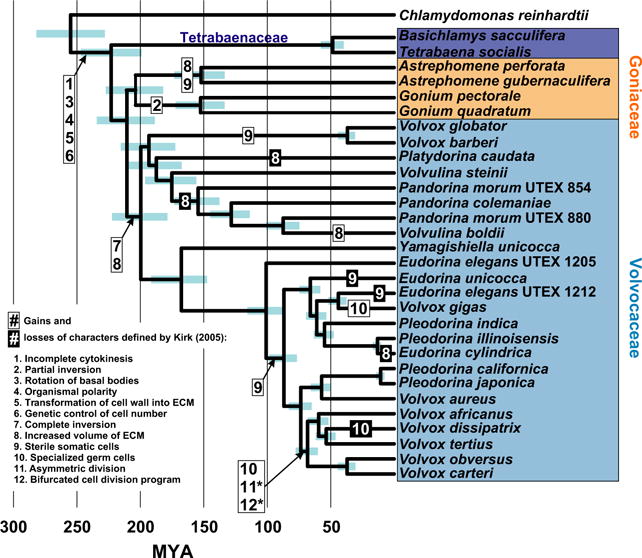
Inferred timeline of developmental evolution in the volvocine algae. Coloured boxes identify the 3 multicellular families. Blue bars are 95% Bayesian Credibility Intervals. Modified from Herron et al. (2009).
The four-celled Tetrabaenaceae (Basichlamys and Tetrabaena) are thought to be sister to the Goniaceae + Volvocaceae, and ancestral character state reconstructions suggest that the most recent common ancestor of the latter two families may have been similar to extant Tetrabaenaceae (Herron & Michod 2008). Yoko Arakaki (University of Tokyo) showed that, contrary to the assumptions underlying those reconstructions, Tetrabaena socialis has two traits previously thought to have originated in the Goniaceae + Volvocaceae. Using immunofluorescence microscopy and ultrastructural observations, Arakaki et al. (2013) showed that T. socialis cells are connected by cytoplasmic bridges and that their basal bodies are rotated so that their flagella beat in parallel. These observations suggest that the MRCA of the colonial volvocine algae may have been more highly integrated than previously suspected. Jonathan Featherston (University of the Witwatersrand) gave an update on the progress of the T. socialis genome, completion of which has the potential to teach us a great deal about the genetics underlying these changes.
Thomas Pröschold (Universidad de Concepción) showed evidence that some unicellular algae classified as Chlamydomonas and Vitreochlamys are derived from multicellular ancestors. If so, this would require a substantial revision of current views of volvocine evolution, as numerous phylogenetic studies have concluded that the colonial volvocines (Tetrabaena + Goniaceae + Volvocaceae) are monophyletic (e.g. Larson et al. 1992; Nozaki et al. 2000; Nozaki 2003; Herron & Michod 2008). None of these previous studies included as many unicellular taxa as Pröschold’s, but his reconstruction provided no clear resolution of backbone relationships, so these results should be treated cautiously.
All members of the family Volvocaceae (Pandorina, Volvulina, Yamagishiella, Platydorina, Eudorina, Pleodorina, Colemanosphaera and Volvox) undergo complete inversion during development. Inversion is crucial for these algae, as they find themselves in an awkward configuration at the end of cell division: with their flagella inside the colony. Cells move relative to the cytoplasmic bridges arising from incomplete cytokinesis, resulting in the entire colony turning inside-out (Marchant 1977; Viamontes & Kirk 1977). At the end of inversion, the flagella are on the surface of the colony, where they are useful for motility. This is a complex developmental process that requires a high degree of coordination among cells. Inversion in Volvox has often been compared to the process of gastrulation in animals, during which the blastula invaginates and forms the three germ layers: endoderm, mesoderm and ectoderm. The simpler process that occurs in Volvox serves as a more tractable model system that can nevertheless add to our understanding of the physical processes involved in gastrulation.
As inversion occurs in all known members of the Volvocaceae, and as the Volvocaceae are, by most indications, monophyletic, it seems probable that the progenitor of the Volvocaceae underwent inversion. It is quite surprising, then, that at least two very different forms of inversion occur within this family (Hallmann 2006; Höhn & Hallmann 2011). The ‘Type A’ inversion of V. carteri moves from anterior to posterior, beginning with the opening of the anterior phialopore, the opening through which the cell sheet moves (Hallmann 2006). The ‘Type B’ inversion of Volvox globator begins at the posterior, with the phialopore opening only after inversion of the posterior hemisphere (Hallmann 2006). In spite of this heterochronic change, the adult forms of V. carteri and V. globator are similar. Stephanie Höhn (University of Cambridge) and colleagues imaged V. globator during inversion using selective plane illumination microscopy and used the resulting measurements to fit a mathematical model of inversion (Höhn et al 2015). The model shows that changes in the curvature of the cell sheet are not enough to explain Type B inversion; contraction of the posterior and expansion of the anterior hemisphere are also needed. Pierre Haas (University of Cambridge) presented a mathematical model of the biomechanics of inversion, which revealed a complex interplay between local changes in cell shape and global geometric constraints imposed by the deformation of an elastic sphere (Haas & Goldstein 2015).
Given the orientation of the flagella at the end of cell division, it would be tempting to conclude that inversion is simply a result of a phylogenetic constraint—that it is the only way that large, motile, spheroidal colonies could have evolved in this group. It would be a tempting conclusion, that is, if it were not for the existence of Astrephomene. Astrephomene is a member of the Goniaceae, the sister group to the Volvocaceae, and manages to form colonies of up to 64 cells without the need for inversion. Shota Yamashita (University of Tokyo) has studied Astrephomene development in detail. Rather than inverting after cell division, Astrephomene achieves a radial arrangement through changes in the plane of cell division. Astrephomene probably represents an independent origin of spheroidal colonies from that in the Volvocaceae, and the fact that they can do this suggests that the configuration that necessitates inversion in the Volvocaceae is not inevitable, but probably more of a frozen accident.
In addition to retrospective approaches to understanding the evolution of multicellularity, several laboratory groups are using prospective strategies. Sarah Cossey (Kansas State University) reported an aggregative response of unicellular C. reinhardtii to the presence of the predator Daphnia. The small multicellular Gonium shows no such response, suggesting the possibility that multicellularity may have evolved through the genetic assimilation of a plastic response in an ancestral unicell. Tara Marriage (Kansas State University) used RNA-Seq to identify genes in Gonium implicated in cell-cycle control. By transforming one of these genes into wild-type C. reinhardtii, Marriage and colleagues were able to produce multicellular C. reinhardtii with cytoplasmic bridges among cells.
I showed recent results from C. reinhardtii evolution experiments. In two such experiments, we have observed the de novo evolution of simple multicellular structures from outcrossed starting populations. In the first experiment, simple multicellular structures evolved in response to selection for increased size by low-speed centrifugation (Ratcliff et al. 2013). Life cycle observations of these nascent multicellular organisms revealed an alternation of unicellular and multicellular stages (Ratcliff et al. 2013). A unicellular stage, or bottleneck, is present in nearly all multicellular life cycles, and it is usually regarded as an adaptation to prevent conflicts among cells (Dawkins 1976; Maynard Smith & Szathmáry 1995). Instead, these results suggest that the unicellular bottleneck was co-opted from the previously existing unicellular life cycle.
In the second experiment, colonial forms reminiscent of Pandorina or Volvulina evolved in response to predation by the ciliate Paramecium tetraurelia. The form of multicellularity observed differs substantially between experiments, suggesting that the particulars of the transition to multicellular life depend not only on the nature of the unicellular ancestor, but on the specific selective pressures driving the transition as well. Jillian Walker (Georgia Institute of Technology) presented life cycle observations on isolates from the predation experiment, which showed that multicellularity persists in the absence of predators and is therefore genetically fixed. In addition, these observations reveal that the life cycles and cell numbers of these isolates are well regulated, with little within-isolate variation.
Evolution of sex and sexes
Volvox and its relatives are an outstanding model for the evolution of sex and mating types. Volvocine algae are facultatively sexual, spanning a wide range of mating systems. Haploid vegetative colonies reproduce asexually through mitosis but occasionally enter a sexual cycle that usually results in a diploid, desiccation-resistant zygote or ‘spore’. Most of the small colonial species and unicellular relatives are isogamous, that is the gametes are of equal size. Nevertheless, each species has two self-incompatible mating types, usually designated as ‘plus’ and ‘minus’. In some of the larger species, the gametes have diverged into a small, motile form that we call sperm and a large, often immotile form that we call eggs. There is good reason to suspect that oogamy (sperm and eggs) has evolved independently from isogamy (equal-sized gametes) in two separate lineages of volvocine algae (or that one lineage has reverted to isogamy).
Furthermore, among the oogamous species, some are heterothallic (i.e. they have genetic sex determination) and some homothallic (i.e. a single genotype can produce both males and females). Among the homothallic species, some are dioecious (i.e. produce separate male and female colonies), others monoecious (i.e. hermaphroditic colonies produce both sperm and eggs) and still others produce both hermaphroditic and male colonies.
Males and females have evolved many times independently from different groups of isogamous ancestors, for example in animals, plants and various groups of multicellular algae. The genetics that determine maleness and femaleness are well understood in most of these groups, but their origins are much more mysterious. In fact, the volvocine algae are the only taxonomic group in which we know which isogamous mating type evolved into males and which into females (minus to male, plus to female; Nozaki et al. 2006).
Sa Geng (Danforth Plant Science Center) investigated the role of the MID gene, which determines isogamous mating type in Chlamydomonas, in mating type and sex determination in colonial species of volvocine algae. Geng’s previous work showed that MID evolved from an ancestral role in mating type determination to a similar role in the oogamous species V. carteri (Geng et al. 2014). By creating transgenic V. carteri strains expressing MID genes from other species, Geng and colleagues showed that MID from Gonium pectorale, an isogamous species, is nevertheless capable of inducing spermatogenesis. These results suggest that changes in gene regulatory networks controlled by MID proteins, rather than in the MID gene itself, underlay the evolution of males and females in the volvocine algae.
Hiroko Kawai-Toyooka (University of Tokyo) described the isolation of mating structures from G. pectorale, a small colonial volvocine alga. Like Chlamydomonas, Gonium is isogamous, and its gametes have cellular protrusions that function in gamete fusion. Chlamydomonas is a model system for gamete fusion, and these mating structures play an important role (Mori et al. 2015). Isolation of these structures is a first step towards characterizing proteins involved in gamete fusion, and it will be interesting to learn how conserved these proteins are across various volvocine species. Sex is likely an ancestral trait in eukaryotes (Goodenough & Heitman 2014), so the relevance of these results may extend beyond volvocine algae.
Takashi Hamaji (Danforth Plant Science Center) presented results of a global gene expression analysis of V. carteri. In both male and female strains, Hamaji and colleagues isolated and sequenced RNA from 64 time points distributed throughout the asexual and sexual life cycles. Genes showing both male-specific and female-specific expressions were identified from the RNA-Seq data, as well as genes expressed in both sexes. Male-specific genes were the most common class and included genes implicated in cell division, cell-cycle control and embryogenesis. The candidate genes thus identified provide clues to the genetic basis and evolution of spermatogenesis.
The outrageous variability of sexual systems in the volvocine algae makes them a valuable system for comparative studies with the potential to bear on one of the toughest problems in evolutionary biology. As with any model system, it is difficult to say how general and how system-specific any results are, but generalizations have to begin with individual data points, and the volvocine algae are emerging as one of the best understood examples of an evolutionary transition from isogamous to oogamous sexual reproduction. As other systems catch up, it will be interesting to see how much of what we have learned from the volvocines turns out to be common to such transitions.
Cellular differentiation
One of the most studied aspects of Volvox development is the differentiation of its 2000 or so cells into two types: a few (usually 12–16) large reproductive cells (germ) and the rest small, biflagellate cells that provide motility (soma). The main genes controlling this differentiation have long been known, but the details of how they control differentiation are still being worked out. Cellular differentiation in the form of sterile somatic cells has evolved at least three times within this clade, and at least two independent lineages evolved further specialization of the reproductive cells (Herron & Michod 2008; Herron et al. 2010).
Zach Grochau-Wright (University of Arizona) explored the evolutionary history of a cluster of genes that includes regA, a transcription factor crucial for the determination of somatic cells in V. carteri (Huskey & Griffin 1979; Kirk et al. 1999). Although Hanschen and colleagues recently showed that regA is present in several other species of Volvox, it is still unknown whether it plays a similar role in these or other volvocine species with cellular differentiation (i.e. Pleodorina and Astrephomene; Hanschen et al. 2014). Grochau-Wright et al. found regA and related genes in volvocine species with and without cellular differentiation, suggesting that important components of the genetic basis for differentiation preceded the evolution of differentiation itself. Whether or not regA controls somatic differentiation in other species is a crucial question. Somatic cells are thought to have evolved multiple times independently in the volvocine algae, and a finding that they are determined by the same genes across these lineages would mean that either the inferred phylogenetic relationships are wrong (and soma only evolved once) or that the repeated origins of somatic cells were not only morphologically convergent but genetically parallel as well. The latter possibility would be particularly surprising, because whatever the developmental basis of somatic determination is in, for example, the members of section Volvox (a.k.a. Euvolvox), Pleodorina, and Astrephomene, it is certainly not the same as in V. carteri.
It has been known for some time that regA prevents expression of genes required for germ cell development, but exactly which genes are differentially expressed in germ vs. somatic cells has not been fully worked out. Arash Kianionmomeni (Bielefeld University) showed that reproductive and somatic cells differ in their response to light (Kianianmomeni & Hallmann 2015). Specifically, the expression of photoreceptor genes differs between the two cell types, including some that are expressed only in one cell type or the other. Light responses serve several critical functions in volvocine life cycles, including phototaxis, control of photosynthesis and circadian control of the cell cycle. Understanding these differences is an important step in determining the mechanistic basis for at least some of the differences between cell types.
Gavriel Matt (Washington University in St. Louis) presented results from a genome-wide analysis comparing gene expression between reproductive and somatic cells. Using RNA-Seq, Matt and Jim Umen (Danforth Plant Science Center) have shown that genes upregulated in somatic cells are more likely than those upregulated in germ cells to be specific to the volvocine algae, suggesting the involvement of novel genes in somatic cell specification. In addition, genes involved in photosynthesis and chlorophyll biogenesis were expressed at appreciable levels in somatic cells. This is a surprising finding, as somatic cell specification is thought to result from suppression of chloroplast machinery by regA.
Alexey Desnitskiy (Saint Petersburg State University) gave a prerecorded talk on Volvox biogeography. Like most aspects of volvocine ecology, this is a topic about which we know surprisingly little. The nominal genus Volvox is almost certainly polyphyletic, with at least two and probably more than two independent origins within the Volvocaceae (Nozaki 2003; Herron et al. 2010). And while these separate lineages have converged on a common body plan, they have not always achieved this in the same way. Desnitskiy proposed that differences in light/dark control of cell division evolved as a result of latitudinal differences in day length during Mesozoic and early Cenozoic warm periods, when suitable Volvox habitat existed in high northern and southern latitudes.
Biophysics and motility
Volvox has proven to be an experimentally tractable model system for several areas of biological physics, including hydrodynamic interactions, flagellar motility, phototaxis, nutrient uptake and flagellar synchronization (reviewed in Goldstein 2015). Volvox colonies can be grown in large numbers (even by physicists!), clonal cultures have relatively little among-colony variation, and they are large enough to be manipulated in ways that most single-celled organisms cannot. Furthermore, their simple structure accommodates the kind of simplifying assumptions physicists are fond of, leading Kirsty Wan, among others at the meeting, to refer to them as ‘spherical cows’.
The ~2000 flagella of a V. carteri colony beat in a synchronized wave that travels from the anterior to the posterior of the colony. Amazingly, these ‘metachronal waves’ appear to result entirely from hydrodynamic coupling (Brumley et al. 2015). In other words, in spite of the apparent high degree of coordination among the flagella of separate cells within a colony, no actual communication among cells is necessary. Synchronization emerges from indirect interactions mediated by the liquid medium. Douglas Brumley (Massachusetts Institute of Technology) showed an elegant demonstration of this, in which somatic cells were physically separated from a colony and held at various distances from each other (Brumley et al. 2014). Despite there being no direct physical connection between the cells, they beat synchronously when close together, with a phase shift that increased with increasing cell-to-cell distance.
Phototaxis in V. carteri depends not only on coordination among flagella, but also on rotation of the colony as well. Swimming Volvox colonies rotate around their anterior–posterior axis, similar to the way a rifle bullet rotates (although likely for a different reason). This is accomplished by having the flagella beat not directly towards the posterior of the colony but at a ~20° angle to the axis of rotation (‘azimuthal offset’). Eyespots in the somatic cells of Volvox sense not only the presence of light but also its direction, and a full rotation gives each cell a full circuit of facing towards and away from the light. The details of how this works are complex and species specific, but the basic idea is that the flagella of cells facing away from the light beat faster (or forward when the others are beating backwards), and this steers the colony towards the light, just as paddling on one side of a canoe will cause it to turn towards the other side (Solari et al. 2011). Timothy Pedley (University of Cambridge) showed simulations of Volvox motility taking this rotation into account, accurately predicting swimming speed and rotation rate and even among-colony interactions that lead to the observed ‘dancing’ of hydrodynamically bound colonies (Drescher et al. 2009).
Rotation is crucial to phototaxis in single-celled algae as well. Noriko Ueki (Tokyo Institute of Technology) showed that the reversed phototactic behaviour of C. reinhardtii motility mutants is due to a ‘cellular lens effect’. In wild-type cells, carotenoid layers block light from one side, so that a cell’s photoreceptor only receives light from one direction (Witman 1993). In mutants lacking the carotenoid layers, the cell acts as a convex lens, focusing light on the side of the photoreceptor that would normally be shielded and resulting in reversed phototaxis.
In contrast to the apparent lack of direct interactions among cells within a Volvox colony, coordination between the flagella of a single cell does appear to require direct interactions. Kirsty Wan (University of Cambridge) showed comparisons of single-celled algae with 2, 4, 8 and 16 flagella per cell (Wan & Goldstein 2015). The flagella of volvocine algae are anchored to the basal bodies, and these are connected by a ‘distal fibre’. This fibre is thus an obvious suspect in the coordination of flagella, and indeed Wan & Goldstein showed that C. reinhardtii mutants without the distal fibre fail to coordinate their flagella (Wan & Goldstein 2015). Patterns of coordination among the flagella of quadriflagellate species turn out to be highly variable, with analogues of the quadruped trot, transverse and rotary gallop, and pronk. A simple experiment shows that these gaits are coordinated by something other than hydrodynamic coupling: using micromanipulation, Wan & Goldstein immobilized one flagellum and showed that the other three beat in the same pattern as normal.
Conclusions and future directions
The relative simplicity of the volvocine algae among multicellular organisms, their diversity of forms and their experimental tractability have made them a valuable model system for a wide variety of biological questions. Perhaps no other system has pulled more empirical and theoretical weight in discussions of the major transitions in evolution. Their range of sizes and degrees of complexity lends itself well to comparative studies of the evolution of complex traits. Similarly, the diversity of their sexual systems has led to important insights into the evolution of males and females from isogamous mating types. The geometric regularity of their body plans has inspired biophysical models of their development and motility for which the few required simplifying assumptions are barely approximations at all. And studies of their morphology, development, genetics, genomics, biophysics and experimental evolution can be understood in a phylogenetic comparative context.
However, research using the volvocine algae is not without its challenges. For a microbe, their genomes are relatively large (~120 Mb), more akin to Drosophila than to Saccharomyces. This will become less of a limitation as sequencing costs fall, but for now it makes whole-genome sequencing and related approaches relatively expensive.
Cryopreservation is expensive and time-consuming compared to other microbial models, and it is limited to unicells and the smallest colonial species. As a result, strains in culture collections must be serially propagated, leading to unintended and unwelcome evolutionary change, frequent loss of sexual competence, and even permanent loss of valuable isolates and type cultures. Several members of the Volvox community have agreed to make strains available, but these arrangements are informal and insufficient. A reliable cryopreservation method that worked for all species and allowed storage in −80° freezers rather than in liquid nitrogen Dewars would be an immense benefit to the research community.
Although the consensus view of volvocine phylogeny has changed substantially with the application of molecular phylogenetic methods, the taxonomy has not been updated to reflect this new understanding. Within the largest family, the Volvocaceae, five nominal genera are polyphyletic (Pandorina, Volvulina, Eudorina, Pleodorina and Volvox). While the morphological convergence that led to these discrepancies is an advantage in comparative studies, the taxonomy should ideally reflect evolutionary relationships. An overhaul of the taxonomy would be a welcome contribution.
One very promising development in volvocine research is the increased attention to nonmodel species. We know an awful lot about the life cycles, developmental genetics and genomics of C. reinhardtii and V. carteri f. nagariensis, but there is a limit to how much we can learn from comparisons involving just these two species. Extension of detailed morphological and developmental studies, whole-genome sequences and techniques for genetic manipulation beyond the canonical two is certain to pay dividends. Several such advances were reported at the meeting, including additional genome projects, cross-species complementation experiments, comparative transcriptomics, proteomics and detailed developmental studies of nonmodel species.
Techniques for genetic characterization and manipulation of V. carteri also continue to advance. Linh Bui (Danforth Plant Science Center) presented a streamlined method for identifying causative mutations through a combination of low-resolution meiotic mapping and whole-genome resequencing. Improved techniques for nuclear transformation include inducible transgene expression using the nitrate reductase promoter by Benjamin Klein (Bielefeld University) and the development of improved selectable transformation vectors by Jose Ortega-Escalante (University of Maryland, Baltimore County).
A fuller understanding of the evolution of multicellular complexity is likely to result from integrating data from diverse fields. Studies of volvocine ecology and biogeography are sorely lacking. As a result, discussions of the evolutionary processes underlying the transition to multicellular life have largely excluded ecological and biogeographic context. Such discussions have nearly always assumed a dominant role of adaptive processes, but nonadaptive hypotheses should also be considered (e.g. Lynch 2007). In addition to systematic ecological studies, we need reliable estimates of basic population biological parameters, for example effective population sizes, rates and mechanisms of migration, and levels of intraspecific genetic diversity. Understanding volvocine population genetics and phylogeography would inform discussions about the relative importance of adaptive and nonadaptive processes (including vicariance) in the evolution of volvocine diversity.
The volvocine algae have already taught us a great deal about the evolution of biological complexity. How many of these results turn out to be generalizable to other major transitions remains to be seen. However, the recent rapid growth of the Volvox research community, along with advances in sequencing technologies, techniques for genetic manipulation and experimental methods, suggests that the potential of the volvocine algae as a model system is far from exhausted. Continued research promises to continue to yield new insights, and the Fourth and subsequent meetings should be exciting. Volvox is on a roll!
Acknowledgments
This work was supported by grants from NASA (Cooperative Agreement Notice 7), NSF (DEB-1457701) and the John Templeton Foundation (43285). I thank James Umen, Kimberly Chen and Gwendolyn Nix for helpful comments on the manuscript.
Footnotes
M.D. Herron wrote the manuscript.
References
- Arakaki Y, Kawai-Toyooka H, Hamamura Y, et al. The simplest integrated multicellular organism unveiled. PLoS ONE. 2013;8:e81641. doi: 10.1371/journal.pone.0081641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brumley DR, Wan KY, Polin M, Goldstein RE. Flagellar synchronization through direct hydrodynamic interactions. eLife. 2014;3:1–15. doi: 10.7554/eLife.02750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brumley DR, Polin M, Pedley TJ, Goldstein RE, Goldstein RE. Metachronal waves in the flagellar beating of Volvox and their hydrodynamic origin. Journal of the Royal Society, Interface. 2015;12:20141358. doi: 10.1098/rsif.2014.1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll SB. Evo-devo and an expanding evolutionary synthesis: a genetic theory of morphological evolution. Cell. 2008;134:25–36. doi: 10.1016/j.cell.2008.06.030. [DOI] [PubMed] [Google Scholar]
- Cheng Q, Fowler R, Edwards L, Miller SM. The role of GlsA in the evolution of asymmetric cell division in the green alga Volvox carteri. Development Genes and Evolution. 2003;213:328–335. doi: 10.1007/s00427-003-0332-x. [DOI] [PubMed] [Google Scholar]
- Darwin CR. The Origin of Species by Means of Natural Selection, or the Preservation of Favoured Races in the Struggle for Life. 6th. John Murray; London: 1872. [PMC free article] [PubMed] [Google Scholar]
- Dawkins R. The Selfish Gene. Oxford University Press; Oxford: 1976. [Google Scholar]
- Dawkins R. Climbing Mount Improbable. W. W. Norton & Company; New York, New York: 1997. [Google Scholar]
- Drescher K, Leptos KC, Tuval I, Ishikawa T, Pedley TJ, Goldstein RE. Dancing Volvox: hydrodynamic bound states of swimming algae. Physical Review Letters. 2009;102:1–4. doi: 10.1103/PhysRevLett.102.168101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng S, De Hoff P, Umen JG. Evolution of sexes from an ancestral mating-type specification pathway. PLoS Biology. 2014;12:e1001904. doi: 10.1371/journal.pbio.1001904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilles R, Gilles C, Jaenicke L. Sexual differentiation of the green alga Volvox carteri. Naturwissenschaften. 1983;70:571–572. [Google Scholar]
- Gilles R, Gilles C, Jaenicke L. Pheromone-binding and matrix-mediated events in sexual induction of Volvox carteri. Zeitschrift für Naturforschung C. 1984;39c:584–592. [Google Scholar]
- Goldstein RE. Green algae as model organisms for biological fluid dynamics. Annual Review of Fluid Mechanics. 2015;47:343–377. doi: 10.1146/annurev-fluid-010313-141426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodenough U, Heitman J. Origins of eukaryotic sexual reproduction. Cold Spring Harbor Perspectives in Biology. 2014;6:a016154. doi: 10.1101/cshperspect.a016154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas P, Goldstein RE. Elasticity and glocality: initiation of embryonic inversion Volvox. arXiv, 1507.01439 [cond–mat.soft] 2015 doi: 10.1098/rsif.2015.0671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallmann A. Morphogenesis in the family Volvocaceae: different tactics for turning an embryo right-side out. Protist. 2006;157:445–461. doi: 10.1016/j.protis.2006.05.010. [DOI] [PubMed] [Google Scholar]
- Hallmann A, Kirk DL. The developmentally regulated ECM glycoprotein ISG plays an essential role in organizing the ECM and orienting the cells of Volvox. Journal of Cell Science. 2000;113:4605–4617. doi: 10.1242/jcs.113.24.4605. [DOI] [PubMed] [Google Scholar]
- Hanschen ER, Ferris PJ, Michod RE. Early evolution of the genetic basis for soma in the Volvocaceae. Evolution. 2014;68:2014–2025. doi: 10.1111/evo.12416. [DOI] [PubMed] [Google Scholar]
- Harris EH. The Chlamydomonas Sourcebook. 2nd. Academic Press; San Diego, California: 2009. [Google Scholar]
- Herron MD. Many from one: lessons from the volvocine algae on the evolution of multicellularity. Communicative and Integrative Biology. 2009;2:368–370. doi: 10.4161/cib.2.4.8611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herron MD, Michod RE. Evolution of complexity in the volvocine algae: transitions in individuality through Darwin’s eye. Evolution. 2008;62:436–451. doi: 10.1111/j.1558-5646.2007.00304.x. [DOI] [PubMed] [Google Scholar]
- Herron MD, Hackett JD, Aylward FO, Michod RE. Triassic origin and early radiation of multicellular volvocine algae. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:3254–3258. doi: 10.1073/pnas.0811205106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herron MD, Desnitskiy AG, Michod RE. Evolution of developmental programs in Volvox (Chlorophyta) Journal of Phycology. 2010;46:316–324. [Google Scholar]
- Hoekstra HE, Coyne JA. The locus of evolution: evo devo and the genetics of adaptation. Evolution. 2007;61:995–1016. doi: 10.1111/j.1558-5646.2007.00105.x. [DOI] [PubMed] [Google Scholar]
- Höhn S, Hallmann A. There is more than one way to turn a spherical cellular monolayer inside out: type B embryo inversion in Volvox globator. BMC Biology. 2011;9:89. doi: 10.1186/1741-7007-9-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höhn S, Honerkamp-Smith AR, Haas PA, Trong PK, Goldstein RE. Dynamics of a Volvox embryo turning itself inside out. Physical Review Letters. 2015;114:1–5. doi: 10.1103/PhysRevLett.114.178101. [DOI] [PubMed] [Google Scholar]
- Huskey RJ, Griffin BE. Genetic control of somatic cell differentiation in Volvox: analysis of somatic regenerator mutants. Developmental Biology. 1979;72:226–235. doi: 10.1016/0012-1606(79)90113-1. [DOI] [PubMed] [Google Scholar]
- Huxley JS. The Individual in the Animal Kingdom. Cambridge University Press; Cambridge, UK: 1912. [Google Scholar]
- Kianianmomeni A, Hallmann A. Transcriptional analysis of Volvox photoreceptors suggests the existence of different cell-type specific light-signaling pathways. Current Genetics. 2015;61:3–18. doi: 10.1007/s00294-014-0440-3. [DOI] [PubMed] [Google Scholar]
- Kindle KL. High-frequency nuclear transformation of Chlamydomonas reinhardtii. Proceedings of the National Academy of Sciences of the United States of America. 1990;87:1228–1232. doi: 10.1073/pnas.87.3.1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King N, Westbrook MJ, Young SL, et al. The genome of the choanoflagellate Monosiga brevicollis and the origin of metazoans. Nature. 2008;451:783–788. doi: 10.1038/nature06617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk DL. Volvox: molecular-Genetic Origins of Multicellularity. Cambridge University Press; Cambridge: 1998. [Google Scholar]
- Kirk DL. A twelve-step program for evolving multicellularity and a division of labor. BioEssays. 2005;27:299–310. doi: 10.1002/bies.20197. [DOI] [PubMed] [Google Scholar]
- Kirk MM, Stark K, Miller SM, et al. regA, a Volvox gene that plays a central role in germ-soma differentiation, encodes a novel regulatory protein. Development. 1999;126:639–647. doi: 10.1242/dev.126.4.639. [DOI] [PubMed] [Google Scholar]
- Larson A, Kirk MM, Kirk DL. Molecular phylogeny of the volvocine flagellates. Molecular Biology and Evolution. 1992;9:85–105. doi: 10.1093/oxfordjournals.molbev.a040710. [DOI] [PubMed] [Google Scholar]
- van Leeuwenhoek A. Part of a letter from Mr Antony van Leeuwenhoek, concerning the worms in sheeps livers, gnats, and animalcula in the excrements of frogs. Philosophical Transactions of the Royal Society of London. 1700;22:509–518. [Google Scholar]
- Lerche K, Hallmann A. Stable nuclear transformation of Gonium pectorale. BMC Biotechnology. 2009;9:64. doi: 10.1186/1472-6750-9-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerche K, Hallmann A. Stable nuclear transformation of Eudorina elegans. BMC Biotechnology. 2013;13:11. doi: 10.1186/1472-6750-13-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerche K, Hallmann A. Stable nuclear transformation of Pandorina morum. BMC Biotechnology. 2014;14:65. doi: 10.1186/1472-6750-14-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnaeus C. Systema naturae per regna tria naturae, secundum classes, ordines, genera, species, cum characteribus, differentiis, synonymis, locis. Vol. 1. Holmiae; Stockholm: 1758. Tomus I. Editio decima, reformata. Editio decima revisa. [Google Scholar]
- Lynch M. The frailty of adaptive hypotheses for the origins of organismal complexity. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(Suppl):8597–8604. doi: 10.1073/pnas.0702207104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning G, Young SL, Miller WT, Zhai Y. The protist, Monosiga brevicollis, has a tyrosine kinase signaling network more elaborate and diverse than found in any known metazoan. Proceedings of the National Academy of Sciences of the USA. 2008;105:9674–9679. doi: 10.1073/pnas.0801314105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant HJ. Colony formation and inversion in the green alga Eudorina elegans. Protoplasma. 1977;93:325–339. [Google Scholar]
- Maynard Smith J, Szathmáry E. The Major Transitions in Evolution. Oxford University Press; Oxford: 1995. [Google Scholar]
- Merchant SS, Prochnik SE, Vallon O, et al. The Chlamydomonas genome reveals the evolution of key animal and plant functions. Science. 2007;318:245–250. doi: 10.1126/science.1143609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori T, Kawai-Toyooka H, Igawa T, Nozaki H. Gamete dialogs in green lineages. Molecular Plant. 2015;8:1442–1454. doi: 10.1016/j.molp.2015.06.008. [DOI] [PubMed] [Google Scholar]
- Nedelcu AM. Environmentally induced responses co-opted for reproductive altruism. Biology Letters. 2009;5:805–808. doi: 10.1098/rsbl.2009.0334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedelcu AM, Michod RE. Evolvability, modularity, and individuality during the transition to multicellularity in Volvocalean green algae. In: Schlosser G, Wagner GP, editors. Modularity in Development and Evolution. Oxford University Press; Oxford: 2004. pp. 466–489. [Google Scholar]
- Nedelcu AM, Michod RE. The evolutionary origin of an altruistic gene. Molecular Biology and Evolution. 2006;23:1460–1464. doi: 10.1093/molbev/msl016. [DOI] [PubMed] [Google Scholar]
- Nedelcu AM, Borza T, Lee RW. A land plant-specific multigene family in the unicellular Mesostigma argues for its close relationship to Streptophyta. Molecular Biology and Evolution. 2006;23:1011–1105. doi: 10.1093/molbev/msj108. [DOI] [PubMed] [Google Scholar]
- Nilsson D-E, Pelger S. A pessimistic estimate of the time required for an eye to evolve. Proceedings of the Royal Society of London B: Biological Sciences. 1994;256:53–58. doi: 10.1098/rspb.1994.0048. [DOI] [PubMed] [Google Scholar]
- Nishii I, Ogihara S, Kirk DL. A kinesin, invA, plays an essential role in Volvox morphogenesis. Cell. 2003;113:743–753. doi: 10.1016/s0092-8674(03)00431-8. [DOI] [PubMed] [Google Scholar]
- Nozaki H. Biologia. Vol. 58. Vydavatelstvo Slovak Academic Press; Bratislava: 2003. Origin and evolution of the genera Pleodorina and Volvox; pp. 425–431. [Google Scholar]
- Nozaki H, Misawa K, Kajita T, Kato M, Nohara S, Watanabe MM. Origin and evolution of the colonial volvocales (Chlorophyceae) as inferred from multiple, chloroplast gene sequences. Molecular Phylogenetics and Evolution. 2000;17:256–268. doi: 10.1006/mpev.2000.0831. [DOI] [PubMed] [Google Scholar]
- Nozaki H, Mori T, Misumi O, Matsunaga S. Males evolved from the dominant isogametic mating type. Current Biology. 2006;16:1017–1018. doi: 10.1016/j.cub.2006.11.019. [DOI] [PubMed] [Google Scholar]
- Nozaki H, Yamada TK, Takahashi F, Matsuzaki R, Nakada T. New “missing link” genus of the colonial volvocine green algae gives insights into the evolution of oogamy. BMC Evolutionary Biology. 2014;14:37. doi: 10.1186/1471-2148-14-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nozaki H, Matsuzaki R, Yamamoto K, Kawachi M, Takahashi F. Delineating a new heterothallic species of Volvox (Volvocaceae, Chlorophyceae) using new strains of “Volvox africanus”. PLoS ONE. 2015;10:e0142632. doi: 10.1371/journal.pone.0142632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pincus D, Letunic I, Bork P, Lim WA. Evolution of the phospho-tyrosine signaling machinery in premetazoan lineages. Proceedings of the National Academy of Sciences of the USA. 2008;105:9680–9684. doi: 10.1073/pnas.0803161105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prochnik SE, Umen J, Nedelcu AM, et al. Genomic analysis of organismal complexity in the multicellular green alga Volvox carteri. Science. 2010;329:223–226. doi: 10.1126/science.1188800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratcliff WC, Herron MD, Howell K, Pentz JT, Rosenzweig F, Travisano M. Experimental evolution of an alternating uni- and multicellular life cycle in Chlamydomonas reinhardtii. Nature Communications. 2013;4:2742. doi: 10.1038/ncomms3742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rokas A. The origins of multicellularity and the early history of the genetic toolkit for animal development. Annual Review of Genetics. 2008;42:235–251. doi: 10.1146/annurev.genet.42.110807.091513. [DOI] [PubMed] [Google Scholar]
- Schiedlmeier B, Schmitt R, Müller W, et al. Nuclear transformation of Volvox carteri. Proceedings of the National Academy of Sciences of the USA. 1994;91:5080–5084. doi: 10.1073/pnas.91.11.5080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimogawara K, Fujiwara S, Grossman A, Usuda H. High-efficiency transformation of Chlamydomonas reinhardtii by electroporation. Genetics. 1998;148:1821–1828. doi: 10.1093/genetics/148.4.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solari CA, Drescher K, Goldstein RE. The flagellar photoresponse in Volvox species (Volvocaceae, Chlorophyceae) Journal of Phycology. 2011;47:580–583. doi: 10.1111/j.1529-8817.2011.00983.x. [DOI] [PubMed] [Google Scholar]
- Suga H, Chen Z, de Mendoza A, et al. The Capsaspora genome reveals a complex unicellular prehistory of animals. Nature Communications. 2013;4:2325. doi: 10.1038/ncomms3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szathmáry E, Maynard Smith J. The major evolutionary transitions. Nature. 1995;374:227–232. doi: 10.1038/374227a0. [DOI] [PubMed] [Google Scholar]
- Umen JG, Olson BJSC. Genomics of volvocine algae. Advances in Botanical Research. 2012;64:185–243. doi: 10.1016/B978-0-12-391499-6.00006-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viamontes GI, Kirk DL. Cell shape changes and the mechanism of inversion in Volvox. Journal of Cell Biology. 1977;75:719–730. doi: 10.1083/jcb.75.3.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wake DB. What salamanders have taught us about evolution. Annual Review of Ecology Evolution and Systematics. 2009;40:333–352. [Google Scholar]
- Wan KY, Goldstein RE. Coordinated beating of algal flagella is mediated by basal coupling. 2015. arXiv, 1510.03272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weismann A. Essays upon Heredity and Kindred Biological Problems. Clarendon Press; Oxford: 1889. [Google Scholar]
- Weismann A. The Evolution Theory. Vol. 1. Edward Arnold; London: 1904. [Google Scholar]
- Witman GB. Chlamydomonas phototaxis. Trends in Cell Biology. 1993;3:403–408. doi: 10.1016/0962-8924(93)90091-e. [DOI] [PubMed] [Google Scholar]
- Yamano T, Iguchi H, Fukuzawa H. Rapid transformation of Chlamydomonas reinhardtii without cell-wall removal. Journal of Bioscience and Bioengineering. 2013;115:691–694. doi: 10.1016/j.jbiosc.2012.12.020. [DOI] [PubMed] [Google Scholar]


