Abstract
Innate lymphoid cells (ILCs) are critical components of tissues in the body, providing a first line of defense against challenges to host integrity. In contrast to strictly cytokine-producing helper ILCs, resident innate lymphocyte populations with cytolytic potential have been identified in multiple tissues in both mouse and human. These cells express the transcription factor Tbet, natural killer (NK) cell receptors, granzymes, perforin and death receptors and can directly kill tumor cells. Signals in the tumor microenvironment may promote this response, including the cytokine IL-15 and stress-associated ligands for activating NK receptors. While there is evidence that these cells are tissue- and tumor-resident, their lineage remains unclear. Whether they are derived from the NK or helper ILC lineages or represent a third differentiation pathway remains to be determined. A better understanding of their lineage will help clarify their regulation and function in the context of anti-tumor immunity.
Introduction
The immune system has evolved to combat many diverse pathogenic threats. Broadly these can be grouped into two categories: intracellular and extracellular threats. Intracellular challenges include pathogens that infect host cells, such as viruses and some bacteria, as well as cell transformation, both of which endanger the integrity of the host cell and can occur anywhere in the body. Cytotoxic type 1 immunity can protect against such challenges by directly killing the infected or stressed cell. Extracellular threats, which include parasites and extracellular bacteria, are often found at barrier sites. These pathogens mainly compete with the host for nutrients but can also cause collateral damage. Type 2 and 3 immunity can combat extracellular threats by mobilizing and coordinating multiple types of immune cells through cytokine production, cell-cell communication and recruitment.
When considering how an immune population combats challenges, knowing the cell’s lineage, activation state and localization is needed to fully understand its function. For instance, CD8 T cells are important effectors in anti-viral responses. Naïve and memory conventional CD8 T cells can be found circulating in lymphatics and blood. Upon activation, they can be recruited into infected tissues, where a subset can differentiate into antigen-specific tissue-resident memory cells (Trms) (1, 2). Trms cannot recirculate, and given their localization, they provide first line defenses against reinfections. There also exist distinct lineages of tissue-resident innate-like T cells (ILTCs), which include intraepithelial lymphocytes in the intestine (3, 4). While both CD8 Trm and ILTCs are tissue resident, they are derived from separate lineages and may play distinct roles in immune responses.
Cancer provides a unique challenge to the immune system, representing both self-tissue yet also an infectious and invasive entity (5, 6). While much of our knowledge of immunology centers on responses against infectious non-self such as bacteria and viruses, the possibility remains that there are distinct types of responses to cancer. Indeed, there is accumulating evidence that type 1 immunity mediated by tissue-resident lymphocytes is critical in combating cancer. Here, we discuss the current understanding of how the lineage, localization and effector programs of resident innate lymphocytes dictate their responses to solid tumors.
Innate Lymphocyte Subsets and Their Characteristics
In addition to T cells, the lymphocyte family also includes innate lymphocytes, which lack Recombination Activating Gene (RAG)-rearranged antigen receptors. The prototypical innate lymphocyte is the natural killer (NK) cell, which circulates throughout the body and can mediate type 1 immune responses through cytotoxicity and cytokine production. Recent work has broadened the definition of innate lymphocytes to include a diverse group of tissue-resident innate lymphoid cells (ILCs) that are found in many tissues throughout the body, including at barrier surfaces. ILCs, along with other resident lymphocytes, provide first-line defenses against pathogens but can also cause immunopathology when dysregulated (7). ILCs are grouped into three types, type 1, 2 and 3, which are assigned based on their distinct transcription factor-driven and functional identities that mirror those ascribed to cytokine-producing helper CD4 T cell subsets.
Type 1 innate lymphocytes, while heterogeneous, are noted for their expression of the T-box transcription factor Tbet and production of type 1 cytokines including interferon gamma (IFNγ). The first member of the type 1 innate lymphocyte family to be described was the NK cell (8-11). NK cells recirculate throughout the body and express cytotoxic molecules including granzymes and perforin. In addition, they express a variety of activating and inhibitory surface receptors, and the balance and integration of signals from these receptors dictates whether cells respond to targets (12). Many of the activating receptors recognize ligands associated with stressed, damaged or infected cells, while the inhibitory receptors can recognize MHC class I family molecules, which are constitutively expressed by healthy self cells but are downregulated by some viruses as an immune evasion mechanism. This receptor repertoire allows NK cells to discriminate between healthy and stressed or infected cells, leading to cell clearance (13).
Type 1 innate lymphocytes also include tissue-resident innate lymphoid cells (ILC1s). In contrast to circulating NK cells, populations of Tbet+ resident ILC1s have been described in many tissues. These cells can express IFNγ, TNFα and NK receptors and have been identified in liver, intestine, spleen, adipose tissue, salivary gland, mammary gland and prostate, among other organs (14). These cells play important roles in maintaining tissue homeostasis; without proper regulation, these cells can lead to immunopathology, as shown in mouse models of ischemic kidney injury and obesity (15, 16).
Type 2 innate lymphoid cells (ILC2s) are helper ILCs that require the transcription factors GATA3 and RORα and produce type 2 cytokines, including IL-4, IL-5, IL-9 and IL-13. ILC2s promote type 2 immune responses, are important for defense against helminths and maintenance of homeostasis and tissue repair, but they can also cause allergic inflammation, as they have been shown to play pathogenic roles in asthma, skin and lung disease (17).
Type 3 innate lymphoid cells (ILC3s) express the transcription factor RORγt, encoded by the gene Rorc, and produce IL-17, IL-22 and lymphotoxin. ILC3s help maintain intestinal homeostasis by responding to intestinal damage and microbiota alterations, and their dysregulation is associated with inflammatory conditions in the gut, including colitis (7). ILC2s and ILC3s are more enriched at barrier surfaces, including intestinal mucosa and lung, while ILC1s have been identified in a wider array of tissues. These subsets perform distinct functions and help maintain homeostasis throughout the body.
Both NK cells and ILCs are derived from the common lymphoid progenitor (CLP) in mouse, after which two distinct progenitors appear: the common helper innate lymphoid progenitor (CHILP), which gives rise to all helper ILCs but not NK cells, and the NK progenitor, which only has NK potential (18). A distinct lineage also arises from the CLP that gives rise to lymphoid tissue-inducer (LTi) cells, which are important early in life for lymphoid tissue development. Parabiosis experiments have demonstrated that mouse ILCs are highly tissue resident (19). Although these studies suggest that ILC populations in mouse tissues are seeded early in life and maintained without much input from the circulation, the possibility remains that tissues maintain multipotent precursor populations, and that circulating ILC precursors (ILCPs) could exist and contribute to tissue ILCs in some circumstances and over time.
The human immune system includes both NK cells and ILC subsets. It has been proposed that there are two distinct subsets of NK cells based on CD56 expression, CD56dimCD16+ (CD56dim) cells, which are found predominantly in the circulation, and CD56brightCD16+/− (CD56bright) cells, which makeup a minority of circulating NK cells but can be found in many human tissues, including lymphoid tissues, liver and uterus (20). However, given that the differentiation of NK and ILC lineages in human remains less well understood, these resident CD56bright cells may in fact be derived from a distinct ILC lineage. There is evidence for an NK-committed precursor with no helper-ILC potential that can be found in the fetal liver, fetal bone marrow, adult bone marrow and tonsils (21, 22). A recent study identified an ILCP with potential for CD56+ NK cells and helper ILC1, 2 and 3 (23). This ILCP could be found in the peripheral blood and seed tissues, where it retained multipotent potential. It remains to be seen whether this ILCP can give rise to CD56dim NK cells and how tissue- and challenge-specific cues drive the differentiation of NK cells and ILCs.
In humans, tissue residency can be difficult to definitively demonstrate, but expression patterns of certain integrins and chemokine receptors offer evidence. Within the NK cell compartment, CD56dim NK cells circulate and are found primarily in the blood. These cells express the trafficking receptors S1PR1 and S1PR5, the chemokine receptor CCR7, and do not express certain adhesion molecules, thus supporting the notion that they are circulating. CD56bright NK cells appear to consist of both circulating and tissue-resident populations. A small proportion of these cells are found in the blood and do not express resident-associated receptors. CD56bright cells can be identified as tissue-resident based on their expression of CD69, which suppresses S1PR1, chemokine receptors CXCR6 and CCR5, and adhesion molecules such as CD49a and CD103 (20). A recent study identified such a subset in human lymphoid tissues (24). CD56bright NK cells in the uterus and liver also share such expression patterns with certain tissue-specific variations (20). Taken together, ILCs and populations of CD56bright NK cells make up important components of the tissue.
Tissue-Resident Cytolytic Innate Lymphocytes
One major question in innate lymphocyte biology revolves around the heterogeneity of type 1 innate lymphocyte populations (14). ILC1s are conventionally defined as helper cells, producing cytokines but lacking cytolytic activity. However, studies have identified populations of resident type 1 innate lymphocytes that express perforin, granzymes, and death receptors such as TNF-related apoptosis-inducing ligand (TRAIL), suggesting their potential to kill. Such populations have been termed tissue-resident NK (TR-NK) cells in organs such as the liver (25), or ILC1-like (ILC1l) in the mammary gland and prostate (26). Given that their lineage remains unclear, we propose to reserve the TR-NK nomenclature if these cells are derived from the NK lineage. CHILP-derived type 1 innate lymphocytes could be divided into two subsets: “helper” ILC1 (ILC1h), which are purely cytokine producing cells with no potential for cytotoxicity, and “killer” ILC1 (ILC1k), which retain cytotoxic potential (Figure 1).
FIGURE 1. Heterogeneity of lymphocyte subsets.
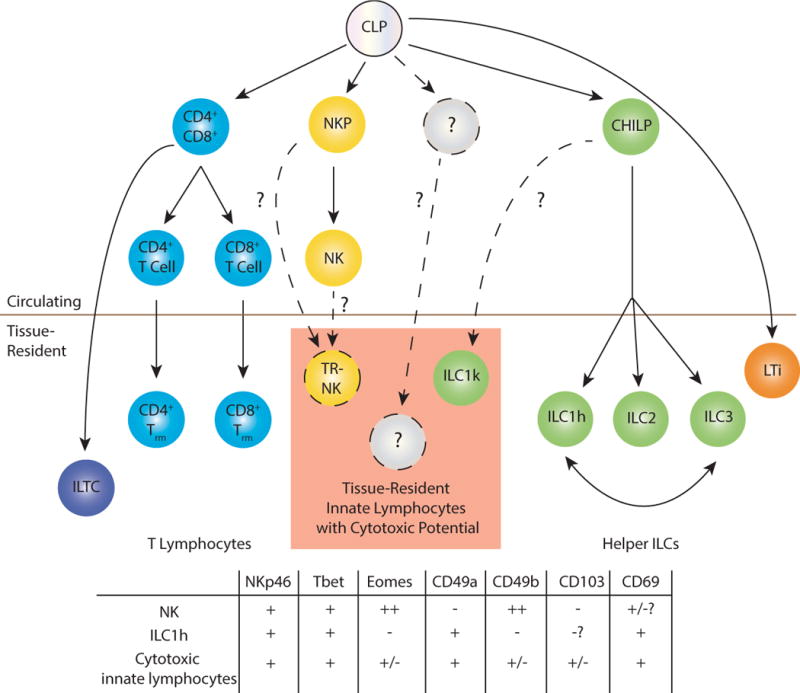
The common lymphoid progenitor (CLP) gives rise to all lymphocyte lineages, including B cells (not pictured) and T cells. In the T cell lineage, the CLP gives rise to the double positive (CD4+CD8+, DP) stage in the thymus. This DP cell can give rise to conventional CD4, CD8 or innate-like T cells. The CLP also gives rise to NK cells through an NK-committed progenitor (NKP), to lymphoid tissue inducer cells (LTi), and to helper ILC lineages through the common helper innate lymphoid progenitor (CHILP). There also exist tissue-resident innate lymphocytes with cytotoxic potential, although their lineage remains unclear. Cells with this phenotype may arise from the NK lineage, which would categorize them as tissue-resident NK cells (TR-NK), from the CHILP, which would categorize them as killer ILC1 (ILC1k), or they may have a distinct progenitor. Marker expression patterns of various type 1 innate lymphocyte subsets are listed.
ILC1h cells, that is, cells with purely cytokine producing potential, have been most clearly identified by “ex”-ILC3s, or Tbet-expressing IFNγ+ cells that can interconvert with RORγt+ ILC3s in response to type 1 cytokines such as IL-12, which can occur both in vitro and in vivo (27-30). This conversion is reminiscent of the malleability of the CD4+ helper lineages, where “ex”-Th17+ T cells can arise in certain pathological conditions such as experimental autoimmune encephalitis (EAE), switching from IL-17 to IFNγ production (31). Whether non-RORγt fate-mapped cells contribute to this purely cytokine-producing ILC1h population or represent a distinct type of ILC1 remains to be determined.
There also exist tissue-resident type 1 innate lymphocytes that are distinct from other helper ILCs, given that they cannot interconvert with ILC3s and do not show history of RORγt expression. In the intestine, these cells can be identified by CD103 expression and absence of CD127 (29). CD103 is expressed by type 1 innate lymphocytes in other tissues including the salivary gland, mammary gland and prostate, and this is associated with an intraepithelial localization (32, 33). The cell death receptor TRAIL is expressed by this group of cells in liver, salivary gland, mammary gland and prostate (25, 26, 33). These cells also show gene and protein expression of granzymes and perforin, although often these molecules are expressed at lower levels than NK cells under homeostatic conditions. The cytolytic activities of these cells have been most clearly demonstrated by the type 1 innate lymphocytes found in the mammary gland, termed ILC1ls (26). Tbet+ innate lymphocytes in the liver have also been reported to have cytotoxic potential when activated, indicating that granzyme and perforin expression levels may be upregulated in certain settings (25, 26, 34, 35). Given the phenotypic overlap of ILC1hs (“ex”-ILC3s) and type 1 cytolytic innate lymphocytes, surface receptors may not be sufficient to differentiate these two populations, and some markers are not uniformly expressed in all tissues. Whether the CHILP only gives rise to helper lineages, as the name implies, or is also capable of giving rise to ILC1ks remains an open question (14). In addition, it is possible that tissue-resident cytolytic innate lymphocytes are differentiated from mature NK cells or NK progenitors, which will qualify them as TR-NK cells, or even from an undefined progenitor distinct from CHILP or NK progenitors (figure 1). Until specific transcription factors or fate-mapping approaches are uncovered to specifically identify the non-Rorc-fate-mapped type 1 innate lymphocytes, the lineage and heterogeneity of this population (or populations) remains unresolved.
Both subsets of human NK cells express cytotoxic molecules such as perforin and granzymes and can mediate cytotoxicity when activated (36). However, at baseline CD56dim cells express higher levels of these molecules and are better able to rapidly produce cytokines in response to IL-12 than many tissue-resident subsets, as shown in the liver and uterus, among other organs (37, 38). Uterine CD56bright cells are capable of tissue remodeling during placentation, suggesting diverse organ-specific functions (39). The lineage relationship between these two groups of NK cells remains unclear. Some evidence supports a linear relationship, with circulating CD56bright cells upstream of CD56dim cells, while other studies suggest circulating and resident populations may be derived from distinct lineages (20, 40). Additionally, circulating CD56bright cells may represent precursors for tissue-resident CD56bright cells. More work is needed to elucidate this relationship.
Human ILC1hs have been identified in multiple organs and are noted for their expression of Tbet and CD127 and production of IFNγ and TNFα. These cells could be converted from ILC2s or ILC3s under certain conditions (28, 29, 41, 42). However, a recent study looking across multiple human tissues using mass cytometry and t-distributed stochastic neighbor embedding (t-SNE) analysis could not find evidence of a distinct ILC1h population, and instead found an intraepithelial-ILC1-like cell (ieILC1l) that clustered closely with NK cells; they expressed cytotoxic molecules and the tissue-resident markers CD69 and CD103. In healthy tissues, ieILC1ls were found in tonsils and intestine (43). Other groups have identified a similar intraepithelial population, which do not interconvert with ILC3s, do not require RORγt and respond to IL-12 and IL-15. These cells accumulate in Crohn’s disease patients, suggesting their potential for immunopathology (29, 32). These findings support the idea that like in mice, ILC1h can be identified as cytokine-producing ILC1s that are converted from other helper ILC subsets, but whether there is a separate lineage of ILC1hs remains unclear (23, 44). There also appears to be a population that expresses granzymes and perforin, although whether ieILC1l cells and resident CD56bright NK cells represent one lineage or distinct types of cells remains to be determined.
Tissue-Resident Cytolytic Innate Lymphocytes in Cancer
The immune system can respond to and clear transformed cells in a process termed cancer immunosurveillance. Immune responses to cancer target both liquid tumors, such as lymphomas, and solid tumors, found in tissues. A cytotoxic type 1 anti-tumor immune response is critical in fighting cancer. While resident cells can express cytolytic effector molecules, they are also expressed by circulating NK and CD8 T cells. Circulating cells are well positioned to carry out potent anti-cancer functions against liquid tumors or tumor metastasis, given their localization and trafficking patterns. In the setting of solid tumors, however, these immune cells would first need be recruited to and infiltrate the tissue and then remain activated to carry out meaningful anti-tumor effects. Tissue-resident cells are better situated to respond to solid tumors and are more likely to be the first responders to transformation.
What are the identities of such resident cells? An investigation of the immune infiltrate into oncogene-driven breast and prostate cancers revealed a cytotoxic type-1 immune response that involves innate lymphocytes (ILC1l) as well as innate-like αβ and γδ T cells, termed type 1 innate-like T cells (ILTC1s). ILC1ls and ILTC1s demonstrate highly overlapping transcriptomes, with many shared receptor expression patterns and similar cytokine requirements, including a dependency on IL-15. Both ILC1ls and ILTC1s are tumor-resident and proliferate, expand and upregulate granzyme expression during tumor progression. Both ILTC1s and ILC1ls are able to directly kill tumor cells in a perforin-dependent manner and to the same extent as circulating NK cells. ILC1ls express Tbet, low levels of Eomes, CD49a, CD103 and CD69, do not express CD127, and do not require Nfil3, unlike circulating NK cells, which are absent from tumors of Nfil3-deficient mice (26). It is possible that this cell population is not limited to the mammary gland, given that they phenotypically resemble the CD103+ intraepithelial ILC1s found in the salivary gland (33), although the anti-tumor activity of salivary gland ieILC1s has not been investigated.
Cytotoxicity is a critical part of anti-tumor immune responses. Mice that lack perforin show accelerated tumor growth in lymphoma and mammary carcinoma (26, 45, 46). While studies have shown expression of perforin and granzymes in cytolytic innate lymphocyte populations in other tissues, it remains to be tested how they respond to tissue-specific spontaneous transformation and whether the cancer setting modulates their effector functions.
NK cells and ILC1ls also express death receptors, such as TRAIL and FasL, and can mediate cell death independently of perforin and granzymes. Mice deficient in TRAIL demonstrate accelerated tumor growth (47, 48), and tumor cells can be killed via these death receptors using pharmacological targeting and in vitro assays (49). However, further studies are needed to determine the extent ILC1ls utilize these mechanisms in vivo.
Given that an understanding of the basic biology of tissue-resident immune populations in humans is an ongoing effort, many questions remain unanswered about the identities of these cells and how they respond to challenges, especially cancer. However, a recent study mentioned above quantified how human ILC populations change in the setting of solid tumors in both lung and colon (43). In healthy mucosal tissues ILCs were more frequent than NK cells. However in both colorectal and lung tumors, NK cells made up the dominant ILC population. Additionally, the cells termed ieILC1ls could be found in both tumor types. These intratumoral cells resembled CD56bright tissue-resident NK cells, as they expressed CD56, Tbet, Eomes, CD94, and the tissue-residence markers. They expressed perforin and granzymes at lower levels than the CD103-negative NK cells, and could produce IFNγ to the same extent as NK cells in response to IL-12, IL-15 and IL-18. Given that the NK cells found in the tumor did not express CD69 or CD103, such populations may have been trafficking through and would not represent resident populations. This study also detected ILC2 and ILC3 populations in lung tumor and a small ILC3 population in colorectal tumors, although the ex vivo functionality of these cells from the tumor was not investigated. This suggests that all subsets of innate lymphocytes may be present in some solid tumors, albeit to varying degrees, with NK cells making up the predominant population. How variable this makeup is in tumors of other solid organs and what their contributions are to anti-tumor immunity remains to be determined.
There is some evidence that tissue-resident lymphocyte populations play important roles in anti-tumor responses in human. CD103 is an integrin that binds E-cadherin, assists in granule positioning and release during tumor cell lysis, and marks many tissue-resident lymphocytes (50). This molecule can be detected on tumor-infiltrating lymphocytes, which includes T cells and innate lymphocytes. The presence of intratumoral CD103+ lymphocytes correlates with favorable prognosis in urothelial cell carcinoma of the bladder, non-small cell lung cancer, ovarian cancer, and endometrial adenocarcinoma (51-55). In many of these studies, CD103 is also expressed by CD8+ T cells, so the specific contribution of resident innate lymphocytes to anti-tumor immunity in humans is difficult to determine. Additionally, ILCs may suppress other immune responses. A recent study described a population of CD56+CD3− ILCs in human tumors which could suppress T cell activation and proliferation in ex vivo cultures (56), although the precise identity of this population, mechanism of action, and signals that drive this response require further study.
Regulation of Tissue-Resident Cytolytic Innate Lymphocytes
While the immune system is well evolved to combat infectious non-self, it also plays an important role in maintaining tissue homeostasis. Cell transformation is one such disruption of homeostasis, and there are multiple types of signals that this may trigger, including cellular stress-induced molecule expression or tissue architecture disruption. Tissue-resident immune populations are important mediators of maintaining healthy tissue, as shown by tissue macrophages in many non-infectious settings (57). Resident lymphocytes have been shown to play important roles, especially at barrier surfaces, in detecting microbial products, cytokines, alarmins and stress signals (58). The localization of tissue-resident lymphocytes requires that their responses to stimuli be tightly controlled in order to avoid collateral damage to the organ. This may explain reduced expression of effector molecules observed at baseline by cells in the uterus and liver, organs that may require tolerogenic responses to the fetus and food antigens, respectively. Tissue-resident cytolytic innate lymphocytes express many activating and inhibitory NK cell receptors, so presumably they can sense and kill stressed or transformed cells in the tissue. It has been shown that the activating receptor NKG2D is important for cancer immunosurveillance in a spontaneous model of prostate cancer (59). Ligands for NKG2D are expressed specifically in the context of cell transformation and viral infection, and in the setting of cancer, ligands can be shed, which leads to NK cell activation and tumor rejection, although how resident populations respond to shed ligands remains unknown (60, 61). Given that multiple cell populations express NKG2D, it remains to be determined whether NKG2D expression in tissue-resident cytolytic innate lymphocytes contributes to the effects seen on prostate tumor growth in the total knockout, whether through interaction with surface-bound or shed ligands. Additionally, other activating NK receptors expressed by these cells may play important roles, and further work is needed to determine which receptors mediate tumor cell killing.
How are tissue-resident cytolytic innate lymphocyte responses modulated? The inhibitory receptors expressed by NK cells are important for mediating NK cell licensing, where interactions with MHC I molecules lead to a functional maturation, allowing NK cells to achieve full effector functions during subsequent activating interactions (62). However, it is not known if tissue-resident cytolytic innate lymphocytes need to experience a similar interaction, or if their effector functions are regulated differently. A clearer understanding of the ontogeny of these cells may help clarify how and where the cells develop and functionally mature.
IL-15 is required for the generation of tissue-resident cytolytic innate lymphocytes, and absence of IL-15 leads to accelerated tumor growth (26). This common gamma chain cytokine is expressed and presented by many cell types, including macrophages, dendritic cells, adipocytes, fibroblasts and epithelial cells. It requires trans-presentation, potentially acting as an alarmin cytokine and promoting immune responses against pathogens or cell transformation (63). It may also be involved in coeliac disease, where epithelial cells in the gut have been shown to express both IL-15 and ligands for activating NK receptors, which could trigger type 1-mediated immunopathology. Intriguingly, high levels of IL-15 in tumors is associated with a high density of intratumoral cytotoxic T cells, and IL15 loss in colon cancer is associated with poor prognosis (64). This raises the possibility that IL-15 in the tissue can promote cytotoxic responses. Another cytokine IL-12, when overexpressed in tumor cells, can promote anti-tumor immune responses by acting on NKp46+ RORγt+ ILCs (65). Given that this protection is perforin-independent, and IL-12 can drive conversion of ILC3s to Tbet+ ILC1hs, this may be an example of anti-tumor immunity mediated by ILC1hs as opposed to cytotoxic type 1 innate lymphocytes. However, given that the source of IL-12 is normally restricted to certain immune populations and not tumor cells, how physiologically relevant this response is remains to be determined.
How might other signals in the tumor microenvironment influence the function of tumor-resident ILCs? TGF-β, for instance, is present in most solid tumors, where it is believed to inhibit NK cell responsiveness. However, this cytokine is also present in many tissues, and it is required for proper differentiation of CD103+ innate lymphocytes in the salivary gland (66) and for tissue-resident memory CD8 T cells (67), which have been associated with better prognosis in melanoma and lung cancer (68, 69). Two recent papers suggest that TGF-β signaling in NK cells can mediate conversion into an ILC1-like population, and that these converted cells appear to have poor anti-tumor activity in transplantable and carcinogen-induced models (70, 71). However, the conversion of NK cells into ILC1s is at odds with other studies that have shown that these two cell types are derived from distinct lineages using fate-mapping and precursor characterization (18, 72). Given that transfer systems may include undefined precursor populations, and that the NKp46-cre system affects NK cells along with type 1 and 3 ILCs, further studies are needed to clarify this potential conversion in other natural tumor settings. The extent to which resident type 1 innate lymphocytes are derived from NK cells versus from a distinct progenitor in the tumor, and what role TGF-β plays in guiding their differentiation and function, requires further exploration (Figure 2).
FIGURE 2. Resident cytolytic type 1 innate lymphocytes in the tumor microenvironment.
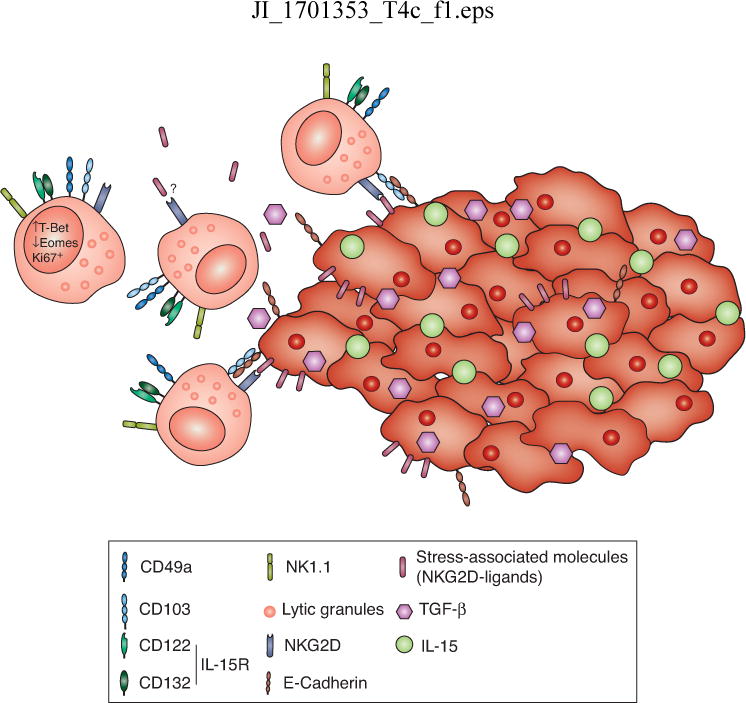
These cells express high levels of Tbet, low levels of Eomes, and proliferate in the setting of the tumor. They have cytotoxic potential directly against tumor cells. The tumor microenvironment may modulate the responses of these cells through the cytokines IL-15 and TGF-β and through ligands for NK receptors, including stress-associated molecules, which can be either surface-bound or shed.
Conclusions
Tissue-resident type 1 innate lymphocytes represent a newly discovered cell type which play critical roles in homeostasis and challenge-associated immune responses. Although the innate lymphocyte field is rapidly evolving, many questions remain unanswered, both about their basic biology and their role in cancer. While there is evidence that type 1 cytolytic innate lymphocytes can be mediators of anti-tumor immunity, the precise identity of the cell population that mediates these responses in every tissue remains unclear. A more comprehensive understanding of the lineage relationships among subsets of type 1 innate lymphocytes may clarify this question. Additionally, what are the tumor-specific signals that drive their activation, and how are their effector responses controlled? Beyond an innate lymphocyte-tumor cell crosstalk in cancer immunosurveillance, how might innate lymphocyte responses influence other types of immune responses to cancer? In this context, it remains possible that cytotoxic responses by tissue-resident populations may also trigger a tissue-repair program, which could in turn promote tumor progression.
Cancer and its accompanying immune responses are complex. However, there have been successful cancer therapeutic approaches that modulate immunity, including the breakthrough in checkpoint blockades, which in addition to altering T cell responses may also impact innate lymphocyte populations that express the targeted co-inhibitory receptors. While these therapies have shown incredible success, they do not demonstrate efficacy for all patients or for all types of cancer. There remains a need for new avenues of immunotherapies, and targeting tissue-resident populations, either to boost their effector programs or to interfere with their pro-tumor responses, holds potential. A better understanding of the basic biology behind tissue-resident cytolytic innate lymphocytes and how they respond to cancer may elucidate novel targets for such therapeutics.
Acknowledgments
We thank members of the Li laboratory for helpful discussions.
Grant Information
This work was supported by NIAID (R01 CA198280-01 to M.O.L), NCI (F31 CA210332-01A1 to B.G.N.), HHMI (Faculty Scholar Award to M.O.L.), Ludwig Center for Cancer Immunology and the Memorial Sloan Kettering Cancer Center Support Grant/Core Grant (P30 CA008748).
References
- 1.Shin H, Iwasaki A. A vaccine strategy that protects against genital herpes by establishing local memory T cells. Nature. 2012;491:463–467. doi: 10.1038/nature11522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schenkel JM, Fraser KA, Vezys V, Masopust D. Sensing and alarm function of resident memory CD8(+) T cells. Nat Immunol. 2013;14:509–513. doi: 10.1038/ni.2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheroutre H, Lambolez F, Mucida D. The light and dark sides of intestinal intraepithelial lymphocytes. Nat Rev Immunol. 2011;11:445–456. doi: 10.1038/nri3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wencker M, Turchinovich G, Di Marco Barros R, Deban L, Jandke A, Cope A, Hayday AC. Innate-like T cells straddle innate and adaptive immunity by altering antigen-receptor responsiveness. Nat Immunol. 2014;15:80–87. doi: 10.1038/ni.2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Epstein B, Jones M, Hamede R, Hendricks S, McCallum H, Murchison EP, Schonfeld B, Wiench C, Hohenlohe P, Storfer A. Rapid evolutionary response to a transmissible cancer in Tasmanian devils. Nat Commun. 2016;7:12684. doi: 10.1038/ncomms12684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 7.Klose CS, Artis D. Innate lymphoid cells as regulators of immunity, inflammation and tissue homeostasis. Nat Immunol. 2016;17:765–774. doi: 10.1038/ni.3489. [DOI] [PubMed] [Google Scholar]
- 8.Kiessling R, Klein E, Wigzell H. "Natural" killer cells in the mouse. I. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Specificity and distribution according to genotype. Eur J Immunol. 1975;5:112–117. doi: 10.1002/eji.1830050208. [DOI] [PubMed] [Google Scholar]
- 9.Kiessling R, Klein E, Pross H, Wigzell H. “Natural” killer cells in the mouse. II. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Characteristics of the killer cell. Eur J Immunol. 1975;5:117–121. doi: 10.1002/eji.1830050209. [DOI] [PubMed] [Google Scholar]
- 10.Herberman RB, Nunn ME, Lavrin DH. Natural cytotoxic reactivity of mouse lymphoid cells against syngeneic acid allogeneic tumors. I. Distribution of reactivity and specificity. Int J Cancer. 1975;16:216–229. doi: 10.1002/ijc.2910160204. [DOI] [PubMed] [Google Scholar]
- 11.Herberman RB, Nunn ME, Holden HT, Lavrin DH. Natural cytotoxic reactivity of mouse lymphoid cells against syngeneic and allogeneic tumors. II. Characterization of effector cells. Int J Cancer. 1975;16:230–239. doi: 10.1002/ijc.2910160205. [DOI] [PubMed] [Google Scholar]
- 12.Long EO, Kim HS, Liu D, Peterson ME, Rajagopalan S. Controlling natural killer cell responses: integration of signals for activation and inhibition. Annu Rev Immunol. 2013;31:227–258. doi: 10.1146/annurev-immunol-020711-075005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pegram HJ, Andrews DM, Smyth MJ, Darcy PK, Kershaw MH. Activating and inhibitory receptors of natural killer cells. Immunol Cell Biol. 2011;89:216–224. doi: 10.1038/icb.2010.78. [DOI] [PubMed] [Google Scholar]
- 14.Spits H, Bernink JH, Lanier L. NK cells and type 1 innate lymphoid cells: partners in host defense. Nat Immunol. 2016;17:758–764. doi: 10.1038/ni.3482. [DOI] [PubMed] [Google Scholar]
- 15.Victorino F, Sojka DK, Brodsky KS, McNamee EN, Masterson JC, Homann D, Yokoyama WM, Eltzschig HK, Clambey ET. Tissue-Resident NK Cells Mediate Ischemic Kidney Injury and Are Not Depleted by Anti-Asialo-GM1 Antibody. J Immunol. 2015;195:4973–4985. doi: 10.4049/jimmunol.1500651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O’Sullivan TE, Rapp M, Fan X, Weizman OE, Bhardwaj P, Adams NM, Walzer T, Dannenberg AJ, Sun JC. Adipose-Resident Group 1 Innate Lymphoid Cells Promote Obesity-Associated Insulin Resistance. Immunity. 2016;45:428–441. doi: 10.1016/j.immuni.2016.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lund S, Walford HH, Doherty TA. Type 2 Innate Lymphoid Cells in Allergic Disease. Curr Immunol Rev. 2013;9:214–221. doi: 10.2174/1573395510666140304235916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klose CSN, Flach M, Mohle L, Rogell L, Hoyler T, Ebert K, Fabiunke C, Pfeifer D, Sexl V, Fonseca-Pereira D, Domingues RG, Veiga-Fernandes H, Arnold SJ, Busslinger M, Dunay IR, Tanriver Y, Diefenbach A. Differentiation of type 1 ILCs from a common progenitor to all helper-like innate lymphoid cell lineages. Cell. 2014;157:340–356. doi: 10.1016/j.cell.2014.03.030. [DOI] [PubMed] [Google Scholar]
- 19.Gasteiger G, Fan X, Dikiy S, Lee SY, Rudensky AY. Tissue residency of innate lymphoid cells in lymphoid and nonlymphoid organs. Science. 2015;350:981–985. doi: 10.1126/science.aac9593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Melsen JE, Lugthart G, Lankester AC, Schilham MW. Human Circulating and Tissue-Resident CD56(bright) Natural Killer Cell Populations. Front Immunol. 2016;7:262. doi: 10.3389/fimmu.2016.00262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Renoux VM, Zriwil A, Peitzsch C, Michaelsson J, Friberg D, Soneji S, Sitnicka E. Identification of a Human Natural Killer Cell Lineage-Restricted Progenitor in Fetal and Adult Tissues. Immunity. 2015;43:394–407. doi: 10.1016/j.immuni.2015.07.011. [DOI] [PubMed] [Google Scholar]
- 22.Montaldo E, Vacca P, Vitale C, Moretta F, Locatelli F, Mingari MC, Moretta L. Human innate lymphoid cells. Immunol Lett. 2016;179:2–8. doi: 10.1016/j.imlet.2016.01.007. [DOI] [PubMed] [Google Scholar]
- 23.Lim AI, Li Y, Lopez-Lastra S, Stadhouders R, Paul F, Casrouge A, Serafini N, Puel A, Bustamante J, Surace L, Masse-Ranson G, David E, Strick-Marchand H, Le Bourhis L, Cocchi R, Topazio D, Graziano P, Muscarella LA, Rogge L, Norel X, Sallenave JM, Allez M, Graf T, Hendriks RW, Casanova JL, Amit I, Yssel H, Di Santo JP. Systemic Human ILC Precursors Provide a Substrate for Tissue ILC Differentiation. Cell. 2017;168:1086–1100 e1010. doi: 10.1016/j.cell.2017.02.021. [DOI] [PubMed] [Google Scholar]
- 24.Lugthart G, Melsen JE, Vervat C, van Ostaijen-Ten Dam MM, Corver WE, Roelen DL, van Bergen J, van Tol MJ, Lankester AC, Schilham MW. Human Lymphoid Tissues Harbor a Distinct CD69+CXCR6+ NK Cell Population. J Immunol. 2016;197:78–84. doi: 10.4049/jimmunol.1502603. [DOI] [PubMed] [Google Scholar]
- 25.Sojka DK, Plougastel-Douglas B, Yang L, Pak-Wittel MA, Artyomov MN, Ivanova Y, Zhong C, Chase JM, Rothman PB, Yu J, Riley JK, Zhu J, Tian Z, Yokoyama WM. Tissue-resident natural killer (NK) cells are cell lineages distinct from thymic and conventional splenic NK cells. Elife. 2014;3:e01659. doi: 10.7554/eLife.01659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dadi S, Chhangawala S, Whitlock BM, Franklin RA, Luo CT, Oh SA, Toure A, Pritykin Y, Huse M, Leslie CS, Li MO. Cancer Immunosurveillance by Tissue-Resident Innate Lymphoid Cells and Innate-like T Cells. Cell. 2016;164:365–377. doi: 10.1016/j.cell.2016.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vonarbourg C, Mortha A, Bui VL, Hernandez PP, Kiss EA, Hoyler T, Flach M, Bengsch B, Thimme R, Holscher C, Honig M, Pannicke U, Schwarz K, Ware CF, Finke D, Diefenbach A. Regulated expression of nuclear receptor RORgammat confers distinct functional fates to NK cell receptor-expressing RORgammat(+) innate lymphocytes. Immunity. 2010;33:736–751. doi: 10.1016/j.immuni.2010.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bernink JH, Peters CP, Munneke M, te Velde AA, Meijer SL, Weijer K, Hreggvidsdottir HS, Heinsbroek SE, Legrand N, Buskens CJ, Bemelman WA, Mjosberg JM, Spits H. Human type 1 innate lymphoid cells accumulate in inflamed mucosal tissues. Nat Immunol. 2013;14:221–229. doi: 10.1038/ni.2534. [DOI] [PubMed] [Google Scholar]
- 29.Bernink JH, Krabbendam L, Germar K, de Jong E, Gronke K, Kofoed-Nielsen M, Munneke JM, Hazenberg MD, Villaudy J, Buskens CJ, Bemelman WA, Diefenbach A, Blom B, Spits H. Interleukin-12 and -23 Control Plasticity of CD127(+) Group 1 and Group 3 Innate Lymphoid Cells in the Intestinal Lamina Propria. Immunity. 2015;43:146–160. doi: 10.1016/j.immuni.2015.06.019. [DOI] [PubMed] [Google Scholar]
- 30.Nussbaum K, Burkhard SH, Ohs I, Mair F, Klose CSN, Arnold SJ, Diefenbach A, Tugues S, Becher B. Tissue microenvironment dictates the fate and tumor-suppressive function of type 3 ILCs. J Exp Med. 2017 doi: 10.1084/jem.20162031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hirota K, Duarte JH, Veldhoen M, Hornsby E, Li Y, Cua DJ, Ahlfors H, Wilhelm C, Tolaini M, Menzel U, Garefalaki A, Potocnik AJ, Stockinger B. Fate mapping of IL-17-producing T cells in inflammatory responses. Nat Immunol. 2011;12:255–263. doi: 10.1038/ni.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fuchs A, Vermi W, Lee JS, Lonardi S, Gilfillan S, Newberry RD, Cella M, Colonna M. Intraepithelial type 1 innate lymphoid cells are a unique subset of IL-12- and IL-15-responsive IFN-gamma-producing cells. Immunity. 2013;38:769–781. doi: 10.1016/j.immuni.2013.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cortez VS, Fuchs A, Cella M, Gilfillan S, Colonna M. Cutting edge: Salivary gland NK cells develop independently of Nfil3 in steady-state. J Immunol. 2014;192:4487–4491. doi: 10.4049/jimmunol.1303469. [DOI] [PubMed] [Google Scholar]
- 34.Cortez VS, Robinette ML, Colonna M. Innate lymphoid cells: new insights into function and development. Curr Opin Immunol. 2015;32:71–77. doi: 10.1016/j.coi.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Daussy C, Faure F, Mayol K, Viel S, Gasteiger G, Charrier E, Bienvenu J, Henry T, Debien E, Hasan UA, Marvel J, Yoh K, Takahashi S, Prinz I, de Bernard S, Buffat L, Walzer T. T-bet and Eomes instruct the development of two distinct natural killer cell lineages in the liver and in the bone marrow. J Exp Med. 2014;211:563–577. doi: 10.1084/jem.20131560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ferlazzo G, Thomas D, Lin SL, Goodman K, Morandi B, Muller WA, Moretta A, Munz C. The abundant NK cells in human secondary lymphoid tissues require activation to express killer cell Ig-like receptors and become cytolytic. J Immunol. 2004;172:1455–1462. doi: 10.4049/jimmunol.172.3.1455. [DOI] [PubMed] [Google Scholar]
- 37.Moffett A, Colucci F. Uterine NK cells: active regulators at the maternal-fetal interface. J Clin Invest. 2014;124:1872–1879. doi: 10.1172/JCI68107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stegmann KA, Robertson F, Hansi N, Gill U, Pallant C, Christophides T, Pallett LJ, Peppa D, Dunn C, Fusai G, Male V, Davidson BR, Kennedy P, Maini MK. CXCR6 marks a novel subset of T-bet(lo)Eomes(hi) natural killer cells residing in human liver. Sci Rep. 2016;6:26157. doi: 10.1038/srep26157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gaynor LM, Colucci F. Uterine Natural Killer Cells: Functional Distinctions and Influence on Pregnancy in Humans and Mice. Front Immunol. 2017;8:467. doi: 10.3389/fimmu.2017.00467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Koopman LA, Kopcow HD, Rybalov B, Boyson JE, Orange JS, Schatz F, Masch R, Lockwood CJ, Schachter AD, Park PJ, Strominger JL. Human decidual natural killer cells are a unique NK cell subset with immunomodulatory potential. J Exp Med. 2003;198:1201–1212. doi: 10.1084/jem.20030305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cella M, Otero K, Colonna M. Expansion of human NK-22 cells with IL-7, IL-2, and IL-1beta reveals intrinsic functional plasticity. Proc Natl Acad Sci U S A. 2010;107:10961–10966. doi: 10.1073/pnas.1005641107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lim AI, Menegatti S, Bustamante J, Le Bourhis L, Allez M, Rogge L, Casanova JL, Yssel H, Di Santo JP. IL-12 drives functional plasticity of human group 2 innate lymphoid cells. J Exp Med. 2016;213:569–583. doi: 10.1084/jem.20151750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Simoni Y, Fehlings M, Kloverpris HN, McGovern N, Koo SL, Loh CY, Lim S, Kurioka A, Fergusson JR, Tang CL, Kam MH, Dennis K, Lim TK, Fui AC, Hoong CW, Chan JK, Curotto de Lafaille M, Narayanan S, Baig S, Shabeer M, Toh SE, Tan HK, Anicete R, Tan EH, Takano A, Klenerman P, Leslie A, Tan DS, Tan IB, Ginhoux F, Newell EW. Human Innate Lymphoid Cell Subsets Possess Tissue-Type Based Heterogeneity in Phenotype and Frequency. Immunity. 2017;46:148–161. doi: 10.1016/j.immuni.2016.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bernink JH, Mjosberg J, Spits H. Human ILC1: To Be or Not to Be. Immunity. 2017;46:756–757. doi: 10.1016/j.immuni.2017.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smyth MJ, Thia KY, Street SE, MacGregor D, Godfrey DI, Trapani JA. Perforin-mediated cytotoxicity is critical for surveillance of spontaneous lymphoma. J Exp Med. 2000;192:755–760. doi: 10.1084/jem.192.5.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Street SE, Zerafa N, Iezzi M, Westwood JA, Stagg J, Musiani P, Smyth MJ. Host perforin reduces tumor number but does not increase survival in oncogene-driven mammary adenocarcinoma. Cancer Res. 2007;67:5454–5460. doi: 10.1158/0008-5472.CAN-06-4084. [DOI] [PubMed] [Google Scholar]
- 47.Zerafa N, Westwood JA, Cretney E, Mitchell S, Waring P, Iezzi M, Smyth MJ. Cutting edge: TRAIL deficiency accelerates hematological malignancies. J Immunol. 2005;175:5586–5590. doi: 10.4049/jimmunol.175.9.5586. [DOI] [PubMed] [Google Scholar]
- 48.Finnberg N, Klein-Szanto AJ, El-Deiry WS. TRAIL-R deficiency in mice promotes susceptibility to chronic inflammation and tumorigenesis. J Clin Invest. 2008;118:111–123. doi: 10.1172/JCI29900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Walczak H. Death receptor-ligand systems in cancer, cell death, and inflammation. Cold Spring Harb Perspect Biol. 2013;5:a008698. doi: 10.1101/cshperspect.a008698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Le Floc’h A, Jalil A, Franciszkiewicz K, Validire P, Vergnon I, Mami-Chouaib F. Minimal engagement of CD103 on cytotoxic T lymphocytes with an E-cadherin-Fc molecule triggers lytic granule polarization via a phospholipase Cgamma-dependent pathway. Cancer Res. 2011;71:328–338. doi: 10.1158/0008-5472.CAN-10-2457. [DOI] [PubMed] [Google Scholar]
- 51.Workel HH, Komdeur FL, Wouters MC, Plat A, Klip HG, Eggink FA, Wisman GB, Arts HJ, Oonk MH, Mourits MJ, Yigit R, Versluis M, Duiker EW, Hollema H, de Bruyn M, Nijman HW. CD103 defines intraepithelial CD8+ PD1+ tumour-infiltrating lymphocytes of prognostic significance in endometrial adenocarcinoma. Eur J Cancer. 2016;60:1–11. doi: 10.1016/j.ejca.2016.02.026. [DOI] [PubMed] [Google Scholar]
- 52.Webb JR, Milne K, Nelson BH. PD-1 and CD103 Are Widely Coexpressed on Prognostically Favorable Intraepithelial CD8 T Cells in Human Ovarian Cancer. Cancer Immunol Res. 2015;3:926–935. doi: 10.1158/2326-6066.CIR-14-0239. [DOI] [PubMed] [Google Scholar]
- 53.Bosmuller HC, Wagner P, Peper JK, Schuster H, Pham DL, Greif K, Beschorner C, Rammensee HG, Stevanovic S, Fend F, Staebler A. Combined Immunoscore of CD103 and CD3 Identifies Long-Term Survivors in High-Grade Serous Ovarian Cancer. Int J Gynecol Cancer. 2016;26:671–679. doi: 10.1097/IGC.0000000000000672. [DOI] [PubMed] [Google Scholar]
- 54.Djenidi F, Adam J, Goubar A, Durgeau A, Meurice G, de Montpreville V, Validire P, Besse B, Mami-Chouaib F. CD8+CD103+ tumor-infiltrating lymphocytes are tumor-specific tissue-resident memory T cells and a prognostic factor for survival in lung cancer patients. J Immunol. 2015;194:3475–3486. doi: 10.4049/jimmunol.1402711. [DOI] [PubMed] [Google Scholar]
- 55.Wang B, Wu S, Zeng H, Liu Z, Dong W, He W, Chen X, Dong X, Zheng L, Lin T, Huang J. CD103+ Tumor Infiltrating Lymphocytes Predict a Favorable Prognosis in Urothelial Cell Carcinoma of the Bladder. J Urol. 2015;194:556–562. doi: 10.1016/j.juro.2015.02.2941. [DOI] [PubMed] [Google Scholar]
- 56.Crome SQ, Nguyen LT, Lopez-Verges S, Yang SY, Martin B, Yam JY, Johnson DJ, Nie J, Pniak M, Yen PH, Milea A, Sowamber R, Katz SR, Bernardini MQ, Clarke BA, Shaw PA, Lang PA, Berman HK, Pugh TJ, Lanier LL, Ohashi PS. A distinct innate lymphoid cell population regulates tumor-associated T cells. Nat Med. 2017;23:368–375. doi: 10.1038/nm.4278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Okabe Y, Medzhitov R. Tissue biology perspective on macrophages. Nat Immunol. 2016;17:9–17. doi: 10.1038/ni.3320. [DOI] [PubMed] [Google Scholar]
- 58.Fan X, Rudensky AY. Hallmarks of Tissue-Resident Lymphocytes. Cell. 2016;164:1198–1211. doi: 10.1016/j.cell.2016.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Savage PA, Vosseller K, Kang C, Larimore K, Riedel E, Wojnoonski K, Jungbluth AA, Allison JP. Recognition of a ubiquitous self antigen by prostate cancer-infiltrating CD8+ T lymphocytes. Science. 2008;319:215–220. doi: 10.1126/science.1148886. [DOI] [PubMed] [Google Scholar]
- 60.Deng W, Gowen BG, Zhang L, Wang L, Lau S, Iannello A, Xu J, Rovis TL, Xiong N, Raulet DH. Antitumor immunity. A shed NKG2D ligand that promotes natural killer cell activation and tumor rejection. Science. 2015;348:136–139. doi: 10.1126/science.1258867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lanier LL. NKG2D Receptor and Its Ligands in Host Defense. Cancer Immunol Res. 2015;3:575–582. doi: 10.1158/2326-6066.CIR-15-0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yokoyama WM, Kim S. Licensing of natural killer cells by self-major histocompatibility complex class I. Immunol Rev. 2006;214:143–154. doi: 10.1111/j.1600-065X.2006.00458.x. [DOI] [PubMed] [Google Scholar]
- 63.Jabri B, Abadie V. IL-15 functions as a danger signal to regulate tissue-resident T cells and tissue destruction. Nat Rev Immunol. 2015;15:771–783. doi: 10.1038/nri3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mlecnik B, Bindea G, Angell HK, Sasso MS, Obenauf AC, Fredriksen T, Lafontaine L, Bilocq AM, Kirilovsky A, Tosolini M, Waldner M, Berger A, Fridman WH, Rafii A, Valge-Archer V, Pages F, Speicher MR, Galon J. Functional network pipeline reveals genetic determinants associated with in situ lymphocyte proliferation and survival of cancer patients. Sci Transl Med. 2014;6:228ra237. doi: 10.1126/scitranslmed.3007240. [DOI] [PubMed] [Google Scholar]
- 65.Eisenring M, vom Berg J, Kristiansen G, Saller E, Becher B. IL-12 initiates tumor rejection via lymphoid tissue-inducer cells bearing the natural cytotoxicity receptor NKp46. Nat Immunol. 2010;11:1030–1038. doi: 10.1038/ni.1947. [DOI] [PubMed] [Google Scholar]
- 66.Cortez VS, Cervantes-Barragan L, Robinette ML, Bando JK, Wang Y, Geiger TL, Gilfillan S, Fuchs A, Vivier E, Sun JC, Cella M, Colonna M. Transforming Growth Factor-beta Signaling Guides the Differentiation of Innate Lymphoid Cells in Salivary Glands. Immunity. 2016;44:1127–1139. doi: 10.1016/j.immuni.2016.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mackay LK, Rahimpour A, Ma JZ, Collins N, Stock AT, Hafon ML, Vega-Ramos J, Lauzurica P, Mueller SN, Stefanovic T, Tscharke DC, Heath WR, Inouye M, Carbone FR, Gebhardt T. The developmental pathway for CD103(+)CD8+ tissue-resident memory T cells of skin. Nat Immunol. 2013;14:1294–1301. doi: 10.1038/ni.2744. [DOI] [PubMed] [Google Scholar]
- 68.Ganesan AP, Clarke J, Wood O, Garrido-Martin EM, Chee SJ, Mellows T, Samaniego-Castruita D, Singh D, Seumois G, Alzetani A, Woo E, Friedmann PS, King EV, Thomas GJ, Sanchez-Elsner T, Vijayanand P, Ottensmeier CH. Tissue-resident memory features are linked to the magnitude of cytotoxic T cell responses in human lung cancer. Nat Immunol. 2017;18:940–950. doi: 10.1038/ni.3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Malik BT, Byrne KT, Vella JL, Zhang P, Shabaneh TB, Steinberg SM, Molodtsov AK, Bowers JS, Angeles CV, Paulos CM, Huang YH, Turk MJ. Resident memory T cells in the skin mediate durable immunity to melanoma. Sci Immunol. 2017;2 doi: 10.1126/sciimmunol.aam6346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cortez VS, Ulland TK, Cervantes-Barragan L, Bando JK, Robinette ML, Wang Q, White AJ, Gilfillan S, Cella M, Colonna M. SMAD4 impedes the conversion of NK cells into ILC1-like cells by curtailing non-canonical TGF-beta signaling. Nat Immunol. 2017;18:995–1003. doi: 10.1038/ni.3809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gao Y, Souza-Fonseca-Guimaraes F, Bald T, Ng SS, Young A, Ngiow SF, Rautela J, Straube J, Waddell N, Blake SJ, Yan J, Bartholin L, Lee JS, Vivier E, Takeda K, Messaoudene M, Zitvogel L, Teng MWL, Belz GT, Engwerda CR, Huntington ND, Nakamura K, Holzel M, Smyth MJ. Tumor immunoevasion by the conversion of effector NK cells into type 1 innate lymphoid cells. Nat Immunol. 2017;18:1004–1015. doi: 10.1038/ni.3800. [DOI] [PubMed] [Google Scholar]
- 72.Constantinides MG, Gudjonson H, McDonald BD, Ishizuka IE, Verhoef PA, Dinner AR, Bendelac A. PLZF expression maps the early stages of ILC1 lineage development. Proc Natl Acad Sci U S A. 2015;112:5123–5128. doi: 10.1073/pnas.1423244112. [DOI] [PMC free article] [PubMed] [Google Scholar]


