Abstract
Background
Follistatin-like protein 1 (FSTL1) is an emerging cardiokine/myokine that is upregulated in heart failure (HF) and found to be cardioprotective in animal models of cardiac injury. We tested the hypothesis that circulating FSTL1 can affect cardiac function and metabolism under baseline physiological conditions and in HF.
Methods and Results
FSTL1 was acutely (10 minutes) or chronically infused (2 weeks) to attain clinically relevant blood levels in conscious dogs with cardiac tachypacing-induced HF. Dogs with no cardiac pacing and FSTL1 infusion served as control. 3H-oleate and 14C-glucose were infused to track the metabolic fate of free fatty acids (FFA) and glucose. Cardiac uptake of lactate and ketone bodies and systemic respiratory quotient (RQ) were also measured. HF caused a shift from prevalent cardiac and systemic fat to carbohydrate oxidation. While acute FSTL1 administration caused minimal hemodynamic changes at baseline, in HF dogs it enhanced cardiac oxygen consumption and transiently reversed the changes in FFA and glucose oxidation and systemic RQ. In HF, chronic FSTL1 infusion stably normalized cardiac FFA, glucose, ketone body consumption and systemic RQ, while moderately improving diastolic and contractile function. Consistently, FSTL1 prevented the downregulation of medium chain acyl-CoA dehydrogenase, a representative enzyme of the FFA oxidation pathway. Complementary in vitro experiments in primary cardiac and skeletal muscle myocytes showed that FSTL1 stimulated oxygen consumption through AMPK activation.
Conclusions
These findings support a novel function for FSTL1 and provide the first direct evidence that a circulating cardiokine/myokine can alter myocardial and systemic energy substrate metabolism, in vivo.
Journal Subject Terms: Animal Model of Human Disease, Growth Factor/Cytokine, Metabolism, Heart Failure
The term “myokines” refers to cytokines or other peptides and proteins produced and secreted by skeletal muscle fibers (1–3). This concept has also been extended to factors secreted by cardiac muscle and termed “cardiokines”(4–5). Well-known cardiac hormones such as atrial and brain natriuretic peptides are classical examples of cardiokines. Intriguingly, some secreted proteins are both myokines and cardiokines and can display endocrine, paracrine and autocrine functions. Cardiokines modulate a variety of biological phenomena, ranging from inflammation, fibrosis and cardiomyocyte growth to apoptosis (4). We previously identified follistatin-like protein 1 (FSTL1), alternatively named TSC36 (TGFβ stimulating clone B), as a candidate cardiokine (6) and later showed that it is also produced by skeletal muscle and can function as a myokine (7). To date, diverse actions have been attributed to FSTL1, including myocardial protection against ischemia-reperfusion injury, post-infarct cardiac rupture and overload-induced hypertrophy, as well as the promotion of revascularization and cardiac regeneration (8–11). FSTL1 has been also proposed as a potential clinical biomarker, since its serum levels increase in systolic and diastolic heart failure (HF) (12–13) and it exhibits prognostic significance in acute coronary syndrome (8).
Despite this wealth of information, no studies have been performed to determine the potential effects of circulating FSTL1 on cardiac function and energy metabolism. The possible influence of FSTL1 on cardiac muscle metabolism was suggested by our prior study (9) showing that FSTL1-deficient mice display reduced TAC-induced AMP-activated kinase (AMPK) activation in heart, whereas FSTL1 overexpression had the opposite effect. AMPK is recognized as a major regulator of glucose and lipid metabolism in various organs, including heart and skeletal muscle, and it also functions to promote mitochondrial biogenesis (14).
In the present study, we used a dog model to test the hypothesis that circulating FSTL1 can affect cardiac function and metabolism under baseline physiological conditions and in HF. Hemodynamics, cardiac function and rates of myocardial oxygen, free fatty acid (FFA), glucose, lactate and ketone body consumption were measured in conscious dogs. Animal size allowed the collection of blood samples from both of the aorta and coronary sinus as well as the pulmonary artery, thus permitting the simultaneous assessment of changes in cardiac and systemic metabolism. Parallel experiments were performed in isolated cardiac and skeletal myocytes to elucidate the influence of FSTL1 on mitochondrial respiration.
METHODS
A more complete description of Methods can be found in Supplements.
Surgical Instrumentation
Twenty-nine male mongrel dogs (age: 9–13 months; weight: 21–27 kg) were chronically instrumented as previously described (15–16). The protocol was approved by the Institutional Animal Care and Use Committee (IACUC) of the Temple University and conformed to the guiding principles for the care and use of laboratory animals published by the National Institutes of Health. The data that support the findings of this study are available from the corresponding author upon reasonable request.
General Protocol
All the experiments were performed in conscious dogs recumbent on the right side on the laboratory table. Two catheters were inserted, respectively, into the coronary sinus and the pulmonary artery, through peripheral veins, under X-ray fluoroscopic guidance. The isotopic tracers [9,10-3H]-oleate (0.7 µCi/min) and [U-14C]-glucose (20 µCi as a bolus, followed by 0.3 µCi/min) were infused through a peripheral vein to track, respectively, the metabolic fate of FFA and glucose used by cardiac muscle as source of energy (15–17). After 40 minutes of tracer infusion, baseline hemodynamics were recorded and paired blood samples withdrawn from aorta, coronary sinus, and pulmonary artery. We could not obtain reliable data from two dogs, due to severe hypotension likely caused by unexpected reaction to radiolabeled tracers infusion. Therefore the group sizes described below correspond to the number of dogs that could effectively undergo a complete study.
Recombinant FSTL1 protein for the infusion protocols
Two types of recombinant human FSTL1 proteins were produced via custom order, namely FSTL1-6His and FSTL1-Fc.
Acute FSTL1 infusion protocol
HF was induced in 7 dogs by pacing the LV at 210 beats/min for 3 weeks and at 240 beats/min for an additional week (15–16). Cardiac pacing was maintained for 28–29 days, when end-diastolic pressure reached ≥25 mmHg. The terminal experiment was performed at that time point. HF dogs were studied at the spontaneous heart rate, with the pacemaker turned off. Seven chronically instrumented dogs were used as normal controls, and during the experiment their hearts were paced to match the spontaneous heart rate measured at baseline in HF dogs. Once the baseline hemodynamic data were collected after 40 minutes of radiotracers infusion, human recombinant FSTL1-6His was infused at the dose of 20 µg/kg over 10 minutes. In pilot experiments, we tested a dose of FSTL1 similar to that used intracoronarily in a prior pig study (8), but found that the changes, although present, were in some cases inconsistent (data not shown). Therefore a dose 5 times higher was chosen, considering that FSTL1 administration to dogs was systemic and not intracoronary. All data and blood sample were collected at 10 minutes, 30 minutes, and 60 minutes after FSTL1 administration. At the end of the protocol, dogs were euthanized with 100 mg/kg of sodium pentobarbital. The heart was then removed and weighed.
Chronic FSTL1 infusion protocol
After 2 weeks of cardiac pacing, 7 dogs were administered a priming bolus of 4 µg/kg followed by a continuous infusion of 10 ng/kg/min of FSTL1-Fc by an external pump connected to a catheter placed in the left atrium. This dose was based on preliminary assessments of FSTL1-Fc half-life in the mouse circulation (data not shown). Our goal was to maintain stable high levels in dog blood, mimicking the FSTL1 levels found in humans with HF (12–13). The infusion and cardiac pacing were maintained for 14 days to match the 28–29 days duration of the pacing protocol in HF dogs not receiving chronic FSTL1 infusion. The terminal experimental was performed at the end of the fourth week of cardiac pacing. Six chronically instrumented dogs were used as control and infused with 4 µg/kg (priming bolus) followed by 10 ng/kg/min FSTL1 for 14 days, with no cardiac pacing. The terminal experiments in both groups were performed without discontinuing FSTL1 infusion. During the experiments, control dog hearts were paced to match the spontaneous heart rate measured at baseline in HF dogs subjected to FSTL1 infusion. Hemodynamic measurements and paired blood samples from aorta, coronary sinus and pulmonary artery were taken at baseline, as described in the general protocol, and during β-adrenergic stimulation with dobutamine infusion at 5, 10 and 15 µg/kg/min, 5 minutes for each dose. The response to dobutamine was compared with that previously recorded in historical groups of normal (n=5) and HF (n=5) dogs not subjected to any chronic treatment.
Once all the hemodynamics and blood samples were taken, dogs were euthanized with 100 mg/kg of sodium pentobarbital. Cardiac tissue samples were freeze-clamped for subsequent molecular analysis and the heart was then removed and weighed.
Hemodynamic Recordings and Calculated Parameters
Directly measured parameters were: heart rate, LV end-diastolic, peak systolic and end-systolic pressure. Calculated parameters were: mean aortic pressure, mean blood flow in the left circumflex coronary artery and the first derivative of LV pressure (dP/dt). dP/dtmax is an index of contractility and dP/dtminis an index of diastolic relaxation rate during the isovolumic phase. The time constant of isovolumic pressure decay (Tau) was calculated as an additional index of LV diastolic relaxation. Data for each time point were obtained by averaging measures over one respiratory cycle. The difference between end-diastolic and end-systolic LV internal diameters was used as a surrogate of stroke volume and multiplied by heart rate to obtain a surrogate of cardiac output. Finally, the area of LV pressure–diameter loops (PDA) was calculated to obtain an index of stroke work (15,18).
Oxygen and Energy Substrate Consumption
Blood gases tension and oxygen concentration were determined to calculate myocardial oxygen consumption (MVO2), which was then normalized by cardiac weight. LV external mechanical efficiency was calculated as the ratio PDA/MVO2/beat (15). Systemic oxygen consumption was calculated by multiplying the difference in oxygen content between aorta and pulmonary artery blood by the surrogate of cardiac output and divided by body weight.
To calculate the cardiac and systemic respiratory quotient (RQ), total CO2 concentration in arterial and CS or pulmonary blood was initially determined according to a method described previously (19). Values of RQ range theoretically between 0.707, corresponding to oxidation of fat as the only substrate, and 1, corresponding to oxidation of carbohydrates as the only substrate.
Cardiac energy substrate consumption
The concentrations of total and labeled FFA, glucose, lactic acid and ketone bodies were determined in arterial and coronary sinus blood samples and the rate of cardiac substrate uptake and oxidation were calculated according to well-established methods (17–18). All the calculated values of cardiac substrate uptake and oxidation were normalized by heart rate and heart weight.
Tagged FSTL1 quantification in plasma
FSTL1-Fc protein concentration was measured using human Fc and IgG ELISA Kit (MednaBio), according to the manufacturer’s instructions.
Western blots
Protein was extracted from frozen tissue to measure citrate synthase (the entry enzyme of the Krebs cycle) medium-chain acyl-CoA dehydrogenase (MCAD, widely assessed as a major representative enzyme of the fatty acid beta-oxidation pathway) and the phosphorylation/activation state of AMPK, as previously described (20–21).
Measurements of mitochondrial O2 consumption Rate, in vitro
Primary cell isolation
Primary canine myoblasts were isolated from the gastrocnemius muscle and grown in complete medium composed of F-10 basal medium, 20% FBS, 1% GlutaMAX™ and antibiotics (22). Primary mouse cardiac myocytes were isolated from the LV free wall and septum of C57BL6 wildtype mice as per the protocol previously described (23).
Mitochondrial oxygen consumption
Mitochondrial oxygen consumption rate (OCR) was measured using the Seahorse Bioscience XF96 Analyzer (Agilent Technologies, Santa Clara, CA), as previously described (23–24).
Statistical Analysis
Statistical analysis was performed by using commercially available software (SigmaStat 4.0). Data were found normally distributed, therefore changes at different time points or for different experimental conditions were compared by one-way ANOVA and between groups comparisons were performed by two-way ANOVA, in both cases followed by Student–Newman post hoc test. Oxygen consumption rates measured in vitro were measured in four independent cell preparations. Data are presented as mean ± SEM (in Figures) or SD (in Tables and text). For all the statistical analyses, significance was accepted at P<0.05.
RESULTS
Body and cardiac weight in the 4 experimental groups
The study protocol was comprised of 4 experimental groups: 1) control and 2) HF dogs with or without acute infusion of FSTL1 and 3) control and 4) HF dogs with or without chronic infusion of FSTL1. Body weight was 23.5±0.6 kg in control dogs and neither HF nor chronic elevation of circulating levels of FSTL1 caused statistically significant differences among the 4 groups. Total cardiac weight was 192.0±17.5 g in control and 224.7±17.9 g in HF (P<0.05) and was not significantly affected by chronic FSTL1 infusion, both in control and HF dogs.
Hemodynamics and cardiac function after acute FSTL1 administration
The ELISA kit could not detect FSTL1 tagged with 6His. Therefore, to assess changes in serum FSTL1 level after acute administration, we infused 20 µg/kg of FSTL1-Fc over 10 minutes in 4 dogs (3 control and 1 in HF), not included in the main protocol, and collected blood samples at 10, 30 and 60 minutes, as described in Methods. As shown in Supplemental Fig. 1A, the concentration of exogenous FSTL1 was highest at the 10 minute time point of infusion (end of infusion) and then slowly declined, but remained relatively high, over the ensuing 50 minutes.
The changes in hemodynamics and cardiac function in control and HF dogs at 10 min, 30 and 60 minutes after the beginning of acute FSTL1 administration are shown in Table 1. FSTL1 infusion in control dogs led to modest decreases in mean arterial pressure and cardiac output, but had no effect on other cardiac parameters. In HF dogs, heart rate was significantly decreased after FSTL1 infusion, while in control dogs the heart was paced at ~143 beats/min to match the baseline heart rate measured in HF and then maintained constant throughout the experiment. In HF dogs, LV end-diastolic pressure, an index of diastolic function relative to the filling phase, was significantly reduced at 60 minutes after the start of FSTL1 infusion, while FSLT1 led to a decline in cardiac output in control and HF dogs. No significant changes were observed in other parameters.
Table 1.
Hemodynamic changes after acute FSTL1 infusion (20 µg/kg over 10 minutes) in Control (n=7) and HF (n=7), and after chronic FSTL1 infusion (10 ng/kg/min for 14 days) in Control (n=6) and HF (n=7).
| Baseline | Acute infusion | Chronic Infusion |
||||
|---|---|---|---|---|---|---|
|
| ||||||
| 10 minutes | 30 minutes | 60 minutes | ||||
| Heart rate (beats/min) | Control | 144.2±1.2 | 144.8±1.3 | 144.5±0.9 | 144.6±2.0 | 134.7±5.3 |
| HF | 142.7±11.6 | 136.3±10.6*† | 134.9±9.8*† | 134.8±12.5*† | 126.0±12.5*† | |
|
| ||||||
| LVSP (mmHg) | Control | 130.7±9.9 | 125.6±10.8 | 121.4±9.1* | 122.4±11.2* | 122.3±11.3 |
| HF | 101.8±11.5† | 97.5±9.5† | 97.2±8.7† | 96.6±11.7† | 102.6±12.5*† | |
|
| ||||||
| dP/dt max (mmHg/s) | Control | 2659±623 | 2461±588 | 2228±348* | 2160±328* | 2530±264 |
| HF | 1499±225† | 1397±182† | 1389±166† | 1390±229† | 1880±208*† | |
|
| ||||||
| LVEDP (mmHg) | Control | 6.0±1.1 | 5.9±1.0 | 5.8±1.0 | 5.7±1.3 | 6.9±1.2 |
| HF | 25.6±1.8† | 21.6±2.7*† | 20.9±3.3*† | 19.6±3.6*† | 19.7±3.5*† | |
|
| ||||||
| LVEDD (mm) | Control | 35.3±2.1 | 35.2±2.3 | 34.5±2.3 | 34.8±2.1 | 34.6±2.4 |
| HF | 43.3±2.1† | 41.7±4.0† | 42.4±4.4† | 42.3±4.4† | 41.8±2.8*† | |
|
| ||||||
| dP/dt min (mmHg/s) | Control | −3191±578 | −3162±655 | −2887±502 | −2817±530* | −3010±427 |
| HF | −1887±377† | −1823±316† | −1820±297† | −1842±345† | −1934±218† | |
|
| ||||||
| Tau (sec) | Control | 25.9±3.3 | 24.5±2.3 | 25.6±4.2 | 25.6±4.1 | 20.9±5.1 |
| HF | 42.0±13.1† | 38.7±10.8† | 41.6±13.9† | 41.2±10.9† | 21.9±6.3* | |
|
| ||||||
| MAP (mmHg) | Control | 111.1±5.1 | 105.3±7.7* | 102.6±5.4* | 103.1±4.3* | 96.3±11.2 |
| HF | 88.3±10.3† | 85.3±13.2† | 85.3±13.6† | 85.6±15.3† | 85.2±11.2*† | |
|
| ||||||
| CO (surrogate units) | Control | 1362±205 | 1310±244 | 1125±178* | 1080±144* | 1358±178 |
| HF | 580±133† | 457±118† | 467±52*† | 462±116*† | 608±65*† | |
|
| ||||||
| PVR (surrogate units) | Control | 0.83±0.18 | 0.79±0.19 | 0.89±0.20 | 0.98±0.16 | 0.72±0.16 |
| HF | 1.42±0.28† | 1.55±0.15† | 1.58±0.20† | 1.62±0.23† | 1.41±0.16*† | |
|
| ||||||
| MCBF (ml/min) | Control | 29.8±2.1 | 29.6±2.1 | 29.3±2.7 | 29.0±3.3 | 29.1±1.0 |
| HF | 28.7±1.6 | 29.0±1.4 | 28.8±2.1 | 29.6±1.7 | 29.4±2.1 | |
P<0.05 vs. Baseline
P<0.05 HF vs. Control
Data are presented as mean ± SD. LVSP, left ventricular systolic pressure; LVEDP, left ventricular end-diastolic pressure; LVEDD, left ventricular end-diastolic diameter; MAP, mean aortic pressure; CO, cardiac output; PVR, total peripheral vascular resistance; and MCBF, mean blood flow in the circumflex coronary artery
Myocardial and systemic oxygen consumption and cardiac mechanical efficiency after acute FSTL1 administration
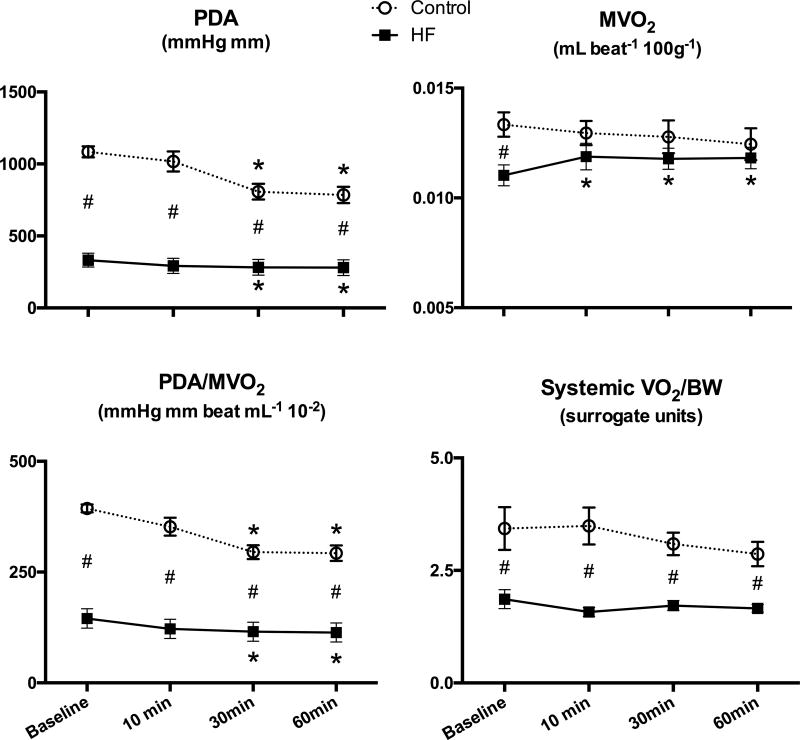
Consistent with the decrease in cardiac output, PDA, an index of stroke work, was decreased after acute FSTL1 administration in control and HF animals (Figure 1). Normalized MVO2 was significantly lower in HF dogs compared to control dogs at baseline, but FSTL1 infusion enhanced this parameter almost to the value observed in control dogs. Cardiac mechanical efficiency, quantified as PDA/MVO2/beat, was significantly decreased at 30 minutes and 60 minutes after FSTL1 infusion in control and HF (Figure 1). A significant difference in systemic oxygen consumption (systemic VO2/BW) was observed at baseline between the control and HF groups, but no changes were observed after FSTL1 administration in either group.
Figure 1. Effects of acute FSTL1 infusion on oxygen consumption and mechanical efficiency.
Changes in pressure-diameter area (PDA), myocardial oxygen consumption (MVO2), mechanical efficiency indexed by the ratio PDA/MVO2 and systemic oxygen consumption (systemic VO2) after acute FSTL1 infusion (20 µg/kg over 10 minutes) in Control (n=7) and HF (n=7). *P<0.05 vs. Baseline, # P<0.05 HF vs. Control.
Energy substrate oxidation, uptake, and respiratory quotient after acute FSTL1 administration
The arterial concentration of FFA was significantly lower and the arterial concentration of total ketone bodies was almost doubled in HF compared to control dogs (Table 2). Glucose and lactate levels were not different between control and HF dogs. In both groups, FSTL1 administration led to late decreases in FFA and glucose, but ketone bodies were reduced only in control dogs. Lactate concentrations were not significantly altered by any experimental condition.
Table 2.
Arterial concentrations of free fatty acids, glucose, lactate and total ketone bodies after acute FSTL1 infusion (20 µg/kg over 10 minutes) in Control (n=7) and HF (n=7), and after chronic FSTL1 infusion (10 ng/kg/min for 14 days) in Control (n=6) and HF (n=7)
| Baseline | Acute infusion | Chronic infusion |
||||
|---|---|---|---|---|---|---|
|
| ||||||
| 10 minutes | 30 minutes | 60 minutes | ||||
| Free fatty acids (mmol/l) | Control | 0.63±0.11 | 0.57±0.12 | 0.49±0.18 | 0.47±0.20* | 0.63±0.16 |
| HF | 0.48±0.11† | 0.52±0.19 | 0.41±0.20 | 0.35±0.15* | 0.58±0.13 | |
|
| ||||||
| Glucose (mg/dl) | Control | 88.3±5.7 | 84.2±7.9 | 83.9±11.1 | 81.7±10.2* | 80.3±6.1 |
| HF | 87.9±5.6 | 84.3±5.4 | 83.0±3.6 | 81.5±6.4* | 81.4±9.3 | |
|
| ||||||
| Lactate (mmol/l) | Control | 0.85±0.21 | 0.83±0.18 | 0.86±0.31 | 0.90±0.37 | 0.90±0.31 |
| HF | 1.03±0.22 | 0.99±0.37 | 1.02±0.33 | 1.09±0.36 | 0.90±0.28 | |
|
| ||||||
| Total ketone bodies (µmol/l) | Control | 50.7±5.6 | 43.6±6.6 | 39.5±2.9* | 35.8±8.5* | 52.7±14.5 |
| HF | 96.2±34.2† | 94.9±26.0† | 91.6±27.9† | 85.7±22.9† | 81.1±28.6 | |
P<0.05 vs. Baseline,
P<0.05 HF vs. Control
Data are presented as mean ± SD
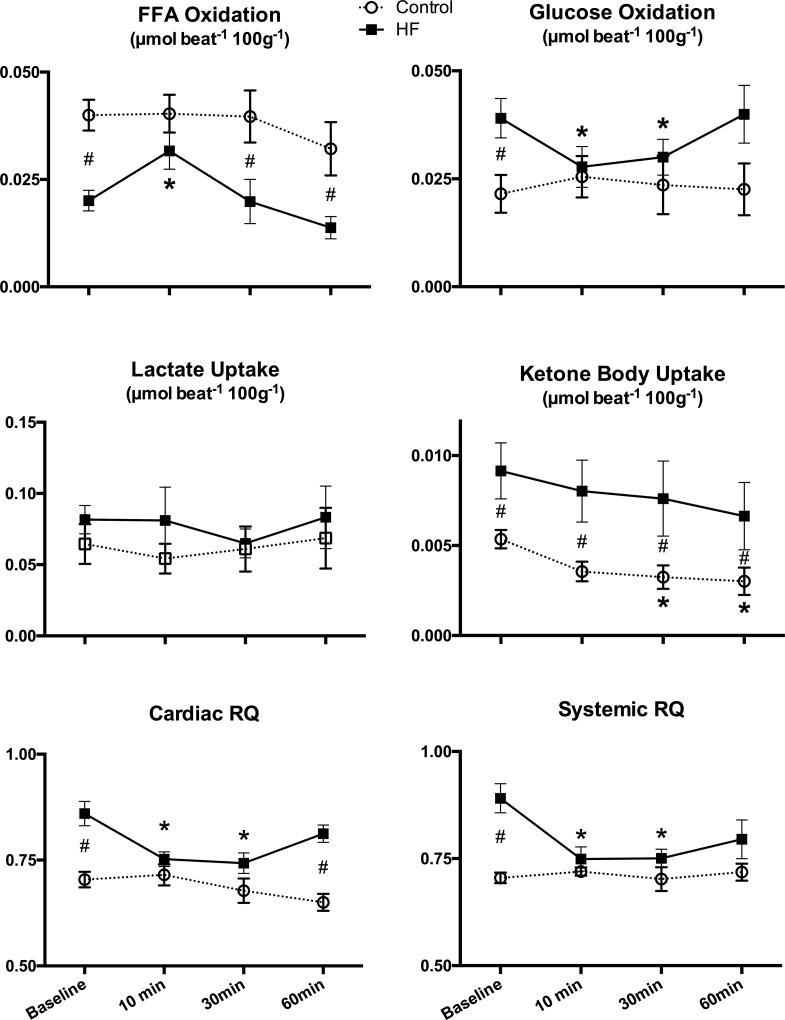
Consistent with previous findings (19–20), failing hearts utilized less FFA and much more glucose than normal hearts at baseline (Figure 2). Acute FSTL1 infusion markedly altered cardiac metabolism in HF. At the 10 minute time point, FSTL1 infusion enhanced FFA oxidation, while glucose oxidation decreased significantly, nullifying the statistical differences between the two groups. Cardiac ketone body uptake was significantly higher in HF compared to control throughout the time course. FSTL1 infusion caused a significant decrease of this parameter, but only in control dogs. Values of cardiac RQ were consistent with changes in FFA and glucose oxidation caused by HF and their reversal by FSTL1. Systemic RQ indicated that carbohydrate oxidation was more prevalent in the entire organism during HF, while acute FSTL1 administration caused a shift to greater fat oxidation.
Figure 2. Effects of acute FSTL1 infusion on substrate uptake and oxidation and respiratory quotient.
Changes in free fatty acid (FFA) oxidation, glucose oxidation, lactate uptake, ketone bodies uptake, cardiac respiratory quotient (RQ), and systemic RQ after acute FSTL1 infusion (20 µg/kg over 10 minutes) in control (n=7) and HF (n=7). * P<0.05 vs. Baseline, # P<0.05 HF vs. CTRL.
Hemodynamics and cardiac function after chronic FSTL1 administration
Supplemental Fig. 1B shows the arterial level of FSTL1 after 2 weeks of continuous infusion. FSTL1 levels in control and HF dogs reached high values and there was no significant difference between the two groups. The hemodynamic and cardiac functional values determined in control and HF dogs at baseline (groups described above) were utilized for comparison to identify the effects of chronic FSTL1 elevation (Table 1). No significant changes were found in any parameters in the control group, whereas FSTL1 infusion caused a significant decrease in heart rate and end-diastolic pressure in HF, indicating a modest amelioration of diastolic filling function and a delayed progression to end-stage failure, consistent with the preservation of physiological Tau values. On the other hand, dP/dtmin decreased in HF even after FSTL1 infusion. β-adrenergic responses are displayed in Supplemental Figure 2. In HF, FSTL1 modestly, but significantly potentiated the contractile response to β-adrenergic stimulation, as indicated by higher dP/dtmax compared to non-treated HF. This effect was not observed in the control group.
Oxygen consumption, mechanical efficiency and energy substrate metabolism after chronic FSTL1 administration
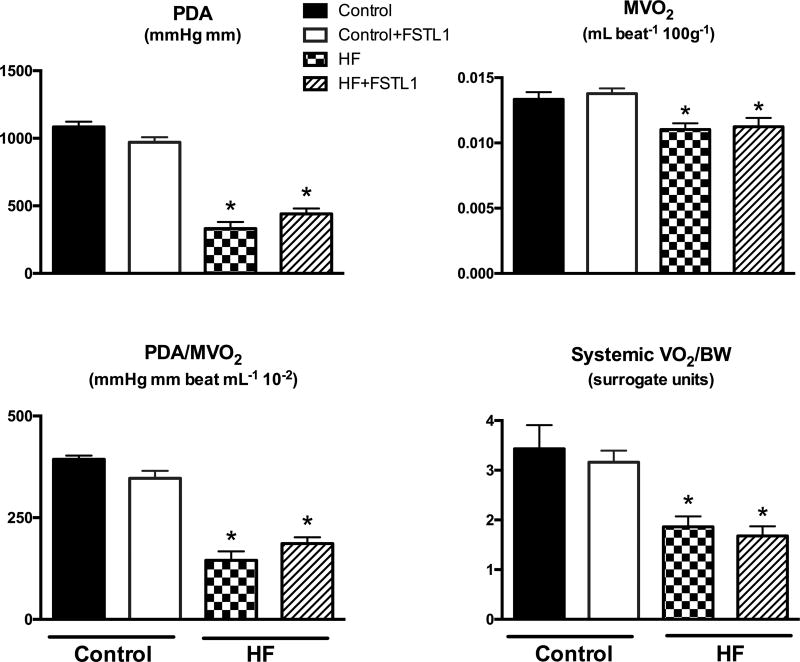
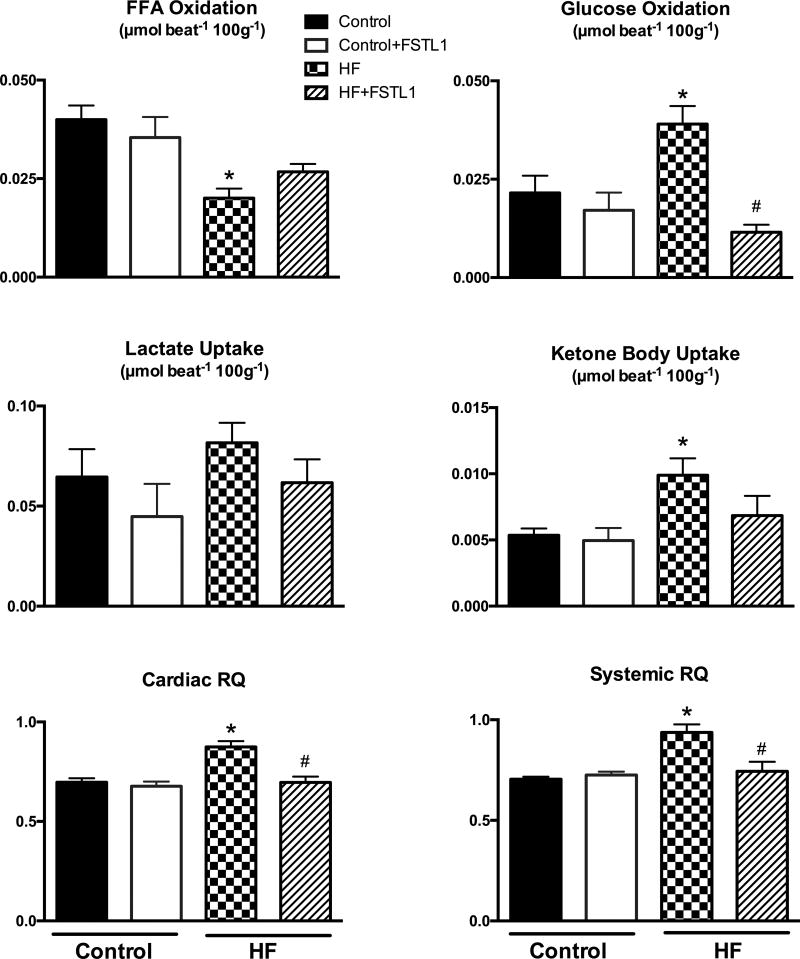
In the control group, chronic FSTL1 infusion did not lead to any significant change in cardiac or systemic oxygen consumption, or cardiac mechanical efficiency (Figure 3). Further, FSTL1 did not affect the arterial levels of energy substrates (Table 2) and no changes were found in cardiac FFA and glucose oxidation or lactate and ketone body uptake in control dogs (Figure 4). In contrast, chronically elevated levels of FSTL1 had a significant impact on cardiac and systemic substrate metabolism in HF. Similar to acute FSTL1 administration, glucose oxidation was inhibited by the chronic increase in FSTL1. Consistent with these data, chronic FSTL1 administration decreased cardiac and systemic RQ in HF. Finally, FSTL1 reversed the increase in cardiac ketone body uptake that results from the HF condition.
Figure 3. Effects of chronic FSTL1 infusion on oxygen consumption and mechanical efficiency.
Pressure-diameter area (PDA), myocardial oxygen consumption (MVO2), mechanical efficiency indexed by the ratio PDA/MVO2, and systemic oxygen consumption (systemic VO2) after chronic FSTL1 infusion (10 ng/kg/min for 14 days) in control (Control+ FSTL1, n=6) and HF (HF+ FSTL1, n=7) compared with non-treated control (n=7) and HF (n=6). *P<0.05 vs. Control, #P<0.05 HF+ FSTL1 vs. HF.
Figure 4. Effects of chronic FSTL1 infusion on substrate uptake and oxidation and respiratory quotient.
Changes in free fatty acid (FFA) oxidation, glucose oxidation, lactate uptake, ketone bodies uptake, cardiac respiratory quotient (RQ), and systemic RQ after chronic FSTL1 infusion (10 ng/kg/min for 14 days) in control (Control+ FSTL1, n=6) and HF (HF+ FSTL1, n=7) compared with non-treated Control (n=7) and HF (n=7). * P<0.05 vs. Control, #P<0.05 HF+ FSTL1 vs. HF.
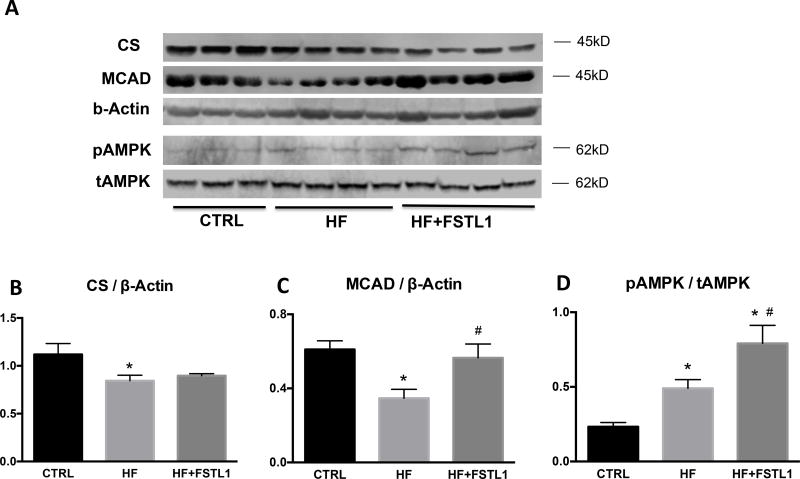
Expression of citrate synthase, MCAD and AMP-dependent kinase
Citrate synthase was downregulated in failing hearts and this alteration was minimally attenuated by chronic FSTL1 infusion (Figure 5). On the other hand, FSTL1 completely prevented the downregulation of myocardial MCAD in HF. The activating phosphorylation of myocardial AMPK was significantly increased in HF vs control as previously shown by others in the same canine model (25), and further increased in HF dogs that were chronically infused with FSTL1.
Figure 5. Effects of chronic FSTL1 infusion on metabolic enzyme expression.
Protein expression of citrate synthase (A,B), medium-chain acyl-CoA dehydrogenase (A,C) and phosphorylated and total AMP activated protein kinase (D,E) in left ventricular tissue from Control (CTRL, n=7), HF (n=7), and HF with chronic FSTL1 infusion (10 ng/kg/min for 14 days) (n=7). *P<0.05 vs. CTRL, # P<0.05 HF+FSTL1 vs. HF. CS, citrate synthase; MCAD, medium-chain acyl-CoA dehydrogenase; AMPK, AMP activated protein kinase (t = total, p = phosphorylated).
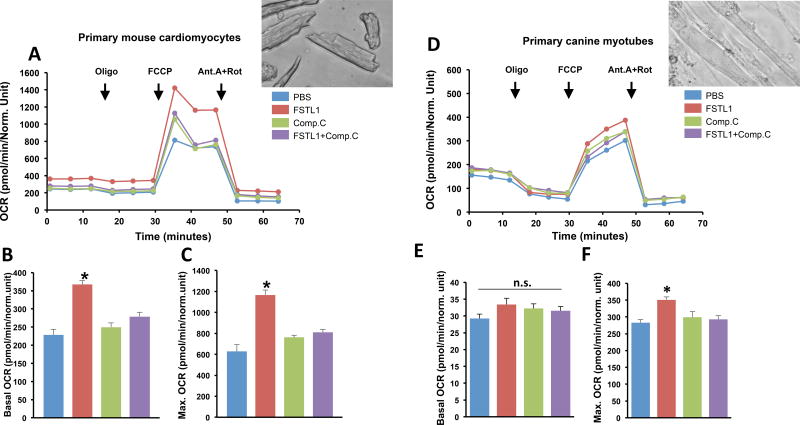
Mitochondrial O2 consumption rate, in vitro
To assess potential effects of FSTL1 on mitochondrial metabolic function in heart and skeletal muscle, the Mito Stress Test was performed in primarily cultured mouse cardiomyocytes and canine myotubes (Figure 6). In adult mouse cardiomyocytes, FSTL1 significantly enhanced the basal (intact cells) and maximal (with uncoupler) oxygen consumption rates (OCR) as well as the spare respiratory capacity (OCRmax − OCRbasal). Similar changes in OCRmax were observed in primary myotubes incubated with FSTL1, but they were smaller than those found in cardiomyocytes. In both cell types, the effect of FSTL1 was abolished when treated in combination with the AMPK inhibitor Compound C, suggesting an essential role of this upstream energy sensor enzyme.
Figure 6. Effects of FSTL1 administration on mitochondrial oxygen consumption.
Incubation with FSTL1 increased mitochondrial oxygen consumption in primary adult mouse cardiomyocytes (A–C) and primary canine myotubes (D–F). Mitochondrial oxygen consumptions were measured in presence of 25 mM glucose and 1 mM sodium pyruvate (pH 7.4). After basal OCR recording, oligomycin, FCCP, and antimycin A + rotenone were added as indicated A. Representative plot of mitochondrial respiration assay in cardiomyocytes. B. Basal mitochondrial oxygen consumption in cardiomyocytes. C. Maximal mitochondrial oxygen consumption in cardiomyocytes. D. Representative plot of mitochondrial respiration assay in myotubes. E. Basal mitochondrial oxygen consumption in myotubes. F. Maximal mitochondrial oxygen consumption in myotubes. Four independent experiments were performed for each cell type. Data indicate mean ± SEM, *P< 0.05 vs. PBS vs. FSTL1. CC, Compound C; Oligo, oligomycin; FCCP, uncoupler; AA, antimycin A; Rot, rotenone.
DISCUSSION
The main finding of the present study is that acute and chronic increases in circulating FSTL1 alter cardiac and systemic metabolism, thus revealing a previously unknown function of this pleiotropic cardiokine. FSTL1 promoted fat oxidation, while carbohydrate oxidation was inhibited. Perhaps due to the enhanced sensitivity of the system to FSTL1 and/or the pre-existing metabolic alterations, these changes were only detectable in dogs with HF, while control dogs were largely refractory to the effects of FSTL1. When FSTL1 was infused acutely, it corrected the HF-induced pathological alterations in cardiac glucose and FFA oxidation and cardiac and systemic RQ, although these changes gradually disappeared in parallel with the clearance of the recombinant protein. Two weeks of FSTL1 infusion caused similar effects. A recent study in rodents has highlighted the importance of ketone bodies as a fuel for the failing heart (26), while a contemporary investigation reported elevated blood levels of ketone bodies in HF patients and provided indirect evidence of higher cardiac consumption of this energy substrate (27). Interestingly, the dog model of tachypacing-induced HF employed here also developed this pathological alteration in ketone body metabolism. Further, our study presents the first direct evidence of increased rate of ketones uptake by failing hearts, a change that was normalized by chronic FSTL1 infusion.
If alterations of substrate utilization have a negative impact on cardiac function in HF (18), their normalization should lead to relevant hemodynamic improvements. However, FSTL1 exerted modest effects on cardiac function and hemodynamics, both in normal and HF dogs. Acute FSTL1 infusion unfavorably affected stoke work and lowered mechanical efficiency in both normal and failing hearts, although these were only transient phenomena. On the other hand, chronic FSTL1 infusion modestly attenuated the progressive systolic and diastolic functional derangement in HF, as indicated by the better preserved dP/dtmax, LV end-diastolic pressure and Tau index. Consistently, chronic FSTL1 infusion led to a ~14% potentiation of the β1-adrenergic contractile response to dobutamine. Taken together, these findings indicate that chronic elevation of circulating FSTL1 induces a mild improvement of both diastolic and systolic dysfunction. The consequent clinical implication is that the sustained high blood levels of FSTL1 observed in HF patients (12) may have a beneficial, albeit insufficient compensatory role. This interpretation could be further validated in the dog HF model by testing FSTL1 receptor blockade, but antagonists to DIP2A, a candidate receptor (28), are not available at this time.
The in vivo metabolic effects of FSTL1 have not been studied previously and in vitro data are limited. Thus, there is little information about potential underlying mechanism, and additional ad hoc studies in different models are warranted. However, it is known that the failing heart is characterized by a downregulation of mitochondrial enzymes responsible for FFA oxidation (20,29). Hence, we tested whether FSTL1 administration could restore the expression of MCAD, a hallmark of beta-oxidation pathway integrity. Consistent with the observed changes in FFA metabolism in vivo, Western blot analysis of failing heart tissue showed that 2 weeks of FSTL1 infusion normalized the expression of MCAD. However, FSTL1 had a minimal effect on the expression of citrate synthase, a marker of mitochondrial all-substrate oxidative capacity, indicating that this cardiokine conferred only partial protection to the energy-producing machinery in cardiomyocytes. In fact, chronic FSTL1 infusion was unable to restore basal MVO2 in the failing heart.
Prior evidence implicating FSTL1 in metabolic modulation was generated in a murine model of TAC-induced cardiac hypertrophy (9): genetic FSTL1 overexpression prevented the reduction in myocardial AMPK activation, yet the metabolic phenotype was not examined. Since AMPK is a major activator of fat and carbohydrate catabolic pathways (14,30), our expectation was to observe a rise in cardiac and systemic oxygen consumption in response to FSTL1 administration, in both healthy and HF dogs. This was found to a modest extent only in failing hearts acutely exposed to the circulating cardiokine. In contrast, chronic FSTL1 infusion did not lead to significant changes in cardiac oxygen consumption, despite the enhanced AMPK phosphorylation. We were therefore prompted to perform complementary, reductionist experiments in isolated cardiac and skeletal myocytes to test whether FSTL1 can indeed directly enhance cellular energy turnover. FSLT1-mediated changes in baseline respiration were only detected in cardiomyocytes, perhaps due to tissue specific response, while a more pronounced enhancement of cellular oxygen consumption was found in both cell types during full mitochondrial uncoupling. Since the “spare capacity” of uncoupled mitochondria is limited by the availability of reducing equivalents deriving from substrate catabolism, it is possible that this was fueled by FSTL1-induced FFA oxidation under the conditions of our experiments. Compound C prevented such changes, consistent with the hypothesis that AMPK signaling mediates the effect of FSTL1 on mitochondrial respiration. In the light of our results showing no significant alterations of baseline skeletal myocytes respiration in vitro, it is not surprising that increases in circulating FSTL1 did not lead to detectable changes in systemic oxygen consumption in any experimental group. Further, we speculate that the significant MVO2 enhancement by acute FSTL1 infusion, measurable only in HF, was perhaps due to mechanisms sensitizing the diseased myocardium that were no longer active after chronic FSTL1 infusion.
Endogenous FSTL1 is upregulated in the heart by diverse injuries including ischemia-reperfusion, transverse aortic constriction and permanent ligation of the LV descending coronary artery (6). FSTL1 expression and secretion in the heart is downstream of Akt signaling (6), a component of a pathway with multiple protective actions in the cardiovascular system. Functionally significant FSTL1 is produced by cardiac myocytes and fibroblasts, and both of these cellular sources can quantitatively contribute to the circulating levels depending upon experimental conditions. In a mouse model of transverse aortic constriction, targeted gene knockout shows that cardiac myocytes are a major source of myocardial and circulating FSTL1, which protects the heart from hypertrophy (9). FSTL1 released by cardiac myocytes has also been shown to protect the kidney from injury in a mouse model of subtotal nephrectomy (31). In contrast, in a murine model of permanent left anterior descending coronary ligation, cardiac fibroblasts are the major source of myocardial and serum FSTL1, which protects the heart from rupture (11). Skeletal muscle accounts for 40% of body mass and ablation of FSTL1 from this compartment leads to a reduction in serum levels of the myokine in a murine model of myocardial infarction. Although studied less extensively, this source of FSTL1 is also functionally relevant as it has been shown to exert protective actions on the vasculature (32).
In our study, we could not measure plasma levels of endogenous FSTL1 in dogs, due to the unavailability of analytical tools sensitive to the canine protein. Another limitation pertains to the experimental design, which was based on single doses for acute and chronic FSTL1 infusion. Nonetheless, plasma levels attained in dogs after FSTL1 infusions can be considered to be within the range observed in patients with systolic and diastolic HF (12–13): in the first study, although the authors showed only relative changes, circulating FSTL1 was found up to four times higher in severe compared to mild HF and it is known that median serum levels of FSTL1 in humans with no HF span from 14 to 90 ng/ml (13,33); in the second study, serum FSTL1 concentration reached values of ~300–400 ng/ml in patients. Therefore, we believe it is reasonable to assume that the doses of FSTL1 used for our dog experiments mimic clinically-relevant conditions, and complement circulating endogenous FSTL1, which is insufficiently protective. This supposition is supported by studies in mice and pig models in which the genetic overexpression of FSTL1 or the infusion of exogenous FSTL1 protected cardiovascular tissues from injury (6–10,32).
Conclusions
We document that FSTL1 can function as a metabolic modulator, thereby assign a novel function to this secreted protein and provide the first direct evidence that a circulating cardiokine/myokine can alter myocardial and systemic substrate energy metabolism, in vivo. Prior studies on the cardio-protective actions of FSTL1 have largely focused on the murine system, primarily using genetic FSTL1 manipulation in models of pressure-overload hypertrophy and myocardial ischemia. Here, it is shown that formulations of recombinant human FSTL1 exhibit a degree of efficacy in the canine tachypacing-induced HF model that typically serves as a critical preclinical model. Further investigation is warranted to draw conclusions about therapeutic implications.
Supplementary Material
Clinical perspective.
What is new?
Follistatin-like protein 1 (FSTL1) is an emerging protein belonging to the family of cardiokines/myokines, mediators secreted both by cardiac and skeletal muscle. The present study is the first to define the effects of FSTL1 on cardiac and systemic metabolism and, more in general, to provide direct evidence that a circulating cardiokine/myokine can alter fatty acid and glucose metabolism, in vivo.
By using a dog model, we found that high levels of FSTL1 can partially reverse the metabolic alterations typically occurring in the failing heart with minor beneficial effects on hemodynamics and cardiac mechanical function.
What are the clinical implications?
It was previously found that FSTL1 can protect the heart against ischemia-reperfusion injury, post-infarct rupture and overload-induced hypertrophy.
It can also promote revascularization and cardiac regeneration. Here we further tested its potential curative effects in a model of dilated cardiomyopathy.
FSTL1 displayed a novel modulatory action on cardiac metabolism and favored a moderate preservation of diastolic and systolic function.
Further investigation is warranted to draw conclusions about the possible therapeutic use of this protein for the treatment of heart failure.
Acknowledgments
Sources of Funding
This work was supported by the NIH grant R01 HL129120 (F.A.R. and K.W.) and in part by NIH grants R21 AG052160 (K.W.) and R01HL126952 (J.Y.P).
Footnotes
Disclosures: None.
References
- 1.Pedersen BK, Steensberg A, Fischer C, Keller C, Keller P, Plomgaard P, Febbraio M, Saltin B. Searching for the exercise factor: is IL-6 a candidate? J. Muscle Res. Cell Motil. 2003;24:113–119. doi: 10.1023/a:1026070911202. [DOI] [PubMed] [Google Scholar]
- 2.Walsh K. Adipokines, myokines and cardiovascular disease. Circ J. 2009;73:13–18. doi: 10.1253/circj.cj-08-0961. [DOI] [PubMed] [Google Scholar]
- 3.Pedersen BK, Febbraio MA. Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat Rev Endocrinol. 2012;8:457–65. doi: 10.1038/nrendo.2012.49. [DOI] [PubMed] [Google Scholar]
- 4.Shimano M, Ouchi N, Walsh K. Cardiokines: Recent progress in elucidating the cardiac secretome. Circulation. 2012;126:e327–332. doi: 10.1161/CIRCULATIONAHA.112.150656. [DOI] [PubMed] [Google Scholar]
- 5.Doroudgar S, Glembotski CC. The cardiokine story unfolds: Ischemic stress-induced protein secretion in the heart. Trends Mol Med. 2011;17:207–214. doi: 10.1016/j.molmed.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oshima Y, Ouchi Y, Sato K, Izumiya Y, Pimentel DR, Walsh K. Follistatin-like 1 is an Akt-regulated cardioprotective factor that is secreted by the heart. Circulation. 2008;117:3099–3108. doi: 10.1161/CIRCULATIONAHA.108.767673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ouchi N, Oshima Y, Ohashi K, Higuchi A, Ikegami C, Izumiya Y, Walsh K. Follistatin-like 1, a secreted muscle protein, promotes endothelial cell function and revascularization in ischemic tissue through a nitric-oxide synthase-dependent mechanism. J Biol Chem. 2008;283:32802–32811. doi: 10.1074/jbc.M803440200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ogura Y, Ouchi N, Ohashi K, Shibata R, Kataoka Y, Kambara T, Kito T, Maruyama S, Yuasa D, Matsuo K, Enomoto T, Uemura Y, Miyabe M, Ishii M, Yamamoto T, Shimizu Y, Walsh K, Murohara T. Therapeutic impact of follistatin-like 1 on myocardial ischemic injury in preclinical models. Circulation. 2012;126:1728–38. doi: 10.1161/CIRCULATIONAHA.112.115089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shimano M, Ouchi N, Nakamura K, van Wijk B, Ohashi K, Asaumi Y, Higuchi A, Pimentel DR, Sam F, Murohara T, van den Hoff MJ, Walsh K. Cardiac myocyte follistatin-like 1 functions to attenuate hypertrophy following pressure overload. Proc Natl Acad Sci U S A. 2011;108:E899–906. doi: 10.1073/pnas.1108559108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wei K, Serpooshan V, Hurtado C, Diez-Cuñado M, ZhaoM, Maruyama S, Zhu W, Noseda M, Nakamura K, Tian X, Liu Q, Matsuura Y, Cai W, Savtchenko A, Mahmoudi M, Schneider MD, vandenHoff M, Butte MJ, Yang PC, Walsh K, Zhou B, Bernstein D, Mercola M, Ruiz-Lozano P. Epicardial Fstl1 reconstitution regenerates the adult mammalian heart. Nature. 2015;525:479–85. doi: 10.1038/nature15372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maruyama S, Nakamura K, Papanicolaou K, Shimizu I, Sano S, Asaumi Y, van den Hoff MJ, Recchia FA, Walsh K. Follistatin-like 1 is required for reparative fibrosis and protects heart from post-infarct cardiac rupture. EMBO Mol Med. 2016;8:949–66. doi: 10.15252/emmm.201506151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.El-Armouche A, Ouchi N, Tanaka K, Doros G, Wittköpper K, Schulze T, Eschenhagen T, Walsh K, Sam F. Follistatin-like 1 in chronic systolic heart failure: a marker of left ventricular remodeling. Circ Heart Fail. 2011;4:621–7. doi: 10.1161/CIRCHEARTFAILURE.110.960625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tanaka K, Valero-Muñoz M, Wilson RM, Essick EE, Fowler CT, Nakamura K, van den Hoff M, Ouchi N, Sam F. Follistatin like 1 Regulates Hypertrophy in Heart Failure with Preserved Ejection Fraction. JACC Basic Transl Sci. 2016;1:207–221. doi: 10.1016/j.jacbts.2016.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zaha VG, Young LH. AMP-activated protein kinase regulation and biological actions in the heart. Circ. Res. 2012;111:800–814. doi: 10.1161/CIRCRESAHA.111.255505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mitacchione G, Powers JC, Grifoni G, Woitek F, Lam A, Ly L, Settanni F, Makarewich CA, McCormick R, Trovato L, Houser SR, Granata R, Recchia FA. The gut hormone ghrelin partially reverses energy substrate metabolic alterations in the failing heart. Circ Heart Fail. 2014;7:643–51. doi: 10.1161/CIRCHEARTFAILURE.114.001167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Woitek F, Zentilin L, Hoffman NE, Powers JC, Ottiger I, Parikh S, Kulczycki AM, Hurst M, Ring N, Wang T, Shaikh F, Gross P, Singh H, Kolpakov MA, Linke A, Houser SR, Rizzo V, Sabri A, Madesh M, Giacca M, Recchia FA. Intracoronary Cytoprotective Gene Therapy: A Study of VEGF-B167 in a Pre-Clinical Animal Model of Dilated Cardiomyopathy. J Am Coll Cardiol. 2015;66:139–53. doi: 10.1016/j.jacc.2015.04.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Recchia FA, Osorio JC, Chandler MP, Xu X, Panchal AR, Lopaschuk GD, Hintze TH, Stanley WC. Reduced synthesis of NO causes marked alterations in myocardial substrate metabolism in conscious dogs. Am J Physiol. 2002;282:E197–206. doi: 10.1152/ajpendo.2002.282.1.E197. [DOI] [PubMed] [Google Scholar]
- 18.Taegtmeyer H, Young ME, Lopaschuk GD, Abel ED, Brunengraber H, Darley-Usmar V, Des Rosiers C, Gerszten R, Glatz JF, Griffin JL, Gropler RJ, Holzhuetter HG, Kizer JR, Lewandowski ED, Malloy CR, Neubauer S, Peterson LR, Portman MA, Recchia FA, Van Eyk JE, Wang TJ. Assessing Cardiac Metabolism: A Scientific Statement From the American Heart Association. Circ Res. 2016;118:1659–701. doi: 10.1161/RES.0000000000000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Recchia FA, McConnell PI, Bernstein RD, Vogel TR, Xu X, Hintze TH. Reduced nitric oxide production and altered myocardial metabolism during the decompensation of pacing-induced heart failure in the conscious dog. Circ Res. 1998;83:969–979. doi: 10.1161/01.res.83.10.969. [DOI] [PubMed] [Google Scholar]
- 20.Osorio JC, Stanley WC, Linke A, Castellari M, Diep QN, Panchal AR, Hintze TH, Lopaschuk GD, Recchia FA. Impaired myocardial fatty acid oxidation and reduced protein expression of retinoid X receptor alpha in pacing-induced heart failure. Circulation. 2002;106:606–612. doi: 10.1161/01.cir.0000023531.22727.c1. [DOI] [PubMed] [Google Scholar]
- 21.Lei B, Matsuo K, Labinskyy V, Sharma N, Chandler MP, Ahn A, Hintze TH, Stanley WC, Recchia FA. Nitric oxide and cyclic GMP reduce glucose transporters translocation and lactate production in ischemic myocardium, in vivo. Proc Natl Acad Sci USA. 2005;102:6966–6971. doi: 10.1073/pnas.0500768102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu W, Wang Y, Qiu G, Chen B. Characterization of the purification and primary culture of adult canine myoblasts in vitro. Mol Med Rep. 2010;3:463–468. doi: 10.3892/mmr_00000281. [DOI] [PubMed] [Google Scholar]
- 23.Wang PY, Ma W, Park JY, Celi FS, Arena R, Choi JW, Ali QA, Tripodi DJ, Zhuang J, Lago CU, Strong LC, Talagala SL, Balaban RS, Kang JG, Hwang PM. Increased oxidative metabolism in the Li-Fraumeni syndrome. N Engl J Med. 2013;368:1027–1032. doi: 10.1056/NEJMoa1214091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller BA, Hoffman NE, Merali S, Zhang XQ, Wang J, Rajan S, Shanmughapriya S, Gao E, Barrero CA, Mallilankaraman K, Song J, Gu T, Hirschler-Laszkiewicz I, Koch WJ, Feldman AM, Madesh M, Cheung JY. TRPM2 channels protect against cardiac ischemia-reperfusion injury: role of mitochondria. J Biol Chem. 2014;289:7615–29. doi: 10.1074/jbc.M113.533851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sasaki H, Asanuma H, Fujita M, Takahama H, Wakeno M, Ito S, Ogai A, Asakura M, Kim J, Minamino T, Takashima S, Sanada S, Sugimachi M, Komamura K, Mochizuki N, Kitakaze M. Metformin prevents progression of heart failure in dogs: role of AMP-activated protein kinase. Circulation. 2009 May 19;119(19):2568–77. doi: 10.1161/CIRCULATIONAHA.108.798561. [DOI] [PubMed] [Google Scholar]
- 26.Aubert G, Martin OJ, Horton JL, Lai L, Vega RB, Leone TC, Koves T, Gardell SJ, Krüger M, Hoppel CL, Lewandowski ED, Crawford PA, Muoio DM, Kelly DP. The Failing Heart Relies on Ketone Bodies as a Fuel. Circulation. 2016;133:698–705. doi: 10.1161/CIRCULATIONAHA.115.017355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bedi KC, Jr, Snyder NW, Brandimarto J, Aziz M, Mesaros C, Worth AJ, Wang LL, Javaheri A, Blair IA, Margulies KB, Rame JE. Evidence for Intramyocardial Disruption of Lipid Metabolism and Increased Myocardial Ketone Utilization in Advanced Human Heart Failure. Circulation. 2016;133:706–16. doi: 10.1161/CIRCULATIONAHA.115.017545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ouchi N1, Asaumi Y, Ohashi K, Higuchi A, Sono-Romanelli S, Oshima Y, Walsh K. DIP2A functions as a FSTL1 receptor. J Biol Chem. 2010 Mar 5;285(10):7127–34. doi: 10.1074/jbc.M109.069468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sack MN, Rader TA, Park S, Bastin J, McCune SA, Kelly DP. Fatty acid oxidation enzyme gene expression is downregulated in the failing heart. Circulation. 1996;94:2837–42. doi: 10.1161/01.cir.94.11.2837. [DOI] [PubMed] [Google Scholar]
- 30.Salt IP1, Hardie DG2. AMP-Activated Protein Kinase: An Ubiquitous Signaling Pathway With Key Roles in the Cardiovascular System. Circ Res. 2017;120:1825–1841. doi: 10.1161/CIRCRESAHA.117.309633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hayakawa S, Ohashi K, Shibata R, Kataoka Y, Miyabe M, Enomoto T, Joki Y, Shimizu Y, Kambara T, Uemura Y, Yuasa D, Ogawa H, Matsuo K, Hiramatsu-Ito M, van den Hoff MJ, Walsh K, Murohara T, Ouchi N. Cardiac myocyte-derived follistatin-like 1 prevents renal injury in a subtotal nephrectomy model. J Am Soc Nephrol. 2015;26:636–46. doi: 10.1681/ASN.2014020210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miyabe M, Ohashi K, Shibata R, Uemura Y, Ogura Y, Yuasa D, Kambara T, Kataoka Y, Yamamoto T, Matsuo K, Joki Y, Enomoto T, Hayakawa S, Hiramatsu-Ito M, Ito M, Van Den Hoff MJ, Walsh K, Murohara T, Ouchi N. Muscle-derived follistatin-like 1 functions to reduce neointimal formation after vascular injury. Cardiovasc Res. 2014;103:111–20. doi: 10.1093/cvr/cvu105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Widera C, Giannitsis E, Kempf T, Korf-Klingebiel M, Fiedler B, Sharma S, Katus HA, Asaumi Y, Shimano M, Walsh K, Wollert KC. Identification of follistatin-like 1 by expression cloning as an activator of the growth differentiation factor 15 gene and a prognostic biomarker in acute coronary syndrome. Clin Chem. 2012;58:1233–41. doi: 10.1373/clinchem.2012.182816. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.