Abstract
Purpose:
Black men are more likely to die as a result of prostate cancer than white men, despite effective treatments that improve survival for clinically significant prostate cancer. We undertook this study to identify gaps in prostate cancer care quality, racial disparities in care, and underlying reasons for poorer quality care.
Methods:
We identified all black men and random age-matched white men with Gleason scores ≥ 7 diagnosed between 2006 and 2013 at two urban hospitals to determine rates of treatment underuse. Underuse was defined as not receiving primary surgery, cryotherapy, or radiotherapy. We then interviewed treating physicians about the reasons for underuse.
Results:
Of 359 black and 282 white men, only 25 (4%) experienced treatment underuse, and 23 (92%) of these were black. Most (78%) cases of underuse were due to system failures, where treatment was recommended but not received; 38% of these men continued receiving care at the hospitals. All men with treatment underuse due to system failures were black.
Conclusion:
Treatment rates of prostate cancer are high. Yet, racial disparities in rates and causes of underuse remain. Only black men experienced system failures, a type of underuse amenable to health information technology–based solutions. Institutions are missing opportunities to use their health information technology capabilities to reduce disparities in cancer care.
INTRODUCTION
In the United States, black men have the highest rates of prostate cancer incidence and mortality.1,2 Black men are 2.4 times more likely than white men to die as a result of prostate cancer. They are younger at diagnosis,2,3 are more likely to have poorly differentiated tumors and to be treated nonsurgically, and have poorer survival than white men.4-6 Much of the racial disparity in survival has been attributed to socioeconomic factors and nonsurgical treatment,7,8 as well as to quality of care, rather than being due to black men’s greater burden of comorbidities.9,10 Yet, randomized trials show equipoise in surgical versus nonsurgical treatment of clinically localized prostate cancer,11-13 and there are conflicting findings regarding racial differences in prostate cancer treatment receipt.4,14,15 Some report worse quality,4,14 attributed to differences in types and volume of hospitals and surgeons treating black men, whereas others find no racial disparity.15 Despite the plethora of studies describing disparate treatment rates between blacks and whites,4,14,16-19 few describe reasons prostate cancer treatment quality varies and what might be done to improve care. To achieve significant reductions in cancer-related racial disparities, hospitals need to know their treatment rates, racial differences in treatments, and reasons for such differences. We undertook this study at two hospitals, an academic and a municipal hospital, serving the Harlem community in New York City, to assess possible racial differences in quality of care and explore reasons for differences in care received.
METHODS
First, we worked with a Steering Committee of local experts in prostate cancer to review the literature to decide on quality measures. Next, we identified men with clinically significant prostate cancer (Gleason score ≥ 7) who could benefit from treatment and abstracted their records to assess care received. We then interviewed the treating physicians of men who experienced treatment underuse to identify possible reasons for underuse and potential interventions to improve outcomes. We contacted patients who experienced underuse for focus groups; these data are presented elsewhere.20 Institutional Review Board approval was obtained at both sites.
Developing Quality Measures and Defining Underuse
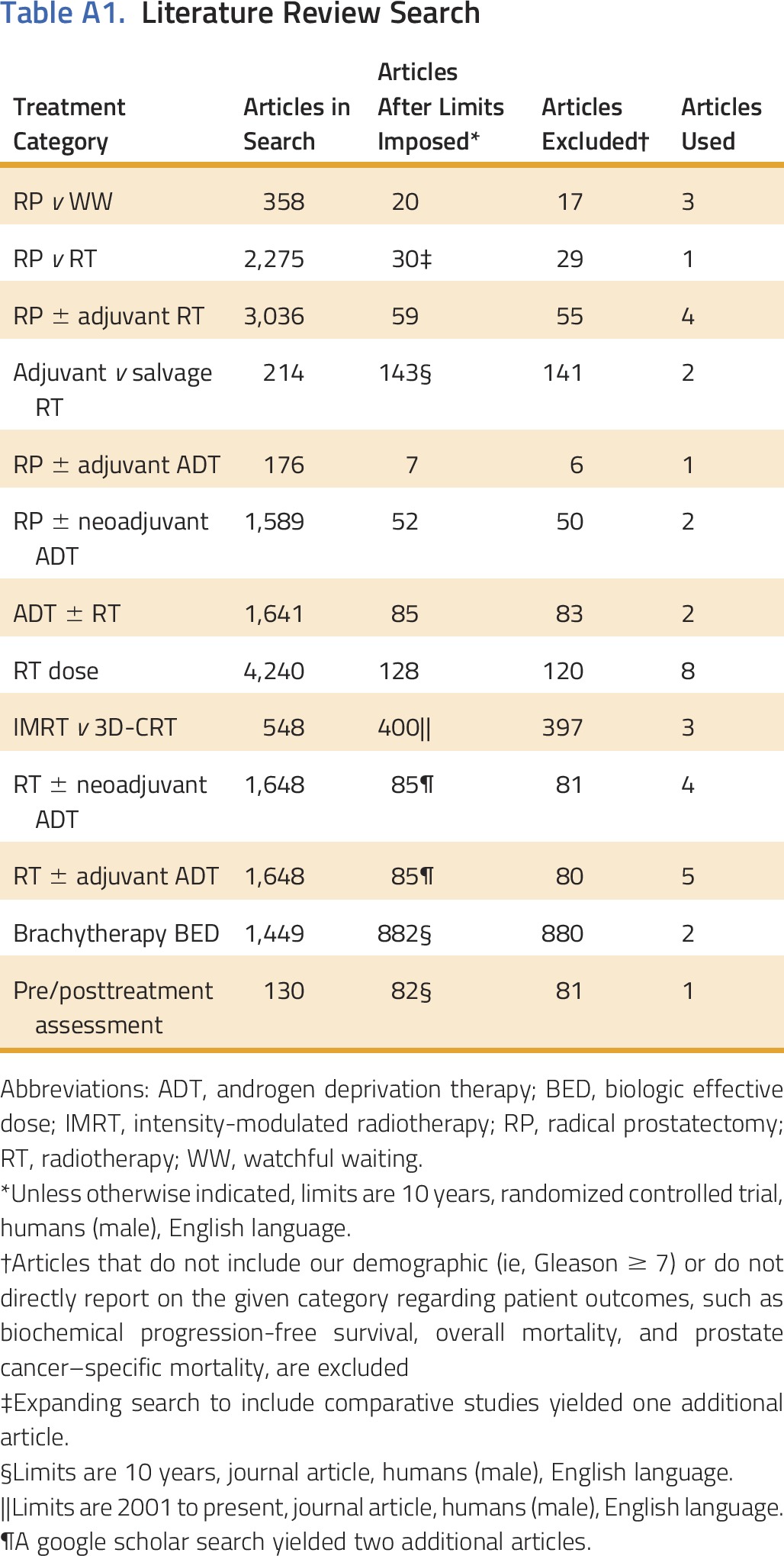
To develop quality measures of locally advanced prostate cancer treatment, we conducted an evidence-based review of randomized trials published in the prior 10 years. We included phase III trials with patients with Gleason score ≥ 7 comparing primary treatments of surgery (open, laparoscopic, or robotic-assisted radical prostatectomy), radiotherapy (external beam radiation therapy [EBRT] and/or brachytherapy), cryotherapy, or androgen deprivation therapy (ADT) and reported results of overall mortality, prostate cancer–specific mortality, or biochemical failure. From 358 articles, 38 met criteria and were reviewed with a steering committee of experts in urology, radiation therapy, medical oncology, general internal medicine, and health services research (Appendix Table A1, online only). We collected information on quality of life, and urinary, bowel, and sexual function. Underuse was defined as: treatment with ADT monotherapy for patients treated after a 2009 seminal publication,21 or no prostatectomy, or no radiotherapy (EBRT or brachytherapy), or no cryotherapy in men with intermediate- or high-risk prostate cancers (Appendix, online only). Definitive treatment included surgery, radiotherapy (RT) with ADT, or cryotherapy.13 We wished to include quality measures of RT dosing and ADT timing, but there was inadequate documentation to reliably assess these measures. Men with intermediate-risk cancer and low life expectancy who received active surveillance, defined as semiannual prostate-specific antigen (PSA) testing, were not considered as underuse. Life expectancy was determined using the Schonberg prognostic index.22
Case Identification
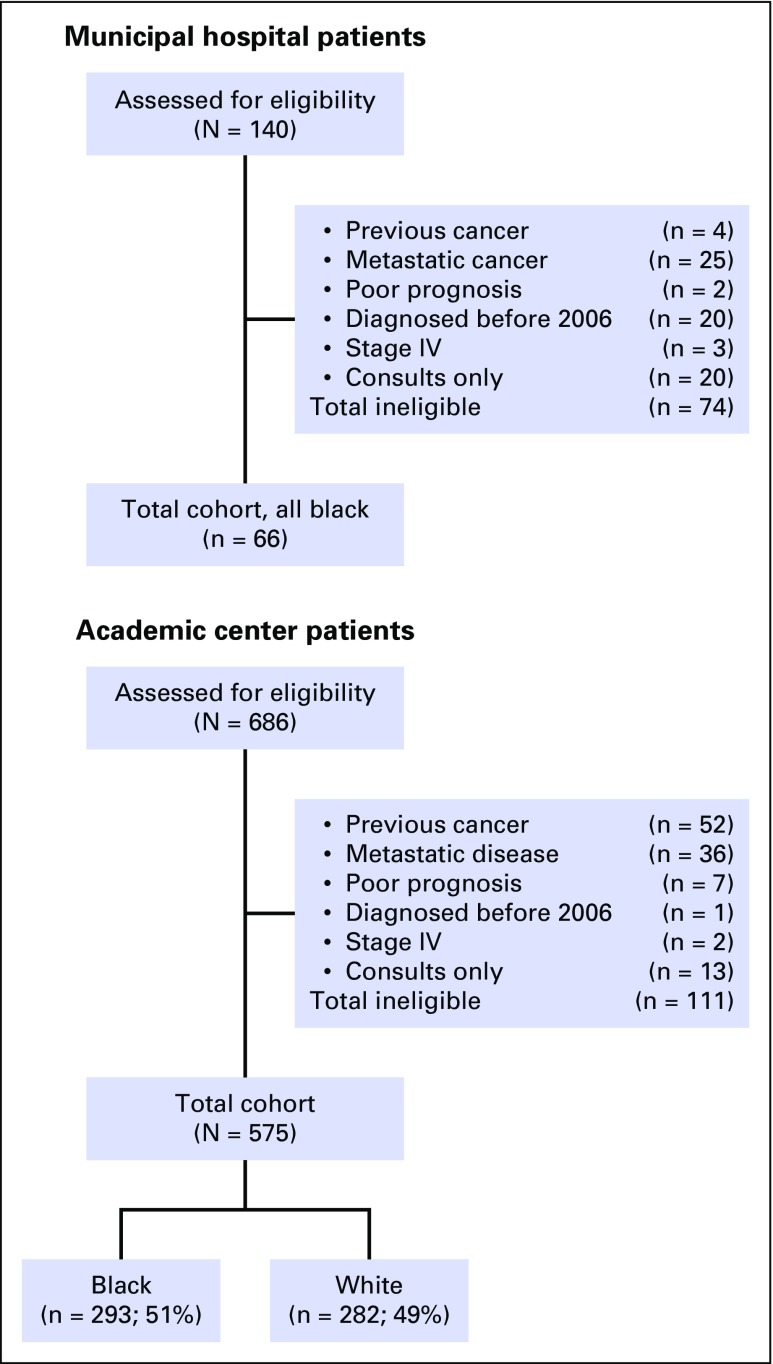
We chose to include only patients with clinically significant disease (eg, Gleason score ≥ 7), because these are locally advanced cancers for which treatment can improve survival; we did not include low-risk cancers (eg, Gleason 6), because there is inadequate evidence to show that treatment (v active surveillance) improves survival.23 We identified all patients with Gleason score 7 to 10 via pathology reports at the academic tertiary referral center (2007 to 2012) and tumor registry at the municipal hospital (2006 to 2013). There were 1,804 (350 black, 1,454 white) and 140 (135 black, 5 white) patients at the respective sites. At the academic site, we retained all black patients and randomly selected Gleason score–matched white patients within 10-year age groups (< 60, 60 to 69, 70 to 79, ≥ 80 years; n = 282), providing a final yield of 641 patients (Fig 1). Patient race was based on administrative reporting.24 Overall, we excluded 185 patients because of previous cancer (n = 56), stage IV disease (n = 66), poor prognosis (n = 9), diagnosis before 2006 (n = 21), or consultation only (n = 33). Poor prognosis was based on physician notes indicating that the patient had a poor prognosis from other comorbidities.
Fig 1.
Patient selection.
We abstracted medical records for clinical characteristics, including comorbidity,25 9-year Schonberg prognostic index22 (a validated tool predicting likelihood of living 9 years among community-dwelling older adults), demographics, pathology, PSA level, smoking, and descriptors of functional status. We estimated each patient’s overall prognosis using the 9-year Schonberg prognostic index. Given the nature of chart data, former smoking status, self-rated health, and number of overnight hospitalizations were not available. With these missing data, our measure of the 9-year Schonberg prognostic index slightly overestimates patient life expectancy overall. There was no difference in missing data between younger and older men or black and white men. High 9-year life expectancy was set at the median score of ≥ 84%. We used D’Amico risk of cancer progression26: high risk included Gleason score 8 to 10, pretreatment PSA > 20, or stage ≥ IIc disease; intermediate risk included Gleason score 7, PSA between 10 and 20, or stage IIb disease.
We collected treatment data from inpatient and outpatient records. When no treatment data were found, progress notes were reviewed for evidence of treatment delivered elsewhere to seek missing treatment information.
Quantitative Analyses
We used bivariate t tests and χ2 tests to compare patient characteristics between the two racial groups. Multivariable conditional logistic regression models determined which clinical and demographic factors most affected the likelihood of underuse in this matched sample.
Qualitative interviews
Three members of the research team (N.A.B., S.R.A., J.J.L.) conducted semistructured interviews with the physicians treating patients who experienced treatment underuse. Interviews, conducted while the treating physician reviewed the patients’ charts, focused on the physician’s general views about treatment options, factors affecting patient decision making, referrals and coordination across specialties, suggestions to improve quality of prostate cancer care, and the specifics of the underuse case. Interviews were audiotaped and reviewed for common themes and reasons for treatment underuse. Building on an a priori framework of reasons for underuse,27 each case was reviewed and discussed, and a primary reason for missed care was identified. The primary reason for underuse was discussed with each physician as a way of validating that the chosen underuse category fit with their view of the cause of treatment underuse.
RESULTS
Hospital Characteristics
Both hospitals in this study are Disproportionate Share Hospitals serving significant proportions of Medicaid patients.28 Surgeons at both sites perform radical prostatectomy, although the municipal hospital did not have robotic surgery capabilities. The academic site had RT and brachytherapy on site; the municipal hospital’s patients received RT at a network hospital 1 mile away. Both had electronic medical records (EMRs) with computerized order entry and the capability to create best practice alerts. Significant numbers of patients at the academic hospital but few from the municipal hospital received care at affiliated private physician offices. The majority of private offices had some patient data in electronic form. The academic referral center was in the process of applying for accreditation by the Commission on Cancer; the municipal hospital lost its accreditation in 2006. By 2013, both hospitals were part of Accountable Care Organizations and Medicaid Health Homes. At the time of this study, the municipal hospital did not have patient portal capabilities; the academic site had such portals, but they were not widely used. Neither hospital had implemented a systematic approach to monitor prostate cancer care performance.
Patient Characteristics
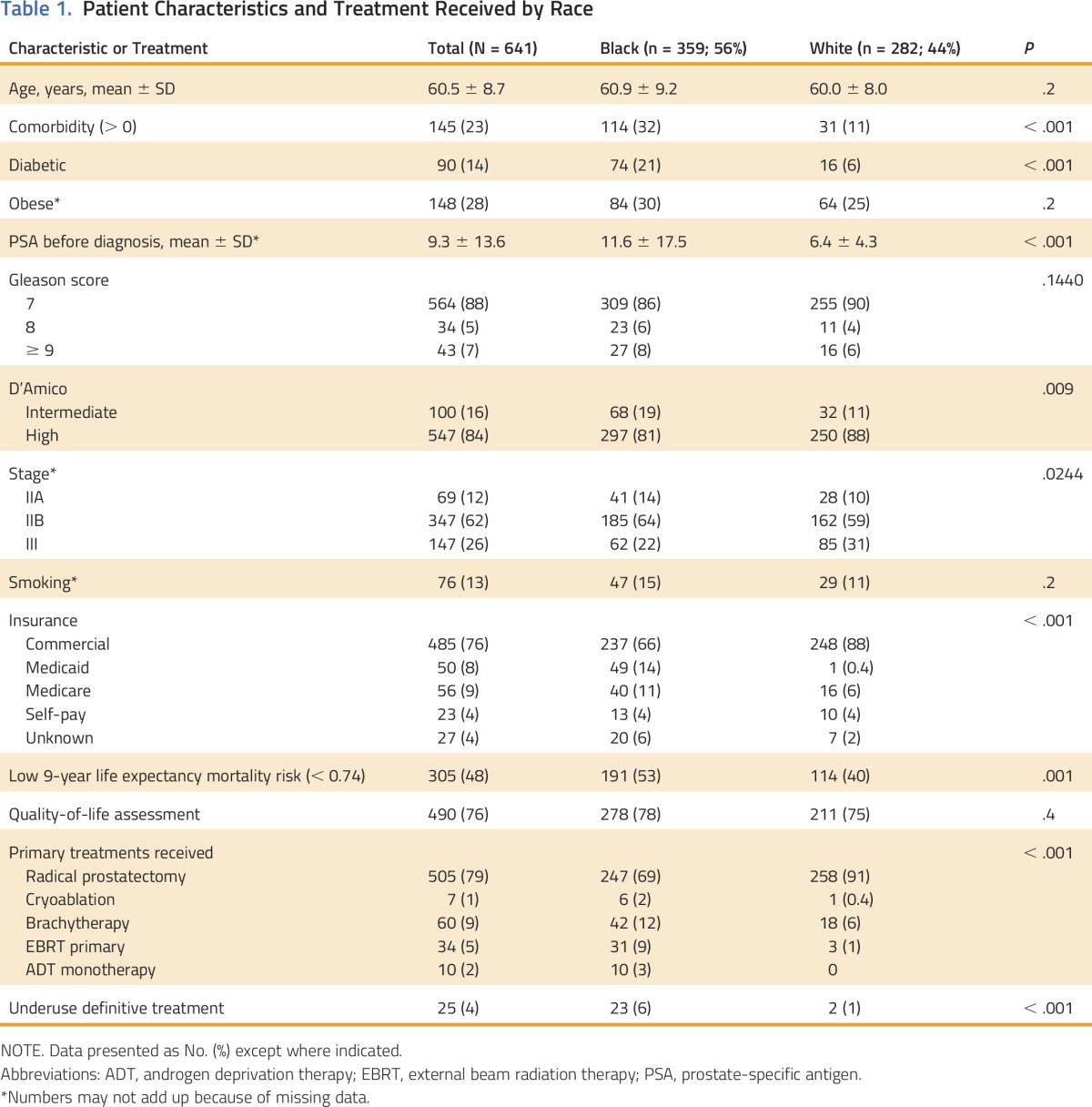
Five hundred seventy-five men at the academic center and 66 men at the municipal hospital were included in this study (Fig 1). Of these 641 men, 56% were black and 84% had high-risk cancer by D’Amico criteria (Table 1). The average age was 60.5 years (± 8.7 years), and there were no differences in age by race. Black men were more likely than white men to have multiple comorbidities (32% v 11%; P < .001), intermediate D’Amico cancer risk (19% v 11%; P < .001), and low 9-year life expectancy (53% v 40%: P < .001) and were less likely to have commercial insurance (66% v 88%; P < .001).
Table 1.
Patient Characteristics and Treatment Received by Race
Physician Characteristics
Seventeen physicians treated the 25 patients who experience treatment underuse. Two urologists treating three patients who experience treatment underuse were unreachable, resulting in 22 cases of underuse for which cause could be categorized. The 15 treating physicians included 10 urologists, two medical oncologists, and three radiation oncologists. They had an average of 20.5 years (range, 4 to 30 years) in practice; 13% practiced at the municipal hospital, 40% practiced at the academic hospital, and 47% were in in private practice affiliated with the academic hospital.
Underuse and Treatments Received
Initially, we identified 53 patients who experience treatment underuse on the basis of chart abstraction data. After interviewing physicians about the patients who experience treatment underuse, 28 were reclassified (13 were treated elsewhere, five had metastatic disease at presentation, four had poor prognosis due to other conditions, four were treated with ADT monotherapy before 2009, and two were clinical exceptions [e.g., advanced age]). There was no racial difference in assessment of quality of life. Only 25 men of the 641 included in this study experienced underuse, and 23 (92%) were black. Sixteen (64%) were treated at the municipal hospital and nine at the academic center. Although underuse was low overall (4%), black men were significantly more likely than white men to experience underuse (6% v 1%; P < .001).
Surgery was the predominant primary treatment received. White men were more likely than black men to undergo surgery (91% v 69%; P < .001), and RT was more prevalent among black than white men (21% v 7%; P < .001).
Multivariate Model
Multivariate conditional logistic model including age, life expectancy, comorbidity, race, insurance, and prostate cancer risk found that underuse was not associated with age (odds ratio [OR], 0.6; 95% CI, 0.2 to 1.8), low life expectancy (OR, 1.3; 95% CI, 0.4 to 3.9), comorbidity (OR, 1.6; 95% CI, 0.6 to 4.1), or black race (OR, 3.9; 95% CI, 0.8 to 36.5). Rather, commercial insurance protected against underuse (OR, 0.09; 95% CI, 0.03 to 0.30) and intermediate D’Amico cancer risk (OR, 3.6; 95% CI, 1.3 to 9.8) increased the risk of treatment underuse. A race-insurance interaction was not significant and was not included in the final model.
Qualitative Interview Findings
All patients who experienced treatment underuse had treatment recommended but not received (Table 2). Seventy-seven percent were classified as system failures—cases in which treatment was recommended, the patient did not refuse treatment, but treatment did not ensue. These men were lost to follow-up. Twenty-three percent of underuse cases were due to patient refusal or financial barriers; half of these occurred at the academic hospital.
Table 2.
Reasons for Underuse of Effective Primary Treatment of Clinically Significant Prostate Cancer (N = 22)
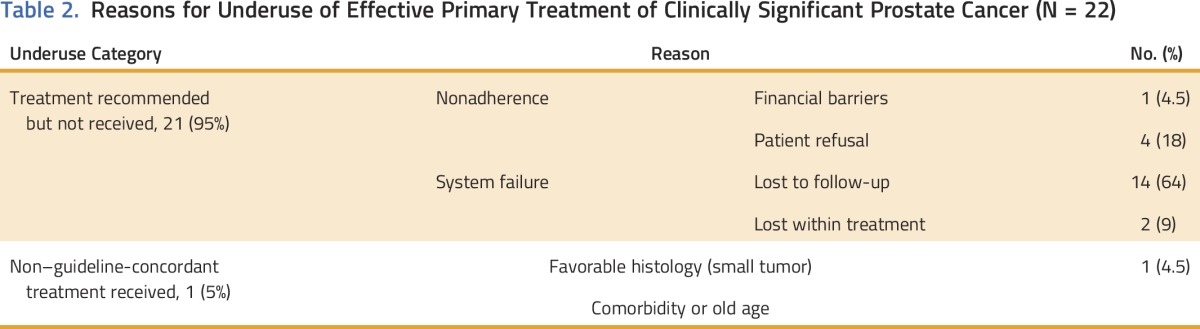
System failures affected black men exclusively (16 of 16) and were more common among men treated in the municipal hospital (14 of 16). The majority of system failures occurred as men underwent work-ups to rule out metastasis or to assess functional status and ability to undergo active treatment. Of those who experienced system failures, 38% continued receiving medical care at the hospital. A small proportion of the system failures (two of 16) included men who were slated to undergo primary EBRT and were started on pre-RT ADT but then did not go on to receive the primary radiation treatment. Reviewing these episodes, the urologists believed it likely that these patients were erroneously treated as patients with metastatic disease who typically receive ongoing ADT if their tumor responds, and their primary treatment, EBRT, never occurred despite the fact that these men continued to receive their ADT and medical care at that hospital. Of the two white men who experienced treatment underuse, one experienced financial barriers to treatment and the other’s physician declined to be interviewed.
DISCUSSION
Overall, we found rates of treatment underuse to be quite low at both academic and municipal hospitals. Although it seems that black men experienced greater rates of underuse, this disparity was related to insurance and cancer risk. Men with commercial insurance were more likely to go to the academic center; those with public insurance were more likely to go to the municipal hospital. Although both institutions had episodes of system failures, such causes of underuse occurred more frequently at the municipal than the academic hospital. Most concerning is the concentration of system failures among black men, the group with the highest mortality from prostate cancer. Men treated at both the municipal and academic hospitals experienced system failures that potentially could have been avoided had the institutions used their EMRs to identify these gaps. Both institutions had EMRs that contained gap report and best practice alert capabilities, yet neither facility used these capabilities to assess and ensure cancer care quality. The fact that several men continued to receive their medical care at these institutions reinforces critical missed opportunities to improve cancer care and potentially reduce racial disparities in cancer outcomes.
We also found that intermediate-risk cancer was associated with underuse. Men with intermediate-risk cancer were more likely to be older and, perhaps, more interested in active surveillance than active treatment, although they were all recommended to undergo active treatment given their higher-risk status. Only one patient in our study was receiving active surveillance. Although seemingly counterintuitive, black men in our study were more likely than white men to have intermediate-risk (v high-risk) prostate cancer. This finding probably results from the large numbers of black men excluded because of metastatic disease at diagnosis.
Men with commercial insurance were more likely to be treated. Decreasing proportions of uninsured patients because of the Affordable Care Act29,30 portend well for men with clinically significant prostate cancer. However, our findings suggest that insurance will not completely solve racial disparities in prostate cancer care.
Health care delivery systems must also change. Health information technology (HIT) is being used to improve population health and chronic disease management and to monitor care performance.31 HIT registries such as the Rapid Quality Reporting System require treatment reporting, but their impact on racial disparities in cancer care is uncertain.32 Oncology Medical Homes (OMH) and Oncology Care Models use HIT to improve communication, coordination, and access, with the goal of reducing variation and improving care at reduced cost.33,34 Yet, the early OMHs served few black and few Medicaid patients—the groups at greatest risk of system failures. Of concern, even within these promising and committed environs, some OMH sites reported challenges implementing the core tracking and coordinating requirements.34 It is still unknown whether tying cancer treatment quality measures to reimbursement will improve treatment rates and reduce disparities. HIT-based interventions that target system failures successfully eliminated racial disparities in breast cancer treatment rates35 but have not been tested for prostate cancer. Disturbingly, both the academic and municipal hospitals in our study had electronic capability to monitor gaps in performance, yet neither used this function in their cancer care delivery—truly, a missed opportunity.
What can hospitals do to eliminate their missed opportunities to ensure all patients with cancer receive optimal care? Some safety-net hospitals are able to provide high-quality cancer care despite their limited resources and can provide useful models.35,36 Successful sites use HIT even when their technology options are limited and assign an accountable person to act on the data generated. Their EMRs have the capacity to create gap reports, identify no-shows, and track patients, and assigned staff then ensure follow-up for needed treatments.35 Such gap reports can be used to monitor receipt of primary therapy for men with elevated Gleason scores and PSAs. Yet, this basic approach is not routinely implemented at many hospitals and may have significant survival implications. Hospitals may wish to charge their Cancer Committees and Quality personnel to work together to identify key measures for gap report analyses and assign personnel to address identified gaps.
Our study sample is small, consisting of just two hospitals serving inner-city New York. However, the hospitals represent different facility types with different patient populations, although both serve relatively large numbers of men with prostate cancer. Although it is possible that a proportion of those classified as experiencing treatment underuse were treated, it is doubtful, because we tracked care wherever chart notes or office/clinical staff suggested patients may have sought other treatments and, with complete chart reviews, nearly half of patients initially classified as experiencing treatment underuse were reclassified as treated. We also reviewed all potential underuse cases with the physicians and contacted patients to ascertain their status and treatments. In some cases, interviews with physicians occurred years after episodes of underuse, and their responses may have been affected by recall bias. To minimize this potential confounder, we had physicians review the patients’ charts before the interview, and they were encouraged to refer to the chart during the interview to refresh their memories. Patient race and hospital are highly correlated in our small sample, making it impossible to separate the impact of these key variables. We do not report previously described differences in patient views of treatment and decision making between men treated at the academic and municipal hospitals.38 Although the Schonberg index is typically used to help make screening decisions, not treatment decisions,39,40 we used this tool because it is frequently used in clinical settings, it provides a more objective measure of patient prognosis, and our patients were all community dwellers.
In conclusion, we found low rates of treatment underuse among men with clinically significant prostate cancer receiving care at an academic referral center or a municipal hospital. The vast majority of underuse was due to system failures, which affected only black men, regardless of institution. These failures occurred despite the presence of integrated EMRs and HIT capabilities to create gap reports in these organizations. Hospitals need to harness their EMRs to track cancer care and intervene when there are care gaps.41 Doing so has tremendous potential to improve cancer care and to reduce disparities in health outcomes.
ACKNOWLEDGMENT
Supported by Department of Defense Grant No. W81XWH-11-1-0540 and National Cancer Institute Grant No. 1K07CA166462 (J.J.L.). The authors thank the physicians and patients who kindly and generously gave their time and expertise to this project, Benjamin Spencer, MD, and members of our Steering Committee, and Timothy Huerta, PhD, for his comments on review of an earlier version of this manuscript.
Appendix
Table A1.
Literature Review Search
Prostate Cancer Quality Measures for Men With Gleason Score ≥ 7
-
Younger men (< 65 years old) or those with Gleason score 8 to 10, PSA > 10 ng/mL, or clinical stage T2c should undergo definitive treatment (ie, radical prostatectomy [RP] or external beam radiation therapy [EBRT], not watchful waiting [WW]) within 12 months of diagnosis.
Exceptions: men with < 10-year life expectancy.
RP and radiotherapy (RT) provide similar cancer control outcomes and should be offered to patients.
For patients undergoing RP with pathologic stage T3/4 (extracapsular extension, seminal vesicle invasion, or invasion of adjacent structures) or positive surgical margins, adjuvant RT should be discussed or the patient should be referred to a radiation oncologist.
Adjuvant androgen deprivation therapy (ADT) should not be received after RP (in patients without PSA > 5 ng/mL, two values > 2 ng/mL more than 3months apart with increasing tendency, three values > 1 ng/mL more than 3 months apart with increasing tendency, or any clinical recurrence).
Neoadjuvant ADT should not be received before RP.
Primary ADT should not be used as monotherapy (apply only to patients treated after 2009).
EBRT dose should be at least 74 Gy.
Intensity-modulated radiotherapy or three-dimensional-conformal radiotherapy should be given with computed tomography treatment planning (or equivalent imaging). If intensity-modulated radiotherapy is available, it is preferable.
For patients who are at intermediate risk, RT should be accompanied by at least 4 months of (neoadjuvant and concurrent) ADT.
For patients who are at high risk, RT should be accompanied by at least 2 years of ADT, with ≤ 2 months given before the start of RT.
If brachytherapy is used, postimplant dosimetry should be performed within 2 months of receiving the implants (30 days for palladium-103 implants, 60 days for 125I implants).
Pre- and post-treatment potency, urinary incontinence, and bowel function should be assessed for all patients. Pretreatment quality of life should be evaluated at least once within 6 months of undergoing definitive treatment; post-treatment quality of life should be evaluated at least once within the first 12 months after treatment.
Because of conflicting studies, there is insufficient evidence to support or contraindicate cryoablation instead of EBRT.
AUTHOR CONTRIBUTIONS
Conception and design: Nina A. Bickell, Gerald P. Hoke
Financial support: Nina A. Bickell
Administrative support: Nina A. Bickell
Provision of study materials or patients: Nina A. Bickell
Collection and assembly of data: Nina A. Bickell, Jenny J. Lin, Sarah R. Abramson, William Oh, Simon J. Hall, Richard Stock, Ann Scheck McAlearney
Data analysis and interpretation: Nina A. Bickell, Jenny J. Lin, William Oh, Simon J. Hall, Richard Stock, Kezhen Fei, Ann Scheck McAlearney
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Racial Disparities in Clinically Significant Prostate Cancer Treatment: The Potential Health Information Technology Offers
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jop/site/ifc/journal-policies.html.
Nina A. Bickell
Honoraria: Merck Foundation Alliance to Advance Patient-Centered Cancer Care
Research Funding: Pfizer
Jenny J. Lin
No relationship to disclose
Sarah R. Abramson
No relationship to disclose
Gerald P. Hoke
No relationship to disclose
William Oh
Leadership: Checkpoint Sciences
Stock or Other Ownership: Bellicum Pharmaceuticals
Consulting or Advisory Role: Sanofi, Janssen, Dendreon, Churchill Pharmaceuticals, Inovio Pharmaceuticals, AstraZeneca
Research Funding: Sotio (Inst)
Simon J. Hall
No relationship to disclose
Richard Stock
No relationship to disclose
Kezhen Fei
No relationship to disclose
Ann Scheck McAlearney
Research Funding: Merck for Mothers Foundation (Inst), Agency for Healthcare Research and Quality (Inst)
REFERENCES
- 1. American Cancer Society: American Cancer Society cancer statistics 2009. https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2009.html.
- 2. American Cancer Society: Cancer facts & figures 2015. http://www.cancer.org/research/cancerfactsstatistics/cancerfactsfigure s2015/
- 3. National Cancer Institute: SEER Cancer Statistics Review 1975-2007. http://seer.cancer.gov/archive/csr/1975_2007/
- 4.Underwood W, III, Jackson J, Wei JT, et al. : Racial treatment trends in localized/regional prostate carcinoma: 1992-1999. Cancer 103:538-545, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Moses KA, Paciorek AT, Penson DF, et al. : Impact of ethnicity on primary treatment choice and mortality in men with prostate cancer: Data from CaPSURE. J Clin Oncol 28:1069-1074, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bill-Axelson A, Holmberg L, Ruutu M, et al. : Radical prostatectomy versus watchful waiting in early prostate cancer. N Engl J Med 364:1708-1717, 2011 [DOI] [PubMed] [Google Scholar]
- 7.Schwartz K, Powell IJ, Underwood W, III, et al. : Interplay of race, socioeconomic status, and treatment on survival of patients with prostate cancer. Urology 74:1296-1302, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Godley PA, Schenck AP, Amamoo MA, et al. : Racial differences in mortality among Medicare recipients after treatment for localized prostate cancer. J Natl Cancer Inst 95:1702-1710, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Albain KS, Unger JM, Crowley JJ, et al. : Racial disparities in cancer survival among randomized clinical trials patients of the Southwest Oncology Group. J Natl Cancer Inst 101:984-992, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Putt M, Long JA, Montagnet C, et al. : Racial differences in the impact of comorbidities on survival among elderly men with prostate cancer. Med Care Res Rev 66:409-435, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. doi: 10.3322/canjclin.46.4.249. American Urological Association: Guideline for the management of clinically localized prostate cancer. https://www.auanet.org/education/guidelines/prostate-cancer.cfm. [DOI] [PubMed]
- 12.Akakura K, Suzuki H, Ichikawa T, et al. : A randomized trial comparing radical prostatectomy plus endocrine therapy versus external beam radiotherapy plus endocrine therapy for locally advanced prostate cancer: Results at median follow-up of 102 months. Jpn J Clin Oncol 36:789-793, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Wilt TJ, MacDonald R, Rutks I, et al. : Systematic review: Comparative effectiveness and harms of treatments for clinically localized prostate cancer. Ann Intern Med 148:435-448, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Barocas DA, Gray DT, Fowke JH, et al. : Racial variation in the quality of surgical care for prostate cancer. J Urol 188:1279-1285, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spencer BA, Miller DC, Litwin MS, et al. : Variations in quality of care for men with early-stage prostate cancer. J Clin Oncol 26:3735-3742, 2008 [DOI] [PubMed] [Google Scholar]
- 16.Jha AK, Shlipak MG, Hosmer W, et al. : Racial differences in mortality among men hospitalized in the Veterans Affairs health care system. JAMA 285:297-303, 2001 [DOI] [PubMed] [Google Scholar]
- 17.Bach PB, Pham HH, Schrag D, et al. : Primary care physicians who treat blacks and whites. N Engl J Med 351:575-584, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Bach PB: Racial disparities and site of care. Ethn Dis 15:S31-S33, 2005. (suppl 2) [PubMed] [Google Scholar]
- 19.Schmid M, Meyer CP, Reznor G, et al. : Racial differences in the surgical care of medicare beneficiaries with localized prostate cancer. JAMA Oncol 2:85-93, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bickell NA, Abramson S, Walker D, et al. : Patient and clinician perspectives on treatment decision-making for African American men with prostate cancer. J Clin Oncol 34:186, 2016. (suppl 7)26527778 [Google Scholar]
- 21.Widmark A, Klepp O, Solberg A, et al. : Endocrine treatment, with or without radiotherapy, in locally advanced prostate cancer (SPCG-7/SFUO-3): An open randomised phase III trial. Lancet 373:301-308, 2009 [DOI] [PubMed] [Google Scholar]
- 22.Schonberg MA, Davis RB, McCarthy EP, et al. : External validation of an index to predict up to 9-year mortality of community-dwelling adults aged 65 and older. J Am Geriatr Soc 59:1444-1451, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilt TJ, Brawer MK, Jones KM, et al. : Radical prostatectomy versus observation for localized prostate cancer. N Engl J Med 367:203-213, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kressin NR: Race/ethnicity identification: Vital for disparities research, quality improvement, and much more than “meets the eye”. Med Care 53:663-665, 2015 [DOI] [PubMed] [Google Scholar]
- 25.Charlson ME, Pompei P, Ales KL, et al. : A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis 40:373-383, 1987 [DOI] [PubMed] [Google Scholar]
- 26.D’Amico AV, Whittington R, Malkowicz SB, et al. : Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA 280:969-974, 1998 [DOI] [PubMed] [Google Scholar]
- 27.Bickell NA, McEvoy MD: Physicians’ reasons for failing to deliver effective breast cancer care: A framework for underuse. Med Care 41:442-446, 2003 [DOI] [PubMed] [Google Scholar]
- 28. Centers for Medicare & Medicaid Services: Disproportional Share Hospital (DSH): The Medicare DSH adjustment. https://www.cms.gov/medicare/medicare-fee-for-service-payment/acuteinpatientpps/dsh.html.
- 29.Sommers BD, Gunja MZ, Finegold K, et al. : Changes in self-reported insurance coverage, access to care, and health under the Affordable Care Act. JAMA 314:366-374, 2015 [DOI] [PubMed] [Google Scholar]
- 30.Obama B: United States health care reform: Progress to date and next steps. JAMA 316:525-532, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chaudhry B, Wang J, Wu S, et al. : Systematic review: Impact of health information technology on quality, efficiency, and costs of medical care. Ann Intern Med 144:742-752, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Halpern MT, Spain P, Holden DJ, et al. : Improving quality of cancer care at community hospitals: Impact of the National Cancer Institute Community Cancer Centers Program pilot. J Oncol Pract 9:e298-e304, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Centers for Medicare & Medicaid Services: Oncology care model webinar. https://innovation.cms.gov/Files/slides/ocm-performancemethod-slides.pdf.
- 34.Tirodkar MA, Acciavatti N, Roth LM, et al. : Lessons from early implementation of a patient-centered care model in oncology. J Oncol Pract 11:456-461, 2015 [DOI] [PubMed] [Google Scholar]
- 35.Bickell NA, Shastri K, Fei K, et al. : A tracking and feedback registry to reduce racial disparities in breast cancer care. J Natl Cancer Inst 100:1717-1723, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bickell NA, Moss AD, Castaldi M, et al: Effect of organizational approaches on safety-net hospitals’ cancer care quality. J Clin Oncol 33, 2015 (suppl; abstr 6511)
- 37. Bickell NA, Lin JJ, Franco R, et al: Breast cancer: Does where you get treated affect survival? J Clin Oncol 34, 2016 (suppl 3S; abstr 28) [Google Scholar]
- 38.Walker DM, McAlearney AS, Sova LN, et al. : Comparing prostate cancer treatment decision making in a resource-rich and a resource-poor environment: A tale of two hospitals. J Natl Med Assoc 108:211-219, 2016 [DOI] [PubMed] [Google Scholar]
- 39.Yourman LC, Lee SJ, Schonberg MA, et al. : Prognostic indices for older adults: A systematic review. JAMA 307:182-192, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Drazer MW, Prasad SM, Huo D, et al. : National trends in prostate cancer screening among older American men with limited 9-year life expectancies: Evidence of an increased need for shared decision making. Cancer 120:1491-1498, 2014 [DOI] [PubMed] [Google Scholar]
- 41.Clauser SB, Wagner EH, Aiello Bowles EJ, et al. : Improving modern cancer care through information technology. Am J Prev Med 40:S198-S207, 2011. (suppl 2) [DOI] [PMC free article] [PubMed] [Google Scholar]