Abstract
PURPOSE
We aimed to compare the diagnostic accuracy and interpretation time of an abbreviated protocol relative to the complete protocol of breast magnetic resonance imaging (MRI) with the use of breast imaging reporting and data system (BI-RADS). Between-reader and between-protocol variability for BI-RADS classification and influence of reader expertise on diagnostic accuracies were also evaluated.
METHODS
We conducted a retrospective reader study in 90 women who underwent breast MRI: 30 benign examinations (graded as American College of Radiology [ACR] 1 or 2), 30 examinations graded as ACR 3 and 30 examinations requiring a histologic proof (graded as ACR 4 or 5). Two radiologists independently reviewed the protocols. The reference standard was 24 months of imaging follow-up (66.6%, n=60), percutaneous biopsy at the12th month imaging follow-up (5.5%, n=5), and breast surgery (27.9%, n=25). Analysis was done on a per-breast basis. There were 26 cancers in 168 breasts (15.1%)
RESULTS
Interpretation time was higher for the complete protocol (mean difference: 84 s, 95% CI [67;101] for senior and 83 s, 95% CI [70;95] for junior reader; P < 0.001). The reliability of BI-RADS classification between both protocols was very good with intraclass correlation coefficient of 0.89 for junior reader and 0.98 for senior reader; the inter-reader reliability was 0.94 and 0.90 for the complete and abbreviated protocols, respectively. For senior reader, the abbreviated and complete protocols yielded 95.1% and 94.4% specificity and 100% sensitivity.
CONCLUSION
Our data provide corroborating evidence that abbreviated protocols decrease interpretation time without compromising sensitivity or specificity. There was a high level of concordance between the abbreviated and complete protocols and between the two readers.
Breast magnetic resonance imaging (MRI) has a dominant and increasingly important role in breast imaging, particularly for screening of women at high risk of developing breast cancer, staging of breast cancers, follow-up after neoadjuvant chemotherapy, and evaluating axillary lymph nodes when a primary site cannot be found by mammography (1–3). At present, it takes about 30 to 40 minutes to perform a breast MRI in accordance with the good practice guidelines of the European Society of Breast Cancer Specialists (EUSOMA) (4). This length of time is relatively long, and the examination presents high direct and indirect costs that limit its wider use (5–11).
Recently, Kuhl et al. (4) has shown that in high-risk women, the use of an abbreviated protocol is a suitable option that does not compromise the sensitivity or the specificity relative to the conventional complete protocol, thanks to specific characteristics of breast cancers that occur in high-risk women. Mango et al. (12) also demonstrated a high sensitivity with an abbreviated protocol for detection of known cancers. Moreover, the use of an abbreviated protocol including the precontrast T1-weighted sequence with fat saturation and single early postcontrast imaging with postprocessing to generate first postcontrast subtraction and subtraction of maximum intensity projection (MIP) sequences allows the time of interpretation to be reduced in addition to decreasing the duration of the examination itself (4, 12). Thus, several authors published on this popular topic and confirmed the ability of an abbreviated MRI protocol to detect breast cancer in screening of high-risk populations as well as in women with proven breast cancers. However, few studies have evaluated the specificity of an abbreviated protocol outside of a high-risk population (13).
Thus, in this reader study on a selected patient population, our aim was to compare the diagnostic accuracy of an abbreviated protocol relative to the complete protocol in terms of the breast imaging reporting and data system (BI-RADS) classification for interpretation of breast MRIs regardless of the indication for the examination. Moreover, we evaluated between-reader variability and influence of reader expertise on diagnostic accuracy.
Methods
From January to June 2013, we retrospectively queried our database to identify the first 90 consecutive MRIs that were classified as American College of Radiology (ACR) category 1 or 2 (n=30), ACR category 3 (n=30), and ACR category 4 or 5 (n=30) in the initial reports. The worst BI-RADS score from either breast was considered to make the selection.
Our institutional review board approved the study. The requirement for informed consent was waived. This retrospective study was conducted according to the Declaration of Helsinki’s “Ethical Principles for Medical Research Involving Human Subjects.”
Inclusion criteria were pathologic correlation or at least 24 months of follow-up after the MRI examination date in the absence of a percutaneous biopsy or 12 months of follow-up after the MRI examination following a percutaneous biopsy. The mean patient age was 50.4 years (range, 27–76 years). The indication for breast MRI was breast cancer staging in 19% (n=17), high-risk women without BRCA 1 or 2 mutations in 37.7% (n=34), women with BRCA 1 or 2 mutations in 19% (n=17), nipple discharge in 1.1% (n=1), lesional characterization in 13.2% (n=12), and ACR category 3 in 10% (n=9).
In our center, a complete breast MRI protocol included an axial T2-weighted acquisition, sagittal three-dimensional Gradient Echo T1-weighted dynamic VIBRANT acquisitions: one before and five after injection (each phase duration was 90 s) of gadolinium and one axial VIBRANT high-resolution acquisition on a 3T MRI device (HDX Twinspeed, GE medical) (Table 1). Images subtracted from the first three series after injection and images of MIP of these subtractions were also available. The abbreviated protocol consisted of only the sagittal sequence VIBRANT acquisition before injection, the first sagittal series VIBRANT acquisition after injection, and the subtracted images (Fig. 1). We did not use axial T2-weighted acquisition and MIP images.
Table 1.
Breast MRI protocol
| Axial T2 SE | Sagittal VIBRANT | Axial VIBRANT HD | |
|---|---|---|---|
| Flip angle (°) | 90 | 10 | 10 |
| Repetition time/Echo time (ms) | 7723/120.12 | 4.89/2.10 | 9.59/4.25 |
| Field of view (cm) | 34×37.4 | 22×24.2 | 29×31.9 |
| Matrix | 320×480 | 224×224 | 416×512 |
| Section thickness (mm) | 3 | 2.2 | 1.8 |
| Number of excitations | 1 | 0.5 | 0.71 |
SE, spin-echo; HD, high-definition.
Figure 1.
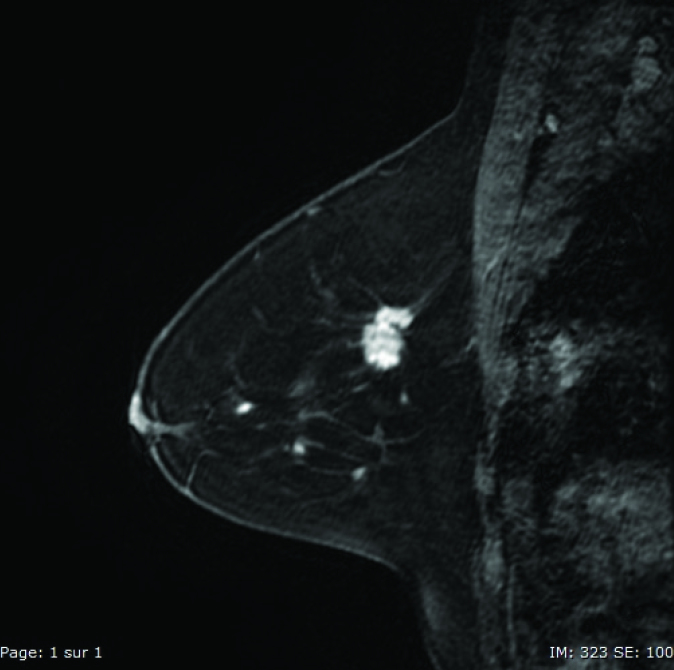
Sagittal VIBRANT® image of an invasive breast carcinoma.
Two radiologists (a junior physician with 6 months of breast MRI experience, and a senior physician with 5 years of experience) individually reviewed the images in two stages separated by at least two weeks with randomization so as to limit all bias. Abbreviated and complete protocols were mixed in the two stages. For both stages, the readers had access to the previous examinations and clinical information, but not to the later examinations, current breast MRI report, and later possible anatomopathology analysis or imaging follow-up. For every reader, the order of the two stages was randomized and the reading of the abbreviated protocol was blinded from the reading of the complete protocol. For each breast, the readers indicated the size, the quadrant, the type of lesion in case of anomaly, and the ACR BI-RADS classification. The duration of read outs were also noted. The BI-RADS classifications were then compiled according to the implication on the care: the benign group not requiring specific care (ACR 1 and 2), the surveillance group (ACR 3), and the group requiring histologic proof (ACR 4 and 5).
At the end of the readings, in case of discordance with regard to the lesion location, a consensus was sought between the two readers to ensure that it was the same lesion in all three cases (two study readings and the prospective clinical reading). If the lesion differed from the one in the prospective clinical reading, it was considered to be a false positive.
Statistical analysis
The quantitative parameters are described as mean ± standard deviation and the qualitative parameters are described as frequency and percentage. For each reader, the comparison of the reading time according to the two reading protocols was performed by paired Student’s t-test. The inter-reader reliability and the reliability between the two reading protocols were assessed using the intraclass correlation coefficient (ICC). For the inter-reader reliability, according to McGraw and Wong (1996) Convention (14), a two-way random effects model, absolute agreement, single rater/measurement (i.e., ICC [2,1]) was performed (15). For intrareader reliability, a two-way mixed-effects model (absolute agreement, single measurement) was computed. Based on the terminology proposed by Landis and Koch (16), an ICC value of 0.6–0.8 indicated substantial agreement and 0.8–1.0 indicated almost full agreement. The discriminant power of the BI-RADS classification to detect a cancer was assessed with the area under the curve (AUC) for each protocol. The AUCs for both protocols were compared using a nonparametric approach (17). The BI-RADS classification was then dichotomized (1/2/3 vs. 4/5), and the sensitivity and specificity for each protocol were computed. Sensitivities were compared using a McNemar’s test in the subpopulation of breasts with a cancer diagnosis. Specificities were compared using a McNemar’s test in the subpopulation of breasts not harboring a malignant lesion.
Thirty patients were included in each group corresponding to a total sample size of 168 breasts. With this sample size, our study had 80% power to detect a change in sensitivity from 0.9 to 0.99 using a two-sided binomial test, and 85% power to detect a change in specificity from 0.8 to 0.9 using a two-sided binomial test.
All analyses were performed using SAS software version 9.3 (SAS Institute Inc.). The level for significance was set at P < 0.05.
Results
Our population consisted of 90 patients, of whom 12 had a personal history of breast cancer with unilateral mastectomy. Thus, ACR ratings were made for 168 breasts. The reference standard was assessed by a follow-up of 24 months for 137 breasts (81.5%), by percutaneous biopsy with at least a 12-month imaging follow-up for 5 breasts (3%), and breast surgery for 26 breasts (15.5%). Out of the total of 168 breasts, breast cancer was present in 26 breasts (15%). Of these, 25 were invasive and one was purely a ductal carcinoma in situ (DCIS). Among the 25 invasive cases of breasts cancer, there were 18 (72%) nonspecific carcinomas (i.e., invasive ductal carcinoma) and 7 (28%) lobular carcinomas. The histology grade was I for 4 lesions (16%), II for 19 lesions (76%), and III for 2 lesions (8%). There were 22 estrogen receptor positive (ER+) lesions (88%) and 3 human epidermal growth factor receptor 2 positive (HER2+) lesions (12%). There were five benign lesions: one adenoma, one fibroadenoma, one papilloadenoma, one breast dystrophy, and one radial scar.
For both readers, the reading time was significantly lower with the abbreviated protocol than with the complete protocol. The average reading time for the junior reader was 247±65 s with the abbreviated protocol and 329±84 s with the complete protocol (mean difference 83 s, 95% CI [70;95] P < 0.001 ), while for the senior reader these were 59±34 s and 14±72 s, respectively (mean difference, 84 s; 95% CI [67;101]; P < 0.001).
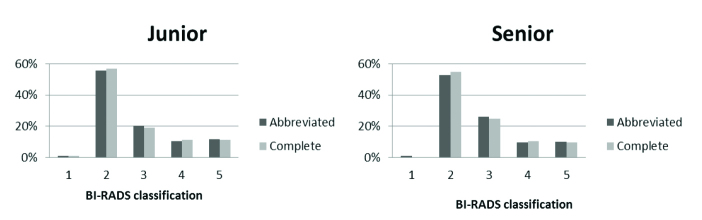
The BI-RADS classifications of the breasts for each reader and each protocol are presented in Table 2 and Fig. 2.
Table 2.
BI-RADS classifications of 168 breasts for each reader and each protocol
| Group | BI-RADS | Junior reader | Senior reader | ||
|---|---|---|---|---|---|
|
|
|
||||
| Abbreviated n (%) |
Complete n (%) |
Abbreviated n (%) |
Complete n (%) |
||
| Benign | 1–2 | 96 (57.2) | 98 (58.3) | 91 (54.2) | 92 (54.8) |
|
| |||||
| ACR 3 | 3 | 34 (20.2) | 32 (19.1) | 44 (26.2) | 42 (25.0) |
|
| |||||
| Biopsy | 4–5 | 38 (22.6) | 38 (22.6) | 33 (19.6) | 34 (20.2) |
BI-RADS, breast imaging reporting and data system; ACR, American College of Radiology.
Figure 2.
BI-RADS classification of 168 breasts by junior and senior readers based on complete and abbreviated protocols.
Considering BI-RADS classification, the inter-reader reliability was 0.941 (0.920–0.956) for the complete protocol and 0.903 (0.871–0.927) for the abbreviated protocol. The reliability between both protocols was 0.895 (0.861–0.922) for senior reader and 0.982 (0.975–0.986) for junior reader.
By pooling the two readers, 14 lesions were classified as “benign” with the complete protocol out of the 78 classified as “ACR 3” with the abbreviated protocol (18%).
Regardless of the reader, the AUC of the BI-RADS classification to detect a cancer was not significantly different between the two protocols (Table 3). For both readers, all cancers were in the group “Biopsy required” using either protocol (sensitivity, 100%).
Table 3.
Comparison of the performance of BI-RADS classification according to complete and abbreviated protocols
| Junior reader | Senior reader | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Abbreviated | Complete | P | Abbreviated | Complete | P | |
| AUC | 0.985 | 0.983 | 0.33 | 0.987 | 0.989 | 0.69 |
|
| ||||||
| Sensitivitya | 100% (26/26) | 100% (26/26) | 1.00 | 100% (26/26) | 100% (26/26) | 1.00 |
|
| ||||||
| Specificityb | 91.5% (130/142) | 91.5% (130/142) | 1.00 | 95.1% (135/142) | 94.4% (134/142) | 0.71 |
|
| ||||||
| False positive rateb | 8.4% (12/142) | 8.4% (12/142) | - | 4.9% (7/142) | 5.6% (8/142) | - |
The reference standard was the diagnosis of cancer. AUC was computed by considering the five levels of BI-RADS classification. Sensitivity and specificity were computed by considering BI-RADS 4/5 against 1/2/3.
BI-RADS, breast imaging reporting and data system; AUC, area under the curve.
Computed on 26 malignant lesions;
Computed on 142 breasts without malignant lesions.
For senior reader, out of the 142 breasts without malignant lesions, 135 were classified as “benign” or “ACR 3” with the abbreviated protocol versus 134 with the complete protocol (specificity, 95.1% vs. 94.4%; P = 0.71). For junior reader, 130 lesions were classified “benign” or “ACR 3,” regardless of the protocol (specificity, 91.5%).
Discussion
In our study, we showed that the reading time was clearly shorter when the abbreviated protocol was used. There was a high level of agreement between the complete and abbreviated protocols for BI-RADS category. The level of sensitivity and specificity was high with the abbreviated protocol and did not differ significantly from the complete protocol.
The use of an abbreviated protocol, stopping at the first series after injection, allows the duration of the examination to be substantially reduced, with an acquisition time of 3 minutes and an occupation time for the scan that varied from 10 to 15 minutes (4, 12, 13). With the complete protocol, the average acquisition time varies from 30 to 60 minutes (4, 12, 18, 19), which does not allow for more than two patients to be processed per hour in the reference centers (20). Use of the abbreviated protocol may lead to improvements in breast MRI screening since it should substantially reduce the indirect costs. Thus, the rate of breast MRIs that can be performed could be increased by at least two folds.
With the abbreviated protocol, the reading time was significantly reduced for both readers relative to the complete protocol. With the abbreviated protocol, reading times for the senior physician were about 60 seconds, as opposed to reading times of 60 to 120 seconds for a typical mammography screening (4, 21, 22). Interpretation time significantly decreased with the abbreviated protocol, allowing for innovative reading options, such as double reading and real-time interpretation (23). In our study, interpretation time is far longer than that published by other abbreviated protocols. This is probably due to the fact that we did not use MIP images and made an interpretation with standard sequence. Indeed, contrary to Kuhl et al. (4) who used only MIP images for the interpretation of abbreviated protocol, we did not use MIP images because we think that it is necessary to make no differences between interpretation of abbreviated and complete protocols. So, we used native images and subtracted images for both interpretations of abbreviated and full protocols.
In our study, there was nearly complete inter-reader agreement for junior and senior readers, with both protocols. The use of an abbreviated protocol was hence not detrimental in terms of reproducibility of the interpretation and the ensuing care.
There was also nearly complete concordance (above 0.80) between both protocols for both readers. Moreover, the sensitivity and the specificity were high for both readers, and they were comparable for both reading protocols. The abbreviated protocol did not influence the sensitivity and the specificity of the examination. We provide corroborating evidence for the equal diagnostic utility of abbreviated versus full multiparametric breast MRI. Indeed, this is in keeping with the findings of Kuhl et al. (4), who demonstrated that the use of an abbreviated protocol for breast screening by MRI is feasible without compromising the sensitivity and the specificity of the examination relative to a complete protocol. It also fits with the findings of Mango et al. (12) who were able to demonstrate a high level of sensitivity for detection of cancers with an abbreviated protocol. Moreover, these results are in agreement with the study by Moschetta et al. (24) who found that abbreviated protocol has the same diagnostic potential as the standard protocol in patients undergoing breast MRI for screening, problem solving, or preoperative staging. In standard clinical situations, the care provided based on findings from an abbreviated protocol corresponded with what was provided when a standard protocol was used. When an abbreviated protocol was used, both readers still detected all cancer lesions. No study previously addressed the concordance between the two protocols and few studies published on abbreviated protocols did not evaluate specificity. Moreover, our study demonstrates that the abbreviated protocol might be used by a junior reader without any impact on the sensitivity and specificity values of the examination.
The percentage of ACR 3 cases with the abbreviated protocol that were reclassified as ACR 2 with the complete protocol was 18% in our study versus 37.7% in the study by Kuhl et al. (4). The utility of the late additional sequences for characterization of the lesion appears to be less clear in our study because we had fewer cases diagnosed as ACR 3 with the abbreviated protocol and reclassified as ACR2 with the complete protocol. Moreover, we did not find a loss of specificity with abbreviated protocol although late VIBRANT acquisitions were not used.
Our study has several limitations. First of all, it is a retrospective study. Second, as the readers were cognizant of the indication for the examination, in 17 cases the cancer was hence known to the readers. This could limit the value of the 100% sensitivity that we encountered in our study with the abbreviated protocol. However, with the exception of tumor staging, detection of all cancer lesions was the same regardless of the examination. Furthermore, detection matched the usual clinical conditions for interpretation of breast MRI, which is integrated into the complete breast imaging process for the patient. Indeed, our goal was to evaluate the impact of standard contextual information in the context of an abbreviated protocol, as done by Heacock et al. (11). In our study, there was only one DCIS, which could have led to an overestimation of the sensitivity due to a better sensitivity for invasive carcinoma than for DCIS. Indeed, diagnosing DCIS on MRI represents the single major diagnostic challenge.
In conclusion, our study indicates that the use of an abbreviated protocol maintained a high level of sensitivity and specificity with decreased examination and reading times. Our results provide corroborating evidence that the abbreviated protocol could be a new diagnostic tool for radiologists instead of full breast MRI protocol.
Main points.
Use of the abbreviated protocol resulted in decreased interpretation time.
There was no difference in sensitivity and specificity between the complete and abbreviated protocols.
There was a high level of concordance between the abbreviated and complete protocols.
Footnotes
Conflict of interest disclosure
The authors declared no conflicts of interest.
References
- 1.Mann RM, Kuhl CK, Kinkel K, Boetes C. Breast MRI: guidelines from the European Society of Breast Imaging. Eur Radiol. 2008;18:1307–1318. doi: 10.1007/s00330-008-0863-7. https://doi.org/10.1007/s00330-008-0863-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nakano S, Kousaka J, Fujii K, et al. Impact of real-time virtual sonography, a coordinated sonography and MRI system that uses an image fusion technique, on the sonographic evaluation of MRI-detected lesions of the breast in second-look sonography. Breast Cancer Res Treat. 2012;134:1179–1188. doi: 10.1007/s10549-012-2163-9. https://doi.org/10.1007/s10549-012-2163-9. [DOI] [PubMed] [Google Scholar]
- 3.Nakano S, Yoshida M, Fujii K, et al. Fusion of MRI and sonography image for breast cancer evaluation using real-time virtual sonography with magnetic navigation: first experience. Jpn J Clin Oncol. 2009;39:552–559. doi: 10.1093/jjco/hyp087. https://doi.org/10.1093/jjco/hyp087. [DOI] [PubMed] [Google Scholar]
- 4.Kuhl CK, Schrading S, Strobel K, Schild HH, Hilgers RD, Bieling HB. Abbreviated breast magnetic resonance imaging (MRI): first postcontrast subtracted images and maximum-intensity projection-a novel approach to breast cancer screening with MRI. J Clin Oncol. 2014;32:2304–2310. doi: 10.1200/JCO.2013.52.5386. https://doi.org/10.1200/JCO.2013.52.5386. [DOI] [PubMed] [Google Scholar]
- 5.Berg WA, Zhang Z, Lehrer D, et al. Detection of breast cancer with addition of annual screening ultrasound or a single screening MRI to mammography in women with elevated breast cancer risk. JAMA. 2012;307:1394–1404. doi: 10.1001/jama.2012.388. https://doi.org/10.1001/jama.2012.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lehman CD, Isaacs C, Schnall MD, et al. Cancer yield of mammography, MR, and US in high-risk women: prospective multi-institution breast cancer screening study. Radiology. 2007;244:381–388. doi: 10.1148/radiol.2442060461. https://doi.org/10.1148/radiol.2442060461. [DOI] [PubMed] [Google Scholar]
- 7.Saslow D, Boetes C, Burke W, et al. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57:75–89. doi: 10.3322/canjclin.57.2.75. https://doi.org/10.3322/canjclin.57.2.75. [DOI] [PubMed] [Google Scholar]
- 8.Tilanus-Linthorst MM, Obdeijn IM, Bartels KC, de Koning HJ, Oudkerk M. First experiences in screening women at high risk for breast cancer with MR imaging. Breast Cancer Res Treat. 2000;63:53–60. doi: 10.1023/a:1006480106487. https://doi.org/10.1023/A:1006480106487. [DOI] [PubMed] [Google Scholar]
- 9.Brennan S, Liberman L, Dershaw DD, Morris E. Breast MRI screening of women with a personal history of breast cancer. AJR Am J Roentgenol. 2010;195:510–516. doi: 10.2214/AJR.09.3573. https://doi.org/10.2214/AJR.09.3573. [DOI] [PubMed] [Google Scholar]
- 10.Kuhl CK, Schmutzler RK, Leutner CC, et al. Breast MR imaging screening in 192 women proved or suspected to be carriers of a breast cancer susceptibility gene: preliminary results. Radiology. 2000;215:267–279. doi: 10.1148/radiology.215.1.r00ap01267. https://doi.org/10.1148/radiology.215.1.r00ap01267. [DOI] [PubMed] [Google Scholar]
- 11.Heacock L, Melsaether AN, Heller SL, et al. Evaluation of a known breast cancer using an abbreviated breast MRI protocol: Correlation of imaging characteristics and pathology with lesion detection and conspicuity. Eur J Radiol. 2016;85:815–823. doi: 10.1016/j.ejrad.2016.01.005. https://doi.org/10.1016/j.ejrad.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 12.Mango VL, Morris EA, David Dershaw D, et al. Abbreviated protocol for breast MRI: Are multiple sequences needed for cancer detection? Eur J Radiol. 2015;84:65–70. doi: 10.1016/j.ejrad.2014.10.004. https://doi.org/10.1016/j.ejrad.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 13.Mann RM, Mus RD, van Zelst J, Geppert C, Karssemeijer N, Platel B. A novel approach to contrast-enhanced breast magnetic resonance imaging for screening: high-resolution ultrafast dynamic imaging. Invest Radiol. 2014;49:579–585. doi: 10.1097/RLI.0000000000000057. https://doi.org/10.1097/RLI.0000000000000057. [DOI] [PubMed] [Google Scholar]
- 14.McGraw K, Wong S. Forming inferences about some intraclass correlation coefficients. Psychol Methods. 1996;17 https://doi.org/10.1037/1082-989X.1.1.30. [Google Scholar]
- 15.Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychol Bull. 1979;86:420–428. doi: 10.1037//0033-2909.86.2.420. https://doi.org/10.1037/0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- 16.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. https://doi.org/10.2307/2529310. [PubMed] [Google Scholar]
- 17.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. https://doi.org/10.2307/2531595. [PubMed] [Google Scholar]
- 18.Carpenter AP, Leemis LM, Papir AS, Phillips DJ, Phillips GS. Managing magnetic resonance imaging machines: support tools for scheduling and planning. Health Care Manag Sci. 2011;14:158–173. doi: 10.1007/s10729-011-9153-z. https://doi.org/10.1007/s10729-011-9153-z. [DOI] [PubMed] [Google Scholar]
- 19.Ivanov I, Yehuda R. Optimizing fitness for duty and post-combat clinical services for military personnel and combat veterans with ADHD-a systematic review of the current literature. Eur J Psychotraumatol. 2014;5 doi: 10.3402/ejpt.v5.23894. https://doi.org/10.3402/ejpt.v5.23894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pinker-Domenig K, Bogner W, Gruber S, et al. High resolution MRI of the breast at 3 T: which BI-RADS(R) descriptors are most strongly associated with the diagnosis of breast cancer? Eur Radiol. 2012;22:322–330. doi: 10.1007/s00330-011-2256-6. https://doi.org/10.1007/s00330-011-2256-6. [DOI] [PubMed] [Google Scholar]
- 21.Tchou PM, Haygood TM, Atkinson EN, et al. Interpretation time of computer-aided detection at screening mammography. Radiology. 2010;257:40–46. doi: 10.1148/radiol.10092170. https://doi.org/10.1148/radiol.10092170. [DOI] [PubMed] [Google Scholar]
- 22.Garg AS, Rapelyea JA, Rechtman LR, et al. Full-field digital mammographic interpretation with prior analog versus prior digitized analog mammography: time for interpretation. AJR Am J Roentgenol. 2011;196:1436–1438. doi: 10.2214/AJR.10.5430. https://doi.org/10.2214/AJR.10.5430. [DOI] [PubMed] [Google Scholar]
- 23.Harvey SC, Di Carlo PA, Lee B, Obadina E, Sippo D, Mullen L. An abbreviated protocol for high-risk screening breast MRI saves time and resources. J Am Coll Radiol. 2016;13:374–380. doi: 10.1016/j.jacr.2015.08.015. https://doi.org/10.1016/j.jacr.2015.08.015. [DOI] [PubMed] [Google Scholar]
- 24.Moschetta M, Telegrafo M, Rella L, Stabile Ianora AA, Angelelli G. Abbreviated combined MR protocol: a new faster strategy for characterizing breast lesions. Clin Breast Cancer. 2016;16:207–211. doi: 10.1016/j.clbc.2016.02.008. https://doi.org/10.1016/j.clbc.2016.02.008. [DOI] [PubMed] [Google Scholar]