Abstract
PURPOSE
We aimed to retrospectively compare the local tumor control rates between low frequency (LF) and high frequency (HF) microwave ablation devices in the treatment of <3 cm lung metastases.
METHODS
A total of 36 patients (55 tumors) were treated with the LF system (915 MHz) and 30 patients (39 tumors) were treated with the HF system (2450 MHz) between January 2011 and March 2016. Computed tomography (CT) scans performed prior to and 24 hours after the ablation were used to measure the size of the ablation zone and to calculate the ablation margin. The subsequent CTs were used to detect local tumor progression. Possible predictive factors for local progression were analyzed. All patients had a minimum follow-up of 3 months with a median of 13.8 months for the LF group and 11.7 months for the HF group.
RESULTS
The ablation margin (P = 0.015), blood vessel proximity (P = 0.006), and colorectal origin (P = 0.029) were significantly associated with the local progression rate. The local progression rates were 36.3% for LF ablations and 12.8% for HF ablations. The 6, 12, and 18 months local progression-free survival rates were 79%, 65.2% and 53% for the LF group and 97.1%, 93.7%, and 58.4% for the HF group, with a significant difference between the survival curves (P = 0.048).
CONCLUSION
HF ablations resulted in larger ablation margins with fewer local progression compared with LF ablations.
Patients with a limited number of lung metastases can hope for a longer disease-free survival if both the primary tumor and the metastases are treated (1). Surgical resection is considered to be the treatment of choice for lung oligometastases (2). If patients are medically inoperable or refuse surgery, less invasive techniques such as stereotactic body radiation therapy or percutaneous thermal ablations can be used. Amongst the thermal ablation techniques, RFA is currently the most widely employed method in the treatment of lung malignancies and has been the subject of most research. MWA is a relatively new thermal ablation technique that has seen an increase in use in the last decade with similar results as RFA in the treatment of lung tumors (3–5).
The MWA systems are powered by either 915 MHz or 2450 MHz generators, with no consensus regarding which one is superior. The size and shape of the ablation zone are highly dependent on the design of the antenna, the power setting, and the treatment duration. At present, 7 MWA systems with different ablation characteristics are commercially available in Europe and the USA; a potential user must be familiar with the performance of these devices in order to be able to choose the best MWA option (6–8). At our institution two of these systems, operating at frequencies of 915 MHz (Evident, Covidien) and 2450 MHz (Emprint, Covidien) have been employed in the treatment of lung tumors. The performance of the low frequency (LF) system which has been commercially available since 2008 is relatively well documented (7–10). The high frequency (HF) system that was launched in 2014 is expected to produce larger, more spherical and predictable ablation zones with the use of a new antenna design (11, 12). The purpose of the present study is to compare the performance of these two systems in the treatment of lung metastases with a focus on prognostic factors and local tumor progression (LTP).
Methods
Patients and treatment protocol
Approval of the institutional review board was obtained for this retrospective study. All patients included in the study provided written informed consent.
The choice to undergo MWA was decided upon the recommendation of the institution’s multidisciplinary tumor board either because the patients were medically inoperable or they refused surgery. The patients had a histologically confirmed and successfully resected primary tumor with new or enlarging pulmonary masses consistent with metastases that were detected on previous computed tomography (CT) examinations. Patients eligible for treatment at our institution should not have mediastinal or extrathoracic spread, underlying coagulopathy and should not have more than 5 pulmonary lesions.
Just before the ablation, an unenhanced planning CT of the thorax (Somatom Sensation 64; Siemens: 5 mm collimation, 30 mAs, 120 kV) was performed. The data sets were acquired unenhanced as a thoracic spiral in craniocaudal direction, with both arms elevated. The purpose of the examination was to confirm the location and size of the tumor, to choose the required antenna length (LF: 12/17/22 cm; HF: 15/20 cm) and to plan the puncture pathway.
The ablations were performed by a radiologist with over 20 years of experience in thoracic CT-guided interventions under conscious analgesia and sedation (15 mg Piritramide + 5 mg Diazepam), pulsoximetric monitoring and local anesthesia with Mepivacainhydrochloride. When necessary, an additional dose of up to 6 mg Piritramide was administered.
The antenna insertion and ablation monitoring were performed under CT guidance; image acquisitions were made at regular intervals to verify the correct position of the applicator and the occurrence of complications. Ablation time and power were adjusted considering the size and location of the tumor, according to the manufacturer’s recommendations, in order to obtain an encompassing ablation margin. All LF ablations were performed at a constant power of 45 W (the maximum power allowed by the device), whereas the HF ablations were started at lower power levels which were steadily increased while taking the location and the size of the tumor into account. If a tumor was small, close to the chest wall or to the mediastinum, lower power levels were used to prevent damage to the surrounding structures. If the lesion was larger and/or the expected ablation zone would not significantly exceed the lung parenchyma, the maximum power was reached and maintained, thus maximizing the chances of a complete ablation. Puncture tract ablation was performed upon removal of the applicator.
The patients received an unenhanced chest CT (Somatom Sensation 64; Siemens: 5 mm collimation, 30 mAs, 120 kV) 24 hours after the ablation to assess the presence of complications and to review the aspect of the ablation zone. The subsequent follow-up using enhanced and unenhanced CTs was performed at 3-month intervals within the first year and at 6-month intervals thereafter.
Study design
In order to be able to verify the homogeneity and comparability of the two groups, the patient’s age, gender, tumor type and size were recorded and compared. The inclusion criteria for the present study were as follows: a) ablation of a lung metastasis performed with either the LF or HF system; b) Imaging follow-up of at least 3 months; c) CT examination performed within 24 hours after ablation. The exclusion criteria were as follows: a) no regular follow-up examinations; b) tumors larger than 3 cm.
A total of 36 patients who underwent LF ablations of 55 lung metastases and 30 patients who underwent HF ablations of 39 metastases were included. Table 1 summarizes patient characteristics for LF and HF ablation groups. The LF ablations took place between January 2011 and May 2015 and the HF ablations took place between June 2014 and March 2016. During the introductory phase of the HF system (from June 2014 to May 2015), 6 ablations were performed with the LF system and 14 were performed with the HF system. As it creates smaller ablation zones, the LF system was preferred during this period for low-diameter tumors located close to the pleura or mediastinum (LF tumor size: 0.7±0.2 cm, 0.3–0.8 cm; HF tumor size: 1.3±0.6 cm, 0.6–2.6 cm).
Table 1.
Patient characteristics
| LF | HF | P | |
|---|---|---|---|
| Patients, n | 36 | 30 | |
|
| |||
| Gender (M/F), n | 19/17 | 13/17 | 0.469 |
|
| |||
| Age, years | 60.3±11.9 (34–78) | 60.4±11.4 (40–78) | 0.915 |
|
| |||
| Metastases, n | 55 | 39 | |
|
| |||
| Primary tumora, n | |||
| Colorectal | 20 (10) | 19 (3) | |
| Breast | 9 (5) | 5 (0) | |
| NSCLC | 5 (1) | 8 (1) | |
| Kidney | 5 (1) | - | |
| Hepatocellular | 4 (2) | 1 (0) | |
| Otherb | 12 (1) | 6 (1) | |
|
| |||
| Tumor size (cm), mean±SD (range) | 1.0±0.4 (0.3–2.5) | 1.1±0.6 (0.4–2.6) | 0.235 |
|
| |||
| Subpleural tumor (<2 cm from pleura), n | 0.137 | ||
| Positive | 24 | 11 | |
| Negative | 31 | 28 | |
|
| |||
| Perivascular tumor (<1 cm from blood vessel), n | 0.197 | ||
| Positive | 8 | 10 | |
| Negative | 47 | 29 | |
|
| |||
| Ablation time (min), mean±SD (range) | 10.1±3.9 (2–20) | 8.1±3.2 (3–17) | <0.001 |
|
| |||
| Power (W), mean±SD (range) | 45.0 | 75.2±16.5 (45.0–96.4) | <0.001 |
LF, low frequency; HF, high frequency; M, male; F, female; NSCLC, non-small cell lung carcinoma.
Numbers in parentheses represent number of metastases that showed local progression at follow-up.
Melanoma, nasopharyngeal, esophagus, thyroid, endometrial, adrenal, adenoid-cystic, and cholangiocarcinoma.
The preinterventional CT (lung window) was used to measure the maximum diameter of the index tumor and to determine if the tumor was in the proximity of a blood vessel. A tumor was considered perivascular when located within 1 cm from a large blood vessel (>3 mm in diameter).
The shape of a microwave ablation zone in ideal conditions is that of a rotational ellipsoid with a long-axis diameter along the antenna shaft and two equal perpendicular short-axis diameters. When considering the size and shape of the ablation zone, the limiting factor is the short-axis diameter which has to be larger than the tumor itself in order to provide a safe ablation margin within the apparently healthy surrounding lung tissue. The short-axis diameter of the ablation zone was measured based on the CT performed on the day following the ablation (lung window).
Because of the low conspicuity of the index tumor within the ablation zone and because of the subsequent tissue contraction, in many cases it was impossible to identify the tumor and to directly measure the ablation margin on the postinterventional CT. Therefore, the ablation margin (AM) was calculated based on the difference between the short-axis diameter of the ablation zone (Dx) and the maximum diameter of the index tumor (Dy) according to the following formula: AM=(Dx−Dy)/2. This method allows an approximation of the ablation margin independently of the lesion conspicuity, but relies on the assumption that the antenna was placed close to the center of the lesion.
The long-term follow-up CTs were used to detect LTP, which was defined as any new growth of the ablation zone detected after 3 months postablation. The selected CT examinations were analyzed independently by two radiologists, with four and two years of experience in thoracic imaging, who were blinded to the device that was used.
The primary purpose of the study was to identify whether there was a statistically significant difference in LTP-free survival (defined as the time period between the ablation and the first detection of LTP) between the two groups. The secondary purpose was to identify if any patient-related (tumor size, proximity to a blood vessel, histologic type) or ablation-related parameters (ablation system, ablation margin) had influenced the occurrence of LTP.
Statistical analysis
The statistical analysis was performed using GraphPad Prism 6 (GraphPad Software) and Minitab 17 (Minitab Inc.). Data were expressed as mean ± standard deviation except for values indicating time where medians were used. Minimum and maximum values were also provided. The parametric Student’s t-test was used to determine whether the differences between the two systems regarding age, tumor size, ablation power, short-axis diameter of the ablation zones and ablation margin were significant. Student’s t-test was also used to determine if predictors (tumor size, ablation margin) were significantly different between LTP and no-LTP groups. The nonparametric Mann-Whitney U test was used determine whether the differences regarding imaging follow-up and time to LTP were significant.
Predictive factors for LTP (tumor size, proximity to a blood vessel, histologic type, ablation system, ablation margin) were evaluated using multiple logistic regression. The effect of the predictive factors was quantified using odds ratios (OR). Kaplan-Meyer curves were used to depict the LTP-free survival. The difference between the curves was analyzed using the log-rank test. The unit of analysis for all the parameters was the lesion (metastasis). A P value of less than 0.05 was considered to indicate a statistically significant difference for all analyses.
Results
With a mean diameter of 1.0±0.4 cm (0.3–2.5 cm) in the LF group and 1.1±0.6 cm (0.4–2.6 cm) in the HF group, the tumor size was not significantly different between the two groups (P = 0.235). The short-axis diameter of the ablation zone was significantly smaller (P < 0.001) after the LF ablations (2.0±0.4 cm; 1.0–3.2 cm) than after the HF ablations (2.9±0.7 cm; 1.2–4.7 cm) (Fig. 1). Consequently, the ablation margins were also significantly smaller (P < 0.001) after LF ablations (0.5±0.2 cm; 0.1–1.0 cm) than after the HF ablations (0.9±0.3 cm; 0.1–1.6 cm).
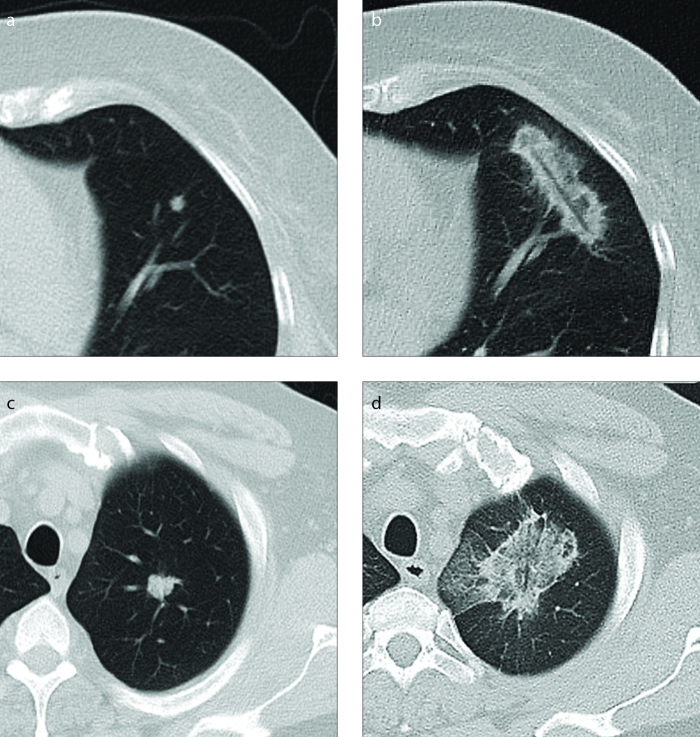
Figure 1. a–d.
Typical aspect of the ablation zones. Panels (a, b) show low frequency (LF) ablation of a 0.4 cm large colorectal carcinoma metastasis with a duration of 12 minutes. The ablation zone has a highly elongated shape. Panels (c, d) show high frequency (HF) ablation of a 1.4 cm large colorectal carcinoma metastasis with a duration of 14 minutes and an average power of 95 W. The ablation zone has a round appearance.
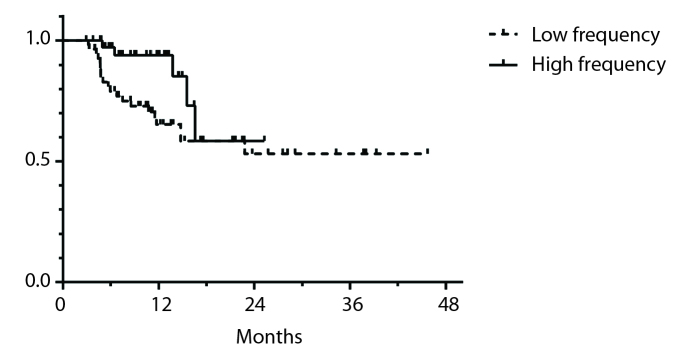
The LF group had a longer follow-up (median, 13.8 months; 3.0–45.7 months) than the HF group (median, 11.7 months; 3.0–28.8 months) (P = 0.006). In the LF group, 36.3% (n=20) of the ablations resulted in LTP whereas in the HF group 12.8% (n=20) of ablation resulted in LTP. The 6-month, 12-month, and 24-month LTP-free survival rates were 79%, 65.2%, and 53% for the LF group and 97.1%, 93.7%, and 58.4% for the HF group, respectively. The difference between the LTP-free survival curves (Fig. 2) was statistically significant (P = 0.048), with better results for the HF group. In the LF group, 85% (n=17) of the LTP occurred within the first year after the treatment. In the HF group 40% (n=2) of the LTP occurred within the first year. The time to LTP was not significantly different (P = 0.108) between the LF group (median, 5.8 months; 3.2–23.0 months) and the HF group (median, 13.7 months; 5.0–16.5 months).
Figure 2.
Local progression-free survival rates. Fewer local progressions can be observed for the HF group.
The ablation margin (P = 0.015), the proximity of a blood vessel (P = 0.006), as well as colorectal origin of the metastasis (P = 0.029) significantly influenced the occurrence of LTP, when the LF and HF groups were pooled together for multivariate logistic regression. The influence of tumor size (P = 0.822), ablation system (P = 0.119) and the other tumor types (P > 0.155) were not statistically significant (Table 2).
Table 2.
Predictive factors for local tumor progression
| No LTP (n=69; 73.4%) | LTP (n=25; 26.5%) | P | |
|---|---|---|---|
| Tumor size (cm), mean±SD (range) | 1.0±0.5 (0.3–2.6) | 1.2±0.5 (0.5–2.5) | 0.201 |
|
| |||
| Primary tumor, n | |||
| Colorectal | 26 | 13 | |
| Breast | 9 | 5 | |
| NSCLC | 11 | 2 | |
| Kidney | 4 | 1 | |
| Other | 19 | 4 | |
|
| |||
| Perivascular tumors, n | 0.018 | ||
| Positive | 9 | 9 | |
| Negative | 60 | 16 | |
|
| |||
| Ablation margin (cm), mean±SD (range) | 1.5±0.6 (0.1–3.2) | 0.8±0.4 (0.0–1.5) | <0.001 |
|
| |||
| Ablation system, n | 0.016 | ||
| LF | 35 | 20 | |
| HF | 34 | 5 | |
LTP, local tumor progression; LF, low frequency; HF, high frequency; NSCLC, non-small cell lung carcinoma.
The presence of a larger ablation was inversely associated with LTP (OR=0.1). In the LF group, the average ablation margin of a treatment with no LTP (0.6±0.2 cm; 0.1–1.1 cm) was significantly larger than that of one with LTP (0.3±0.2 cm; 0–0.7 cm) (P = 0.001). Similarly, in the HF group, the average ablation margin of a treatment with no LTP (0.9±0.4 cm; 0.1–1.6 cm) was significantly larger than that of an unsuccessful one with LTP (0.6±0.1 cm; 0.5–0.7 cm) (P = 0.037). When pooling the LF and the HF groups together, 66% of the ablations with LTP and only 22.8% of the ablations without LTP had an ablation margin of less than 0.5 cm.
The perivascular location of a tumor was positively associated with LTP (OR=9.3). The ablation of a perivascular lesion using the LF system led to LTP in 75% of cases (n=6), whereas the ablation of a perivascular lesion using the HF system led to LTP in 30% of cases (n=3). Most of the lesions (60%; n=3) that resulted in LTP following HF ablations were perivascular.
The colorectal origin of the metastases was positively associated with LTP (OR=7.4). The individual LTP rates for each tumor type are shown in Table 2.
Discussion
Six different MWA systems are commercially available in the USA and Europe: three using 915 MHz (LF) generators (Evident, Covidien; MicroThermX, BSD Medical; Avecure, MedWaves) and three using 2450 MHz (HF) generators (Certus 140, Neuwave; AMICA, HS medical; Acculis MTA, Microsulis) (6, 7). In addition to the previously mentioned devices, the Covidien HF Emprint system was released in Europe and the USA in 2014 (12). Other devices are available only on the Asian markets (6). There is no consensus upon the superiority of neither LF nor HF systems as some authors have reported faster and larger ablations as well as higher temperatures with LF generators compared with HF generators, (13–15) while others have demonstrated the opposite (8, 16). The data available to this point suggests that the performance of the MWA devices is rather a consequence of a combination between the frequency, antenna design, cooling system, power output and other technical factors specific to each system.
The HF system analyzed in the present study operates at a power output of up to 100 W and has an antenna designed to provide tissue-independent spherical and predictable ablations. The innovations of the system consist of thermal, field and wavelength control. In brief, the active tip of the antenna has a geometry that enables the creation of a spherical microwave field (field control). The antenna is completely cooled with circulating sterile solution as opposed to the conventional antennas where the active tip remains uncooled. Completely cooling the active tip increases antenna reliability and prevents tissue sticking (thermal control). The homogeneous environment around the radiator also prevents the wavelength changes caused by tissue desiccation close to the active tip, thus avoiding comet-shaped ablations (wavelength control) (11, 12, 17).
The publications currently available concerning the HF system are scarce and consist of an ex vivo temperature monitoring study and one percutaneous and two laparoscopic liver ablation patient series (11, 18–20). To the best of our knowledge, no reports regarding lung ablations with the new HF system are available. Saccomandi et al. (20) compared the same two devices as in our study using ex vivo livers and showed that when controlled for power and time, the HF system produces larger ablation zones (20). In line with these observations, the LF treatments in our study resulted in smaller ablation zones than the ones created by the HF treatments (2.0 vs. 2.9 cm), requiring longer ablation times (10.1 min vs. 8.1 min). As the average tumor size was similar between the two groups (1.0 cm for LF group vs. 1.1 cm for HF group), the smaller LF ablation zones resulted in narrower ablation margins for this system (0.5 cm for LF vs. 0.9 cm for HF) (Fig. 3).
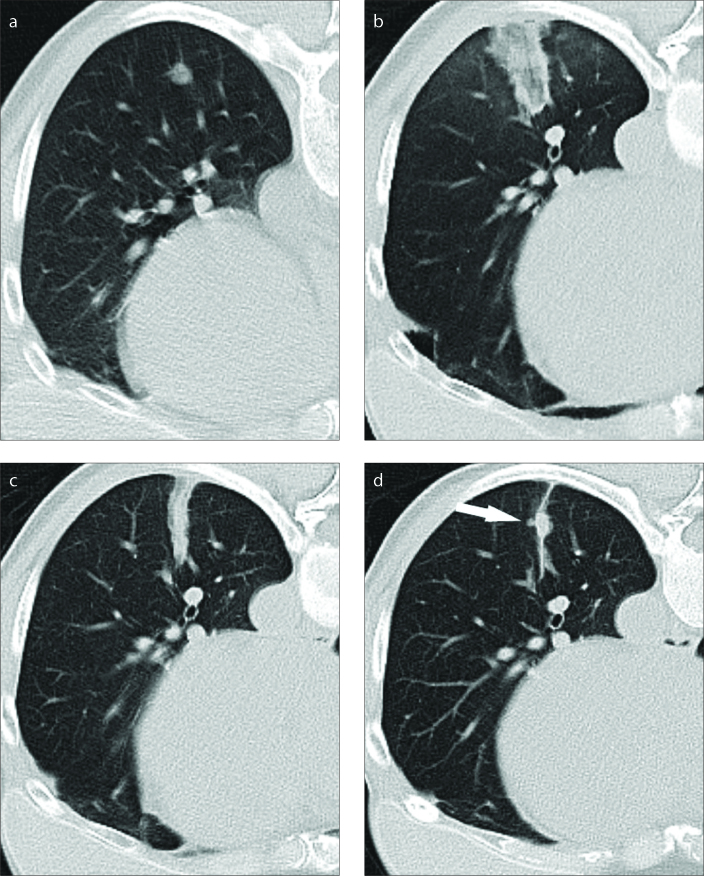
Figure 3. a–d.
LF ablation of a lung metastasis originating from breast carcinoma (10 minutes; 45 W). Image (a) shows the preinterventional aspect of the 0.8 cm large tumor. There are no large blood vessels close to the tumor. Image obtained 24 hours after the ablation (b) shows a narrow ablation zone typical for the system. The ablation margin was 0.8 cm. Image obtained 3 months after ablation (c) shows contraction of the ablation zone. Image obtained 6 months after ablation (d) shows new tumoral growth within the ablation zone (arrow).
The ground glass opacity on the postablation CT has been previously proven to correspond to necrosis except for a peripheral rim of 2–4 mm that may contain viable cells (21). Based on this observation and the fact that some tumors might have microscopic extensions in the surrounding tissue, it is important to provide an ablation margin to include them (22–25). In a study by Anderson et al. (25) only the width of the ablation margin and the proximity of a blood vessel >3 mm were able to predict LTP, whereas tumor size, lobar location, or adjacent bronchi had no significant influence. The cutoff value for a safe ablation margin recommended by the authors was >5 mm (25). Our results confirm these findings, as a smaller ablation margin was significantly associated with LTP. Neither the tumor size, nor the use of a specific ablation system were significantly associated with LTP. This means that as long as a sufficiently large ablation margin is created, the tumor size does not have an impact on LTP and that the ability of the HF system to create larger ablation zones, and consecutively more encompassing ablation margins is the main reason of the improved LTP-free survival noticed for this system rather than its other properties.
Although microwave ablations have been shown to be less susceptible towards the heat-sink effect, its influence on the therapy success is still significant using the current generation of MWA devices (26, 27). The significantly higher LTP rate after the ablation of perivascular tumors with both systems confirms these observations. Despite its improvements, three out of five local progressions occurred after HF ablations of perivascular tumors and at least one case could be attributed with a reasonable degree of certainty to the heat sink effect (Fig. 4).
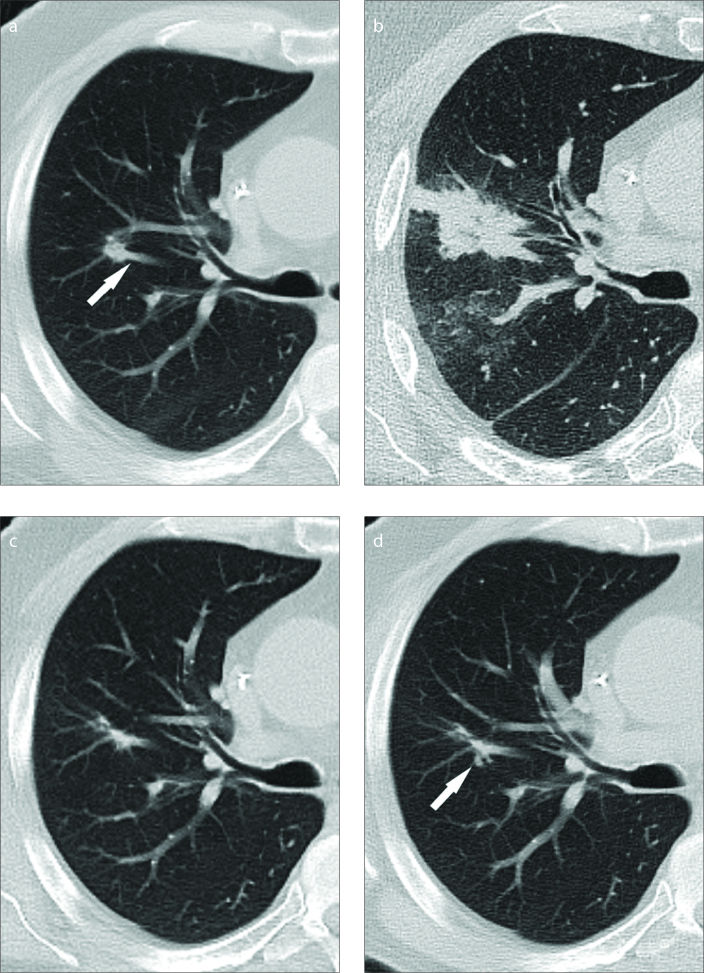
Figure 4. a–d.
HF ablation of a lung metastasis originating from colorectal carcinoma (10 minutes; 72 W). Image (a) shows preinterventional aspect of the 0.7 cm large tumor. The tumor is in direct contact with a large blood vessel (arrow). Image obtained 24 hours after the treatment (b) shows an irregular shaped ablation zone and signs of intraparenchymal bleeding. The ablation margin was 2.1 cm. Image obtained 7 months after ablation (c) indicates a strong contraction of the ablation zone. Image obtained 13 months after ablation (d) shows new tumor growth within the ablation zone (arrow).
The significant influence of tumor histology on the LTP rate is difficult to interpret. De Baère et al. (28) demonstrated in a study involving radiofrequency ablations of 1037 lung metastases that rectal carcinoma metastases were associated with a higher LTP (30.7%). Colon carcinomas (16.2%), kidney carcinomas (25.1%), sarcomas (8.3%), and other metastases(16.4%) all had smaller LTP rates (28). In our study, the LTP rates after the LF ablations were higher for colorectal carcinoma (50%) and breast carcinoma (55.5%) than for other tumor types (Table 1). When both groups were pooled, only colorectal metastases were significantly associated with higher LTP rates. Although, as shown by de Baère et al. (28), some tumor types might lead to higher LTP rates, the number of patients in our study was small and as such, these results should be interpreted with caution.
At similar median follow-up times (13.8 vs. 11.7 months), the LF ablations showed more LTP than the HF ablations (36.3% vs. 12.8%). The difference between the survival curves of the two groups was significant and this is most likely a consequence of the larger ablation margins obtained with the HF system. In other studies, LTP rates after MWA range between 21% and 33% (3, 9, 10, 29–31) which are lower than the rates for the LF system, but higher than the rates for the HF system.
As it is a retrospective nonrandomized study, based on groups that were treated at different time periods, the present work has some limitations. Therefore, factors such as differences in operator experience or systemic chemotherapy that could have influenced the LTP rate could not be accounted for. Another limitation is the fact that the LTPs were identified only according to imaging features without histologic or PET-CT confirmation and that a few LTPs might have been missed due to shorter follow-up times in some patients. The ablation of smaller tumors with the LF system during the one year when both devices were in use could be in favor of this system, but the overall tumor size was not significantly different between the two groups.
In conclusion, HF system provides larger ablation zones and more encompassing ablation margins compared with the LF system. This is likely the reason for the fewer LTPs noticed after the HF ablations, as the ablation margin was significantly associated with LTP. However, further randomized studies are required to confirm these differences and their impact on the overall survival of the patients.
Main points.
The high frequency MWA system allows creation of larger ablation zones and, more encompassing ablation margins.
Insufficient ablation margins and the proximity to a blood vessel can lead to a higher LTP rate.
Patients treated with the high frequency ablation system can have fewer local tumor progressions.
Footnotes
Conflict of interest disclosure
The authors declared no conflicts of interest.
References
- 1.Reyes DK, Pienta KJ. The biology and treatment of oligometastatic cancer. Oncotarget. 2015;6:8491–8524. doi: 10.18632/oncotarget.3455. https://doi.org/10.18632/oncotarget.3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Van Cutsem E, Cervantes A, Adam R, et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol. 2016;27:1386–1422. doi: 10.1093/annonc/mdw235. https://doi.org/10.1093/annonc/mdw235. [DOI] [PubMed] [Google Scholar]
- 3.Wolf FJ, Grand DJ, Machan JT, DiPetrillo TA, Mayo-Smith WW, Dupuy DE. Microwave ablation of lung malignancies: effectiveness, CT findings, and safety in 50 patients. Radiology. 2008;247:871–879. doi: 10.1148/radiol.2473070996. https://doi.org/10.1148/radiol.2473070996. [DOI] [PubMed] [Google Scholar]
- 4.Vogl TJ, Naguib NN, Gruber-Rouh T, Koitka K, Lehnert T, Nour-Eldin NE. Microwave ablation therapy: clinical utility in treatment of pulmonary metastases. Radiology. 2011;261:643–651. doi: 10.1148/radiol.11101643. https://doi.org/10.1148/radiol.11101643. [DOI] [PubMed] [Google Scholar]
- 5.Egashira Y, Singh S, Bandula S, Illing R. Percutaneous high-energy microwave ablation for the treatment of pulmonary tumors: a retrospective single-center experience. J Vasc Interv Radiol. 2016;27:474–479. doi: 10.1016/j.jvir.2016.01.001. https://doi.org/10.1016/j.jvir.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 6.Lubner MG, Brace CL, Hinshaw JL, Lee FT., Jr Microwave tumor ablation: mechanism of action, clinical results, and devices. J Vasc Interv Radiol. 2010;21:S192–203. doi: 10.1016/j.jvir.2010.04.007. https://doi.org/10.1016/j.jvir.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ward RC, Healey TT, Dupuy DE. Microwave ablation devices for interventional oncology. Expert Rev Med Devices. 2013;10:225–238. doi: 10.1586/erd.12.77. https://doi.org/10.1586/erd.12.77. [DOI] [PubMed] [Google Scholar]
- 8.Hoffmann R, Rempp H, Erhard L, et al. Comparison of four microwave ablation devices: an experimental study in ex vivo bovine liver. Radiology. 2013;268:89–97. doi: 10.1148/radiol.13121127. https://doi.org/10.1148/radiol.13121127. [DOI] [PubMed] [Google Scholar]
- 9.Carrafiello G, Mangini M, Fontana F, et al. Microwave ablation of lung tumours: single-centre preliminary experience. Radiol Med. 2014;119:75–82. doi: 10.1007/s11547-013-0301-z. https://doi.org/10.1007/s11547-013-0301-z. [DOI] [PubMed] [Google Scholar]
- 10.Vogl TJ, Worst TS, Naguib NN, Ackermann H, Gruber-Rouh T, Nour-Eldin NE. Factors influencing local tumor control in patients with neoplastic pulmonary nodules treated with microwave ablation: a risk-factor analysis. AJR Am J Roentgenol. 2013;200:665–672. doi: 10.2214/AJR.12.8721. https://doi.org/10.2214/AJR.12.8721. [DOI] [PubMed] [Google Scholar]
- 11.Ierardi AM, Mangano A, Floridi C, et al. A new system of microwave ablation at 2450 MHz: preliminary experience. Updates Surg. 2015;67:39–45. doi: 10.1007/s13304-015-0288-1. https://doi.org/10.1007/s13304-015-0288-1. [DOI] [PubMed] [Google Scholar]
- 12.Alonzo M, Bos A, Bennett S, Ferral H. The emprint ablation system with thermosphere technology: one of the newer next-generation microwave ablation technologies. Semin Intervent Radiol. 2015;32:335–338. doi: 10.1055/s-0035-1564811. https://doi.org/10.1055/s-0035-1564811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun Y, Cheng Z, Dong L, Zhang G, Wang Y, Liang P. Comparison of temperature curve and ablation zone between 915- and 2450-MHz cooled-shaft microwave antenna: results in ex vivo porcine livers. Eur J Radiol. 2012;81:553–557. doi: 10.1016/j.ejrad.2011.02.013. https://doi.org/10.1016/j.ejrad.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 14.Sun Y, Wang Y, Ni X, et al. Comparison of ablation zone between 915- and 2,450-MHz cooled-shaft microwave antenna: results in in vivo porcine livers. AJR Am J Roentgenol. 2009;192:511–514. doi: 10.2214/AJR.07.3828. https://doi.org/10.2214/AJR.07.3828. [DOI] [PubMed] [Google Scholar]
- 15.Liu FY, Yu XL, Liang P, Wang Y, Zhou P, Yu J. Comparison of percutaneous 915 MHz microwave ablation and 2450 MHz microwave ablation in large hepatocellular carcinoma. Int J Hyperthermia. 2010;26:448–455. doi: 10.3109/02656731003717574. https://doi.org/10.3109/02656731003717574. [DOI] [PubMed] [Google Scholar]
- 16.Simo KA, Tsirline VB, Sindram D, et al. Microwave ablation using 915-MHz and 2.45-GHz systems: what are the differences? HPB (Oxford) 2013;15:991–996. doi: 10.1111/hpb.12081. https://doi.org/10.1111/hpb.12081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brannan JD. Thermal ablation: understanding the breakthrough to predictably spherical ablations with the Thermosphere™ technology. 2014 [Google Scholar]
- 18.Berber E. Laparoscopic microwave thermosphere ablation of malignant liver tumors: an initial clinical evaluation. Surg Endosc. 2016;30:692–698. doi: 10.1007/s00464-015-4261-3. https://doi.org/10.1007/s00464-015-4261-3. [DOI] [PubMed] [Google Scholar]
- 19.Zaidi N, Okoh A, Yigitbas H, Yazici P, Ali N, Berber E. Laparoscopic microwave thermosphere ablation of malignant liver tumors: An analysis of 53 cases. J Surg Oncol. 2016;113:130–134. doi: 10.1002/jso.24127. https://doi.org/10.1002/jso.24127. [DOI] [PubMed] [Google Scholar]
- 20.Saccomandi P, Schena E, Massaroni C, et al. Temperature monitoring during microwave ablation in ex vivo porcine livers. Eur J Surg Oncol. 2015;41:1699–1705. doi: 10.1016/j.ejso.2015.08.171. https://doi.org/10.1016/j.ejso.2015.08.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamamoto A, Nakamura K, Matsuoka T, et al. Radiofrequency ablation in a porcine lung model: correlation between CT and histopathologic findings. AJR Am J Roentgenol. 2005;185:1299–306. doi: 10.2214/AJR.04.0968. https://doi.org/10.2214/AJR.04.0968. [DOI] [PubMed] [Google Scholar]
- 22.Ahmed M, Solbiati L, Brace CL, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria--a 10-year update. J Vasc Interv Radiol. 2014;25:1691–1705. doi: 10.1016/j.jvir.2014.08.027. https://doi.org/10.1016/j.jvir.2014.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim Y-S, Lee WJ, Rhim H, Lim HK, Choi D, Lee JY. The minimal ablative margin of radiofrequency ablation of hepatocellular carcinoma (> 2 and < 5 cm) needed to prevent local tumor progression: 3D quantitative assessment using CT image fusion. AJR Am J Roentgenol. 2010;195:758–765. doi: 10.2214/AJR.09.2954. https://doi.org/10.2214/AJR.09.2954. [DOI] [PubMed] [Google Scholar]
- 24.Wang X, Sofocleous CT, Erinjeri JP, et al. Margin size is an independent predictor of local tumor progression after ablation of colon cancer liver metastases. Cardiovasc Intervent Radiol. 2013;36:166–175. doi: 10.1007/s00270-012-0377-1. https://doi.org/10.1007/s00270-012-0377-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anderson EM, Lees WR, Gillams AR. Early indicators of treatment success after percutaneous radiofrequency of pulmonary tumors. Cardiovasc Intervent Radiol. 2009;32:478–483. doi: 10.1007/s00270-008-9482-6. https://doi.org/10.1007/s00270-008-9482-6. [DOI] [PubMed] [Google Scholar]
- 26.Pillai K, Akhter J, Chua TC, et al. Heat sink effect on tumor ablation characteristics as observed in monopolar radiofrequency, bipolar radiofrequency, and microwave, using ex vivo calf liver model. Medicine (Baltimore) 2015;94:e580. doi: 10.1097/MD.0000000000000580. https://doi.org/10.1097/MD.0000000000000580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ringe KI, Lutat C, Rieder C, Schenk A, Wacker F, Raatschen HJ. Experimental evaluation of the heat sink effect in hepatic microwave ablation. PLoS One. 2015;10:e0134301. doi: 10.1371/journal.pone.0134301. https://doi.org/10.1371/journal.pone.0134301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Baere T, Auperin A, Deschamps F, et al. Radiofrequency ablation is a valid treatment option for lung metastases: experience in 566 patients with 1037 metastases. Ann Oncol. 2015;26:987–991. doi: 10.1093/annonc/mdv037. https://doi.org/10.1093/annonc/mdv037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vogl TJ, Naguib NNN, Gruber-Rouh T, Koitka K, Lehnert T, Nour-Eldin N-EA. Microwave ablation therapy: clinical utility in treatment of pulmonary metastases. Radiology. 2011;261:643–651. doi: 10.1148/radiol.11101643. https://doi.org/10.1148/radiol.11101643. [DOI] [PubMed] [Google Scholar]
- 30.Lu Q, Cao W, Huang L, et al. CT-guided percutaneous microwave ablation of pulmonary malignancies: Results in 69 cases. World J Surg Oncol. 2012;10:80. doi: 10.1186/1477-7819-10-80. https://doi.org/10.1186/1477-7819-10-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Belfiore G, Ronza F, Belfiore MP, et al. Patients’ survival in lung malignancies treated by microwave ablation: our experience on 56 patients. Eur J Radiol. 2013;82:177–181. doi: 10.1016/j.ejrad.2012.08.024. https://doi.org/10.1016/j.ejrad.2012.08.024. [DOI] [PubMed] [Google Scholar]