Abstract
Most pancreatic ductal adenocarcinomas (PDAs) show solid growth pattern, but ductal adenocarcinomas may demonstrate intratumoral cystic appearance or accompany peritumoral non-neoplastic cystic lesions, thus mimicking cystic pancreatic tumors on imaging studies. The histopathologic findings for PDA with cystic feature are divided into neoplastic and non-neoplastic cysts. Neoplastic cystic changes include large-duct type cysts (microcystic appearance), neoplastic mucin cysts (macrocystic appearance), colloid carcinomas (mucinous noncystic adenocarcinomas), and degenerative cystic change usually caused by hemorrhagic necrosis of tumor. Non-neoplastic cystic changes include retention cysts caused by ductal obstruction and pseudocysts caused by tumor-associated pancreatitis. Depending on the presence, size, number, and configuration of cystic changes, PDA should be differentiated from various types of cystic neoplasms. This pictorial essay provides histopathologic classification of PDAs with cystic features along with the corresponding cross-sectional imaging findings, and their differential diagnosis.
Pancreatic ductal adenocarcinoma (PDA) accounts for 90% of all pancreatic neoplasms and is the fourth leading cause of cancer-related death in the western world (1). PDA typically presents as an infiltrative hypovascular solid mass, frequently associated with pancreatic and/or common bile duct obstruction and local vascular invasion (2). However, PDAs may demonstrate intratumoral cystic features or accompany peritumoral non-neoplastic cystic lesions (2–5). Depending on histopathologic types of PDAs with cystic features, PDAs may mimic various types of cystic neoplasms on imaging. Therefore, radiologists should be aware of pathologic background and corresponding imaging findings of PDAs with cystic features. In this review, imaging findings and differential diagnosis of each pathologic type of PDA with cystic features are discussed.
Classification of PDAs with cystic features based on histopathologic types
There are several different histopathologic types of the cystic changes associated with PDAs (2–5). Cystic features of PDAs are largely divided into neoplastic cysts and non-neoplastic cysts. Neoplastic cystic changes include large-duct type cysts, neoplastic mucin cysts, and colloid carcinomas formed by neoplastic glands themselves and degenerative cystic changes usually caused by hemorrhagic necrosis of tumor. Non-neoplastic cystic changes include retention cysts caused by ductal obstruction and pseudocysts caused by tumor-associated pancreatitis.
Neoplastic cysts
PDAs with large-duct type cysts
Pathologically, conventional PDAs are composed of small, irregular shaped ductal elements infiltrating the desmoplastic stroma (6). Large-duct type is one of the morphologic variations of PDAs, which is characterized by more dilated malignant ducts forming a microcystic feature (3). The pathologic criteria are as follows: more than 50% of tumor sections available for examination contain infiltrative ducts with a diameter >5 mm or have macroscopically identifiable microcystic pattern (7). With these criteria, large-duct type ductal adenocarcinomas account for 7% of ductal adenocarcinomas in a review of 230 pancreatectomy specimens (7). Large-duct type PDAs manifest as a predominantly solid tumor with several small intratumoral cysts on imaging (Figs. 1, 2). The size of cysts is small, usually 0.5–0.7 cm in diameter, occasionally exceeding 1 cm (3, 4). Although rare, multiple small intratumoral cysts of large-duct type can show honeycomb-like appearance on cross-sectional images (Fig. 2) (8). PDA with cystic structures showing honeycomb-appearance may be mistaken with serous cystadenoma (Fig. 3) or branch duct type intraductal papillary mucinous neoplasm (IPMN) (2).
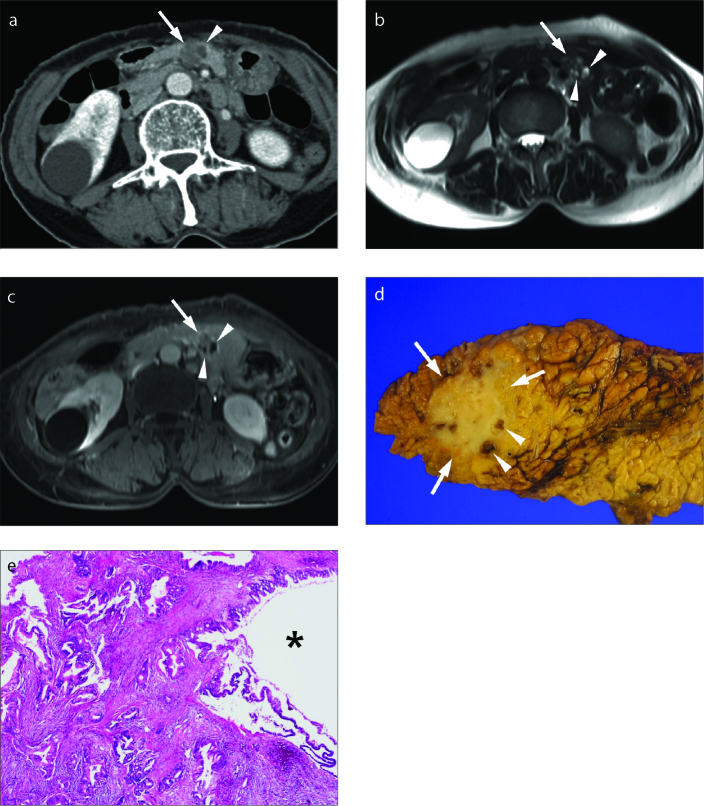
Figure 1. a–e.
Pancreatic ductal adenocarcinoma (PDA) with large-duct type cyst in a 67-year-old female. Axial contrast-enhanced CT scan (a), axial T2-weighted (b) and axial contrast-enhanced T1-weighted (c) images show a 2 cm poorly enhancing mass (arrow) in the pancreas body with several small well-defined intratumoral cystic components (arrowheads) within the tumor. The tumor mimics serous cystadenoma or branch-duct type intraductal papillary mucinous tumor. Photograph of the gross specimen (d) reveals an ill-marginated solid tumor (arrows) with multiple small intratumoral cysts (arrowheads). Photograph of the microscopic examination (hematoxylin-eosin [H-E] stain, original magnification ×40) (e) demonstrates a cystic structure (asterisk) composed of irregular large-duct type glands embedded in desmoplastic stroma.
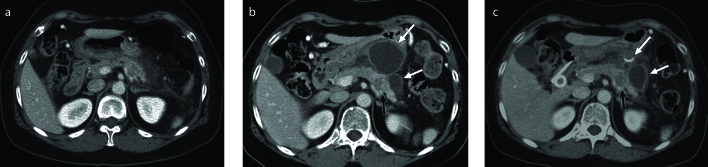
Figure 2. a–d.
PDA with large-duct type cyst in a 72-year-old female. Axial contrast-enhanced CT scan (a), axial fat-suppressed T2-weighted (b) and axial fat-suppressed contrast-enhanced T1-weighted (c) images show a 2.8 cm heterogeneously enhancing solid mass (white arrow) containing several small cystic components (white arrowheads) in the pancreas head. Photograph of the microscopic examination (d) shows cystic lesion (asterisk) lined by dilated mucin-producing tumor cells (black arrows) within the PDA.
Figure 3. a, b.
Serous cystadenoma in a 55-year-old female. Axial contrast-enhanced CT scan (a) shows a 2 cm lobulated low attenuated mass (arrows) in the pancreas tail. Axial fat-suppressed T2-weighted image (b) shows a multiseptated high signal intensity cystic mass with honeycomb-like appearance (arrows).
PDAs with neoplastic mucin cysts
Yoon et al. (4) proposed neoplastic mucin cyst as a new classification of intratumoral cystic lesion of PDA. Both neoplastic mucin cysts and large-duct cysts are lined by mucin-producing neoplastic cells. The difference between neoplastic mucin cysts and large-duct type cysts is the size of cyst and the amount of mucin secretion (5). The size of large-duct type cysts is small, usually <1 cm, but the size of neoplastic mucin cysts can be large, up to 7 cm. Neoplastic mucin cysts in PDAs (Fig. 4) have smooth margin and they are usually solitary, but occasionally a few cysts are seen (4).
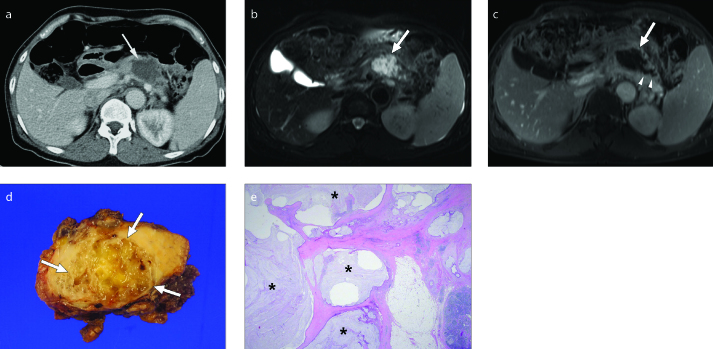
Figure 4. a–d.
PDA with neoplastic mucin cyst in a 62-year-old female. Axial fat-suppressed T2-weighted image (a) shows a 1.5 cm heterogeneous hyperintense mass (arrow) in the pancreas body. The upstream pancreatic duct is mildly dilated. Axial contrast-enhanced fat-suppressed T1-weighted image (b) shows a predominantly cystic mass (arrow) with eccentric enhancing solid portion (black arrows) along the right lateral wall of the mass. Photograph of the gross specimen (c) reveals an ill-marginated solid tumor (arrows) with several small intratumoral cysts (arrowheads) in the pancreas body. Approximately 50% of the tumor is composed of cysts. Photomicrograph of a histologic specimen (H-E stain, x12.5) (d) demonstrates cystic lesions (asterisk) lined by mucin-secreting malignant cells within the PDA.
PDA with neoplastic mucin cyst should be distinguished from IPMN with an associated invasive carcinoma. When a solid mass in the pancreatic parenchyma surrounding the dilated ducts appears, IPMN progressing to invasive carcinoma should be considered (9). PDA with neoplastic mucin cyst differs from invasive carcinoma of IPMN (Fig. 5), because neoplastic mucin cyst does not appear around a solid mass but within a solid mass (4).
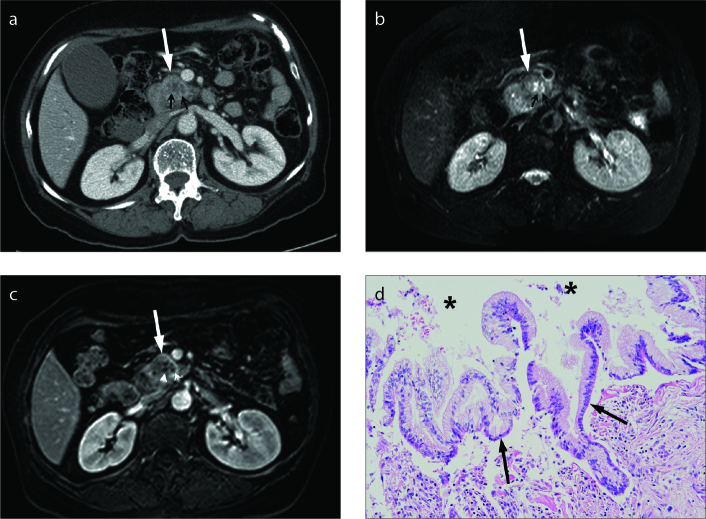
Figure 5. a–c.
Invasive carcinoma arising in intraductal papillary mucinous neoplasm in a 75-year-old female. Axial fat-suppressed T2-weighted image (a) shows a 2 cm hypointense solid tumor (large arrow) surrounding the clustered cystic lesions (arrowhead) in the uncinate process of the pancreas. Common bile duct and main pancreatic duct (small arrows) are also dilated. Solid pancreatic mass (arrow) shows delayed enhancement on axial contrast-enhanced fat-suppressed T1-weighted image (b). Coronal maximum-intensity projection image from 3D magnetic resonance cholangiopancreatography (c) shows dilated branch duct (arrowhead) and the main pancreatic duct with abrupt cutoff of distal common bile duct (arrow). Pylorus preserving pancreaticoduodenectomy confirmed invasive carcinoma arising in intraductal papillary mucinous neoplasm.
Colloid carcinomas
According to the World Health Organization classification of pancreatic tumors, colloid carcinoma, also known as mucinous noncystic adenocarcinoma is a histologic variant of PDA, accounting for 1%–3% (5, 10). Colloid carcinoma is not a true cystic tumor, but this tumor shows predominantly cystic appearance on imaging due to abundant mucin. Pathologically, colloid carcinoma is defined as an infiltrating ductal epithelial neoplasm of pancreas characterized by the presence, in at least 50% of the neoplasm, of abundant extracellular stromal mucin pools and a scant amount of neoplastic cells floating in the center (5, 10). As the mucin pool acts as a physical barrier, colloid carcinoma has better prognosis than ordinary PDA (10).
Colloid carcinomas of the pancreas show poorly enhancing low attenuated mass on contrast-enhanced computed tomography (CT) and very high signal intensity on T2-weighted magnetic resonance imaging (MRI) due to abundant extracellular mucinous components (Fig. 6). On dynamic contrast-enhanced imaging, the colloid carcinomas show central poorly enhancing mucin pools with gradual peripheral and internal mesh-like enhancement of the intervening stroma (10).
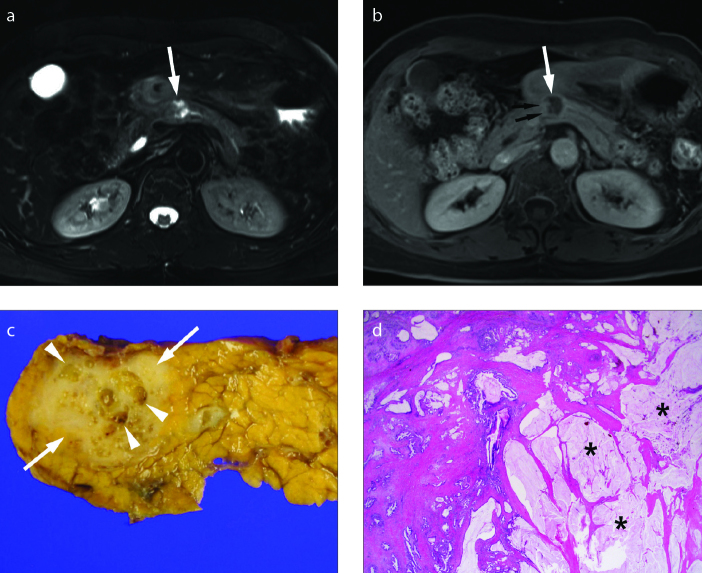
Figure 6. a–e.
Colloid carcinoma in a 62-year-old female. Axial contrast-enhanced CT scan (a) shows a 4 cm lobulated hypodense cystic lesion (arrow) in the proximal tail of the pancreas. Axial T2-weighted image (b) shows a lobulated very high signal intensity mass (arrow), mimicking cystic tumor. Axial contrast-enhanced delayed phase T1-weighted image (c) shows poor enhancement of the mass (arrow). The main pancreatic duct is mildly dilated (arrowheads). Surgical specimen (d) reveals a solid, firm and gelatinous mass characteristic of colloid carcinoma. Photomicrograph of a histologic specimen (H-E stain, x12.5) (e) shows well-defined pools of mucin (asterisk) embedded in the stroma of the gland with floating malignant epithelial cells.
Colloid carcinoma can be confused with mucinous cystic neoplasm (MCN) or IPMN with invasive carcinomas due to its predominantly cystic imaging features. MCN with an associated invasive carcinoma manifests as a large unilocular or septated cystic lesion with intracystic enhancing soft tissue (Figs. 7, 8). In contrast, colloid carcinomas show lobulated contours, indistinct margin and gradual internal enhancement. Intraductal papillary component, dilatation of the downstream pancreatic duct, or spillage of mucin from the ampulla of Vater at endoscopic retrograde cholangiopancreatography are the features of IPMNs, but not of colloid carcinomas (11).
Figure 7. a, b.
Mucinous cystic neoplasm in a 62-year-old female. Axial contrast-enhanced CT scan (a) shows a 4 cm well-defined lobulated cystic lesion (arrow) without enhancing solid portion in the pancreatic body. Axial T2-weighted image (b) shows septated bright signal intensity cystic lesion with a locule of fluid-fluid level. Distal pancreatectomy confirmed mucinous cystic neoplasm.
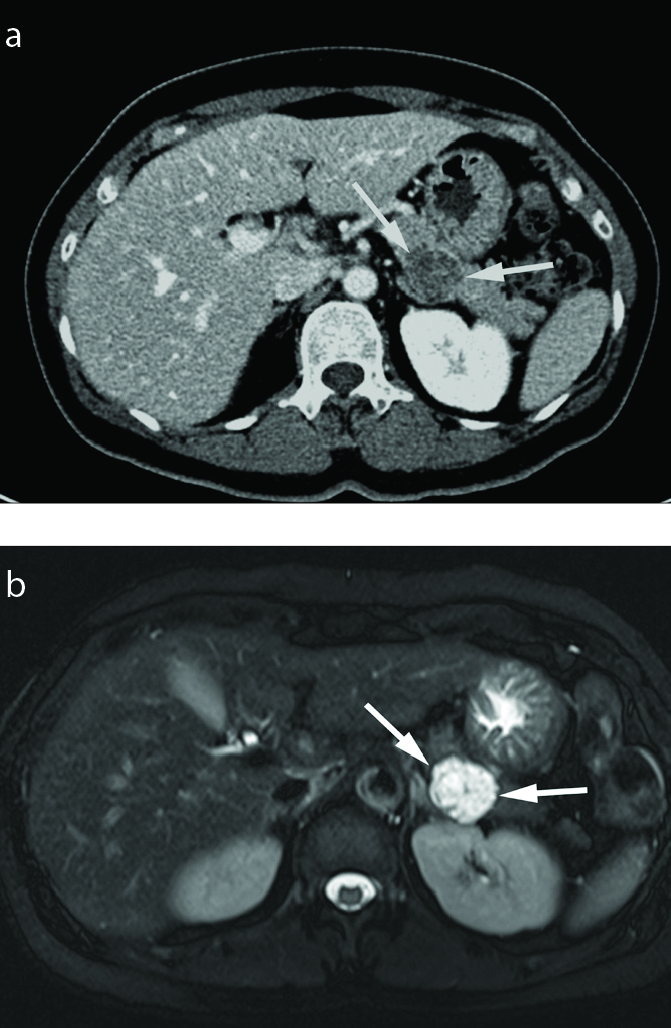
Figure 8.
Mucinous cystic neoplasm with borderline malignant potential in an 85-year-old female. Axial contrast-enhanced CT scan shows an 11 cm septated cystic lesion (arrows) in the pancreas tail. This cystic lesion also contains an enhancing solid portion of 1 cm (arrowhead). Distal pancreatectomy confirmed mucinous cystic neoplasm with borderline malignant potential.
PDAs with degenerative cystic changes
Pathologically, degenerative cystic change is formed by tumor necrosis containing necrotic and hemorrhagic tissue in the cavity. Degenerative cystic cavities in PDAs are usually characterized by a large solitary intratumoral cystic lesion with irregular margin and central location of the tumor (Fig. 9) (3, 4). The incidence of degenerative cystic change is increased in larger tumors, and most tumors tend to have a high proliferation rate and be classified as moderately or poorly differentiated adenocarcinomas (3, 12).
Figure 9. a–c.
PDA with degenerative necrosis in a 74-year-old female. Axial T2-weighted image (a) shows about 3 cm mass (arrows) with central high signal intensity area (arrowhead) in the pancreas head. Axial contrast-enhanced T1-weighted image (b) demonstrates central nonenhancing necrotic area (arrowhead) and poorly enhancing solid portion with irregular outer margin (arrows). Photomicrograph of a histologic specimen (H-E stain, x12.5) (c) shows extensive necrosis of PDA.
PDAs with degenerative cystic change should be differentiated from other pancreatic tumors with intratumoral cystic degeneration such as solid-pseudopapillary neoplasm and cystic neuroendocrine tumor (4). Solid pseudopapillary neoplasm is usually seen as a large well-defined predominantly solid mass with cystic components of variable size in young women. Well-circumscribed tumor margin and homogeneously enhancing soft-tissue components are clues to differentiate solid pseudopapillary neoplasms from PDA. Pancreatic neuroendocrine tumor (Fig. 10) is typically seen as a well-circumscribed hypervascular solid tumor, with cystic components reported in 17% (11). The solid component of neuroendocrine tumor shows intense arterial enhancement, which helps to differentiate neuroendocrine tumor from PDA.
Figure 10.
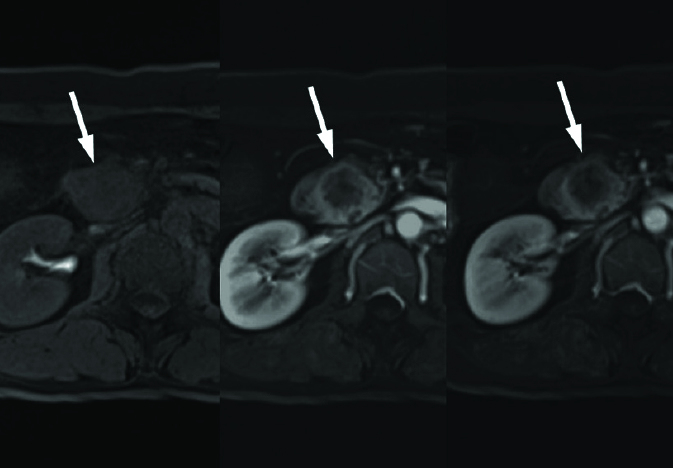
Pancreatic neuroendocrine tumor in a 53-year-old female. Axial dynamic contrast-enhanced T1-weighted images show an approximately 3 cm predominantly solid mass with central poorly enhancing necrotic area in the pancreas head. The peripheral solid portion of the mass shows strong contrast enhancement in the arterial phase (middle).
Non-neoplastic cysts
PDAs with retention cysts
Obstruction of pancreatic duct by tumor may result in the formation of retention cysts in PDAs (8). Due to its mechanism of formation, retention cyst is located at the tumor periphery, not inside the tumor. Pathologically, retention cysts are lined by normal flat ductal epithelium without atypia (3). On CT or MRI, retention cysts have smooth margins and are located outside of the tumor in the pancreas (Figs. 11, 12). The cysts are usually unilocular and small (0.5–1.5 cm), but can be large, up to 10 cm. When a cyst suspected to be a retention cyst is observed, it is important to ensure carefully whether obstruction of the pancreatic duct is caused by a stone, stricture, or duct-obstructing tumor (13). Careful evaluation of the peripheral portion of the cyst may reveal poorly enhancing ductal adenocarcinoma near the retention cyst (Figs. 11, 12).
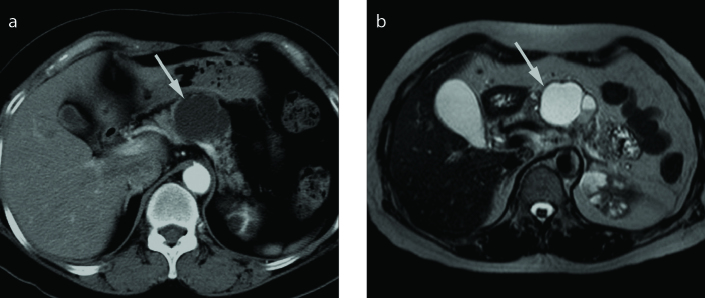
Figure 11. a–c.
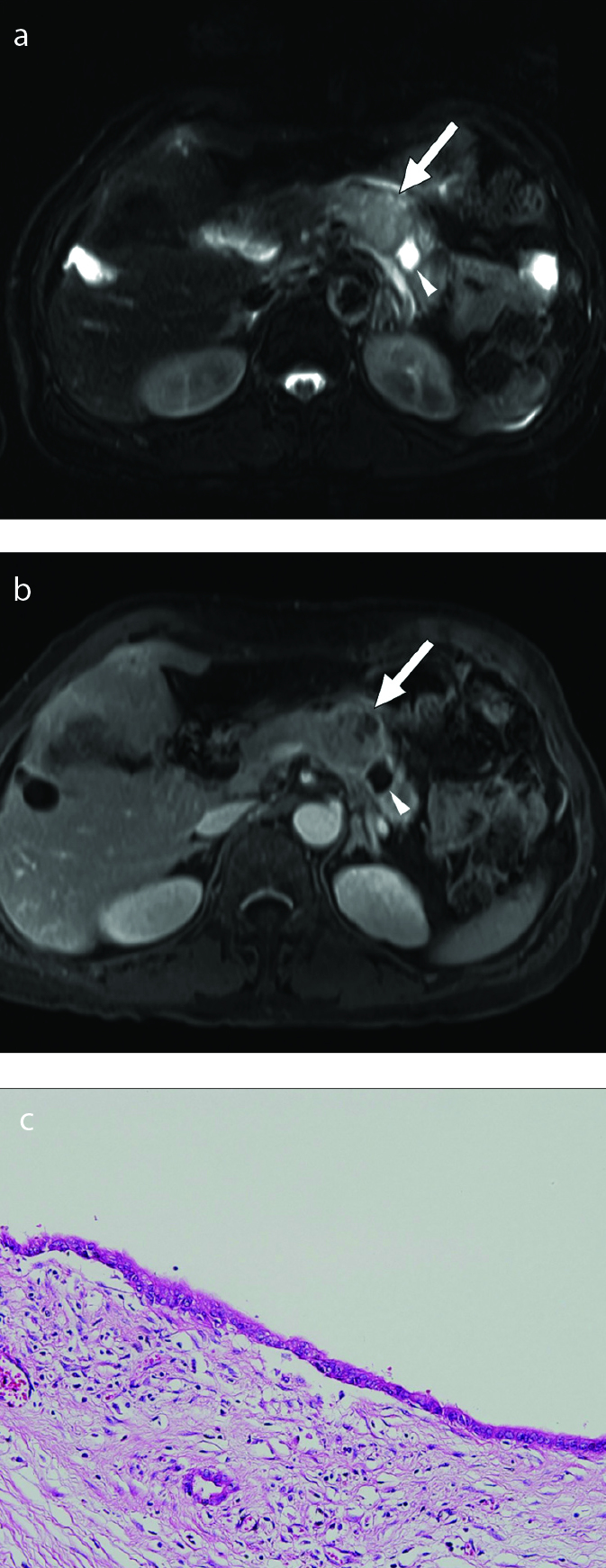
PDA with retention cyst in a 67-year-old female. Axial T2-weighted (a) and contrast-enhanced T1-weighted (b) images show a small well-defined peripheral cyst in pancreas tail (arrowhead) and a lobulated poorly enhancing solid mass (arrow) adjacent to a cyst. Photograph of the microscopic examination (H-E stain, ×200) (c) reveals a retention cyst lined by normal ductal epithelium.
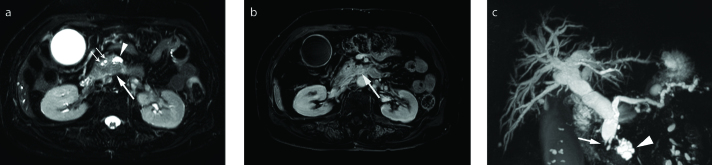
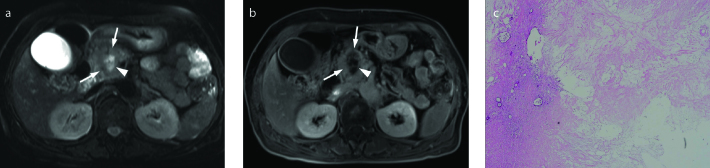
Figure 12. a–c.
PDA with retention cyst in a 47-year-old male. Axial T2-weighted (a) and axial contrast-enhanced T1-weighted (b) images show a large oligolocular cystic lesion (asterisk) in the tail of pancreas. The preoperative differential diagnosis included mucinous cystic neoplasm or pseudocyst. (c) Photograph of the gross specimen (left) reveals a large retention cyst (asterisk) and a 2 cm solid ductal adenocarcinoma (arrow) adjacent to the cyst. Correlating with the pathologic specimen, a poorly enhancing low signal intensity mass (arrows) abutting on retention cyst (asterisk) is outlined retrospectively on contrast-enhanced T1-weighted image (right, magnified image of (b)).
PDAs with attached pseudocysts
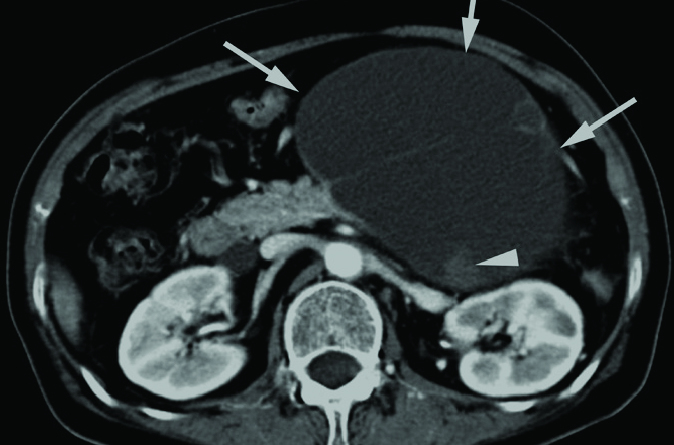
Idiopathic pancreatitis can be an initial presentation of pancreatic adenocarcinoma (14, 15). Tumor-related pancreatitis can occur by ductal obstruction due to PDA, which may result in pseudocyst formation. Histopathologically, pseudocyst is lined by necrotic tissue with hemorrhagic material and turbid fluid, which is surrounded by inflammatory tissue. Pseudocysts are usually unilocular and always located outside of the tumor or even outside of the pancreas on CT and MRI (Fig. 13). Pseudocysts are often irregularly marginated in the early stage of their formation, but gradually become well-defined with a thick enhancing wall formed by granulation tissue and a fibrous capsule over time. Internal blood products and necrotic debris are commonly noted as high signal intensity on T1-weighted images (11).
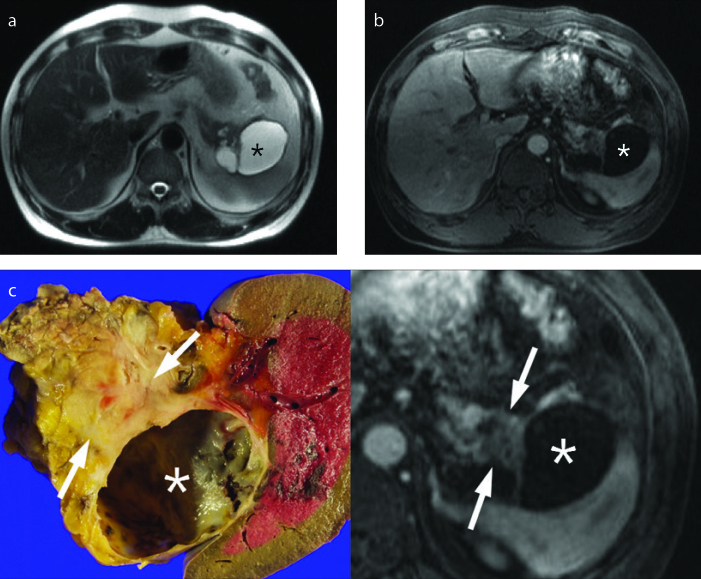
Figure 13. a–c.
PDA presenting with acute pancreatitis and pseudocyst in a 72-year-old female. Initial axial contrast-enhanced CT (a) shows enlarged pancreas with peripancreatic fluid collection, suggestive of acute pancreatitis. Axial contrast-enhanced CT scan (b) obtained 2 weeks after diagnosis of acute pancreatitis shows two large cystic lesions (white arrows) in peripancreatic area and a suspicious poorly enhancing mass-like lesion (black arrow) in the tail of pancreas. On follow-up contrast-enhanced CT scan (c) obtained 4 weeks after initial CT scan (a), the size of pseudocyst (white arrow) is decreased after insertion of drainage catheter, but a low attenuated mass in the pancreas tail becomes more prominent. Distal pancreatectomy confirmed PDA and attached pseudocyst.
When acute pancreatitis occurs concurrently with PDA, overlapping clinical findings and confusing imaging findings often delay correct diagnosis and treatment. If there are no predisposing factors for acute pancreatitis in old-aged patients, hidden PDA should be considered in the differential diagnosis of pseudocyst.
Summary of imaging features
Depending on the histopathologic types of cystic features, PDAs demonstrate differences in number, size, location and margin of cysts on cross-sectional images (Fig. 14). Large-duct type cysts usually represent as several small cysts, while neoplastic mucin cysts, colloid carcinomas, and degenerative necrotic cysts usually present as a single cyst. Large-duct type cysts and neoplastic mucin cysts are characterized by well-defined margins. Colloid carcinomas and degenerative necrotic cysts are characterized by ill-defined margins. Retention cysts and pseudocysts are located outside the tumor and can be of variable sizes. Pseudocysts may even be located outside the pancreas.
Figure 14.
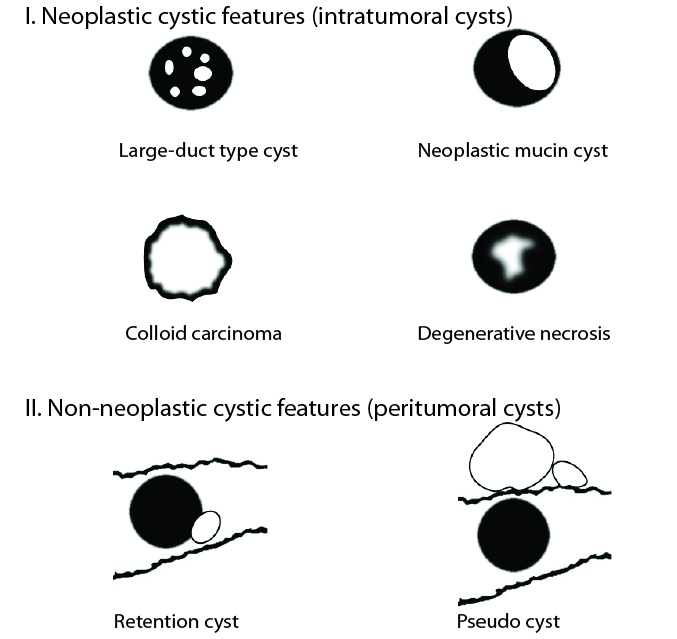
Schematic drawings of PDAs with six cystic features based on different histopathologic types. The gray portion represents solid component of the PDA and the white portion represents cystic components of the tumor in each drawing. In the drawings of the retention cyst and pseudocyst, the wavy outline indicates the edge of the pancreas. Large-duct type cyst shows several small intratumoral cysts. Neoplastic mucin cyst is characterized by a well-defined relatively larger intratumoral cyst than large-duct type cysts. Colloid carcinoma is seen as a predominant cystic mass with gradual and peripheral enhancement due to central poorly enhancing mucin pools. Degenerative cystic cavity is characterized by an irregular marginated intratumoral necrotic lesion. Retention cyst is located outside of the tumor. Pseudocysts are located outside the tumor or even outside the pancreas.
Conclusion
Depending on the histopathologic types of cystic features, PDAs demonstrate different imaging findings. PDAs with large-duct type cyst usually show a predominantly solid tumor with several small intratumoral cysts. PDAs with neoplastic mucin cyst or colloid carcinoma may mimic mucinous cystic neoplasm or IPMN with an associated invasive carcinoma. PDAs with degenerative cystic change should be differentiated from solid pseudopapillary tumor and cystic neuroendocrine tumor. Combined retention cyst or pseudocyst may delay the diagnosis of PDA, because a small poorly enhancing solid tumor can be masked by the large cystic lesion. The radiologists should be aware that PDAs can show cystic features on imaging and PDA must be considered in the differential diagnosis of cystic pancreatic lesions.
Main points.
Pancreatic ductal adenocarcinomas (PDAs) may show intratumoral neoplastic cystic features or accompany peritumoral non-neoplastic cystic lesions, mimicking cystic pancreatic tumors.
Neoplastic cystic changes include large-duct type cysts, neoplastic mucin cysts, colloid carcinomas and degenerative cystic cavities.
Non-neoplastic cystic changes include retention cysts caused by ductal obstruction and pseudocysts caused by tumor-related pancreatitis.
Depending on the histopathologic type of cystic features, PDAs may demonstrate different imaging findings in the number, size, location and margin of cysts on cross-sectional imaging.
Footnotes
Conflict of interest disclosure
The authors declared no conflicts of interest.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. https://doi.org/10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Cho HW, Choi JY, Kim MJ, et al. Pancreatic tumors: emphasis on CT findings and pathologic classification. Korean J Radiol. 2011;12:731–739. doi: 10.3348/kjr.2011.12.6.731. https://doi.org/10.3348/kjr.2011.12.6.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kosmahl M, Pauser U, Anlauf M, Kloppel G. Pancreatic ductal adenocarcinomas with cystic features: neither rare nor uniform. Mod Pathol. 2005;18:1157–1164. doi: 10.1038/modpathol.3800446. https://doi.org/10.1038/modpathol.3800446. [DOI] [PubMed] [Google Scholar]
- 4.Yoon SE, Byun JH, Kim KA, et al. Pancreatic ductal adenocarcinoma with intratumoral cystic lesions on MRI: correlation with histopathological findings. Br J Radiol. 2010;83:318–326. doi: 10.1259/bjr/69770140. https://doi.org/10.1259/bjr/69770140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hruban RH, Boffetta P, Hiraoka N, et al. Ductal adenocarcinoma of the pancreas. In: Bosman FT, editor. World Health Organization Classification of tumours of the digestive system. 4th ed. Lyon, France: International Agency for Research on Cancer; 2010. pp. 281–295. [Google Scholar]
- 6.Chen J, Baithun SI. Morphological study of 391 cases of exocrine pancreatic tumours with special reference to the classification of exocrine pancreatic carcinoma. J Pathology. 1985;146:17–29. doi: 10.1002/path.1711460103. https://doi.org/10.1002/path.1711460103. [DOI] [PubMed] [Google Scholar]
- 7.Bagci P, Andea AA, Basturk O, Jang KT, Erbarut I, Adsay V. Large duct type invasive adenocarcinoma of the pancreas with microcystic and papillary patterns: a potential microscopic mimic of non-invasive ductal neoplasia. Mod Pathol. 2012;25:439–448. doi: 10.1038/modpathol.2011.181. https://doi.org/10.1038/modpathol.2011.181. [DOI] [PubMed] [Google Scholar]
- 8.Hori S, Shimada K, Ino Y, et al. Macroscopic features predict outcome in patients with pancreatic ductal adenocarcinoma. Virchows Arch. 2016;469:621–634. doi: 10.1007/s00428-016-2026-6. https://doi.org/10.1007/s00428-016-2026-6. [DOI] [PubMed] [Google Scholar]
- 9.Yamada Y, Mori H, Matsumoto S, Kamei N, Hongo N. Invasive carcinomas derived from intraductal papillary mucinous neoplasms of the pancreas: a long-term follow-up assessment with CT imaging. J Comput Assist Tomogr. 2006;30:885–890. doi: 10.1097/01.rct.0000220801.76276.0f. https://doi.org/10.1097/01.rct.0000220801.76276.0f. [DOI] [PubMed] [Google Scholar]
- 10.Yoon MA, Lee JM, Kim SH, et al. MRI features of pancreatic colloid carcinoma. AJR Am J Roentgenol. 2009;193:W308–W313. doi: 10.2214/AJR.09.2347. https://doi.org/10.2214/AJR.09.2347. [DOI] [PubMed] [Google Scholar]
- 11.Kalb B, Sarmiento JM, Kooby DA, Adsay NV, Martin DR. MR imaging of cystic lesions of the pancreas. Radiographics. 2009;29:1749–1765. doi: 10.1148/rg.296095506. https://doi.org/10.1148/rg.296095506. [DOI] [PubMed] [Google Scholar]
- 12.Adsay NV, Klimstra DS. Cystic forms of typically solid pancreatic tumors. Semin Diagn Pathol. 2000;17:81–88. [PubMed] [Google Scholar]
- 13.Basturk O, Coban I, Adsay NV. Pancreatic cysts: pathologic classification, differential diagnosis, and clinical implications. Arch Pathol Lab Med. 2009;133:423–438. doi: 10.5858/133.3.423. [DOI] [PubMed] [Google Scholar]
- 14.Dzeletovic I, Harrison ME, Crowell MD, et al. Pancreatitis before pancreatic cancer: clinical features and influence on outcome. J Clin Gastroenterol. 2014;48:801–805. doi: 10.1097/MCG.0b013e3182a9f879. https://doi.org/10.1097/MCG.0b013e3182a9f879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kimura W, Sata N, Nakayama H, et al. Pancreatic carcinoma accompanied by pseudocyst: report of two cases. J Gastroenterol. 1994;29:786–791. doi: 10.1007/BF02349289. https://doi.org/10.1007/BF02349289. [DOI] [PubMed] [Google Scholar]