Summary
The current Iraq and Afghanistan conflicts have seen the highest survival rates in US service members ever, despite staggering numbers of traumatic brain injury and limb loss cases. The improvement in survival can be attributed at least in part to advances in far-forward, rapid medical treatment, including the administration of hypertonic saline solutions and decompressive craniectomies to manage elevated intracranial pressure. After evacuation to military hospitals in the continental United States, service members who have had limb loss face extensive rehabilitation. The growing amputee population has led to a burgeoning interest in the treatment of phantom limb pain and in the development of advanced prostheses.
Operations Iraqi Freedom and Enduring Freedom have seen the highest wartime percentage of service members who survive battle injuries. The enemy's weapon of choice from these conflicts is the improvised explosive device. The types of injuries currently seen were largely fatal in previous conflicts, but the effects of protective gear and the rapid nature of battlefield medical treatment and evacuation has resulted in a survival rate of nearly 95% for those who live long enough to receive medical treatment.1 The medical advancements achieved during efforts to save individuals are substantial, particularly to the treatment of traumatic brain injury (TBI) and to the field of neurorehabilitation. Here we discuss 5 new advances that concern mild TBI diagnosis, severe TBI treatment, and postamputation rehabilitation.
DoD guidelines: A new approach to the identification and management of concussion/mild TBI
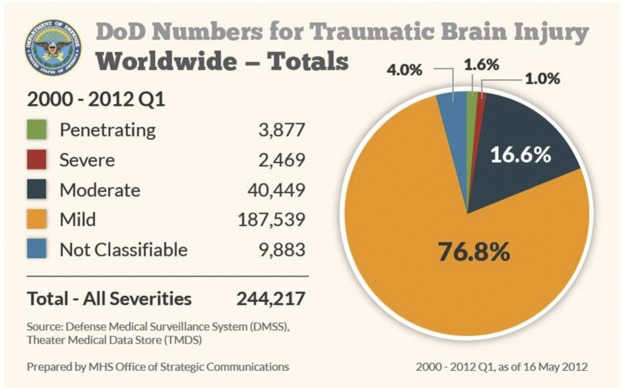
From 2000 to the first quarter of 2012, a total of 244,217 cases of TBI were documented, with 187,539 (76.9%) being mild and with approximately 16% of all head injuries identified as due to combat. Mild TBI (mTBI) is characterized by a “confused or disoriented state which lasts less than 24 hours; loss of consciousness for up to 30 minutes; memory loss lasting less than 24 hours; and structural brain imaging (MRI or CT scan) yielding normal results.” Prior to 2010, the Department of Defense (DoD) adhered to a symptom-based protocol of concussion identification and management, which required service members in the war zone to seek medical attention if and when they experienced symptoms such as dizziness, trouble thinking, or headaches following a potentially concussive event. However, concussion, also known as mTBI, may not have externally apparent signs, and some symptoms overlap with other conditions. Furthermore, given the possibility that cognitive impairment due to concussion might not be recognized in a self-reporting medical system, there was a suspicion that a large proportion of injured service members never sought medical care.2 In order to better identify service members who may have sustained a concussion/mTBI, the DoD issued new guidelines in July 2010 that shifted concussion care to a mandatory event-based protocol as opposed to symptom-based. These new guidelines have fundamentally changed the approach to concussion care. Under the new guidelines, all service members who are involved in 4 kinds of events must be screened for concussion, even if they do not appear to have visible injuries. The 4 mandatory events are as follows: being in a vehicle accident (e.g., blast, collision, or rollover), being within 50 meters of an explosive blast (indoor, in vehicle, or outdoor), sustaining a direct blow to the head, or being directed to be evaluated by a commander. Anyone involved in 1 or more of these 4 events is screened for concussion using the Military Acute Concussion Evaluation (MACE), consisting of a set of history questions, a screening neurologic examination, and a 10-minute cognitive test which measures orientation, immediate memory, concentration, and memory recall. The MACE is modeled after the Standard Assessment of Concussion used by the National Football League. In addition, all service members who are evaluated are given a mandatory 24-hour rest period, even if they are diagnosed as not having a concussion. This would be equivalent to sidelining a football player for the rest of the game after a suspected concussion.3 A service member who is diagnosed with concussion is allowed to fully recover before returning to duty. For a service member who has sustained a second concussion within a 12-month period, the minimal 24-hour rest period is extended to 7 days, and a third concussion requires an evaluation by a neurologist prior to return to full duty. For recalcitrant patients, care by a neurologist is available at the base hospitals in Kandahar and Bagram in Afghanistan. The purpose of these stringent guidelines is to give each service member the chance to fully recover before returning to duty in order to avoid the risk of reinjury. Of note, from January through December 2011, >8,700 events were recorded from Afghanistan and Iraq, which resulted in 16.5% medically documented concussions. The military has also established concussion care centers in Afghanistan, with a 99.7% return to duty rate (equaling >1,000 personnel from August 2010 through July 2012) at one Marine Corps center. Most recently, a blast gauge has been issued to some troops (figure 1). This is a very small device that is attached to each warfighter. It measures the blast pressure and acceleration. If these are at a low level, the gauge's yellow indicator lights up yellow. If high, a red indicator illuminates. When there is no exposure, it is green. A yellow or red light indicates that this individual needs to be screened for injury.4

Blast gauge
Figure 1. A gauge attached to the back of a soldier's helmet. A gauge will also be placed on the breast and shoulder of the soldier's body armor.
Management of increased intracranial pressure
Events that cause mild brain injury can also cause moderate to severe TBI that requires more extensive medical care. For severe TBI, the diagnostic criteria include a Glasgow Coma Scale (GCS) score of <8, altered or loss of consciousness for more than 24 hours, or posttraumatic amnesia for longer than 1 week. Since 2000, approximately 1% of all TBI cases in the military has been classified as severe (figure 2).5 Severe TBI comes with a great concern for secondary brain injury, which can be due to hypotension, hypoxia, fever, hyperglycemia, hypercapnia, seizures, trauma-related vasospasm, and increased intracranial pressure (ICP). Elevation of ICP, in particular, is one of the most serious threats following severe TBI because of its correlation with increased mortality.6 Relief and control of ICP, therefore, represent the cornerstone management of TBI patients. Here we review 2 measures—hypertonic saline solutions and decompressive craniectomies—as a means of ICP reduction in the military population.
Incidences of traumatic brain injury in the US military since 2000
Figure 2. Numbers represent the medical diagnoses of traumatic brain injury that occurred in the US military, including those in the continental United States. Injuries were classified by severity and represent all 4 branches of the armed forces.
Hypertonic saline solution
In the combat setting, the ideal resuscitation fluid is nontoxic, easy to transport and administer on the field of battle, can be effectively administered in small volumes, and has minimal systemic side effects. Since 2000, the preferred fluid in the combat casualty resuscitation setting has been Hextend. Its long presence in the vascular system after administration prevents the need for additional fluid administrations in the case of delayed evacuation. However, Hextend requires substantial fluid volumes to maintain the desired blood pressure, which presents a tactical burden for combat medics who must carry large volumes of fluid. One bag (500 mL) of Hextend is currently administered as part of standard protocol for initial resuscitation, with no more than 1,000 mL given per casualty. More recent laboratory and clinical evidence has shown the successful use of hypertonic saline (HTS) solution7 as opposed to Hextend to maximize the volume-to-benefit ratio. Compared to 500–1,000 mL of Hextend, 250 mL of 7.5% HTS is likely to have the same physiologic effect of reducing ICP and improving cerebrovascular function.8 Bolus dosing has also shown some success in reducing the acute elevation of ICP. A retrospective cohort study has demonstrated that administration of 30 mL of 23.4% saline results in reduction in pupil size, return of pupil reactivity to light exposure, and an increase of 2 or more on the GCS score within an hour of administration.9 HTS has the additional benefits of improving brain oxygenation and microcirculation and restoring resting membrane potential and cell volume to injured neurons.10 Most importantly, HTS has the unique benefit of increasing serum osmolality without any accompanying volume contraction. This is particularly important in service members who may have hemorrhagic shock. The small dose administration of HTS allows military medics to carry smaller, lighter packages that reduce the tactical burden of Hextend bags and have the potential to improve functional outcome. Individual medics could effectively quadruple their resuscitation capacity without increasing the volume or weight carried.
Decompressive craniectomies
Many patients with severe TBI have raised ICP that is refractory to other treatments. In such cases, surgical decompressive craniectomy (DC) is performed with increasing frequency to control ICP.
In the treatment of severe TBI in the civilian population, recent reports offer conflicting views on the management of ICP by DC. Some suggest that DC offers no beneficial effect on patient outcome or results in unfavorable outcomes as compared to standard care.11 Others demonstrate ICP, functional and long-term outcomes, and mortality may all be improved by DC. But all see it as a salvage procedure performed after all other options of ICP management have been exhausted. No clear consensus yet exists in civilian medicine as to the timing, necessity, and usefulness of DC in severe TBI cases.
The military, however, has adopted an aggressive approach, advocating the use of rapid (within a few hours), far-forward decompressive craniectomies following the most severe penetrating or closed head injuries. Depressive craniectomies allow for optimal management of ICP prior to transfer. This is ideal for combat injuries as service members with severe TBI must be evacuated via a long medical flight, often without neurosurgical expertise on board, to a tertiary military hospital that may be 8–18 hours away. A 2008 study examining service members with penetrating head injury transferred from Iraq and Afghanistan back to the United States showed that early DC (within the first 2–4 hours from injury) was successful in mitigating the effects of brain edema and increased ICP during the long air evacuation transfer to the continental United States. By 1 year follow-up, 83% of patients who received early DC had a Glasgow Outcome Scale score of greater than 3.12 Another study on blast-related neurotrauma in the Iraq war further showed that although patients who had a hemicraniectomy were neurologically worse initially, they improved to a status that was statistically indistinguishable from the non-craniectomy group.13
Phantom limb pain and cortical reorganization
Research indicates that up to 85% of service members who require amputation as a result of battlefield trauma will experience phantom limb pain14: the perception of active pain in a missing limb. Despite the abundance of theories proposed for the phenomenon, ranging from purely physiologic (neuromas) to purely psychological (denial), the mechanisms behind phantom limb pain remain unclear.
Two increasingly popular theories of phantom limb pain are cortical reorganization and proprioceptive memory. The cortical reorganization theory posits that maladaptive plasticity after amputation creates the perception of pain in the missing limb. This theory arises from research showing that the magnitude of cortical reorganization of the primary somatosensory and motor cortices has a strong positive correlation with phantom limb pain.15 Further evidence for this theory comes from studies showing that when a treatment reduces phantom limb pain, there is a corresponding reduction in cortical reorganization.15 The proprioceptive memory theory posits that memories of limb position become embedded in the subconscious over the course of one's life. With amputation, a patient loses a limb but not the memories of this limb, leading to the perception that the limb remains.16
While as of yet there is no study indicating that cortical reorganization is not simply a correlate but rather the cause of phantom limb pain, researchers have suggested that treatments be directed at the brain rather than the peripheral nervous system. One treatment is mirror therapy. Mirror therapy creates the illusion of 2 intact limbs through strategic placement of a mirror between an intact and a residual limb (figure 3). The patient looks at the reflection of the intact limb while performing synchronized movements with the phantom and intact limb, providing the patient with visual feedback that the amputated limb is healthy and able to perform motor movements.17 Recent evidence showing the efficacy of mirror therapy18 (figure 4), as well as the relatively small risk associated with this treatment, make it one of the promising therapies to date for this perplexing phenomenon.
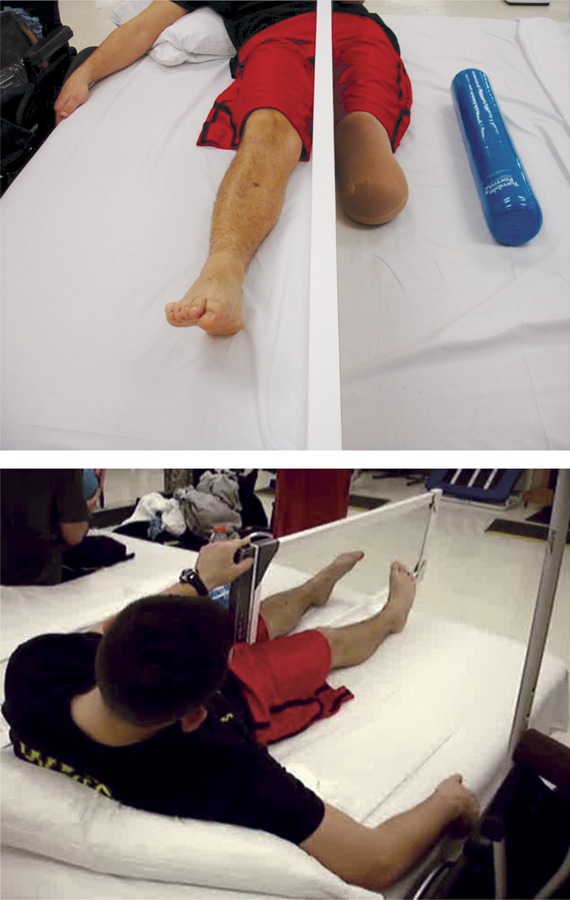
Mirror therapy in a patient with unilateral lower limb amputation
Figure 3. The mirror is placed between the intact and amputated limb to give the illusion of 2 intact limbs.
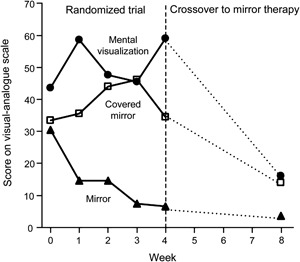
Changes in phantom limb pain, as assessed by a study on mirror therapy
Figure 4. Phantom limb pain was measured on a 100-mm visual analogue scale, with a higher score indicating a greater severity of pain. The dotted lines represent the weeks during which patients in all 3 groups used mirror therapy, and data points show medians. From Chan BL, Witt R, Charrow AP, et al. Mirror therapy for phantom limb pain. N Engl J Med 2007;357:2206–2007. Copyright © 2007 Massachusetts Medical Society. Reprinted with permission.
Advanced upper extremity prosthetics
In 2009, the Defense Advanced Research Projects Agency (DARPA) Revolutionizing Prosthetics program funded a 4-year effort to develop a Modular Prosthetic Limb (MPL) system which would improve the state of upper extremity prosthetics (originally a 3-prong hook and a myoelectric with a thumb which could open and close against 4 fixed fingers). The prosthetic arm must fulfill a number of specifications: the final design must have 22° of motion (including independent control of each finger), weigh approximately the same as a biological arm (9 pounds), and be contained within a cosmetic casing (figure 5). However, most importantly, the arm must use a brain-controlled interface that gives the limb both sensation and intuitive motor control.19
The modular prosthetic limb
Figure 5. The modular prosthetic limb has the same number of degrees of freedom as the human arm and is currently being tested on a small number of amputees at Walter Reed National Military Medical Center in conjunction with the Johns Hopkins Applied Physics Laboratory.
Currently myoelectric prostheses offer some intuitive control over a prosthetic arm using signals from electrodes placed on the surface of the residual limb. However, the new technology is inherently limited because the electrical signals present on the surface of an arm cannot capture the complexity of natural movements. The DARPA MPL ultimately seeks to offer enhanced control over the prosthetic arm through implantable electrodes to be inserted into the residual muscles or through neural control.19
While neural control over prosthetic limbs is still in the preliminary stages, remarkable progress has already been made in a number of domains. Velliste et al.20 developed a system that enabled monkeys to feed themselves via a neurally controlled prosthetic arm. The primary motor cortices of the monkeys were implanted with intracortical microelectrode arrays, which picked up single- and multi-unit spiking activity configured to manipulate the prosthetic arm. O’Doherty et al.21 developed a brain-machine-brain interface that allowed monkeys to move and feel with a virtual-reality arm, where feeling was tested by the ability to distinguish between virtual objects based on artificial texture. Touching different virtual objects with the virtual arm induced distinct intracortical microstimulation to the primary somatosensory cortex, allowing the monkey to distinguish between the objects. In the human model, Hochberg et al.22 recently developed a system that gave 2 people with tetraplegia the ability to neurally control a robotic arm to perform reach and grasp movements.
The Revolutionizing Prosthetics program recently began working with patients at Walter Reed National Military Medical Center to test the latest limb prototype. The prototype can adapt to multiple levels of amputation, can perform several complex grasps, and has more than 22 degrees of freedom, including independent articulation of the fingers.19
The DoD is committed to improving the medical and restorative care of injured service members. From our personal perspectives, as military medical care providers who have served in Iraq and Afghanistan (G.S.F.L.) and devoted careers to providing and improving care (J.W.T. and G.S.F.L.), we can emphatically state that there is nothing more professionally rewarding that delivering and advancing medical care of our most deserving patients—fellow citizens who have been injured defending the principles that define our great nation and, thus, all of us.
Neurology and the military: Five new things
The DoD has implemented a new concussion care protocol changing from a symptom-based to a mandatory event-based clinical evaluation.
Hypertonic saline solution can be administered in small doses with minimal systemic side effects, making it ideal for in-theater intracranial pressure management.
Decompressive craniectomies are increasingly used to stabilize intracranial pressure following the most severe head injuries and before lengthy evacuation to tertiary military hospitals.
Mirror therapy has shown promise as an inexpensive therapy to alleviate phantom limb pain in amputees.
Advanced prostheses offering neural interfaces may allow amputee service members to regain intuitive motor control and sensation in their limb.
STUDY FUNDING
No targeted funding reported.
DISCLOSURES
J.W. Tsao is Director of Traumatic Brain Injury Programs for the US Navy Bureau of Medicine and Surgery and is responsible for crafting brain injury policy for the US Navy. A. Alphonso, S. Griffin, and I. Yurkiewicz report no disclosures relevant to the manuscript. G. Ling is a traumatic brain injury subject matter expert for the US Department of Defense. Go to Neurology.org/cp for full disclosures.
Correspondence to: jack.tsao@med.navy.mil
Footnotes
Correspondence to: jack.tsao@med.navy.mil
REFERENCES
- 1.Cullison A. On distant battlefields, survival odds rise sharply. 2010. Available at: http://online.wsj.com/article/SB10001424052748704655004575114623837930294.html. Accessed July 18, 2012.
- 2.Tanielian TL, Rand Corporation. Invisible Wounds of War: Summary and Recommendations for Addressing Psychological and Cognitive Injuries. Santa Monica: RAND; 2008.
- 3.McCrea M, Kelly JP, Kluge J, Ackley B, Randolph C. Standardized assessment of concussion in football players. Neurology. 1997;48:586–588. doi: 10.1212/wnl.48.3.586. [DOI] [PubMed] [Google Scholar]
- 4.Zoroya G. Army device will gauge blast hits on soldiers. USA Today [serial online]. July 18, 2011. Available at: http://usatoday30.usatoday.com/news/military/2011-07-18-army-device-measures-brain-injuries_n.htm. Accessed January 11, 2013.
- 5.Center DaVBI. DoD TBI worldwide 2000-2012 Q1. 2012. Available at: http://www.dvbic.org/sites/default/files/uploads/dod-tbi-2000-2012.pdf. Accessed July 16, 2012.
- 6.Saul TG, Ducker TB. Effect of intracranial pressure monitoring and aggressive treatment on mortality in severe head injury. J Neurosurg. 1982;56:498–503. doi: 10.3171/jns.1982.56.4.0498. [DOI] [PubMed] [Google Scholar]
- 7.Ling GS, Marshall SA. Management of traumatic brain injury in the intensive care unit. Neurol Clin. 2008;26:409–426. doi: 10.1016/j.ncl.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 8.Riha GM, Kunio NR, Van PY. Hextend and 7.5% hypertonic saline with Dextran are equivalent to Lactated Ringer's in a swine model of initial resuscitation of uncontrolled hemorrhagic shock. J Trauma. 2011;71:1755–1760. doi: 10.1097/TA.0b013e3182367b1c. [DOI] [PubMed] [Google Scholar]
- 9.Koenig MA, Bryan M, Lewin JL, Mirski MA, Geocadin RG, Stevens RD. Reversal of transtentorial herniation with hypertonic saline. Neurology. 2008;70:1023–1029. doi: 10.1212/01.wnl.0000304042.05557.60. [DOI] [PubMed] [Google Scholar]
- 10.Meyer MJ, Megyesi J, Meythaler J. Acute management of acquired brain injury part II: an evidence-based review of pharmacological interventions. Brain Inj. 2010;24:706–721. doi: 10.3109/02699051003692126. [DOI] [PubMed] [Google Scholar]
- 11.Cooper DJ, Rosenfeld JV, Murray L. Decompressive craniectomy in diffuse traumatic brain injury. N Engl J Med. 2011;364:1493–1502. doi: 10.1056/NEJMoa1102077. [DOI] [PubMed] [Google Scholar]
- 12.Bell RS, Vo AH, Neal CJ. Military traumatic brain and spinal column injury: a 5-year study of the impact blast and other military grade weaponry on the central nervous system. J Trauma. 2009;66:S104–S111. doi: 10.1097/TA.0b013e31819d88c8. [DOI] [PubMed] [Google Scholar]
- 13.Armonda RA, Bell RS, Vo AH. Wartime traumatic cerebral vasospasm: recent review of combat casualties. Neurosurgery. 2006;59:1215–1225. doi: 10.1227/01.NEU.0000249190.46033.94. [DOI] [PubMed] [Google Scholar]
- 14.Sherman RA, Sherman CJ. Prevalence and characteristics of chronic phantom limb pain among American veterans: results of a trial survey. Am J Phys Med. 1983;62:227–238. [PubMed] [Google Scholar]
- 15.MacIver K, Lloyd DM, Kelly S, Roberts N, Nurmikko T. Phantom limb pain, cortical reorganization and the therapeutic effect of mental imagery. Brain. 2008;131:2181–2191. doi: 10.1093/brain/awn124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anderson-Barnes VC, McAuliffe C, Swanberg KM, Tsao JW. Phantom limb pain: a phenomenon of proprioceptive memory? Med Hypotheses. 2009;73:555–558. doi: 10.1016/j.mehy.2009.05.038. [DOI] [PubMed] [Google Scholar]
- 17.Ramachandran VS, Rogers-Ramachandran D. Synaesthesia in phantom limbs induced with mirrors. Proc Biol Sci. 1996;263:377–386. doi: 10.1098/rspb.1996.0058. [DOI] [PubMed] [Google Scholar]
- 18.Chan BL, Witt R, Charrow AP. Mirror therapy for phantom limb pain. N Engl J Med. 2007;357:2206–2207. doi: 10.1056/NEJMc071927. [DOI] [PubMed] [Google Scholar]
- 19.Burck JM, Zeher MJ, Armiger RS, Beaty J. Developing the world's most advanced prosthetic arm using model-based design. 2009. Available at: http://www.mathworks.com/company/newsletters/articles/developing-the-worlds-most-advanced-prosthetic-arm-using-model-based-design.html. Accessed July 24, 2012.
- 20.Velliste M, Perel S, Spalding MC, Whitford AS, Schwartz AB. Cortical control of a prosthetic arm for self-feeding. Nature. 2008;453:1098–1101. doi: 10.1038/nature06996. [DOI] [PubMed] [Google Scholar]
- 21.O'Doherty JE, Lebedev MA, Ifft PJ. Active tactile exploration using a brain-machine-brain interface. Nature. 2011;479:228–231. doi: 10.1038/nature10489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hochberg LR, Bacher D, Jarosiewicz B. Reach and grasp by people with tetraplegia using a neurally controlled robotic arm. Nature. 2012;485:372–375. doi: 10.1038/nature11076. [DOI] [PMC free article] [PubMed] [Google Scholar]