Abstract
We examined the reliability, validity and factor structure of the Eye Contact Avoidance Scale (ECAS), a new 15-item screening tool designed to measure eye contact avoidance in individuals with fragile X syndrome (FXS). Internal consistency of the scale was acceptable to excellent and convergent validity with the Social Responsiveness Scale, 2nd Edition (SRS-2) and the Anxiety Depression and Mood Scale (ADAMS) was good. Boys with a comorbid ASD diagnosis obtained significantly higher scores on the ECAS compared to boys without ASD, when controlling for communication ability. A confirmatory factor analysis indicated that a two-factor model (Avoidance and Aversion) provided an excellent fit to the data. The ECAS appears to be a promising reliable and valid tool that could be employed as an outcome measure in future pharmacological/behavioral treatment trials for FXS.
Keywords: fragile X syndrome, screening tool, eye gaze, factor analysis, psychometrics
Fragile X syndrome (FXS) is the most common known inherited cause of intellectual disability affecting approximately 1 in 3,000 individuals in the general population (Hagerman, 2008). The syndrome is caused by an expansion of a CGG trinucleotide repeat in the promoter region of the FMR1 gene at location q27.3 on the X chromosome (Verkerk et al., 1991) resulting in reduced expression of FMRP, the protein product of the gene. As a consequence, affected individuals display a variety of cognitive and behavioral features including deficits in sensory processing and impairments in social-communication skills (Reiss & Dant, 2003). Deficits in social behaviors occur in children with FXS from an early age, particularly in males, including eye gaze avoidance, social avoidance, and social withdrawal during social interactions (Hall, DeBernardis, & Reiss, 2006; Hagerman & Hagerman, 2002; Kaufmann et al., 2004).
Over the past few decades, research in FXS has shown that different antecedents, settings, familiarity of people, and social demands can result in eye gaze avoidance in FXS (Hall et al., 2006). For instance, studies have shown that individuals with FXS exhibit greater eye gaze avoidance and escape behaviors when interacting with an unfamiliar person versus a familiar person (Cohen et al., 1988; Wolff, Gardner, Paccla, & Lappen, 1989). Further, compared to children with ASD, children with FXS may demonstrate greater sensitivity to social gaze when it is initiated by their parents (Cohen, Vietze, Sudhalter, Jenkins, & Brown, 1989). It has also been shown that children with FXS appear to engage in social escape behaviors such as eye-rubbing and face-hiding in an attempt to avoid eye contact during social interactions (Hall et al., 2006). Multimodal task demands have also been shown to influence gaze avoidance in FXS regardless of whether the task involves placing social or non-social demands (Murphy, Abbeduto, Schroder, & Serlin, 2007). Although many factors may influence eye contact avoidance in FXS, Cohen and colleagues (1991) reported that eye contact avoidance in FXS did not appear to be associated with communication ability or age.
Males with FXS have also been shown to exhibit numerous symptoms of anxiety such as excessive shyness, gaze aversion, compulsive behaviors, difficulty separating from a parent, avoidance of social situations, and hypervigilence (Cordeiro, Ballinger, Hagerman, & Hessl, 2011; Hall, Lightbody, Huffman, Lazzeroni, & Reiss, 2009; Tranfaglia, 2011). Additional research also points to abnormal functioning of the hypothalamic-pituitary-adrenal (HPA) axis in FXS, including elevated insula activation, neuroanatomical differences (i.e. enlargement of the hippocampus), and increased cortisol levels as contributing to the experience of hyperarousal during eye contact in children with FXS (Hessl et al., 2002; Reiss & Dant, 2003). Because of these findings, anxiety appears to be a key construct to explore when assessing eye contact avoidance in this population.
Over the past decade, investigators have begun to evaluate targeted pharmacological agents designed to reduce symptomatology in FXS (Hagerman et al., 2009). Although these initial trials showed promise, there are currently few instruments designed to accurately capture changes in symptomatology in this population, as reported by the NICHD Working Groups 2013 report (Berry-Kravis et al., 2013). These researchers identified several issues related to outcome measures in clinical trials of FXS including lack of measures designed or validated for FXS, few measures to assess individuals across multiple levels of functioning, and limited measures designed specifically to capture specific symptoms of FXS. Furthermore, the Working Groups concluded that the majority of outcome tools currently available demonstrate a moderate quality level for clinical trials and there continues to be limited data on the sensitivity, reliability, and validity of these measures in FXS (Budimirovic et al., in press). Therefore, it is essential for researchers to focus on creating and validating measures that are sensitive to changes in prominent symptoms in FXS. For example, several authors have employed eye-tracking systems to quantify eye gaze avoidance in individuals with FXS (Hall et al. 2015). Using a customized setup of the Tobii X120 eye-tracker that allowed eye-tracking to be conducted during a live face-to-face social interaction, Hall and colleagues reported that individuals with FXS, particularly males, demonstrated significant deficits in looking to the eye region of the face. Although eye tracking is clearly an objective method to quantify eye gaze avoidance, these systems are extremely expensive to set up and require significant expertise to acquire and analyze the resulting data. Thus, eye tracking may therefore not be a feasible method to employ in a large clinical trial. To our knowledge, there are no questionnaire-based measures designed to specifically quantify eye gaze avoidance in children with FXS. A questionnaire designed to assess eye gaze avoidance in FXS has the potential to assist in tailoring interventions to meet the individual's needs and track changes in eye gaze avoidance over time in this population. Furthermore, feedback from parents about the function of eye gaze avoidance in FXS could be helpful in targeting associated symptoms, such as anxiety.
In order to quantify eye contact avoidance in children with FXS and to address the need for valid and sensitive outcome measures in FXS, we developed the Eye Contact Avoidance Scale (ECAS). This parent-report measure was designed to assess eye contact avoidance in social situations (e.g., eye contact while listening versus speaking) as well as quantify how making eye contact with others may be aversive (e.g., difficulty maintaining eye contact versus anxiety when required to make eye contact). The ECAS was administered to caregivers of children with FXS in order to collect normative data and to examine the psychometric properties of the measure.
Methods
Participants
Participants were recruited for this study as part of a larger investigation examining the efficacy of behavioral interventions for social gaze avoidance in FXS. Advertisements were posted and emailed via the National Fragile X Foundation inviting parents to complete an online screening survey about their child (see below). The introduction to the survey stated: “We are conducting a study to evaluate and teach eye contact and social skills to male children ages 8 to 16 years with fragile X syndrome. To determine your eligibility for the study, please complete the brief survey by clicking below”. Informed consent was obtained from caregivers included in the study prior to completing the survey.
Survey responses were received for 157 individuals with FXS, two of which were incomplete, two had been completed on individuals who were female, and five had been completed on individuals who were outside the study age range (8 to 16 years). The study sample therefore consisted of 148 boys with FXS aged 8 to 16 years. The mean age of the participants was 11.56 years (SD = 2.60 years). 81 (54.7%) boys were reported to have a comorbid diagnosis of ASD and 8 (5.4%) boys were reported to have a comorbid diagnosis of ADHD.
Measures
Eye Contact Avoidance Scale (ECAS)
The ECAS is divided into two sections. In the first section, caregivers completed basic demographic information concerning the child's sex, age, diagnosis of FXS, current level of support at school, and whether their child had been diagnosed with another genetic disorder, significant motor disability or neurological disorder (e.g., Autism Spectrum Disorder, Down syndrome, cerebral palsy etc). To obtain information about the child's communication ability, we also included the following question: “Does your child use full sentences to communicate things he wants or needs?” (0 =never; 1 = rarely; 2 = sometimes, 3 = often, 4 = always).
The second part of the ECAS contains questions specifically designed to measure eye contact avoidance in FXS. We devised questions on the ECAS in the following five domains of social functioning: 1). Avoidance when speaking to others; 2). Avoidance when listening to others; 3). Inability to maintain eye contact with others; 4). Difficulty maintaining eye contact with others; and 5). Anxiety if required to maintain eye contact with others. Items in each domain were preceded by an introductory explanation (e.g., We are interested in the extent to which your child avoids eye contact when he/she talks to people). For each domain, items were worded to elicit ratings from the perspective of the informant (e.g., “Does your child avoid eye contact when he/she talks to you?”), friends and family (e.g., “Does your child avoid eye contact when he/she talks to friends and family?”) and unfamiliar people (e.g., “Does your child avoid eye contact when he/she talks to unfamiliar people?”). Thus, given that there were 5 domains with 3 items per domain, the ECAS contains 15 items in total.
Each item on the ECAS is rated on a 0 to 4-point Likert scale (0 = never, 1 = rarely, 2 = sometimes, 3 = often, and 4 = always) with items 3a, 3b and 3c being reverse scored so that higher scores reflect higher levels of eye contact avoidance. Total domain scores (i.e., Speaking, Listening, Inability, Difficulty and Anxiety) can be obtained by summing the scores across the three levels of familiarity in each question block (maximum possible score for each domain = 12). Total subscale scores (i.e., Informant, Friends/family and Unfamiliar people) can be obtained by summing the scores for the five items at each level of familiarity (maximum possible score for each subscale = 20). Finally, a total score can be obtained by summing the scores across all 15 items (maximum possible total score = 60). The ECAS is provided in the Appendix.
Statistical analyses
Statistical analyses were conducted using SPSS Version 20 (SPSS, Inc) and AMOS Version 6.0 (Arbuckle 2005). To examine the internal consistency of the scale, we computed Cronbach's alpha coefficients for each domain, subscale and the total score. We then examined the effect of communication ability on ECAS scores. Given that communication ability scores were significantly related to the ECAS scores, we included communication ability as a covariate in subsequent analyses. To examine the effect of ASD diagnostic status on ECAS scores, we conducted a series of one-way analyses of covariance (ANCOVA) and to check that the assumptions for ANCOVA were met, we conducted tests for the homogeneity of variances (Levene's Test) and for the homogeneity of regression slopes. To examine the convergent validity of the ECAS scores we computed partial correlations between the ECAS total score and the scores obtained on the domains of the Social Responsiveness Scale, Second Edition (SRS-2; Constantino & Gruber, 2012) and the Anxiety, Depression, and Mood Scale (ADAMS; Esbensen, Rojahn, Aman, & Ruedrich, 2003) for a subsample of participants. For each analysis, the alpha level was set at .05.
Finally, to examine the factor structure of the ECAS, we conducted a confirmatory factor analysis of the data using structural equation modeling (SEM) techniques (AMOS, Version 6.0; Arbuckle 2005). SEM allows the investigator to estimate measurement models, to assess the adequacy of each theorized model, and to compare competing models (Bollen 1989; Burns and Nolen-Hoeksema 1992; Tomarken and Waller 2003). We used the chi-square goodness of fit test to examine the fit of each model. Changes in chi-square values relative to changes in degrees of freedom (chi-square difference tests) were used to compare nested models (Arbuckle, 2005).
Results
Internal consistency
Table 1 shows Cronbach's alpha coefficients computed for each domain, subscale and the total score of the ECAS (Table 1). Domain coefficients ranged from .79 to .89, subscale coefficients ranged from .82 to .87 and the total score coefficient was .92, indicating that the internal consistency of the scale was acceptable to excellent.
Table 1.
Internal consistency of the domain, subscale and total scores of the ECAS.
| ECAS domain/subscale | Cronbach's alpha | N of items |
|---|---|---|
| Domain | ||
| Speaking | .79 | 3 |
| Listening | .79 | 3 |
| Inability | .86 | 3 |
| Difficulty | .82 | 3 |
| Anxiety | .89 | 3 |
| Subscale | ||
| Informant | .83 | 5 |
| Friends/family | .82 | 5 |
| Unfamiliar people | .87 | 5 |
| Total score | .92 | 15 |
ECAS score distributions
Figure 1 shows the distribution of scores obtained by the 148 boys with FXS for each domain, subscale and the total score of the ECAS. Mean domain scores ranged from 7.1 (SD = 2.5) to 8.7 (SD = 2.2), mean subscale scores ranged from 11.5 (SD = 3.5) to 16.1 (SD = 3.5) and the mean total score was 41.2 (SD = 8.9). Planned comparisons of the scores obtained on the subscales indicated that boys obtained significantly higher scores on the Unfamiliar people subscale compared to the Friends/family and Informant subscales (F(2,290) = 198.0, p < .001).
Figure 1.
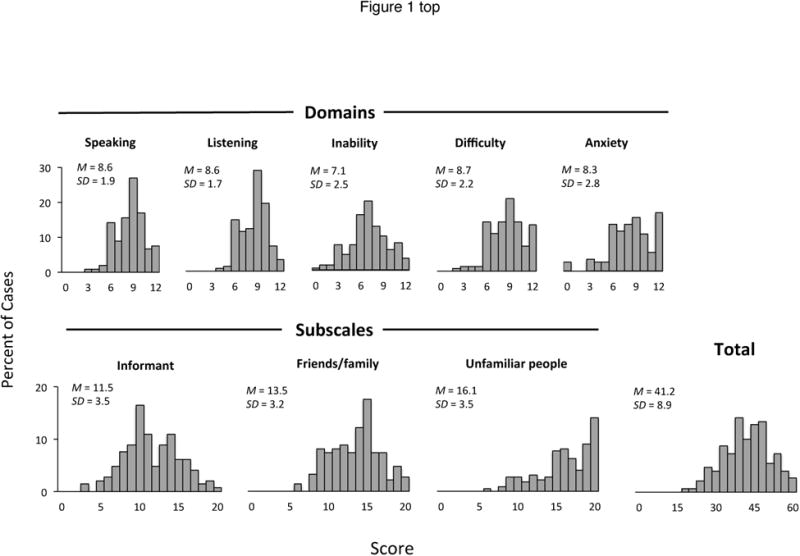
Score distributions obtained on each domain, subscale and total score of the ECAS.
Effect of communication ability on ECAS scores
In response to the question “Does your child speak in full sentences to indicate his/her wants and needs”, 21 boys (14.2%) were reported to “never” use full sentences to communicate wants/needs, 25 (16.9%) “rarely”, 43 (29.1%) “sometimes”, 27 (18.5%) “often” and 30 (20.3%) “always” (Note that data were missing for 2 cases). Table 2 shows the mean scores obtained on the ECAS for each domain, subscale and on the total score at each level of communication ability. One-way ANOVA analyses indicated that there was a significant effect of communication ability on the Speaking (F(1,143) = 2.53, p = .043), Listening (F(1,143) = 2.45, p = .049) and Inability (F(1,143) = 3.01, p = .020) domains of the ECAS as well as on the Unfamiliar people (F(1,143) = 3.09, p = .018) subscale and on the total ECAS score (F(1,143) = 2.82, p = .027). In each case, planned comparisons indicated that individuals with lower levels of communication ability obtained higher scores (p's < .05).
Table 2.
Mean scores obtained on each domain, subscale, and total score of the ECAS at each level of communication ability. SD's are shown in parentheses. Results of one-way ANOVA analyses, together with results of Levene's Test for homogeneity of variances, are also shown.
| Communication level1 | ||||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| ECAS domain/subscale | 0 (N=21) | 1 (N=25) | 2 (N=43) | 3 (N=27) | 4 (N=30) | Levene's Test (p) | F | p |
| Domain | ||||||||
| Speaking | 9.00 (2.30) | 9.28 (1.72) | 8.74 (1.77) | 8.44 (1.64) | 7.83 (1.87) | .751 | 2.53 | .043 |
| Listening | 8.71 (2.03) | 9.24 (1.51) | 8.58 (1.71) | 8.56 (1.67) | 7.83 (1.56) | .220 | 2.45 | .049 |
| Inability | 7.61 (2.77) | 7.76 (2.01) | 6.93 (2.36) | 7.67 (2.80) | 5.83 (2.53) | .348 | 3.01 | .020 |
| Difficulty | 8.67 (2.76) | 9.52 (1.83) | 8.67 (1.94) | 8.81 (2.40) | 7.87 (2.03) | .194 | 2.03 | .094 |
| Anxiety | 9.43 (2.13) | 8.64 (2.43) | 8.05 (2.84) | 7.78 (2.53) | 7.83 (3.43) | .461 | 1.50 | .206 |
| Subscale | ||||||||
| Informant | 12.76 (3.43) | 12.68 (2.98) | 11.02 (3.50) | 11.48 (3.69) | 11.55 (3.46) | .867 | 2.24 | .068 |
| Friends/family | 14.05 (3.12) | 14.56 (3.04) | 13.33 (3.15) | 13.78 (3.56) | 12.30 (2.71) | .590 | 2.08 | .087 |
| Unfamiliar people | 16.62 (3.69) | 17.20 (2.81) | 16.63 (3.34) | 16.00 (3.68) | 14.33 (3.39) | .643 | 3.09 | .018 |
| Total score | 43.43 (9.19) | 44.44 (7.87) | 40.98 (8.77) | 41.26 (9.29) | 37.20 (8.21) | .829 | 2.82 | .027 |
Uses full sentences to communicate wants/needs: 0 = never; 1 = rarely; 2 = sometimes; 3 = often; 4 = always
Effect of ASD diagnosis
Table 3 shows the mean scores obtained on each domain, subscale and on the total score for those with and without a comorbid diagnosis of ASD (Table 3). ANCOVA analyses showed that there was a significant effect of ASD diagnosis on the Speaking (F(1,143) = 5.36, p = .022), Listening (F(1,143) = 6.97, p = .009), and Difficulty (F(1,143) = 7.36, p = .007) domains of the ECAS, as well as on the Informant (F(1,143) = 4.54, p = .035) and Unfamiliar people (F(1,143) = 4.02, p = .047) subscales of the ECAS. Finally, there was a significant effect of ASD diagnosis on the ECAS total score (F(1,143) = 5.48, p = .021). In each case, individuals with an ASD diagnosis obtained significantly higher scores than those without an ASD diagnosis (p's < .05).
Table 3.
Mean scores obtained on each domain, subscale, and total score of the ECAS by ASD diagnostic status. SD's are shown in parentheses. Results of ANCOVA analyses with communication level included as a covariate, together with tests of ANCOVA assumptions, are also shown.
| ECAS domain/subscale | No ASD (N=67) | ASD (N=81) | Levene's Test (p) | Homogeneity of regression slopes (p) | F | p |
|---|---|---|---|---|---|---|
| Domain | ||||||
| Speaking | 8.17 (1.90) | 9.01 (1.77) | .284 | .118 | 5.36 | .022 |
| Listening | 8.09 (1.69) | 8.94 (1.66) | .381 | .902 | 6.97 | .009 |
| Inability | 6.71 (2.53) | 7.39 (2.55) | .976 | .629 | 1.38 | .242 |
| Difficulty | 8.09 (1.98) | 9.16 (2.25) | .408 | .671 | 7.36 | .007 |
| Anxiety | 7.91 (2.95) | 8.54 (2.63) | .650 | .777 | .95 | .331 |
| Subscale | ||||||
| Informant | 10.76 (3.03) | 12.20 (3.67) | .070 | .260 | 4.54 | .035 |
| Friends/family | 12.85 (3.22) | 14.06 (3.04) | .345 | .600 | 3.84 | .052 |
| Unfamiliar people | 15.36 (3.72) | 16.78 (3.15) | .484 | .395 | 4.02 | .047 |
| Total score | 38.97 (8.88) | 43.08 (8.52) | .786 | .766 | 5.48 | .021 |
Factor Structure
Table 4 shows the correlations obtained between the domain scores (Table 4). Correlations ranged from .46 to .82 for boys with FXS and .42 to .80 for controls. Figure 2 shows a two-factor measurement model of the data.
Table 4.
Correlations obtained between domain scores on the ECAS.
| ECAS | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| Domain | ||||
| 1. Speaking | 1.00 | |||
| 2. Listening | .826** | 1.00 | ||
| 3. Inability | .537** | .509** | 1.00 | |
| 4. Difficulty | .677** | .708** | .557** | 1.00 |
| 5. Anxiety | .498** | .502** | .401** | .452** |
p < .001
Figure 2.
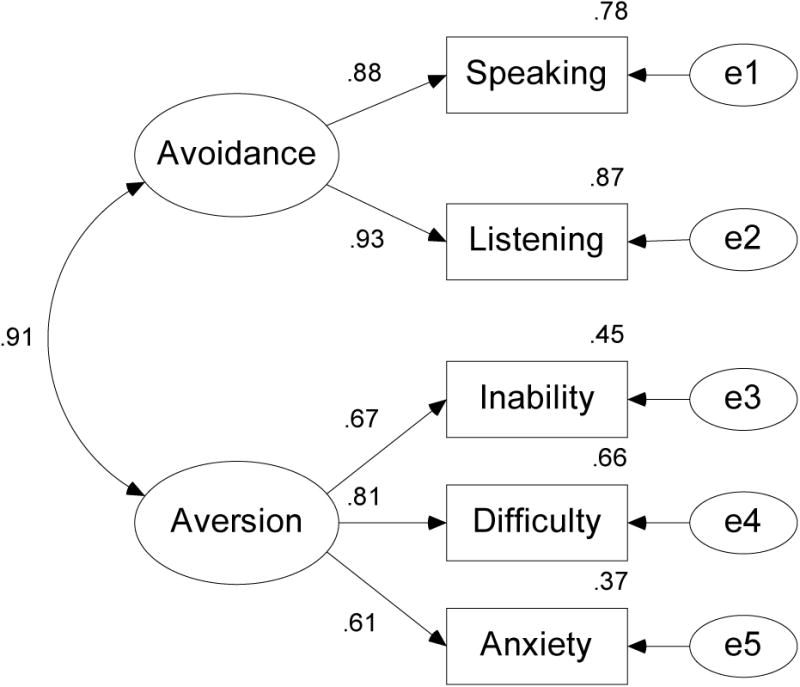
Two-factor measurement model of the ECAS domain scores.
In the model, circles represent unobserved variables (factors and error terms) and rectangles represent observed variables (i.e., domain scores). The observed Speaking and Listening domain scores load on a factor labeled Avoidance and the observed Inability, Difficulty and Anxiety domain scores load on a factor labeled Aversion. E1 to E5 are the error terms for the domain scores.
The measurement model was identified by setting the unstandardized factor loadings for each factor to 1.0 and the unstandardized factor loadings for the error terms to be 1.0. The overall fit of the measurement model was excellent (χ2(7, N = 148) = 5.75, p = 0.57). The factor loadings ranged from .61 to .93 and the communalities (i.e., estimates of the lower bound of the reliabilities) ranged from .37 to .87. To investigate whether a one-factor solution would provide a better fit to the data, we estimated a model in which all five domains loaded on a single factor. The fit of this model was also acceptable (χ2(9, N = 148) = 11.95, p = .21) with the factor loadings ranging from .57 to .92 and the communalities ranging from .33 to .85.
Convergent validity
For a representative sample of 48 participants with FXS, caregivers also completed the Social Responsiveness Scale 2 (SRS-2; Constantino & Gruber, 2012), and the Anxiety, Depression, and Mood Scale (ADAMS; Esbensen, Rojahn, Aman, & Ruedrich, 2003). Table 5 shows partial correlations obtained between the ECAS total score and the SRS-2 domain and ADAMS scores, controlling for communication level (Table 5).
Table 5.
Partial correlations obtained between the ECAS total score and scores obtained on the SRS-2 and ADAMS for a subsample of 48 boys with FXS.
| SRS-2 domain T score | r | ADAMS domain score | r |
|---|---|---|---|
| Social Awareness | .254 | Manic/hyperactive | .114 |
| Social Cognition | .106 | Depressed mood | .150 |
| Social Communication | .405** | Social avoidance | .551** |
| Social Motivation | .446** | General anxiety | .017 |
| Restricted Interests | .207 | Obsessive/compulsive | .360** |
p < .05;
p < .01
ECAS total scores were significantly correlated with scores obtained on the social communication (r = .41, p < .01) and social motivation (r = .47, p < .01) domains of the SRS-2. ECAS total scores were also significantly correlated with the social avoidance (r = .55, p < .01) and obsessive compulsive (r =. 36, p < .01) subscales of the ADAMS.
Discussion
We evaluated the reliability, validity and factor structure of a new parent-report screening tool designed to measure eye contact avoidance in individuals with FXS, the most common known inherited form of intellectual disability. Eye contact avoidance is a hallmark behavioral feature of individuals with FXS, yet to our knowledge, there are few measures available to quantify this socially significant behavior in this population. We therefore developed the scale to quantify eye contact avoidance in five domains of functioning based on the familiarity level of the person during social interactions (i.e., the informant, friends/family and unfamiliar people). Given previous research suggesting that eye contact may be aversive for individuals with FXS, we included questions about the child's ability to maintain eye contact for a short duration of time, the child's difficulty maintaining eye contact, and whether the child appeared upset or anxious when eye contact was required. Furthermore, we examined the psychometric properties of the ECAS to determine whether this instrument could be employed as a valid and reliable measure of eye gaze avoidance in FXS in clinical trials.
Overall, our findings indicated that the internal consistency of the ECAS scores was acceptable to excellent, with alpha coefficients ranging from .79 to .89 for the domain scores, .82 to .87 for the subscale scores, and .92 for the total score. Furthermore, the ECAS was sensitive to detecting differences in eye gaze avoidance based on the familiarity of the person during social interactions. For instance, in concordance with the current literature, results indicated that eye contact avoidance occurred more frequently towards unfamiliar people than toward more familiar people (Cohen et al., 1988; Wolff et al., 1989).
Considering that children with ASDs display deficits in eye contact, it was important to examine the effect of ASD diagnosis on eye contact avoidance. We therefore compared boys who had a comorbid diagnosis of ASD to those who did not, when controlling for communication ability. As expected, we found that those with an ASD diagnosis obtained higher mean scores on the Speaking, Listening and Difficulty domains and on the Informant and Unfamiliar People subscales of the ECAS compared to those without an ASD diagnosis. This is consistent with the current literature showing greater social impairments in children with FXS that also meet criteria for ASD (Kaufman et al., 2004; Hatton et al., 2006).
We employed a confirmatory factor analysis to examine the underlying factor structure of the ECAS and found that a two-factor solution provided an excellent fit to the data. The observed variables Speaking and Listening loaded on a latent construct we labeled Avoidance and the observed variables Inability, Difficulty, and Anxiety loaded on a latent construct we labeled Aversion. For the Avoidance factor, the factor loadings of the Speaking and Listening variables were extremely high in both groups. On the Aversion factor, the factor loadings were highest for the Difficulty variable, and slightly lower for the Inability and Anxiety variables.
Finally, as a novel measure of a singular behavior of social impairment, it is promising that the ECAS demonstrated good convergent validity with commonly used measures of social impairment (i.e., the SRS-2 and ADAMS). As predicted due to the value of eye gaze in social interactions, the ECAS was moderately correlated with the social communication and social motivation subscales of the SRS-2. The ECAS was not associated with the social cognition subdomain of the SRS-2 likely because this domain taps into the interpretation of social behavior in a more abstract capacity (i.e. understanding humor, using imaginative play) that is not captured in a measure on eye contact avoidance. When considering using screening measures such as the SRS-2 to capture social impairment in research or clinical studies, the ECAS could be included in a screening battery in order to better capture a prominent behavioral symptom for children with FXS and ASD.
Considering that high hyperarousal is thought to be the underlying mechanism influencing eye gaze avoidance in FXS (Hall et al., 2009; Holsen et al., 2008; Cohen, 1995), it was encouraging to find that the ECAS was significantly correlated with the social avoidance subscale on the ADAMS. The general anxiety subscale was not correlated with the ECAS providing stronger evidence of anxiety/hyperarousal present in a social context in FXS as compared to generalized anxiety. Given that the Anxiety domain moderately loaded on the Aversion factor in the confirmatory factor analysis and that the ECAS was correlated with the social avoidance subscale on the ADAMS, this provides evidence of the essential role anxiety may play in eye gaze avoidance in FXS. As behavioral interventions targeting social skills are designed for children with FXS, it will be important to incorporate techniques that target anxiety symptoms in order to decrease hyperarousal. The ECAS may be utilized in this context as a way to track observed eye gaze avoidance in order to monitor progress in decreasing anxiety during social interactions.
Since the SRS-2 and ADAMS, or similar measures are often administered in research as well as clinical settings to assess social impairment and psychiatric symptoms in children with FXS, the ECAS may be considered to be included with these measures as a supplemental assessment. With the current emphasis on validating existing measures and developing new measures for the FXS population (Berry-Kravis et al., 2013), the field has seen a rise of FXS validated measures such as the Pediatric Anxiety Rating Scale –Revised (Russo-Ponsaran, Yesensky, Hessl, & Berry-Kravis, 2014), the Aberrant Behavior Checklist utility index (Kerr et al., 2015) and modified scoring of the Aberrant Behavior Checklist (Sansone et al., 2012). Additionally, as novel paradigms in eye tracking studies are taking shape such as assessing social gaze during a naturalistic face-to-face social interactions as opposed to passive viewing of social stimuli (Hall et al., 2015), the ECAS could be incorporated into the study protocol as a way to screen for eye contact and/or collect pre and post intervention data.
There are several limitations to the study that should be noted. Since the ECAS was administered online, the caregivers involved in the present study may have been restricted to higher SES families. Furthermore, parents reported on the ASD diagnosis status of their children. Without a verifiable way to assess for the presence and severity of ASD symptoms, the analyses examining the effect of ASD diagnosis should therefore be interpreted cautiously. As data are collected in the larger study, a thorough diagnostic assessment will be performed, and the effect of ASD symptom severity on ECAS scores will likely be examined in the future. Additionally, expanding the validation of this measure to include females with FXS and children with social phobia will provide more normative data to use as a comparison to other diagnosis groups. Given that only 3 items on the ECAS require a child to be able to talk, it is also possible that the scale could be used for children who are mute or very low functioning. Ideally, the ECAS would be administered to different raters to gain a more reliable picture of the child's eye contact difficulties across raters. Furthermore, it is unknown to what extent eye contact difficulties may be stable over time. Future collection of adaptive abilities and IQ on a greater number of participants will contribute to identifying other factors that could be influencing eye gaze avoidance in FXS.
The goal of this study was to explore the psychometrics of a novel instrument to assess eye gaze avoidance in FXS and to provide normative data. Based on our findings, the ECAS demonstrates promise as an outcome measure considering its sensitivity to examine eye gaze avoidance and aversion across varying levels of familiarity of people and task demands. The sound reliability and convergent validity with established assessments in the field make the ECAS a potential measure for future pharmacological trials for FXS. Additionally, the inclusion of anxiety items on the ECAS may assist in deepening our understanding of underlying mechanisms contributing to the unique expression of social avoidance in this population. As clinical trials in this population continue, it is hoped that additional outcome measures will be designed specifically for FXS.
Acknowledgments
The authors would like to thank the families who participated in the study and Rebecca Barnett for her assistance in drafting the measure and setting up the online survey. This research was funded by award number R01HD081336 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (PI: S.S. Hall).
Appendix 1
Eye Contact Avoidance Scale (ECAS)
© 2017, Scott Hall and Kaitlin Venema
Your name:___________________________
Child's name:_______________________________
Child's sex: Male / Female
Child's age (years):_________
Does your child have a diagnosis of fragile X syndrome?: Yes / No
Please indicate whether your child had been diagnosed with another genetic disorder, significant motor disability or neurological disorder (e.g., Autism Spectrum Disorder, Down syndrome, cerebral palsy etc):
________________________________________________________________
Current level of support at school:_____________________________________
Instructions: For each question, please circle the response that best describes your child's behavior.
We are interested in your child's ability to communicate his/her wants and needs
| Never | Rarely | Sometimes | Often | Always | |
|---|---|---|---|---|---|
| Does your child use full sentences to communicate things he/she wants or needs? | 0 | 1 | 2 | 3 | 4 |
1. We are interested in the extent to which your child avoids eye contact when he/she talks to people.
| Never | Rarely | Sometimes | Often | Always | |
|---|---|---|---|---|---|
| a) Does your child avoid eye contact when he/she talks to you? | 0 | 1 | 2 | 3 | 4 |
| b) Does your child avoid eye contact when he/she talks to friends and family? | 0 | 1 | 2 | 3 | 4 |
| c) Does your child avoid eye contact when he/she talks to unfamiliar people? | 0 | 1 | 2 | 3 | 4 |
2. We are interested in the extent to which your child avoids eye contact when people talk to him/her.
| Never | Rarely | Sometimes | Often | Always | |
|---|---|---|---|---|---|
| a) Does your child avoid eye contact when you look at or speak to him/her directly? | 0 | 1 | 2 | 3 | 4 |
| b) Does your child avoid eye contact when friends and family look at or speak to him/her directly? | 0 | 1 | 2 | 3 | 4 |
| c) Does your child avoid eye contact when unfamiliar people look at or speak to him/her directly? | 0 | 1 | 2 | 3 | 4 |
3. We are interested in your child's ability to maintain eye contact with people.
| Never | Rarely | Sometimes | Often | Always | |
|---|---|---|---|---|---|
| a) Is your child able to maintain eye contact with you for up to 5 seconds? | 4 | 3 | 2 | 1 | 0 |
| b) Is your child able to maintain eye contact with friends and family for up to 5 seconds? | 4 | 3 | 2 | 1 | 0 |
| c) Is your child able to maintain eye contact with unfamiliar people for up to 5 seconds? | 4 | 3 | 2 | 1 | 0 |
4. We want to know how difficult it is for your child to maintain eye contact with people.
| Never | Rarely | Sometimes | Often | Always | |
|---|---|---|---|---|---|
| a) Does it appear difficult for your child to maintain eye contact with you? | 0 | 1 | 2 | 3 | 4 |
| b) Does it appear difficult for your child to maintain eye contact with friends and family? | 0 | 1 | 2 | 3 | 4 |
| c) Does it appear difficult for your child to maintain eye contact with unfamiliar people? | 0 | 1 | 2 | 3 | 4 |
5. We want to know whether your child gets anxious or upset if you require him/her to maintain eye contact with people.
| Never | Rarely | Sometimes | Often | Always | |
|---|---|---|---|---|---|
| a) Does your child get anxious or upset if you require him/her to maintain eye contact with you? | 0 | 1 | 2 | 3 | 4 |
| b) Does your child get anxious or upset if you require him/her to maintain eye contact with friends and family? | 0 | 1 | 2 | 3 | 4 |
| c) Does your child get anxious or upset if you require him/her to maintain eye contact with unfamiliar people? | 0 | 1 | 2 | 3 | 4 |
SCORING
| Subscale | Domain totals | |||
|---|---|---|---|---|
| Domain | Informant | Friends/family | Unfamiliar people | |
| 1. Speaking | 1a: | 1b: | 1c: | Speaking total: |
| 2. Listening | 2a: | 2b: | 2c: | Listening total: |
| 3. Inability | 3a: | 3b: | 3c: | Inability total: |
| 4. Difficulty | 4a: | 4b: | 4c: | Difficulty total: |
| 5. Anxiety | 5a: | 5b: | 5c: | Anxiety total: |
| Informant total: | Friends/family total: | Unfamiliar people total: | Total ECAS score: ___/60 | |
References
- Arbuckle JL. AMOS 6.0 User's Guide. Spring House, PA: AMOS Development Corporation; 2005. [Google Scholar]
- Bollen KA. Structural Equations with Latent Variables. Hoboken, NJ: John Wiley & Sons; 1989. [Google Scholar]
- Berry-Kravis E, Hessl D, Abbeduto L, Reiss AL, Beckel-Mitchener A, Urv TK The Outcome Measures Working Groups. Outcome measures for clinical trials in fragile X syndrome. Journal of Developmental and Behavioral Pediatrics. 2013;34(7):508–522. doi: 10.1097/DBP.0b013e31829d1f20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollen KA. A new incremental fit index for general structural equation models. Sociological Methods & Research. 1989;17(3):303–316. [Google Scholar]
- Burns DD, Nolen-Hoeksema S. Therapeutic empathy and recovery from depression in cognitive-behavioral therapy: a structural equation model. Journal of Consulting and Clinical Psychology. 1992;60(3):441–449. doi: 10.1037//0022-006x.60.3.441. [DOI] [PubMed] [Google Scholar]
- Budimirovic DB, Berry-Kravis E, Erickson CA, Hall SS, Hessl D, Reiss AL, King MK, Abbeduto L, Kaufmann WE. Updated report on tools to measure outcomes of clinical trials in fragile X syndrome. Journal of Neurodevelopmental Disorders. doi: 10.1186/s11689-017-9193-x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen IL. A theoretical analysis of the role of hyperarousal in the learning and behavior of fragile X males. Developmental Disabilities Research Reviews. 1995;1(4):286–291. [Google Scholar]
- Cohen IL, Fisch GS, Sudhalter V, Wolf-Schein EG, Hanson D, Hagerman R, Jenkins EC, Brown WT. Social gaze, social avoidance, and repetitive behavior in fragile X males: a controlled study. American Journal on Mental Retardation. 1988;92(5):436–446. [PubMed] [Google Scholar]
- Cohen IL, Vietze PM, Sudhalter V, Jenkins EC, Brown WT. Parent-child dyadic gaze patterns in fragile X males and in non-fragile X males with autistic disorders. Journal of Child Psychology and Psychiatry. 1989;30(6):845–856. doi: 10.1111/j.1469-7610.1989.tb00286.x. [DOI] [PubMed] [Google Scholar]
- Cohen IL, Vietze PM, Sudhalter V, Jenkins EC, Brown WT. Effects of age and communication level on eye contact in fragile X males and non-fragile X autistic males. American Journal of Medical Genetics. 1991;38(2-3):498–502. doi: 10.1002/ajmg.1320380271. [DOI] [PubMed] [Google Scholar]
- Constantino JN, Gruber CP. Social Responsiveness Scale: Second Edition (SRS-2) Los Angeles, CA: Western Psychological Services; 2012. [Google Scholar]
- Cordeiro L, Ballinger E, Hagerman R, Hessl D. Clinical assessment of DSM-IV anxiety disorders in fragile X syndrome: Prevalence and characterization. Journal of Neurodevelopmental Disorders. 2011;3(1):57–67. doi: 10.1007/s11689-010-9067-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esbensen AJ, Rojahn J, Aman MM, Ruedrich S. Reliability and validity of an assessment instrument for anxiety, depression, and mood among individuals with mental retardation. Journal of Autism and Developmental Disorders. 2003;33(6):617–629. doi: 10.1023/b:jadd.0000005999.27178.55. [DOI] [PubMed] [Google Scholar]
- Hagerman PJ. The fragile X prevalence paradox. Journal of Medical Genetics. 2008;45(8):498–499. doi: 10.1136/jmg.2008.059055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagerman RJ, Berry-Kravis E, Kaufmann WE, Ono MY, Tartaglia N, Lachiewicz A, et al. Advances in the treatment of fragile X syndrome. Pediatrics. 2009;123(1):379–390. doi: 10.1542/peds.2008-0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagerman RJ, Hagerman PJ. Fragile X syndrome: Diagnosis, treatment, and research. Baltimore: The John Hopkins University Press; 2002. [Google Scholar]
- Hall SS, DeBernardis M, Reiss A. Social escape behaviors in children with fragile X syndrome. Journal of Autism and Developmental Disorders. 2006;36:935–947. doi: 10.1007/s10803-006-0132-z. [DOI] [PubMed] [Google Scholar]
- Hall SS, Frank MC, Pusiol GT, Farzin F, Lightbody AA, Reiss AL. Quantifying naturalistic social gaze in fragile X syndrome using a novel eye tracking paradigm. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2015;168(7):564–572. doi: 10.1002/ajmg.b.32331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall SS, Lightbody AA, Huffman LC, Lazzeroni LC, Reiss AL. Physiological correlates of social avoidance behavior in children and adolescents with fragile X syndrome. Journal of the American Academy of Child and Adolescent Psychiatry. 2009;48(3):320–329. doi: 10.1097/CHI.0b013e318195bd15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatton DD, Sideris J, Skinner M, Mankowski J, Bailey DB, Roberts J, Mirrett P. Autistic behavior in children with fragile X syndrome: Prevalence, stability, and impact of FMRP. American Journal of Medical Genetics. 2006;140A:1804–1813. doi: 10.1002/ajmg.a.31286. [DOI] [PubMed] [Google Scholar]
- Hessl D, Glaser B, Dyer-Friedman J, Blasey C, Hastie T, Gunnar M, Reiss AL. Cortisol and behavior in fragile X syndrome. Psychoneuroendocrinology. 2002;27:855–872. doi: 10.1016/s0306-4530(01)00087-7. [DOI] [PubMed] [Google Scholar]
- Holsen LM, Dalton KM, Johnstone T, Davidson RJ. Prefrontal social cognition network dysfunction underlying face encoding and social anxiety in fragile X syndrome. Neuroimage. 2008;43:592–604. doi: 10.1016/j.neuroimage.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann WE, Cortell R, Kau ASM, Bukelis I, Tierney E, Gray RM, Cox C, Capone GT, Stanard P. Autism spectrum disorder in fragile X syndrome: Communication, social interaction, and specific behaviors. American Journal of Medical Genetics. 2004;129A:225–234. doi: 10.1002/ajmg.a.30229. [DOI] [PubMed] [Google Scholar]
- Kerr C, Breheny K, Lloyd A, Brazier J, Bailey DB, Jr, Berry-Kravis E, Cohen J, Petrillo J. Developing a utility index for the Aberrant Behavior Checklist (ABCC) for fragile X syndrome. Quality of Life Research. 2015;24(2):305–314. doi: 10.1007/s11136-014-0759-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy MM, Abbeduto L, Schroeder S, Serlin R. Contribution of social and information-processing factors to eye-gaze avoidance in fragile X syndrome. American Journal of Mental Retardation. 2007;112(5):349–360. doi: 10.1352/0895-8017(2007)112[0349:COSAIF]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Reiss AL, Dant CC. The behavioral neurogenetics of fragile X syndrome: Analyzing gene-brain-behavior relationships in child developmental psychopathologies. Developmental and Psychopathology. 2003;15:927–968. doi: 10.1017/s0954579403000464. [DOI] [PubMed] [Google Scholar]
- Russo-Ponsaran NM, Yesensky J, Hessl D, Berry-Kravis E. Feasibility, reproducibility, and clinical validity of the pediatric anxiety rating scale-revised for fragile X syndrome. American Journal on Intellectual and Developmental Disabilities. 2014;119(1):1–16. doi: 10.1352/1944-7558-119.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansone SM, Widaman KF, Hall SS, Reiss AL, Lightbody A, Kaufmann WE, Berry-Kravis E, Lachiewicz A, Brown EC, Hessl D. Psychometric study of the aberrant behavior checklist in fragile X syndrome and implications for targeted treatment. Journal of Autism and Developmental Disorders. 2012;42:1377–1392. doi: 10.1007/s10803-011-1370-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomarken AJ, Waller NG. Potential problems with“ well fitting” models. Journal of Abnormal Psychology. 2003;112(4):578. doi: 10.1037/0021-843X.112.4.578. [DOI] [PubMed] [Google Scholar]
- Tranfaglia MR. The psychiatric presentation of fragile X: Evolution of the diagnosis and treatment of the psychiatric comorbidities of fragile X syndrome. Developmental Neuroscience. 2011;33:337–348. doi: 10.1159/000329421. [DOI] [PubMed] [Google Scholar]
- Verkerk AJ, Pieretti M, Sutcliffe JS, Fu YH, Kuhl DP, Pizzuti A, et al. Identification of a gene (FMR-1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell. 1991;65(5):905–914. doi: 10.1016/0092-8674(91)90397-h. [DOI] [PubMed] [Google Scholar]
- Wolff PH, Gardner J, Paccla J, Lappen J. The greeting behavior of fragile X males. American Journal of Mental Retardation. 1989;93:406–411. [PubMed] [Google Scholar]


