Abstract
Inexpensive, noninvasive immunoassays can be used to quickly detect disease in humans. Immunoassay sensitivity and specificity are decidedly dependent upon high-affinity, antigen-specific antibodies. Antibodies are produced biologically. As such, antibody quality and suitability for use in immunoassays cannot be readily determined or controlled by human intervention. However, the process through which high-quality antibodies can be obtained has been shortened and streamlined by use of genetic engineering and recombinant antibody techniques. Antibodies that traditionally take several months or more to produce when animals are used can now be developed in a few weeks as recombinant antibodies produced in bacteria, yeast, or other cell types. Typically most immunoassays use two or more antibodies or antibody fragments to detect antigens that are indicators of disease. However, a label-free biosensor, for example, a quartz-crystal microbalance (QCM) needs one antibody only. As such, the cost and time needed to design and develop an immunoassay can be substantially reduced if recombinant antibodies and biosensors are used rather than traditional antibody and assay (e.g. enzyme-linked immunosorbant assay, ELISA) methods. Unlike traditional antibodies, recombinant antibodies can be genetically engineered to self-assemble on biosensor surfaces, at high density, and correctly oriented to enhance antigen-binding activity and to increase assay sensitivity, specificity, and stability. Additionally, biosensor surface chemistry and physical and electronic properties can be modified to further increase immunoassay performance above and beyond that obtained by use of traditional methods. This review describes some of the techniques investigators have used to develop highly specific and sensitive, recombinant antibody-based biosensors for detection of antigens in simple or complex biological samples.
Keywords: Recombinant antibody, Biosensors, Piezoimmunosensors, QCM
Introduction
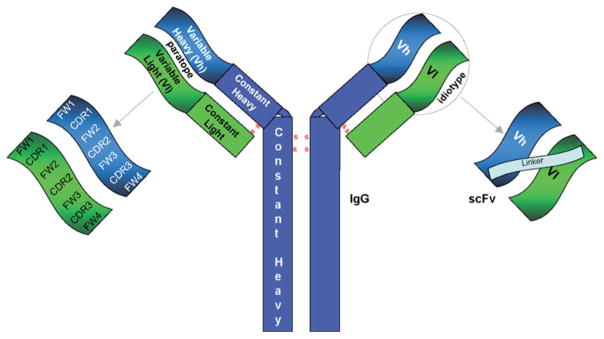
Chemical sensors and biosensors are defined as sensors that use chemical and/or biological molecules as recognition elements to quantitatively measure chemical or biomolecular analytes with sufficient sensitivity and selectivity. The most used biological recognition elements are antibodies. Antibodies are produced by vertebrate animals as part of their defensive immune response when exposed to foreign organisms or toxic substances. For example, the human immune system contains approximately one trillion B-cells. When the immune system encounters foreign antigens, B-cells respond by producing Y-shaped immunoglobulins called antibodies. Each arm of an antibody contains a pair of antibody heavy (~55 kDa) and light (~25 kDa) chains that are connected by interchain disulfide bonds. An antibody can be divided into two distinct regions, variable (located at the top of the Y) and constant (located at the bottom of the Y) regions. An antigen binds to the paratope of an antibody that is located at the top of the Y in a region called the antibody’s idiotype (discussed below). The amino acids present within the variable region vary from one antigen-specific antibody to another, whereas the amino acids present within the constant region are quite similar from one class (e.g. an IgA, IgD, IgE, IgG, or IgM) of antibody to another within an animal species. The variable region of an antibody heavy chain is known as the Vh (variable heavy) chain region whereas the variable light chain region is known as the Vl region. Together, the Vh and Vl regions structurally make up the antibody’s idiotype. The idiotype contains six complementary regions with three CDRs (i.e. CDR1, CDR2 and CDR3) each being found on the Vh and Vl chains. The amino acids present within the CDRs can complement and interact with the amino acids present on an antigen. Interactions between a positively charged amino acid on an antibody with a negatively charged amino acid on an antigen would be an example of a complementary interaction. The Vh CDR3 region is generally responsible for antigen-binding specificity whereas Vl CDR3 contributes to antibody affinity (antigen binding strength). The CDR regions are also known as hypervariable (HV) regions, because the amino acids present within these regions vary substantially from one antigen-specific antibody to another. The idiotype amino acids that do not directly interact with the antigen provide a framework (FW) to support the amino acids that are primarily involved in antigen binding. Four FW regions support the three CDRs found on each of the Vh and Vl antibody chains. The antigenic site on an antigen that is bound by an antibody is referred to as an epitope and is usually 4–10 amino acids in size; however, some epitopes can be much larger.
An antibody binds to an antigen specifically to form an immune complex. This is the basis for antibody-based immunoassay and immunosensor procedures. The IgG class of antibody is typically used in immunoassays because they bind antigens with greater affinity and specificity than other classes of antibodies. For biosensors and other immunoassays, the constant region of an antibody does not usually contribute to antibody–antigen binding and can non-specifically bind to other molecules present in biological samples. Researchers have created smaller, antibody fragments (Fabs, scFvs, minibodies, nanobodies, etc.) through genetic engineering to enhance antibody performance and reduce assay costs [1, 2, 3]. In this review, we will focus on the analytical aspects of antibody fragments (e.g. scFvs) as recognition elements and their applications in biosensing.
ScFv recognition elements and their productions
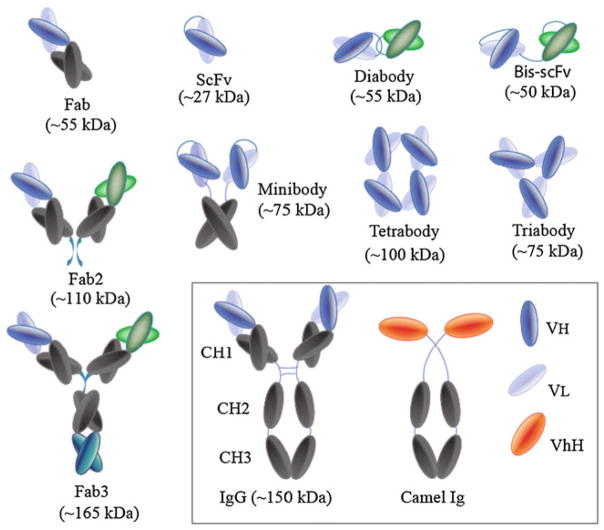
ScFv recombinant antibodies (~27 kDa) were first developed in the 1980s as an alternative to the larger (~150 kDa), natural antibodies produced in animals and humans [2]. An scFv is made up of an antibody’s variable light (Vl) and heavy (Vh) chain fragments, usually joined together by a peptide amino acid sequence termed a linker (Fig. 1). The scFv represents the idiotype of an antibody and is one of the smallest antibody fragments that retains the antigen-binding specificity of the parent antibody (e.g. an IgG, Fig. 1) [4]. The advantage of using small scFvs is that they can be genetically engineered to contain, for instance, metal-binding amino acids (e.g. cysteines, histidines, etc.) in the linker peptide so that the scFv can self-assemble on sensor (e.g. gold) surfaces, at high density, in the proper orientation to enhance scFv antigen-binding activity. Most scFvs use a 15–20 amino acid glycine (G), serine (S) linker peptide (e.g. GGGGSGGGGSGGGGS) to tether the Vh and Vl chains together as a monomeric fragment [2, 3]. ScFvs bearing either fewer than 15 amino acids or no linker amino acids cannot fold over to form an scFv, but can become bound to other scFv or intertwined with other Vh-Vl fragments to form diabodies (2 scFvs), triabodies (3 scFvs), tetramers (4 scFvs), bis-scFvs (two different scFvs joined in tandem), etc. (Fig. 2). The scFv multimers can be useful in enhancing assay sensitivity when used to detect antigens (e.g. viruses) with multivalent epitopes or antigen sites. As the number of interactions between a multimeric scFv and a multi-valent antigen increases, the avidity and stability of the scFv–antigen interaction will also increase to enhance the sensitivity of the immunoassay [1, 5]. ScFvs can also be engineered as minibodies (e.g. scFv engineered to contain the Fc region of an antibody) or can be conjugated to radionuclides, cytotoxic drugs, peptides, lipids, nanoparticles, and other molecules to generate bi-functional scFvs for diagnostic or therapeutic applications [6, 7].
Fig. 1.
Configuration of IgG and scFv recombinant antibodies, and locations of the antibody heavy and light-chain variable, constant, and idiotype regions, the antibody paratope framework (FW), and complementary determining (CDR) regions
Fig. 2.
The antibody, and different antibody fragments [1]
Antigen-specific scFvs can be selected from large phage (bacterial virus) ribosome or cell (e.g. E. coli, yeast) displayed recombinant antibody libraries, then expressed in large quantities by use of bacterial or eukaryotic expression systems [8]. The source of antibody genetic information used to make these libraries is the B-cells. Total or messenger RNA (m-RNA) is isolated from B-cells and reverse transcribed to make complementary DNA (c-DNA). The c-DNA is amplified using the polymerase chain reaction (PCR) using DNA primers that anneal to antibody Vh and Vl chains. The amplified Vh and Vl chain DNAs are joined together to form a single chain fragment by using, for instance, synthetic DNA oligo-nucleotides that encode for the peptides found in the scFv linker. The single-chain DNA is cloned into a bacterial or yeast DNA vector (e.g. a plasmid or phagemid) and then introduced into bacterial or yeast cells by a process called transformation to produce a library of many (between 1×108 and 1×1012) different antigen-specific scFv antibodies. For phage display, the transformed bacteria are rescued with a helper virus to establish an active phage (viral) infection that results in the production of phage particles. Each phage particle contains the scFv DNA and displays on the phage surface the scFv protein encoded by the scFv DNA. To select for antigen-specific scFv, the phage library is applied to an immobilized antigen. The immobilized antigen is washed to remove unbound phage. The antigen-bound phage-displayed scFvs are then used to infect bacteria (typically E. coli) to introduce the recombinant antibody DNA into the bacteria. The bacteria are plated on to agar in Petri dishes. Each colony that grows on the Petri dish produces a unique scFv. The colonies are picked and induced to express scFv that can then be assayed by an enzyme-linked immunosorbant assay (ELISA) or similar assay to identify scFv specific for the antigen [9].
Antigen-specific scFv can also be obtained by using E. coli or yeast cell display. ScFvs are displayed on the surface of the cell. The antigen is labeled with a fluorescent dye and mixed with the cell-displayed scFv library. Cells that display scFv reactive with the antigen will become fluorescently labeled and can be selected by use of a fluorescence-activated cell sorter (FACS) [10, 11]. Alternatively, cells displaying antigen-binding scFv can be selected by use of biotinylated antigen. Biotin binds to avidin, streptavidin or neutravidin with high affinity. Biotin binds to avidin, streptavidin or neutravidin with high affinity. Cells bearing scFv that bind to the biotinylated antigen can be selected from a library of cells by using magnetic beads bearing streptavidin and a magnet [10, 11].
The ribosomal display technique involves the formation of a stable scFv–ribosome–mRNA complex that enables selection of the scFv. The ribosome-displayed scFv library is applied to immobilized or biotinylated antigen to select for antigen-specific scFv. The scFv mRNA coupled to the ribosome is reverse transcribed and amplified by PCR to obtain scFv DNA sufficient for cloning. The scFv DNA is cloned into a DNA vector and used to transform cells that can express larger quantities of scFv [11]. Of the three techniques, phage display is most popular because of its high-throughput capabilities. This methodology is simple, inexpensive, scalable, and has been used to generate large numbers of scFvs, Fabs, and peptides [12, 13]. Compared with standard hybridoma monoclonal antibody technology, antigen-specific antibodies can be readily selected using phage display without the need to immunize animals to obtain antibodies [14].
The selected scFv fragments can be expressed in both prokaryotes (mainly E. coli) and eukaryotes (yeast, insect cells, plant cells, and mammalian cells). Expression in E coli is mainly used for small, non-glycosylated scFv and very large expression levels of 2 gL−1 have been achieved. ScFv expression in yeast cells can be carried out as rapidly as in E. coli. Expression of larger recombinant antibodies in plant and mammalian cells has also been demonstrated [7].
Recombinant DNA and library display techniques have been used to identify single-domain antibodies (sdAbs) that arise from camelids (e.g. camels and llamas) [15], sharks, etc. Antibodies from these animals contain heavy chains only. The heavy chains pair with one another to form homodimers that can bind to antigens [1, 16, 17]. Shark new antigen receptor antibodies (IgNAR) are 12–13 kDa in size and contain only two CDR regions rather than the six CDRs normally found in humans, mice, rats, etc. Shark IgNAR are highly soluble and thermally stable and are an alternative source of engineered antibodies for a wide range of antigen recognition and binding applications [18]. Phage-displayed IgNAR antibody libraries can be obtained from either antigen-immunized or naive (non-immunized) sharks to obtain highly soluble, high affinity, nano-sized sdAbs [19]. Phage antibody libraries derived from antigen-immunized animals, camels, sharks, etc., are usually biased and more specific for the immunizing antigens, whereas libraries derived from naive (non-immunized) animals have greater antigen-binding diversity and are more suitable for obtaining antibodies to a wide variety of antigens.
ScFvs may be developed for virtually any antigen by use of the display techniques described above. By use of a phage display peptide library, phage display techniques can also be used to select peptides that mimic antigen epitopes [13]. Economic production of scFvs creates a number of opportunities that might be more difficult to accomplish with classical antibodies. ScFvs have found widespread use as the recognition element in therapy and imaging procedures (e.g. cancer imaging) because of their small size and cellular penetration ability [7]. However, the literature describes few applications in which scFvs were used as components of bioassays and biosensors [5]. This review focuses on the assessment and evaluation of the scFvs as molecular recognition elements for immunosensor and immunoassay development.
ScFv immunosensors and immunoassays
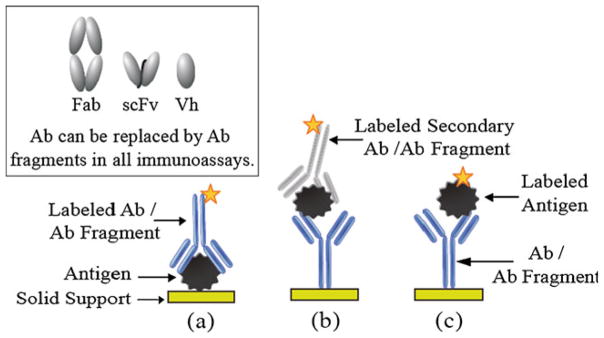
Antibodies including scFv and sdAbs have been used in a wide variety of immunoassays. Immunoassays are often classified into two general types—heterogeneous and homogeneous [20]. In the heterogeneous assay format (e.g. an enzyme-linked immunoassay, or ELISA), an antigen-specific antibody is immobilized on a solid support (e.g. a microtiter plate, bead, etc.) and used to capture an antigen from a sample (e.g. human serum). The solid support is washed to remove unbound antigen or contaminants, and a labeled (e.g. fluorescent dye, enzyme, radioactive isotope, etc.) antigen-specific antibody directed to a different epitope on the same antigen is used to detect the captured antigen. The solid support is washed to remove any unbound secondary antibody, and an assay signal (e.g. fluorescence, enzyme-induced color change, radioactivity, etc.) is generated and picked up by a fluorescence, color, or radioactivity detector in a microtiter plate reader to yield a quantifiable result. The strength of the assay signal in these non-competitive assays generally correlates with the antigen concentration in the sample. Examples of traditional direct, sandwich and competitive heterogeneous assay formats are presented in Fig. 3. In a homogeneous immunoassay format, immunoreactions take place in solution and do not require a separation step. An example of a homogenous assay is a FRET (fluorescence energy resonance transfer) assay. In a FRET assay, two different antibodies specific for different epitopes on the same antigen are labeled with two different FRET compatible fluorescent dyes. When the sample containing the antigen is excited with light that excites the first dye-labeled antibody, but not the other fluorescent dye-labeled antibody, the light emitted by the dye on the first antibody can excite the dye on the second antibody if both antibodies are close to one another (i.e. bound to the same antigen). If the excited second dye-labeled antibody emits light that can be picked up by a fluorescence microtiter plate reader, then both dye-labeled antibodies are bound to the same antigen. Homogenous assays such as FRET assays do not require washing steps and can be prone to sample assay interference [21]. The interactions between an antibody and an antigen can be detected directly by use of label-free transducers (e.g. quartz crystal microbalance (QCM), surface plasmon resonance (SPR) [22] and electrochemical impedance spectroscopy (EIS). In Table 1 we list a few examples of engineered antibody (e.g. scFv, Vh, Fab)-based immunoassays for detection of viruses, cytochrome P450s (catalytic cellular enzymes that oxidize organic substances), IgG by use of a QCM [23, 24], small molecules, toxins, prostate-specific antigen (PSA), and bacteria by SPR [22], and Her2 by use of a piezoelectric microcantilever [25] and other biosensors [26–28].
Fig. 3.
(a) Direct immunoassay: an antigen immobilized on a solid support is detected and quantified by use of an antigen-specific antibody conjugated with a reporter molecule; b) Sandwich immunoassay: an antibody specific for an antigen captures the antigen from a sample. The captured antigen is detected and quantified by use of an antibody labeled with a reporter molecule (e.g. dye, enzyme, isotope, etc.); c) Competition immunoassay: a capture antibody specifically captures an antigen from a sample. The antigen in the sample competes with a purified labeled antigen for binding to the capture antibody. As the concentration of unlabeled antigen in a sample increases, assay signal decreases
Table 1.
Representative engineered antibody (scFv, VHH, Fab)-based immunoassays
| Detection methods | Examples (targets) | Ref. | ||
|---|---|---|---|---|
| scFv | Label | Colorimetric (ELISA) | Rabbit IgG, ricin toxoid | [29–31] |
| Fluorescence | Human epidermal growth factor receptor 2(HER2)/neu, c-Met protein (on lung cancer cell), Salmonella O-polysaccharide, B. anthracis spore (exosporium) | [31–34] | ||
| Chemiluminescence | MUC1 (on breast cancer cells), HER2 | [35, 36] | ||
| Luminescence | Salmonella antigen | [37] | ||
| Label-free | Immunoelectron microscopy | Hemocyanin of the Tunisian scorpion Androctonus australis | [38] | |
| Electrochemical | L. monocytogenes, Venezuelan equine encephalitis virus | [39, 40] | ||
| QCM | Parathion, HIV-1 virin infectivity factor, cytochrome P4501B1, rabbit IgG | [23, 41, 42] | ||
| SPR | Morphine-3-glucuronide, prostate-specific antigen, aflatoxin B1, L. monocytogenes, | [22, 40, 43–45] | ||
| Piezoelectric microcantilever | HER2 | [25] | ||
| Vh | Label | Radiochemical | Lysozyme-expressing tumors and metastatic lesions | [46] |
| Crystallography | Carbonic anhydrase | [47] | ||
| ELISA | Tetanus toxoid and lysozyme, caffeine | [48, 49] | ||
| Label-free | SPR | Human prostate-specific antigen | [15] | |
| Fab | Label | ELISA | Rabies virus | [50] |
| Electrochemiluminescence assay (ECLA) | Ranibizumab | [51] | ||
| Luminescence | Prostatic acid phosphatase (PAP) | [52] | ||
| Label-free | Electrochemical | Deoxynivalenol (DON), atrazine, testosterone | [53–56] | |
| QCM | Cocaine | [57] | ||
| SPR | Simazine, diphenylurea | [58, 59] | ||
Abbreviations/acronyms: Her2/Neu-a molecule found on the surfaces of cells, for example breast cancer cells; c-Met: a small molecule found within cells that controls cell signaling; Salmonella O-polysaccharide: a carbohydrate–lipid polymer found on the surface of Gram-negative bacteria such as Salmonella; exosporium: the outer coat of spores found in some bacteria, for example Bacillus anthracis; MUC1: a glycoprotein found on the surface of some cancer cells; parathion: an insecticide; atrazine: a herbicide; lysozyme: a proteolytic enzyme; carbonic anhydrase: catalytic enzyme that converts water and CO2 to bicarbonate and H+; ranibizumab: a therapeutic antibody used to treat macular degeneration; deoxynivalenol, a fungal toxin; simazine: a herbicide
ScFv-based direct label-free heterogeneous immunosensors
One of the simplest immunoassay formats is the direct label-free immunosensor in which the interaction between a single immobilized antibody and a captured antigen is detected by use of an electrical, mechanical, electromagnetic (including light), chemical, acoustic, or thermal transducer to produce a quantifiable signal. The most common label-free transducers are QCM, SPR, and EIS, in which binding between an immobilized antibody and an antigen leads to a change of resonance frequency (QCM), refractive index (SPR), or electrical impedance (EIS). Direct label-free scFv-based immunosensors combine the high sensitivity and high selectivity rendered by the scFv or sdAb, as a capture agent, that is immobilized on a sensor surface. The binding between the antibody and antigen can be directly transduced to a signal for readout. The problem most often encountered in immunosensor or assay development is the failure to immobilize antibodies at high, uniform density in a correct orientation to reduce assay insensitivity and eliminate inter-assay variability and inefficiency. Our laboratory and others have developed several approaches to genetically engineer scFv to address such problems. These will be briefly summarized in the review.
Engineering scFv for sensor surface immobilization
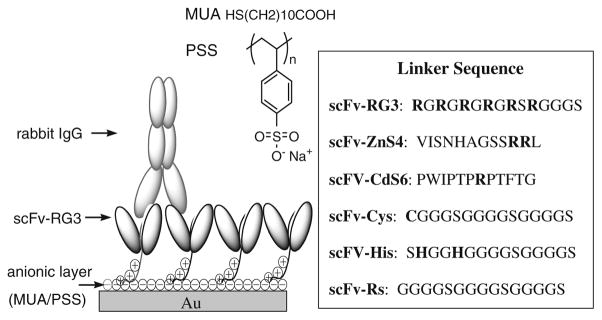
ScFv can be immobilized on transducer surfaces by use of a variety of approaches (Fig. 4). ScFv can be engineered to contain metal-binding cysteine (Cys) or histidine (His) amino acids in the peptide linker so that the scFv will correctly self-assemble on to metal surfaces of many transducers at high density (Fig. 4a) [24]. ScFv can be engineered to contain positively charged amino acids (e.g. arginine or Arg) in the peptide linker for scFv immobilization on to negatively charged self assembled monolayer (SAM)-modified transducer surfaces (Fig. 4b). The 6-histidine (6×His) amino acid sequence engineered on to the end (carboxy or C-terminus) of some scFvs can be used to non-covalently immobilize scFv to Ni-NTA sensor surfaces (Fig. 4c). ScFv can be conjugated to biotin via scFv-free amines (e.g. the ε-amino group on lysine amino acids) and immobilized on to streptavidin sensor surfaces (Fig. 4d). The ε-amino group on scFv genetically engineered to contain C-terminal or naturally occurring lysines can be used to couple scFv to the sensor surface with carboxyl functional group using carbodiimide-mediated covalent coupling chemistry (Fig. 4e) [35].
Fig. 4.
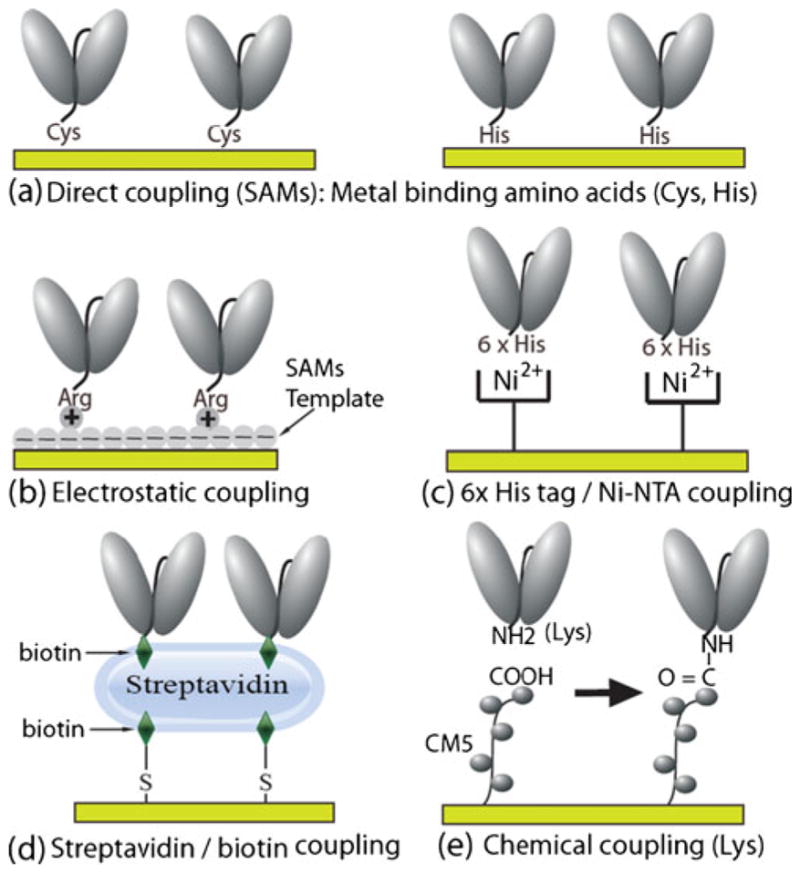
Coupling of scFvs to the Au QCM sensor surface
Engineered scFv Piezoimmunosensors (QCM)
Antibody and antigen interactions can be characterized through the use of a variety of transducers. Piezoimmunosensors use a QCM to measure antibody–antigen interaction by monitoring a change in frequency due to the mass change when an antibody, immobilized on a quartz crystal Au sensor surface, captures an antigen from a sample (e.g. serum). Piezoimmunosensor assays are simple, rapid, sensitive, and typically only require a single antibody to carry out the analyses. Thus, we will focus on discussing piezoimmunosensors as representative label-free biosensors to illustrate methods of using engineered scFvs as recognition elements for immunosensor development. As with any immunoassay, assay sensitivity and specificity are directly correlated with, and dependent upon, antibody sensitivity and specificity. Assay performance also depends on the components present in samples such as serum. Components such as complement and heterophilic antibodies (e.g. human serum antibodies that bind to mouse monoclonal antibodies) can bind to and interfere with the antigen-specific antibodies used in the assays [11]. In some cases, these components bind to the constant, but not the variable antigen-binding site of an assay’s antibodies. One way of overcoming this problem is to remove the constant regions of the antibodies used in the assays and just use the antigen-binding variable region (e.g. scFv, sdAb, etc.). To demonstrate the suitability of this approach, we used a model system involving the mouse IgG monoclonal antibody designated A10B and its antigen, rabbit IgG, a relatively inexpensive and abundant protein. The A10B was engineered as an scFv by using the hybridoma cells producing the A10B monoclonal antibody as the source of genetic material for the scFv. The A10B scFv, similar to the monoclonal antibody, bound rabbit IgG. Additionally, the A10B IgG monoclonal antibody was digested with papain and passed over a protein-A affinity column (to remove A10B IgG Fc constant regions) to obtain rabbit IgG-binding A10B Fab fragments that contain constant light and constant heavy chain 1 regions. The A10B scFv contained a cysteine amino acid within the linker peptide joining the A10B Vh and Vl regions. The A10B IgG, Fab and scFv fragments were immobilized on QCM gold sensor surfaces and used to capture rabbit IgG and to determine non-specific binding activity to serum components. Non-specific serum-binding activity was greatest on the A10B IgG and Fab-fragment-coupled sensor surfaces and nearly non-existent on the A10B scFv-cys sensor. Antigen-binding activity was greatest on the A10B scFv-cys sensor and much less on the A10B IgG and Fab fragment sensors [60].
The amino acids that make up the linker region of the A10B scFv were re-engineered to contain amino acids such as histidine, which can also bind metal (e.g. gold) [61], and arginine, which can couple to a negatively charged surface to form a self-assembled monolayer (SAM) [24]. Similar to cysteine, the histidine and arginine amino acids enabled the scFv to self-assemble as a monolayer and to assume optimum antigen-binding orientation on the QCM gold sensor surface. In previously published studies, average surface coverage of 1.7×10−11 mol cm−2 and 5.3×10−12 mol cm−2 have been reported for Fab′-SH fragment-modified and whole IgG antibody-modified surfaces, respectively. By comparison, surfaces modified with scFv containing a linker cysteine (scFv-Cys) show nearly ten times (1.74 ± 0.53 ×10−10 mol cm−2) as many antigen-binding sites per square centimeter as those modified with Fab′-SH fragments, and 35 times as many binding sites as surfaces modified with whole IgG antibody [60]. Results demonstrated that the A10B scFv engineered to contain arginine linker amino acids (A10B scFv-RG3) coupled to a negatively charged SAM template gave the highest antigen (i.e. rabbit IgG)-binding activity with a 42-fold improvement in binding activity over the A10B Fab fragment sensor (Figs. 5 and 6). Protein-A-coated gold nanoparticles (AuNP) bind to rabbit IgG. When used to detect rabbit IgG bound to A10B scFv-RG3 on SAM sensor surfaces, assay sensitivity was enhanced from nanomolar to picomolar and subpicomolar levels [24]. Assay sensitivity could also be increased five-fold by incorporating QCM sensors with a 25 MHz rather than 10 MHz oscillation frequency. Recently, we have shown that the CGSGSGS and RGRGRG peptide linker can be added to short synthetic peptide recognition elements for piezoimmunosensor development. We demonstrated the generic applicability of these linker peptide sequences for immobilization of a Her2 mimotope-derived synthetic peptide, which is only 2007 (g/mol) in molecular weight and 19 amino acids long, on Au and successfully used it for detection of Herceptin in human serum with high sensitivity and specificity by use of a QCM piezoimmunosensor [62].
Fig. 5.
A10B scFv sensors with different linker sequences
Fig. 6.
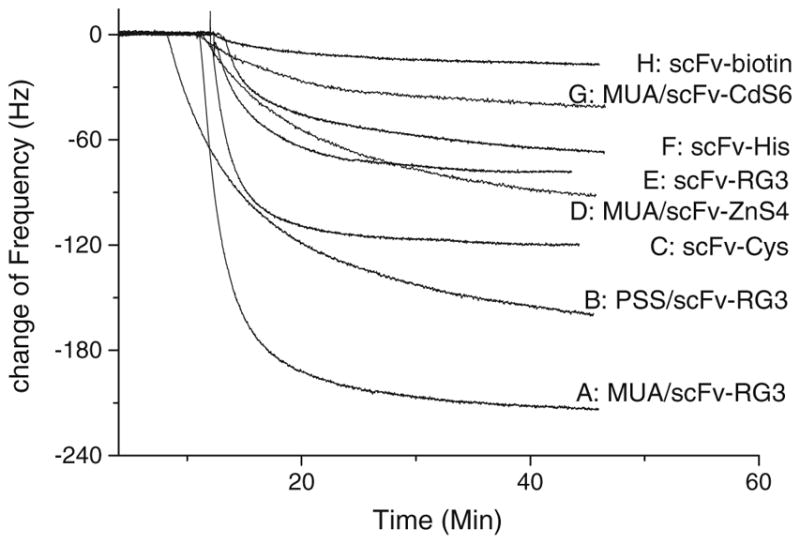
Signal vs. time curves of different A10B scFv QCM sensors for detection of 132 nmol L−1 rabbit IgG
Antigen–antibody interactions are based on the spatial complementarities of groups of amino acids within the antigenic site or epitope of an antigen with those in the paratope or antigen-binding site of the antibody. In favorable cases, antibodies can be used to recognize a single antigenic site in the presence of several thousand different antigenic sites. The problem with immunoassays is that individual monoclonal antibodies target antigenic sites or epitopes, not the whole antigen. Antibodies that bind to the same or similar epitopes on two or more relatively unique molecules can cross-react with and bind to different molecules bearing similar epitopes to produce a false-positive assay signal [63, 64]. However, similar but distinct molecules seldom share two or more epitopes and can be distinguished from one another by using a pair of antigen-specific capture and detecting antibodies. A true positive assay signal will only occur if both antibodies simultaneously bind the same antigen at the same time. We demonstrated that this approach was feasible using a QCM and human CYP1B1 (a P450 enzyme) as an antigen [23]. Six different CYP1B1-specific scFvs were biotinylated and coupled to neutravidin (immobilized on gold QCM sensor surfaces) and used to capture CYP1B1 from solution. Verification of positive CYP1B1 binding was based on at least two different CYP1B1-specific scFvs simultaneously binding to CYP1B1. The specificity of the CYP1B1 QCM assay was confirmed by utilizing negative control antigens—rabbit IgG, fetal bovine serum, and an irrelevant cytochrome P450 (designated BM-3, a P450 obtained from Bacillus megatarium) in place of CYP1B1. This approach successfully reduced false-positive assay signals even for molecules (e.g. BM-3) closely related to CYP1B1.
ScFv used in other immunoassays
ScFvs and sdAbs can be used in lieu of whole antibodies as capture agents in sandwich-based immunoassays [65, 66] with the benefits of increased sensitivity, flexibility, and higher intrinsic stability of the antibody recognition elements. These benefits enable scFv based immunoassays to be robust so that they have low cross-activity and less non specific adsorption from sample matrix. Different scFvs selected from phage display are ideal for use as capture antibodies to develop multiplexed immunosensor microarrays. ScFv-based immunosensors have been used to detect bacteria in real biological samples [31] and small molecules, for example UO2, in environmental water samples [67].
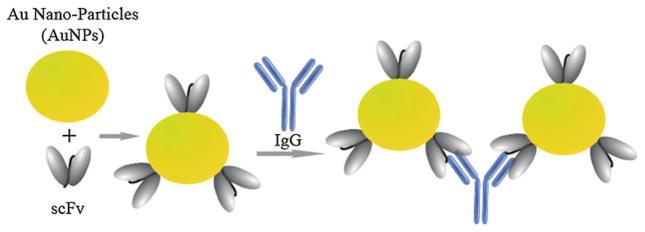
ScFvs have been used to develop biological (e.g. enzyme) sensors [68]. Biological sensors are functional proteins engineered to display peptides to which antibodies bind. When antibodies bind to the peptides on the proteins, the biological activity of the proteins is altered to produce a measurable signal that can be quantified. Protein sensors have been described for β-lactamase [69], β-galactosidase [70], alkaline phosphatase [71], green fluorescent protein [72], and the tail spike protein of the P22 bacterial virus [73]. Conceivably, an enzyme similar to alkaline phosphatase can be engineered or chemically modified to display a peptide representing a domain on HIV. If an scFv specific for the HIV peptide binds to the peptide on the enzyme and the enzyme loses catalytic activity, the enzyme will not produce a visible color change when alkaline phosphatase substrates, for example p-nitrophenol phosphate are used in an assay. However, if a sample contains HIV, then the peptide-specific scFv will bind to HIV and not to the peptide on the enzyme. In such a situation, the enzyme will produce a color change whenever HIV is present. Such approaches have potential in new, simple, and inexpensive assays. ScFv can be conjugated with nanoparticles to develop sensitive colorimetric scFv immunosensors. We have used IgG-specific scFvs coupled to Au nanoparticles to detect IgG in solution (Fig. 7). In the presence of IgG, Au nanoparticles bearing scFvs aggregate and undergo a visible color change [29].
Fig. 7.
Schematic diagram depicting the interactions and aggregations of scFv-conjugated gold nanoparticles with the antigen, IgG
Affinity is the measure of the binding strength between a single paratope on an antibody and a single epitope on an antigen. High-affinity antibodies bind antigens more quickly and release antigens more slowly than low-affinity antibodies, and are more suited to use in assays to detect trace concentrations of antigens. Avidity measures the cumulative binding strength that occurs between multiple paratopes on one antibody (e.g. the ten paratopes on one IgM antibody) with multiple repeating epitopes on one particle (e.g. a virus with ten or more epitopes).
A monomeric scFv has only one paratope. If a monomeric scFv has high antigen-binding specificity but low affinity, it may not be suitable for use in assays to detect small amounts of antigen (e.g. a virus) simply because it cannot rapidly bind and hang on to an antigen flowing by it in solution. However, if a large number of monomeric scFv are grouped together (e.g. when engineered as dimers, trimers, etc.) or immobilized as a layer of scFv on a sensor surface, the avidity of the scFv and the sensitivity of the assay to detect an antigen (e.g. a virus) can be increased.
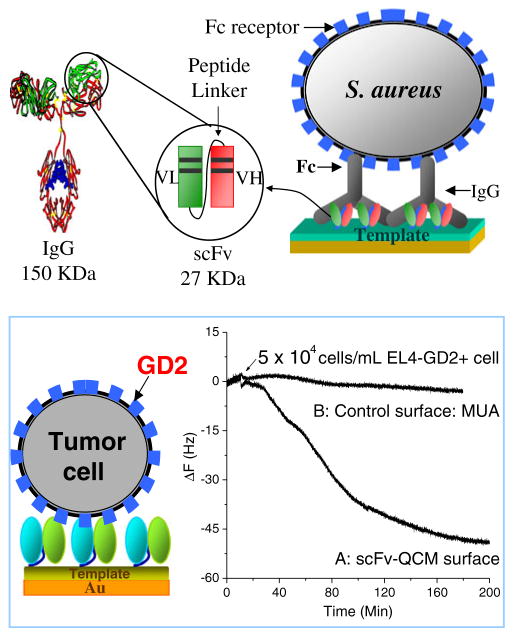
Many multivalent scFvs have been successfully developed to target cell surface receptors for cancer imaging and therapy [7, 74, 75]. These scFvs can be used to develop cell-based immunosensors to detect, capture or enrich cells from complex biological fluids or biopsy suspensions. An anti-GD2 scFv tetravalent multimer that binds to a disialosyl ganglioside receptor (denoted GD2) [75] was immobilized on the Au QCM surface to prepare a QCM-based immunosensor. GD2 is highly expressed (e.g. 5×106 molecules per NB cell) in a wide variety of human tumors including neuroblastoma (NB) and melanoma cells. The scFv multimer provides more binding sites which can increase antigen-binding avidity and assay sensitivity. As shown in Fig. 8, the scFv-bearing immunosensor successfully captures GD2-positive cells whereas a sensor without scFv does not. This preliminary work demonstrated that a multivalent scFv immobilized on an immunosensor can be used to detect cancer cell-surface receptors such as GD2 [76].
Fig. 8.
(a) scFv-based Fc sensor; (b) scFv sensor for detection of cancer cell surface receptor
Monovalent scFvs can be used to immobilize the constant Fc region of an antibody so that the Fc domain can bind to the Fc receptor on a cell’s surface [77]. As illustrated in Fig. 8, A10B scFv was immobilized on a preformed 11-mercaptoundecanoic acid (MUA) sensor surface to form a SAM that bound to the CH1 region of rabbit IgG, leaving the rabbit IgG Fc region exposed and available for binding to an Fc receptor on a eukaryotic cell and to protein A on the surface of the Staphylococcus aureus bacterium.
Conclusions
One of the most important components of any sensor device is the recognition element; this is the part of the sensor which detects a specific analyte. Antigen-specific antibodies (e.g. IgG) or antibody fragments (e.g. Fab) are probably the most difficult component of an immunoassay to acquire, simply because they are derived biologically and their binding specificities and affinities cannot be readily controlled. Additionally, when labeled with a reporter molecule (e.g. biotin, dye, enzyme, etc.) or immobilized as a capture agent on to a surface, their binding activity and specificity can change. Some of the challenges in selecting high affinity, antigen-specific antibodies can be overcome by use of recombinant antibody techniques, for example phage or yeast display. Once selected, recombinant antibodies (scFv, sdAb, peptides [62], etc) can be re-engineered to contain metal-binding or positively charged amino acids that will not interfere with the antibody’s antigen-binding specificity. These amino acids can be used to correctly orient an antibody on a surface so that it can retain antigen-binding activity.
There are several distinct advantages in using recombinant antibodies (scFv, sdAb, etc) to develop immunosensors or immunoassays. Antigen-specific scFv and sdAb can be readily selected from large bacterial virus (phage) displayed recombinant antibody libraries and can be readily formatted by genetic engineering or chemical conjugation for scFv coupling to the sensors used to detect antigens. Whether it is a protein released by an infectious agent or a protein produced by our bodies in response to the disease, recombinant antibodies can be produced to specifically bind to them. Additionally, scFv-based label-free immunosensors, for example piezoimmunosensors, only need a single recombinant antibody to detect an antigen with high specificity, thus eliminating the need for a second (i.e. detecting) antigen-specific antibody. This greatly simplifies assay procedure, because in some cases it can be extremely difficult to generate and produce just one antigen-specific antibody, let alone two. The surface of the sensor can be fabricated and chemically modified under controlled conditions so that scFv can be correctly oriented (e.g. on Au) at high density to enhance assay avidity and sensitivity. A wide variety of sensing transducers and imaging technology can be developed by using recombinant antibodies to replace traditional whole antibodies for use in antigen detection. Techniques such as cyclic voltammetry (CV), surface plasmon resonance (SPR), surface acoustic wave (SAW), quartz crystal microbalance (QCM), and many other detection techniques offer researchers options needed to optimize conditions to achieve high assay sensitivity and specificity even when complex samples (e.g. blood) are being analyzed. For example, a QCM can readily sense a change in mass on the sensor’s surface. As such, an scFv-based piezoimmunosensor surface can be readily interrogated to ensure that the same concentration of scFv is immobilized on the sensor surface every time, to enhance inter-assay reproducibility, which is something that cannot readily be achieved for most traditional immunoassays (e.g. ELISAs) [78, 79]. Although scFvs have found widespread use in clinical therapy and imaging procedures, the same cannot be said for their uses in biosensors. Here we have described the situations in which scFvs have been used as the recognition elements for biosensors to detect antigens or cells bearing antigens. With advances in biomarker research, a combination of several good biomarkers that correlate well with disease state will enable scFv-based biosensors to be developed for use in disease diagnosis and detection. Because antibody-based sensing platforms are so common, it is only a matter of time before we begin to take advantage of scFvs for routine use as component in biosensors.
Acknowledgments
Xiangqun Zeng is grateful for the support of NIH grant (R21 EB006495) and OU-Beaumont Multidisciplinary Research Awards. Ray Mernaugh was supported in part by the Vanderbilt University Institute for Chemical Biology.
Contributor Information
Xiangqun Zeng, Department of Chemistry, Oakland University, 2200 Squirrel Road, Rochester, MI 48309, USA.
Zhihong Shen, Department of Chemistry, Oakland University, 2200 Squirrel Road, Rochester, MI 48309, USA.
Ray Mernaugh, Department of Biochemistry, Vanderbilt University, Nashville, TN 37232, USA.
References
- 1.Holliger P, Hudson PJ. Engineered antibody fragments and the rise of single domains. Nat Biotechnol. 2005;23:1126–1136. doi: 10.1038/nbt1142. [DOI] [PubMed] [Google Scholar]
- 2.Borrebaeck CA, Wingren C. Recombinant antibodies for the generation of antibody arrays. Methods Mol Biol. 2011;785:247–262. doi: 10.1007/978-1-61779-286-1_17. [DOI] [PubMed] [Google Scholar]
- 3.Roitt IB, Brostoff J, Male D. Immunology. 5. Mosby International Limited; London: 1998. [Google Scholar]
- 4.Padlan EA. Anatomy of the antibody molecule. Mol Immunol. 1994;31:169–217. doi: 10.1016/0161-5890(94)90001-9. [DOI] [PubMed] [Google Scholar]
- 5.Conroy PJ, Hearty S, Leonard P, O’Kennedy RJ. Antibody production, design and use for biosensor-based applications. Semin Cell Dev Biol. 2009;20:10–26. doi: 10.1016/j.semcdb.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 6.Kreitman RJ, Wilson WH, Bergeron K, Raggio M, Stetler-Stevenson M, FitzGerald DJ, Pastan I. Efficacy of the anti-CD22 recombinant immunotoxin BL22 in chemotherapy-resistant hairy-cell leukemia. N Eng J Med. 2001;345:241–247. doi: 10.1056/NEJM200107263450402. [DOI] [PubMed] [Google Scholar]
- 7.Weisser NE, Hall JC. Applications of single-chain variable fragment antibodies in therapeutics and diagnostics. Biotechnol Adv. 2009;27:502–520. doi: 10.1016/j.biotechadv.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 8.O’Brien PM, Aitken R. Methods in Molecular Biology. Humana Press; Totowa, New Jersey: 2002. [Google Scholar]
- 9.Leong SSJ, Chen WN. Preparing recombinant single chain antibodies. Chemical Engineering Science. 2008;63:1401–1414. [Google Scholar]
- 10.Breitling F, Dubel S. Recombinant Antibodies. John Wiley & Sons, Inc; New York: Spektrum Akademischer Verlag; 1999. [Google Scholar]
- 11.Kontermann R, DUbel S. Antibody engineering. Springer-Verlag; Berlin Heidelberg: Spring-Verlag Berlin Heidelberg; New York: 2001. [Google Scholar]
- 12.Pini A, Viti F, Santucci A, Carnemolla B, Zardi L, Neri P, Neri D. Design and Use of a Phage Display Library. HUMAN ANTIBODIES WITH SUBNANOMOLAR AFFINITY AGAINST A MARKER OF ANGIOGENESIS ELUTED FROM A TWO-DIMENSIONAL GEL. J Biol Chem. 1998;273:21769–21776. doi: 10.1074/jbc.273.34.21769. [DOI] [PubMed] [Google Scholar]
- 13.Jiang B, Liu WL, Qu H, Meng L, Song S, Ouyang T, Shou C. A Novel Peptide Isolated from a Phage Display Peptide Library with Trastuzumab Can Mimic Antigen Epitope of HER-2. J Biol Chem. 2005;280:4656–4662. doi: 10.1074/jbc.M411047200. [DOI] [PubMed] [Google Scholar]
- 14.Carmen S, Jermutus L. Concepts in antibody phage display. Brief Funct Genomic Proteomic. 2002;1:189–203. doi: 10.1093/bfgp/1.2.189. [DOI] [PubMed] [Google Scholar]
- 15.Saerens D, Frederix F, Reekmans G, Conrath K, Jans K, Brys L, Huang L, Bosmans E, Maes G, Borghs G, Muyldermans S. Engineering camel single-domain antibodies and immobilization chemistry for human prostate-specific antigen sensing. Anal Chem. 2005;77:7547–7555. doi: 10.1021/ac051092j. [DOI] [PubMed] [Google Scholar]
- 16.De Genst E, Handelberg F, Van Meirhaeghe A, Vynck S, Loris R, Wyns L, Muyldermans S. Chemical basis for the affinity maturation of a camel single domain antibody. J Biol Chem. 2004;279:53593–53601. doi: 10.1074/jbc.M407843200. [DOI] [PubMed] [Google Scholar]
- 17.Streltsov VA, Varghese JN, Carmichael JA, Irving RA, Hudson PJ, Nuttall SD. Structural evidence for evolution of shark Ig new antigen receptor variable domain antibodies from a cell-surface receptor. Proc Natl Acad Sci USA. 2004;101:12444–12449. doi: 10.1073/pnas.0403509101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu JL, Anderson GP, Delehanty JB, Baumann R, Hayhurst A, Goldman ER. Selection of cholera toxin specific IgNAR single-domain antibodies from a naive shark library. Mol Immunol. 2007;44:1775–1783. doi: 10.1016/j.molimm.2006.07.299. [DOI] [PubMed] [Google Scholar]
- 19.Shao CY, Secombes CJ, Porter AJ. Rapid isolation of IgNAR variable single-domain antibody fragments from a shark synthetic library. Mol Immunol. 2007;44:656–665. doi: 10.1016/j.molimm.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 20.Van Emon JM. Immunoassay and other bioanalytical Techniques. CRC press, Taylor & Francis Group; Roca Raton: 2007. [Google Scholar]
- 21.Ohiro Y, Ueda H, Shibata N, Nagamune T. Enhanced fluorescence resonance energy transfer immunoassay with improved sensitivity based on the Fab′-based immunoconjugates. Anal Biochem. 2007;360:266–272. doi: 10.1016/j.ab.2006.10.025. [DOI] [PubMed] [Google Scholar]
- 22.Townsend S, Finlay WJJ, Hearty S, O’Kennedy R. Optimizing recombinant antibody function in SPR immunosensing - The influence of antibody structural format and chip surface chemistry on assay sensitivity. Biosens Bioelectron. 2006;22:268–274. doi: 10.1016/j.bios.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 23.Shen Z, Yan H, Parl FF, Mernaugh RL, Zeng X. Recombinant Antibody Piezoimmunosensors for the Detection of Cytochrome P450 1B1. Anal Chem. 2007;79:1283–1289. doi: 10.1021/ac061211a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shen Z, Yan H, Zhang Y, Mernaugh RL, Zeng X. Engineering Peptide Linkers for scFv Immunosensors. Anal Chem. 2008;80:1910–1917. doi: 10.1021/ac7018624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Capobianco JA, Shih WY, Yuan QA, Adams GP, Shih WH. Label-free, all-electrical, in situ human epidermal growth receptor 2 detection. Review of Scientific Instruments. 2008:79. doi: 10.1063/1.2949831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stich N, Gandhum A, Matyushin V, Raats J, Mayer C, Alguel Y, Schalkhammer T. Phage display antibody-based proteomic device using resonance-enhanced detection. J Nanosci Nanotechnol. 2002;2:375–381. doi: 10.1166/jnn.2002.111. [DOI] [PubMed] [Google Scholar]
- 27.Emanuel PA, Dang J, Gebhardt JS, Aldrich J, Garber EAE, Kulaga H, Stopa P, Valdes JJ, Dion-Schultz A. Recombinant antibodies: a new reagent for biological agent detection. Biosens Bioelectron. 2000;14:751–759. doi: 10.1016/s0956-5663(99)00058-5. [DOI] [PubMed] [Google Scholar]
- 28.Ferreira GNM, Encarnação JM, Rosa L, Rodrigues R, Breyner R, Barrento S, Pedro L, Aires da Silva F, Gonçalves J. Recombinant single-chain variable fragment and single domain antibody piezoimmunosensors for detection of HIV1 virion infectivity factor. Biosens Bioelectron. 2007;23:384–392. doi: 10.1016/j.bios.2007.04.022. [DOI] [PubMed] [Google Scholar]
- 29.Liu Y, Liu Y, Mernaugh RL, Zeng XQ. Single chain fragment variable recombinant antibody functionalized gold nanoparticles for a highly sensitive colorimetric immunoassay. Biosens Bioelectron. 2009;24:2853–2857. doi: 10.1016/j.bios.2009.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anderson GP, Bernstein RD, Swain MD, Zabetakis D, Goldman ER. Binding Kinetics of Antiricin Single Domain Antibodies and Improved Detection Using a B Chain Specific Binder. Anal Chem. 2010;82:7202–7207. doi: 10.1021/ac100961x. [DOI] [PubMed] [Google Scholar]
- 31.Mechaly A, Zahavy E, Fisher A. Development and implementation of a single-chain Fv antibody for specific detection of Bacillus anthracis spores. Appl Environ Microbiol. 2008;74:818–822. doi: 10.1128/AEM.01244-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zdobnova TA, Dorofeev SG, Tananaev PN, Vasiliev RB, Balandin TG, Edelweiss EF, Stremovskiy OA, Balalaeva IV, Turchin IV, Lebedenko EN, Zlomanov VP, Deyev SM. Fluorescent immunolabeling of cancer cells by quantum dots and antibody scFv fragment. J Biomed Opt. 2009;14:021004. doi: 10.1117/1.3122775. [DOI] [PubMed] [Google Scholar]
- 33.Lu RM, Chang YL, Chen MS, Wu HC. Single chain anti-c-Met antibody conjugated nanoparticles for in vivo tumor-targeted imaging and drug delivery. Biomaterials. 2010;32:3265–3274. doi: 10.1016/j.biomaterials.2010.12.061. [DOI] [PubMed] [Google Scholar]
- 34.Mackenzie CR, Clark ID, Evans SV, Hill IE, Macmanus JP, Dubuc G, Bundle DR, Narang SA, Young NM, Szabo AG. Bifunctional Fusion Proteins Consisting of a Single-Chain Antibody and an Engineered Lanthanide-Binding. Immunotechnology. 1995;1:139–150. doi: 10.1016/1380-2933(95)00015-1. [DOI] [PubMed] [Google Scholar]
- 35.Singh R, Samant U, Hyland S, Chaudhari PR, Wels WS, Bandyopadhyay D. Target-specific cytotoxic activity of recombinant immunotoxin scFv(MUC1)-ETA on breast carcinoma cells and primary breast tumors. Mol Cancer Ther. 2007;6:562–569. doi: 10.1158/1535-7163.MCT-06-0604. [DOI] [PubMed] [Google Scholar]
- 36.Zhang WY, Yip TC, Kwok CS. Rapid purification of a new humanized single-chain Fv antibody/human interleukin-2 fusion protein reactive against HER2 receptor. Acta Biochim Biophys Sinica. 2004;36:707–712. doi: 10.1093/abbs/36.10.707. [DOI] [PubMed] [Google Scholar]
- 37.Wang JQ, Ensor CM, Dubuc GJ, Narang SA, Daunert S. Genetically fused single-chain anti-Salmonella antibody with aequorin: a bioluminescence immunoassay for a Salmonella antigen. Anal Chim Acta. 2001;435:255–263. [Google Scholar]
- 38.Mousli M, Goyffon M, Billiald P. Production and characterization of a bivalent single chain Fv alkaline phosphatase conjugate specific for the hemocyanin of the scorpion Androctonus australis. Biochim Biophys Acta-Gen Subj. 1998;1425:348–360. doi: 10.1016/s0304-4165(98)00090-7. [DOI] [PubMed] [Google Scholar]
- 39.Hu WG, Thompson HG, Alvi AZ, Nagata LP, Suresh MR, Fulton RE. Development of immunofiltration assay by light addressable potentiometric sensor with genetically biotinylated recombinant antibody for rapid identification of Venezuelan equine encephalitis virus. J Immunol Methods. 2004;289:27–35. doi: 10.1016/j.jim.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 40.Le HQA, Sauriat-Dorizon H, Korri-Youssoufi H. Investigation of SPR and electrochemical detection of antigen with polypyrrole functionalized by biotinylated single-chain antibody: A review. Anal Chim Acta. 2010;674:1–8. doi: 10.1016/j.aca.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 41.Horacek J, Garrett SD, Skladal P, Morgan MRA. Characterization of the interactions between immobilized parathion and the corresponding recombinant scFv antibody using a piezoelectric biosensor. Food Agric Immunol. 1998;10:363–374. [Google Scholar]
- 42.Ferreira GNM, Encarnacao JM, Rosa L, Rodrigues R, Breyner R, Barrento S, Pedro L, da Silva FA, Goncalves J. Recombinant single-chain variable fragment and single domain antibody piezoimmunosensors for detection of HIV1 virion infectivity factor. Biosens Bioelectron. 2007;23:384–392. doi: 10.1016/j.bios.2007.04.022. [DOI] [PubMed] [Google Scholar]
- 43.Nanduri V, Bhunia AK, Tu SI, Paoli GC, Brewster JD. SPR biosensor for the detection of L-monocytogenes using phage-displayed antibody. Biosens Bioelectron. 2007;23:248–252. doi: 10.1016/j.bios.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 44.Dunne L, Daly S. Development of ELISA and biacore-based immunoassays for the detection of aflatoxin B1 using a genetically engineered dimeric SCFV. Abstr Pap Am Chem Soc. 2004;228:U60–U60. [Google Scholar]
- 45.Dillon PP, Manning BM, Daly SJ, Killard AJ, O’Kennedy R. Production of a recombinant anti-morphine-3-glucuronide single-chain variable fragment (scFv) antibody for the development of a “real-time” biosensor-based immunoassay. J Immunol Methods. 2003;276:151–161. doi: 10.1016/s0022-1759(03)00099-1. [DOI] [PubMed] [Google Scholar]
- 46.Cortez-Retamozo V, Lauwereys M, Gh GH, Gobert M, Conrath K, Muyldermans S, De Baetselier P, Revets H. Efficient tumor targeting by single-domain antibody fragments of camels. Int J Cancer. 2002;98:456–462. doi: 10.1002/ijc.10212. [DOI] [PubMed] [Google Scholar]
- 47.Desmyter A, Decanniere K, Muyldermans S, Wyns L. Antigen specificity and high affinity binding provided by one single loop of a camel single-domain antibody. J Biol Chem. 2001;276:26285–26290. doi: 10.1074/jbc.M102107200. [DOI] [PubMed] [Google Scholar]
- 48.Ghahroudi MA, Desmyter A, Wyns L, Hamers R, Muyldermans S. Selection and identification of single domain antibody fragments from camel heavy-chain antibodies. FEBS Lett. 1997;414:521–526. doi: 10.1016/s0014-5793(97)01062-4. [DOI] [PubMed] [Google Scholar]
- 49.Ladenson RC, Crimmins DL, Landt Y, Ladenson JH. Isolation and characterization of a thermally stable recombinant anti-caffeine heavy-chain antibody fragment. Anal Chem. 2006;78:4501–4508. doi: 10.1021/ac058044j. [DOI] [PubMed] [Google Scholar]
- 50.Liu XJ, Lin H, Tang Q, Li C, Yang ST, Wang ZC, Wang CJ, He Q, Cao B, Feng ZQ, Guan XH, Zhu J. Characterization of a Human Antibody Fragment Fab and Its Calcium Phosphate Nanoparticles that Inhibit Rabies Virus Infection with Vaccine. Plos One. 2011:6. doi: 10.1371/journal.pone.0019848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lowe J, Maia M, Wakshull E, Siguenza P, Liu P, Lakhani S, Rusit J, Elliott R, Quarmby V. Development of a novel homogenous electrochemiluminescence assay for quantitation of ranibizumab in human serum. J Pharm Biomed Anal. 2010;52:680–686. doi: 10.1016/j.jpba.2010.01.048. [DOI] [PubMed] [Google Scholar]
- 52.Ito K, Nishimura W, Maeda M, Gomi K, Inouye S, Arakawa H. Highly sensitive and rapid tandem bioluminescent immunoassay using aequorin labeled Fab fragment and biotinylated firefly luciferase. Anal Chim Acta. 2007;588:245–251. doi: 10.1016/j.aca.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 53.Romanazzo D, Ricci F, Volpe G, Elliott CT, Vesco S, Kroeger K, Moscone D, Stroka J, Van Egmond H, Vehniainen M, Palleschi G. Development of a recombinant Fab-fragment based electrochemical immunosensor for deoxynivalenol detection in food samples. Biosens Bioelectron. 2010;25:2615–2621. doi: 10.1016/j.bios.2010.04.029. [DOI] [PubMed] [Google Scholar]
- 54.Ionescu RE, Gondran C, Bouffier L, Jaffrezic-Renault N, Martelet C, Cosnier S. Label-free impedimetric immunosensor for sensitive detection of atrazine. Electrochim Acta. 2010;55:6228–6232. [Google Scholar]
- 55.Lu HH, Kreuzer MP, Takkinen K, Guilbault GG. A recombinant Fab fragment-based electrochemical immunosensor for the determination of testosterone in bovine urine. Biosens Bioelectron. 2007;22:1756–1763. doi: 10.1016/j.bios.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 56.Helali S, Martelet C, Abdelghani A, Maaref MA, Jaffrezic-Renault N. A disposable immunomagnetic electrochemical sensor based on functionalised magnetic beads on gold surface for the detection of atrazine. Electrochim Acta. 2006;51:5182–5186. [Google Scholar]
- 57.Halamek J, Makower A, Skladal P, Scheller FW. Highly sensitive detection of cocaine using a piezoelectric immunosensor. Biosens Bioelectron. 2002;17:1045–1050. doi: 10.1016/s0956-5663(02)00098-2. [DOI] [PubMed] [Google Scholar]
- 58.Harris RD, Luff BJ, Wilkinson JS, Piehler J, Brecht A, Gauglitz G, Abuknesha RA. Integrated optical surface plasmon resonance immunoprobe for simazine detection. Biosens Bioelectron. 1999;14:377–386. doi: 10.1016/s0956-5663(99)00014-7. [DOI] [PubMed] [Google Scholar]
- 59.Stocklein WFM, Warsinke A, Micheel B, Kempter G, Hohne W, Scheller FW. Diphenylurea hapten sensing with a monoclonal antibody and its Fab fragment: Kinetic and thermodynamic investigations. Anal Chim Acta. 1998;362:101–111. [Google Scholar]
- 60.Shen Z, Stryker GA, Mernaugh RL, Yu L, Yan HP, Zeng X. Single-chain fragment variable antibody piezoimmunosensors. Anal Chem. 2005;77:797–805. doi: 10.1021/ac048655w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shen Z, Mernaugh RL, Yan NP, Yu L, Zhang Y, Zeng X. Engineered recombinant single-chain fragment variable antibody for immunosensors. Anal Chem. 2005;77:6834–6842. doi: 10.1021/ac0507690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shang Y, Singh R, Mernaugh AR, Chisti MM, Zeng X. Immobilization of a Human Epidermal Growth Factor Receptor 2 Mimotope-Derived Synthetic Peptide on Au and Its Potential Application for Detection of Herceptin in Human Serum by Quartz Crystal Microbalance. Anal Chem. 2011;83:8928–8936. doi: 10.1021/ac201430p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vanemon JM, Lopezavila V. Immunochemical Methods - for Environmental-Analysis. Anal Chem. 1992;64:A79–A88. [Google Scholar]
- 64.Walters RR. Affinity-Chromatography. Anal Chem. 1985;57:1102A–1114A. doi: 10.1021/ac00288a001. [DOI] [PubMed] [Google Scholar]
- 65.Matarraz SMS, Gonzalez-Gonzalez M, Jara M, Orfao A, Fuentes M. New technologies in cancer. Protein microarrays for biomarker discovery. Clin Transl Oncol. 2011;13:156–161. doi: 10.1007/s12094-011-0635-8. [DOI] [PubMed] [Google Scholar]
- 66.Borrebaeck CAK, Ohlin M. Antibody evolution beyond Nature. Nat Biotechnol. 2002;20:1189–1190. doi: 10.1038/nbt1202-1189. [DOI] [PubMed] [Google Scholar]
- 67.Zhu XX, Kriegel AM, Boustany CA, Blake DA. Single-Chain Variable Fragment (scFv) Antibodies Optimized for Environmental Analysis of Uranium. Anal Chem. 2010;83:3717–3724. doi: 10.1021/ac200159x. [DOI] [PubMed] [Google Scholar]
- 68.Deshpande SS. Enzyme Immunoassays From Concept to Product Development. Chapman & Hall; New York: 1996. [Google Scholar]
- 69.Legendre D, Soumillion P, Fastrez J. Engineering a regulatable enzyme for homogeneous immunoassays. Nat Biotechnol. 1999;17:67–72. doi: 10.1038/5243. [DOI] [PubMed] [Google Scholar]
- 70.Benito A, Feliu JX, Villaverde A. beta-galactosidase enzymatic activity as a molecular probe to detect specific antibodies. J Biol Chem. 1996;271:21251–21256. doi: 10.1074/jbc.271.35.21251. [DOI] [PubMed] [Google Scholar]
- 71.Brennan CA, Christianson K, Lafleur MA, Mandecki W. A Molecular Sensor System Based on Genetically-Engineered Alkaline-Phosphatase. Proc Natl Acad Sci USA. 1995;92:5783–5787. doi: 10.1073/pnas.92.13.5783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Doi N, Yanagawa H. Design of generic biosensors based on green fluorescent proteins with allosteric sites by directed evolution. FEBS Lett. 1999;453:305–307. doi: 10.1016/s0014-5793(99)00732-2. [DOI] [PubMed] [Google Scholar]
- 73.Ramirez E, Mas JM, Carbonell X, Aviles FX, Villaverde A. Detection of molecular interactions by using a new peptide-displaying bacteriophage biosensor. Biochem Biophys Res Commun. 1999;262:801–805. doi: 10.1006/bbrc.1999.1268. [DOI] [PubMed] [Google Scholar]
- 74.Schultz J, Lin Y, Sanderson J, Zuo Y, Stone D, Mallett R, Wilbert S, Axworthy D. A Tetravalent Single-chain Antibody-Streptavidin Fusion Protein for Pretargeted Lymphoma Therapy. Cancer Res. 2000;60:6663–6669. [PubMed] [Google Scholar]
- 75.Cheung N-KV, Modak S, Lin Y, Guo H, Zanzonico P, Chung J, Zuo Y, Sanderson J, Wilbert S, Theodore LJ, Axworthy DB, Larson SM. Single-Chain Fv-Streptavidin Substantially Improved Therapeutic Index in Multistep Targeting Directed at Disialoganglioside GD2. J Nucl Med. 2004;45:867–877. [PubMed] [Google Scholar]
- 76.Shen Z, Cheung N-KV, Zeng X. Single-Chian Fv multimers for label free detection of Disialoganglioside GD2 on cancer cells. (In preparation) [Google Scholar]
- 77.Yan HP, Shen ZH, Mernaugh R, Zeng XQ. Single Chain Fragment Variable Recombinant Antibody as a Template for Fc Sensors. Anal Chem. 2011;83:625–630. doi: 10.1021/ac102087w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Riechmann L, Muyldermans S. Single domain antibodies: comparison of camel VH and camelised human VH domains. J Immunol Methods. 1999;231:25–38. doi: 10.1016/s0022-1759(99)00138-6. [DOI] [PubMed] [Google Scholar]
- 79.Nuttall SD, Irving RA, Hudson PJ. Immunoglobulin VH domains and beyond: design and selection of single-domain binding and targeting reagents. Curr Pharm Biotechnol. 2000;1:253–263. doi: 10.2174/1389201003378906. [DOI] [PubMed] [Google Scholar]